Abstract
Cystathionine β-synthase (CBS) domains have been identified in a wide range of proteins of unrelated functions such as, metabolic enzymes, kinases and channels, and usually occur as tandem re-peats, often in combination with other domains. In plants, CBS Domain-Containing Proteins (CDCPs) form a multi-gene family and only a few are so far been reported to have a role in development via regu-lation of thioredoxin system as well as in abiotic and biotic stress response. However, the function of majority of CDCPs still remains to be elucidated in plants. Here, we report the cloning, characterization and functional validation of a CBS domain containing protein, OsCBSCBSPB4 from rice, which pos-sesses two CBS domains and one PB1 domain. We show that OsCBSCBSPB4 encodes a nucleo-cytoplasmic protein whose expression is induced in response to various abiotic stress conditions in salt-sensitive IR64 and salt-tolerant Pokkali rice cultivars. Further, heterologous expression of OsCBSCB-SPB4 in E. coli and tobacco confers marked tolerance against various abiotic stresses. Transgenic tobac-co seedlings over-expressing OsCBSCBSPB4 were found to exhibit better growth in terms of delayed leaf senescence, profuse root growth and increased biomass in contrast to the wild-type seedlings when subjected to salinity, dehydration, oxidative and extreme temperature treatments. Yeast-two hybrid stud-ies revealed that OsCBSCBSPB4 interacts with various proteins. Of these, some are known to be in-volved in abiotic stress tolerance. Our results suggest that OsCBSCBSPB4 is involved in abiotic stress response and is a potential candidate for raising multiple abiotic stress tolerant plants.
Keywords: Cystathionine β-synthase (CBS) domain, Phox/Bemp1 (PB1) domain, Abiotic stress, Stress tolerance, Transgenic plants
1. INTRODUCTION
Identified for the first time in archaebacteria Methanococcus janaschii by Alexander Bateman [1], the Cystathionine β-Synthase (CBS) domain or the Bateman domain has been thereafter discerned in wide range of proteins in all kingdoms of life. This domain comprises of ~60 amino acids and is present in either pairs or quads in proteins, with each pair forming a tight association, referred to as CBS pair or Bateman module. They may exist either as a lone module (e.g. OsCBSX3) or fused to other diverse domains (e.g. OsCBSCLC6) in the protein [2, 3]. In humans, many hereditary diseases have been linked to mutations in the CBS domain of various proteins such as homocystinuria, caused by mutation in cystathionine beta synthase [4] and retinitis pigmentosa, caused by mutation in IMPDH [5], thereby emphasizing the significant role of this domain in the living systems. CBS domains are known to bind specific nucleotides (mostly AMP) and form energy sensing modules which either activate or inhibit the other associated or interacting domains of various proteins [3, 6]. However, the precise role and regulation of proteins harboring this domain is still concealed, especially in plant systems.
The studies on CBS domain-containing proteins (CDCPs) have been initiated only recently in plants. In an effort to improve the stress tolerance in plants, Kumari et al. [7] analyzed the differential regulation of salinity stress-responsive genes among salt-tolerant (Pokkali) and salt-sensitive (IR64) genotypes of rice by subtractive cDNA approach wherein one of the potential genes was found to encode CBS domain-containing protein. The transcript accumulation of this hypothetical gene was differentially regulated in the contrasting genotypes of rice, indicating towards their probable role in salinity tolerance. This observation encouraged us to address the question ‘whether CBS Domain Containing Proteins (or CDCPs) are correlated to abiotic stress tolerance in plants?’
Previously we have identified 34 CDCPs in Arabidopsis thaliana and 59 in Oryza sativa. Our in silico expression analysis clearly indicated a potential role of some CDCPs in stress tolerance [2]. In this context, we have reported that OsCBSX4, a single CBS domain containing protein from rice when over-expressed, imparts salinity, oxidative and heavy metal tolerance to transgenic tobacco plants [8]. Further in Arabidopsis, single CBS domain-containing protein, AtCBSX1 has also been reported to maintain cellular redox homeostasis via thioredoxin systems in response to changes in ATP:AMP ratio [9]. Recently, the role of CDCPs has also been indicated in resistance to Magnaporthe oryzae in rice [10] and tolerance to low nitrogen stress in soybean [11]. These reports indicate that CDCPs might play an important role in various cellular processes in plants.
In this study, we have characterized and functionally validated OsCBSCBSPB4, a CDCP containing two pairs of CBS domains and a Phox/Bemp1 (PB1) domain. OsCBSCBSPB4 is specifically induced in response to salinity, oxidative and extreme temperature stresses in salt-sensitive IR64 and salt-tolerant Pokkali cultivars of rice. Our results show that over-expression of OsCBSCBSPB4 in tobacco enhances multiple abiotic stress tolerance, thereby suggesting an important role of this protein in plant stress response.
2. MATERIALS AND METHODS
2.1. Cloning and Sequence Analysis of OsCBSCBSPB4
The coding region of OsCBSCBSPB4 (LOC_Os12 g07190, RGAP 7 database) was amplified as 1,629 bp fragment from cDNA, prepared from salt-tolerant Pokkali rice. The amplicon was then cloned in TOPO-TA vector (Invitrogen) and sequenced (Macrogen, Korea).
ScanProsite tool [12] was used for analyzing the domain organization of OsCBSCBSPB4. For homology analysis, BLAST search was conducted using GenBank. Multiple sequence alignment was performed using Clustal W2 [13]. Neighbour joining method [14] was used to generate unrooted phylogenetic tree for different CBSCBSPB4 domain-containing proteins reported in various organisms using the MEGA7 software [15].
2.2. Stress Treatments
Seeds of IR64 and Pokkali rice were germinated hydroponically and grown at 28±1°C. For salinity stress, 14-day-old rice seedlings of both the cultivars were transferred to 200 mM NaCl solution. For desiccation stress, seedlings were air dried on blotting paper, whereas, exposing the seedlings to 4°C and 42°C provided low and high temperature stresses, respectively. For oxidative stress, the seedlings were kept in 1 µM methyl viologen (MV) solution. Seedlings grown in water for the same period were taken as control. The shoot tissues were harvested after 6 h of the stress treatments.
2.3. RNA Isolation and qRT-PCR
Total RNA was extracted using RaFlexTM (GeNei, India) from the two week old IR64 and Pokkali rice seedlings exposed to different abiotic stresses (cold, salinity, heat, MV and desiccation) as per the manufacturer’s protocol. For preparation of cDNA, approximately 5 µg of total RNA from the stressed and non-stressed samples was reverse transcribed using oligo (dT) primer and the first strand cDNA Synthesis Kit (Fermentas, Life Sciences). Primers for real-time PCR were designed using Mac Vector 8.0 software. The reaction was performed using StepOne™ Real-Time PCR System (Applied Biosystems). eEF-1α was used as the internal control. Three technical replicates were analyzed for each sample. The relative expression ratio of OsCBSCBSPB4 was calculated using comparative Ct value method [16]. Heatmaps were generated using MeV software [17].
