Abstract.
Peptide vaccine strategies using Plasmodium-derived antigens have emerged as an attractive approach against malaria. However, relatively few studies have been conducted with malaria-exposed populations from non-African countries. Herein, the seroepidemiological profile against Plasmodium falciparum of naturally exposed individuals from a Brazilian malaria-endemic area against synthetic peptides derived from vaccine candidates circumsporozoite protein (CSP), liver stage antigen-1 (LSA-1), erythrocyte binding antigen-175 (EBA-175), and merozoite surface protein-3 (MSP-3) was investigated. Moreover, human leukocyte antigen (HLA)-DRB1* and HLA-DQB1* were evaluated to characterize genetic modulation of humoral responsiveness to these antigens. The study was performed using blood samples from 187 individuals living in rural malaria-endemic villages situated near Porto Velho, Rondônia State. Specific IgG and IgM antibodies and IgG subclasses were detected by enzyme-linked immunosorbent assay, and HLA-DRB1* and HLA-DQB1* low-resolution typing was performed by PCR-SSP. All four synthetic peptides were broadly recognized by naturally acquired antibodies. Regarding the IgG subclass profile, only CSP induced IgG1 and IgG3 antibodies, which is an important fact given that the acquisition of protective immunity appears to be associated with the cytophilicity of IgG1 and IgG3 antibodies. HLA-DRB1*11 and HLA-DQB1*7 had the lowest odds of responding to EBA-175. Our results showed that CSP, LSA-1, EBA, and MSP-3 are immunogenic in natural conditions of exposure and that anti-EBA antibody responses appear to be modulated by HLA class II antigens.
INTRODUCTION
Malaria remains a major public health problem around the world, affecting approximately 212 million people annually in tropical areas and taking an estimated 429,000 lives, mostly those of children under 5 years of age.1 In high-endemic malaria areas, young children are particularly susceptible, whereas with exposure, older children and adults develop considerable protection from severe malaria and death, although sterile immunity is likely never achieved.2,3 These changes are thought to reflect the parasitological and clinical immunity, collectively referred to as naturally acquired immunity, which generally determines not only the age-specific incidence and the prevalence of infections but also the expression of pathological processes that underlie the clinical manifestations of the infection.
Because experiments demonstrating that adoptive transferred serum from naturally immune individuals protects against clinical malaria or significantly attenuates the severity and disease burden,4,5 it is widely assumed that antibodies play an important role in malarial protective immunity. In this regard, several studies have demonstrated that cytophilic antibodies are particularly critical for immunity to malaria infection.6–17 Therefore, efforts have been made to identify target antigens that induce antibodies in sufficient quantity and, above all, are functionally capable of participating in the development of an antimalarial immunity. Collectively, these data reflect the importance of studies of the humoral immune response to malaria vaccine candidates in individuals naturally exposed to malaria infection. Moreover, this type of evaluation is a crucial point for field trials of potentially protective vaccine candidates.
In this scenario, studies assessing the immune response against Plasmodium falciparum vaccine candidates in naturally exposed individuals from the Brazilian Amazon were conducted by our group.10,18–22 In Brazil, malaria is hypo- to meso-endemic, present throughout the year with clear seasonal fluctuations and is frequently associated with migration movements of nonimmune individuals to areas where malaria is endemic.23 The exposed populations are composed of autochthonous and migrant individuals from the nonendemic regions of Brazil. Asymptomatic and or paucisymptomatic infections by P. falciparum and Plasmodium vivax were detected in epidemiological studies in the states of Rondônia (RO) and Amazonas, which likely indicates a pattern of clinical immunity in both populations.24–28 Seropidemiological studies investigating the type of immune responses elicited in naturally exposed populations to several malaria vaccine candidates in Brazilian populations provide important information on whether immune responses specific to these antigens are generated in natural infections and their immunogenic potential as vaccine candidates.
In the present work, the natural acquired humoral immune response against four synthetic antigens derived from P. falciparum sporozoites, liver and blood stage antigens in malaria-exposed individuals from the Brazilian Amazon was analyzed, and HLA-DRB1* and -DQB1* were used as molecular markers in an attempt to determine the presence of a genetic modulation of the humoral responsiveness to the P. falciparum antigens. The identification of antigens recognized by antibodies of such individuals may help to establish the best targets for a potential multipeptide-based vaccine for future use in this population.
MATERIALS AND METHODS
Study area and subjects.
The study described here was performed using blood samples obtained from individuals from rural malaria-endemic villages situated near Porto Velho, the capital of the state of RO, and a malaria-endemic region in the Brazilian Amazon (63°54′13″W 8°45′43″S). The population of Porto Velho, sampled in this study, is composed both of autochthonous individuals and migrants from several nonendemic areas of Brazil who have lived in the region for 10 years or more.
Serum samples were obtained from 187 malaria exposed (autochthonous and migrant) individuals (77 women and 110 men) with ages ranging from 8 to 74 years (average age 31 ± 16 years). The average time of residence in the malaria-endemic area was 24 ± 16 years. These individuals composed the exposed group. Additionally, serum samples from 109 individuals (61 women and 48 men with an average age of 38 ± 10 years) living in downtown Porto Velho, where malaria transmission does not occur, were included in our study as noninfected individuals. These individuals composed the nonexposed group. This population was also composed of autochthonous and Brazilian migrants inhabiting the region for 26 ± 18 years. All of the individuals from the nonexposed group were negative for malaria parasites as assessed by thick blood films. Most of the individuals (64%) denied prior malaria infection, and 36% reported an average of 1.8 ± 1.4 past episodes of malaria occurring at least 5 years before the collection of the samples. Nonendemic control blood samples from 15 individuals of the laboratory staff (Rio de Janeiro, Brazil) who had neither a history of malaria nor contact with malaria transmission were included in our study as controls. Written informed consent was obtained from all of the individuals before admission to the study. This study was reviewed and approved by the Oswaldo Cruz Foundation Ethical Committee (258/04).
The samples and the survey data were collected 2–4 times during the dry months (June to August), from 1996 to 2007, coinciding with the period of increased malaria transmission in the RO State. The longitudinal study of antibody response allowed us to discriminate more precisely between responders and nonresponders. To evaluate the degree of exposure to malaria, the survey data used were age, time of residence in the endemic area and the number of malaria episodes reported by each individual.
Venous peripheral blood (10 mL) was collected into EDTA tubes for both an antibody analysis and HLA class II typing. The plasma was stored at −20°C, and the pellets, containing peripheral blood cells, were mixed with equal volumes of a cryopreservation solution (0.9% NaCl/4.2% sorbitol/20% glycerol) and were stored in liquid nitrogen until use. Thin and thick blood smears were examined for the identification of the malaria parasite by a technician experienced in malaria diagnosis from the Brazilian Malaria Health Services and from the Laboratory of Malaria Research (Fiocruz), which is the headquarters of the Center for Malaria Research and Training, a reference center for malaria diagnosis in the Extra-Amazonian Region for the Brazilian Ministry of Health. Thick blood smears from all of the subjects were stained with Giemsa, and a total of 200 microscopic fields were examined under a 1,000-fold magnification. Thin blood smears of the positive samples were examined for species identification. The parasite density was determined by counting the parasites in a predetermined number of white blood cells in the thick blood films, and the number of blood parasites per milliliter was calculated.29 To increase the sensitivity of the parasite detection, molecular analyses using specific primers for genus (Plasmodium sp.) and species (P. falciparum and P. vivax) were performed in all of the samples as previously described.30 Donors positive for P. vivax and/or P. falciparum at the time of blood collection were subsequently treated per the chemotherapeutic regimen recommended by the Brazilian Ministry of Health.
Synthetic peptides.
The evaluation of the antibody response was performed using synthetic peptides derived from P. falciparum sporozoite-, liver- and blood-stages antigens. Peptides were synthesized by fluorenylmethoxycarbonyl (F-moc) solid-phase chemistry.31 Analytical chromatography of the peptides demonstrated a purity of > 90%. Peptide sequences were the following:
Circumsporozoite protein.
A long synthetic peptide consisting of 50 repeat sequences of asparagine–alanine–asparagine–proline of the circumsporozoite protein (CSP), which is the predominant surface antigen on the sporozoites (GenBank: AKU89585.1)
Liver stage antigen-1.
A 41-mer peptide containing the epetitive epitope of the pre-erythrocytic stage Liver Stage Antigen-1 (LSA-1). The sequence is LAKEKLQEQQSDLEQERLAKEKLQEQQSDLEQERLAKEKLQ (GenBank: CZT98619.1
Erythrocyte binding antigen.
A 42-mer peptide containing region IV of the erythrocyte binding antigen 175 (EBA-1751089-1130). The sequence is SNNEYKVNEREDERTLTKEYEDIVLKSHMNRESDDGELYDEN (GenBank: 001349207)
Merozoite surface protein-3.
A 27-mer peptide containing the central region of the merozoite surface protein-3 (MSP-3211-237). The sequence is AKEASSYDYILGWEFGGGVPEHKKEEN (GenBank: CZT98611.1)
Enzyme-linked immunosorbent assay.
