Abstract
Currently, the most effective treatment for recurrent urinary tract infections in women is antibiotics. However, the limitation for this treatment is the duration and dosage of antibiotics and the resistance that bacteria develop after a long period of administration. With the aim of identifying mainly novel natural agents with antibacterial activity, the present study was undertaken to investigate the biological and phytochemical properties of extracts from the leaves Taraxacum officinale. The structural identification of compounds present in hexane (Hex) and ethyl acetate (AcOEt) extracts was performed by mass spectrometry (GC-MS) spectroscopic techniques and nuclear magnetic resonance (NMR) with the major compounds corresponding to different sesquiterpene lactones (α-santonin, glabellin, arborescin, and estafiatin), monoterpene (9,10-dimethyltricycle [4.2.1.1 (2,5)]decane-9,10-diol), phytosterol (Stigmasta-5,22-dien-3β-ol acetate), terpenes (lupeol acetate, pregn-5-en-20-one-3β-acetyloxy-17-hydroxy, 2-hydroxy-4-methoxy benzaldehyde), and coumarin (benzofuranone 5,6,7,7-a-tetraaldehyde-4,4,7a-trimethyl). The results obtained show that the Hex extract was highly active against Staphylococcus aureus showing a MIC of 200 μg/mL and moderately active against Escherichia coli and Klebsiella pneumoniae with MIC values of 400 μg/mL and 800 μg/mL for the other Gram-negative strains tested with Proteus mirabilis as uropathogens in vitro. Therefore, the effective dandelion extracts could be used in the development of future products with industrial application.
1. Introduction
The increasing resistance of uropathogens to antibiotics and the recognition of the generally self-limiting nature of uncomplicated urinary tract infection (UTI) suggest that it is time to reconsider the empirical treatment of UTI by using antibiotics. Limitation for this treatment is the duration and dosage of antibiotics and the resistance that bacteria develop after a long period of administration [1]. Therefore, alternatives to the pharmaceutical industry approaches need to be considered. During our continuous exploratory search for new antibacterial extracts, we have selected the Chilean medicinal plant Taraxacum officinale Weber ex F. H. Wigg. (Asteraceae), commonly known as dandelion, for a systematic study on the chemical composition and potential antibacterial properties.
T. officinale is an herbaceous perennial plant of the family Asteraceae, native to Asia; it can be found growing in temperate regions of the world, including Chile in the III-IX region. Dandelion is considered a weedy species [2]; it has numerous antioxidant, anti-inflammatory, antidiabetic, antimicrobial, and anticancer properties [3, 4]. There are several references to treating bacterial infections and the use of traditional botanical remedies [5–7]. These properties have been attributed to the large number of bioactive compounds in their tissues, and several studies have reported a wide range of compounds, including terpenes, flavonoids, and phenolic compounds, which are mentioned as responsible for the medicinal activity of different plants [8, 9]. For the genus Taraxacum, only few studies concerning its antimicrobial properties consider chemical identification of the extracts obtained and, most of the time, this identification is qualitative (e.g., using colorimetric methods indicating presence or absence). Authors report the presence of terpenoids, triterpenoids, steroids, coumarins, phenols, saponins, flavonoids, flavones, flavonols, chalcones, phlobatannins, and cardiac glycosides in antimicrobial extracts [10–18], but neither compound isolation nor further identification was performed.
Therefore, based on the above reasoning and observations, the aim of the study was to explore the phytochemical of n-hexane and ethyl acetate extracts of the Taraxacum officinale plant and its antibacterial activity against selected uropathogenic bacterial strains.
2. Results and Discussion
2.1. Chromatographic Analysis
Results of the gas chromatography analysis of hexane and ethyl acetate extracts from leaves of T. officinale are summarized in Tables 1 and 2, respectively.
Table 1.
Main components of Hex extract.
| No. | RT (min) |
Main components | RIa | % areab |
|---|---|---|---|---|
| 1 | 14.74 | 2-Cyclohexen-1-ol | 892 | 1.81 |
| 2 | 16.47 | 2-Cyclohexen-1-one | 942 | 0.74 |
| 3 | 29.8 | α-Ionene | 1372 | 0.29 |
| 4 | 34.01 | Brassicasterol acetate | 1538 | 0.25 |
| 5 | 35.61 | Diethyl phthalate | 1605 | 4.25 |
| 6 | 40.77 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 1841 | 13.08 |
| 7 | 40.91 | 4-Octadecenal | 1848 | 0.39 |
| 8 | 43.15 | n-Hexadecanoic acid | 1960 | 1.05 |
| 9 | 43.82 | Hexadecanoic acid, ethyl ester | 1994 | 0.41 |
| 10 | 46.13 | Phytol | 2118 | 3.48 |
| 11 | 47.13 | Ethyl 9,12,15-octadecatrienoate | 2174 | 1.49 |
| 12 | 71.44 | Lupeol | 3443 | 23.31 |
| 13 | 75.13 | Lupeol acetate | 3516 | 25.09 |
| 14 | 75.26 | Betulin | 3518 | 23.81 |
aRI: retention indices relative to C8–C36 n-alkanes on the Rtx-5MS capillary column. bSurface area of GC peak.
Table 2.