2.4. Heterologous Expression of OsCBSCBSPB4 Protein
For expression in E. coli, the coding region was amplified from the cloned cDNA for OsCBSCBSPB4 in TOPO-TA vector using forward primer containing EcoRI site: 5´ GGAATTCATGGTTCAAGGTAAATTTAGAC 3´ and reverse primer containing XhoI site: 5´ CCGCTCGAGTCACACTTTAGCTCGTTTCAG 3´ and cloned into pET28a vector (Novagen) at EcoRI / XhoI sites to create pET28a-OsCBSCBSPB4 plasmid. The resulting plasmid was then transformed into BL21 (DE3) E. coli cells. Expression of OsCBSCBSPB4 protein was induced using 0.5 mM IPTG at 37°C and cells were harvested after 4 h. To check the expression of OsCBSCBSPB4 protein, the cells were lysed and protein concentration was determined by Bradford’s method [18]. The protein was then analyzed on 12% SDS PAGE and visualized by Coomassie staining.
2.5. Preparation of Polyclonal Antibodies Against OsCBSCBSPB4
For raising anti-OsCBSCBSPB4 antibodies, essentially same protocol was followed as described by Singh et al. [8]. In brief, the OsCBSCBSPB4 cDNA was cloned in pET28a and overexpressed in E. coli (BL21-DE3) following which the recombinant protein was purified using Ni–NTA agarose as per the manufacturer’s protocol (Qiagen, Germany) and used to raise polyclonal antibodies in rabbit.
2.6. Assessing Growth Pattern of OsCBSCBSPB4-transformed E. coli Under Various Abiotic Stresses
E. coli BL21 (DE3) cells transformed with either pET28a plasmid or pET28a-OsCBSCBSPB4 construct were grown in liquid LB medium at 37°C (till OD600 ~0.4) followed by induction with 0.5 mM IPTG and further grown for 13 h. At the time of IPTG induction, for salinity stress, NaCl was added to the growth medium to a final concentration of 200 mM. Likewise, for high and low temperature treatments, the bacterial cells were transferred to respective 42°C and 15°C, and for oxidative and dehydration treatments, 1 mM MV and 10% PEG 8000 were respectively added to the growth medium. Cell aliquots (1 mL) were taken after every 1 h and cell survival was estimated by measuring the absorbance at 600 nm. The O.D. represents the mean of three replicates of at least two independent recombinant bacterial cultures.
2.7. Preparation of OsCBSCBSPB4 Construct, Plant Transformation and Transgenic Screening
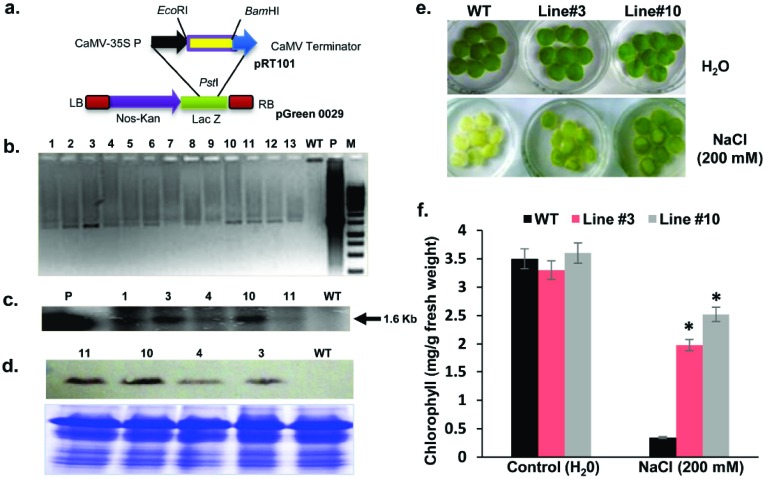
For plant transformation, OsCBSCBSPB4 was initially cloned in pRT101 at BamH1/EcoR1 sites. The 35S CaMV promoter-OsCBSCBSPB4 gene cassette was then excised from pRT101 and cloned in pGREEN0029 vector which carries an NPT (kanamycin) gene as the selectable marker. Tobacco (Nicotiana tabacum cv. petit havana) leaf discs were transformed with Agrobacterium tumefaciens (LBA4404 strain) cells harboring pGREEN-OsCBSCBSPB4 construct following the procedure described previously [19]. Putative T0 transgenic plants were regenerated from independent calli in the presence of kanamycin and further screened by PCR and Southern blotting. For PCR based screening method, genomic DNA from wild-type (WT) and transgenic lines was used as template. Forward primer was designed from 35S CaMV promoter sequence and OsCBSCBSPB4-specific sequence was used as reverse primer. For Southern hybridization, 15 µg of genomic DNA from PCR positive lines was digested with EcoRI and BamHI, blotted and probed using α32 P-dCTP labeled OsCBSCBSPB4 gene, as described [8]. In addition, anti-OsCBSCBSPB4 antibodies were used to confirm OsCBSCBSPB4 protein expression in the transgenic plants through western blotting as described by Singla-Pareek et al. [20].
The seeds from the transgenic plants were germinated on kanamycin-containing medium to select transgenic T1 seedlings. The positive transgenic plants were used for further growth analysis.
2.8. Leaf Disc Assay
Leaf discs of 1.0 cm diameter were excised from healthy and fully expanded tobacco leaves of similar age from the transgenic and WT plants (60 day old). The discs were floated in 6 mL solution of 200 mM NaCl (for stress) or water (for control) for 5 days. The treatment was carried out at 25°C and the experiment was repeated at least three times with different transgenic lines.
2.9. Stress-survival Assays of T1 Transgenic Tobacco Plants
Seeds of two independent transgenic tobacco lines (line 3 and 10) over-expressing rice OsCBSCBSPB4 gene were selected by germinating the seeds on kanamycin containing half-strength MS medium selection plates. After 7 days of germination, the seedlings were transferred to medium supplemented with 1 µM MV, 5% mannitol and 200 mM NaCl for oxidative, dehydration and salt stress, respectively. Seedlings were exposed to 4°C to provide low temperature stress and 42°C for high temperature stress. After 24 h of heat stress and 3 d of cold stress, plants were kept at 26±1°C for recovery.
2.10. Yeast Two-Hybrid Assay
The yeast two-hybrid screening was performed as described earlier with some modifications [21]. The OsCBSCBSPB4 coding region was cloned in-frame at EcoRI site of pBD-GAL4 vector to make the bait plasmid pBD-OsCBSCBSPB4. Plasmid DNA of bait and prey (containing rice cDNA library) constructs were transformed into the Saccharomyces cerevisiae strain AH109. The transformants were grown on synthetic minimal medium plates lacking tryptophan, uracil, and leucine and adenine (4-DO). Colonies which were able to grow on 4-DO plates were then streaked on YPD medium plates and transferred to a filter paper to carry out the filter lift assay. After transfer, positive clones were identified using X-gal based screening and plasmid was isolated from these positive clones, followed by sequencing to identify the putative interacting partners.
