Abstract
Paracetamol has recently been suggested to affect emotion processing in addition to alleviating pain in humans. We investigated in adult male Hannover–Wistar rats whether acute intraperitoneally administrated paracetamol affects behavior in tests measuring anxiety, mood, motor activity, and memory. Unoperated rats received saline or a low (50 mg/kg) or high (300 mg/kg) dose of paracetamol, while rats with a spared nerve injury (SNI) model of neuropathy and sham‐operated rats received saline or the low dose of paracetamol. Rats were tested on open‐field (OFT), elevated plus‐maze (EPM), light‐dark box (LDB), novel‐object recognition (NOR), sucrose preference, rotarod, and monofilament tests. In unoperated rats, both the low and high dose of paracetamol reduced line crossings, and grooming time in the OFT, and novel preference in NOR. The high dose of paracetamol increased the time spent in the closed arm in EPM, reduced the number of rearings and leanings in OFT, the time spent in the light box in LDB, and sucrose preference. Paracetamol had no significant effect on the rotarod test measuring motor activity. The low dose of paracetamol suppressed mechanical pain hypersensitivity in SNI rats, without influencing pain behavior in sham‐operated rats. Saline‐ but not paracetamol‐treated SNI rats spent more time than sham‐operated rats in the closed arm in the EPM test. Together the results suggest that a high dose of paracetamol increases anxiety‐like and anhedonic behavior, and impairs recognition memory in unoperated controls, while in neuropathy, a low dose of paracetamol reduces nerve injury‐associated anxiety probably by reducing neuropathic pain.
Keywords: anhedonia, anxiety, neuropathic pain, paracetamol, recognition memory
Abbreviations
- EPM
elevated plus‐maze
- LDB
light‐dark box
- NOR
novel‐object recognition
- OFT
open‐field test
- SNI
spared nerve injury
1. INTRODUCTION
Paracetamol, also known as acetaminophen, is a commonly used medication to alleviate pain and reduce fever. It was accepted to be safe at the therapeutic dosage and toxic to liver at acute overdose administration. Although most studies focus on the hepatotoxicity, recent studies transferred from hepatotoxicity on neurotoxicity.1 Several recent studies on humans suggest that, in addition to its well‐known pain relieving and fever‐reducing effects, paracetamol may affect a wide variety of cognitive functions. It was shown to alter how human subjects evaluate negative and positive experiences.2, 3, 4, 5 A brain imaging study showed that, compared to a placebo, daily doses of paracetamol for 3 weeks reduced neural responses to social rejection in the insula and dorsal anterior cingulate cortex.2 This finding is in line with the suggestion that the pain of social rejection and physical pain share similar underlying neural mechanisms.3 In addition to social and physical pain, many kinds of unexpected events can produce anxiety and unease in humans and, for that matter, pain. Acute doses of paracetamol have been shown to ameliorate negative reactions to threats,4 and to reduce discomfort that people experience when making difficult decisions5 and empathy for pain.6
Paracetamol produces antinociception through mechanisms that block prostaglandin synthesis by inhibiting the cyclooxygenase enzymes.7 Also, other mechanisms of action have been suggested that link paracetamol with the cannabinoid,8, 9 opioid, and serotonergic neurotransmitter systems.10 In this study, we investigated whether acute intraperitoneal administration of paracetamol affects the behavior of healthy adult rats in tests measuring anxiety, anhedonia, motor performance, and memory. Painkillers, however, are normally taken when subjects experience pain, and not when there is no pain. We therefore also tested the effect of paracetamol on rats with a spared nerve injury model (SNI) of neuropathy in the left hind limb and on sham‐operated rats.11 As neuropathy in SNI rats causes pain and anxiety,12 we investigated how a low dose of paracetamol that has been shown to reduce mechanical hypersensitivity in neuropathic rats13 affects their pain behavior and performance in the tests that measure anxiety and anhedonia. Based on earlier reports on healthy human subjects, we hypothesized that acute effects of systemic paracetamol might reduce anxiety and distress in healthy rats. We also expected that paracetamol reduces anxiety‐ and distress‐related behavior as well as pain behavior in neuropathic rats.
2. MATERIALS AND METHODS
2.1. Animals
Adult male Hannover–Wistar rats (weight 200–300 g; age approximately 7–10 weeks; Harlan, Horst, Netherlands) were used in this study. The experimental protocols were approved by the Experimental Animal Ethics Committee of the Provincial Government of Southern Finland (Hämeenlinna, Finland), and the experiments were performed according to the guidelines of European Communities Council Directive of 22 September 2010 (2010/63/EU). All efforts were made to limit distress and to use only the number of animals necessary to produce reliable scientific data. All rats were first housed in pairs in individual cages in standard laboratory conditions (room temperature 22°C, humidity 55%) in a 12‐h light/dark cycle (lights on between 6 am to 6 pm) with ad libitum access to rat chow and tap water. The rats that underwent sham or SNI operation were housed singly after the operation. The transparent cages were kept in the same room, so the rats could hear, see, and smell the other rats. After their arrival, the rats were first accustomed to the new living environments for 1 week in a room next to the laboratory where the tests were run. Then, they were accustomed on 3 days to the laboratory environment next door. During each of the 3 days, at about the same time of the day (between 12:00 and 14:00), the rats spent approximately 2 hours in the laboratory environment and were handled by the experimenter several times during this period.
2.2. Surgical procedures for producing neuropathy
The spared nerve injury (SNI) model, as described by Decosterd and Woolf,11 was adopted for inducing neuropathy. The rat was anaesthetized with intraperitoneal (ip) administration of sodium pentobarbital (60 mg/kg, Orion Pharma, Espoo, Finland). Additional doses (15–20 mg/kg ip) were administered if necessary to keep the depth of anesthesia, so that the animal did not react to noxious stimulation. During surgery, an incision was made into the skin on the lateral surface of the left thigh, followed by a section through the biceps femoris muscle to expose the sciatic nerve and its terminal branches: the sural, common peroneal, and tibial nerves. The common peroneal and tibial nerves were then tightly ligated with 4–0 silk, sectioned distal to the ligation, and 3–4 mm of the distal nerve stump was removed. The sural nerve was left intact and care was exercised not to stretch it. In sham‐operated rats, the surgical procedure was identical, except that the tibial and common peroneal nerves were not ligated or sectioned. To prevent postoperative pain, animals were treated with 0.01 mg/kg of buprenorphine (Orion Pharma, Espoo, Finland) twice daily for 3 days and they were allowed to recover for at least a week before the experiments.
