Abstract
3,4-Methylenedioxypyrovalerone (MDPV) and its structural parent, α-pyrrolidinovalerophenone (α-PVP), are two of the best studied new synthetic cathinones. Unlike methcathinone and certain other cathinones, MDPV and α-PVP act primarily as reuptake inhibitors at dopamine and norepinephrine membrane transporters rather than as substrates. The structure-activity relationships for this action have been investigated. The metabolism of these two agents has also been extensively investigated, but it is not known which, or if any, of the metabolites contribute to their pharmacological actions. MDPV and α-PVP have been examined in a variety of behavioral assays including rodent locomotor assays, self-administration studies, intracranial self-stimulation, conditioned place preference, and drug discrimination. The results of these studies are consistent with the agents acting as potent cocaine-like central stimulants with abuse potential.
Keywords: Synthetic cathinones, Mechanism of action, Structure-activity relationships (SAR), Metabolism, Behavioral studies
1. Introduction
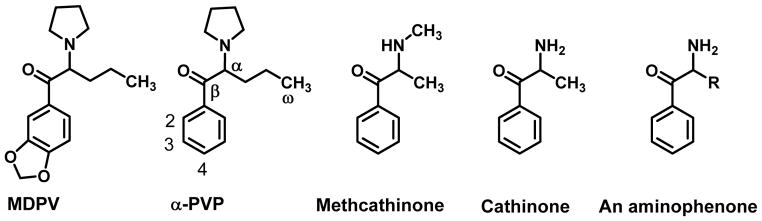
3,4-Methylenedioxypyrovalerone (MDPV) (Figure 1) is one of the best known of the synthetic cathinones and an original constituent of psychoactive “bath salts”. α-Pyrrolidinovalerophenone (α-PVP or flakka), its structural parent, is currently quite popular on the clandestine market (Ackerman, 2015; Storrs, 2015). The former is simply the 3,4-methylenedioxy counterpart of α-PVP. Both agents are structurally related to methcathinone which, in turn, is structurally derived from cathinone (Figure 1). Cathinone, an aminophenone (where R = -CH3), is a natural product found in the plant Catha edulis, and is the only known naturally-occurring cathinone. Other cathinone analogs are considered “synthetic cathinones”. Nomenclature for these agents can be confusing at times. However, these cathinone analogs are derivatives of “aminophenone”. When the R substituent of the aminophenone (Figure 1) is a methyl (-CH3) group, they are referred to as propiophenones. Methcathinone, then, is a propiophenone. As the length of the R group is increased from one carbon atom to two, three, or four, these are termed butyrophenones, valerophenones (sometimes, pentiophenones or pentanophenones), and hexanophenones, respectively, and so on. The first “P” in α-PVP indicates the presence of a pyrrolidine moiety; hence, if the α-side chain is shortened by one carbon atom, this becomes α-pyrrolidinobutyrophenone (α-PBP), and if shortened by two carbon atoms, α-pyrrolidinopropiophenone (α-PPP). Extending the side chain of α-PVP by a single carbon atom results in α-pyrrolidinohexanophenone (α-PHP). Because this review primarily considers MDPV and α-PVP, the general numbering system for these agents is shown for α-PVP in Figure 1.
Figure 1.
Chemical structures of MDPV, α-PVP, methcathinone, cathinone, and the general structure of an aminophenone which serves as the scaffolding of the synthetic cathinones.