2.11. Sub-cellular Localization of OsCBSCBSPB4
OsCBSCBSPB4 was cloned in pMBPII vector as translational fusion with GFP at the C-terminus under the control of 35S CaMV promoter at BamHI and XbaI site. Particle bombardment was used to introduce OsCBSCBSPB4:GFP fusion plasmid into the onion epidermal cells with a Biolistic PDS-1000/He system (BioRad) as described by Singh et al. [8]. The transformed onion epidermis was incubated at 28°C in dark for 16 h and GFP was detected in cells under a confocal microscope. For staining the nucleus, onion epidermal peels were incubated for 5 min with 100 nM of 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen, Eugene, OR) prior to microscopic analysis. Cellular structure was visualized using bright-field optics.
3. RESULTS
3.1. Sequence Analysis of OsCBSCBSPB4
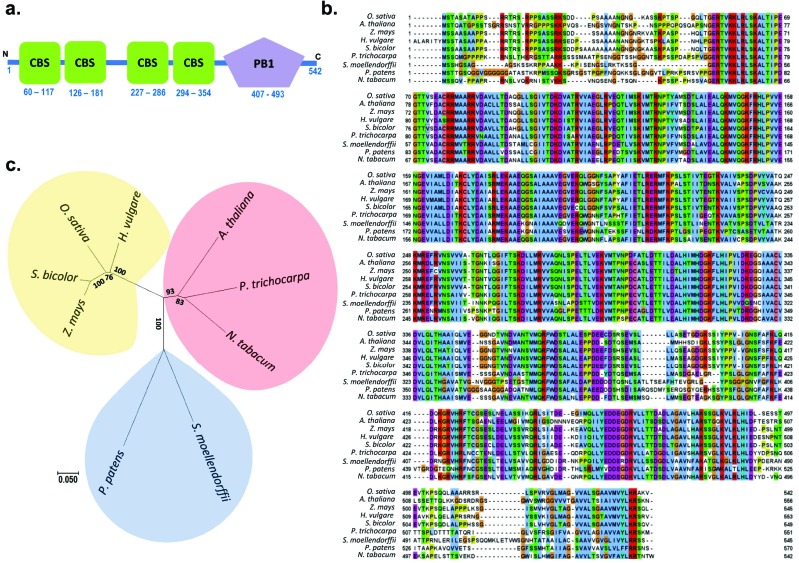
OsCBSCBSPB4 (LOC_Os12g07190) was PCR amplified as 1629 bp fragment from salt tolerant rice “Pokkali” cDNA and showed 100% sequence identity to O. sativa ssp. japonica. OsCBSCBSPB4 is located on chromosome 12 and encodes a protein of 542 amino acids with a molecular mass of 58.3 kDa and a pI of 6.95. Domain analysis of the deduced amino acid sequence using ScanProsite database [12] confirmed the presence of two pairs of CBS domains, first pair lying in the region from 60-181 amino acids and the second pair being present within 227-354 amino acid residues (Fig. 1a). Along with CBS domains, a Phox/Bemp1 (PB1) domain was also predicted in the OsCBSCBSPB4 protein at 407-493 amino acid residues.
Fig. (1).
Sequence analysis and comparison of OsCBSCBSPB4 with corresponding proteins from other species. (a) Schematic depiction of domains present in OsCBSCBSPB4 protein as determined by ScanProsite. Two pairs of CBS domains along with single PB1 domain have been indicated. (b) Multiple alignment of OsCBSCBSPB4 with other reported CBSCBSPB4 proteins. Clustal W2 was used for multiple alignment and Jalview was used to view the alignment. (c) The phylogenetic relation between CBSCBSPB4 domain containing proteins from different species. The evolutionary history was inferred using the Neighbor-Joining method. Evolutionary analysis was conducted in MEGA7.
Multiple sequence alignment revealed high amino acid sequence identity of OsCBSCBSPB4 with corresponding proteins from other species (Fig. 1b). OsCBSCBSPB4 shared 60% amino acid identity with AtCBSCBSPB4 from Arabidopsis and exhibited respectively 68%, 84%, 85% and 86% identity with corresponding proteins from Nicotianatabacum, Hordeum vulgare, Zea mays and Sorghum bicolor (Fig. 1b). Furthermore, the phylogenetic tree revealed a closer relationship of OsCBSCBSPB4 with other grass family members than Arabidopsis (Fig. 1c).
3.2. OsCBSCBSPB4 is a Multiple-stress Inducible Gene
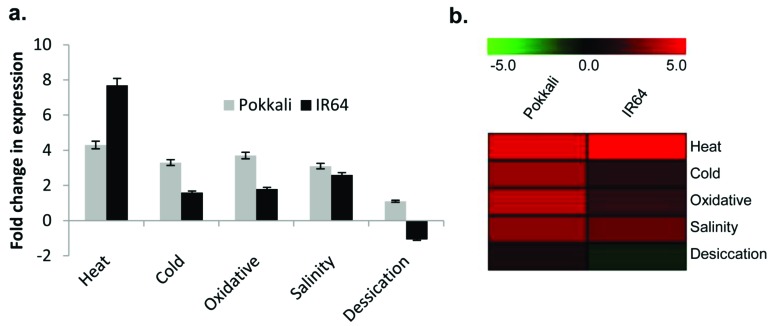
Quantitative Real time PCR (qRT-PCR) studies were carried out to determine the expression profile of OsCBSCBSPB4 in the salt-sensitive IR64 and salt-tolerant Pokkali rice varieties in response to different abiotic stress conditions. For this, two-week old IR64 and Pokkali seedlings were subjected to different stress treatments viz. salinity, desiccation, oxidative, heat and cold stress for 6 h.
Under control (non-stressed) conditions, the expression of OsCBSCBSPB4 was found to be slightly higher in Pokkali (1.26-fold) than in IR64. However, OsCBSCB SPB4 expression increased several folds in both IR64 and Pokkali under stress conditions. In Pokkali rice, a 3.1-fold increase in OsCBSCBSPB4 expression was observed after
6 h of NaCl (200 mM) treatment. Oxidative stress and low temperature treatment also induced OsCBSCBSPB4 expression to 3.7- and 3.3-fold, respectively. However, desiccation treatment led to only a marginal increase in OsCBSCBSPB4 transcript levels. Highest induction, up to 4.3-fold in transcript levels could be seen in response to heat treatment (Fig. 2a,b).
Fig. (2).
