Abstract
Objective
Whether activation or inhibition of the mTOR pathway is beneficial to ischemic injury remains controversial. It may result from the different reaction of ischemic penumbra and core to modulation of mTOR pathway after cerebral ischemia–reperfusion injury in rats.
Methods
Longa's middle cerebral artery occlusion (MCAO) method was conducted to induce the focal cerebral ischemia–reperfusion. Western blot analysis was used to examine the protein expression involving mTOR pathway, apoptosis, and autophagy‐related proteins. TTC staining and Fluoro‐Jade B staining was conducted to detect the infarct volume and cell apoptosis, respectively. Neurological function was measured by modified neurological severity score and left‐biased swing.
Results
mTOR signaling pathway was activated in ischemic penumbra and decreased in ischemic core after ischemia and ischemia–reperfusion. Ischemia–reperfusion injury induced the increase in cleaved caspase 9 and caspase 3 both in ischemic penumbra and in ischemic core, whereas the expression of phosphorylated ULK1, Beclin 1 and LC3‐II was decreased. Rapamycin pre or postadministration inhibited the overactivation of mTOR pathway in ischemic penumbra. Ameliorated neurological function and reduced infarct volume were observed after pre or postrapamycin treatment. Rapamycin markedly decreased the number of FJB‐positive cells and the expression of cleaved caspase‐3 and cleaved caspase‐9 proteins as well as increased the activation of autophagy reflected by ULK1, Beclin‐1 and LC3.
Interpretation
mTOR signaling pathway was activated in ischemic penumbra after cerebral ischemia–reperfusion injury in rats. mTOR inhibitor rapamycin significantly decreased the mTOR activation and infarct volume and subsequently improved neurological function. These results may relate to inhibition of neuron apoptosis and activation of autophagy.
Introduction
Stroke is the second most common cause of death and major cause of disability around the world. Brain ischemic stroke is caused by pathophysiological thrombosis and thromboembolism, which give rise to cerebral arterial occlusion with subsequent ischemia of partial brain tissue supplied by that artery.1, 2 Although tremendous progress has been made in the study of pathophysiology on ischemic stroke, translation of basic research into clinical therapies has not successed. Currently, systemic thrombolysis with recombinant intravenous tissue plasminogen activator (rtPA) remains a frequently used therapy which can improve clinical outcome of patients with acute ischemic stroke.3, 4 However, rtPA has an increased risk of hemorrhage beyond a few hours of poststroke, and only less than 4% of stroke patients can benefit from it.5, 6 Therefore, it is urgent to clarify the mechanism of ischemic stroke and explore new potential targeted strategies for the treatment of stroke.
The mammalian target of rapamycin (mTOR) pathway plays an essential role in a number of important physiological functions including cell growth, proliferation, protein synthesis, metabolism, and autophagy.7 In the brain, the mTOR pathway regulates synaptic plasticity, neuronal transmission, axon outgrowth, neuronal size, and spine morphology, thus loss of homeostasis of mTOR pathway is involved in a variety of neurologic diseases such as epilepsy, Alzheimer's disease, and Parkinson's disease.7, 8, 9, 10 Numerous reports have demonstrated that mTOR pathway is involved in brain ischemia–reperfusion induced injury.11, 12, 13, 14, 15, 16 However, whether upregulating or downregulating mTOR pathway has a protective effect on neurons in ischemia–reperfusion injury remains controversial. Some studies have shown that upregulating mTOR offers neuronprotection,11, 12, 13 whereas others have reported that inhibiting mTOR by rapamycin administration reduces neurological damage and promotes neuronal viability in some ischemic models.14, 15, 16 The contradictory results indicate that the subtle balance of mTOR signaling pathway in cerebral ischemia is critical to cellular physiology. However, the change in the mTOR signaling pathway in different brain areas and in different time intervals after ischemia–reperfusion has not been examined in detail, thus it is possible that regulating the mTOR pathway at different stages results in opposing effects. Therefore, in this study, we explored the changes of key proteins of mTOR signaling pathway in different regions and different stages after cerebral ischemia–reperfusion (I/R) injury using middle cerebral artery occlusion (MCAO) in rats, and observed the effects and possible mechanism of action of rapamycin on cerebral I/R injury in rats.
Materials and Methods
Animals
Male Sprague–Dawley (SD) rats weighing 230–270 g were purchased from Shanghai Slac Laboratory Animal Corporation (certificate: SCXK2007‐0005) and housed at Zhejiang University City College Animal Care Facility. The animal procedures were approved by the Committee on the Ethics of Animal Experiments of Zhejiang University and were carried out in accordance with Zhejiang University Institutional Animal Care and Use Committee (Permit number: ZJU2015‐489‐02).