2.3. Assessment of the limb‐withdrawal threshold
The assessment of the limb‐withdrawal threshold was conducted in a plexiglas chamber (21 × 15 × 15 cm), which was inverted upon an elevated metal grid. Before the assessment, the rat was habituated in the chamber for 2 hours daily for 2 days. The neuropathic hypersensitivity was verified on the 3rd day by measuring the hind limb‐withdrawal threshold with monofilaments to stimulate the lateral foot pad of hind paws, which is the terminal area of the spared sural nerve, using methods described in detail elsewhere.14 Briefly, a series of monofilaments were applied with increasing force ranging from 0.4 to 60 g (North Coast Medical, Inc., Morgan Hill, CA). For each force level, the stimulus was repeated five times in a row and one withdrawal response to the stimulus was considered as 20% of hypersensitivity. To reduce the bias from experimenter, we measured the withdrawal response using a blinded test. The percentage of withdrawal responses at each stimulus intensity represented an index of hypersensitivity. When assessing the drug‐induced effect on the limb‐withdrawal response rate, the difference in the postdrug–predrug response was calculated at each test stimulus force and experimental condition. A drug‐induced change in the response rate that is below < 0 % represents a drug‐induced attenuation of the response. Limb‐withdrawal responses were assessed in the nerve‐injured/sham‐operated hind limb.
2.4. Behavioral tests
2.4.1. Open‐field test
The open‐field test (OFT) permits assessment of anxiety‐like exploratory and locomotor behaviors.15 The arena was circular with an 85‐cm diameter white floor and a 50‐cm high white wall.16, 17, 18 The floor had three concentric black circles, and the two outer circles were divided into segments by six radial lines. Each segment in the outermost circle was subdivided to two equally large segments by an additional short radial line, resulting in a total of 19 floor sections of equal size. The testing was conducted in a bright environment at the light level of 300 lux. Each animal was placed on a starting point in the center and then recorded for 5 minutes by a digital video camera. The number of line crossings, the number of rearings and leanings, the time spent grooming, and the number of defecations were counted by the experimenter from the video recording for the statistical analysis. The time spent in the central area (segments in the center and middle circle) and the time in the outer area (segments in the outermost circle) was measured to calculate the percentage (%) of time spent in the central area for statistics.
2.4.2. Elevated plus‐maze
The elevated plus‐maze (EPM) was used to test anxiolytic/anxiogenic effects of the drugs.19 It consisted of two open arms 45 × 10 cm and two closed arms 45 × 10 × 35 cm with an open roof, elevated to 50 cm from the floor and arranged so that the open arms were opposite to each other.17 The testing was conducted in a bright environment at the light level of 300 lux. For testing, each animal was placed in the center of the maze and recorded for 5 minutes by a digital video camera. The time spent in the center and in the open and closed arms of the maze was measured. The assessment was determined by calculating the percentage of time spent in the closed arm.
2.4.3. Light‐dark box
The plexiglas light‐dark box (LDB; 30 × 30 × 30 cm) was constructed of two chambers separated by a plexiglas board (30 × 30 × 15 cm), one of which was covered with black masking tape, the other was covered with white paper and illuminated by a cold light source of xenon lamp (100 lux). The device records automatically time spent in each chamber using a computer‐controlled 4 × 16 array of photo beams. The box was placed in a dark room, the illumination being provided exclusively by the xenon lamp. Half of the rats were individually placed in the center of the white compartment facing the opening, whereas the other half of the rats were individually placed in the center of the dark compartment. The test lasted for 5 minutes, and the time spent in each compartment was measured. The LDB assessment was determined by calculating the percentage of the time spent in light box. A reduction in time spent in the light box was considered to represent increased anxiety‐like behavior.
2.4.4. Novel‐object recognition
The novel‐object recognition (NOR) task was based on the protocols described previously20, 21, 22 with some modifications. Testing was carried out in a circular arena with a white 85 cm diameter floor and 50 cm high wall. The arena was illuminated by four 40 W fluorescent lamps which provided a constant light level of 300 lux. A digital video camera was positioned above the arena and was used to record behavior during testing for subsequent analysis. The objects were plastic Coca‐Cola® bottles (filled with water) with a base diameter of 6.5 and 23.5 cm height and two stacked plastic Rubik's cubes with the side length of 5.7 cm and height of 11.4 cm. The objects had no apparent natural significance to the rats, and were secured to the base of the arena with adhesive plaster. Animals were habituated to the arena in the absence of objects for 20 minutes on the day before the test day. The test day comprised of three stages: habituation, exposure 1 and exposure 2. Rats were first introduced to the arena for a 3‐minutes habituation period and then returned to their home cage for 7 minutes. During exposure 1, two identical objects (Coca‐Cola® bottles) were placed in opposite quadrants of the arena, 16 cm from the perimeter. The rat was allowed to freely explore the arena and the objects for a period of 3 minutes, after which the animal was removed from the arena and returned to its home cage for an interval of 5 minutes. Prior to exposure 2, one of the bottles was replaced with the novel object (two stacked plastic Rubik's cubes). The animal was again allowed to freely explore the objects for a period of 3 minutes in the arena and then returned to its home cage. The arena was cleaned with 70 % ethanol between rats to remove odors and olfactory cues, and fecal pellets were removed between exposures. Exploration of an object was defined as sniffing the object, rearing against the object, or having the head directed toward the object within a 2 cm annulus of the object. The NOR assessment was determined by calculating a discrimination ratio as follows: (Total time spent for exploring either object)/(Total time spent for exploring both objects).
