Abstract
The recent technology of 3D cultures of cellular aggregates derived from human stem cells have led to the emergence of tissue‐like structures of various organs including the brain. Brain organoids bear molecular and structural resemblance with developing human brains, and have been demonstrated to recapitulate several physiological and pathological functions of the brain. Here we provide an overview of the development of brain organoids for the clinical community, focusing on the current status of the field with an critical evaluation of its translational value.
Introduction
The promise of human disease modeling and regenerative therapy relies on the ability to faithfully reconstruct the disease‐relevant cellular ensemble ex vivo. While immortalized cell lines and animal models have commonly been used for pathophysiological studies and for testing therapeutic interventions, numerous limitations exist. Virtually all cell lines used for drug screening consist of a singular cell type, and they are grown in two‐dimensional (2D) cultures, an artificial environment that is vastly different from living organs. Indeed, the inadequacy of such cultures to recapitulate the complex in vivo cell–cell interactions, tissue structures, and physiological functions is increasingly been recognized as one major reason accounting for drug failures.1 Furthermore, many human pathologies are human specific and hence cannot be effectively reproduced in rodents. This is particularly true when it comes to the human brain, the most complex organ of the body.2
The need for more accurate ex vivo models have led to the development of 3D culture systems. A commonly employed 3D culture method involves generating cell aggregates and growing them in suspension cultures as spheroids. This approach is gaining popularity, particularly in cancer research, where a large body of literature has demonstrated improved accuracy of therapeutic responses.3, 4 Nevertheless, the in vivo cellular compartmentations and cell–cell interactions are not preserved in these systems. Recently, a stem‐cell‐based 3D tissue model, termed organoid, that better recapitulates tissue composition, architecture, and functionality has been developed.5 Organoids are in vitro 3D grown organized structures that mimic their respective organs (Fig. 1).6, 7 They contain multiple organ‐specific cell types that are spatially configured and connected in a manner reminiscent of their in vivo counterparts. Importantly, organoids are capable of exhibiting key physiological functions of the organs, making them attractive as the next‐generation ex vivo model for disease studies and drug screenings.5
Figure 1.
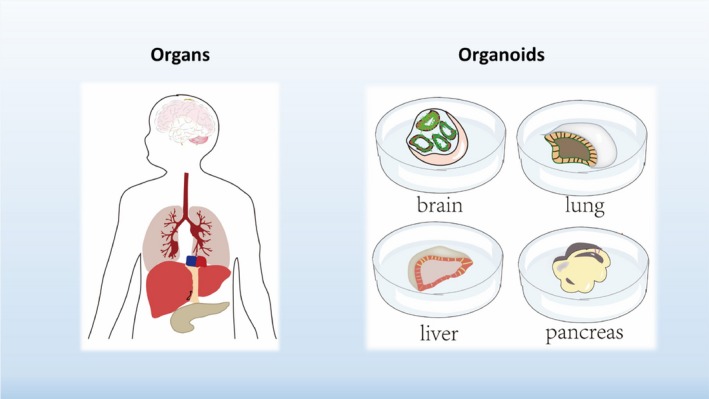
Organoids: organs in a dish. A diagram illustrates the concept of organoids, 3D cultured mini organ‐like structures that resemble their respective organs in terms of cell types, structures, and functions.
The marriage between the abovementioned organoid method and human pluripotent stem cells (hPSCs, including both human embryonic stem cells [hESCs] and human‐induced pluripotent stem cells [hiPSCs]) have led to the creation of a number of mini‐human “organs in a dish”; and the list is rapidly growing.6 Developing brain organoids is technically challenging due to the diversity of neuronal subtypes and the complexity of functional circuitry. Recently large 3D globular structures (organoids) of specific brain regions or the whole brain have been developed, and some of these models have been used to study neurological disorders and Zika virus‐linked brain infections. While the detailed technical aspects of brain organoids have been reviewed elsewhere,8 here we provide a concise review for the medical community by focusing on their potential clinical applications, evaluating current limitations, and highlighting critical issues that need to be resolved before the brain organoid model can be maximally utilized for clinical translation.