Microtiter 96-well plates (Maxisorp, NUNC, Denmark) were coated with the antigens at an optimal dilution using phosphate-buffered saline at pH 7.4 (PBS) or a carbonate-bicarbonate buffer at pH 9.6 at 100 µL/well overnight at 4°C (see Table 1). The plates were washed, and the uncoated sites were blocked and then reacted for 1 hour with sera in duplicate that was diluted (1/100) in dilution buffer. The plates were washed, and mouse antihuman IgG or IgM (Sigma, St. Louis, MO) was diluted 1/2,000 in dilution buffer and incubated for 1 hour. To detect specific IgG subclasses plates were incubated with mouse anti-human IgG1, IgG2, IgG3, or IgG4 antibodies peroxidase conjugate (Sigma) diluted 1/1,000 in dilution buffer for 2 hours. After washing, 100 µL of a solution of orthophenylenediamine (Sigma) and H2O2 (Sigma) in citrate-phosphate buffer at pH 5.0 was added to each well, and the plate was incubated for 15–30 minutes at room temperature in the dark, and then 50 µL/well of H2SO4 (Sigma) 2 N was used to stop the reaction. The plates were read at 492 nm in a spectrophotometer (Spectramax 250, Molecular Devices). Sera from the 15 Rio de Janeiro controls were used to establish the normal range for the assay. The cut-off value was determined as the mean optical density (OD) + 3 standard deviations of the Rio controls. To standardize the OD data obtained in the different experiments, an OD index was calculated for each immunoglobulin determination as the ratio of the observed OD/cut-off values. Samples with an OD index > 1.0 were considered positive. The cut off values for CSP, LSA-1, EBA, and MSP-3 were, respectively, 0.094, 0.089, 0.067, and 0.075 for IgG, 0.195, 0.164, 0.101, and 0.131, for IgM, 0.083, 0.086, 0.084, and 0.072 for IgG1, 0.179, 0.151, 0.104, and 0.131 for IgG2, 0.099, 0.105, 0.120, and 0.128, for IgG3 and 0.109, 0.110, 0.123, and 0.18 for IgG4.
Table 1.
Antigens and enzyme-linked immunosorbent assay antibody assays
| Antigen | Coating | Washing | Blocking | Serum | Anti-serum |
|---|---|---|---|---|---|
| (NANP)50 | 1 µg/mL in PBS | 3 times with PBST | 1 hour with 5% (wt/vol) BSA in PBST at 37°C | 1 hour diluted in 2.5% (wt/vol) BSA in PBST at 37°C | Diluted in 2.5% (wt/vol) BSA in PBST at 37°C |
| LSA-1 | 2 µg/mL in PBS | 3 times with PBST | Overnight with 2.5% (wt/vol) powdered-milk in PBS at RT | 1 hour diluted in 1.25% (wt/vol) powdered milk in PBS at RT | Diluted in 1.25% (wt/vol) powdered milk in PBS at RT |
| EBA | 2 µg/mL in carbonate-bicarbonate buffer | 3 times with PBST | 1 hour with 5% (wt/vol) powdered-milk in PBST at 37°C | 1 hour diluted in 1.25% (wt/vol) powdered milk in PBST at 37°C | Diluted in 1.25% (wt/vol) powdered milk in PBST at 37°C |
| MSP-3 | 2 µg/mL in carbonate-bicarbonate buffer | 3 times with PBST | 1 hour with 3% (wt/vol) powdered-milk in PBST at RT | 1 hour diluted in 1% (wt/vol) powdered milk in PBST at RT | Diluted in 1% (wt/vol) powdered milk in PBST at RT |
EBA-157 = erythrocyte binding antigen-175; LSA-1 = liver stage antigen-1(LSA-1); MSP-3 = merozoite surface protein-3; PBST = PBS-Tween 20 0.05%; wt/vol = weight/volume; RT = room temperature.
HLA typing.
HLA typing was performed in the 107 malaria naturally exposed individuals and the 77 malaria nonexposed individuals. Genomic DNA was isolated from frozen peripheral blood by the phenol-chloroform extraction procedure as previously described.32 HLA-DRB1* and –DQB1* low-resolution typing was performed by polymerase chain reaction with sequence-specific primers on all of the samples as previously described.33,34
Statistical analysis.
The data were stored in the dBASE data bank software (Ashton Tate, Borland, CA). Statistica (Microsoft, Redmond, WA) and the EpiInfo version 6 (Centers for Disease Control and Prevention, Atlanta, GA) statistical software programs were used for the data analysis. Student’s t test was used to analyze the differences in the mean values, and χ2 analysis was applied to compare the prevalence of the positive responses. The Spearman rank coefficient test was used to analyze the correlation between the variables. The antigen frequencies were calculated by the formula af = n/N, where n is the number of samples positive for the antigen and N is the total number of samples, and the gene frequencies were calculated by the formula gf = 1 − √ (1 − af).35 The HLA antigen-specific associations with the responders and non-responders were analyzed by multiple logistic regression using R version 2.14.0 statistical software (The R Foundation for Statistical Computing, Vienna, Austria, available at http://www.R-project.org/) and were corrected by the time of residence in a malaria-endemic area and the number of previous malaria attacks. The HLA unidentified specificities (blank) as well as the HLA specificities with frequencies equal to zero in at least one of the studied groups were pooled into one group as other specificities.
RESULTS
Clinical and epidemiological characteristics of the studied population.
Individuals from the exposed group claimed to have experienced an average of 1.2 malaria episodes in the previous 12 months. All except 13 individuals reported at least one malaria episode during their life, and 44 (23.5%) of the subjects reported more than 10 episodes. Forty-eight (26%) of the individuals had detectable parasitemia (4,389 ± 9,610 parasites/µL) at the time of the blood sampling, and 37 were infected with P. falciparum, nine were infected with P. vivax and two were infected with P. falciparum and P. vivax.
IgG and IgM antibody responses against CSP, LSA-1, EBA, and MSP-3.
The prevalence of individuals presenting antibodies, regardless of their IgM or IgG type and IgG isotype against CSP, LSA-1, EBA, and MSP-3 were, respectively, 67% (125/187), 73% (136/187), 78% (146/187), and 61% (115/187). Antibodies against EBA and LSA-1 were significantly more prevalent than antibodies against MSP-3 (χ2 = 11.413; P = 0.0007, MSP-3 × EBA; χ2 = 4.693; P = 0.03, MSP-3 × LSA-1). The antibody responses to CSP, LSA-1, EBA, and MSP-3 were strongly associated with the time of residence in a malaria-endemic area (CSP: P = 0.01; LSA-1: P = 0.0003; EBA: P = 0.01; MSP-3: P = 0.01) (Table 2).
Table 2.
Characteristics of the CSP, LSA-1, EBA, and MSP-3 responders and non-responders in the exposed group
| CSP | LSA-1 | EBA | MSP-3 | |||||
|---|---|---|---|---|---|---|---|---|
| R | NR | R | NR | R | NR | R | NR | |
| N | 125/187 | 62/187 | 136/187* | 51/187 | 146/187** | 41/187 | 115/187 | 72/187 |
| (66.8%) | (33.2%) | (72.7%) | (27.3%) | (78.1%) | (21.9%) | (61.5%) | (38.5%) | |
| Sex | ||||||||
| Male (%) | 63.2 | 50 | 61.5 | 52.9 | 61.6 | 48.8 | 58.3 | 59.7 |
| Female (%) | 36.8 | 50 | 38.5 | 47.1 | 38.4 | 51.2 | 41.7 | 40.3 |
| AgeΩ | 32 ± 16 | 30 ± 16 | 32 ± 16 | 29 ± 14 | 32 ± 16 | 31 ± 14 | 34 ± 16 | 28 ± 14 |
| Time of residence in malaria-endemic area† | 26 ± 16*** | 21 ± 16 | 27 ± 19**** | 18 ± 13 | 26 ± 17***** | 20 ± 13 | 28 ± 17‡ | 19 ± 13 |
| Reported number of previous malaria attacks | 15 ± 16 | 13 ± 15 | 15 ± 16 | 14 ± 14 | 14 ± 15 | 15 ± 17 | 16 ± 16 | 13 ± 14 |
CSP = circumsporozoite protein; EBA-157 = erythrocyte binding antigen-175; HLA = human leukocyte antigen; LSA-1 = liver stage antigen-1(LSA-1); MSP-3 = merozoite surface protein-3; N = number of positive individuals/number of tested individuals; R = responders; NR = nonresponders; † = years. * P = 0.0007, LSA-1 vs MSP-3; ** P = 0.03, EBA vs MSP-3; *** P = 0.01, CSP responders vs nonresponders; **** P = 0.0008, LSA-1 responders vs nonresponders; ***** P = 0.04, EBA responders vs nonresponders; ‡ MSP-3 responders vs nonresponders.