2.4.5. Sucrose preference test
The rat was kept in the home cage and, during a time period of 20:00–8:00, was presented with two bottles: one filled with tap water and the other with 0.8 % sucrose solution (200 mL each). The 12‐hour consumption of water and sucrose solution was calculated by weighing the bottles both before and after the test, and by subtracting the weight of the bottle after test from the weight before the test. Sucrose preference percentage (%) was the ratio of the sucrose solution consumption divided by the total fluid consumption (i.e., water plus sucrose), multiplied by 100. A reduction in sucrose preference was considered to represent anhedonia.23
2.4.6. Rotarod test
The locomotor activity of the rats was assessed in the rotarod test, in which animals walk on a rotating drum (Ugo Basile, Varese, Italy). After starting the rotarod test device, the speed was increased to the maximum revolution speed (26 revolutions/min) in 2 revolutions/s. The rat was trained 5 minutes per day for 2 days to stay on the drum. On the third day, performance was assessed once by measuring the duration (seconds) that the rat stayed up on the drum at the maximum drum speed.
2.5. Course of the study
Totally 10 separate groups (n = 6) of rats were used in this study. Groups 1, 2, and 3 received an ip injection of saline (10 mL/kg), a low dose of paracetamol (50 mg/kg, 10 mg/mL, Orion Pharma, Espoo, Finland), or a high dose of paracetamol (300 mg/kg, 10 mg/mL), respectively. The behavioral tests, in the order of OFT, EPM and LDB, with a 10‐minute interval between the tests, were started 90 minutes after the injection. All behavioral tests, except the sucrose preference test, were conducted during the light cycle phase. The drug injections were given in the early afternoon between 12:00 and 13:00. For the sucrose preference test, the drug injection was given at 19:45 in the evening. The paracetamol doses and the time point of testing after drug injection were based on an earlier study reporting dose‐dependent antinociceptive effects of intraperitoneally injected paracetamol.13 After an interval of 3 days, the rats received an ip injection of saline (10 mL/kg), paracetamol (50 mg/kg) or paracetamol (300 mg/kg), respectively, at 19:45 on the fourth day and were put back to their home cages for the sucrose preference test.
Groups 4, 5, and 6 were trained for the rotarod test on 2 days (the first and second day) and were habituated for NOR on the second day. On the third day, the rats received an ip injection of saline (10 mL/kg), paracetamol (50 mg/kg), or paracetamol (300 mg/kg), respectively. The NOR started at 90 minutes after the injection. After an interval of 10 minutes in the home cage, the rat performed the rotarod test.
Groups 7 and 9 received sham surgery and Groups 8 and 10 the SNI surgery. After a recovery period of 1–2 weeks, all rats were trained for the assessment of the limb‐withdrawal threshold on 2 days. On the third day, the limb‐withdrawal threshold was assessed five times at each force level. After the assessment, Groups 7 and 8 received an ip injection of saline (10 mL/kg), and Groups 9 and 10 received an ip injection of a low dose of paracetamol (50 mg/kg) and, at 80 minutes after the injection, the limb‐withdrawal threshold was again assessed to test the analgesic effect of paracetamol.
The behavioral tests, in the order of OFT, EPM, and LDB, with a 10‐minute interval between the tests, started 90 minutes after the injection. After an interval of 3 days, the rats received an ip injection of saline (10 mL/kg) or paracetamol (50 mg/kg) at 19:45 on the fourth day and were put back to the home cage for the sucrose preference test.
2.6. Statistical analysis
Statistical evaluation of the data was performed using a one‐, two‐ or three‐way ANOVA followed by Bonferroni‐corrected t tests, or a t test when comparing only two groups. In all tests, P < .05 was considered to represent a statistically significant difference.
3. RESULTS
Tables 1 and 2 summarize the results of the behavioral tests in unoperated and operated rats.
Table 1.
Behavioral tests in unoperated rats
| Tests | Saline | Low dose | Effect | High dose | Effect |
|---|---|---|---|---|---|
| OFT | |||||
| Line crossing (#) | 52.33 ± 4.00 | 28.33 ± 7.18 | ↓ | 20.67 ± 2.04 | ↓↓ |
| Rearing and Leaning (#) | 19.33 ± 1.52 | 10.83 ± 3.57 | ↔ | 5.67 ± 1.65 | ↓↓ |
| Grooming time (s) | 15.83 ± 3.66 | 2.33 ± 1.31 | ↓↓ | 2.50 ± 2.50 | ↓↓ |
| Defecation (#) | 1.00 ± 1.00 | 1.83 ± 1.08 | ↔ | 2.00 ± 0.58 | ↔ |
| Center time (%) | 7.50 ± 1.62 | 5.72 ± 2.52 | ↔ | 5.83 ± 3.10 | ↔ |
| EPM | |||||
| Closed Arm Time (%) | 61.3 ± 6.76 | 64.88 ± 5.42 | ↔ | 89.06 ± 4.54 | ↑ |
| LDB | |||||
| Light box time (%) | 54.61 ± 3.48 | 43.44 ± 2.89 | ↔ | 38.94 ± 4.96 | ↓ |
| Sucrose preference | |||||
| Sucrose preference (%) | 90.79 ± 2.78 | 92.29 ± 1.66 | ↔ | 38.06 ± 11.46 | ↓↓↓ |
| Rotarod | |||||
| Drop latency (s) | 112.17 ± 31.73 | 143.83 ± 24.03 | ↔ | 65.33 ± 12.22 | ↔ |
OFT, Open‐Field Test; EPM, Elevated Plus Maze; LDB, Light‐Dark Box; Effect, effect vs saline; ↑, significant increase; ↓, significant decrease; ↔, no significant change. # = number. One arrow = P < .05; two arrows = P < .01; three arrows = P < .001.
Table 2.