2. Mechanism of action
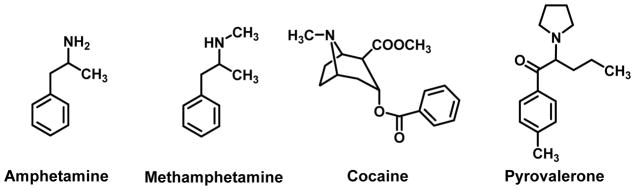
The mechanism of action of MDPV and α-PVP as central (i.e., CNS) stimulants remains to be fully elucidated. Cathinone and methcathinone (Figure 1) long have been known to act as releasing agents (i.e., as substrates) at dopamine (DA) and norepinephrine (NE) transporters (DAT and NET, respectively) (reviewed: Glennon, 2014). However, once MDPV was identified on the clandestine market and its structure elucidated, new studies quickly revealed that it was unique. That is, rather than behaving as a DAT/NET releasing agent, MDPV behaved as a reuptake inhibitor. In other words, rather than acting as an amphetamine- or methamphetamine-like releasing agent – and the structures of cathinone analogs are quite similar to those of the corresponding amphetamine analogs – cathinone being β-keto amphetamine, and methcathinone being β-ketomethamphetamine (Figure 2) – MDPV behaved like cocaine, a structurally unrelated agent. In fact, MDPV was found to be up to 100-fold more potent than cocaine as a DA reuptake inhibitor. This might have been considered a novel discovery except that, in retrospect, the substance (now known as MDPV, a valerophenone) was patented as a stimulant some years ago (Adorjan, 1967; Köppe et al., 1969) and, furthermore, Meltzer et al., (2006) had already reported that certain related valerophenones behaved as DA reuptake inhibitors.
Figure 2.
Chemical structures of amphetamine, methamphetamine, cocaine, and pyrovalerone.
α-PVP (although not known by that name at the time) also had been reported in the patent literature as a CNS stimulant (Wander, 1963; Seeger, 1964); see Section 5 for further discussion of the patent literature. It was later demonstrated that this agent was a potent reuptake inhibitor at DAT and NET (IC50 = 52.3 and 56.0 nM) and lacked action at the serotonin transporter (SERT) (Meltzer et al., 2006) relative to cocaine (IC50 = 461 nM, 378 nM, and 494 nM, for DAT, NET, and SERT, respectively). Its 4-methyl counterpart, termed pyrovalerone (see Figure 2 for chemical structure), behaved likewise (IC50 = 52.0 nM, 28.3 nM, and 1,070 nM at DAT, NET, and SERT, respectively). In fact, pyrovalerone (known in the early literature as 84/F 1983) had been identified as a central stimulant in the 1960s (Stille et al., 1963; Holliday et al., 1964) and was one of the first cathinone analogs whose mouse, rabbit, and human metabolism was investigated (Michaelis et al., 1970). Due to different and varied names and acronyms being applied to these agents, it took some time to unravel this puzzle.
The Meltzer et al., (2006) study was innovative and aimed at identifying therapeutic agents for the possible treatment of cocaine abuse. It was not until MDPV and α-PVP made their appearance on the illicit market that other investigators turned their attention to these aminophenones. MDPV was quickly shown to be a DA reuptake inhibitor, and the DAT/NET blocking actions of α-PVP reported by Meltzer et al., (2006) were confirmed (Bauman et al., 2013; Cameron et al., 2013; Eshleman et al, 2013; Simmler at al., 2013) soon after they were identified on the clandestine market. It is currently thought that MDPV and α-PVP act primarily as DAT/NET reuptake inhibitors, and that the 3,4-methylenedioxy group of MDPV, absent in α-PVP, imparts somewhat enhanced action at SERT (see structure-activity discussion to follow). Various cathinone analogs have been examined for their binding at several different neurotransmitter receptors and, although a few bind with low (i.e., μM) affinity at certain receptors, there is no evidence that such receptor actions are common to more than an individual agent or two, or are related to their central stimulant actions as a group.
3. Structure-activity Relationships
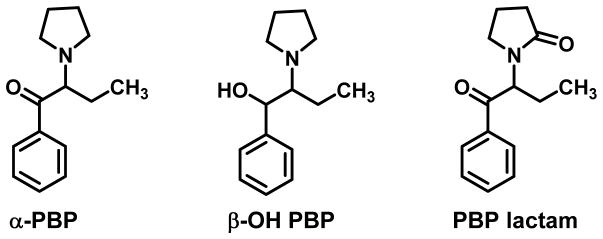
In electrophysiological studies, MDPV produced cocaine-like hyperpolarization of Xenopus laevis oocytes transfected with hDAT; hyperpolarization is the signature of a DA reuptake inhibitor. One of the first dedicated structure-activity relationship (SAR) studies of MDPV involved its “deconstruction” using this technique (Cameron et al., 2013). That is, each structural feature of MDPV was systematically eliminated, one at a time, to identify its role on activity, and it was found that both the pyrrolidine ring and an extended side chain were optimal for inhibition of DAT. Opening of the pyrrolidine ring or truncation of its side chain resulted in reduced potency. One of the deconstructed MDPV analogs was α-PVP (Cameron et al., 2013).
