Abstract.
House structure may influence the risk of malaria by affecting mosquito entry and indoor resting. Identification of construction features associated with protective benefits could inform vector control approaches, even in low-transmission settings. We examined the association between house structure and malaria prevalence in a cross-sectional analysis of 2,788 children and adults residing in 866 houses in a low-transmission area of Southern Province, Zambia, over the period 2008–2012. Houses were categorized according to wall (brick/cement block or mud/grass) and roof (metal or grass) material. Malaria was assessed by point-of-care rapid diagnostic test (RDT) for Plasmodium falciparum. We identified 52 RDT-positive individuals residing in 41 houses, indicating an overall prevalence in the sample of 1.9%, ranging from 1.4% to 8.8% among the different house types. Occupants of higher quality houses had reduced odds of P. falciparum malaria compared with those in the lowest quality houses after controlling for bed net use, indoor insecticide spraying, clustering by house, cohabitation with another RDT-positive individual, transmission season, ecologic risk defined as nearest distance to a Strahler-classified third-order stream, education, age, and gender (adjusted odds ratio [OR]: 0.26, 95% confidence interval [CI]: 0.09–0.73, P = 0.01 for houses with brick/cement block walls and metal roof; OR: 0.22, 95% CI: 0.09–0.52, P < 0.01 for houses with brick/cement block walls and grass roof). Housing improvements offer a promising approach to vector control in low-transmission settings that circumvents the threat posed by insecticide resistance, and may confer a protective benefit of similar magnitude to current vector control strategies.
INTRODUCTION
Malaria remains an important cause of morbidity and mortality in endemic regions worldwide, and vector control strategies are vital to control and elimination efforts.1,2 The two predominantly deployed vector control measures are indoor residual spraying (IRS) of insecticides and insecticide-treated bed nets (ITNs).3 The emergence of insecticide resistance and changes in behavior of mosquitoes to avoid contact with insecticides may threaten the efficacy of IRS and ITNs, creating appeal for additional approaches to prevent malaria.4
Malaria is transmitted by female anopheline mosquito vectors that generally prefer to feed in the late evening and night and exhibit endophagic (indoor feeding) behavior, making the house a potentially high-risk transmission environment.5 Housing features that impede mosquito entry and indoor mosquito resting are, therefore, likely to diminish occupants’ risk of malaria.6,7 Indeed, housing improvements such as window and door screening played an important role in malaria control programs during the first half of the twentieth century in North America and Europe before the widespread use of insecticides.8 The first such experiments were conducted over a century ago by Angelo Celli in Italy, who recognized malaria as a disease of poverty and identified poor housing as a modifiable risk factor.9,10 More recently, his results were recapitulated in a small number of trials done in sub-Saharan Africa where malaria remains endemic, showing reduced numbers of indoor anopheline mosquitos and lower prevalence of childhood anemia in houses that received window screening or other entry barriers compared with those that did not.11–14
However, studies in sub-Saharan Africa that examined associations of wall and roof construction with malaria have yielded equivocal results. About half of the studies demonstrated an association, among which wall material appeared to be more influential than roof material.15–38 Results of studies that applied adjusted models to account for age, gender, ITN use, ecologic variables, and socioeconomic indicators were somewhat more conclusive; most demonstrated a significant protective effect of high-quality walls ranging from 24% to 63% reduction in the risk or odds of malaria, and half showed a protective effect of high-quality roofs ranging from 15% to 62% reduction.27–37
Results of a cross-sectional analysis of housing, grouped by wall and roof type, and malaria in a low-transmission area of southern Zambia are presented. Survey data and field observations were analyzed from participants living in various house types to inform potential approaches to housing interventions for vector control against malaria in Zambia and similar low-transmission settings in sub-Saharan Africa. Higher quality housing was hypothesized to correlate with reduced prevalence of malaria compared with lower quality housing.
METHODS
Study site.
The study was conducted in a 1,200 km2 region east of Macha Hospital in Choma District, Southern Province, Zambia. The area lies at an altitude of 1,000 m above sea level and the local biome is mainly Miombo woodland. The rainy season is from November to April, followed by a cool, dry season from April to August and a hot, dry season from August to November.39 The inhabitants are traditional villagers living in homesteads consisting of one or more houses where members of a family or extended family reside. In general, the houses in the study area have doors or other makeshift barriers. Windows, when present, rarely have glass or screens but some windows have curtains. Eaves, a gap between the roof and top edge of the wall, are open in nearly all houses constructed with grass roofs, whereas most houses with metal roofs have closed eaves.