Behavioral tests in operated Sham and SNI rats
| Tests | Sham | SNI | ||||
|---|---|---|---|---|---|---|
| Saline | Paracet. | Eff. | Saline | Paracet. | Eff. | |
| OFT | ||||||
| Line crossing (#) | 52.17 ± 13.46 | 41.33 ± 5.48 | ↔ | 18.83 ± 5.24 | 27.83 ± 7.76 | ↔ |
| Rear/Lean (#) | 19.50 ± 5.43 | 8.00 ± 1.84 | ↓ | 4.17 ± 1.22 | 1.33 ± 0.42 | ↔ |
| Grooming (s) | 6.33 ± 3.00 | 4.50 ± 2.92 | ↔ | 4.33 ± 2.93 | 2.67 ± 2.67 | ↔ |
| Defecation (#) | 2.83 ± 0.98 | 1.67 ± 0.42 | ↔ | 2.00 ± 2.61 | 1.00 ± 1.06 | ↔ |
| Center time (%) | 11.33 ± 3.30 | 6.06 ± 2.04 | ↔ | 6.22 ± 3.17 | 3.22 ± 1.14 | ↔ |
| EPM | ||||||
| Closed Arm Time (%) | 59.94 ± 4.85 | 57.94 ± 5.51 | ↔ | 85.56 ± 3.49 | 60.11 ± 6.41 | ↓ |
| LDB | ||||||
| Light box time (%) | 52.22 ± 4.40 | 53.67 ± 3.19 | ↔ | 33.72 ± 3.15 | 35.5 ± 1.89 | ↔ |
| Sucrose Pref. | ||||||
| Sucrose pref. (%) | 91.34 ± 2.38 | 94.17 ± 1.06 | ↔ | 96.73 ± 0.60 | 93.78 ± 1.08 | ↔ |
SNI, spared nerve injury; OFT, Open‐Field Test; EPM, Eleveated Plus Maze; LDB, Light‐Dark Box; Rear/Lean, Rearings and Leanings; Pref., preference; Paracet., Paracetamol 50 mg/kg; # = number; Eff., effect vs saline; ↑, significant increase; ↓, significant decrease; ↔, no significant change. One arrow = P < .05.
3.1. Behavioral tests in unoperated rats
3.1.1. Open‐field test (OFT)
The number of line crossings differed significantly between the drug treatment groups (F (2,15) = 11.414, P = .001, one‐way ANOVA, Figure 1A). Both the low‐ and high‐dose paracetamol groups (50 and 300 mg/kg, respectively) made fewer line crossings than the saline group (low‐dose group vs saline, P = .010; high‐dose group vs saline, P = .001, Bonferroni test), but the paracetamol‐treated groups did not differ from each other. Treatment also affected the number of rearings and leanings (Figure 1B; F (2,15) = 8.003, P = .004, one‐way ANOVA); the high‐dose group had lower scores than the saline group (P = .004), whereas the low‐dose group did not differ significantly from the saline (P = .078) or high‐dose (P = .463) groups. Also, the grooming time differed significantly between the groups (Figure 1C; F (2,15) = 8.443, P = .003, one‐way ANOVA); both the low‐ (P = .008) and the high‐dose (P = .009) groups had shorter grooming times than the saline group. There was no statistically significant difference between the groups in the number of defecations (F (2,15) = 0.345, P = .714) and the percentage of the time spent in the central area (F (2,15) = 0.160, P = .853, one‐way ANOVA).
Figure 1.
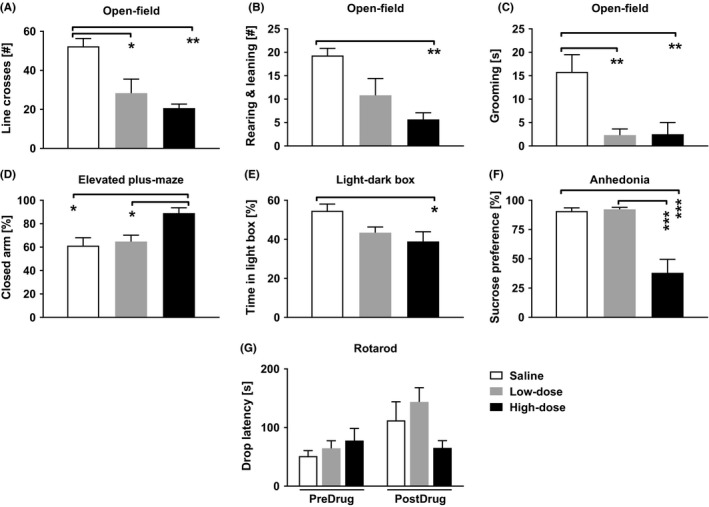
Results of behavioral tests in unoperated rats. (A) The number of line crosses in the open‐field test (OFT) in saline, low‐dose and high‐dose groups. (B) The number of rearings and leanings in OFT. (C) The grooming time in OFT. (D) The percentage of time in the closed arm in the elevated plus‐maze test. (E) The percentage of time spent in the light box in the light‐dark box test. (F) Consumption of sucrose‐containing water as a percentage of total water consumption in the sucrose preference test. (G) Locomotion in the rotarod test, measured as the time (s) on the drum (=drop latency). Low dose: 50 mg/kg of paracetamol, High dose: 300 mg/kg of paracetamol. Error bars indicate SEM (n = 6). *P < .05, **P < .01, ***P < .001 (Bonferroni‐corrected t test)
3.1.2. Elevated plus‐maze (EPM)
Treatment affected the performance in the EPM test, so that the time spent in the closed arm differed between the groups (F (2,15) = 7.151, P = .007, one‐way ANOVA, Figure 1D). The high‐dose group (300 mg/kg) spent more time in the closed arm than the low‐dose (50 mg/kg; P = .025) and saline groups (P = .010), but the low‐dose group did not differ significantly from the saline group.
3.1.3. Light‐dark box (LDB)
Treatment had a significant effect on the time the groups spent in the light box (F (2,15) = 4.333, P = .033 one‐way ANOVA, Figure 1E). The high‐dose paracetamol group (300 mg/kg) spent a significantly shorter time in the light box than the saline group, (P = .032) but the low‐dose group (50 mg/kg) did not differ significantly from the high‐dose or saline groups.
3.1.4. Sucrose preference test
Treatment had a main effect on the sucrose preference (F (2,15) = 20.813, P < .001, one‐way ANOVA, Figure 1F). Post hoc tests showed that the high dose of paracetamol (300 mg/kg) significantly decreased sucrose preference compared to both the low dose of paracetamol (50 mg/kg) and saline groups (P < .001 in both tests), but the low‐dose group did not differ significantly from the saline group. After balancing the volume of saline and paracetamol injected, the rats in the high‐dose group still preferred water over sucrose (t 12 = 4.472, P = .001, t test) indicating that the volume injected did not affect the preference.
