Abstract.
After the report of an outbreak of dengue virus serotype 2 in 2014 in Nampula and Pemba cities, northern Mozambique, a surveillance system was established by the National Institute of Health. A study was performed during 2015–2016 to monitor the trend of the outbreak and confirm the circulating serotype of dengue virus (DENV). After the inclusion of consenting patients who met the case definition, samples from 192 patients were tested for the presence of nonstructural protein 1 antigen, and 60/192 (31%) samples were positive. Further analysis included DENV IgM antibodies, with 39 (20%) IgM positive cases. Reverse transcriptase (RT) PCR was performed for identification of the prevailing DENV serotype; 21/23 tested samples were DENV-2 positive, with DENV-2 present in both affected cities. When sequencing DENV, phenotype Cosmopolitan was identified. The surveillance indicates ongoing spread of DENV-2 in northern Mozambique 2 years after the first report of the outbreak.
Dengue is an expanding mosquito-borne viral disease, with an increasing impact on global health. Over 50% of the population in subtropical and tropical regions are at risk of contracting the disease.1 Dengue virus (DENV) is considered to be the most important arbovirus based on its fast spread combined with its high burden of morbidity and mortality.2
DENV is a positive single-stranded RNA virus belonging to the genus flavivirus and the family Flaviviridae. It has four antigenically related but distinct serotypes DENV-1, DENV-2, DENV-3, and DENV-4.2 DENV diversity is vast, and within each serotype, distinct genotypes can be identified and have been correlated with geographical distribution of the virus. An association between disease severity and genetical differences within the genotype has also been reported.3
The primary vector of the DENV is the Aedes aegypti mosquito, but it is also transmitted by Aedes albopictus. The disease is self-limiting but can sometimes be severe. Clinical symptoms include fever, headache, retro-orbital pain, nausea, vomiting, myalgia, arthralgia, thrombocytopenia, rash, and mucosal bleeding from the gums, nose, and the gastrointestinal system.4 Dengue is endemic in parts of Africa, Asia, and the Americas with favorable conditions for vector multiplication, disease transmission, and spread. High temperature, heavy rainfall, inadequate waste disposal management, unplanned urbanization, high population density, traveling, and global mobility are some of the factors that have contributed to the increasing global spread of DENV.5
In Africa, simultaneous circulation of DENV-1, DENV-2, and DENV-3 has been described in Gabon.6 DENV outbreaks have been reported from several countries in East Africa, the earliest reports dating back as far as 1823.7 The first recorded outbreak of DENV in Mozambique occurred in Pemba between late 1984 and early 1985.8 The circulating serotype in this outbreak was also the first documented case of DENV-3 in Africa. Not until about three decades later, in March 2014, a DENV outbreak was reported for the second time in Mozambique, again in the northern parts of the country.9 The circulating serotype was identified as DENV-2. In 2014, the National Institute of Health in Mozambique established a surveillance system of DENV in the affected cities Nampula and Pemba, with the objective to monitor the trend in DENV prevalence and provide evidence for decision making on interventions for controlling DENV in northern Mozambique.
Nampula and Pemba are the capital cities of Nampula and Cabo Delgado provinces in northern Mozambique, with a population of approximately 470,000 in Nampula and 140,000 inhabitants in Pemba. Both cities are strategic urban centers and melting points of different cultures, with heavy traffic of people from and to different countries and booming commercial activities. The tropical climate is favorable for mosquitoes to breed especially during the rainy season from December to March.
A cross-sectional study on patients with suspected DENV infection was conducted at Nampula Central Hospital (NCH) and Pemba Provincial Hospital (PPH) from January 2015 until March 2016. Both hospitals are tertiary health facilities that offer health care services to a cross section of the society, ranging from high income earners to middle- and low-income earners.
To be eligible for inclusion in this study, patients had to have fever 37.5°C or above (axillary temperature) for a maximum of 7 days duration and of unknown etiology. Cases diagnosed with malaria, cellulitis, otitis media, and pneumonia, which are readily identifiable infections, were excluded. In addition, participants had to be above 5 years of age. A written informed consent was obtained from all patients, or parents of children, included at the emergency unit of NCH and PPH during the study period. For all consenting participants, a questionnaire on clinical and sociodemographic data was filled in by trained health personnel.