Transmission intensity in the study area is low. During the study period, the entomological inoculation rate was < 1 infective bite per person per season.40 The predominant malaria vector is Anopheles arabiensis.40 Vector control efforts include distribution of ITNs, with little IRS having been carried out in the Macha area. Malaria control efforts include case management with artemisinin-based combination therapy, introduced in Zambia in 2002 and into the study area in 2004.41,42
Study design.
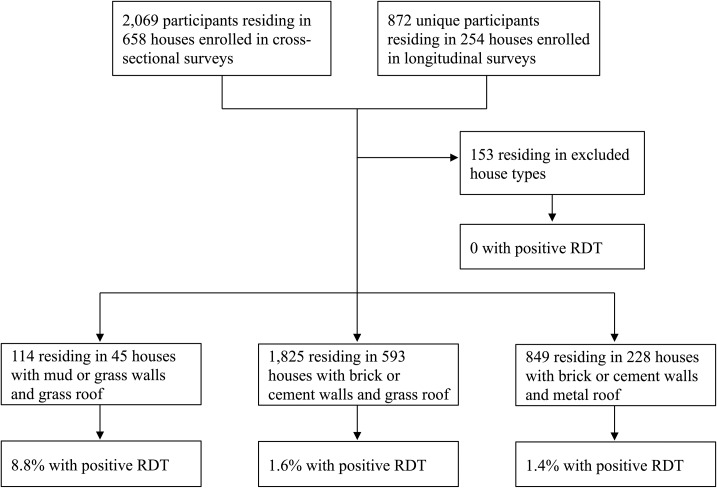
The study was conducted within the context of an epidemiologic survey of malaria using data collected from February 2008 to February 2012.43 Homesteads in the study region were randomly assigned to either a cross-sectional sample or longitudinal cohort. Cross-sectional homesteads were visited once during surveys carried out five times per calendar year to account for temporal differences in transmission. Homesteads in the longitudinal cohort were surveyed every other month five times per calendar year on average. The current analysis is restricted to participants residing in homesteads enrolled in the cross-sectional survey and to the first study visit of participants in homesteads enrolled in the longitudinal cohort (Figure 1). The study was approved by the Tropical Diseases Research Center Ethics Review Committee and the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health. Approvals were also obtained from community leaders.
Figure 1.
Study flow diagram of participant recruitment from cross-sectional and longitudinal surveys in Southern Province, Zambia from 2008 to 2012, showing the proportion of participants with a positive P. falciparum rapid diagnostic test (RDT).
Data collection.
Quickbird™ satellite images acquired from DigitalGlobe Services, Inc. (Denver, CO) were used to construct a sampling frame for the random selection of homesteads. Images were imported into ArcGIS 9.2 (Redlands, CA) and homestead locations were identified, manually enumerated, and randomly selected from the sampling frame for assignment to either the cross-sectional survey or the longitudinal cohort. The field team was provided with maps and Global Positioning System coordinates of the randomly selected homesteads.
For each study visit, permission was obtained from the head of household, individual residents of the homestead were enumerated, and written informed consent was obtained from each adult participant, or from the participant’s parent or guardian for children ≤ 18 years. Surveys were administered to gather individual-level demographic information and ITN use, and house- and homestead-level information including prior application of IRS, educational achievement of the head of the household, and availability of flush toilet and electricity. ITN use was determined by an affirmative answer to the survey item, “Do you sleep under a bed net?” Homestead distance to Strahler-classified third-order water streams (i.e., formed by the convergence of two second-order streams, which in turn are formed by two first-order streams arising de novo), which previous analyses have shown to be predictive of malaria risk in this region,44 was estimated from a digital elevation model. Directly observed house features were recorded, including wall composition (fired or unfired brick, cement block, mud brick, grass, mud, and wooden pole), roof material (iron sheet or corrugated tin, grass, thatch, asbestos sheets), and floor (cement, dirt, vinyl, other). Each participant was assessed for Plasmodium falciparum infection by rapid diagnostic test (RDT) (ICT Diagnostics, Cape Town, South Africa). Individuals who tested positive were offered treatment with artemether–lumefantrine (Coartem®) per World Health Organization and national guidelines.3 Homesteads with multiple contemporaneous RDT-positive individuals were delineated to account for clustering of cases at the homestead level.