Focal cerebral ischemia models
To induce focal cerebral ischemia, rats were subjected to MCAO as reported previously.17 Briefly, rats were fasted overnight before operation but had free access to water. The next day, rats were anesthetized with 10% isoflurane and the common carotid artery, external carotid artery, and internal carotid artery were exposed through a midline incision. A monofilament nylon suture was inserted through the ICA toward the cranial base until feeling a mild resistance. At this stage, cerebral blood flow value was detected by LDF measurement and an approximately 80% drop of the baseline blood flow value was considered MCA occlusion. The suture was withdrawn after 1–6 h to allow reperfusion. Similar surgical procedure was conducted in sham animals without introducing the occluding suture. Rectal temperature was maintained at 37 ± 0.5°C during the operation and during recovery from the operation.
Drug treatment
Rapamycin (LC Laboratories, USA) stock solution was prepared by dissolving 30 mg in 1 mL of 100% ethanol and stored at −20°C. It was diluted in 5% Tween 80, 5% polyethyleneglycol 400 and 4% ethanol immediately before use.18 Rapamycin was injected intraperitoneal once a day at the dose of 3.0 mg/kg. For rapamycin pretreatment group (prerapa), rats were administrated rapamycin for consecutive 3 day, and MCAO was conducted 24 h after the last injection. For rapamycin posttreatment group (post‐rapa), rats were administrated rapamycin 6 h after MCAO and another consecutive 3 day. For vehicle‐treated group (veh), rats received the same volume of vehicle in all experiments.
Neurobehavioral testing
Modified neurological severity score (NSS) was used to access their neurobehavioral change.19 Neurological function included motor tests, sensory tests, beam balance test, and reflex absence and abnormal movements, which all were graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18). Rats were trained before operation, and then were assessed 1, 3, 7, 10, and 14 days after MCAO. The observers were blinded to the treatment group.
Infarct volume measurement
Rats were decapitated 4 days after MCAO and brains were rapidly removed. Coronal sections with the thickness of 2 mm were stained with 2% 2,3,5‐triphenyltetrazolium chloride (TTC) (Solarbio, China) for 30 min at 37°C followed by immersion in 4% paraformaldehyde solution overnight and photographed by a digital camera. Microscope image‐analysis software (Image‐Pro plus, USA) was used to calculate the infarct volumes as previously reported.20 Briefly, infarct area of each brain section was added to obtain the total infarct areas. The infarct volume was obtained by multiplying the total infarct areas to the thickness of the sections. The percentage of infarct volume to total brain volume represents the degree of cerebral infarction.
Western blotting analysis
Proteins expression in the ischemic core, ischemic penumbra, and contralateral normal brain were detected by standard methods as previously described.21 Proteins were separated and transferred onto polyvinylidene fluoride membrane (Millpore, USA). The membrane was blocked with 5% skim milk followed by the primary antibody of phospho‐Akt(Ser473), phospho‐mTOR(Ser2448), phospho‐p70s6 kinase (Thr389), phospho‐S6 (Ser240/244), cleaved caspase‐9, cleaved caspase‐3, phospho‐ULK1(Ser757), Beclin1(Abcam, UK), and LC3(Abcam) overnight at 4°C, respectively. The membrane was then incubated with the peroxidase conjugated anti‐rabbit or anti‐mouse secondary antibody (Pierce, 1:5000, USA). Enhanced chemiluminescence reagent (Pierce) was used to visualize the protein signals. The membranes were reprobed and incubated with the rabbit Akt antibody, rabbit mTOR antibody, rabbit p70S6 kinase antibody, rabbit S6 antibody, rabbit ULK1 antibody, and mouseβ‐actin, respectively. Except Beclin 1 and LC3 antibody were obtained from Abcam, all other antibodies were purchased from Cell Signaling Technology and diluted according to the ratio 1:1000. Intensity of the signals was measured by Image J (NIH, USA). The results were presented as a ratio of phosphorylation to total protein or to actin. Each experiment was conducted independently at least three times.