3.1.5. Rotarod test
Figure 1G shows the performance of the rats in the rotarod test. The three groups of rats performed the rotarod test in a comparable manner during the predrug period, as the performances of the groups did not differ statistically significantly from each other (F (2,15) = 0.781, P = 0.476, one‐way ANOVA). Moreover, treatment had no significant effect on their performance (postdrug; F (2,15) = 2.699, P = .100, one‐way ANOVA).
3.1.6. Novel‐object recognition (NOR)
There were no statistically significant main effects of exposure, drug or object on the NOR test, but the three‐way interaction of exposure, drug, and object was significant (F (2,15) = 4.665, P = .017, mixed design ANOVA; within‐subjects variable: exposure; between‐subjects variable: drug and object, Figure 2). Tests of simple effects of this interaction showed that the saline‐treated group recognized the novel from familiar objects in the second exposure (F (1,5) = 8.81, P = .006).
Figure 2.
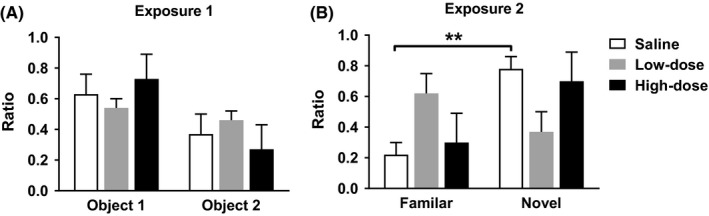
Novel‐object recognition in unoperated rats. Exposure 1 shows the difference of discrimination ratio between Object 1 and Object 2. Exposure 2 shows the difference of discrimination ratio between the Familiar object and the Novel object. Low dose: 50 mg/kg of paracetamol, High dose: 300 mg/kg of paracetamol. Error bars indicate SEM (n = 6). **P < .01 (Bonferroni‐corrected t test)
3.2. Behavioral tests in operated rats
3.2.1. Verification of neuropathic hypersensitivity
The SNI rats showed significant hypersensitivity to the monofilament stimulus in the limb‐withdrawal test compared to sham‐operated rats (main effect of operation: F (1,10) = 6.85, P = .026, two‐way mixed design ANOVA, Figure 3A).
Figure 3.
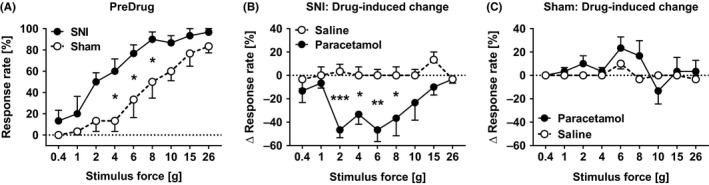
Verification of the spared nerve injury (SNI) model and the effect of paracetamol on mechanical hypersensitivity. The limb‐withdrawal response rate (A) in the hind limb ipsilateral to the operation in sham and SNI rats before drug treatment, and the drug treatment‐induced change in the limb‐withdrawal response rate in SNI (B) and sham‐operated animals (C). Paracetamol was given at the dose of 50 mg/kg. In a, the Y‐axis shows the response rate at different test stimulus forces; the higher the response rate, the stronger the hypersensitivity. In B and C, the Y‐axis shows the difference in the postdrug–predrug response at each test stimulus force; 0% represents the corresponding predrug value and differences <0% represent drug‐induced reductions in the response. All tests were performed in the nerve‐injured or sham‐operated hind limb. Error bars indicate SEM (n = 6). *P < .05, **P < .01, ***P < .005 (reference: the corresponding value in the saline‐treated group; Bonferroni‐corrected t test)
3.2.2. Effect of paracetamol on mechanical hypersensitivity
In SNI rats, paracetamol at a low dose (50 mg/kg) significantly decreased mechanical hypersensitivity (Figure 3B) in the injured hind limb (main effect treatment: F (1,10) = 14.94, P = .003, two‐way mixed design ANOVA). In sham‐operated rats, the treatment did not affect the response rate (main effect of treatment: F (1,10) = 3.245, P = 0.102, Figure 3C).
Further behavioral tests assessing effect of paracetamol on anxiety and anhedonia in SNI rats were performed only at a low dose of paracetamol (50 mg/kg), compared with saline that proved high enough to suppress the SNI‐induced hypersensitivity.
3.2.3. Open‐field test (OFT)
Operation, but not the drug treatment, had a significant main effect on the number of line crossings (main effect of operation: F (1,20) = 7.333, P = .014, two‐way ANOVA, Figure 4A) in the OFT. SNI rats made fewer line crossings than sham‐operated rats independent on drug treatment. Operation had a significant main effect also on the number of rearings and leanings (F (1,20) = 13.993, P = .001, two‐way ANOVA, Figure 4B) that was smaller in SNI than sham‐operated rats. Also, drug treatment had a significant main effect on the number of rearings and leanings (F (1,20) = 5.910, P = .024, two‐way ANOVA) that was smaller in rats treated with the low dose of paracetamol compared to saline.
Figure 4.