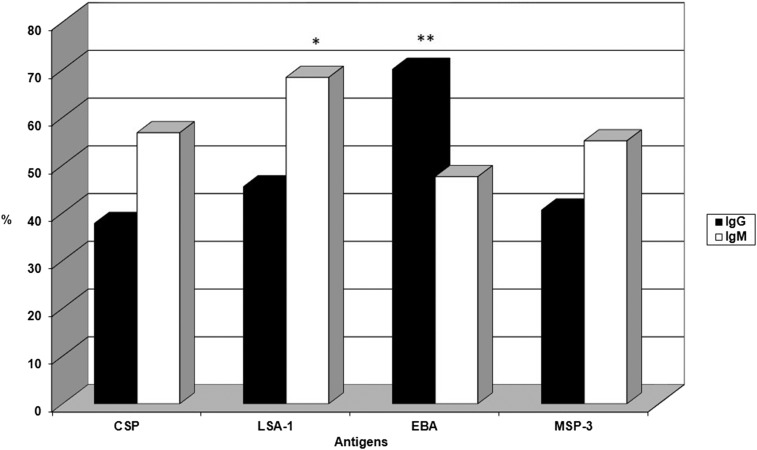
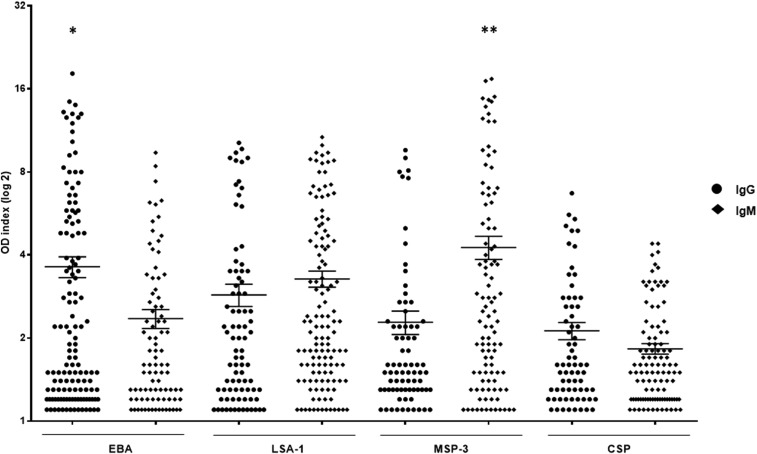
The frequencies of individuals presenting IgG and IgM antibodies are shown in Figure 1. IgG antibodies against EBA were significantly more prevalent (70%) than antibodies against LSA1 (45%), MSP-3 (41%), and CSP (38%) (P < 0.0001, χ2 − 22.191, EBA × LSA-1; P < 0.0001, χ2 = 31.548, EBA × MSP-3; P < 0.0001, χ2 = 37.573, EBA × CSP). Not only was the frequency of the responders to EBA higher, but EBA also induced higher levels of IgG antibodies than LSA-1, MSP-3, and CSP (P = 0.03, EBA × LSA-1; P = 0.0006, EBA × MSP-3; P < 0.0001, EBA × CSP) (Figure 2). The frequency of the responders showed that IgM antibodies to LSA-1 were significantly more prevalent (68%) than IgM antibodies to EBA (48%), MSP-3 (55%), and CSP (57%) (P < 0.0001, χ2 = 15.852, LSA-1 × EBA; P = 0.01, χ2 = 6.521, LSA-1 × MSP-3; P = 0.02, χ2 = 4.944, LSA-1 × CSP). Interestingly, MSP-3 showed higher levels of an IgM antibody response than EBA, LSA-1, and CSP (P < 0.0001, MSP-3 × EBA; P = 0.03, MSP-3 × LSA-1; P < 0.0001, MSP-3 × CSP) (Figure 2). The levels of IgG and IgM antibodies to LSA-1, EBA, and MSP-3 increased with age (LSA-1: P = 0.02, r = 0.2433 for IgG, P = 0.006, r = 0.2384 for IgM; EBA: P = 0.007, r = 0.2344 for IgG, P = 0.001, r = 0.3343 for IgM; MSP-3: P = 0.03, r = 0.2451 for IgG, P = 0.004, r = 0.2767 for IgM) and time of residence in a malaria-endemic area (LSA-1: P = 0.02, r = 0.2510 for IgG, P = 0.006, r = 0.2407 for IgM; EBA: P = 0.03, r = 0.1822 for IgG, P = 0.005, r = 0.2944 for IgM; MSP-3: P = 0.001, r = 0.3693 for IgG, P = 0.01, r = 0.3464 for IgM).
Figure 1.
Frequency of individuals from the exposed group with IgG and IgM antibodies to circumsporozoite protein (CSP), liver stage antigen-1 (LSA-1), erythrocyte binding antigen (EBA), and merozoite surface protein-3 (MSP-3) antigens. * P < 0.0001, IgM LSA-1 vs IgM EBA; P = 0.02, IgM LSA-1 vs IgM CSP; P = 0.01, IgM LSA-1 vs IgM MSP-3; ** P < 0.0001, IgG EBA vs IgG CSP, IgG LSA-1, and IgG MSP-3.
Figure 2.
Distribution of the IgG and IgM antibody response (OD index) against erythrocyte binding antigen (EBA), liver stage antigen-1 (LSA-1), merozoite surface protein-3 (MSP-3), and circumsporozoite protein (CSP) in the exposed group. * P < 0.0001, IgG EBA vs IgG CSP; P = 0.03, IgG EBA vs IgG LSA-1; P = 0.0006, IgG EBA vs IgG MSP-3. ** P < 0.0001, IgM MSP-3 vs IgM EBA; P < 0.0001, IgM MSP-3 vs IgM CSP; P = 0.03, IgM MSP-3 vs IgM LSA-1.
Twenty-one of the 109 individuals (19.3%) from the nonexposed group presented antibodies against P. falciparum antigens. Of these, 16 individuals (14.7%) presented antibodies against a single antigen, three individuals (2.7%) presented antibodies against two antigens and two individuals (1.8%) presented antibodies against three antigens. The frequencies of the antibodies against individual antigens were low: 10 (9.2%) individuals presented antibodies against CSP, 10 (9.2%) presented antibodies against LSA-1, five (4.6%) presented antibodies against EBA and four (3.7%) presented antibodies against MSP-3. None of the 15 Rio-controls, who had neither a history of malaria nor contact with malaria transmission areas, had detectable antibodies to CSP, LSA-1, EBA, or MSP-3 (OD index values for IgG and IgM, respectively: CSP: 0.586 and 0.765; LSA-1: 0.476 and 0.698; EBA: 0.627 and 0.821; MSP-3: 0.611 and 0.876).
IgG isotypes against CSP, LSA-1, EBA, and MSP-3.
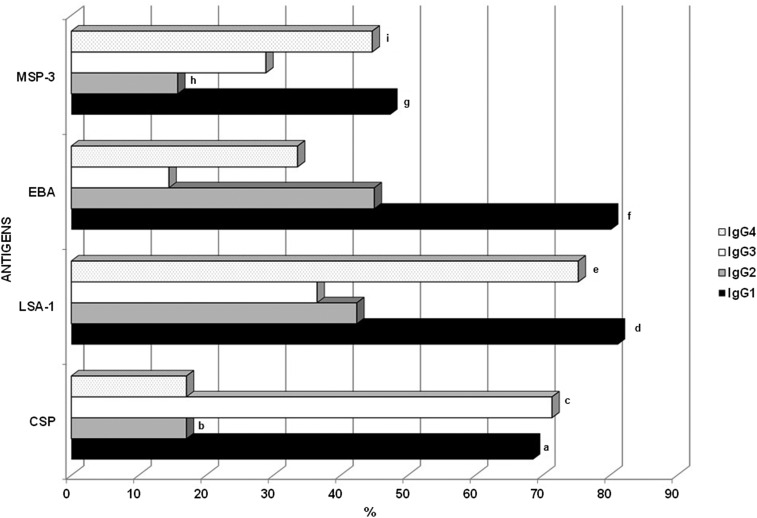
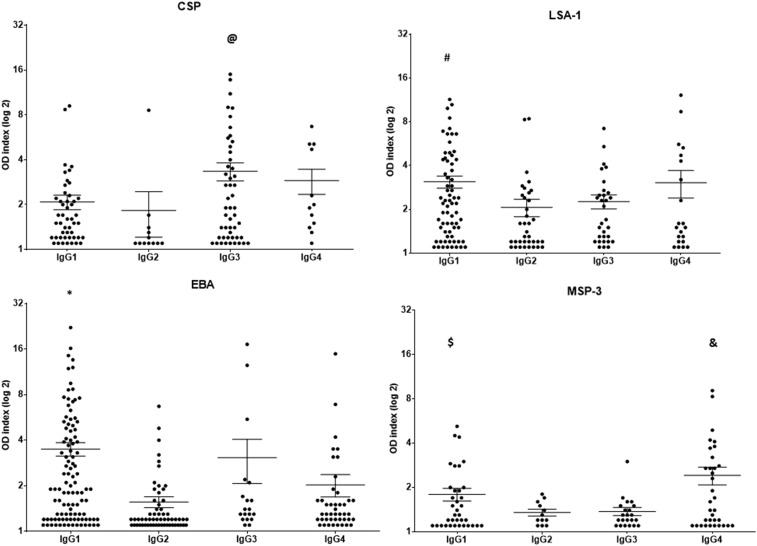
IgG isotypes were analyzed in all of the IgG positive samples. The frequencies of the individuals presenting IgG1, IgG2, IgG3, and IgG4 antibodies against CSP, LSA-1, EBA, and MSP-3 are shown in Figure 3. The results show that CSP mainly induced cytophilic IgG1 and IgG3 antibodies, and LSA-1 and MSP-3 preferentially induced an IgG1 and IgG4 antibody response, whereas EBA mainly induced an IgG1 antibody response (P < 0.05, for all analyses). As shown in Figure 4, EBA and LSA-1 induced higher levels of IgG1 antibodies, whereas CSP induced higher levels of IgG3 antibodies, and MSP-3 induced higher levels of IgG4 antibodies. The levels of IgG1 antibodies against LSA-1 and EBA and the levels of IgG3 antibodies against CSP showed a positive correlation with age (LSA-1: P = 0.04, r = 0.2344; EBA: 0.004, r = 0.2764; CSP = 0.03, r = 0.2656). No correlation between the level of the isotypes and the time of exposure or parasitemia was detected.