Main components of AcOEt extract.
| No. | RT (min) |
Main components | RIa | % areab |
|---|---|---|---|---|
| 1 | 13.64 | 1,2-Epoxycyclohexane | 865 | 0.19 |
| 2 | 14.72 | 2-Cyclohexen-1-ol | 892 | 0.90 |
| 3 | 16.44 | 2-Cyclohexen-1-one | 941 | 0.41 |
| 4 | 40.68 | Hexahydrofarnesol | 1837 | 0.76 |
| 5 | 40.79 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 1842 | 15.57 |
| 6 | 43.19 | n-hexadecanoic acid | 1962 | 0.83 |
| 7 | 43.83 | Hexadecanoic acid, ethyl ester | 1994 | 0.37 |
| 8 | 46.15 | Phytol | 2119 | 0.75 |
| 9 | 46.65 | Linolenic acid | 2147 | 0.67 |
| 10 | 47.01 | Linoleic acid ethyl ester | 2167 | 0.23 |
| 11 | 47.15 | Ethyl 9,12,15-octadecatrienoate | 2175 | 0.94 |
| 12 | 47.98 | 2-[(Z)-9-octadecenyloxyethanol] | 2222 | 0.48 |
| 13 | 51.17 | 3-Ethyl-3-hydroxy-5α-androstan-17-one | 2413 | 1.61 |
| 14 | 70.82 | (22Z)-Stigmasta-5,22-diene-3β-ol acetate | 3426 | 2.98 |
| 15 | 72.95 | β-sitosterol | 3483 | 4.55 |
| 16 | 73.43 | α-Amyrin | 3496 | 4.78 |
| 17 | 75.19 | Lupeol acetate | 3517 | 19.95 |
| 18 | 79.09 | Betulin | 3557 | 6.17 |
aRI: retention indices relative to C8–C36 n-alkanes on the Rtx-5MS capillary column. bSurface area of GC peak.
Forty components were identified in the hexane extract: 72.46% were triterpenoids, 16.56% terpenes, 4.25% phthalate ester, 1.46% fatty acids and derivatives, 1.42% aldehydes and ketones, 1.81% alcohols, and 0.55% unknown compounds. The hexane extract was mainly characterized by lupeol acetate (25.09%), betulin (23.81%), lupeol (23.31%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (13.08%), diethyl phthalate (4.25%), and phytol (3.48%). On the other hand, eighty components were identified in the ethyl acetate extract: 37.06% were triterpenoids, 17.08% terpenes, 3.04% fatty acids and derivatives, 1.50% ketones and alcohols, and 37.86% unknown compounds. The ethyl acetate extract was mainly characterized by lupeol acetate (19.95%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (15.57%), betulin (6.17%), α-Amyrin (4.78%), β-sitosterol (4.55%), and (22Z)-Stigmasta-5,22-dien-3β-ol acetate (2.98%). In addition, the composition of both extracts of leaves from T. officinale in which triterpenoids were the predominant portion found was reported, unlike other studies in which it has been described that most compounds constituting the leaf part are polyphenols and flavonoids glycosides [4].
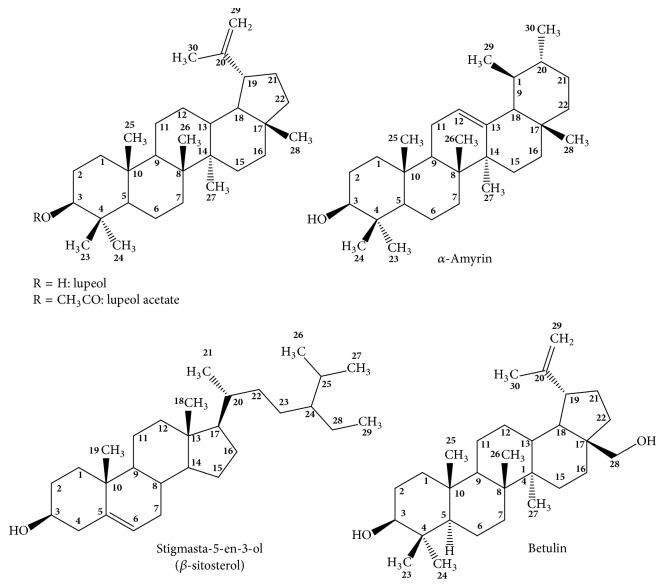
Most of the metabolites isolated belonging to the group of terpenes (lupeol, lupeol acetate, α-Amyrin, β-sitosterol, and betulin) from both extracts were characterized and identified by different NMR experiments (1H-NMR, 13C NMR 1D, and 2D). In addition, all the spectra of these products were compared with literature data and with authentic samples acquired from Sigma-Aldrich. The structures of these terpenes are shown in Figure 1.
Figure 1.
Structures of main terpenes isolates of n-hexane and ethyl acetate extracts.
2.2. Spectroscopic NMR Characterization
2.2.1. Lupeol
1 H-NMR. 4.68 (1H, bs, H-29a); 4.56 (1H, bs, H-29b); 3.18 (1H, t, H-3); 2.36 (1H, m, H-19); 1.67 (3H, s, H-30); 1.62 (2H, m, H-2); 1.38 (2H, m, H-6); 1.35 (1H, dd, H-18); 1.25 (1H, t, H-9); 1.02 (3H, s, H-26); 0.96 (3H, s, H-23); 0.94 (3H, s, H-27); 0.82 (3H, s, H-25); 0.78 (3H, s, H-28); 0.67 (1H, t, H-5).