Outcome and exposure.
The primary outcome was malaria infection in individual house occupants, defined as a positive RDT result. A house typology scheme was developed according to wall and roof construction materials. House types in which ≤ 3% of the total study population resided were excluded from the analysis due to insufficient statistical power to examine associations between those house types and malaria prevalence (Supplemental Table 1). Houses were assigned to one of three groups: fired brick or cement block walls with metal roof (high quality), fired brick or cement block walls with grass roof (medium quality), or mud or grass walls with grass roof (low quality).
Statistical analysis.
Statistical comparisons of baseline characteristics across house types and malaria prevalence across seasons were done using one-way analysis of variance or χ2 tests. Generalized estimating equations logistic regression models clustered by house were fitted to the data, adjusted for age, gender, bed net use, prior indoor residual spraying, transmission season, distance to a third-order stream, education level of the household head, and presence of other individuals in the homestead with a contemporaneously positive RDT. Collinearity was determined by evaluation of the variance inflation factor, with values > 10 interpreted as evidence of collinearity. Statistical analyses were conducted using Stata 14.0 (StataCorp, College Station, TX).
RESULTS
Study participants.
The study sample consisted of 2,788 participants residing in 866 houses among 488 homesteads. Occupants of mud and grass houses were generally younger in age and their houses were less likely to have received IRS, have electricity, or have a head of household with greater than sixth grade education compared with residents of brick or cement block houses (Table 1, Figure 2). ITN use among those in low-quality houses was less common, although ITN use was not significantly associated with malaria in our sample (adjusted odds ratio [OR]: 0.60, 95% confidence interval [CI]: 0.30–1.20, P = 0.15). Higher quality houses had a slightly smaller proportion of male occupants compared with the lowest quality houses. Distance to third-order streams was similar among the different house types. IRS coverage was low, with 6.8% of high-, 2.6% of medium-, and none of the low-quality houses reporting ever having their house sprayed.
Table 1.
Sociodemographic and household characteristics of the study sample
| House type | ||||
|---|---|---|---|---|
| Brick or cement walls and metal roof | Brick or cement walls and grass roof | Mud or grass walls and grass roof | ||
| Characteristic | n = 228 | n = 593 | n = 45 | P value |
| No. participants (%) | 849 (30.5) | 1,825 (65.5) | 114 (4.1) | – |
| Positive RDT, n (%) | 12 (1.4) | 30 (1.6) | 10 (8.8) | < 0.01* |
| Age, years, mean (SD) | 23.0 (21.5) | 21.0 (19.5) | 17.0 (14.1) | < 0.01* |
| Children ≤ 5 years old, n (%) | 153 (18.0) | 392 (21.5) | 26 (22.8) | 0.10* |
| Male gender, n (%) | 375 (44.2) | 899 (49.3) | 56 (49.1) | 0.05* |
| ITN use, n (%)† | 255 (30.0) | 622 (34.0) | 30 (26.3) | 0.04* |
| Transmission season, n (%) | < 0.01‡ | |||
| February 2008 to July 2008 | 22 (11.3) | 161 (82.6) | 12 (6.2) | – |
| August 2008 to July 2009 | 166 (27.4) | 393 (65.0) | 46 (7.6) | – |
| August 2009 to Jul 2010 | 267 (31.6) | 548 (64.9) | 29 (3.4) | – |
| August 2010 to July 2011 | 254 (32.4) | 512 (65.3) | 18 (2.3) | – |
| August 2011 to February 2012 | 140 (38.9) | 211 (58.6) | 9 (2.5) | – |
| Head of household with > sixth grade education, n (%) | 582 (68.6) | 1,221 (66.9) | 65 (57.0) | 0.05* |
| Distance to category 3 stream, meters, mean (SD) | 4,595 (2,550) | 4,560 (2,270) | 4,960 (2,100) | 0.20* |
| Floor type, n (%) | < 0.01‡ | |||
| Cement | 444 (52.3) | 159 (8.7) | 0 (0.0) | – |
| Dirt | 405 (47.7) | 1,665 (91.3) | 114 (100.0) | – |
| House with prior IRS, n (%) | 58 (6.8) | 47 (2.6) | 0 (0.0) | < 0.01* |
| House with electricity, n (%) | 32 (3.8) | 1 (0.1) | 0 (0.0) | < 0.01* |
| House with flush toilet, n (%) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0.32* |
IRS = indoor residual spraying; ITN = insecticide-treated bed net; RDT = rapid diagnostic test for P. falciparum; SD = standard deviation. The number below each house type refers to the number of houses among the 488 homesteads within the southern Zambia study site, surveyed between 2008 and 2012.