Organogenesis in a dish: an overview
Classical experiments in developmental biology have unveiled important principles of tissue morphogenesis: stem cells self‐organization with simultaneous lineage development and functional specialization, guided by specific factors and signaling molecules.7 Remarkably, these abilities remain intact even when the stem cells are taken out of their naïve environment – this forms the molecular basis of growing organ‐like structures from stem cells ex vivo.7, 9 In 2009, Hans Clevers's group described the first long‐term cultures of self‐organizing intestinal crypt‐like structures from mouse gut stem cells.10 This landmark paper established a novel experimental paradigm for generating tissue organoids. Subsequent studies worldwide confirmed the generalizability of such approach to human stem cells, and produced organoids of a variety of organ types including colon,11 intestine,12 prostate,13, 14 fallopian tube,15 stomach,16 liver,17, 18 kidney,19 lung,20 and brain.8
Human neural cultures: historical aspects
Unlike rodents, of which various brain parts of either embryonic or adult stages are readily available, human neural tissues are largely inaccessible. The derivation of hESCs21 and more recently hiPSCs22 open the door for generating human neural models as these stem cells can be differentiated into any cell types of the body including neural cells. Prior to the advent of brain organoids, neuroscientists have strived to establish neural differentiation protocols from the hPSCs. Drawing on the insights learned from early embryogenesis, Chambers et al. discovered that blocking TGFβ/BMP signal pathways in hESCs resulted in highly efficient neural differentiation.23 This led them to identify specific inhibitors of the SMAD proteins (downstream effectors of TGFβ/BMP signaling) as potent neural fate inducers, constituting the basis of the so‐called dual‐SMAD inhibition protocol that is widely used for directed neural differentiation today.23, 24 Upon extended differentiation in culture, these neural stem cells gave rise to functionally mature neurons expressing markers of different cortical layers.24 It is noteworthy that astrocytes appeared later in culture than neurons, reflecting the correct developmental timing of gliogenesis after neurogenesis.23, 24 Independently Yoshiki Sasai's group explored directed differentiation of hPSCs after embryoid body (EB) formation, and developed a method known as the serum‐free culture of embryoid bodies after quick reaggregation (SEEBq).25, 26 When specific neural inductive molecules were added, they generated polarized neural tissues.25, 26
Collectively, these pioneering studies revealed the neural‐default lineage competence of hPSCs showed that key aspects of embryonic neurogenesis could be recapitulated in vitro and demonstrated the feasibility of guided differentiation toward brain regions via manipulation of extrinsic signaling, thus paving the way for the development of 3D brain organoids discussed below.
Brain organoids: current technologies
Building on these earlier works, a recent flurry of papers described the generation of various neural organoids (Table 1), ranging from the whole‐brain organoids,27, 28, 29 to large sub‐brain regions such as cortical organoids,30, 31 to specific regions, including cerebellum,32 midbrain,33 adenohypophysis,34 hypothalamus,35 and hippocampus.36 Comparing with the classical neurospheres, these organoids are generally much larger. More importantly, within these organoids the neural progenitors and neurons are arranged in an organized manner, a critical feature that is absent from the neurospheres.
Table 1.
Summary of published human brain organoids papers discussed in this paper
| Protocols of brain organoids | This set consists of research studies that described generation of organoids of different brain regions. Note that for some brain parts more than one method have been reported. | 27, 29, 30, 31, 32, 33, 34, 35, 36, 56, 57, 68, 69 |
| Characterizations of brain organoids | This set consists of studies that primarily focus on detailed molecular as well as functional analyses of existing brain organoid models. | 28, 33, 58, 66 |
| Applications of brain organoids | This set consists of studies that apply brain organoids to either reproduce neuropathological phenotypes, understand disease mechanism, or screen therapeutics. | 31, 41, 42, 43, 44, 47, 50, 51, 71 |
Among the published studies, Lancaster et al. reported the first human brain organoid model that was entirely cultured in 3D.27 One remarkable feature of their cerebral organoids is that a plethora of brain regions, including cortex, hippocampus, hindbrain, and even retina, was observed within these large 3D globular structures (Fig. 2A). In addition to the increased size, cerebral organoids showed highly thickened neuronal lobes within which neural progenitors and neurons reside. Immunostaining analysis revealed the presence of distinct zones such as the ventricular zone (VZ), the intermediate zone (IZ), and even the cortical plate‐like structure, similar to the developing brain.27, 29 Particularly exciting is the presence of so‐called outer subventricular zone (oSVZ), an embryonic structure that is uniquely expanded in humans but absent in rodents. OSVZ is thought to contribute to our bigger cortex.37 Interestingly, when Lancaster et al. subjected mouse embryonic stem cells to the same protocol, organoids could form but did not contain distinguishable oSVZ, suggesting that this 3D organoid system can recapitulate at least some human‐specific aspects of neurogenesis.