13 C-NMR. 151.2 (C-20); 109.5 (C-29); 79.2 (C-3); 55.5 (C-5); 50.6 (C-9); 48.5 (C-18); 48.2 (C-19); 43.2 (C-17); 43.0 (C-14); 41.0 (C-8); 40.2 (C-22); 39.0 (C-4); 38.9 (C-1); 38.2 (C-13); 37.3 (C-10); 35.8 (C-16); 34.4 (C-7); 30.0 (C-21); 28.2 (C-23); 27.2 (C-15); 27.6 (C-2); 25.3 (C-12); 21.1 (C-11); 19.5 (C-30); 18.5 (C-6); 18.1 (C-28); 16.3 (C-26); 16.2 (C-25); 15.6 (C-24); 14.7 (C-27) [19–22].
2.2.2. Lupeol Acetate
1 H-NMR. 4.69 (1H, d, J = 2.1 Hz, H-29a); 4.57 (1H, m, H-29b); 4.48 (1H, dd, J = 10.5 and 6.6 Hz, H-3); 2.37 (1H, dt, J = 11.1 and 5.7 Hz, H-19); 2.04 (3H, s, CH3CO); 1.68 (3H, s, H-30); 1.03 (3H, s, H-26); 0.94 (3H, s, H-27); 0.88 (3H, s, H-24); 0.88 (3H, s, H-25); 0.70 (3H, s, H-28).
13 C-NMR. 171.4 (CO); 151.4 (C-20); 109.7 (C-29); 81.3 (C-3); 55.7 (C-5); 50.7 (C-9); 48.3 (C-18); 48.3 (C-19); 43.2 (C-17); 42.8 (C-14); 41.2 (C-8); 40.3 (C-22); 38.7 (C-1); 38.4 (C-4); 38.1 (C-13); 37.4 (C-10); 34.5 (C-16); 34.5 (C-7); 30.2 (C-21); 28.3 (C-23); 27.8 (C-2); 25.4 (C-15); 25.4 (C-12); 21.3 (C-11); 21.1 (CH3CO); 19.6 (C-30); 18.5 (C-6); 18.3 (C-28); 16.8 (C-24); 16.5 (C-25); 16.3 (C-26); 14.8 (C-27) [23–25].
2.2.3. α-Amyrin
1 H-NMR. 5.06 (1H, t, J = 3.2 Hz, H-12); 3.16 (1H, dd, J = 11.2 and 5.1 Hz, H-3); 1.94 (1H, dt, J = 13.5 and 4.5 Hz, H-15β); 1.85 (1H, dt, J = 7.0 and 3.0 Hz, H-22); 1.76 (1H, dt, J = 13.5 and 5.0 Hz, H-16β); 1.01 (3H, s, H-27); 0.94 (3H, s, H-28); 0.93 (3H, s, H-23); 0.89 (3H, s, H-26); 0.85 (3H, d, J = 6.0 Hz, H-29); 0.73 (3H, d, J = 7.0 Hz, H-30); 0.67 (1H, d, J = 11.6 Hz, H-5).
13 C-NMR. 139.5 (C-13); 124.4 (C-12); 79.6 (C-3); 59.0 (C-18); 55.1 (C-5); 47.7 (C-9); 42.0 (C-14); 41.5 (C-22); 40.0 (C-8); 39.6 (C-19); 39.6 (C-20); 38.7 (C-1); 38.7 (C-4); 36.9 (C-10); 33.7 (C-17); 32.2 (C-7); 31.2 (C-21); 28.7 (C-2); 28.1 (C-28); 28.1 (C-23); 27.2 (C-15); 26.6 (C-16); 23.3 (C-11); 23.2 (C-27); 21.4 (C-30); 18.4 (C-6); 17.4 (C-29); 16.8 (C-26); 15.6 (C-25); 15.6 (C-24) [20, 22, 26, 27].
2.2.4. Stigmasta-5-en-3-ol (β-Sitosterol)
1 H-NMR. 5.36 (1H, t, J = 6.4 Hz, H-6); 3.53 (1H, tdd, J = 4.5, 4.2 and 3.8 Hz, H-3); 1.01 (3H, d, J = 7.2 Hz, H-29); 0.93 (3H, d, J = 6.5 Hz, H-19); 0.84 (3H, t, J = 7.2 Hz, H-24); 0.83 (3H, d, J = 6.4 Hz, H-26); 0.81 (3H, d, J = 6.4 Hz, H-27); 0.68 (3H, s, H-18).
13 C-NMR. 140.9 (C-5); 121.9 (C-6); 72.0 (C-3); 56.9 (C-14); 56.3 (C-17); 50.3 (C-9); 46.1 (C-22); 42.6 (C-13); 42.5 (C-4); 39.9 (C-12); 37.5 (C-1); 36.7 (C-10); 36.3 (C-18); 34.2 (C-20); 32.1 (C-7); 32.1 (C-8); 31.9 (C-2); 29.4 (C-25); 28.5 (C-16); 26.3 (C-15); 26.3 (C-21); 23.3 (C-23); 21.3 (C-11); 20.1 (C-26); 19.6 (C-27); 19.2 (C-19); 19.0 (C-28); 12.2 (C-24); 12.0 (C-29) [28, 29].
2.2.5. Betulin
1 H-NMR. 4.70 (1H, bs, H-29b); 4.58 (1H, bs, H-29a); 3.79 (1H, d, J = 10.8 Hz, H-28b); 3.33 (1H, d, J = 10.8 Hz, H-28a); 3.18 (1H, dd, J = 10.2 and 5.3 Hz, H-3); 1.67 (3H, s, H-30); 0.99 (3H, s, H-27); 0.97 (3H, s, H-26); 0.96 (3H, s, H-23); 0.80 (3H, s, H-25); 0.75 (3H, s, H-24).