Transcript profile of OsCBSCBSPB4 in shoots of salt-sensitive (IR64) and salt-tolerant (Pokkali) rice cultivars. (a) Fold change in transcript levels (expression level relative to untreated control plants) of OsCBSCBSPB4 in two-week old IR64 and Pokkali seedlings subjected to 6 h of salinity (NaCl 200 mM), oxidative (methyl viologen 1 μM), desiccation (air drying) and high (42°C) and low (4°C) temperature stresses. The experiment was repeated twice with three replicates in each case. (b) Corresponding heat map of the OsCBSCBSPB4 transcript in IR64 and Pokkali. Scale bar at the top represents low (green), intermediate (black) and high (red) expression levels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Likewise in IR64 rice seedlings, OsCBSCBSPB4 expression was also induced in response to heat (7.7-fold), cold (1.6-fold), oxidative (1.8-fold) and salinity stress (2.6-fold) (Fig. 2a,b). However, OsCBSCBSPB4 expression declined marginally after desiccation stress in IR64. Thus, OsCBSCBSPB4 was found to be highly stress-inducible gene being induced to different levels in IR64 and Pokkali rice genotypes, as can also be visualized through the heatmap (Fig. 2b).
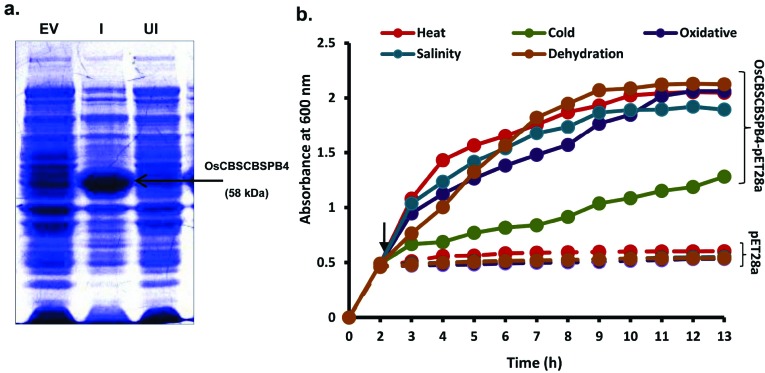
3.3. Over-expression of OsCBSCBSPB4 in E. coli Enhances Multiple Abiotic Stress Tolerance
To get an insight into the role of OsCBSCBSPB4 in stress response, OsCBSCBSPB4 cDNA was cloned in pET28a vector to generate pET28a-OsCBSCBSPB4 construct followed by transformation in E. coli (BL21-DE3). The expression of the recombinant OsCBSCBSPB4 protein in E. coli was confirmed by SDS-PAGE. A band corresponding to OsCBSCBSPB4 protein (~58 kDa) could be observed in the induced sample compared to the uninduced one (Fig. 3a). For stress treatments, E. coli cells were grown in liquid medium containing different stress-inducing agents after IPTG induction, such as 200 mM NaCl for salinity, 1 mM MV for oxidative and 10% PEG for dehydration stress. For high and low temperature treatments, bacterial cells were transferred to 42°C and 15°C, respectively immediately after IPTG induction. The survival rate of the cells (expressed as bacterial O.D.) upon treatment with different stresses is shown in (Fig. 3b). The bacterial cells transformed with pET28a plasmid (or empty vector) were used as control and these cells exhibited much slower growth rate under tested stress conditions. In contrast, OsCBSCBSPB4-transformed bacterial cells possessed higher growth rates even after stress imposition and could continuingly grow till 9 – 10 h before reaching a plateau (Fig. 3b). However, bacterial cells grown at 15°C exhibited slower growth when compared to that under other stresses but the growth rate was still more when compared to the empty vector-transformed bacterial cells. Thus, comparison of
Fig. (3).
Heterologous expression of OsCBSCBSPB4 in E. coli and assessing the response to various abiotic stresses. (a) Coomassie stained SDS-PAGE showing crude protein extract from E. coli transformed with empty vector (EV), or OsCBSCBSPB4 where ‘UI’ indicates uninduced protein and ‘I’ indicates protein induced with 0.5 mM IPTG. The induced band corresponding to OsCBSCBSPB4 after 4 h of IPTG induction is visualized (marked by arrow). (b) Survival rate of E. coli BL21 (DE3) transformants in LB broth in response to various abiotic stress inducing agents such as, 200 mM NaCl for salinity, 10% PEG 8000 for dehydration, 1 mM MV for oxidative, 42°C for high temperature and 15°C for low temperature stress. pET28a-OsCBSCBSPB4 (represented by solid lines) and the empty pET28a vector (taken as control; represented by dotted lines) were grown at 37°C for 2 h followed by 0.5 mM IPTG induction and stress treatments. Cell aliquots (1 mL) were taken after every 1 h. Cell survival was estimated by monitoring absorbance (O.D.) at 600 nm. O.D. represents the mean of three replicates of at least two independent recombinant bacterial cultures. Time point of addition of IPTG and stress imposition is marked by arrow. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
growth pattern of OsCBSCBSPB4-transformed bacterial cells under stress conditions against pET28a-transformed cells revealed an important role of OsCBSCBSPB4 in cellular response to abiotic stress.