Fluoro‐Jade B staining
Seven days after operation, rats were deeply anesthetized and perfused transcardially with saline, subsequently followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). Brains were postfixed with 4% paraformaldehyde, and then dehydrated in ascending concentration of sucrose. After embedded in Tissue‐Tek OCT, brains were sectioned at 20 μm thickness. Fluoro‐Jade B staining was conducted as described previously.22 Tissues were first placed in 0.1 mol/L phosphate buffer. They were then treated in turn with 1% NaOH in 80% ethanol for 5 min, 70% ethanol for 2 min, ddH2O for 2 min, 0.06% KMnO4 for 10 min, and ddH2O for 2 min. Tissues were transferred into 0.001% Fluoro‐Jade B in 0.1% acetic acid for 20 min and washed three times with ddH2O for 1 min each and dried overnight. Finally, the sections were mounted on glass slides and imaged using a fluorescence microscope. FJB‐positive cells in each field were calculated.
Statistical analysis
Quantitative results were expressed as mean ± SEM. One‐way ANOVA with SNK‐Q test for post hoc multiple comparisons (version 16.0, SPSS Inc., Chicago, IL) was used to analyze the differences among groups. P < 0.05 was considered statistically significant.
Results
mTOR signaling pathway was changed differentially in different brain zones following MCAO
The area lying just outside the ischemic core region (tissue most severely damaged by stroke or ischemic event) was termed the ischemic penumbra. It has the potential for recovery and therefore is the target for interventional therapy in acute ischemic stroke.23 In this study, we first stained the coronal brain section by TTC staining at 3 h after ischemia without reperfusion and partitioned it into three parts: (I) normal zone; (II) ischemic penumbra; (III) ischemic core (Fig. 1A). Then, we analyzed the changes in the four key proteins in these three parts. The results showed that the expression of Akt, mTOR, S6K, and S6 phosphorylation was significantly increased in the ischemic penumbra but markedly decreased in ischemic core as compared with the normal zone (Fig. 1B–C). Similar results were also demonstrated at other time points of 1 h and 6 h (data not shown). These results suggested that mTOR signaling pathway was abnormally activated in ischemic penumbra while inhibited in ischemic core.
Figure 1.
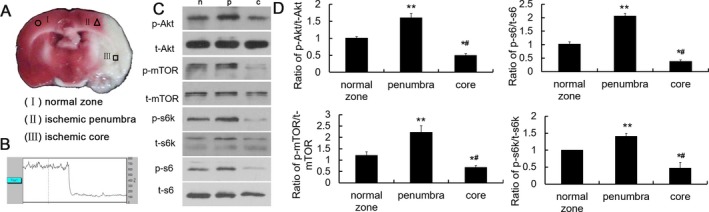
mTOR signaling pathway was activated in ischemic penumbra but inhibited in ischemic core. (A) Partition of brain slices by TTC staining after MCAO. (I) normal zone; (II) ischemic penumbra; (III) ischemic core. (B) Representative LDF image during MCAO. (C) Representative western blots of Akt, mTOR, S6K, and S6 in normal zone, ischemic penumbra, and ischemic core. (D) Quantitative summary demonstrated that the phosphorylation of the four proteins was significantly increased in ischemic penumbra but decreased in ischemic core. (*P < 0.05, **P < 0.01vs. normal zones, # P < 0.05 vs. ischemic penumbra, n = 6/group).
Changes in mTOR signaling pathway at different ischemic intervals with or without reperfusion
Next, we observed the changes in mTOR signaling pathway reflected by S6 phosphorylation in the ischemic penumbra at different ischemia/reperfusion intervals after MCAO. The expression of p‐S6 phosphorylation levels did not change significantly after 30 min of ischemia, however, it increased markedly starting from 1 h and maintained at a high level until 6 h after ischemia (Fig. 2A–B). Since the mortality rate of rats was increased along with the prolongation of ischemic time, we determined the ischemia time as 1.5 h followed by reperfusion for the following experiments.
Figure 2.
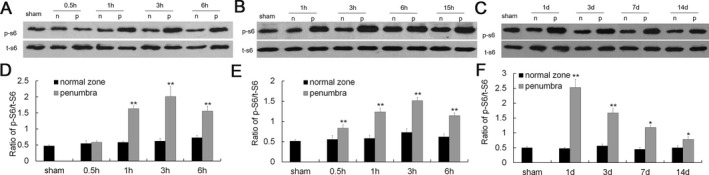
Expression of S6 phosphorylation at different ischemia/reperfusion intervals after MCAO. (A) Representative western blots of p‐S6 expression at different ischemia time after MCAO. (B) Quantitative summary demonstrated that the expression of p‐S6 was significantly increased in ischemic penumbra 1 h after MCAO and remained elevated for 6 h. (C, E) Representative western blots of p‐S6 expression at different intervals of reperfusion after 1 h ischemia. (D, F) Quantitative summary demonstrated that the expression of p‐S6 was significantly increased in ischemic penumbra after 1–24 h reperfusion and gradually reduced thereafter. (*P < 0.05, **P < 0.01 vs. normal zones, n = 6/group).