Marusich et al., (2014) examined truncation of the α-PVP n-propyl side chain to its ethyl and methyl counterparts (i.e., α-PBP and α-PPP, respectively) and found that shortening of the chain resulted in reduced potency at DAT and NET (Table 1; see Table 2 for structures). Kolanos et al., (2015a) reported similar results (Table 2) at DAT. Stepwise shortening of the α-side chain resulted in decreased potency; however, lengthening the chain to afford α-PHP resulted in retention of potency at DAT. A quantitative SAR (i.e., QSAR) investigation of a series of eight such analogs, analogs that differed only with respect to their α-substituent, revealed a significant relationship between the potency of the agents as reuptake inhibitors at DAT and both the volume (Å3) and lipophilicity (π) of the α-substituent (Kolanos et al., 2015a). Although not explicitly addressing either MDPV or α-PVP per se, others have also reported that shortening the α-side chain of cathinone-related agents decreases their potency as DA reuptake inhibitors (Meltzer et al., 2006; Carroll et al., 2009).
Table 1.
Effect of pyrrolidinophenones on inhibition of synaptosomal monoamine transporters (Marusich et al., 2014); see Table 2 for chemical structures.
| IC50 (nM) | |||
|---|---|---|---|
|
| |||
| DAT | NET | SERT | |
| MDPV | 4.1 | 25.9 | 3,305 |
| α-PVP | 12.8 | 14.2 | >10,000 |
| α-PBP | 63.3 | 91.5 | >10,000 |
| α-PPP | 196.7 | 444.7 | >10,000 |
Table 2.
Effect of α chain length on the ability of pyrrolidinophenones to inhibit synaptosomal reuptake at DAT and SERT (Kolanos et al., 2015a).
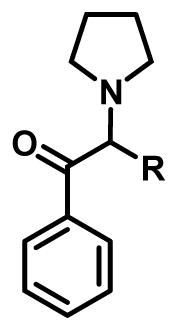
| |||
|---|---|---|---|
| R | IC50 (nM) | ||
|
| |||
| DAT | SERT | ||
| α-PHP | -CH2CH2CH2CH3 | 11.6 | >10,000 |
| α-PVP | -CH2CH2CH3 | 17.5 | >10,000 |
| α-PBP | -CH2CH3 | 63.3 | >10,000 |
| α-PPP | -CH3 | 196.7 | >10,000 |
| -H | 3,250 | >10,000 | |
Both MDPV and α-PVP possess a pyrrolidine ring. Abbreviation of the pyrrolidine ring of MDPV to its simplest tertiary amine analog (i.e., its N,N-dimethylamine), secondary amine analog (i.e., its N-methylamine), and primary amine (i.e., its NH2 analog) resulted in a progressive decrease in potency; nevertheless, all analogs retained action as reuptake inhibitors at DAT when the extended side chain was present. Ring expansion of the pyrrolidine ring of α-PVP (Kolanos et al., 2015a) or the 3,4-dichloro analog of α-PVP (Meltzer et al., 2006) resulted in a several-fold reduction in potency as DAT reuptake inhibitors. It would appear that the pyrrolidine ring, as found in MDPV and α-PVP, is optimal for action as a DAT reuptake inhibitor.