13 C-NMR. 150.6 (C-20); 109.8 (C-29); 79.2 (C-3); 60.6 (C-28); 55.4 (C-5); 50.5 (C-9); 48.8 (C-19); 47.9 (C-17); 47.9 (C-18); 42.8 (C-14); 41.0 (C-8); 38.9 (C-1); 38.8 (C-4); 37.4 (C-10); 37.2 (C-13); 34.3 (C-7); 34.1 (C-22); 29.8 (C-21); 29.2 (C-16); 28.1 (C-23); 27.5 (C-2); 27.1 (C-15); 25.3 (C-12); 20.9 (C-11); 19.2 (C-30); 18.4 (C-6); 16.2 (C-25); 16.1 (C-26); 15.4 (C-24); 14.8 (C-27) [30, 31].
Recently, other authors have determined from ethanol extract three novel lupane-, bauerane-, and euphane-type triterpenoids and other known triterpenes present in dandelion roots, using spectroscopic analysis, which have potential anti-inflammatory activity [32, 33]. Dandelion leaves in this study were found to be rich in triterpenes such as betulin, lupeol acetate, lupeol, α-Amyrin, and β-sitosterol from both extracts with potential antibacterial activity against pathogens that infect the urinary tract. In several investigations, the toxicity of dandelion was found to be low, due to absence of any significant toxins or alkaloids [4]. Therefore, it would be interesting to improve the biological activity by enhancing the extract with other additives to develop a pharmaceutical product for medicinal purposes.
2.3. Determination of the Fatty Acids
It was determined that the total fatty acid content of the dandelion leaves, using the Soxhlet method, is 4.8 ± 0.5% raw extract/dry matter, composed of different fatty acids as shown in Figure 2 and Table 3.
Figure 2.
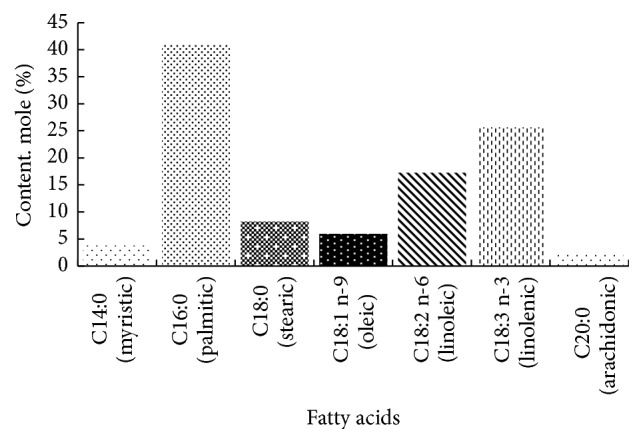
Fatty acid (% w/w of total fatty acids) composition in the leaves of T. officinale from Temuco region, Chile. The values represent the means of three samples, analysed individually in triplicate.
Table 3.
Fatty acids content in the T. officinale leaves collected from Temuco region, Chile (mg/g dry weight).
| Fatty acid | mg/g dry weight of T. officinale, Temuco, Chile |
|---|---|
| C14 : 0 (myristic) | 0,1 |
| C16 : 0 (palmitic) | 1,5 |
| C18 : 0 (stearic) | 0,3 |
| C18 : 1 n-9 (oleic) | 0,2 |
| C18 : 2 n-6 (linoleic) | 0,7 |
| C18 : 3 n-3 (linolenic) | 1,0 |
| C20 : 0 (arachidonic) | 0,1 |
Figure 2 shows the fatty acid composition of leaves sampled from T. officinale; seven fatty acids were identified in the raw extracts, from which the most abundant was palmitic acid (16 : 0) with 41 ± 0.8%. The next most abundant fatty acids were linolenic acid (18 : 3n-3) and linoleic acid (18 : 2n-6), corresponding to 26 ± 1.9% and 17 ± 1.0%, respectively. Small and very small amounts of stearic (18 : 0), oleic (18 : 1n-9), myristic (14 : 0), and arachidic (20 : 0) acids were also detected.
Manning 2001 [34] mentioned that pollen lipids, dominated by long-chain unsaturated (linoleic and linolenic) and saturated (myristic and lauric) fatty acids, have bactericidal and antifungal properties, playing an important role in inhibiting the growth of the spore forming bacteria Paenibacillus (American foulbrood), Melissococcus pluton (European larvae foulbrood), and other microorganisms that inhabit the brood combs of beehives.
The total fatty acid content in leaves, relative to dry plant weight, is 3,9 mg per gram and palmitic acid 1.5 mg per gram as the prevailing fatty acid in the sample. The next two most abundant fatty acids were 18 : 2n-6 and 18 : 3n-3 (Table 1). These values are in accordance with those reported by Imai et al. (1995), Liu et al. (2002), and USDA (2016) [35–37].
2.4. In Vitro Antimicrobial Assay
The antibacterial effects of the extracts of T. officinale were evaluated against four uropathogenic bacteria (Table 4). Evaluation of the dandelion extracts revealed that the extract Hex inhibited the growth of S. aureus by 89% at 200 μg/mL and was a more effective inhibitor for the Gram-positive than for the Gram-negative strains (Table 4). K. pneumoniae was the lowest inhibited by the Hex extracts.
Table 4.