We then detect the change in p‐S6 expression after different reperfusion intervals. The expression of phospho‐S6 was markedly elevated within 6 h after reperfusion (Fig.2C–D), it reached a peak value at 1 d and decreased gradually thereafter but still maintained at an elevated level till 14 d (Fig. 2E–F) or even 6 w (data not shown). The above results suggested that mTOR signaling pathway was activated after ischemia with and without reperfusion.
Changes in apoptosis and autophagy in different brain zones
Many cross‐talk pathways are observed between apoptosis and autophagy involving mTOR signaling. We also observed changes in apoptosis‐ and autophagy‐related proteins after MCAO in different brain zones. As compared to normal zone, the expression of cleaved caspase‐9 and caspase‐3 was elevated both in ischemic penumbra and in ischemic core as detected 24 h and 7 day after MCAO (Fig. 3A and B). In contrast, the level of phosphorylated ULK1, Beclin 1, and LC3‐II was decreased both in ischemic penumbra and in ischemic core as detected 24 h and 7 day after MCAO (Fig. 3C–F). These data indicate that I/R injury results in neuronal apoptosis and inhibition of autophagy activity.
Figure 3.
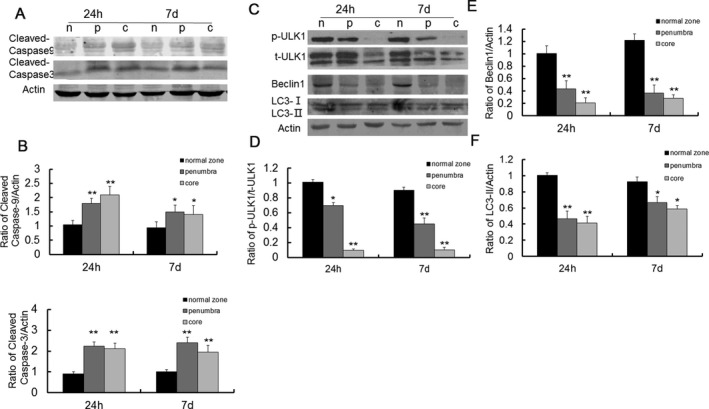
Expression of apoptosis and autophagy‐related proteins after MCAO. (A) Representative western blots of cleaved caspase 9 and caspase 3 at 24 h and 7 days after MCAO. (B) Quantitative summary demonstrated that the expression of cleaved caspase 9 and caspase 3 was significantly increased both in ischemic penumbra and ischemic core at 24 h and 7 day after MCAO. (C) Representative western blots of p‐ULK1, beclin 1, and LC3 expression at 24 h and 7 day after MCAO. (D‐F) Quantitative summary demonstrated that the expression of p‐ULK1, beclin 1, and LC3 was significantly decreased both in ischemic penumbra and ischemic core at 24 h and 7 day after MCAO. (*P < 0.05, **P < 0.01 vs. normal zones, n = 6/group).
Rapamycin inhibited mTOR signaling pathway and improved neurologic deficits
To detect whether activation of mTOR signaling pathway in ischemic penumbra is beneficial or detrimental to I/R injury, we treated rats with mTOR inhibitor rapamycin before or after MCAO and killed the rats for western blot analysis 3 day after MCAO. Compared with the vehicle‐treated model group, the expression of p‐S6 was significantly inhibited both in pre and postrapamycin‐treated group (Fig. 4A–B). Another cohort of rats was evaluated for neurological function at 1, 3, 7, 10, and 14 day by NSS score. Left‐biased swing was also tested to evaluate the asymmetry of movement at 7 day and 14 day. Compared with the sham group, the vehicle‐treated group showed an obvious increase in NSS score at all time points. However, the NSS score in pre and postrapamycin‐treated group were significantly decreased in 7, 10, and 14 days after MCAO (Fig. 4C). Similar results were also exhibited in the left‐biased swing test (Fig. 4D). The results indicate that rapamycin reverses the abnormal activation of mTOR signaling pathways caused by cerebral ischemia–reperfusion and significantly improves the associated neurological deficits.