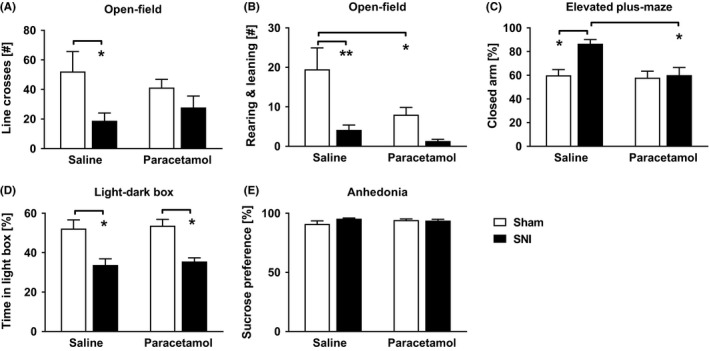
Results of behavioral tests in nerve‐injured (SNI) and sham‐operated rats. (A) The number of line crosses in the open‐field test (OFT). (B) The number of rearings and leanings in OFT. (C) The percentage of the time spent in the closed arm in the elevated plus‐maze test. (D) The percentage of time spent in the light box in the light‐dark box test. (E) Consumption of sucrose‐containing water as a percentage of total water consumption in the sucrose preference test. Paracetamol: 50 mg/kg. Error bars indicate SEM (n = 6). *P < .05, **P < .01 (Bonferroni‐corrected t test)
Operation had no significant main effect on the grooming time (F (1,20) = 0.443, P = .513), the number of defecations (F (1,20) = 0.935, P = .345), or duration in the central area (F (1,20) = 2.393, P = .138). Also, drug treatment had no significant main effect on the grooming time (F (1,20) = 0.369, P = .550), number of defecations (F (1,20) = 1.952, P = .178), or time in the central area (F (1,20) = 2.598, P = .123).
3.2.4. Elevated plus‐maze (EPM)
There was a significant main effect of operation (F (1,20) = 7.726, P = .012, two‐way ANOVA) and drug treatment (F (1,20) = 7.548, P = .012), and a significant operation × drug treatment interaction (F (1,20) = 5.575, P = .028) on the time the rats spent in the closed arm in the EPM test. Saline‐treated SNI rats spent a significantly longer time in the closed arm compared to saline‐treated sham‐operated rats (P = .022) and to SNI rats treated with paracetamol (50 mg/kg; P = .01; Figure 4C).
3.2.5. Light‐dark box (LDB)
Operation had a main effect on activity in the light‐dark box test (F (1,20) = 31.272, P < .001, two‐way ANOVA). The percentage of time spent in the light box was significantly smaller in SNI rats than in sham‐operated rats (P = .002, Figure 4D). Drug treatment, on the other hand, had no significant main effect on activity in the light‐dark box test (F (1,20) = .242, P = .628, two‐way ANOVA, Figure 4D).
3.2.6. Sucrose preference test
Neither the operation (between‐subject effect: F (1,10) = 1.499, P = .249, mixed design two‐way ANOVA) nor the drug treatment (within‐subject effect: F (1,10) = 0.349, P = .568) affected the sucrose preference (Figure 4E).
4. DISCUSSION
4.1. Effect of paracetamol on healthy control rats
In general, paracetamol influenced emotional and cognitive behavior of the unoperated rats in a way that varied with the paracetamol dose and the behavioral test. Two parameters presumably gauging some aspects of emotionality in the OFT, the center time and defecation,24 were not influenced by paracetamol (50 or 300 mg/kg). The number of line crossings, rearings and leanings as well as the time used for grooming in the OFT were suppressed in a dose‐related fashion by paracetamol treatment. Motor impairment is not likely to explain these decreases in the OFT behavior, as locomotor performance in the rotarod test was not suppressed by paracetamol. In the EPM test, paracetamol increased the time spent in the closed arm and in the LDB test paracetamol decreased the time spent in light. Both of these changes are considered to represent an increase in anxiety‐like behavior.25 Sucrose preference was significantly reduced by the high but not low dose of paracetamol suggesting that paracetamol has a dose‐related anhedonic effect. All these results are consistent with the interpretation that a high (300 mg/kg) but not a low (50 mg/kg) dose of paracetamol produces in healthy control rats a mood change that has a negative valence as reflected by increased anxiogenic‐like and anhedonic behavior. Moreover, paracetamol impaired cognitive performance as suggested by the finding that the performance in the NOR test that assesses recognition memory was suppressed in paracetamol‐treated healthy control animals. However, it has been suggested that the NOR test involves two cognitive processes, “familiarity recognition” and “novelty preference,”26 of which only “familiarity recognition” involves memory. Therefore, further studies using additional memory tests are still needed to confirm whether the paracetamol‐induced change in cognitive performance in the NOR test was due to an action on mechanisms underlying familiarity recognition or novelty preference. Anyway, the present result showing impaired cognitive performance of unoperated rats in the NOR test following a high dose of paracetamol is in line with clinical observations in elderly humans whose cognitive capacity is reduced by high acute doses of paracetamol.27
Earlier, paracetamol was shown to have a dose‐related (up to 200 mg/kg) anxiolytic‐like effect on the mouse Vogel conflict, social interaction,28 and EPM tests.29 The paracetamol‐induced anxiolytic‐like effect on mice was mediated by the endocannabinoid system, as it was reversed by a cannabinoid type‐1 receptor antagonist.28, 29 It should be noted that the behavioral assessments in the mouse studies showing anxiolytic‐like effects by low to moderate doses of paracetamol were performed 30 minutes after drug administration.28, 29 In the present rat study showing anxiogenic‐like effects by a high dose of paracetamol, the time point of testing was 90–120 minutes after drug administration that represents the time point for the peak effect of paracetamol.13, 30
4.2. Effect of paracetamol on animals with experimental neuropathy
In SNI rats, paracetamol had a significant antihypersensitivity effect at a low dose (50 mg/kg) that was subantinociceptive in sham‐operated animals. This finding is in line with an earlier study showing that 50 mg/kg of paracetamol reduced mechanical allodynia in neuropathic rats,13 and with a recent study that used a diabetic mouse model and showed that paracetamol (5‐100 mg/kg) alleviated diabetic nociceptive pain in a dose‐dependent manner.31 It is known that paracetamol is potentially hepatotoxic especially when used in high doses.1 The finding of this study that a low dose of paracetamol that does not elevate hepatic enzymes in rats13 alleviated neuropathic pain without increasing anxiety‐like or anhedonic behavior may be relevant also for human medicine. In the future, the effects of higher paracetamol doses on pain and anxiety‐like behavior in neuropathic rats should be tested to investigate whether the anxiogenic‐like and anhedonic behavior that was observed in healthy control rats after a high dose of paracetamol might also appear in neuropathic rats.