Antibacterial activity of n-hexane and ethyl acetate extract of Taraxacum officinale against the tested bacteria.
| Extracts | Percentage of growth inhibition (%)/MIC (µg/mL) at 24 h∗ | |||
|---|---|---|---|---|
| E. coli | S. aureus | K. pneumoniae | P. mirabilis | |
| Hex | 72 ± 2.1 400 |
89 ± 3.3 200 |
52 ± 0.0 400 |
70 ± 0.8 800 |
| EtOAc | 97 ± 0.9 1600 |
0 ± 0.0 | - | - |
| Chloramphenicol | 95 ± 0.0 25 |
- | 94 ± 0.0 100 |
89 ± 0.0 200 |
| Streptomycin | - | 88 ± 1.1 25 |
- | - |
∗Mean of triplicates ± standard deviation of three replicates; (-) not tested.
EtOAc extract showed low activity against E. coli. with 1600 μg/mL and growth inhibition with a 97% value. However, this extract did not exert an antibiotic effect on Gram-positive bacteria S. aureus in the concentrations tested (Table 4). Similar results have been reported where the ethanol extract of leaves of T. officinale had low antimicrobial activity against S. aureus, E. coli, and Salmonella abony [38].
In addition, it was observed that as the extract concentration increased, so does the bacteria growth inhibition. This inhibitory effect may be due to the presence of phenolic compounds, terpenes, tannins, flavonoids, alkaloids, and/or proteins in the plant extracts. Such compounds had been reported to have an active effect on the bacterial cells membrane, which may destroy these microorganisms [39, 40].
In studying the antipathogenic properties of genus Taraxacum to combat infectious diseases, T. officinale is the most studied species, but it has shown various results depending on the extraction characteristics or on the bioassay performed. For example, a methanolic extract of T. officinale at 0.2 mg/mL was effective as antibacterial against Micrococcus luteus and Vibrio cholera with MIC values of 1.0 mg/mL and 12.5 mg/mL, respectively, but did not show any activity against S. aureus, Enterobacter faecalis, Enterococcus bacteria, V. cholera, Bacillus subtilis, Pseudomonas aeruginosa, K. pneumonia, or E. coli [12]. In another work, methanolic extracts of T. officinale show an activity between 0.003 and 0.5 mg/mL on S. aureus, P. aeruginosa, B. cereus, Shigella sonnei, S. enterica serovar Typhimurium, E. coli, K. pneumonia, Candida albicans, and C. neoformans with MIC values ranging from 0.04 to 5.0 mg/mL [11]. So, these extracts could be active or improve their antibacterial activity against other Gram-negative and Gram-positive strains, and this work provides preliminary information for the development and use of natural medicine with extracts of T. officinale in the control of disease against uropathogenic bacteria, as antimicrobial agents.
3. Materials and Methods
3.1. Collection and Plant Material
The leaves of T. officinale were collected in Temuco, Cautín province (IX region), Chile, in May 2014 and authenticated by agronomist Lorena Jorquera, Ph.D., from “Pontificia Universidad Católica de Valparaíso”, V region, Chile. The leaves were transported to the laboratory, washed 3 times with water and once with sterile distilled water (WDS), then air-dried on kraft paper, and placed in hot air oven at a temperature of 45.0°C for a period of 4 days until the weight became constant. The dried plant material was converted to a powdered form with the help of clean grinder.
3.2. Preparation of the Extracts
The extracts of T. officinale (1,5 g, 1,5%) were obtained from dried plant material (100 g) suspended first in 400 mL of n-hexane and then the residues were further extracted with ethyl acetate (EtOAc) separately; that is, an extraction was performed sequentially and serially. The extraction was carried out by using an orbital shaker (150 rpm) at 25°C for 48 h at room temperature; then the extract was filtered through Whatman N° 1 filter paper (Sigma-Aldrich, Darmstadt, Germany), concentrated under reduced pressure with a rotatory evaporator, and finally kept in the refrigerator (−4°C) until the analyses were made.
3.3. Isolation of Natural Compounds from Extracts
The n-hexane and EtOAc extracts were subjected to chromatography over a silica gel column (400 g) and eluted with mixtures of increasing polarity n-hexane and EtOAc. Fractions were combined based on TLC monitoring and purified by repeated CC on silica gel columns. Compounds were identified by chromatographic analysis and spectroscopic data, including 1H- and 13C-NMR, respectively, on a Bruker Avance 400 Digital NMR spectrometer operating at 400.1 MHz for 1H and 100.6 MHz for 13C, and comparisons with data reported in the literature were made.
3.4. Chromatographic Analysis
The n-hexane and ethyl acetate extract was diluted with acetone, and analysis by gas chromatography (Hewlett Packard, Palo Alto, CA, USA) was carried out according to the method detailed elsewhere [41]. The operating conditions were as follows: on-column injection; injector temperature, 250°C; detector temperature, 280°C; carrier gas, He at 1.0 mL/min; oven temperature program, 40°C increased to 260°C at 4°C/min and then 260°C for 5 min, to achieve the best separation through a capillary Rtx-5MS column. The mass detector ionization employed an electron impact of 70 eV. Compounds in the chromatograms were identified by comparison of their mass spectra with those in the NIST11 library database [42]. Chromatographic peaks were considered “unknown” when their similarity index (MATCH) and reverse similarity index (RMATCH) were less than 850 and discarded in this identification process [43]. These parameters referred to the degree in which the target spectrum matches the standard spectrum in the NIST Library (the value 1000 indicates a perfect fit), by comparison of their retention index with those reported in the literature [44], for the same type of column or those of commercial standards, when available. The retention indices were determined under the same operating conditions in relation to a homologous n-alkanes series (C8–C36) by
| (1) |
where n is the number of carbon atoms in the smaller n-alkane, N is the number of carbon atoms in the larger n-alkane, and Tr is the retention time. Components relative concentrations were obtained by peak area normalization.