P value was computed by one-way analysis of variance.
Determined by affirmative answer to the survey item, “Do you sleep under a bed net?”
P value was computed by χ2 test.
Figure 2.
Representative photographs of house types in the study area.
House construction.
Most participants (65%) resided in houses constructed of brick or cement block walls with grass roofs. Thirty percent lived in brick or cement block houses with metal roofs, and 4% lived in houses of mud or grass walls and grass roofs. Nearly all (96%) houses lacked electricity and plumbing. All of the low-quality houses and almost all (91%) of the medium-quality houses had dirt floors, compared with 48% of high-quality houses. Over the study period 2008–2012, the proportion of participants residing in high-quality houses increased from 11% to 39%, and the percentage of those living in low-quality houses decreased from 6% to 2%.
Malaria prevalence.
A total of 52 RDT-positive individuals (1.9% of the sample) were identified among 41 of the 866 houses in 36 of the 488 homesteads. RDT positivity ranged from 1.4% among participants residing in high-quality houses to 8.8% among those in low-quality houses. Malaria prevalence declined significantly throughout the study period from 7% in 2008 to 4% in 2009, and < 1% each subsequent transmission season from 2010 to 2012 (P < 0.001).
Seven of the 36 homesteads had multiple RDT-positive residents: one homestead had six positive individuals, another homestead had five, two homesteads had three, and three homesteads had two. In the single homestead with six RDT-positive participants, five of the six resided in the same mud-and-grass house. Within the other homesteads, 13 of the 18 houses were medium quality (mud or grass walls and metal roof), and the remaining five houses were high quality (brick or cement block walls and metal roof).
Association between housing quality and malaria prevalence.
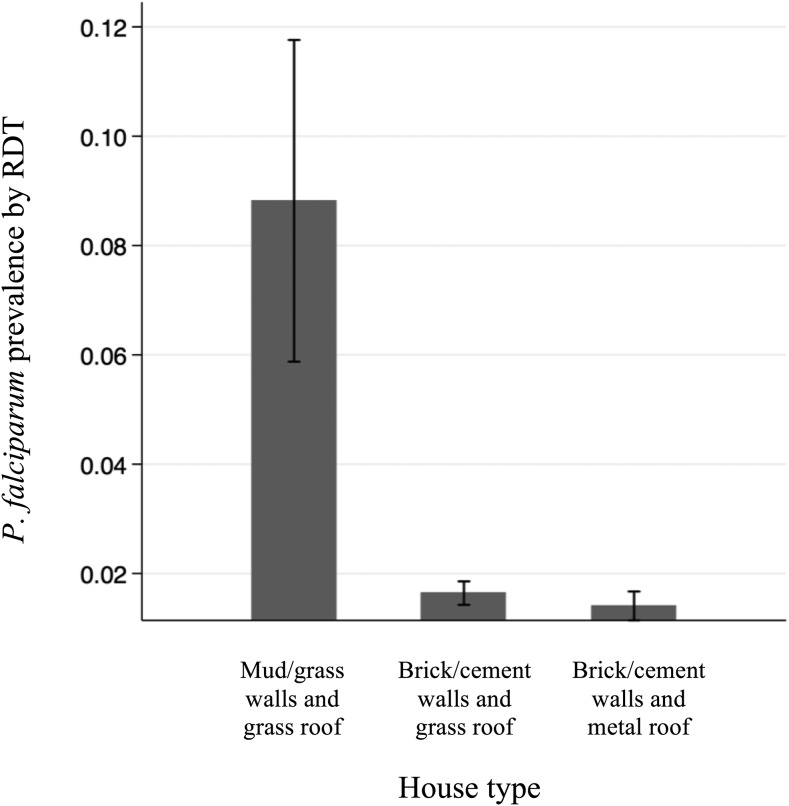
Compared with low-quality houses constructed of mud or grass walls with grass roofs, residing in a medium- or high-quality house was associated with significantly reduced odds of malaria (OR: 0.26, 95% CI: 0.09–0.73, P = 0.01 for houses with brick/cement block walls with metal roofs; OR: 0.22, 95% CI: 0.09–0.52, P < 0.01 for houses with brick/cement block walls with grass roofs) (Table 2, Figure 3).