Figure 2.
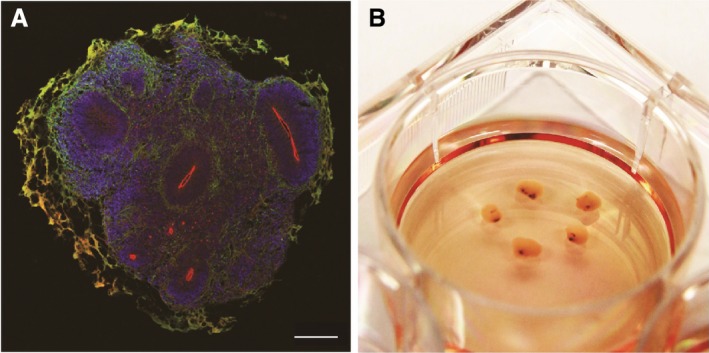
A model of human midbrain organoids. (A) Developing (day 35) human midbrain organoids contained multiple polarized neuroepithelia. Apical side is marked by aPKC (red), basal side is enriched with neuronal cells marked by MAP2 (green). Note that the green‐orange color staining lining the exterior of the organoid is due to nonspecific staining of Matrigel. Scale bar: 500 um. (B) Long‐term cultures of human midbrain organoids accumulate neuromelanin (black spots).
3D brain organoids may better capture some important physiological features. We have recently succeeded in generating human midbrain‐like organoids (hMLOs) from hPSCs following a guided self‐organization principle.33 These organoids grew to more than 2 mm by 30 days and contained layered neuroepithelia similar to the developing midbrain (Fig. 2B). Furthermore, gene expression, immunostaining analysis, and electrophysiological recording of pacemaking firing indicated the presence of cardinal midbrain dopaminergic neurons.33 Most importantly, hMLOs released dopamine, and over long term culture reliably and robustly produced neuromelanin, a dark pigment found in human substantia nigra (Fig. 2C).33, 38 Given none of previous studies reported production of neuromelanin in vitro, the 3D midbrain organoid model uniquely allows preservation of important physiological features of the human midbrains, and may represent a useful platform to study human midbrain development and associated diseases.
Translational applications of brain organoids
Neurodevelopmental disorders
Leveraging on the remarkable recapitulation of early events of brain development in organoids, Lancaster and her colleagues applied their cerebral organoids to model microcephaly, a neurodevelopmental disorder.27 Loss‐of‐function mutation in CDK5RAP2 has been implicated in microcephaly, but mouse models failed to reproduce the full spectrum of human phenotypes.39, 40 When brain organoids were generated from microcephaly patients harboring truncating mutations of CDK5RAP2, they were found to be significantly smaller than the ones generated from controls.27 The authors went on to show that precocious neuronal differentiation likely contributed to the overall smaller dimensions of the patient‐derived organoids.27 Using the same system, Li et al. demonstrated folding of the brain organoids, a major feature that distinguish us from rodents. They further used it to model expanded cortical growth and macrocephaly.41
Two groups independently adapted the brain organoid method to study Miller–Dieker syndrome using patient iPSCs.42, 43 Their results identified several cellular features of this classical form of lissencephaly, including aberrant cell cycle of outer radial glia and non–cell‐autonomous alteration of WNT signaling.42, 43
In another example, Mariani et al. used forebrain organoids to study idiopathic autism spectrum disorder (ASD), a disease with a strong link to dysregulated neurogenesis.31 They found several differences, including an overall overproduction of inhibitory neurons (likely as a result of increased FOXG1 expression), altered cell cycle of the neural stem cell, and abnormal synapses and neurites.31 This study extends our understanding of early molecular and gene expression changes in sporadic ASD.
Neurodegeneration
Raja et al. explored iPSC‐derived forebrain organoids to model the Alzheimer's disease (AD).44 Their results indicated that major hallmarks of AD‐associated pathological phenotypes, such as amyloid aggregation and tau hyperphosphorylation, could be sufficiently reproduced in organoids derived from familial AD (fAD) patients.44 Importantly, these phenotypes emerged after long‐term culture, with amyloid aggregation preceding tau pathology. These findings lend support to the amyloid hypothesis of AD, and are in agreement with another recent paper45 which utilized a 3D culture of dissociated neural stem cells. Raja and his colleagues further presented evidence that β‐ and γ‐secretase inhibitors treatment of AD patient‐derived organoids significantly attenuated amyloid aggregation and subsequently tau pathology.44 Overall, this study demonstrates the capability of brain organoids in elucidating key pathological phenotypes of AD and illustrates their potential utility as a preclinical drug discovery model.