Figure 3.
Frequency of the individuals from the exposed group with IgG1, IgG2, IgG3 and IgG4 antibodies against the circumsporozoite protein (CSP), liver stage antigen-1 (LSA-1), erythrocyte binding antigen (EBA), and merozoite surface protein-3 (MSP-3) antigens. a: P < 0.0001, CSP IgG1 × CSP IgG2 and CSP IgG4; b: P < 0.0001, CSP IgG2 × EBA IgG2; P = 0.0009, CSP IgG2 × LSA-1 IgG2; c: P < 0.0001, CSP IgG3 × CSP IgG2 and CSP IgG3 × CSP IgG4; P < 0.0001, CSP IgG3 × LSA-1 IgG3, MSP-3 IgG3, and EBA IgG3; d: P < 0.0001, LSA-1 IgG1 × LSA-1 IgG2 and LSA-1 IgG3; P = 0.0001, LSA-1 IgG1 × MSP-3 IgG1; e: P < 0.0001, LSA-1 IgG4 × LSA-1 IgG2 and LSA-1 IgG3; P < 0.0001, LSA-1 IgG4 × CSP IgG4, EBA IgG4, and MSP-3 IgG4; f: P < 0.0001, EBA IgG1 × EBA IgG2, EBA IgG3 and EBA IgG4; P = 0.0001, EBA IgG1 × MSP-3 IgG1; g: P < 0.0001, MSP-3 IgG1 × MSP-3 IgG2; P = 0.02, MSP-2 IgG1 × IgG3; h: P < 0.0001, MSP-3 IgG2 × EBA IgG2; P = 0.0003, MSP-3 IgG3 × LSA-1 IgG2; i: P = 0.0002, MSP-3 IgG4 × MSP-3 IgG2.
Figure 4.
Distribution of the IgG1, IgG2, IgG3, and IgG4 antibody response (OD index) against the circumsporozoite protein (CSP), liver stage antigen-1 (LSA-1), erythrocyte binding antigen (EBA), and merozoite surface protein-3 (MSP-3) antigens in the exposed group. @ P = 0.01, CSP IgG3 vs CSP IgG1. # P = 0.01, LSA-1 IgG1 vs LSA-1 IgG2; P = 0.03, LSA-1 IgG1 vs LSA-1 IgG3. * P < 0.0001, EBA IgG1 vs EBA IgG2; P = 0.003, EBA IgG1 vs EBA IgG4). $ P = 0.02, MSP-3 IgG1 vs MSP-3 IgG2; P = 0.03, MSP-3 IgG1 vs MSP-3 IgG3. & P = 0.003, MSP-3 IgG4 vs MSP-3 IgG2; P = 0.004, MSP-3 IgG4 vs MSP-3 IgG3.
HLA class II typing.
The majority of the individuals from the exposed group (107/187–57%) and from the nonexposed group (77/109–70%) were typed for HLA class II, and there were no differences in the HLA-DR and HLA-DQ antigen frequencies between these groups (χ2 = 23.376, df = 14, P > 0.05 for HLA-DR; (χ2= 6.064, df = 7, P > 0.05 for HLA-DQ; data not shown). To evaluate the effect of class II antigens on the immune responses to CSP, LSA-1, EBA, and MSP-3 antigens in the exposed group, the individuals were regrouped into responder and non-responder groups. The individuals from the exposed group who were classified as nonresponders were those who had no detectable antibodies in the different time-points in the study period. Using a multiple logistic regression analysis, corrected for the time of residence in a malaria-endemic area and the number of previous malaria attacks, it was possible to observe that HLA-DRB1*11 (OR = 0.16; P value: 0.006) and HLA-DQB1*7 (OR: 0.24; P value: 0.034) had the lowest odds ratios for responding to EBA-175 (Table 3).
Table 3.
Distribution of the HLA II alleles (DRB1 and DQB1) and the odds ratios (ORs) for the association among the HLA alleles and antigens
| EBA-175 | MSP3 | LSA-1 | CSP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA alleles | NR (%) N = 17 | R (%) N = 91 | OR (95% CI) | P value | NR (%) N = 36 | R (%) N = 72 | OR (95% CI) | P value | NR (%) N = 23 | R (%) N = 85 | OR (95% CI) | P value | NR (%) N = 22 | R (%) N = 86 | OR (95% CI) | P value | ||
| DR | *01 | Y | 15 13.9) | 78 (72.2) | 1.13 (0.21–6.13) | 0.881 | 32 (29.6) | 61 (56.4) | 1.97 (0.49–7.96) | 0.337 | 18 (16.6) | 75 (69.4) | 0.53 (0.15–1.88) | 0.334 | 17 (15.7) | 77 (71.3) | 0.4 (0.11–1.44) | 0.166 |
| N | 2 (1.8) | 13 (12) | 4 (3.7) | 11 (10.1) | 5 (4.6) | 10 (9.2) | 5 (4.6) | 9 (8.3) | ||||||||||
| *04 | Y | 15 (13.9) | 65 (60.2) | 2.9 (0.59–14.53) | 0.186 | 28 (25.9) | 52 (48.1) | 1.47 (0.53–4.1) | 0.452 | 18 (16.6) | 62 (57.4) | 1.58 (0.51–4.89) | 0.427 | 18 (16.6) | 63 (58.3) | 1.8 (0.53–6.05) | 0.342 | |
| N | 2 (1.8) | 26 (24.1) | 8 (7.4) | 20 (18.5) | 5 (4.6) | 23 (21.3) | 4 (3.7) | 23 (21.3) | ||||||||||
| *07 | Y | 14 (13) | 78 (72.2) | 0.65 (0.14–2.97) | 0.588 | 34 (31.4) | 58 (53.7) | 3.76 (0.72–19.47) | 0.113 | 21 (19.4) | 72 (66.6) | 1.88 (0.36–9.61) | 0.446 | 18 (16.6) | 73 (67.5) | 0.61 (0.16–2.27) | 0.463 | |
| N | 3 (2.8) | 13 (12.04) | 2 (1.8) | 14 (12.9) | 2 (1.9) | 13 (12) | 4 (3.7) | 13 (12) | ||||||||||
| *08 | Y | 16 (14.8) | 76 (70.4) | 1.79 (0.20–15.94) | 0.598 | 30 (27.7) | 62 (57.4) | 0.3 (0.08–1.15) | 0.082 | 22 (20.3) | 70 (64.8) | 3.43 (0.41–28.66) | 0.254 | 21 (19.4) | 71 (65.7) | 2.89 (0.34–24.35) | 0.328 | |
| N | 1 (0.9) | 15 (13.8) | 6 (5.5) | 10 (9.2) | 1 (0.9) | 15 (13.8) | 1 (0.9) | 15 (13.8) | ||||||||||
| *09 | Y | 16 (14.8) | 86 (79.6) | 0.61 (0.05–6.63) | 0.687 | 34 (31.4) | 68 (62.9) | 0.51 (0.06–3.92) | 0.522 | 22 (20.3) | 81 (75) | 0.73 (0.06–8.03) | 0.803 | 21 (19.4) | 80 (74.) | 1.3 (0.14–12) | 0.812 | |
| N | 1 (0.9) | 5 (4.6) | 2 (1.8) | 4 (3.7) | 1 (0.9) | 4 (3.7) | 1 (0.9) | 6 (5.5) | ||||||||||
| *10 | Y | _ | 35 (32.4) | 71 (65.7) | 1.85 (0.1–33.44) | 0.676 | 22 (20.3) | 84 (77.7) | 0.38 (0.02–6.81) | 0.517 | 21 (19.4) | 85 (78.7) | 0.42 (0.02–7.74) | 0.563 | ||||
| N | 1 (0.9) | 1 (0.9) | 1 (0.9) | 1 (0.9) | 1 (0.9) | 1 (0.9) | ||||||||||||
| *11 | Y | 9 (8.3) | 77 (71.3) | 0.16 (0.04–0.59) | 0.006 | 27 (25) | 59 (54.6) | 0.63 (0.22–1.8) | 0.397 | 19 (17.5) | 73 (67.5) | 0.89 (0.25–3.17) | 0.867 | 17 (15.7) | 70 (64.8) | 0.75 (0.23–2.4) | 0.633 | |
| N | 8 (7.4) | 14 (12.9) | 9 (8.3) | 13 (12) | 4 (3.7) | 12 (11.1) | 5 (4.6) | 16 (14.8) | ||||||||||
| *12 | Y | 15 (13.8) | 84 (77.7) | 0.91 (0.09–8.83) | 0.937 | 32 (29.6) | 67 (62) | 0.96 (0.18–5.13) | 0.966 | 22 (20.3) | 78 (72.2) | 1.71 (0.18–15.9) | 0.636 | 20 (18.5) | 79 (73.1) | 0.78 (0.13–4.46) | 0.788 | |
| N | 2 (1.8) | 7 (6.4) | 4 (3.7) | 5 (4.6) | 1 (0.9) | 7 (6.4) | 2 (1.8) | 7 (6.4) | ||||||||||
| *13 | Y | 12 (11.1) | 63 (58.3) | 1.32 (0.36–4.82) | 0.673 | 20 (18.5) | 54 (50) | 0.5 (0.19–1.27) | 0.148 | 14 (12.9) | 60 (55.5) | 0.69 (0.25–1.86) | 0.465 | 14 (12.9) | 59 (54.6) | 0.88 (0.31––2.47) | 0.818 | |
| N | 5 (4.6) | 28 (25.9) | 16 (14.8) | 18 (16.6) | 9 (8.3) | 25 (23.1) | 8 (7.4) | 27 (25) | ||||||||||