Table 2.
Adjusted associations between house construction and malaria prevalence by rapid diagnostic test in the southern Zambia study site from 2008 to 2012
| Wall material | Roof material | No. of participants (% total) | Malaria prevalence, % (n) | Adjusted OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Fired brick/cement | Iron sheet/corrugated tin | 849 (30) | 1.4 (12) | 0.26 | 0.09–0.73 | 0.01 |
| Fired brick/cement | Grass | 1,825 (65) | 1.6 (30) | 0.22 | 0.09–0.52 | < 0.01 |
| Mud/grass | Grass | 114 (4) | 8.8 (10) | REF |
CI = confidence interval; OR = odds ratio. Generalized estimating equations logistic regression model adjusted for transmission season, clustering by house, distance to third-order stream, participant age, participant gender, participant bed net use, prior indoor residual spraying, head of household level of education, and homestead with > 1 contemporaneous case.
Figure 3.
Prevalence of P. falciparum infection in 2,788 participants residing among three different house types in the southern Zambia study site from 2008 to 2012, as determined by rapid diagnostic test (RDT). Error bars represent 95% confidence intervals estimated from the adjusted model.
No difference was observed between houses with the same wall type (brick/cement block) but different roof type (metal or grass), despite the presumed presence of open eaves in houses with grass roofs (OR: 1.2, 95% CI: 0.58–2.61, P = 0.58). There was a paucity of houses with mud or grass walls and metal roofs in the sample (< 1% of the total), precluding statistical testing for effect measure modification between wall and roof type. Adjusted models with wall and roof type as separate variables showed a significant reduction in the odds of malaria prevalence between wall types (OR: 0.22, 95% CI: 0.09–0.52, P < 0.01 for cement/brick versus mud) but not roof types (OR: 1.21, 95% CI: 0.57–2.55, P = 0.62). Floor type was not significantly associated with RDT positivity and displayed collinearity with house type, hence it was omitted from the adjusted model.
DISCUSSION
This observational study of the association between malaria prevalence and house structure showed that in a low-transmission area of Southern Province, Zambia, better housing was associated with reduced odds of P. falciparum infection (defined as a positive RDT). Housing improvements may offer an effective addition to current vector control approaches.
Malaria prevalence among occupants of mud-and-grass houses was 8.8% compared with 1.4–1.6% among those living in houses with brick or cement block walls and metal or grass roofs. Controlling for clustering by house, transmission season, and individual- and house-level variables of age, gender, ITN use, proximity to third-order streams, prior IRS application, level of education, and cohabitation with an RDT-positive person, residing in a house of cement or brick walls and metal or grass roof was associated with a significant reduction in the odds of malaria by approximately 75% compared with residing in a house with mud or grass walls and a grass roof. These results are similar to previous reports of housing and malaria in sub-Saharan Africa, which ranged from 15% to 63% risk or odds reduction for high-quality wall and roof types.29–36 The magnitude of the association appears comparable to IRS, for which a Cochrane review estimated a 74% reduction in parasite prevalence, and ITNs, estimated by a Cochrane review to confer a protective efficacy of 13%.45,46
The observed reduction in odds of malaria infection may be attributable to fewer gaps in houses constructed of brick or cement blocks and absence of eaves in houses with metal roofs, leading to reduced mosquito entry.47–49 Previous studies in similar settings have shown higher quality walls and roofs to be associated with lower indoor mosquito numbers compared with houses with gaps in the walls and grass roofs.15,50 Grass roofs have also been associated with longer mosquito survival compared with other roof types,51 and may promote indoor resting and parasite development in the mosquito, perhaps due to lower temperatures inside houses with grass roofs compared with metal roofs.52 Different house types may encourage or discourage ITN use by promoting or impeding ventilation throughout the house via windows and open eaves,53 although the current analysis showed ITN use to be lowest in houses expected to have greater ventilation (i.e., low-quality houses).