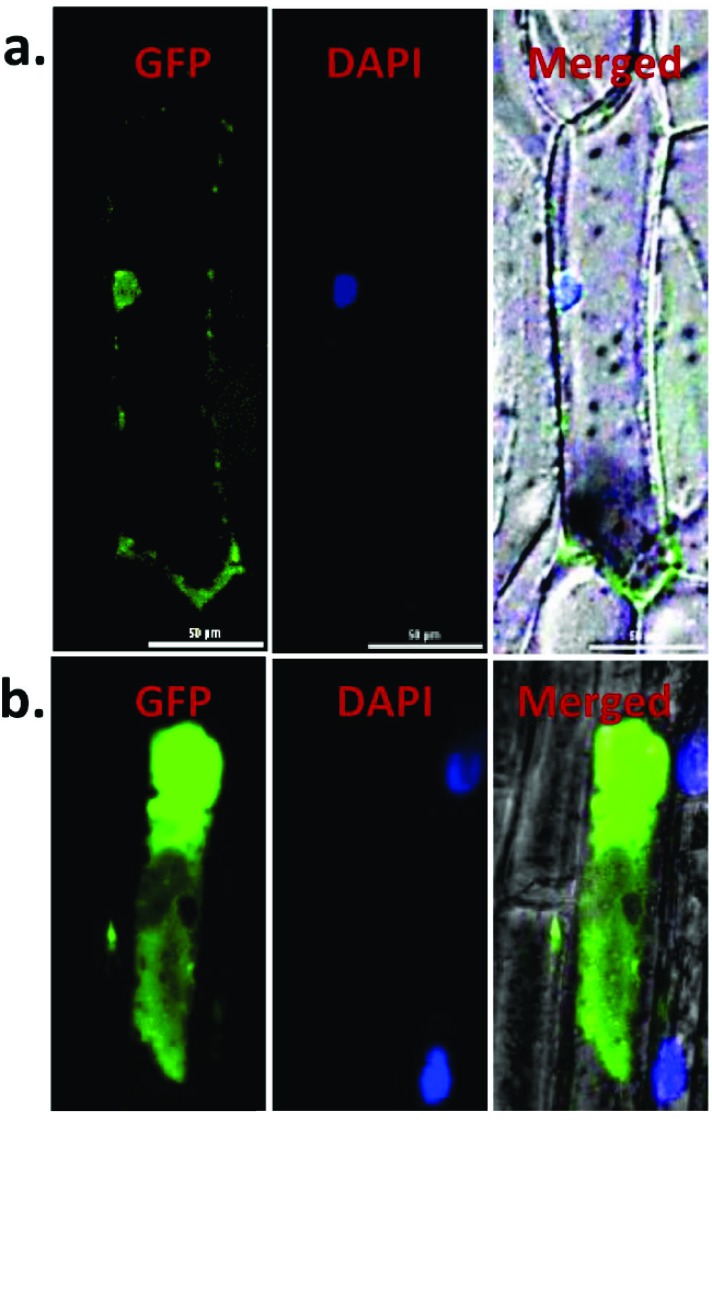
3.4. The OsCBSCBSPB4 protein is Localized in the Nucleus and Cytoplasm
To determine the sub-cellular localization of OsCBSCBSPB4, fusion construct of corresponding cDNA was fused in frame with GFP at C-terminus to give OsCBSCBSPB4:GFP fusion construct driven by the 35S CaMV promoter. The resulting chimeric protein (OsCBSCBSPB4-GFP) was transiently expressed in onion peel epidermal cells. Expression of OsCBSCBSPB4:GFP fusion protein was analyzed by confocal microscopy. As shown in the (Fig. 4), OsCBSCBSPB4-GFP fusion protein was found to be localized majorly in the nucleus though some expression could be observed in the cytoplasm as well (Fig. 4a). On the other hand, free GFP expression could be seen in the whole cell (Fig. 4b). However, online nuclear localization prediction tools such as NLS mapper, did not predict any strong Nuclear Localization Signal (NLS) sequence in the OsCBSCBSPB4 protein.
Fig. (4).
OsCBSCBSPB4 localizes to both nucleus and cytoplasm. Onion peel epidermal cells were transiently transformed with constructs containing either (a) OsCBSCBSPB4:GFP or (b) GFP alone by particle bombardment method. Subcellular localization of OsCBSCBSPB4:GFP fusion protein or GFP alone was viewed under confocal microscope, 16 h after bombardment. DAPI was used to detect nuclei.
3.5. Over-expression of OsCBSCBSPB4 in Transgenic Tobacco Confers Tolerance Towards Salinity Stress
To determine the functional significance of OsCBSCBSPB4 in planta, full-length OsCBSCBSPB4 cDNA was cloned in the plant transformation vector pGREEN via the shuttle vector pRT101 (Fig. 5a). The resulting recombinant plasmid harboring OsCBSCBSPB4 gene was transformed into tobacco via Agrobacterium-mediated transformation. The kanamycin resistant tobacco seedlings obtained after transformation were then screened for transgene by PCR (Fig. 5b). The morphological and growth characteristics of T0 plants were found to be similar to the untransformed WT plants under control conditions. For subsequent analysis, ten T0 transgenic lines were grown to maturity. A total of five independent PCR positive lines were confirmed by Southern hybridization for stable integration of the transgene. A band corresponding to 1.6 kb fragment could be observed in the transgenic plants but not in the wild-type (Fig. 5c). Further, the transgenic lines were checked for the expression of OsCBSCBSPB4 protein by western blot using anti-OsCBSCBSPB4 specific antibodies. The expression of OsCBSCBSPB4 protein in the transgenic lines could be confirmed through western blot (Fig. 5d).
Fig. (5).
Overexpression of OsCBSCBSPB4 in transgenic tobacco confers salinity stress tolerance. (a) Schematic representation of OsCBSCBSPB4-pGREEN0029 construct used for tobacco transformation. (b) Confirmation of transgenic lines for the presence of OsCBSCBSPB4 transgene by PCR (1-13, putative transgenic lines; WT, wild-type plant). (c) Southern blot analysis showing a band corresponding to 1.6 kb (marked by an arrow). Genomic DNA extracted from PCR lines (1, 3, 4, 10 and 11) was used for analysis along with WT. ‘P’ indicates positive control (taken as OsCBSCBSPB4 amplicon). (d) Western blot analysis for detection of OsCBSCBSPB4 protein in the transgenic tobacco lines using anti-OsCBSCBSPB4 antibodies; corresponding coomassie-stained protein gel has been included below to show equal loading of protein. (e) Leaf disc senescence assay of wild-type (WT) and transgenic lines (line 3 and line 10) under 200 mM NaCl stress (5 days). Leaf discs floated on water served as control. (f) Chlorophyll content (mg/g fresh weight) of the corresponding leaf discs after 5 d of 200 mM NaCl stress. Experiment was repeated thrice and error bars indicate standard error. ‘*’ indicates significant differences in comparison with the WT under respective conditions at p<0.05 (Student’s t-test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
The ability of transgenic plants (T0) to tolerate high levels of salinity stress was evaluated using leaf disc bioassay. The leaf discs from Southern positive transgenic lines, namely, line 3 and 10 along with WT plants were floated onto 200 mM NaCl solution. Visual differences in the ‘greenness’ were observed after 5 days (Fig. 5e).
Bleaching of leaf tissues was also analyzed by measuring the remaining chlorophyll content of the leaf discs after the stress treatment (Fig. 5f). Under control conditions, no significant difference in chlorophyll content of the leaf discs could be noticed between WT and transgenic lines. However under salt stress, chlorophyll content drastically reduced by 90% in the leaf discs of WT plants. While in transgenic lines 3 and 10, a less significant decrease (by 40% for line 3 and 30% for line 10) was observed. Importantly, measured differences in the chlorophyll content of transgenic and WT lines were in conformity with the observed differences in ‘greenness’ in these plants.