| *14 | Y | 15 (13.89) | 78 (72.22) | 0.69 (0.12–3.82) | 0.679 | 33 (30.5) | 65 (60.1) | 0.58 (0.11–2.89) | 0.513 | 21 (19.4) | 72 (66.6) | 1.43 (0.28–7.23) | 0.662 | 21 (19.4) | 73 (67.5) | 2.51 (0.29–21.55) | 0.4 | |
| N | 2 (1.85) | 13 (12.04) | 3 (2.7) | 7 (6.4) | 2 (1.8) | 13 (12) | 1 (0.9) | 13 (12.) | ||||||||||
| *15 | Y | 14 (12.9) | 83 (76.8) | 0.74 (0.13–4.04) | 0.728 | 32 (29.6) | 65 (60.1) | 0.81 (0.19–3.43) | 0.78 | 20 (18.5) | 78 (72.22) | 0.8 (0.18–3.51) | 0.776 | 20 (18.5) | 77 (71.3) | 1.25 (0.24–6.56) | 0.786 | |
| N | 3 (2.7) | 8 (7.4) | 4 (3.7) | 7 (6.4) | 3 (2.78) | 7 (6.48) | 2 (1.8) | 9 (8.3) | ||||||||||
| *16 | Y | _ | 34 (31.4) | 62 (57.4) | 3.19 (0.59–17.2) | 0.177 | 21 (19.4) | 75 (69.4) | 1.38 (0.26–7.23) | 0.698 | 20 (18.5) | 76 (70.3) | 1.27 (0.24–6.61) | 0.771 | ||||
| N | 2 (1.8) | 10 (9.2) | 2 (1.8) | 10 (9.2) | 2 (1.8) | 10 (9.2) | ||||||||||||
| *17 | Y | _ | 31 (28.7) | 68 (62.9) | 0.56 (0.11–2.72) | 0.478 | 21 (19.4) | 78 (72.2) | 1.02 (0.18–5.76) | 0.975 | 20 (18.5) | 79 (73.1) | 2.25 (0.25–20.06) | 0.466 | ||||
| N | 5 (4.63) | 4 (3.7) | 2 (1.8) | 7 (6.4) | 2 (1.8) | 7 (6.4) | ||||||||||||
| *18 | Y | _ | _ | _ | _ | |||||||||||||
| N | ||||||||||||||||||
| DQ | *02 | Y | 14 (13) | 71 (65.7) | 1.66 (0.33–8.35) | 0.536 | 28 (25.9) | 57 (52.7) | 0.86 (0.28–2.65) | 0.799 | 18 (16.6) | 67 (62) | 0.89 (0.27–2.88) | 0.854 | 16 (14.8) | 69 (63.8) | 0.75 (0.23–2.44) | 0.644 |
| N | 3 (2.8) | 20 (18.52) | 8 (7.4) | 15 (13.8) | 5 (4.6) | 18 (16.6) | 6 (5.5) | 17 (15.7) | ||||||||||
| *04 | Y | 16 (14.8) | 73 (67.6) | 2.42 (0.28–20.5) | 0.416 | 31 (28.7) | 57 (52.7) | 1.03 (0.30–3.48) | 0.952 | _ | 19 (17.5) | 70 (64.8) | 1.14 (0.29–4.53) | 0.845 | ||||
| N | 1 (0.9) | 18 (16.7) | 5 (4.6) | 14 (12.9) | 3 (2.7) | 16 (14.1) | ||||||||||||
| *05 | Y | 13 (12) | 64 (59.3) | 1.12 (0.29–4.3) | 0.866 | 27 (25) | 50 (46.3) | 2.01 (0.68–5.94) | 0.204 | 17 (15.7) | 67 (62.) | 0.87 (0.27–2.74) | 0.818 | 13 (12) | 64 (59.2) | 0.51 (0.18–1.43) | 0.204 | |
| N | 4 (3.7) | 27 (25) | 9 (8.3) | 22 (20.3) | 6 (5.5) | 18 (16.6) | 9 (8.3) | 22 (20.3) | ||||||||||
| *06 | Y | 9 (8.3) | 60 (55.6) | 0.82 (0.25–2.67) | 0.744 | 21 (19.4) | 48 (44.4) | 0.83 (0.33–2.08) | 0.7 | 11 (10.1) | 56 (51.8) | 0.52 (0.2–1.37) | 0.189 | 16 (14.8) | 53 (49) | 1.76 (0.6–5.16) | 0.298 | |
| N | 8 (7.4) | 31 (28.7) | 15 (13.8) | 24 (22.2) | 12 (11.1) | 29 (26.8) | 6 (5.5) | 33 (30.5) | ||||||||||
| *07 | Y | 7 (6.5) | 56 (51.8) | 0.24 (0.06–0.89) | 0.034 | 20 (18.5) | 44 (40.7) | 0.5 (0.19–1.3) | 0.158 | 12 (11.1) | 50 (46.3) | 0.62 (0.23–1.67) | 0.351 | 12 (11.1) | 51 (47.2) | 0.63 (0.23–1.72) | 0.372 | |
| N | 10 (9.3) | 35 (32.4) | 16 (14.8) | 28 (25.9) | 11 (10.1) | 35 (32.4) | 10 (9.2) | 35 (32.4) | ||||||||||
| *08 | Y | 15 (13.9) | 67 (62) | 2.5 (0.5–12.4) | 0.26 | 29 (26.8) | 54 (50) | 1.56 (0.54–4.51) | 0.408 | 18 (16.6) | 64 (59.2) | 1.35 (0.43–4.21) | 0.602 | 19 (17.5) | 63 (58.3) | 2.41 (0.64–9.1) | 0.193 | |
| N | 2 (1.8) | 24 (22.2) | 7 (6.4) | 18 (16.6) | 5 (4.6) | 21 (19.4) | 3 (2.7) | 23 (21.3) | ||||||||||
| *09 | Y | 14 (12.9) | 83 (76.8) | 0.23 (0.04–1.27) | 0.094 | 34 (31.4) | 63 (58.3) | 1.29 (0.23–7.27) | 0.768 | 21 (19.4) | 76 (70.3) | 0.92 (0.16–5.06) | 0.927 | 20 (18.5) | 77 (71.3) | 0.84 (0.15–4.57) | 0.842 | |
| N | 3 (2.8) | 8 (7.4) | 2 (1.8) | 9 (8.3) | 2 (1.8) | 9 (8.3) | 2 (1.8) | 9 (8.3) | ||||||||||
CSP = circumsporozoite protein; EBA = erythrocyte binding antigen; HLA = human leukocyte antigen; LSA-1 = liver stage antigen-1; MSP-3 = merozoite surface protein-3; Y = yes (presence of an HLA allele); N = no (absence of an HLA allele); R = responder; NR = nonresponder; N = number of individuals; OR = odds ratio; CI = confidence interval Bold values shows the significant results.
DISCUSSION
Over the years, peptide vaccine strategies using P. falciparum derived antigens have reemerged as an attractive approach against malaria in Africa. However, few studies have been conducted on malaria-exposed populations from non-African countries, particularly in Brazil. Therefore, the seroepidemiological profiles of the naturally exposed individuals from a malaria-endemic setting of the Brazilian Amazon against four synthetic peptides derived from the main vaccine candidates against P. falciparum were investigated. The association between the immunological recognition of a given antigen and a certain degree of protection against malaria may indicate the potential value of the antigen as a candidate vaccine. Moreover, is important to identify the baseline of the naturally acquired immune response to distinguish it from the vaccine-induced response in future clinical trials.36
The results indicate that all four synthetic peptides were broadly recognized by naturally acquired antibodies and that these antibody responses were associated with the time of residence in a malaria-endemic area, indicating that CSP, LSA-1, EBA, and MSP-3 are immunogenic in natural conditions of exposure and appear to be dependent of the time of exposure. Curiously, no association was recorded between the anti-CSP, -LSA-1, -EBA, and -MSP-3 antibody prevalence and the number of previous malaria episodes in the studied population. This finding may be limited by the donor-reported data, particularly for individuals born in the area who do not recall childhood infections. Moreover, people living for longer periods of time in the region may have acquired some degree of immunity to the clinical disease after experiencing a number of infections and, therefore, report less episodes of clinical malaria in the more recent years, leading to a bias in the analysis. In fact, studies have reported a high frequency of asymptomatic malaria infections among individuals in this area.25,26,37–40 In this regard, the population in the present study has been living in the Amazon region for an average of 24 years. Most likely, given the high prevalence of P. vivax in relation to P. falciparum in the area, the number of malaria infections does not necessarily mean stimulation of responses to P. falciparum antigens.