These results suggest that wall construction may have a greater influence on malaria risk than roof construction, although the low number of house types consisting of low-quality walls and high-quality roofs limited the analysis. Some but not all previous studies of housing in sub-Saharan Africa have similarly found a protective effect of brick walls compared with mud walls, but not of metal roofs compared with grass or thatch roofs.15,19,30,35 Two studies showed a trend toward significance, but appeared underpowered to conclusively examine roof type due to low numbers of grass or thatch roofs among the sample (4% and 12% of sampled houses).15,35 Grass roofs on houses in Macha tend to be densely packed and hence may pose an effective barrier to entry, whereas walls made of mud or grass may have gaps permitting mosquito ingress. It may therefore be that gaps in walls associated with mud and grass construction are a greater determinant of mosquito vector entry than grass roofs in the study site.
The strengths of the study include its large sample size, random selection from a satellite-imaged sampling frame, adjustments for several confounders, and generalizability to similar low-transmission areas in sub-Saharan Africa. There are also limitations. This was an observational study, limiting causal inferences between housing and malaria. The study site did not have a sufficient number of houses with the combination of mud or grass walls and metal roof, limiting the ability to isolate the effect of roof type. Interpretation of the model for wall and roof type as distinct variables was limited by collinearity because nearly all houses with high-quality roofs had high-quality walls (Supplemental Table 1). Wealth indicators were measured but the overall level of poverty in the sample precluded additional analyses of household wealth, house structure, and malaria. There were no data on eaves, although houses in the study area with grass roofs typically have open eaves while most houses with metal roofs have closed or partially blocked eaves. Nor were there data on other potentially influential house features such as windows, number of rooms, or ceiling.
At the end of the nineteenth century, the Italian malariologist Angelo Celli recognized malaria as a disease of rural poverty and conducted the first interventional trial against malaria,9 demonstrating the effectiveness of house modifications that reduced mosquito entry. With the advent of chemical insecticides in the first half of the last century, interest in the basic outfitting of houses with screens and structural improvements waned.54 Today, insecticide resistance threatens malaria control and elimination.4 Interventions not reliant on insecticides, such as housing improvements, could aid in sustaining progress toward malaria control and elimination.6,11,13,36 Although governments and aid organizations cannot wholesale raise the socioeconomic status of people living in malarious areas, they can nonetheless opt to direct resources toward combatting malaria in a manner that also elevates standards of living, offering the downstream health, social, and economic benefits accompanying that rise. The findings of this study support housing improvements as a worthwhile consideration for malaria control efforts, particularly in the face of emerging insecticide resistance, and corroborate prior studies’ findings of a protective benefit of comparable magnitude to current vector control strategies.