3.5. Fatty Acids Determination
The gas chromatography analysis was performed on an Agilent Technologies 7890B gas chromatograph using a 30 m capillary column, Supelco Omega-Wax, 0.25 mm (Agilent Corp. CA). Hydrogen was used as a carrier gas at a flow rate of 1 mL/min. Detection was with flame-ionization detection and areal quantitation was made with an auxiliary automatic integrator. A temperature of 170°C was used at the beginning, which was maintained for two minutes, rising from 2,5°C/min to a final temperature of 240°C, which was maintained for 3 min. Injector and detector remained at 250°C and 270°C, respectively. Methyl esters were prepared according to our previously described procedure [45] by treating the extracted oil with 0.5 M NaOH in methanol, followed by 14% BF3 in methanol treatment. The acid identification was made by comparing the relative retention times of FAME peaks from samples with standards. The results were recorded, processed, and expressed in relative percentage of each fatty acid.
3.6. Bacterial Strains
The uropathogenic bacteria were clinical isolates belonging to the Chemistry Department, Biological Tests Laboratory (Universidad Técnica Federico Santa María) collection. They comprised Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, and Proteus mirabilis. The identification of all isolates was confirmed through genomic DNA analysis by sequencing (Macrogen USA, Cambridge, MA). Strains were cultured in Mueller-Hinton Broth (MHB) and Mueller-Hinton agar at 37°C.
3.7. In Vitro Antimicrobial Assay
The minimum inhibitory concentration (MIC) of the plant extract was determined using the broth serial dilution method with modifications, following Andrews 2001 [46]. Briefly, the extracts were first dissolved in ethanol (ET) and the final concentration of ET in each microwell was less than 1%, which did not affect the growth of the test strain. A dilution range of the extracts from 12.5 μg/mL to 1600 μg/mL was tested. Later, 1.5 μL of microbial suspension (1 × 106 CFU/mL) of each strain was inoculated into sterile 96-well microplates with equal volume of MHB broth and plant extracts. Then, the 96-well microplates were incubated aerobically at 37°C for 24 h in a shaker at 120 rpm. MIC was determined according to the OD600 obtained in an Accu Reader M965 spectrophotometer. After the cultures were incubated at 37°C for 24 h, the minimum inhibitory concentration (MIC) was confirmed as the lowest concentration of the test extract that demonstrated no visible growth. The plates were incubated for 24 h at 37°C. Chloramphenicol and streptomycin were used as positive control tested at the same concentrations for bacterial strains and 1% ET was used as negative control with inoculum. Also, a negative control was used with 1% ET without inoculum to subtract the OD600 obtained. Each concentration of the extracts was tested in triplicate. Each value represents the mean ± SD of three experiments performed in triplicate, for which the mean and standard deviation for each value were calculated.
Finally, the percentage of bacterial growth inhibition (GI) was calculated using the following formula:
| (2) |
where C Abs is the absorbance of the control treatment and T Abs is the absorbance of samples treated with different extracts.
4. Conclusions
Different secondary metabolites of the n-hexane and ethyl acetate extracts were isolated from T. officinale (Compositae) leaves, mainly known as triterpenoids, and other unknown compounds to a lesser extent. Their presence and structures were elucidated by spectroscopic analyses. In addition, the fatty acids content was determined showing a higher content of palmitic and linolenic acids. Both extracts present antibacterial activity against uropathogenic clinical bacteria, being more active against Gram-positive bacteria (MIC 200 μg/mL, 89% of bacterial inhibition). These results suggest that both extracts have potential as antibacterial uropathogenic disease agents.
Acknowledgments
The authors thank FCR-CSB, CORFO-INNOVA project Grant no. 09CEII-6991, and the Department of Chemistry of Universidad Técnica Federico Santa María.
Contributor Information
Katy Díaz, Email: katy.diaz@usm.cl.
Rolando Chamy, Email: rchamy@ucv.cl.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Katy Díaz Peralta designed the research, performed extractions, separation, and purification of the compounds, performed the bioassays, and wrote the biological component of this document. Rolando Chamy Maggi contributed to research design, copywriting, and corrections. Luis Espinoza Catalán contributed to the structures determination by spectroscopic methods (1D, 2D NMR) and document writing. Alejandro Madrid Villegas contributed to the interpretation of the results (GC-M) and document writing. Leonardo Pizarro Dasso contributed to the determination of fatty acids by chromatographic methods. All authors read and approved the final document.