Figure 4.
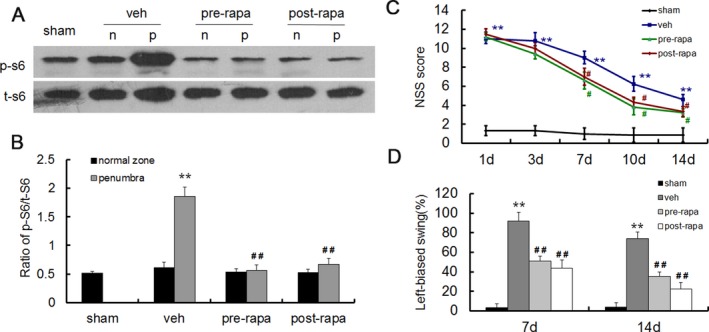
Rapamycin administration inhibited mTOR signaling pathway and improved neurologic deficits. (A) Representative western blots of p‐S6 expression after pre or posttreatment of rapamycin. (B) Quantitative summary demonstrated that the expression of p‐S6 was significantly reduced with pre and postrapamycin treatment. (C) Assessment of neurological severity scores in different groups within 14 days after MCAO. The scores were significantly lower in pre and postrapamycin group as compared with the vehicle group at 7, 10, and 14 day. (D) Rapamycin treatment improved asymmetrical motor function at 7 days and 14 days after MCAO in the elevated body swing test. (**P < 0.01 vs. sham group, # P < 0.05, ## P < 0.01 vs. vehicle group, n = 6 in A and B, n = 10 in C and D per group).
Rapamycin treatment reduced the infarct volume in MCAO rats
Rats were killed 3 day after MCAO and the infarct volume was calculated after TTC staining. No obvious infarct area was noticed in sham group. The infarct volume was 28.4 ± 3.1% of total brain volume in the vehicle‐treated group, whereas rapamycin pre and posttreated groups exhibited markedly decrease in infarct volume of 15.8 ± 2.9% and 20.1 ± 1.8%, respectively (Fig. 5A–B).
Figure 5.

Rapamycin treatment decreased infarct volume. (A) Representative slices of TTC staining. (B) Quantitative analyses of infarct volume. Rapamycin treatment significantly reduced infarction volumes. (**P < 0.01 vs. sham group, ## P < 0.01 vs. vehicle group, n = 12 per group).
Rapamycin treatment reduced neuronal apoptosis and increased activation of autophagy
To characterize the apoptosis of neurons after ischemia–reperfusion injury in different groups, we performed Fluoro‐Jade B staining 7 day after MCAO. No obvious FJB‐positive cells were shown in sham group, however, it was robust in vehicle‐treated I/R modeling rats in ischemic penumbra. Although FJB‐positive cells were also abundant in both pre and postrapamycin‐treated group as compared with sham group, it was significantly less than that in the vehicle‐treated group (Fig. 6A–B). Similar results were also obtained at other time points of 3 days and 2 week after MCAO (data not shown). Accordingly, cleaved caspase‐9 and cleaved caspase‐3 was markedly increased in vehicle‐treated group in ischemic penumbra, whereas pre and postrapamycin administration alleviated the increase in cleaved caspase‐9 and cleaved caspase‐3 (Fig. 6C–D). Similarly, pre and postrapamycin administration increased the expression of phosphorylated ULK1, Beclin 1, and LC3‐II proteins (Fig. 6E–F).
Figure 6.
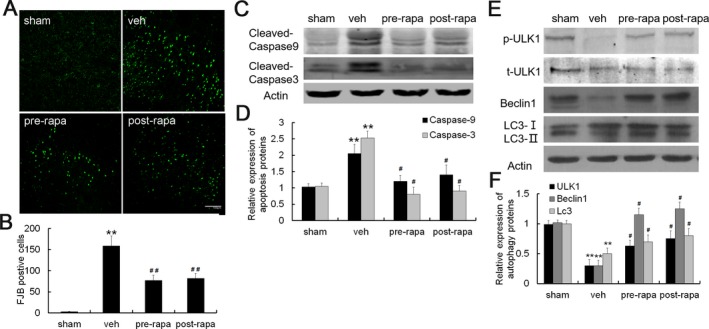
Rapamycin treatment reduced neuronal apoptosis and activated autophagy. (A) Representative photographs of FJB staining. (B) Quantitative analyses of FJB staining. The number of FJB‐positive cells was markedly decreased after rapamycin treatment. (C) Representative western blots of cleaved caspase 9 and caspase 3 expression after rapamycin treatment. (D) Quantitative summary demonstrated that the expression of cleaved caspase 9 and caspase 3 was significantly decreased in pre and postrapamycin treatment. (E) Representative western blots of p‐ULK1, Beclin 1 and LC 3 expression after rapamycin treatment. (F) Quantitative summary demonstrated that the expression of p‐ULK1, Beclin 1 and LC 3 was significantly increased in pre and postrapamycin treatment. (**P < 0.01 vs. sham group,#p < 0.05,## P < 0.01 vs. vehicle group, n = 6 per group, Scale bar = 100 μm).