Both MDPV and α-PVP also possess a common β-carbonyl group (see Figure 1) and a chiral center. Reduction of the carbonyl group of either agent has yet to be examined; however, reduction of the carbonyl group of the α-PVP analog pyrovalerone abolished its actions as a reuptake inhibitor at DAT and NET (Meltzer et al., 2006). Likewise, optical isomers of α-PVP have yet to be examined; however, the S-isomer of pyrovalerone was 100 times more potent than its R-isomer as a reuptake inhibitor at DAT (Meltzer et al., 2006). Similarly, the S-isomer of MDPV was 190 times more potent as a DAT reuptake inhibitor, and 70 times more potent as a NET reuptake inhibitor, than its R-enantiomer (Kolanos et al., 2015b).
4. Metabolism
An understanding of the metabolism of synthetic cathinones is valuable not only for anti-doping and forensic purposes, but to also assist with the identification of metabolites that could potentially retain abuse potential and require additional pharmacological evaluation. For example, several synthetic cathinones, including α-PVP, have been added to a list of banned substances at sporting events (World Anti-Doping Agency, 2016). The metabolism of α-PVP and MDPV has been examined, but only Phase I metabolism will be described here; many of the metabolites can form sulfates or, more frequently, glucuronides, or other conjugates as Phase II metabolites.
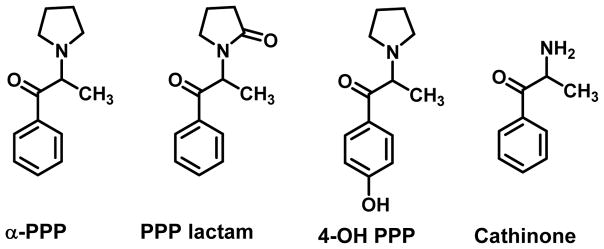
Metabolic studies with α-PVP and MDPV were preceded by investigation of some structurally simpler counterparts that aided studies with the more complex cathinones. For example, several metabolites were identified in rat urine following the administration of α-PPP (Figure 3), a truncated version of α-PVP, including a lactam (PPP lactam) resulting from oxidation of the carbon atom adjacent to the pyrrolidine nitrogen, aromatic hydroxylation to 4-hydroxy-α-PPP (4-OH PPP), and dealkylation of the pyrrolidine ring to cathinone (Figure 3) (Springer et al., 2003a)
Figure 3.
Metabolites of α-PPP in rats (Springer et al., 2003a)
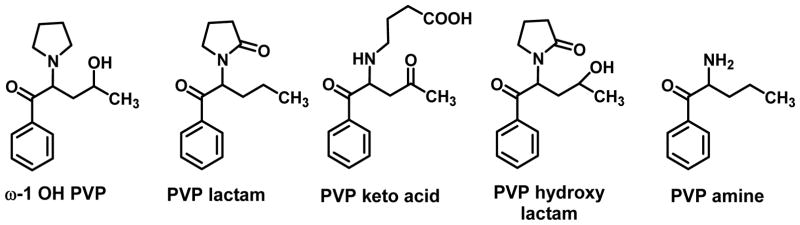
Sauer et al., (2009) identified a number of α-PVP metabolites in rat urine and proposed a metabolic scheme to account for their formation. α-PVP underwent hydroxylation of the penultimate side chain carbon atom to form ω-1 hydroxy PVP (ω-1 OH PVP; Figure 4), hydroxylation of the pyrrolidine ring followed by oxidation to PVP lactam, and subsequent ring-opening to the keto acid (PVP keto acid). The lactam also underwent hydroxylation to PVP hydroxy lactam, and the pyrrolidine ring was degraded to the primary amine (PVP amine) (Figure 4). Another route of metabolism involved aromatic hydroxylation, presumably at the 4- or para-position, such that each of the metabolites shown in Figure 4 had a corresponding 4-hydroxy counterpart (structures not shown). Hence, some of the metabolic routes for α-PVP were common to those of α-PPP except that α-PVP, being structurally more complex than the latter, has greater opportunity for additional metabolism.
Figure 4.
α-PVP metabolites identified in rat urine; the corresponding 4-hydroxy metabolites are not shown (Sauer et al., 2009).