Neuroinfection
Several research groups applied the brain organoid model to study Zika infection, a current epidemic that has been strongly linked to newborn microcephaly.35, 46, 47, 48, 49, 50 These studies have provided evidence that human neural stem cells are selectively vulnerable35, 48 and more susceptible to premature differentiation50 upon Zika virus infection, providing a molecular explanation for the observed microcephaly. A further study suggested activation of innate immune responses as a possible mechanism for Zika‐induced neuroprogenitor cell death.47 In addition, organoid model has been used to identify putative receptor proteins mediating Zika entry into the nervous system.49 Most recently, brain organoids were used to validate potential repurposed drugs for treating Zika infections.51
Current limitations of brain organoids
Heterogeneity
Although the whole‐brain organoids pioneered by Lancaster et al. contain a palette of different brain parts, their spatial positions relative to one another are often haphazard and do not correlate with the in vivo configuration.27, 52 In addition, even within the same batch of organoids, there is variation among the organoids in terms of the parts of brain represented and their spatial distributions.28 As a result, current methods are most suited to investigate strong or overt phenotypes. Given that cerebral organoids develop in the absence of any inductive factors, this high level of heterogeneity is somehow expected, and may be largely explained by the lack of reproducible body axes.
Consistent with that notion, improved uniformity is seen in the region‐specific organoid models which employ soluble neural inductive factors, but still there exist considerable variabilities.30, 31, 35 At least three reasons could account for them. One reason stems from the fact that the starting cells, either aggregates of dissociated hPSCs or larger clumps, are not sufficiently quantified. It is also reasonable to assume that autocrine signaling exist within these starting cells – such signals remain poorly defined and can be another source of variation between and within batches of organoid preparations. Given that most brain organoids are long‐term cultures, subtle differences at the beginning of culture are likely to be propagated and amplified to give rise to huge variations at the end. Finally, the embedding material, Matrigel, is a poorly defined proteinaceous mixture secreted by Engelbreth–Holm–Swarm (EHS) mouse sarcoma cells. Matrigel is not only rich in laminin, but also contains a host of other proteins and growth factors. Thus, variability from Matrigel may also contribute to the heterogeneity of brain organoids.
Potential solutions
The key issue is to reconstruct the proper patterning factors axes in a spatiotemporally defined manner reminiscent of the developmental program. One possible way to establish a continuous morphogen gradient in the organoids is through the use of slow‐releasing microbeads.53 Bioengineered scaffolds with regulated signal release may represent another promising method to install external body axes and guide localized differentiation.29, 54 Second, regarding the variability of starting cells, a deeper understanding of stem cell biology may enable more precise control of the cells, thereby enhancing reproducibility. Finally, synthetic biomaterials, such as hydrogels, with biophysical and biochemical properties optimized for neural tissues, could replace Matrigel as the embedding matrices.55
Absence of physiological neural cell types
Besides neurons, glial cells including astrocytes and oligodendrocytes are essential for normal brain integrity and physiology. Nevertheless, among all published organoids reports only a handful described the presence of functional astrocytes and oligodendrocytes, and they emerge late in culture.30, 56, 57, 58 The inadequate presence of functional astrocytes and oligodendrocytes thus limits organoids’ use to study glial‐related aspects of neuropathology.
In addition to the abovementioned bona fide neural cell types, intact brains also contain many other cell types such as microglia, pericytes, and even meningeal cells.59 These cells play critical roles in maintaining functional homeostasis of the brain and safeguarding it against infections or traumas. Given the non‐neuroectodermal origin of many of them, it is perhaps unsurprising that prevailing methods of generating organoids do not produce these cells.