References
- 1.Foxman B., Buxton M. Alternative approaches to conventional treatment of acute uncomplicated urinary tract infection in women. Current Infectious Disease Reports. 2013;15(2):124–129. doi: 10.1007/s11908-013-0317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart-Wade S. M., Neumann S., Collins L. L., Boland G. J. The biology of Canadian weeds. 117. Taraxacum officinale G. H. Weber ex Wiggers. Canadian Journal of Plant Science. 2002;82(4):825–853. doi: 10.4141/P01-010. [DOI] [Google Scholar]
- 3.González-Castejón M., Visioli F., Rodriguez-Casado A. Diverse biological activities of dandelion. Nutrition Reviews. 2012;70(9):534–547. doi: 10.1111/j.1753-4887.2012.00509.x. [DOI] [PubMed] [Google Scholar]
- 4.Schütz K., Carle R., Schieber A. Taraxacum—a review on its phytochemical and pharmacological profile. Journal of Ethnopharmacology. 2006;107(3):313–323. doi: 10.1016/j.jep.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z. Clinical study of 96 cases with chronic hepatitis B treated with jiedu yanggan gao by a double-blind method. Chinese Journal of Integrated Traditional and Western Medicine. 1990;10(2):71–74. [PubMed] [Google Scholar]
- 6.Lardos A. The botanical materia medica of the Iatrosophikon - A collection of prescriptions from a monastery in Cyprus. Journal of Ethnopharmacology. 2006;104(3):387–406. doi: 10.1016/j.jep.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Silva N. C. C., Fernandes Júnior A. Biological properties of medicinal plants: a review of their antimicrobial activity. Journal of Venomous Animals and Toxins including Tropical Diseases. 2010;16(3):402–413. doi: 10.1590/s1678-91992010000300006. [DOI] [Google Scholar]
- 8.Chadwick M., Trewin H., Gawthrop F., Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. International Journal of Molecular Sciences. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mir M., Sawhney S., Jassal M. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker Journal of Pharmacy and Pharmacology. 2013;2(1):1–5. [Google Scholar]
- 10.Ragasa C., Apuada M., Rideout J. Terpenoids from Taraxacum officinale. National Research Council of the Philippines Research Journal. 2009;10(1):17–26. [Google Scholar]
- 11.Fabri R. L., Nogueira M. S., Dutra L. B., Bouzada M. L. M., Scio E. Antioxidant and antimicrobial potential of asteraceae species. Revista Brasileira de Plantas Medicinais. 2011;13(2):183–189. doi: 10.1590/S1516-05722011000200009. [DOI] [Google Scholar]
- 12.Khan A. M., Qureshi R. A., Gillani S. A., Ullah F. Antimicrobial activity of selected medicinal plants of Margalla hills, Islamabad, Pakistan. Journal of Medicinal Plant Research. 2011a;5(18):4665–4670. [Google Scholar]
- 13.Khan A. M., Qureshi R. A., Ullah F., et al. Phytochemical analysis of selected medicinal plants of Margalla hills and surroundings. Journal of Medicinal Plant Research. 2011b;5(25):6017–6023. doi: 10.5897/JMPR11.869. [DOI] [Google Scholar]
- 14.Abdul K., Jassim N., Safanah A., Omar M. Identification of Dandelion Taraxacum officinale Leaves Components and Study Its Extracts Effect on Different Microorganisms. Journal of Al-Nahrain University. 2012;15(3):7–14. [Google Scholar]
- 15.Lateef O., Issah Y. Screening ethanolic and aqueous leaf extracts of Taraxacum offinale for in vitro bacteria growth inhibition. Journal of Pharmaceutical and Biomedical Sciences Vol. 2012;20(6):1–4. [Google Scholar]
- 16.Ghaima K. K., Hashim N. M., Ali S. A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale) Journal of Applied Pharmaceutical Science. 2013;3(5):96–99. doi: 10.7324/JAPS.2013.3518. [DOI] [Google Scholar]
- 17.Kenny O., Smyth T. J., Walsh D., Kelleher C. T., Hewage C. M., Brunton N. P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chemistry. 2014;161:79–86. doi: 10.1016/j.foodchem.2014.03.126. [DOI] [PubMed] [Google Scholar]
- 18.Tettey C. O., Ocloo A., Nagajyothi P. C. N., Lee K. D. An in vitro analysis of antiproliferative and antimicrobial activities of solvent fractions of Taraxacum officinale (Dandelion) leaf. Journal of Applied Pharmaceutical Science. 2014;4(3):41–45. doi: 10.7324/JAPS.2014.40309. [DOI] [Google Scholar]
- 19.Suryati S., Nurdin H., Dachriyanus D., Hj Lajis M. Structure elucidation of antibacterial compound from Ficus deltoidea Jack leaves. Indonesian Journal of Chemistry. 2011;11(1):67–70. [Google Scholar]
- 20.Rodríguez-Ortega M., Chumpitaz Z., Ríos S., et al. Actividad antiviral contra el virus de la fiebre amarilla, cepa vacunal 17D, de extractos de hojas de Taraxacum officinale GH Weber ex Wiggers. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2013;12:346–355. [Google Scholar]
- 21.Imam S., Azhar I., Hasan M. M., Ali M. S., Ahmed S. W. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica linn. Pakistan Journal of Pharmaceutical Sciences. 2007;20(2):125–127. [PubMed] [Google Scholar]
- 22.Mahato S. B., Kundu A. P. 13C NMR Spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry. 1994;37(6):1517–1575. doi: 10.1016/S0031-9422(00)89569-2. [DOI] [Google Scholar]
- 23.Perfeito J., Santos M., López K., Paula J., Silveira D. Characterization and biological properties of Pouteria torta extracts: a preliminary study. Revista Brasileira de Farmacognosia. 2005;15(3):183–186. doi: 10.1590/S0102-695X2005000300002. [DOI] [Google Scholar]