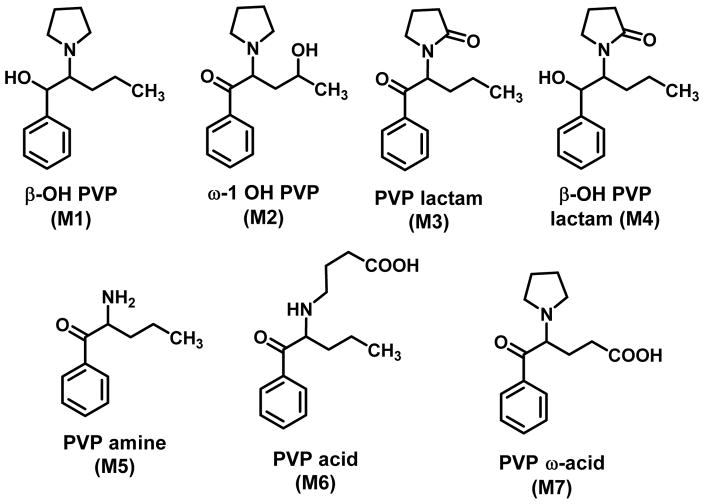
Tyrkkö et al., (2013) examined urine from eight α-PVP users collected at autopsy or from clinical toxicology cases; seven metabolites were identified (M1-M7; Figure 5). Three of the rat metabolites shown in Figure 4 were identified: ω-1 OH PVP (M2), PVP lactam (M3) and PVP amine (M5), as well as four additional metabolites (M1, M4, M6, and M7). It was speculated that reduction of the β-keto group was not identified in the Sauer et al., (2009) study because of species differences. It was also noted that although α-PVP underwent significant metabolism in humans, the drug itself was the most abundant component in all urine samples (see discussion of the Uralets et al., 2014 study below).
Figure 5.
α-PVP metabolites identified in human urine (Tyrkkö et al., 2011).
Negreira et al., (2015) examined the metabolism of α-PVP using human liver microsomes and liver cytosol and identified six Phase I metabolites; the major metablolite was PVP lactam. Other metabolites included (using the identifiers employed in Figure 5) β-OH PVP (M1), β-OH PVP lactam (M4) and PVP amine (M5); another metabolite was an aldehyde that, upon oxidation, might give rise to PVP acid (M6).
Urine specimens obtained from nineteen human α-PVP users confirmed that the two major metabolic pathways involved diastereomeric β-hydroxylation (i.e., reduction of the β-carbonyl group to afford a diastereomeric mixture) and lactam formation (i.e., metabolites labeled M1 and M3 in Figure 5) (Shima et al., 2014). Although other (conjugated) metabolites were found, no lactam ring-open products were identified (Shima et al., 2014). Recently (Grapp et al., 2016), toxicological analysis in an acute poisoning case identified unmetabolized α-PVP and three metabolites: M1, M3, and M5 (as designated in Figure 5). Uralets et al., (2014) have also studied α-PVP and this will be discussed below.
There would appear to be similarities between the metabolism of α-PVP in rats and humans (apart from 4-hydroxylation) and consistency among the different studies with humans. β-OH PVP and PVP lactam are likely the two major human metabolites of α-PVP; other metabolites were derived from further metabolism of these metabolites. Aromatic (presumably 4- or para-) hydroxylation seems common in rats, but no such metabolites were identified in studies with humans. This is not an unexpected finding in that it has been reported that although 4-hydroxylation is a major route of metabolism of related agents (e.g. amphetamine) in rats, it represents a minor route in humans (Cho and Kumagai, 1994).
α-PBP (Figure 6) is structurally similar to α-PVP, being truncated by a single methylene group. A recent investigation of Phase I α-PBP metabolites in urine specimens of eleven drug abusers indicated the same two major routes of metabolism: β;-hydroxylation and lactam formation (Figure 6), and no 4-hydroxy metabolites were detected (Matsuta et al., 2015).
Figure 6.
Human metabolites of α-PBP (Matsuta et al., 2015).