Paracetamol is thought to have a central analgesic and antihyperalgesic effect through multiple mechanisms.7, 8, 9, 32, 33 The analgesic effect has been suggested to be mediated by inhibiting the prostaglandin synthesis7 and through modulation of the serotonergic and other monoaminergic neurotransmissions.34 Additionally, a peripheral antiallodynic and antihyperalgesic effect of paracetamol that involves adenosine A135 and cannabinoid36 receptors has been demonstrated. The present results do not allow concluding whether the attenuation of neuropathic hypersensitivity in SNI rats was due to central, peripheral, or both of these mechanisms. In SNI animals, the OFT test showed reductions in line crossings, and rearings and leanings, which may be explained by SNI rather than paracetamol treatment, as the reductions were not different between saline‐ and paracetamol‐treated SNI animals.
SNI per se increased anxiety as revealed by the comparison of saline‐treated SNI and sham animals in the EPM and LDB tests. This is in line with earlier results showing that SNI produces anxiety‐like behavior in the EPM test that is not explained by motor impairment.12 The development of anxiety‐like behavior in the SNI model may, however, vary with the time point of testing after nerve injury, as an earlier study reported that it may take up to 5–9 weeks to observe an anxiety‐like behavior in the EPM test,37 while in this study it was observed during the third postoperative week. The model of experimental neuropathy also influences the development of anxiety‐like behavior as indicated by the earlier finding that both the partial sciatic nerve ligation model and the chronic constriction injury model induced pain hypersensitivity but only the latter model induced anxiety‐like behavior.38
In SNI animals, administration of paracetamol at a low (50 mg/kg) dose reduced anxiety‐like behavior in the EPM but not LDB test. This discrepancy in the EPM and LDB results may reflect different sensitivity of these two tests of anxiety‐like behavior. Concerning the paracetamol‐induced reduction in anxiety‐like behavior in the EPM test, a plausible explanation is that paracetamol reduced anxiety‐like behavior indirectly by attenuating neuropathic pain that per se was causing anxiety. This explanation is in line with earlier results showing that impairment of visual attention by visceral pain was reversed by suppressing pain using a moderate (200 mg/kg) dose of paracetamol.39 In this study, however, we cannot exclude the possibility that in the brain of SNI animals, paracetamol had a direct anxiolytic effect through action on mood mechanisms, although this explanation is not supported by the finding that in unoperated rats, paracetamol produced a dose‐related anxiogenic‐like rather than anxiolytic‐like effect. In the sucrose preference test, neither SNI per se nor a low (50 mg/kg) dose of paracetamol induced a change in the behavior of SNI animals. This is in line with some earlier results reporting that during the third postoperative week, SNI did not influence performance in the forced‐swimming test that, as the sucrose preference test, is used to assess depression‐like behavior.12, 24 Time course for the development of depression‐like behavior or anhedonia in the SNI model may be longer than the current postoperative time point of testing as suggested by the earlier finding that depression‐like behavior in the forced‐swimming test was described in the eighth postoperative week.40
5. CONCLUSIONS
In healthy rats, acute administration of paracetamol produced a dose‐related increase of anxiety‐like and anhedonic behavior. This was accompanied by reduced cognitive performance in the novel‐object recognition test. In neuropathic rats, a low dose of paracetamol that was subantinociceptive in sham‐operated controls had a marked antihypersensitivity effect that was associated with anxiolytic‐like effect. Anxiolytic‐like effect of paracetamol on neuropathic animals may be explained by attenuation of neuropathic pain that was driving anxiogenesis.
DISCLOSURE
Zuyue Chen reports grants from The Academy of Finland, during the conduct of the study. Hong Wei reports grants from The Sigrid Juselius Foundation, during the conduct of the study. Antti Pertovaara reports grants from The Sigrid Jusélius Foundation, Helsinki, Finland, during the conduct of the study. Jianhong Wang declares no conflict of interest. Synnöve Carlson reports grants from The Academy of Finland, during the conduct of the study.
ACKNOWLEDGEMENTS
This work was supported by the Academy of Finland, Helsinki, Finland [13273147, International Program/China‐Finland, 2014‐2016], and the Sigrid Jusélius Foundation, Helsinki, Finland.
Chen Z, Wei H, Pertovaara A, Wang J‐H, Carlson S. Anxiety‐ and activity‐related effects of paracetamol on healthy and neuropathic rats. Pharmacol Res Perspect. 2018;e00367 https://doi.org/10.1002/prp2.367
Chemical compounds: paracetamol (Pub Chem CID: 1983).
REFERENCES
- 1. Ghanem CI, Pérez MJ, Manautou JE, Mottino AD. Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol Res. 2016;109:119‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeWall CN, MacDonald G, Webster GD, et al. Acetaminophen reduces social pain behavioral and neural evidence. Psychol Sci. 2010;21:931‐937. [DOI] [PubMed] [Google Scholar]
- 3. Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford: Oxford University Press; 1998. [Google Scholar]
- 4. Randles D, Heine SJ, Santos N. The common pain of surrealism and death acetaminophen reduces compensatory affirmation following meaning threats. Psychol Sci. 2013;24:966‐973. [DOI] [PubMed] [Google Scholar]
- 5. DeWall CN, Chester DS, White DS. Can acetaminophen reduce the pain of decision‐making? J Exp Soc Psychol. 2015;56:117‐120. [Google Scholar]
- 6. Mischkowski D, Crocker J, Way BM. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc Cogn Affect Neurosci. 2016;11:1345‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79:9‐19. [DOI] [PubMed] [Google Scholar]
- 8. Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB 1 receptors. Eur J Pharmacol. 2006;531:280‐281. [DOI] [PubMed] [Google Scholar]