Potential solutions
During neural development neurogenesis precedes gliogenesis, thus extended time of culture may be required to generate more astrocytes and oligodendrocytes. Alternatively, methods to accelerate the appearance and maturation of astroglial cells should be developed. For the inclusion of non‐neural cell types, one way may be to first generate them separately, and later add them to the brain organoids.60, 61, 62
Lack of vascularization
In all brain organoid models described so far, there is no vasculature. This deficiency severely limits the size of viable organoids can grow to, beyond which nutrient and oxygen delivery become insufficient and necrosis inevitably ensues. The normal diffusion penetration distance is much shorter than the thickness of the neonatal cortical zone (over 2 mm).63 Indeed this could be a critical reason for the lack of a more elaborated and structured cortical plate in established organoids. Vasculature is also important for later stage of SVZ development during which it serves as the neurogenic niche.64, 65
Potential solutions
Better designed bioreactors may facilitate more efficient nutrient delivery and has been recently shown to be a promising approach to generate organoids with improved quality.35 Alternatively, microfluidic perfusion network may be implemented to facilitate oxygen, nutrient, and waste exchange. Furthermore, with coculture of microvascular endothelial cells and other supportive cell types, artificial blood–brain barriers may be incorporated within the organoids.
Immaturity
Current brain organoids closely resemble fetal brains,28, 30, 66 and can only partially represent the diverse functional cell types of the brain, making them less ideal for studying complex network activities and circuit formations. It also raises the question as in to what extent can brain organoids effectively model neuropsychiatric or neurodegenerative diseases.
Potential solutions
The ability to maintain healthy and functional organoids allows long‐term culturing, during which spontaneous maturation will occur.58 This will likely be driven by technical innovations.35 The existence of genetic forms of premature aging diseases (including Hutchinson–Gilford progeria syndrome and Werner syndrome), provides us with another means of accelerating aging in a dish. Studies have shown that introduction of these disease‐causing genetic variants into otherwise wild‐type cells recapitulates many aspects of cellular aging.67 Taking this approach into 3D brain organoids may be potentially very useful for inducing adult or aged phenotypes within an experimentally feasible time frame.
Brain in a dish: future promises
From the discussions above, it is clear that the emerging field of brain organoids is imperfect, but it holds great promises and may recapitulate novel disease phenotypes that classical 2D neural cultures cannot achieve. To facilitate an easy evaluation of the relative strengths and weakness of the 3D organoids technology, we have compared several relevant aspects between 3D organoids and 2D neural stem cell cultures in Table 2. Organoid technology may bridge the gap between disease genetics and clinical applications, complement conventional cell‐line‐based drug screenings, and can potentially foster development of personalized therapy. Below we present a perspective on the potential of brain organoids, and we anticipate that, through a concerted effort between stem cell biologists, neurologists, bioengineers, and material scientists, brain organoids can be a revolutionizing tool for neuroscience research that ultimately lead to better clinical practices (Fig. 3).
Table 2.
A comparison between 2D neural stem cell cultures and 3D brain organoid cultures
| 2D cultures | 3D organoids | |
|---|---|---|
| Homogeneity and purity | Relatively homogenous, mostly with a few cell types | More heterogeneous, with a diverse set of neuronal cells (and some non‐neuronal cells) |
| Functional maturity | Cell mature after long‐term culture | Cell show enhanced maturity after long‐term culture |
| Culture period | Shorter (weeks to a few months [<6 months]) | Longer (months to > a year) |
| Cell transplantation | Yes | Not reported yet, more challenging |
| Probing disease mechanisms | More suitable for investigating diseases involving singular cell types; less ideal for complex interactions or neural circuits | May be utilized for structural abnormalities, multicell‐type interactions, or functional connectivity. |
| Drug screening | More amenable | Less amenable |
| Personalized medicine | Yes | Yes |
Figure 3.
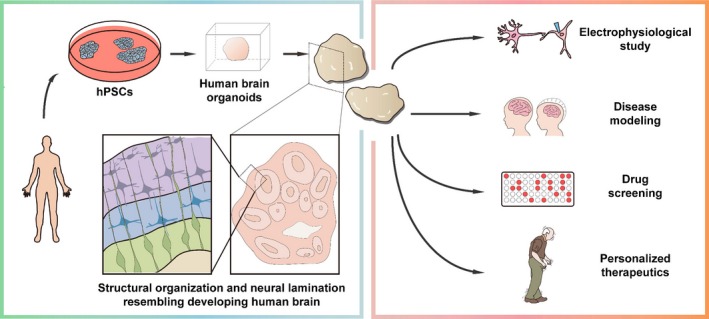
A schematic of generating human brain organoids (left) and their potential clinical applications (right). Either human embryonic stem cells or induced pluripotent stem cells can be used to generate human neural cell aggregates in 3D. As these neural organoids mature in culture over time, they typically contain laminated neuronal cells similar to the human brains. The human brain organoids may be used for electrophysiological studies, disease modeling, drug screening, or personalized therapeutics.