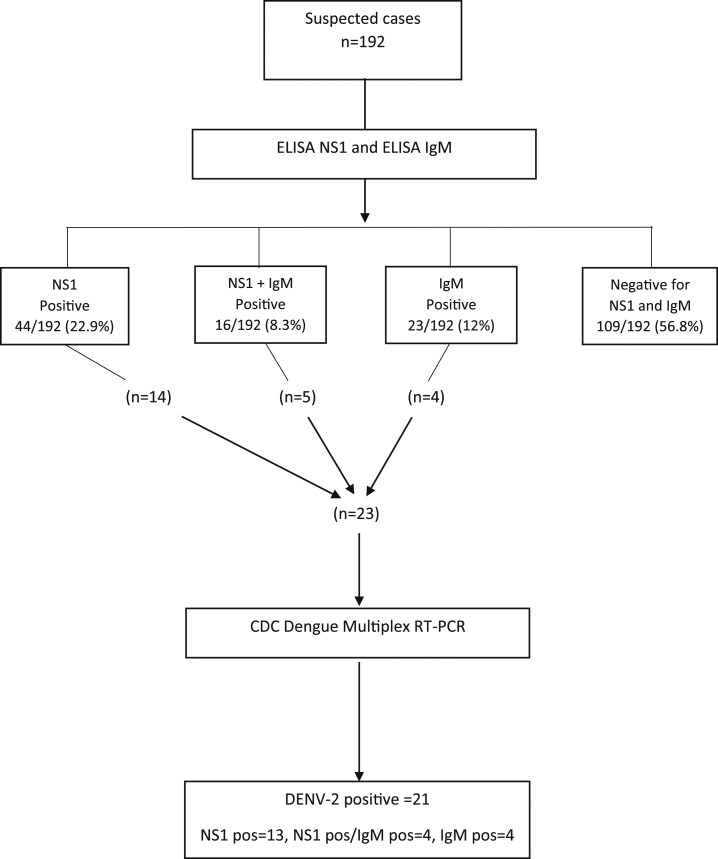
A total of 290 samples were collected from the 290 patients initially included in the study, (152 from Pemba and 138 from Nampula) between January 2015 and March 2016. Based on the availability of rapid tests at the emergency unit of both study sites (PPH and NCH), 192/290 patients were analyzed for nonstructural protein 1 (NS1) antigen, 108 from Pemba and 84 from Nampula. These 192 patients with available NS1 antigen results were then included in the further analysis according to the test algorithm in Figure 1.
Figure 1.
Study test algorithm for 192 suspected cases of dengue virus (DENV) infection in northern Mozambique 2015–2016. Samples were tested by ELISA NS1 and ELISA IgM. In addition, the CDC Dengue Multiplex RT-PCR was performed on 23 samples positive for nonstructural protein 1 (NS1) alone or NS1 combined with IgM, alternatively IgM alone.
Acute sera were examined for the presence of dengue NS1 antigen using the Panbio NS1 ELISA kit, (Standard Diagnostics, Inc, Yongin-si, Gyeonggi-do, South Korea), following the instructions of the manufacturer. Sera were handled within the cold chain and sent to the Virus Isolation Laboratory at the National Institute of Health in Maputo for additional analysis. For detection of dengue-specific IgM antibodies, the ELISA commercial kit of PanBio, was used.
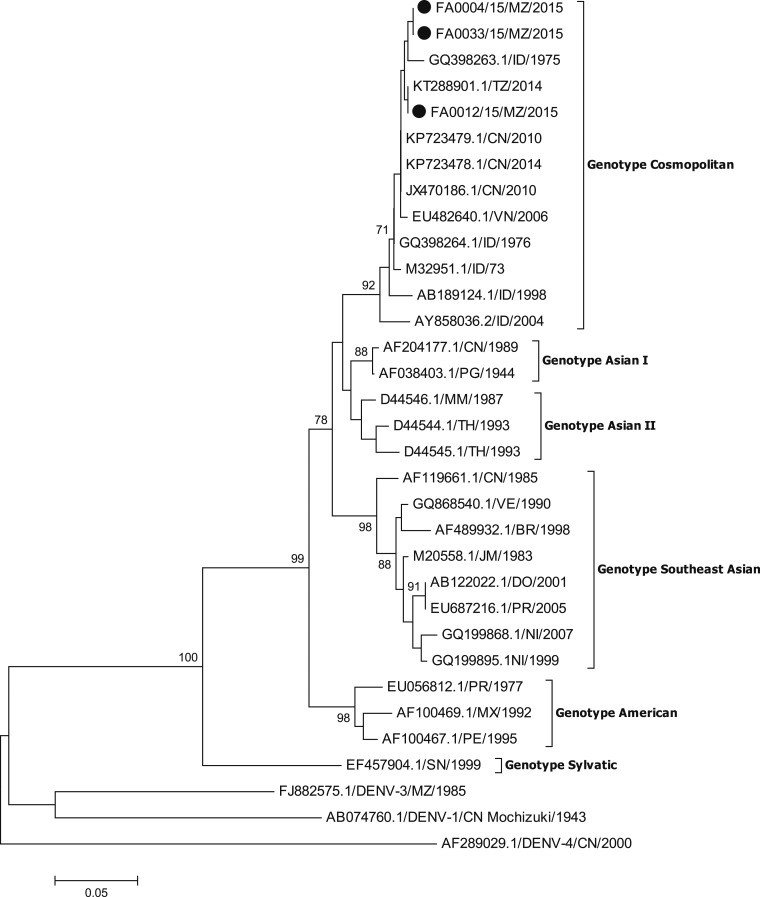
To identify the prevailing dengue virus serotype, PCR was performed on a subset of included samples (Figure 1). The Centers for Disease Control and Prevention (CDC) DENV-1-4 Real-Time RT-PCR Assay was used according to the manufacturer’s instructions for amplification and serotype analysis of DENV.10 For the subsequent DENV-2 sequencing, E/NS1 junction gene containing 240 nucleotides, one pair of primers (4A-4B) was used to amplify fragment of approximately 900 bp as described by Faria.11 Sequences analysis was performed using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.htmL). The identity of sequences was performed using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the alignment using CLUSTAL OMEGA software (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was built considering a bootstrap of 1,000 replications (Figure 2).
Figure 2.