- 9. Andersson DA, Gentry C, Alenmyr L, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)‐tetrahydrocannabinol. Nat Commun. 2011;2:1‐20. [DOI] [PubMed] [Google Scholar]
- 10. Ruggieri V, Vitale G, Pini LA, Sandrini M. Differential involvement of opioidergic and serotonergic systems in the antinociceptive activity of N‐arachidonoyl‐phenolamine (AM404) in the rat: comparison with paracetamol. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:219‐229. [DOI] [PubMed] [Google Scholar]
- 11. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149‐158. [DOI] [PubMed] [Google Scholar]
- 12. Leite‐Almeida H, Almeida‐Torres L, Mesquita AR, et al. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57‐65. [DOI] [PubMed] [Google Scholar]
- 13. Im K‐S, Jung H‐J, Kim J‐B, et al. The antinociceptive effect of acetaminophen in a rat model of neuropathic pain. Kaohsiung J Med Sci. 2012;28:251‐258. [DOI] [PubMed] [Google Scholar]
- 14. Wei H, Wu HY, Chen Z, et al. Mechanical antihypersensitivity effect induced by repeated spinal administrations of a TRPA1 antagonist or a gap junction decoupler in peripheral neuropathy. Pharmacol Biochem Behav. 2016;150–151:57‐67. [DOI] [PubMed] [Google Scholar]
- 15. Hall CS (1934). Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 18: 385‐403. [Google Scholar]
- 16. Kauppila T, Tanila H, Carlson S, Taira T. Effects of atipamezole, a novel α2‐adrenoceptor antagonist, in open‐field, plus‐maze, two compartment exploratory, and forced swimming tests in the rat. Eur J Pharmacol. 1991;205:177‐182. [DOI] [PubMed] [Google Scholar]
- 17. Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation‐induced neuropathy. Pain. 1999;80:341‐346. [DOI] [PubMed] [Google Scholar]
- 18. Pertovaara A, Kauppila T, Tukeva T. The effect of medetomidine, an α2‐adrenoceptor agonist, in various pain tests. Eur J Pharmacol. 1990;179:323‐328. [DOI] [PubMed] [Google Scholar]
- 19. Handley SL, Mithani S. Effects of alpha‐adrenoceptor agonists and antagonists in a maze‐exploration model of ‘fear’‐motivated behaviour. Naunyn‐Schmiedeberg's Arch Pharmacol. 1984;327:1‐5. [DOI] [PubMed] [Google Scholar]
- 20. Moriarty O, Gorman CL, McGowan F, et al. Impaired recognition memory and cognitive flexibility in the rat L5–L6 spinal nerve ligation model of neuropathic pain. Scand J Pain. 2016;10:61‐73. [DOI] [PubMed] [Google Scholar]
- 21. Bevins RA, Besheer J. Object recognition in rats and mice: a one‐trial non‐matching‐to‐sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306‐1311. [DOI] [PubMed] [Google Scholar]
- 22. King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KCF. 5‐HT6 receptor antagonists reverse delay‐dependent deficits in novel object discrimination by enhancing consolidation—an effect sensitive to NMDA receptor antagonism. Neuropharmacology. 2004;47:195‐204. [DOI] [PubMed] [Google Scholar]
- 23. Bessa JM, Mesquita AR, Oliveira M, et al. A trans‐dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gould TD, Dao DT, Kovacsics CE. The open field test In: Gould TD, ed. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Totowa, NJ: Humana Press; 2009:1‐20. [Google Scholar]
- 25. Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55‐65. [DOI] [PubMed] [Google Scholar]
- 26. Ennaceur A. One‐trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244‐254. [DOI] [PubMed] [Google Scholar]
- 27. Karplus TM, Saag KG. Nonsteroidal anti‐inflammatory drugs and cognitive function: do they have a beneficial or deleterious effect? Drug Saf. 1998;19:427‐433. [DOI] [PubMed] [Google Scholar]
- 28. Umathe SN, Manna SSS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic‐like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1191‐1199. [DOI] [PubMed] [Google Scholar]
- 29. Zaitone SA, El‐Wakeil AF, Abou‐El‐Ela SH. Inhibition of fatty acid amide hydrolase by URB597 attenuates the anxiolytic‐like effect of acetaminophen in the mouse elevated plus‐maze test. Behav Pharmacol. 2012;23:417‐425. [DOI] [PubMed] [Google Scholar]
- 30. Siepsiak M, Szałek E, Karbownik A, et al. Pharmacokinetics of paracetamol in patients with chronic pancreatitis. Pharmacol Rep. 2016;68:733‐736. [DOI] [PubMed] [Google Scholar]
- 31. Micov A, Tomic M, Pecikoza U, Ugresic N, Stepanovic‐Petrovic R. Levetiracetam synergises with common analgesics in producing antinociception in a mouse model of painful diabetic neuropathy. Pharmacol Res. 2015;97:131‐142. [DOI] [PubMed] [Google Scholar]
- 32. Högestätt ED, Jönsson BAG, Ermund A, et al. Conversion of acetaminophen to the bioactive N‐acylphenolamine AM404 via fatty acid amide hydrolase‐dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405‐31412. [DOI] [PubMed] [Google Scholar]
- 33. Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology. 2006;50:814‐823. [DOI] [PubMed] [Google Scholar]
- 34. Courade J‐P, Caussade F, Martin K, et al. Effects of acetaminophen on monoaminergic systems in the rat central nervous system. Naunyn‐Schmiedeberg's Arch Pharmacol. 2001;364:534‐537. [DOI] [PubMed] [Google Scholar]
- 35. Liu J, Reid AR, Sawynok J. Antinociception by systemically‐administered acetaminophen (paracetamol) involves spinal serotonin 5‐HT 7 and adenosine A 1 receptors, as well as peripheral adenosine A 1 receptors. Neurosci Lett. 2013;536:64‐68. [DOI] [PubMed] [Google Scholar]
- 36. Dani M, Guindon J, Lambert C, Beaulieu P. The local antinociceptive effects of paracetamol in neuropathic pain are mediated by cannabinoid receptors. Eur J Pharmacol. 2007;573:214‐215. [DOI] [PubMed] [Google Scholar]
- 37. Seminowicz DA, Laferriere AL, Millecamps M, Jon SC, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long‐term neuropathic pain. NeuroImage. 2009;47:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roeska K, Doods H, Arndt K, Treede R‐D, Ceci A. Anxiety‐like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349‐357. [DOI] [PubMed] [Google Scholar]
- 39. Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D. Decrease in non‐selective, non‐sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain. 2004;109:214‐224. [DOI] [PubMed] [Google Scholar]
- 40. Goncalves L, Silva R, Pinto‐Ribeiro F, et al. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008;213:48‐56. [DOI] [PubMed] [Google Scholar]