IgG antibodies to a number of pre-erythrocytic and erythrocytic malaria vaccine candidates are associated with protection against malaria in areas with different levels of transmission.10,13,19,41–46 In the present study, the results showed that both the prevalence of the responders and the levels of IgG antibodies were higher for EBA than for LSA1, MSP-3, and CSP. These results differ from those in a study performed by Ford et co-workers who observed a low immunoreactivity to EBA-175 in individuals living in different states of the Brazilian Amazon region, including Mato Grosso, Amapá, Rondonia, Pará, and Acre.47 These differences may be a result of dissimilarities in exposure, age, and time of residence of the studied populations in the endemic area because several studies have demonstrated a direct effect of these parameters on the antibody response to several plasmodial antigens.18,19,45,48 Given that the repertoire of the host erythrocyte receptors available in specific populations will select for the expression of specific parasite ligands that can mediate a successful erythrocyte invasion, the immune response to such ligands may vary in different populations, correlating to their expression. We can also hypothesize that the difference in immunoreactivity may be due the differential usage of the EBA-175-glycophorin A invasion pathway. Interestingly, the prevalence of IgG antibodies against EBA-175 in our study was similar to that observed in Gambia and Nigeria.49 Notably, high levels of IgG antibodies against EBA have been consistently associated with protection from clinical malaria in previous studies.15,50–52
IgM antibodies to LSA-1 were significantly more prevalent than IgM antibodies to EBA, MSP-3 and CSP. A high frequency of IgM antibodies against LSA-1 was observed in individuals living in Kenya; however, in this study, IgM antibodies to LSA-1 were more frequent in individuals with parasitemia than in individuals without parasitemia.53 In our study, no association was observed between the IgM antibodies against LSA-1 and the presence or levels of parasitemia. The elevated prevalence of IgM against LSA-1 in this population may be related with the genetic background of the studied individuals. A recent study compared the breadth and magnitude of P. falciparum-specific IgM and IgG responses in two distinct ethnic groups, Fulani and Dogon, with a microarray containing 1,087 P. falciparum proteins. The authors observed that IgM response more strongly distinguished the two ethnic groups. Therefore, the authors suggest that the selective pressure of malaria may result in host genetic polymorphisms that yield more robust IgM responses in certain ethnic groups, influencing the antigen specificity of IgM responses to infection.54
The specific antibodies induced by natural infections to most proteins are associated with age and time of exposure in endemic regions, a phenomenon that has been frequently reported for various antigens.10,18,19,41–46,55,56 In the present work, the levels of IgG and IgM antibodies to LSA-1, EBA, and MSP-3 increased with age and time of residence in a malaria-endemic area, reflecting the most likely exposure to the malaria parasites and, possibly, the maturation of the immune system over time.
Evidence that protective immunity to P. falciparum malaria is associated with different classes and subclasses of antibodies reveals the importance of considering the quality of the response. Cytophilic IgGs play an important role in protection against malarial disease.6,57–61 The acquisition of protective immunity is associated with the cytophilicity of IgG1 and IgG3 antibodies and the reduced proportion of IgG2 and IgG4 noncytophilic subclasses of the same specificity that can block the effector mechanisms. The binding of the Fc portions of IgG1 and IgG3 cytophilic antibodies to Fc receptors on phagocytic cells triggers a range of effector functions, such as phagocytosis, the production of cytokines and chemokines, cytotoxicity, and the generation of reactive oxygen and nitrogen species.62 However, the contribution of parasite-reactive IgG2 antibodies to protection against clinical malaria is suggested in some epidemiological settings.61,63,64 By contrast, several studies have suggested that lgG4 antibodies most likely do not protect against the disease.65–68 In Brazil, an analysis of the antibody isotypes specific for several P. falciparum proteins revealed that all four IgG subclasses are present and, for some proteins, such as the N-terminal region of the P126 protein, individuals with higher levels of the anti-Nt47 cytophilic IgG antibody had significantly lower parasitemia levels.10 In the present work, the IgG1, IgG2, IgG3, and IgG4 subclasses against LSA-1, EBA, CSP, and MSP-3 were observed.
The results of the present study, which show different IgG subclass profiles in response to the four studied antigens, may be related to the antigen properties because it has been suggested that the characteristics of antigens themselves influence class switching in B cells.69–71 The results show that CSP induced mainly cytophilic IgG1 and IgG3 antibodies. Similar data were obtained by Oluwasogo and coworkers, which confirmed the prevalence of the cytophilic IgG1 and IgG3 antibodies over the noncytophilic IgG2 and IgG4 subclasses specific to P. falciparum CSP in individuals living in Nigeria.72 Furthermore, the results presented here showed that the levels of IgG3 antibodies against CSP showed a positive correlation with age. Significant age-related increases in IgG3 antibodies to CSP have also been reported in areas of stable malaria transmission, but none were identified in unstable malaria transmission where IgG3 responses to CSP declined with age.73
Here, the IgG1 and IgG4 isotypes were preferentially induced by the LSA-1 antigen. In a study performed in Kenya, John and others reported different data: most antibodies against LSA-1 belonged to the IgG1 and IgG3 subclasses, although IgG2 was observed at lower frequencies. However, the IgG4 isotype was not found.44,74,75 The difference observed between that study and ours may be a result of the cumulative exposure to antigens, which varies according to the intensity of malaria transmission, because IgG1/IgG3 class switching is independently affected by the cumulative exposure to the antigen.71
Studies performed by independent research groups have demonstrated the dominance of the MSP-3-specific IgG1 and IgG3 antibodies in different settings.60,76,77 In our study, MSP-3 preferentially induced an IgG1 isotype, but MSP-3-specific IgG3 antibodies were infrequent. Interestingly, a relatively high frequency of individuals presenting IgG4 antibodies was observed. The high frequency and levels of IgG4 may be related to helminthic infections; it has been suggested that some allergens or helminths are known to induce IgG4 expression and stimulate both IgG4 and IgE induction.78 However, except for LSA-1, both the frequency and the levels of IgG4 to other studied antigens were low.
In the present study, we noted that EBA mainly induced an IgG1 antibody response, and the levels of this IgG1 antibody response showed a positive correlation with age, consistent with previous reports showing that this P. falciparum protein predominantly induced the IgG1 subclass and that the levels of IgG1 against EBA correlated positively with age.79,80 However, our results differ from those described by others who reported a predominance of the IgG1 and IgG3 subclasses in children living in malaria-endemic areas of Gabon and Mozambique.15,79
The HLA class II molecules, originally called immune response genes, play an essential role in stimulating the immune response by binding and presenting antigen peptides to CD4+ T lymphocytes. Differences in HLA binding affinities can result in a decreased binding and an inefficient presentation of peptides to CD4+ T lymphocytes, leading to a decreased cytokine production by CD4+ T lymphocytes and, consequently, a decreased production of antibodies by B lymphocytes, because B lymphocytes must interact with CD4+ T lymphocytes to be activated. Therefore, HLA allelic forms can influence the host capacity to mount both a naturally acquired and an artificially induced antiplasmodial humoral immune response.18,19,81–89 In view of these data, we hypothesized the possible involvement of HLA class II molecules in the modulation of the antibody specificity profiles induced by P. falciparum antigens in the exposed group. In our study, the analysis of the presence of the HLA-DRB1* and HLA-DQB1* allelic groups and the antibody response to CSP, LSA-1, and MSP-3 did not show any association. However, using a multiple logistic regression analysis, corrected for the time of residence in a malaria-endemic area and the number of previous malaria attacks, we observed that HLA-DRB1*11 and HLA-DQB1*7 had the lowest odds ratios for responding to EBA-175. Considering the importance of anti-EBA-175 antibodies in the development of antiparasite immunity and its possible inclusion in a subunit vaccine, additional studies should focus on the evaluation of the genetic restriction of the anti-EBA-175 humoral response.
In conclusion, the results from the present study show that the CSP, LSA-1, EBA, and MSP-3 antigens evoke an antibody response, particularly of the IgG1 subclass, in a high percentage of individuals naturally exposed to P. falciparum infections. The results also show that the anti-EBA IgG antibody response appears to be modulated by HLA class II antigens. Given the increasing focus on the use of subunit malaria vaccines, evaluating the influence of class II allele frequencies in ethnically diverse populations may be important before vaccine trials are conducted among people naturally exposed to malaria parasites.
Acknowledgments:
We are grateful to all of the patients who agreed to participate in this study for their cooperation and generous donation of blood, which made this study possible.
REFERENCES
- 1.World Health Organization , 2016. World Malaria Report (2016). Available at: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/.