Neighbor-joining phylogenetic tree of DENV-2 circulating in Mozambique during the 2015 DENV outbreak. Isolates described in this study are marked with a black dot. The phylogenetic tree was constructed using the MEGA 6 software (http://www.megasoftware.net/), by the neighbor-joining method and the kimura II parameter model based on the analysis of the best-fit model for each dataset as provided by the software. The phylogeny was inferred based on envelope-nonstructural (E/NS1) junction gene nucleotide sequences (240 positions) from 33 sequences. The nucleotide sequences identified are accessible in GenBank, accession numbers: BanKIt 203333 Seq1- FA0004/15/MZ/2015: KY815103, BanKIt 203333 Seq2- FA0012/15/MZ/2015: KY815104, BanKIt 203333 Seq3- FA0033/15/MZ/2015: KY815105.
Confirmed dengue cases are defined as participants with a positive PCR result; also, detection of NS1 antigen in combination with the finding of IgM antibodies against DENV by enzyme-linked immunosorbent assay in a patient’s sample constitutes a confirmed case.
Laboratory results showed a positive finding for NS1 among 60/192 (31%) NS1 tested samples, with a total of 39/192 (20%) samples positive for DENV IgM antibody. CDC DENV-RT-PCR was performed on 23 of the NS1 and/or IgM positive samples with 21 being PCR positive, all for DENV-2 serotype. A sequence analysis performed for three of the DENV-2 strains showed the genotype Cosmopolitan (Figure 2).
Among the 192 included suspected cases of DENV infection with an NS1 test performed (Figure 1), 33 (17%) were confirmed; 21 based on a positive PCR result and 12 on the combined positive findings of NS1 antigen and DENV IgM. The presence of either NS1 antigen (31 subjects) or DENV IgM (19 subjects) in patients without a positive PCR result were not considered confirmatory. These 50 patients (26%) were thus instead classified as probable cases.
Confirmed positive cases were most frequently found in the age group between 16 and 35 years. Male patients showed a slightly higher positivity as compared with females. Headache, myalgia, and athralgia were the most common symptoms of the patients. There were no cases of hemorrhagic symptoms such as bleeding in the gums. There was no record of death, including patients confirmed positive for DENV (data not shown).