Optimizing the model system capable of showing relevant phenotypes
Systematic optimization of the SFEBq method with different regimes of neural inductive factors will likely expand our toolbox of region‐specific brain organoids, and lead to establishment of brain tissues with subregions/domains adjoining with appropriate orientation. As a proof‐of‐principle, three recent studies have reconstituted forebrain organoids by fusing dorsalized and ventralized cortical organoids that were independently generated, and observed substantial interneuron migration within the organoids.56, 68, 69 These new models will enable us to study the diverse collection of neurodevelopmental disorders, many of which could not be possibly studied with current methods, such as corpus callosum agenesis.
With more mature organoids it would be possible to study aspects of neuronal connectivity, synaptic plasticity, and network activity, which are inevitably altered in neuropsychiatric diseases including schizophrenia and depression.70 Likewise, neurodegenerative diseases may be studied if the organoids can be endowed with an aging phenotype. In this regard, it is encouraging that (1) a model of human midbrain organoids produced neuromelanin, a dark pigment found in adult midbrains, after 2 months of culture;33 (2) long‐term cultured AD iPSC‐based brain organoids recapitulated both Aβ and tau pathology in a sequential manner.44 Further development in incorporation of other relevant cell types60 and accelerating maturation and/or aging in a dish67 would be extremely exciting and important.
Modeling environmental challenges, toxicity, and infections
Compared with 2D neural cultures, 3D brain organoids have demonstrated more accurate cellular behaviors and have the potential to be used for modeling viral infection or for neural toxicity studies.51, 71 At present a major technical barrier is the heterogeneity of organoid cultures, which might be addressed by innovative bioengineered approaches such as scaffolds, micropatterning, and bioreactors.35, 72 With improved uniformity and reduced intra‐ and interbatch variations, we anticipate to find increased usage of brain organoids in phenotypic screening against various agents such as toxins, stressors, viruses, and drugs. In line with this, the recent series of studies following Zika outbreak, starting from identifying target cells, locating possible route of entry, illuminating downstream cellular mechanisms, to screening candidate drug molecules, provided a nice illustration of the power of the brain organoid model in our endeavor to combat a global health threat.
Enabling personalized disease modeling and precision medicine
Coupling iPSC technology with brain organoids opens up opportunities to model neural diseases in a personalized manner. Individual‐derived brain organoids could serve as a potential source for cell therapy, or can be used for drug testing either in a therapeutic or preventive manner. Comparison between organoids derived from patients and healthy controls should reveal disease‐relevant insights that are informative to both clinicians and researchers. Moreover, for complex diseases organoids may serve as an in vitro surrogate to allow better patient stratification based on more defined endophenotypes.
Recent advances in genome editing technologies, such as clustered regularly interspaced short palindromic repeats (CRISPR), enable precise DNA alterations.73, 74 These methods would create isogenic lines which differ exclusively at a particular DNA sequence(s), thus permitting unequivocal delineation from genotype to phenotype. This powerful approach shall uncover the pathophysiological roles of many known causal mutations and guide targeted therapeutic interventions. Considering many neurological diseases are genetically complex, as evidenced by the large number of risk (and protective) genetic variants identified by GWAS and sequencing studies,75 we envision that this approach would also serve as the foundation of a platform to vigorously interrogate the function of these disease‐associated genetic loci, either individually or in a combinatorial manner.
Conclusion
While still being a relatively recent discovery, brain organoid is rapidly evolving as the next‐generation in vitro model system. As outlined in our review, current brain organoids are far from being perfect, and have limited value in studying overt phenotypes mostly related to early neurodevelopment. Nevertheless, we envision that with joint progress made by bioengineers, material scientists, and molecular biologists, improved brain organoids will soon be generated, and they will likely to bring significant benefits in every level, from basic research to drug discovery, and to clinical practices.
Author Contribution
A.X.S and E.K.T wrote the manuscript. All authors contributed to the ideas and edited the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgment
We thank Ms. Danlei Wang and Ms. Pei Zhuang for assistance with figures. This article is dedicated to Dr. Tao‐Shih Hsieh (1948–2016), a biochemist from Duke University, USA, and Dr. Yoshiki Sasai (1962–2014), a stem cell biologist from RIKEN, Japan. We thank the support from the National Medical Research Council.