Supplementary Material
Acknowledgments:
We thank members of the community for their volunteer participation in the survey and the Macha Research Trust field team for conducting the survey, without whom this research would not be possible.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Bhatt S, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organzation, 2015. World Malaria Report. Geneva, Switzerland: WHO Global Malaria Programme. [Google Scholar]
- 3.Reyburn H, 2010. New WHO guidelines for the treatment of malaria. BMJ 340: c2637. [DOI] [PubMed] [Google Scholar]
- 4.Quinones ML, et al. 2015. Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: a challenge for malaria control and elimination. Am J Trop Med Hyg 93: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huho B, et al, 2013. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol 42: 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, Lindsay SW, 2015. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J 14: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, Gething PW, 2017. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med 14: e1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane C, 1931. Housing and Malaria: A Critical Summary of the Literature Dealing with This Subject. Geneva, Switzerland: League of Nations: Health Organisation. [Google Scholar]
- 9.Celli A, 1900. The new prophylaxis against malaria in Lazio. Lancet 156: 1603–1606. [Google Scholar]
- 10.Harrison G, 1978. Mosquitoes, Malaria, and Man: A History of the Hostilities Since 1880. Dutton. [Google Scholar]
- 11.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana WR, Killeen GF, Moore SJ, 2010. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl Trop Dis 4: e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massebo F, Lindtjorn B, 2013. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: a randomized trial. Malar J 12: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby MJ, Ameh D, Bottomley C, Green C, Jawara M, Milligan PJ, Snell PC, Conway DJ, Lindsay SW, 2009. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet 374: 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampango A, Braganca M, Sousa B, Charlwood JD, 2013. Netting barriers to prevent mosquito entry into houses in southern Mozambique: a pilot study. Malar J 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adiamah JH, Koram KA, Thomson MC, Lindsay SW, Todd J, Greenwood BM, 1993. Entomological risk factors for severe malaria in a peri-urban area of The Gambia. Ann Trop Med Parasitol 87: 491–500. [DOI] [PubMed] [Google Scholar]
- 16.Koram KA, Bennett S, Adiamah JH, Greenwood BM, 1995. Socio-economic determinants are not major risk factors for severe malaria in Gambian children. Trans R Soc Trop Med Hyg 89: 151–154. [DOI] [PubMed] [Google Scholar]
- 17.Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, Muchiri E, Magnussen P, Cox J, 2004. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health 9: 757–766. [DOI] [PubMed] [Google Scholar]
- 18.Nkuo-Akenji T, Ntonifor NN, Ndukum MB, Abongwa EL, Nkwescheu A, Anong DN, Songmbe M, Boyo MG, Ndamukong KN, Titanji VP, 2006. Environmental factors affecting malaria parasite prevalence in rural Bolifamba, South West Cameroon. Afr J Health Sci 13: 40–46. [DOI] [PubMed] [Google Scholar]
- 19.Somi MF, Butler JR, Vahid F, Njau J, Kachur SP, Abdulla S, 2007. Is there evidence for dual causation between malaria and socioeconomic status? Findings from rural Tanzania. Am J Trop Med Hyg 77: 1020–1027. [PubMed] [Google Scholar]
- 20.Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, John CC, Wilson ML, 2009. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study. Trop Med Int Health 14: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siri JG, Wilson ML, Murray S, Rosen DH, Vulule JM, Slutsker L, Lindblade KA, 2010. Significance of travel to rural areas as a risk factor for malarial anemia in an urban setting. Am J Trop Med Hyg 82: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Louis VR, Sie A, Sauerborn R, 2010. Household risk factors for clinical malaria in a semi-urban area of Burkina Faso: a case-control study. Trans R Soc Trop Med Hyg 104: 61–65. [DOI] [PubMed] [Google Scholar]
- 23.Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ, 2011. Malaria risk factors in north-east Tanzania. Malar J 10: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, Berhane Y, Keating J, 2013. Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J 12: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magalhaes RJ, Langa A, Sousa-Figueiredo JC, Clements AC, Nery SV, 2012. Finding malaria hot-spots in northern Angola: the role of individual, household and environmental factors within a meso-endemic area. Malar J 11: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townes LR, Mwandama D, Mathanga DP, Wilson ML, 2013. Elevated dry-season malaria prevalence associated with fine-scale spatial patterns of environmental risk: a case-control study of children in rural Malawi. Malar J 12: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilaye T, Deressa W, 2007. Prevalence of urban malaria and associated factors in Gondar Town, Northwest Ethiopia. Ethiop Med J 45: 151–158. [PubMed] [Google Scholar]