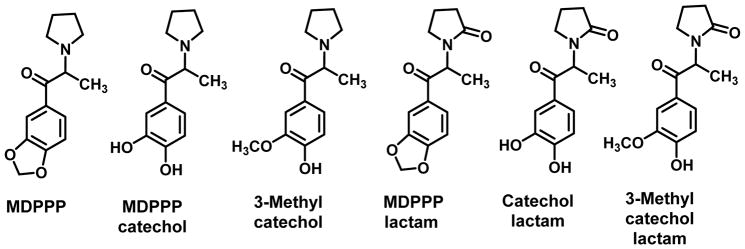
Because MDPV possesses aromatic substituents (i.e., a 3,4-methylenedioxy group) and an extended side chain, it would be useful to examine the metabolism of a structurally simpler analog that possesses only a methylenedioxy ring. The metabolism of MDPPP, or the 3,4-methylenedioxy counterpart of α-PPP (Figure 7), has been examined in rats. The methylenedioxy group of MDPPP underwent ring-opening to a catechol which was subsequently O-methylated to the 3-methyl catechol; all three of these metabolites can also form lactams (Figure 7) (Springer et al., 2003b). These metabolites also underwent oxidative deamination to their corresponding di-keto analogs.
Figure 7.
Metabolites of MDPPP in rats (Springer et al., 2003b).
The metabolism of MDPV, evaluated in vitro using human liver microsomes, resulted in identification of two Phase I metabolites: MDPV catechol and its corresponding 3-OMe derivative presumably arising from the action of catechol-O-methyl transferase (Strano-Rossi et al., 2010). Approximately 80% of MDPV remained unchanged; however, the authors offered the caveat that the high percentage of unmetabolized parent compound could have been due to the excess drug added to the liver microsome samples.
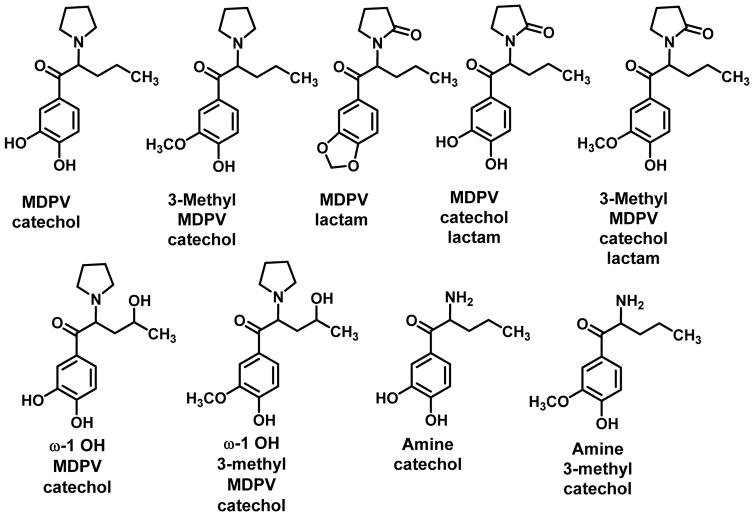
Meyer et al., (2010) examined the metabolism of MDPV in rats and humans, found a significant amount of un-metabolized MDPV, and showed some species differences in metabolism; partly overlapping pathways were postulated for the two species. The metabolism of MDPV in humans (Figure 8) was not unlike what was shown in Figure 7 for MDPPP.
Figure 8.
Human metabolites of MDPV (Meyer et al., 2010).
Ring-opening of the methylenedioxy group followed by O-methylation gave MDPV catechol and 3-methyl MDPV catechol; three lactams were also formed, one from MDPV and one from each of the ring-open products. In addition, both the catechol and 3-methyl catechol metabolites were identified as their ω-1 hydroxyl analogs and as their primary amines (Meyer et al., 2010).