3.6. OsCBSCBSPB4 Over-expressing Tobacco Plants Show Tolerance Towards Various Abiotic Stress Conditions
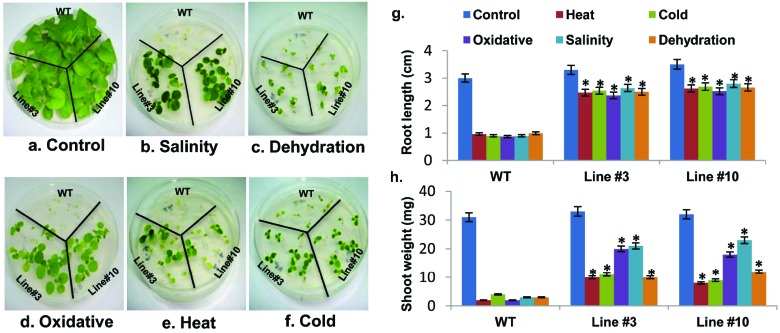
To further evaluate the role of OsCBSCBSPB4 in stress tolerance, the T1 transgenic seedlings were analyzed for stress response. In order to carry out systematic phenotypic comparison, WT and T1 transgenic plants were germinated on half-strength MS plates and later transferred to medium supplemented with different stress inducers for salinity, dehydration, oxidative, heat and cold stress treatments. Under non-stress conditions, WT and transgenic plants exhibited no morphological differences in growth (Fig. 6a). However, in response to the applied stress conditions, the growth of WT plants was severely retarded (Fig. 6b-6f). In contrast, OsCBSCBSPB4 over-expressing T1 transgenic plants exhibited better growth under stress conditions (Fig. 6b-6f).
Fig. (6).
Transgenic T1 seedlings over-expressing OsCBSCBSPB4 protein confers multiple abiotic stress tolerance. Seven day old seedlings of WT and transgenic lines (line 3 and line 10) were transferred to half strength MS medium for (a) control; or medium supplemented with either (b) NaCl (200 mM) for salinity or (c) Mannitol (5%) for dehydration or (d) MV (1 μM) for oxidative stress treatments. Seedlings were maintained under culture room conditions at 26±1°C and pictures taken after 15 d are shown. For high (e) and low (f) temperature stress, seedlings were exposed to 42°C for 24 h and 4°C for 72 h, respectively followed by recovery at 26±1°C. Graph showing comparative root length (g) and shoot weight (h) indicating severe inhibition in WT seedlings as compared to the transgenic seedlings under stress conditions. Experiment was repeated thrice and error bars indicate standard error. ‘*’ indicates significant differences in comparison with the WT under respective conditions at p<0.05 (Student’s t-test). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Under salt stress, the root length was greatly reduced in the WT plants showing 70% decline, whereas both the transgenic lines exhibited only 20% reduction in root length under salinity conditions (Fig. 6g). Since OsCBSCBSPB4 transcript levels were significantly increased under other stresses such as, high/low temperature, desiccation and oxidative stress, seedlings were also subjected to these stress treatments and the response was evaluated. The results indicated a response similar to that observed under salinity stress for both WT and T1 transgenic lines under these stress conditions. Under all tested stress conditions, the reduction in shoot weight and root length was less in transgenic seedlings as compared to the WT seedlings. The root length was reduced by 23%, 25%, 28% and 24% under low temperature, high temperature, oxidative and dehydration stress, respectively in transgenic seedlings as compared to 70%, 68%, 71% and 67% reduction in WT plants (Fig. 6g). Similarly, the reduction in shoot weight in response to applied stress conditions was also less in the transgenic seedlings as compared to the WT seedlings (Fig. 6h).
3.7. Identification of Potential Interacting Partners of OsCBSCBSPB4
In order to identify the potential interacting partners of OsCBSCBSPB4, the yeast two-hybrid approach was used. We isolated and identified a total of 100 clones, out of which a few have been listed in (Table 1). These included transcription factors, such as RING-H2 finger protein and also the
Table 1.
Potential interacting partners of OsCBSCBSPB4 identified through screening of rice library in yeast two-hybrid assay.
| Sl. No. | Putative Interacting Partners | Predicted Function |
|---|---|---|
| 1 | Glutamine synthetase, catalytic domain containing protein | Glutamine biosynthetic process, nitrogen compound metabolic process |
| 2 | RING-H2 finger protein | Regulating growth/developmental processes, hormone signaling and responses to biotic and abiotic stresses in plants |
| 3 | Elongation factor protein | Protein biosynthesis |
| 4 | T-complex protein | Cytosolic protein folding machinery |
| 5 | LTPL9 - Protease inhibitor/seed storage/LTP family protein precursor | Protein of unknown function, induced in abiotic stress |
| 6 | Heat shock protein (Hsp 90) | For protein folding, assembly, translocation and degradation in many normal cellular processes, stabilize proteins and membranes, and can assist in protein refolding under stress conditions |
| 7 | Dihydrodipicolinate reductase | Dihydrodipicolinate reductase activity, catalyzes the second step of lysine biosynthesis |
| 8 | AMP-binding protein (or 4-coumarate-CoA ligase) | Biosynthesis of secondary metabolites |
| 9 | Amino acid permease | Regulates phloem amino acid composition; involved in the transport of amino acids into the cell |
| 10 | 60S ribosomal protein L31 | Structural constituent of ribosome |
| 11 | Magnesium-protoporphyrin IX monomethyl ester cyclase, chloroplast precursor | Involved in chlorophyll biosynthesis |
| 12 | Rieske domain containing protein | Involved in electron transfer chains of mitochondria and chloroplast |
| 13 | Histone H3 | Involved in the structure of chromatin |
| 14 | Acyl-desaturase, chloroplast precursor | Participates in polyunsaturated fatty acid biosynthesis in plastids |
| 15 | Lissencephaly type-1-like homology motif | Required for normal fertility and the first inter-node elongation |
| 16 | Photosystem II 10 kDa polypeptide, chloroplast precursor | Photosynthesis |
| 17 | DnaK family protein | Involved in protein folding, and help to protect cells from stress |
| 18 | Elongation factor Tu | Protein synthesis |
| 19 | Cysteine proteinase inhibitor precursor protein | Inhibition of proteases |
| 20 | Nitrogen regulatory protein P-II | Involved in the regulation of nitrogen metabolism |
| 21 | Phosphate carrier protein, mitochondrial precursor | Transporter activity |
| 22 | Metallothionein-like protein 3B | Metal binding proteins |
| 23 | Fructose-1,6-bisphosphatase | Carbohydrate metabolism |
proteins involved in stress response such as, heat shock proteins (Hsp90). Besides, we identified an AMP-binding domain containing protein as one of the potential interacting partner of OsCBSCBSPB4.
4. DISCUSSION
The mechanisms of stress tolerance exist in all living systems but these mechanisms may not be exactly same and may vary from species to species. Despite variations, the format of cellular response to stress is generally believed to be conserved to large extent across prokaryotes as well as eukaryotes, including plants [22]. As abiotic stress ultimately affects the cellular gene-expression machinery, it is evident that a large number of genes are up or down regulated. Most of the genes involved in these pathways have not been characterized to date and have remained hypothetical. Therefore, it is essential to understand and execute functional approaches to establish the role of these genes with unknown or hypothetical functions, in order to improve our understanding of plant stress tolerance mechanisms [23]. Importantly, functionally analogous stress tolerant genes exist in both unicellular organisms and plants and hence, examining the functional significance of plant genes through their over-expression in simple organisms, such as yeast or bacteria seems to be promising. This approach has been tested by various groups [24-26].