- 28.Ong’echa JM, et al. 2006. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg 74: 376–385. [PubMed] [Google Scholar]
- 29.Sintasath DM, Ghebremeskel T, Lynch M, Kleinau E, Bretas G, Shililu J, Brantly E, Graves PM, Beier JC, 2005. Malaria prevalence and associated risk factors in Eritrea. Am J Trop Med Hyg 72: 682–687. [PubMed] [Google Scholar]
- 30.Bousema T, et al. 2010. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis 201: 1764–1774. [DOI] [PubMed] [Google Scholar]
- 31.Ouma P, van Eijk AM, Hamel MJ, Parise M, Ayisi JG, Otieno K, Kager PA, Slutsker L, 2007. Malaria and anaemia among pregnant women at first antenatal clinic visit in Kisumu, western Kenya. Trop Med Int Health 12: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 32.Mmbando BP, Kamugisha ML, Lusingu JP, Francis F, Ishengoma DS, Theander TG, Lemnge MM, Scheike TH, 2011. Spatial variation and socio-economic determinants of Plasmodium falciparum infection in northeastern Tanzania. Malar J 10: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Y, Hoshen M, Louis V, Seraphin S, Traore I, Sauerborn R, 2006. Housing conditions and Plasmodium falciparum infection: protective effect of iron-sheet roofed houses. Malar J 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temu EA, Coleman M, Abilio AP, Kleinschmidt I, 2012. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS One 7: e31409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rulisa S, et al. 2013. Malaria prevalence, spatial clustering and risk factors in a low endemic area of eastern Rwanda: a cross sectional study. PLoS One 8: e69443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanzirah H, et al. 2015. Mind the gap: house structure and the risk of malaria in Uganda. PLoS One 10: e0117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P, 2000. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg 94: 17–21. [DOI] [PubMed] [Google Scholar]
- 38.Snyman K, et al. 2015. Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg 92: 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutcliffe CG, Kobayashi T, Hamapumbu H, Shields T, Mharakurwa S, Thuma PE, Louis TA, Glass G, Moss WJ, 2012. Reduced risk of malaria parasitemia following household screening and treatment: a cross-sectional and longitudinal cohort study. PLoS One 7: e31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss WJ, et al. 2015. Malaria epidemiology and control within the International Centers of Excellence for Malaria Research. Am J Trop Med Hyg 93: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chanda E, Kamuliwo M, Steketee RW, Macdonald MB, Babaniyi O, Mukonka VM, 2013. An overview of the malaria control programme in Zambia. ISRN Prev Med 2013: 495037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chizema-Kawesha E, Miller JM, Steketee RW, Mukonka VM, Mukuka C, Mohamed AD, Miti SK, Campbell CC, 2010. Scaling up malaria control in Zambia: progress and impact 2005–2008. Am J Trop Med Hyg 83: 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss WJ, Norris DE, Mharakurwa S, Scott A, Mulenga M, Mason PR, Chipeta J, Thuma PE, Southern Africa IT, 2012. Challenges and prospects for malaria elimination in the southern Africa region. Acta Trop 121: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss WJ, Hamapumbu H, Kobayashi T, Shields T, Kamanga A, Clennon J, Mharakurwa S, Thuma PE, Glass G, 2011. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: a cross-sectional and longitudinal community survey. Malar J 10: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lengeler C, 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev CD000363. [DOI] [PubMed] [Google Scholar]
- 46.Pluess B, Tanser FC, Lengeler C, Sharp BL, 2010. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev CD006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirby MJ, West P, Green C, Jasseh M, Lindsay SW, 2008. Risk factors for house-entry by culicine mosquitoes in a rural town and satellite villages in The Gambia. Parasit Vectors 1: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsay SW, Snow RW, 1988. The trouble with eaves; house entry by vectors of malaria. Trans R Soc Trop Med Hyg 82: 645–646. [DOI] [PubMed] [Google Scholar]
- 49.Njie M, Dilger E, Lindsay SW, Kirby MJ, 2009. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol 46: 505–510. [DOI] [PubMed] [Google Scholar]
- 50.Animut A, Balkew M, Lindtjorn B, 2013. Impact of housing condition on indoor-biting and indoor-resting Anopheles arabiensis density in a highland area, central Ethiopia. Malar J 12: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Hill N, Lines JD, Curtis CF, 1991. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop 49: 97–108. [DOI] [PubMed] [Google Scholar]
- 52.Murdock CC, Sternberg ED, Thomas MB, 2016. Malaria transmission potential could be reduced with current and future climate change. Sci Rep 6: 27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Seidlein L, Ikonomidis K, Bruun R, Jawara M, Pinder M, Knols BG, Knudsen JB, 2012. Airflow attenuation and bed net utilization: observations from Africa and Asia. Malar J 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindsay SW, Emerson PM, Charlwood JD, 2002. Reducing malaria by mosquito-proofing houses. Trends Parasitol 18: 510–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.