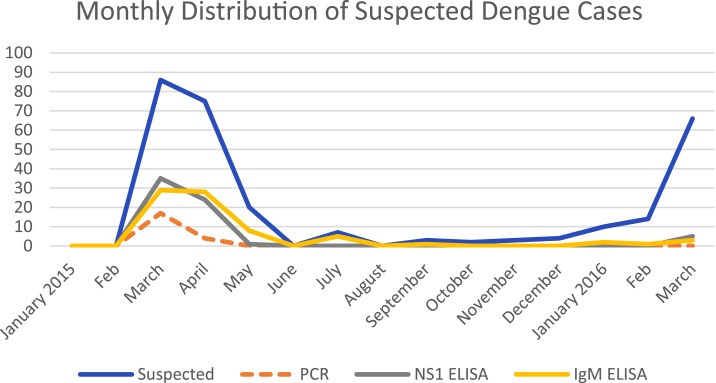
The highest incidence of DENV infection occurred between February and May (Figure 3), a period of heavy rainfall with favorable conditions for breeding, multiplication, and spread of the vector mosquito. The peak of high transmission for DENV was in March, thereafter it gradually diminished until June.
Figure 3.
The monthly distribution of the 290 suspected dengue cases and the resulting positivity in relation to PCR, nonstructural protein 1 (NS1), and IgM. This figure appears in color at www.ajtmh.org.
In the current DENV-2 outbreak in Mozambique, strains belonging to the genotype Cosmopolitan have been identified. In Africa, Cosmopolitan DENV-2 strains have earlier been found in Somalia and Burkina Faso during the 1980s, and more recently in Ghana and Gabon.6 The same genotype was also present in 2014 during a DENV outbreak in Dar es Salaam, Tanzania.12
In conclusion, the ongoing surveillance shows that, still 2 years after the first reports of the outbreak, DENV-2 of the genotype Cosmopolitan is circulating, and DENV-2 has thus become endemic in northern Mozambique. Further spread to other regions in Mozambique are probable, demanding urgent implementation of public health interventions to control the disease. Continued surveillance of DENV infection will be important in monitoring the burden of DENV disease.
This study was approved by the National Bioethical Committee in Mozambique. The permit number is IRB00002657.
Acknowledgments:
Our sincere gratitude goes to the staff at the Provincial Health Directorates in Pemba and Nampula for their unrelenting assistance during the investigation. We are thankful to all the staff and study patients at the health facilities, who have contributed to this investigation. We particularly thank Virgilio Antonio, Sãozinha Agostinho, Siasa Mendes, Inocêncio Chongo, Gabriela Pinto, and Almiro Tivane for their participation in data collection.
REFERENCES
- 1.Guzman MG, et al. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8 (Suppl): S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmaseda A, et al. 2006. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg 74: 449–456. [PubMed] [Google Scholar]
- 3.Nunes PC, et al. 2016. Dengue severity associated with age and a new lineage of dengue virus-type 2 during an outbreak in Rio De Janeiro, Brazil. J Med Virol 88: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization , 2012. Global Strategy for Dengue Prevention and Control 2012–2020 ISBN 978 92 4 150403 4 [Internet]. Geneva, Switzerland: WHO. Available at: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf. Accessed January 30, 2017.
- 5.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS, 2011. Dengue virus infection in Africa. Emerg Infect Dis 17: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caron M, Grard G, Paupy C, Mombo IM, Bikie Bi Nso B, Kassa Kassa FR, Nkoghe D, Leroy EM, 2013. First evidence of simultaneous circulation of three different dengue virus serotypes in Africa. PLoS One 8: e78030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba M, Villinger J, Masiga DK, 2016. Repetitive dengue outbreaks in East Africa: a proposed phased mitigation approach may reduce its impact. Rev Med Virol 26: 183–196. [DOI] [PubMed] [Google Scholar]
- 8.Gubler DJ, Sather GE, Kuno G, Cabral JR, 1986. Dengue 3 virus transmission in Africa. Am J Trop Med Hyg 35: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 9.Massangaie M, et al. 2016. Clinical and epidemiological characterization of the first recognized outbreak of dengue virus-type 2 in Mozambique, 2014. Am J Trop Med Hyg 94: 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Disease Control , 2013. CDC DENV-1-4 Real-Time RT-PCR Assay [Internet] (updated December 4, 2013). Available at: https://www.cdc.gov/dengue/resources/rt-pcr/cdcpackageinsert.pdf. Accessed February 6, 2017.
- 11.Faria NR, Nogueira RM, de Filippis AM, Simoes JB, Nogueira Fde B, da Rocha Queiroz Lima M, dos Santos FB, 2013. Twenty years of DENV-2 activity in Brazil: molecular characterization and phylogeny of strains isolated from 1990 to 2010. PLoS Negl Trop Dis 7: e2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vairo F, et al. 2016. Clinical, virologic, and epidemiologic characteristics of dengue outbreak, Dar es Salaam, Tanzania, 2014. Emerg Infect Dis 22: 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]