Until now, only a few CDCPs have been characterized mainly from bacteria, humans and other animal systems. It is only recently that the identification and functional characterization of plant CDCPs is being undertaken [8, 10, 11]. In the present study, we have isolated an OsCBSCBSPB4 gene from salt-tolerant rice cv. Pokkali and identified its novel function in multiple abiotic stress tolerance in bacteria as well as in model plant tobacco. OsCBSCBSPB4 protein possesses two pairs of CBS domains which are known to be involved in a wide range of regulatory activities. For instance, single-CBS domain containing proteins, CBSX1 and CBSX2, from Arabidopsis have been identified as redox regulators that influence plant development via regulation of thioredoxin systems by sensing changes in adenosine-containing ligands [9]. Detailed studies on CBSX2 have revealed that it modulates the H2O2 status, which is linked to jasmonic acid response and in turn controls secondary wall thickening of the endothecial cells in anthers for dehiscence to occur [27]. Also, the four-CBS-domain (or two pairs of CBS domains) containing protein KINβγ, functions as the energy-sensing module of plant SnRK1 kinases through adenosine nucleotide binding [28].
In addition to CBS domains, OsCBSCBSPB4 also possesses a PB1 domain, a protein interaction module that is conserved in animals, fungi, amoeba and plants. This domain is mainly involved in heterodimerization via interactions with PB1 domains of other proteins or with other protein domains. The canonical PB1 dimerization is required for the formation of complexes between proteins which are reported to be involved in activation of NADPH oxidase, polarity establishment in yeasts, cell polarization in animals and also in early cardiovascular development in mammals [29]. PB1 domain has also been observed in Auxin Response Factor (ARF) activators and Auxin/Indole 3-acetic acid inducible (Aux/IAA) repressors regulating the auxin responsive genes in plants [30]. Thus, presence of PB1 domain in OsCBSBSPB4 is indicative of the ability of this protein to interact with other proteins, thereby possibly transmitting cellular signals in the system.
CONCLUSION
Our results show that heterologous expression of OsCBSCBSPB4 conferred tolerance to bacterial cells against multiple abiotic stresses. In agreement, we could observe an increase in the expression of OsCBSCBSPB4 under these stresses in the two rice genotypes, IR64 and Pokkali. Thus, this salinity-responsive OsCBSCBSPB4 gene may also be involved in conferring cold, heat and oxidative stress tolerance to plants as could be seen from the response of OsCBSCBSPB4-overexpressing transgenic lines to these stress conditions. In support of this, reports describing the ability of other CDCPs, such as OsCBSX4, in improving the tolerance of transgenic plants towards oxidative, salinity and heavy metal stress already exist [8].
In order to investigate the underlying mechanism behind observed stress tolerance conferred by OsCBSCBSPB4, yeast two hybrid studies were carried out which led to the identification of various nuclear and cytoplasmic proteins involved in stress response and tolerance as potential interacting partners of OsCBSCBSPB4. To name few, heat shock protein (Hsp90) [31], T-complex protein [32], LTPL9, a protease inhibitor/ seed storage/ LTP family protein precursor [33], metallothionein-like protein [34] and RING-H2 finger protein [35, 36] were identified as the potential interacting partners of OsCBSCBSPB4. Heat shock proteins and T-complex proteins are very well known molecular chaperones that assist protein folding and are induced under stress conditions [31, 32]. The transcription factor RING-H2 finger protein, is also associated with key roles in regulating growth/develop-mental processes, hormone signaling and responses to biotic and abiotic stresses in plants [35, 36]. In addition, we could also detect an AMP binding domain containing protein (or 4-coumarate-CoA ligase) as one of the interacting partners that is probably involved in the biosynthesis of plant secondary compounds [37]. It is induced by wounding and UV irradiation or upon fungal infection [38]. The observed interaction of OsCBSCBSPB4 with an AMP binding domain containing protein indicates towards a role of OsCBSCBSPB4 similar to that proposed for few CDCPs, where they bind adenosine nucleotides and transmit signals to the catalytic units. We believe that OsCBSCBSPB4 can shuttle between nucleus and cytoplasm, as evident from its nucleo-cytoplasmic localization, and regulate the functions of various proteins involved in stress response which is further supported by the observed stress tolerance conferred by over-expression of OsCBSCBSPB4 in bacteria as well as tobacco. Though no strong NLS sequence was predicted in the OsCBSCBSPB4 protein but nuclear localization has been otherwise predicted for 4 CDCPs in soybean [11].
We hypothesize that OsCBSCBSPB4, like other CDCPs, regulates the activation of other proteins, specifically stress-proteins. This, we believe, may be the reason why its over-expression imparts tolerance against abiotic stress conditions. Taken together, OsCBSCBSPB4 represents a potential candidate gene from rice, which may be playing an important role in stress tolerance mechanism in plants.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
This work was supported by internal grants of International Centre for Genetic Engineering and Biotechnology (ICGEB), and Department of Biotechnology (DBT), Government of India. TUA acknowledges ICGEB for providing a pre-doctoral fellowship.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 2.Kushwaha H.R., Singh A.K., Sopory S.K., Singla-Pareek S.L., Pareek A. Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics. 2009;10:200–205. doi: 10.1186/1471-2164-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ereño-Orbea J., Oyenarte I., Martínez-Cruz L.A. CBS domains: Ligand binding sites and conformational variability. Arch. Biochem. Biophys. 2013;540:70–81. doi: 10.1016/j.abb.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Shan X., Dunbrack R.L., Christopher S.A., Kruger W.D. Mutations in the regulatory domain of cystathionine beta synthase can functionally suppress patient-derived mutations in cis. Hum. Mol. Genet. 2001;10:635–643. doi: 10.1093/hmg/10.6.635. [DOI] [PubMed] [Google Scholar]
- 5.Kennan A., Aherne A., Palfi A., Humphries M., McKee A., Stitt A., Simpson D.A., Demtroder K., Orntoft T., Ayuso C., Kenna P.F., Farrar G.J., Humphries P. Identification of an IMPDH1 mutation in autosomal dominant retinitis pigmentosa (RP10) revealed following comparative microarray analysis of transcripts derived from retinas of wild-type and Rho (-/-) mice. Hum. Mol. Genet. 2002;11:547–557. doi: 10.1093/hmg/11.5.547. [DOI] [PubMed] [Google Scholar]