- 2.Langhorne J, Ndungu FM, Sponaas AM, Marsh K, 2008. Immunity to malaria: more questions than answers. Nat Immunol 9: 725–732. [DOI] [PubMed] [Google Scholar]
- 3.White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC, 2014. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis 210: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 4.McGregor IA, 1964. The passive transfer of human malarial immunity. Am J Trop Med Hyg 13: 237–239. [DOI] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P, 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun HP, Druilhe P, 1992. P. falciparum malaria: evidence for an isotype imbalance may be responsible for the delayed acquisition of protective immunity. Infect Immun 60: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luty AJ, Mayombo J, Lekoulou F, Mshana R, 1994. Immunologic responses to soluble exoantigens of Plasmodium falciparum in Gabonese children exposed to continuous intense infection. Am J Trop Med Hyg 51: 720–729. [DOI] [PubMed] [Google Scholar]
- 8.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P, 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 182: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aribot G, Rogier C, Sarthou JL, Trape JF, Balde AT, Druilhe P, Roussilhon C, 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa). Am J Trop Med Hyg 54: 449–457. [DOI] [PubMed] [Google Scholar]
- 10.Banic DM, Oliveira-Ferreira J, Pratt-Riccio LR, Conseil V, Gonçalves D, Fialho RR, Gras-Masse H, Daniel-Ribeiro CT, Camus D, 1998. Immune response and lack of immune response to Plasmodium falciparum P126 antigen and its amino-terminal repeat in malaria-infected humans. Am J Trop Med Hyg 58: 768–774. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RR, Allen SJ, Greenwood BM, Riley EM, 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg 58: 406–413. [DOI] [PubMed] [Google Scholar]
- 12.Ndungu FM, Bull PC, Ross A, Lowe BS, Kabiru E, Marsh K, 2002. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol 24: 77–82. [DOI] [PubMed] [Google Scholar]
- 13.Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P, 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun 72: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima-Junior JC, et al., 2011. B cell epitope mapping and characterization of naturally acquired antibodies to the Plasmodium vivax merozoite surface protein-3α (PvMSP-3α) in malaria exposed individuals from Brazilian Amazon. Vaccine 29: 1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobaño C, et al., 2012. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin Vaccine Immunol 19: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balogun HA, Awah N, Nilsson S, Rogier C, Trape JF, Chen Q, Roussilhon C, Berzins K, 2013. Pattern of antibodies to the Duffy binding like domain of Plasmodium falciparum antigen Pf332 in Senegalese individuals. Acta Trop 130: 80–87. [DOI] [PubMed] [Google Scholar]
- 17.Osier FH, et al., 2014. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banic DM, Goldberg AC, Pratt-Riccio LR, Oliveira-Ferreira J, Santos F, Gras-Masse H, Camus D, Kalil J, Daniel-Ribeiro CT, 2002. Human leukocyte antigen class II control of the immune response to p126-derived amino terminal peptide from Plasmodium falciparum. Am J Trop Med Hyg 66: 509–515. [DOI] [PubMed] [Google Scholar]
- 19.Pratt-Riccio LR, Lima-Junior JC, Carvalho LJ, Theisen M, Espíndola-Mendes EC, Santos F, Oliveira-Ferreira J, Goldberg AC, Daniel-Ribeiro CT, Banic DM, 2005. Antibody response profiles induced by Plasmodium falciparum glutamate-rich protein in naturally exposed individuals from a Brazilian area endemic for malaria. Am J Trop Med Hyg 73: 1096–1103. [PubMed] [Google Scholar]
- 20.Pratt-Riccio LR, et al., 2008. Evaluation of the genetic polymorphism of Plasmodium falciparum P126 protein (SERA or SERP) and its influence on naturally acquired specific antibody responses in malaria-infected individuals living in the Brazilian Amazon. Malar J 7: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt-Riccio LR, et al., 2011. Antibodies against the Plasmodium falciparum glutamate-rich protein from naturally exposed individuals living in a Brazilian malaria-endemic area can inhibit in vitro parasite growth. Mem Inst Oswaldo Cruz 106 (Suppl 1): 34–43. [DOI] [PubMed] [Google Scholar]
- 22.Riccio EK, Totino PR, Pratt-Riccio LR, Ennes-Vidal V, Soares IS, Rodrigues MM, Souza JM, Daniel-Ribeiro CT, Ferreira-da-Cruz MF, 2013. Cellular and humoral immune responses against the Plasmodium vivax MSP-119 malaria vaccine candidate in individuals living in an endemic area in north-eastern Amazon region of Brazil. Malar J 12: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT, 2010. Malaria in Brazil: an overview. Malar J 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade AL, Martelli CM, Oliveira RM, Arias JR, Zicker F, Pang L, 1995. High prevalence of asymptomatic malaria in gold mining areas in Brazil. Clin Infect Dis 20: 475. [DOI] [PubMed] [Google Scholar]
- 25.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP, 2002. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg 66: 641–648. [DOI] [PubMed] [Google Scholar]
- 26.Tada MS, Ferreira RG, Katsuragawa TH, Martha RC, Costa JD, Albrecht L, Wunderlich G, Silva LH, 2012. Asymptomatic infection with Plasmodium falciparum and Plasmodium vivax in the Brazilian Amazon Basin: to treat or not to treat? Mem Inst Oswaldo Cruz 107: 621–629. [DOI] [PubMed] [Google Scholar]
- 27.Gomes LR, et al., 2013. Asymptomatic infection in individuals from the municipality of Barcelos (Brazilian Amazon) is not associated with the anti-Plasmodium falciparum glycosylphosphatidyinositol antibody response. Mem Inst Oswaldo Cruz 108: 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendonça VR, Souza LC, Garcia GC, Magalhães BM, Lacerda MV, Andrade BB, Gonçalves MS, Barral-Netto M, 2014. DDX39B (BAT1), TNF and IL6 gene polymorphisms and association with clinical outcomes of patients with Plasmodium vivax malaria. Malar J 13: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shute GT, 1988. The microscpopic diagnosis of malaria. Wernsdorfer WH, McGregor SI, eds. Malaria: Principles and Practice of Malariology. New York, NY: Churchill Livingstone, 781–814. [Google Scholar]
- 30.Snounou G, 1996. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol 50: 263–291. [DOI] [PubMed] [Google Scholar]
- 31.Gausepohl H, Boulin C, Kraft M, Frank RW, 1996. Automated multiple peptide synthesis. Pept Res 5: 315–320. [PubMed] [Google Scholar]
- 32.Oliveira-Ferreira J, Pratt-Riccio LR, Arruda M, Santos F, Daniel-Ribeiro CT, Goldberg AC, Banic DM, 2004. HLA class II and antibody responses to circumsporozoite protein repeats of P. vivax (VK210, VK247 and P. vivax-like) in individuals naturally exposed to malaria. Acta Trop 92: 63–69. [DOI] [PubMed] [Google Scholar]
- 33.Olerup O, Zetterquist H, 1992. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens 39: 225–235. [DOI] [PubMed] [Google Scholar]
- 34.Bunce M, O’Neil CM, Barnardo MCNM, Krausa P, Browning MJ, Morris PJ, Welsh KI, 1995. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46: 355–367. [DOI] [PubMed] [Google Scholar]
- 35.Baur MP, Danilovs JA, 1980. Population analysis of HLA-A, B, C, DR and other genetic markers. Terasaki PI, ed. Histocompatibility Testing. Los Angeles, CA: UCLA Tissue Typing Laboratory, 955–1210. [Google Scholar]
- 36.Dodoo D, et al., 2011. Measuring naturally acquired immune responses to candidate malaria vaccine antigens in Ghanaian adults. Malar J 10: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camargo LM, Noronha E, Salcedo JM, Dutra AP, Krieger H, Pereira da Silva LH, Camargo EP, 1999. The epidemiology of malaria in Rondonia (western Amazon region, Brazil): study of a riverine population. Acta Trop 72: 1–11. [DOI] [PubMed] [Google Scholar]
- 38.Tada MS, Marques RP, Mesquita E, Dalla Martha RC, Rodrigues JA, Costa JDN, Pepelascov RR, Katsuragawa TH, Pereira-da-Silva LH, 2007. Urban malaria in the Brazilan western Amazon Region. I. High prevalence of asymptomatic carriers in an urban riverside district is associated with a high level of clinical malaria. Mem Inst Oswaldo Cruz 102: 263–269. [DOI] [PubMed] [Google Scholar]
- 39.Costa JD, Zanchi FB, Rodrigues FL, Honda ER, Katsuragawa TH, Pereira DB, Taborda RL, Tada MS, Ferreira RG, Pereira-da-Silva LH, 2013. Cross-reactive anti-PfCLAG9 antibodies in the sera of asymptomatic parasite carriers of Plasmodium vivax. Mem Inst Oswaldo Cruz 108: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fratus AS, Cabral FJ, Fotoran WL, Medeiros MM, Carlos BC, Martha R, da Silva LH, Lopes SC, Costa FT, Wunderlich G, 2014. Antibody recognition of Plasmodium falciparum infected red blood cells by symptomatic and asymptomatic individuals in the Brazilian Amazon. Mem Inst Oswaldo Cruz 109: 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM, 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173: 765–769. [DOI] [PubMed] [Google Scholar]
- 42.Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, Nahlen BL, Bloland PB, Kaslow DC, Lal AA, 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg 58: 211–219. [DOI] [PubMed] [Google Scholar]