[Correction added on 14 February 2018 after first online publication: Missing data was added in the Funding information nd Figure 2 was replaced with the correct image.]
Funding Statement
This work was funded by Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme grant ; Singapore Ministry of Health’s National Medical Research Council grant ; National Medical Research Council grant .
References
- 1. Horvath P, Aulner N, Bickle M, et al. Screening out irrelevant cell‐based models of disease. Nat Rev Drug Discov 2016;15:751–769. [DOI] [PubMed] [Google Scholar]
- 2. Watase K, Zoghbi HY. Modelling brain diseases in mice: the challenges of design and analysis. Nat Rev Genet 2003;4:296–307. [DOI] [PubMed] [Google Scholar]
- 3. Thoma CR, Zimmermann M, Agarkova I, et al. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 2014;69–70:29–41. [DOI] [PubMed] [Google Scholar]
- 4. Zanoni M, Piccinini F, Arienti C, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 2016;6:19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol 2016;18:246–254. [DOI] [PubMed] [Google Scholar]
- 6. Clevers H. Modeling development and disease with organoids. Cell 2016;165:1586–1597. [DOI] [PubMed] [Google Scholar]
- 7. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014;345:1247125. [DOI] [PubMed] [Google Scholar]
- 8. Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell 2016;18:736–748. [DOI] [PubMed] [Google Scholar]
- 9. Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self‐organising stem cells. Development 2012;139:4111–4121. [DOI] [PubMed] [Google Scholar]
- 10. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–265. [DOI] [PubMed] [Google Scholar]
- 11. Sato T, Stange DE, Ferrante M, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011;141:1762–1772. [DOI] [PubMed] [Google Scholar]
- 12. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011;470:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karthaus WR, Iaquinta PJ, Drost J, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014;159:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kessler M, Hoffmann K, Brinkmann V, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 2015;6:8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCracken KW, Catá EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem‐cell‐derived gastric organoids. Nature 2014;516:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huch M, Gehart H, van Boxtel R, et al. Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell 2015;160:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC‐derived organ bud transplant. Nature 2013;499:481–484. [DOI] [PubMed] [Google Scholar]
- 19. Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–568. [DOI] [PubMed] [Google Scholar]
- 20. Dye BR, Hill DR, Ferguson MAH, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015;4:e05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 23. Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi Y, Kirwan P, Smith J, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 2012;15:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eiraku M, Watanabe K, Matsuo‐Takasaki M, et al. Self‐organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008;3:519–532. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 2005;8:288–296. [DOI] [PubMed] [Google Scholar]
- 27. Lancaster MA, Renner M, Martin C‐A, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quadrato G, Nguyen T, Macosko EZ, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017;545:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lancaster MA, Corsini NS, Wolfinger S, et al. Guided self‐organization and cortical plate formation in human brain organoids. Nat Biotechnol 2017;35:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paşca AM, Sloan SA, Clarke LE, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015;12:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mariani J, Coppola G, Zhang P, et al. FOXG1‐dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 2015;162:375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muguruma K, Nishiyama A, Kawakami H, et al. Self‐organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 2015;10:537–550. [DOI] [PubMed] [Google Scholar]
- 33. Jo J, Xiao Y, Sun AX, et al. Midbrain‐like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin‐producing neurons. Cell Stem Cell 2016;19:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suga H, Kadoshima T, Minaguchi M, et al. Self‐formation of functional adenohypophysis in three‐dimensional culture. Nature 2011;480:57–62. [DOI] [PubMed] [Google Scholar]
- 35. Qian X, Nguyen HN, Song MM, et al. Brain‐region‐specific organoids using mini‐bioreactors for modeling ZIKV exposure. Cell 2016;165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self‐organizing human embryonic stem cell‐derived dorsomedial telencephalic tissue. Nat Commun 2015;6:8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell 2011;146:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zucca FA, Basso E, Cupaioli FA, et al. Neuromelanin of the human substantia nigra: an update. Neurotox Res 2014;25:13–23. [DOI] [PubMed] [Google Scholar]
- 39. Buchman JJ, Tseng H‐C, Zhou Y, et al. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron 2010;66:386–402. [DOI] [PubMed] [Google Scholar]