- 6.Baykov A.A., Tuominen H.K., Lahti R. The CBS domain: A protein module with an emerging prominent role in regulation. ACS Chem. Biol. 2011;6:1156–1163. doi: 10.1021/cb200231c. [DOI] [PubMed] [Google Scholar]
- 7.Kumari S., Sabharwal V.P., Kushwaha H.R., Sopory S.K., Singla-Pareek S.L., Pareek A. Transcriptome map for seedling stage specific salinity stress response indicates a specific set of genes as candidate for saline tolerance in Oryza sativa L. Funct. Integr. Genomics. 2009;9:109–123. doi: 10.1007/s10142-008-0088-5. [DOI] [PubMed] [Google Scholar]
- 8.Singh A.K., Kumar R., Pareek A., Sopory S.K., Singla-Pareek S.L. Overexpression of rice CBS domain containing protein improves salinity, oxidative and heavy metal tolerance in transgenic tobacco. Mol. Biotechnol. 2012;2:9847–9852. doi: 10.1007/s12033-011-9487-2. [DOI] [PubMed] [Google Scholar]
- 9.Yoo K.S., Ok S.H., Jeong B.C., Jung K.W., Cui M.H., Hyoung S., Lee M.R., Song H.K., Shin J.S. Single Cystathionine β-Synthase domain containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell. 2011;23:3577–3594. doi: 10.1105/tpc.111.089847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mou S., Shi L., Lin W., Liu Y., Shen L., Guan D., He H. Over-expression of rice CBS domain containing protein, OsCBSX3, confers rice resistance to Magnaporthe oryzae inoculation. Int. J. Mol. Sci. 2015;16:15903–15917. doi: 10.3390/ijms160715903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Q., Shang W., Zhang C., Chen H., Chen L., Yuan S., Chen S., Zhang X., Zhou X. Identification and comparative analysis of CBS domain-containing proteins in soybean (Glycine max) and the primary function of GMCBS21 in enhanced tolerance to low nitrogen stress. Int. J. Mol. Sci. 2016;17:620–637. doi: 10.3390/ijms17050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Castro E., Sigrist C.J., Gattiker A., Bulliard V., Langendijk-Genevaux P.S., Gasteiger E., Bairoch A., Hulo N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 14.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Eisen M.B., Spellman P.T., Brown P.O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Mustafiz A., Sahoo K.K., Singla-Pareek S.L., Sopory S.K. Metabolic engineering of glyoxalase pathway for enhancing stress tolerance in plants. Methods Mol. Biol. 2010;639:95–118. doi: 10.1007/978-1-60761-702-0_6. [DOI] [PubMed] [Google Scholar]
- 20.Singla-Pareek S.L., Reddy M.K., Sopory S.K. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc. Natl. Acad. Sci. USA. 2003;100:14672–14677. doi: 10.1073/pnas.2034667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T., Chiba T., Ozawa R., Yoshida M., Hattori M., Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 23.Diédhiou C.J., Golldack D. Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci. 2006;170:793–800. [Google Scholar]
- 24.Mundree S.G., Whittaker A., Thomson J.A., Farrant J.M. An aldose reductase homolog from the resurrection plant Xerophytaviscosa. Planta. 2000;211:693–700. doi: 10.1007/s004250000331. [DOI] [PubMed] [Google Scholar]
- 25.Yamada A., Saitoh T., Mimura T., Ozeki Y. Expression of mangrove allene oxide cyclase enhances salt tolerance in Escherichia coli, yeast and tobacco cells. Plant Cell Physiol. 2002;4:903–910. doi: 10.1093/pcp/pcf108. [DOI] [PubMed] [Google Scholar]
- 26.Yamada A., Tsutsumi K., Tanimoto S., Ozeki Y. Plant RelA/SpoT homolog confers salt tolerance in Escherichia coli and Saccharomyces cerevisiae. Plant Cell Physiol. 2003;44:3–9. doi: 10.1093/pcp/pcg001. [DOI] [PubMed] [Google Scholar]
- 27.Jung K.W., Kim Y.Y., Yoo K.S., Ok S.H., Cui M.H., Jeong B.C., Yoo S.D., Jeung J.U., Shin J.S. A cystathionine-β-synthase domain-containing protein, CBSX2, regulates endothecial secondary cell wall thickening in anther development. Plant Cell Physiol. 2013;54:195–208. doi: 10.1093/pcp/pcs166. [DOI] [PubMed] [Google Scholar]
- 28.Ramon M., Ruelens P., Li Y., Sheen J., Geuten K., Rolland F. The hybrid Four-CBS-Domain KINbc subunit functions as the canonical c subunit of the plant energy sensor SnRK1. Plant J. 2013;75:11–25. doi: 10.1111/tpj.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumimoto H., Kamakura S., Ito T. Structure and function of the PB1 domain, a protein interaction module conserved in animals, fungi, amoebas, and plants. Sci. STKE. 2007;401:re6. doi: 10.1126/stke.4012007re6. [DOI] [PubMed] [Google Scholar]
- 30.Guilfoyle T.J. The PB1 domain in Auxin response factor and Aux/IAA proteins: A versatile protein interaction module in the Auxin response. Plant Cell. 2015;27:33–43. doi: 10.1105/tpc.114.132753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., Vinocur B., Shoseyov O., Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Yokota S.I., Yanagi H., Yura T., Kubota H. Upregulation of cytosolic chaperonin CCT subunits during recovery from chemical stress that causes accumulation of unfolded proteins. Eur. J. Biochem. 2000;267:1658–1664. doi: 10.1046/j.1432-1327.2000.01157.x. [DOI] [PubMed] [Google Scholar]
- 33.Jung H.W., Kim W., Hwang B.K. Three pathogen‐inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell Environ. 2003;26:915–928. doi: 10.1046/j.1365-3040.2003.01024.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumar G., Kushwaha H.R., Panjabi-Sabharwal V., Kumari S., Joshi R., Karan R., Mittal S., Singla-Pareek S.L., Pareek A. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol. 2012;12:107. doi: 10.1186/1471-2229-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko J.H., Yang S.H., Han K.H. Upregulation of an Arabidopsis RING‐H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006;47:343–355. doi: 10.1111/j.1365-313X.2006.02782.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu H., Zhang H., Yang Y., Li G., Yang Y., Wang X., Basnayake B.M., Li D., Song F. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol. Biol. 2008;68:17–30. doi: 10.1007/s11103-008-9349-x. [DOI] [PubMed] [Google Scholar]
- 37.Lindl T., Kreuzaler F., Hahlbrock K. Synthesis of p-coumaroyl coenzyme a with a partially purified p-coumarate:CoA ligase from cell suspension cultures of soybean (Glycine max). Biochim. Biophys. Acta. 1973;302:457–464. doi: 10.1016/0005-2744(73)90174-5. [DOI] [PubMed] [Google Scholar]
- 38.Ehlting J., Büttner D., Wang Q., Douglas C.J., Somssich I.E., Kombrink E. Three 4‐coumarate: coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999;19:9–12. doi: 10.1046/j.1365-313x.1999.00491.x. [DOI] [PubMed] [Google Scholar]