- 24.Ben nejma A., Besbes M., Guérineau V., Touboul D., Ben jannet H., Hamza M. A. Isolation and structure elucidation of acetylcholinesterase lipophilic lupeol derivatives inhibitors from the latex of the Tunisian Periploca laevigata. Arabian Journal of Chemistry. 2013;10:S2767–S2772. doi: 10.1016/j.arabjc.2013.10.026. [DOI] [Google Scholar]
- 25.Ragasa C., Apuada M. J., Rideout J. A. Terpenoids from Taraxacum officinale. National Research Council of the Philippines Research Journal. 2009;10:17–26. [Google Scholar]
- 26.Bandeira P. N., Lemos T. L. G., Costa S. M. O., Dos Santos H. S. Obtenção de derivados da mistura triterpenoídica α- e β-amirina. Brazilian Journal of Pharmacognosy. 2007;17(2):204–208. doi: 10.1590/S0102-695X2007000200012. [DOI] [Google Scholar]
- 27.Dias M. O., Hamerski L., Pinto A. C. Separação semipreparativa de α e β-amirina por cromatografia líquida de alta eficiência. Química Nova. 2011;34(4):704–706. doi: 10.1590/S0100-40422011000400026. [DOI] [Google Scholar]
- 28.Chaturvedula V. S., Prakash I. Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus . International Current Pharmaceutical Journal. 2012;1(9):239–242. doi: 10.3329/icpj.v1i9.11613. [DOI] [Google Scholar]
- 29.Rajput A. P., Rajput T. A. Isolation of Stigmasterol and (β-Sitosterol from Chloroform Extract of Leaves of Corchorus fascicularis Lam. International Journal of Biological Chemistry. 2012;6(4):130–135. doi: 10.3923/ijbc.2012.130.135. [DOI] [Google Scholar]
- 30.Green B., Bentley M. D., Chung B. Y., Lynch N. G., Jensen B. L. Isolation of betulin and rearrangement to allobetulin. A biomimetic natural product synthesis. Journal of Chemical Education. 2007;84(12):1985–1987. doi: 10.1021/ed084p1985. [DOI] [Google Scholar]
- 31.Tijjani A., Ndukwe I. G., Ayo R. G. Isolation and characterization of lup-20(29)-ene-3, 28-diol (Betulin) from the stem-bark of Adenium obesum (Apocynaceae) Tropical Journal of Pharmaceutical Research. 2012;11(2):259–262. doi: 10.4314/tjpr.v11i2.12. [DOI] [Google Scholar]
- 32.Saeki D., Yamada T., In Y., et al. Officinatrione: An unusual (17S)-17,18-seco-lupane skeleton, and four novel lupane-type triterpenoids from the roots of Taraxacum officinale. Tetrahedron. 2013;69(5):1583–1589. doi: 10.1016/j.tet.2012.12.001. [DOI] [Google Scholar]
- 33.Kikuchi T., Tanaka A., Uriuda M., Yamada T., Tanaka R. Three novel triterpenoids from taraxacum officinale roots. Molecules. 2016;21(9, article no. 1121) doi: 10.3390/molecules21091121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning R. Fatty acids in pollen: a review of their importance for honey bees. Bee World. 2001;82(2):60–75. doi: 10.1080/0005772X.2001.11099504. [DOI] [Google Scholar]
- 35.Imai H., Ohnishi M., Kinoshita M., Kojima M., Ito S. Structure and Distribution of Cerebroside Containing Unsaturated Hydroxy Fatty Acids in Plant Leaves. Bioscience, Biotechnology, and Biochemistry. 1995;59(7):1309–1313. doi: 10.1271/bbb.59.1309. [DOI] [Google Scholar]
- 36.Liu L., Howe P., Zhou Y.-F., Hocart C., Zhang R. Fatty acid profiles of leaves of nine edible wild plants: An Australian study. Journal of Food Lipids. 2002;9(1):65–71. doi: 10.1111/j.1745-4522.2002.tb00209.x. [DOI] [Google Scholar]
- 37. USDA National Nutrient Database for Standard Reference Release 28 slightly revised May, 2016. Software v.3.7 2017-02-01. Online document at: https://www.ars.usda.gov.
- 38.Ionescu D., Predan G., Rizea G. D., et al. Antimicrobial activity of some hydroalcoholic extracts of artichoke (cynara scolymus), burdock (arctium lappa) and dandelion (taraxacum officinale) Bulletin of the Transilvania University of Brasov, Series II: Forestry, Wood Industry, Agricultural Food Engineering. 2013;6(2):113–120. [Google Scholar]
- 39.Jassim A. M. N. Study of Some Eucalptus Rostrata Leaves Components and Effect of Its Extract on Different Microorganisms. Al-Mustansiriyah Journal of Science. 2005;16(2):62–71. [Google Scholar]
- 40.Kitts D. D., Hu C. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine. 2005;12(8):588–597. doi: 10.1016/j.phymed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Canales N., Montenegro I., Párraga M., et al. In Vitro Antimicrobial Activity of Embothrium coccineum Used as Traditional Medicine in Patagonia against Multiresistant Bacteria. Molecules (Basel, Switzerland) 2016;21(11) doi: 10.3390/molecules21111441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. NIST/EPA/NIH Mass Spectral Library with Search Program (Data Version: NIST 11, Software Version 2.0 g). http://webbook.nist.gov/chemistry/name-ser.html.
- 43.Santander R., Creixell W., Sánchez E., Tomic G., Silva J. R., Acevedo C. A. Recognizing Age at Slaughter of Cattle from Beef Samples Using GC/MS-SPME Chromatographic Method. Food and Bioprocess Technology. 2013;6(12):3345–3352. doi: 10.1007/s11947-012-0998-z. [DOI] [Google Scholar]
- 44.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th. IL, USA: Allured Publishing Corporation: Carol Steam; 2007. [Google Scholar]
- 45.Haraldsson G. G., Almarsson Ö. Studies on the positional specificity of lipase from mucor miehei during interesterification reactions of cod liver oil with n-3 polyunsaturated fatty acid and ethyl ester concentrates. Acta chemica Scandinavica. 1991;45:723–730. doi: 10.3891/acta.chem.scand.45-0723. [DOI] [Google Scholar]
- 46.Andrews J. M. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy. 2001;48(1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]