- 40. Lizarraga SB, Margossian SP, Harris MH, et al. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development 2010;137:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Muffat J, Omer A, et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 2017;20:385–396.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bershteyn M, Nowakowski TJ, Pollen AA, et al. Human iPSC‐derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 2017;20:435–449.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iefremova V, Manikakis G, Krefft O, et al. An organoid‐based model of cortical development identifies non‐cell‐Autonomous defects in Wnt signaling contributing to Miller‐Dieker syndrome. Cell Rep 2017;19:50–59. [DOI] [PubMed] [Google Scholar]
- 44. Raja WK, Mungenast AE, Lin Y‐T, et al. Self‐organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer's disease phenotypes. PLoS ONE 2016;11:e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi SH, Kim YH, Hebisch M, et al. A three‐dimensional human neural cell culture model of Alzheimer's disease. Nature 2014;515:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016;534:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dang J, Tiwari SK, Lichinchi G, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 2016;19:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016;352:816–818. [DOI] [PubMed] [Google Scholar]
- 49. Nowakowski TJ, Pollen AA, Di Lullo E, et al. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 2016;18:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gabriel E, Ramani A, Karow U, et al. Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 2017;20:397–406.e5. [DOI] [PubMed] [Google Scholar]
- 51. Xu M, Lee EM, Wen Z, et al. Identification of small‐molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 2016;22:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 2014;9:2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee K, Silva EA, Mooney DJ. Growth factor delivery‐based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011;8:153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carlson AL, Bennett NK, Francis NL, et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat Commun 2016;7:10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature 2016;539:560–564. [DOI] [PubMed] [Google Scholar]
- 56. Birey F, Andersen J, Makinson CD, et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017;545:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Monzel AS, Smits LM, Hemmer K, et al. Derivation of human midbrain‐specific organoids from neuroepithelial stem cells. Stem Cell Rep 2017;8:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sloan SA, Darmanis S, Huber N, et al. Human astrocyte maturation captured in 3d cerebral cortical spheroids derived from pluripotent stem cells. Neuron 2017;95:779–790.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jakel RJ, Schneider BL, Svendsen CN. Using human neural stem cells to model neurological disease. Nat Rev Genet 2004;5:136–144. [DOI] [PubMed] [Google Scholar]
- 60. Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell‐driven condensation. Cell Stem Cell 2015;16:556–565. [DOI] [PubMed] [Google Scholar]
- 61. Haenseler W, Sansom SN, Buchrieser J, et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal‐co‐culture‐specific expression profile and inflammatory response. Stem Cell Rep 2017;8:1727–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muffat J, Li Y, Yuan B, et al. Efficient derivation of microglia‐like cells from human pluripotent stem cells. Nat Med 2016;22:1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex 1991;2009(19 Suppl 1):i70–i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lange C, Turrero Garcia M, Decimo I, et al. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J 2016;35:924–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Camp JG, Badsha F, Florio M, et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci 2015;112:15672–15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miller JD, Ganat YM, Kishinevsky S, et al. Human iPSC‐based modeling of late‐onset disease via progerin‐induced aging. Cell Stem Cell 2013;13:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bagley JA, Reumann D, Bian S, et al. Fused cerebral organoids model interactions between brain regions. Nat Methods 2017;14:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiang Y, Tanaka Y, Patterson B, et al. Fusion of regionally specified hPSC‐derived organoids models human brain development and interneuron migration. Cell Stem Cell 2017;21:383–398.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Penzes P, Buonanno A, Passafaro M, et al. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem 2013;126:165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schwartz MP, Hou Z, Propson NE, et al. Human pluripotent stem cell‐derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci USA 2015;112:12516–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yin X, Mead BE, Safaee H, et al. Engineering stem cell organoids. Cell Stem Cell 2016;18:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR‐Cas9. Science 2014;346:1258096. [DOI] [PubMed] [Google Scholar]
- 74. Muffat J, Li Y, Jaenisch R. CNS disease models with human pluripotent stem cells in the CRISPR age. Curr Opin Cell Biol 2016;43:96–103. [DOI] [PubMed] [Google Scholar]
- 75. Foo J‐N, Liu J‐J, Tan E‐K. Whole‐genome and whole‐exome sequencing in neurological diseases. Nat Rev Neurol 2012;8:508–517. [DOI] [PubMed] [Google Scholar]