Discussion
The results presented in this work revealed that mTOR signaling pathway is abnormally activated in ischemic penumbra and inhibited in ischemic core after MCAO. Increased apotosis and decreased autophagy activity are demonstrated both in ischemic penumbra and ischemic core. Rapamycin administration significantly reduces lesion volumes and neuronal apoptosis and improves autophagy and motor function, implying a new direction for the treatment of cerebral ischemia.
A complicated cascade of pathophysiological events is involved in the development of I/R injury including excitotoxicity, oxidative stress, inflammation, and apoptosis which is proposed in the ischemic penumbra zone. Since mTOR signaling pathway plays a pivotal role regarding all the above pathophysiological process, it has been widely studied in brain ischemia. Activation of mTOR signaling pathway in nude mice significantly reduces cerebral ischemia injury as well as reduce neurological deficit, infarct areas and brain water content.11, 12, 13, 24, 25 However, inhibiting mTOR signaling pathway using rapamycin or knockdown of mTOR promotes autophagy and attenuates ischemia‐induced neuronal death.14, 15, 16, 26 Reports also demonstrate that inhibition of mTOR pathway suppresses autophagy, prevents cytochromecrelease and reduces ischemic brain damage.3, 15, 27 This discrepancy may result from different animal strains, ischemic model, the time and route of drug administration. In this study, we first performed a more detailed temporal analysis of mTOR signaling changes in normal zone, ischemic penumbra and ischemic core of the MCAO model in rat. We found that mTOR pathway was activated in ischemic penumbra and suppressed in ischemic core for at least 2 weeks. According to our results, it is reasonable to hypothesize that upregulating the mTOR pathway in ischemic core and downregulating the mTOR pathway in ischemic penumbra may benefit the treatment of ischemia–reperfusion injury. Since the neurons in ischemic core are difficult to rescue, we used rapamycin as an inhibitor to suppress the over activation of mTOR in ischemic penumbra, a zone which can be transferred to normal zone under appropriate treatment. As we expected, rapamycin protected the ischemia–reperfusion injury by reducing the infarct volume and neurological deficit.
Rapamycin has diverse effect by inhibiting mTOR pathway. First, rapamycin has dual effects on apoptosis.28 On one hand, rapamycin is proved to be proapoptotic, especially in cancers, resulting in apoptotic death of the cancer cells.29 On the other hand, some evidence has demonstrated that rapamycin can be antiapoptotic.30, 31 For example, rapamycin inhibits death of syncytia via inhibition of proapoptotic Bax and inhibition of the mitochondrial cell death pathway.32, 33 In neurodegenerative diseases, rapamycin inhibited high expression of mTOR and improved the level of antiapoptotic protein Bcl‐2. It also induced autophagy and inhibited cell apoptosis, thus playing the effect of neuroprotection.34, 35 Second, rapamycin has anti‐inflammatory effects. Rapamycin apparently attenuates behavioral deficits after MCAO by shifting the microglia phenotype from M1 type to M2 type.36 It can attenuate secondary injury after focal ischemia by enhancing anti‐inflammatory activity of Tregs to restrain neuroinflammation.4 In addition, rapamycin can inhibit astrocyte proliferation, migration and production of inflammatory mediators after oxygen‐glucose deprivation/reoxygenation.37 Third, rapamycin has a paradoxical effect on oxidative stress. On one hand, rapamycin can reduce oxidative stress in a rat of Parkinson model and restores the mitophagy inhibited by monoamine oxidase B (Mao‐B) in an inducible cell model.38, 39 On the other hand, rapamycin increases oxidative stress response in adult stem cells40 and nucleolar disruption leads to oxidative damage and Parkinsonism through mTOR repression.41 Fourth, rapamycin can induce autophagy. Rapamycin activates autophagy to rescue the neurons destined to die in global ischemia and in neonatal hypoxia‐ischemia.42, 43 In our study, mTOR signaling pathway is abnormally activated in ischemic penumbra, and neurnal apoptosis is activated, whereas autophagy was inhibited. Rapamycin inhibits the activation of mTOR pathway, reduced cell apoptosis and activate autophagy in ischemic penumbra, suggested that rapamycin has a protective effect on cerebral ischemia–reperfusion injury in SD rats.