Uralets et al., (2014) analyzed 34,561 human urine specimens for designer drugs, and 16 cathinone analogs were detected. The three most prevalent synthetic cathinones identified were αPVP, MDPV, and pentedrone. Simple cathinone analogs with small N-alkyl groups (e.g. mephedrone, ethcathinone, flephedrone) were typically converted to their corresponding alcohols by reduction of the β-keto group, and to N-dealkylated products. However, the presence of a pyrrolidine ring, as found in α-PVP and MDPV, hindered this reduction due, presumably, to steric hindrance. A 3,4-methylenedioxy group, such as found in MDPV, also hindered reduction. α-PVP was detected unchanged and as its metabolites, whereas MDPV was detected in urine with no free excreted metabolites. The authors acknowledged the limitations of their study, including their consideration only of non-conjugated forms of agents and metabolites; hence, other metabolites might have been present but were undetected.
As earlier mentioned, one reason to investigate the metabolism of synthetic cathinones is to identify metabolites that might retain central stimulant activity. Unfortunately, most of the metabolites described above have yet to be pharmacologically characterized, although it might be noted that cathinone (Figure 1), a metabolite of α-PPP, is centrally active and the namesake of the class of agents termed synthetic cathinones. Lactam metabolites, because they lack a protonatable amine, and the aromatic hydroxy analogs, because they should not readily penetrate the blood-brain barrier (depending upon where hydroxylation occurs), are unlikely to be potent stimulants. But, this remains to be investigated.
Recently, Anizan et al., (2014) conducted a relevant study with MDPV. Rats were administered doses of MDPV and blood was collected using in-dwelling jugular catheters; plasma specimens were collected and analyzed for MDPV levels, as well as for levels of its two major metabolites (MDPV catechol and 3-methyl MDPV catechol) (see Figure 8) following different doses and times of administration. A positive correlation was found between plasma concentration of MDPV and both drug-induced locomotor activity and stereotypy, but not with metabolite levels. In fact, where metabolite levels were at their maximum, locomotor activity and stereotypy were at their lowest; it was speculated that the metabolites might even exert effects that suppress motor activity (Anizan et al., 2014).
The metabolism of MDPV, α-PVP, and several other synthetic cathinones has now been investigated. However, it is not yet known how many, which, and if any of the metabolites contribute to their overall central actions and/or side effects.
5. Behavioral Studies
Early History from the Patent Literature
α-PVP, although not termed as such at the time, was described in numerous European and U. S. patents from 1963 to 1967. Its synthesis was described in a British patent GB 927475 (Wander, May 29, 1963) with assignment to Dr. A. Wander S.A. (Bern, Switzerland) and claimed for “central stimulating action”. Later that year, British patent GB 933507 (Thomae, August 8, 1963) was issued with claims for α-pyrrolidino ketones as “central nervous system stimulants, and, in some cases, hypertensive and spasmolytic agents”. Subsequent patents that included α-PVP were issued in Germany (DE 1161274, January 16th 1964), Switzerland (CH 395998/CH 395999, January 14th, 1966 and CH 401054, April 30, 1966), and the United States (US 3287217; Seeger, November 22, 1966 and US 3314970; Seeger, April 18, 1967). Although none of these patents included biological data, claims included “excellent central nervous system stimulating as well as hypertensive activities, coupled with low toxicity”. In addition, α-PVP was projected to produce effects at an “estimated human dose from 10 – 50 mg, preferably 20 mg for adults” (Seeger, 1967).
MDPV and analogs were first described in French patent FR 5502 (Adorjan, December 4, 1967) with assignment to Boehringer, C. H. & Sohn and claimed as therapeutic central nervous system stimulants. Subsequent MDPV patents (all assigned to Boehringer Ingelheim G.M.B.H.) were issued in Great Britain (Boehringer Ingelheim G.M.B.H., GB 1,149,366; April 23, 1969), Germany (Köppe and Zeile, DE 1,545,591; August 7, 1969) and the United States (Köppe, et al., U.S. 3,478,050; November 11, 1969). The first three patents included animal data from a small series of substances that compared changes in the α-alkyl substituent of the MDPV structure to support favorable claims (compared to racemic amphetamine) for efficacy-like versus toxicity ratios (Table 3). Efficacy-like activity (Z) was characterized as stimulation of motor activity in mice, and toxicity was defined as acute lethal dose 50% (LD50) in mice. A potential therapeutic index score (LD50/Z = J) was determined for each drug and, as shown, MDPV exhibited the most favorable therapeutic ratio (i.e. 875).