- 43.Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Perignon JL, Druilhe P, 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4: e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW, 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis 197: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nebie I, et al., 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinicalmalaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun 76: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osier FH, et al., 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford L, Lobo CA, Rodriguez M, Zalis MG, Machado RL, Rossit AR, Cavasini CE, Couto AA, Enyong PA, Lustigman S, 2007. Differential antibody responses to Plasmodium falciparum invasion ligand proteins in individuals living inmalaria-endemic areas in Brazil and Cameroon. Am J Trop Med Hyg 77: 977–983. [PubMed] [Google Scholar]
- 48.Sarr JB, et al., 2012. Differential acquisition of human antibody responses to Plasmodium falciparum according to intensity of exposure to Anopheles bites. Trans R Soc Trop Med Hyg 106: 460–467. [DOI] [PubMed] [Google Scholar]
- 49.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, Daugherty JR, Lanar DE, Conway DJ, 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun 68: 5559–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards JS, et al., 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51: e50–e60. [DOI] [PubMed] [Google Scholar]
- 51.Villasis E, Lopez-Perez M, Torres K, Gamboa D, Neyra V, Bendezu J, Tricoche N, Lobo C, Vinetz JM, Lustigman S, 2012. Anti-Plasmodium falciparum invasion ligand antibodies in a low malaria transmission region, Loreto, Peru. Malar J 11: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nhabomba AJ, et al., 2014. Impact of age of first exposure to Plasmodium falciparum on responses to malaria in children: a randomized, controlled trial in Mozanbique. Malar J 13: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW, 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 73: 222–228. [PubMed] [Google Scholar]
- 54.Arama C, et al., 2015. Genetic resistance to malaria is associated with greater enhancement of immunoglobulin (Ig)M than IgG responses to a broad array of Plasmodium falciparum antigens. Open Forum Infect Dis 2: ofv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, Takacs B, Schönfeld HJ, Holder AA, Greenwood BM, 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol 14: 321–337. [DOI] [PubMed] [Google Scholar]
- 56.Al-Yaman F, Genton B, Anders RF, Falk M, Triglia T, Lewis D, Hii J, Beck HP, Alpers MP, 1994. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg 51: 593–602. [DOI] [PubMed] [Google Scholar]
- 57.Cavanagh DR, Dodoo D, Hviid L, Kurtzhals JA, Theander TG, Akanmori BD, Polley S, Conway DJ, Koram K, McBride SJ, 2004. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect Immun 72: 6492–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metzger WG, Okenu DM, Cavanagh DR, Robinson JV, Bojang KA, Weiss HA, McBride JS, Greenwood BM, Conway DJ, 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with reduced prospective risk of malaria. Parasite Immunol 25: 307–312. [DOI] [PubMed] [Google Scholar]
- 59.Polley SD, Conway DJ, Cavanagh DR, McBride JS, Lowe BS, Williams TN, Mwangi TW, Marsh K, 2006. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 24: 4233–4246. [DOI] [PubMed] [Google Scholar]
- 60.Stanisic DI, et al., 2009. Immunoglobulin-G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherif MK, et al., 2015. Is Fc gamma receptor IIA (FcγRIIA) polymorphism associated with clinical malaria and Plasmodium falciparum specific antibody levels in children from Burkina Faso? Acta Trop 142: 41–46. [DOI] [PubMed] [Google Scholar]
- 62.Ortega E, Soto-Cruz I, 2007. Early biochemical events in leukocyte activation through receptors for IgG. Signal Transduct 7: 415–426. [Google Scholar]
- 63.Aucan C, Traoré Y, Tall F, Nacro B, Traoré-Leroux T, Fumoux F, Rihet P, 2000. Infect High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Immun. 68: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasr A, Iriemenam NC, Troye-Blomberg M, Giha HA, Balogun HA, Osman OF, Montgomery SM, ElGhazali G, Berzins K, 2007. Fc gamma receptor IIa (CD32) polymorphism and antibody responses to asexual blood-stage antigens of Plasmodium falciparum malaria in Sudanese patients. Scand J Immunol 66: 87–96. [DOI] [PubMed] [Google Scholar]
- 65.Dubois B, Deloron P, Astagneau P, Chougnet C, Lepers JP, 1993. Isotypic analysis of Plasmodium falciparum-specific antibodies and their relation to protection in Madagascar. Infect Immun 61: 4498–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucchi NW, et al., 2008. Antibody responses to the merozoite surface protein-1 complex in cerebral malaria patients in India. Malar J 7: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarthou JL, Angel G, Aribot G, Rogier C, Dieye A, Toure Balde A, Diatta B, Seignot P, Roussilhon P, 1997. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun 65: 3271–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tangteerawatana P, Krudsood S, Chalermrut K, Looareesuwan S, Khusmith S, 2001. Natural human IgG subclass antibodies to Plasmodium falciparum blood stage antigens and their relation to malaria resistance in an endemic area of Thailand. Southeast Asian J Trop Med Public Health 32: 247–254. [PubMed] [Google Scholar]
- 69.Snapper CM, Finkelman FD, 1999. Immunoglobulin class switching. Paul WE, ed., Fundamental Immunology, 4th edition. Philadelphia, PA: Lippincott-Raven, 831–861. [Google Scholar]
- 70.Stavnezer J, 1996. Immunoglobulin class switching. Curr Opin Immunol 8: 199–205. [DOI] [PubMed] [Google Scholar]
- 71.Tongren JE, et al., 2006. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun 74: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oluwasogo OA, Ebenezer OO, Chiaka A, 2012. Evaluation of host humoral antibody production against Plasmodium falciparum recombinant circumsporozoite antigen in Nigerian children. J Vector Borne Dis 49: 151–156. [PubMed] [Google Scholar]
- 73.Noland GS, Jansen P, Vulule JM, Park GS, Ondigo BN, Kazura JW, Moormann AM, John CC, 2015. Effect of transmission intensity and age on subclass antibody responses to Plasmodium falciparum pre-erythrocytic and blood-stage antigens. Acta Trop 142: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.John CC, Ouma JH, Sumba PO, Hollingdale MR, Kazura JW, King CL, 2002. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am J Trop Med Hyg 66: 372–378. [DOI] [PubMed] [Google Scholar]
- 75.John CC, Zickafoose JS, Sumba PO, King CL, Kazura JW, 2003. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect Immun 71: 4320–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Courtin D, et al., 2009. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One 4: e7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mamo H, Esen M, Ajua A, Theisen M, Mordmüller B, Petros B, 2013. Humoral immune response to Plasmodium falciparum vaccine candidate GMZ2 and its components in populations naturally exposed to seasonal malaria in Ethiopia. Malar J 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garraud O, Nkenfou C, Bradley JE, Perler FB, Nutman TB, 1995. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol 155: 1316–1325. [PubMed] [Google Scholar]
- 79.Toure FS, Deloron P, Migot-Nabias F, 2006. Analysis of human antibodies to erythrocyte binding antigen 175 peptide 4 of Plasmodium falciparum. Clin Med Res 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mewono L, Matondo-Maya DW, Matsiegui PB, Agnandji ST, Kendjo E, Barondi F, Issifou S, Kremsner PG, Mavoungou E, 2008. Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of Plasmodium falciparum in Gabonese children with acute falciparum malaria. Eur Cytokine Netw 19: 30–36. [DOI] [PubMed] [Google Scholar]
- 81.Patarroyo ME, et al., 1991. Genetic control of the immune response to a synthetic vaccine against Plasmodium falciparum. Parasite Immunol 13: 509–516. [DOI] [PubMed] [Google Scholar]
- 82.Beck HP, Felger I, Genton B, Alexander N, al-Yaman F, Anders RF, Alpers M, 1995. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect Immun 63: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stephens HA, Brown AE, Chandanayingyong D, Webster HK, Sirikong M, Longta P, Vangseratthana R, Gordon DM, Lekmak S, Rungruang E, 1995. The presence of the HLA class II allele DPB1*0501 in ethnic Thais correlates with an enhanced vaccine-induced antibody response to a malaria sporozoite antigen. Eur J Immunol 25: 3142–3147. [DOI] [PubMed] [Google Scholar]
- 84.Nardin EH, Oliveira GA, Calvo-Calle JM, Castro ZR, Nussenzweig RS, Schmeckpeper B, Hall BF, Diggs C, Bodison S, Edelman R, 2000. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J Infect Dis 182: 1486–1496. [DOI] [PubMed] [Google Scholar]
- 85.Johnson AH, et al., 2004. Human leukocyte antigen class II alleles influence levels of antibodies to the Plasmodium falciparum asexual-stage apical membrane antigen 1 but not to merozoite surface antigen 2 and merozoite surface protein 1. Infect Immun 72: 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q, Xue X, Xu X, Wang C, Chang W, Pan W, 2009. Influence of HLA-DRB1 alleles on antibody responses to PfCP-2.9-immunized and naturally infected individuals. J Clin Immunol 29: 454–460. [DOI] [PubMed] [Google Scholar]
- 87.Storti-Melo LM, et al., 2012. Influence of HLA-DRB-1 alleles on the production of antibody against CSP, MSP-1, AMA-1, and DBP in Brazilian individuals naturally infected with Plasmodium vivax. Acta Trop 121: 152–155. [DOI] [PubMed] [Google Scholar]
- 88.Lima-Junior JC, et al., 2012. Influence of HLA-DRB1 and HLA-DQB1 alleles on IgG antibody response to the P. vivax MSP-1, MSP-3α and MSP-9 in individuals from Brazilian endemic area. PLoS One 7: e36419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lima-Junior J, Pratt-Riccio LR, 2016. Major histocompatibility complex and malaria: focus on Plasmodium vivax infection. Front Immunol 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]