This study also demonstrated that both rapamycin pre and posttreatment had similar effect, indicating that rapamycin exerts its neuroprotective effect in cerebral ischemia may be associated with the later development of ischemia–reperfusion injury. Since currently available treatment of tPA has a strict time window, rapamycin may be considered as an alternative drug that used for brain stroke treatment when tPA is counterindicated. Additional studies are needed to determine the specific mechanisms of rapamycin's neuroprotective effect in stroke and its potential translational applications.
In conclusion, our data showed that mTOR signaling pathway was abnormally activated in ischemic penumbra. mTOR inhibitor rapamycin administrated before or after MCAO improves the neurologic function and decreases the infarct volume by decreasing the number of neurons apoptosis and activating of autophagy process. These results indicate that mTOR signaling pathway is a potential target for neuroprotection in ischemic brain treatment, but the specific mechanism needs further study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81371429), and Science and Technology R&D Program of Hangzhou (20140633B37), Hangzhou Science and Technology Major Project (20152013A02).
Funding Statement
This work was funded by National Natural Science Foundation of China grant 81371429; Science and Technology R&D Program of Hangzhou grant 20140633B37; Hangzhou Science and Technology Major Project grant 20152013A02.
References
- 1. Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Flynn RWV, Macwalter PSM, Doney ASF, et al. The cost of cerebral ischaemia. Neuropharmacology 2008;55:250–256. [DOI] [PubMed] [Google Scholar]
- 3. Yang X, Hei C, Liu P, et al. Inhibition of mTOR pathway by rapamycin reduces brain damage in rats subjected to transient forebrain ischemia. Int J Biol Sci 2015;11:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie L, Sun F, Wang J, et al. mTOR signaling inhibition modulates macrophage/microglia‐mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol 2014;192:6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Zhang Z, Chow N, et al. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke 2012;43:2444–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wechsler LR, Jovin TG, Jovin TG. Intravenous recombinant tissue‐type plasminogen activator in the extended time window and the US Food and Drug Administration: confused about the time. Stroke 2012;43:2517–2519. [DOI] [PubMed] [Google Scholar]
- 7. Wong M. Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomedical 2013;36:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bové J, Martinez‐Vicente M, Vila M, et al. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 2011;12:437–452. [DOI] [PubMed] [Google Scholar]
- 9. Santini E, Heiman M, Greenqard P, et al. 2009. Inhibition of mTOR signaling in Parkinson's disease prevents L‐DOPA‐induced dyskinesia. Sci Signal 2: ra36. [DOI] [PubMed] [Google Scholar]
- 10. Chong ZZ, Shang YC, Zhang L, et al. Mammalian target of rapamycin: hitting the bull's‐eye for neurological disorders. Oxid Med Cell Longev 2010;3:374–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koh PO, Cho JH, Won CK, et al. Estradiol attenuates the focal cerebral ischemic injury through mTOR/p70S6 kinase signaling pathway. Neurosci Lett 2008;436:62–66. [DOI] [PubMed] [Google Scholar]
- 12. Zare Mehrjerdi F, Aboutaleb N, Habibey R, et al. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res 2013;1526:94. [DOI] [PubMed] [Google Scholar]
- 13. Sanghera KP, Mathalone N, Baiqi R, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci 2011;47:145–153. [DOI] [PubMed] [Google Scholar]
- 14. Chauhan A, Sharma U, Jaqannathan NR, et al. Rapamycin protects against middle cerebral artery occlusion induced focal cerebral ischemia in rats. Behav Brain Res 2011;225:603–609. [DOI] [PubMed] [Google Scholar]
- 15. Yin L, Ye S, Chen Z, et al. Rapamycin preconditioning attenuates transient focal cerebral ischemia/reperfusion injury in mice. Int J Neurosci 2012;122:748–756. [DOI] [PubMed] [Google Scholar]
- 16. Urbanek T, Kuczmik W, Basta‐Kaim A, et al. Rapamycin induces of protective autophagy in vascular endothelial cells exposed to oxygen–glucose deprivation. Brain Res 2014;1553:1–11. [DOI] [PubMed] [Google Scholar]
- 17. Shahjouei S, Cai PY, Ansari S, et al. Middle cerebral artery occlusion model of stroke in rodents: a step‐by‐step approach. J Vasc Interv Neurol 2016;8:1–8. [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng LH, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 2008;63:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001;32:1005–1011. [DOI] [PubMed] [Google Scholar]
- 20. Wang T, Li Y, Wang Y, et al. Lycium barbarum polysaccharide prevents focal cerebral ischemic injury by inhibiting neuronal apoptosis in mice. PLoS ONE 2014;9:e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu Z, Liu F, Chen L, et al. Effect of chronic administration of low dose rapamycin on development and immunity in young rats. PLoS ONE 2015;10:e0135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmued LC, Hopkins KJ. Fluoro‐Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 2000;874:123–130. [DOI] [PubMed] [Google Scholar]
- 23. Genova HM. Ischemic Penumbra. New York: Springer, 2011. [Google Scholar]
- 24. Xie R, Wang P, Ji X, et al. Ischemic post‐conditioning facilitates brain recovery after stroke by promoting Akt/mTOR activity in nude rats. J Neurochem 2013;127:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fu L, Huang L, Cao C, et al. Inhibition of AMP‐activated protein kinase alleviates focal cerebral ischemia injury in mice: interference with mTOR and autophagy. Brain Res 2016;1650:103. [DOI] [PubMed] [Google Scholar]
- 26. Hwang JY, Gerhner M, Pontarelli F, et al. Global ischemia induces lysosomal‐mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ 2017;24:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu Q, Gao L, Huang L, et al. Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat. J Neuroinflammation 2014;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castedo M, Ferri KF, Kroemer G, et al. Mammalian target of rapamycin (mTOR): pro‐ and anti‐apoptotic. Cell Death Differ 2002;9:99. [DOI] [PubMed] [Google Scholar]
- 29. Harada H, Andersen JS, Mann M, et al. p70S6 kinase signals cell survival as well as growth, inactivating the pro‐apoptotic molecule BAD. Proc Natl Acad Sci USA 2001;98:9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calastretti A, Rancati F, Ceriani MC, et al. Rapamycin increases the cellular concentration of the BCL‐2 protein and exerts an anti‐apoptotic effect. Eur J Cancer 2001;37:2121–2128. [DOI] [PubMed] [Google Scholar]
- 31. Thyrell L, Hiortsberg L, Arulampalam V, et al. Interferon alpha‐induced apoptosis in tumor cells is mediated through the phosphoinositide 3‐kinase/mammalian target of rapamycin signaling pathway. J Biol Chem 2004;279:24152–24162. [DOI] [PubMed] [Google Scholar]
- 32. Castedo M, Ferri KF, Blanco J, et al. Human immunodeficiency virus 1 envelope glycoprotein complex‐induced apoptosis involves mammalian target of rapamycin/Fkbp12‐Rapamycin–associated protein–mediated P53 phosphorylation. J Exp Med 2001;194:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferri KF, Jacotot E, Blanco J, et al. Apoptosis control in syncytia induced by the HIV type 1–envelope glycoprotein complex. J Exp Med 2000;192:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res 2014;11:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chong ZZ, Shang YC, Wang S, et al. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol 2012;99:128–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li D, Wang C, Yao Y, et al. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J 2016;30:3388–3399. [DOI] [PubMed] [Google Scholar]
- 37. Li CY, Li X, Liu SF, et al. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen‐glucose deprivation and reoxygenation. Neurochem Int 2015; s83–84:9–18. [DOI] [PubMed] [Google Scholar]
- 38. Jiang J, Jiang J, Zuo Y, et al. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson's disease. Int J Mol Med 2013;31:825–832. [DOI] [PubMed] [Google Scholar]
- 39. Siddiqui A, Hanson I, Andersen JK, et al. MAO‐B elevation decreases parkin's ability to efficiently clear damaged mitochondria: protective effects of rapamycin. Free Radical Res 2012;46:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kofman AE, McGraw MR, Payne CJ, et al. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging 2012;4:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rieker C, Engblom D, Kreiner G, et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci 2011;31:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hwang JY, Gertner M, Pontarelli F, et al. Global ischemia induces lysosomal‐mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ 2017;24:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carloni S, Girelli S, Scopa C, et al. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia‐ischemia. Autophagy 2010;6:366. [DOI] [PubMed] [Google Scholar]