Table 3.
Behavioral and toxic effects of MDPV and MDPV analogs compared to racemic amphetamine (Benzedrine®). Data obtained and adapted from patents related to MDPV (FR 5502, GB 1,149,366 and DE 1,545,591).
| a MDPV α-alkyl group | Za–c | LD50d | Je |
|---|---|---|---|
| n-Propyl (i.e. MDPV) | 0.20 | 175 | 875 |
| isoPropyl | 0.96 | 285 | 296 |
| n-Butyl | 0.54 | 250 | 463 |
| f Benzedrine | 1.95 | 80 | 42 |
Doses refer to base dose.
Z = dose required for stimulation of the central nervous system mg/kg (mouse, s.c.).
It is unclear if value represents minimal effective dose (MED), effective dose 50% (ED50), or other representative score.
LD50 = lethal dose 50%, toxicity in mg/kg (mouse) s.c.
J = (potential) therapeutic index = LD50/Z.
Benzedrine® [(±)-amphetamine] was used as reference CNS stimulant.
In the U.S. patent, these α-substituted ketones were stated to “exhibit extraordinarily powerful central nervous system stimulating activities in warm-blooded animals, while their toxicity is very low. Thus, their therapeutic ratio is very high and therefore unexpectedly favorable (pg. 4)”. Moreover, the inventors projected that for human consumption “each dosage unit may conveniently contain from 2 to 40 mg, preferably from 10 to 20 mg of active ingredient” (e.g., see GB 1,149,366). MDPV is the most potent substance in this series and, as such, the projected dose would probably be 2 to 10 mg (total dose) for human consumption. In comparison, the U.S. Drug Enforcement Administration (DEA) stated in 2013 that “users of MDPV anecdotally reported that they take 25 mg or less per session” (DEA 2013). Thus, humans might currently use or abuse doses of MDPV that are approximately 2 to 10 times higher than those envisioned by the inventors of this drug. Lastly, Table 3 indicates that MDPV is approximately 10 times more potent than racemic amphetamine as a stimulant and exhibits a more favorable therapeutic ratio.
Behavioral Protocols
MDPV and α-PVP have been evaluated in various animal models to better understand their neurobiology and behavioral actions. For example, the effects of these drugs on motor activities in rodents can be evaluated quickly and do not involve learning/conditioning; this likely explains why the most often reported results come from such studies. Other procedures, however, have also been employed such as wheel-running and rotorod performance. In addition, these drugs (like other CNS stimulants) may induce changes in animals’ body temperature, which may influence behavioral responses. Other animal models have evaluated the effects of MDPV and α-PVP in procedures that involve training or conditioning. The major advantages of the latter procedures are that they provide very stable baselines of activity to study the effects of drugs and the results of such studies are very reproducible. Such assays include (a) drug self-administration studies in which animals will perform tasks to inject themselves with drugs, (b) intracranial self-stimulation in which subjects will self-stimulate certain brain areas that activate circuits that are probably related to “natural reward” or reinforcement mechanisms, (c) conditioned place preference in which an animal that chooses to spend more time in a location that has been coupled with drug injections is assumed to experience positive reward in that area and (d) drug discrimination in which the internal stimulus effects of a drug serve as a reflection of the subjective effects of that drug.
a. Locomotor Activity
Rodents’ motor activity is considered spontaneous or unconditioned and is arguably the most often used initial test to assess for pharmacological effects of a drug. In general, evaluation of this behavior falls into two categories: automated monitoring (including radio-telemetry) of behavior and direct observational techniques. A widely used device to investigate the behavioral reaction of an animal to a drug is an enclosed chamber that is equipped with photo- or infrared-beams. It has been argued, however, that animals show a behavioral repertoire in an arena that is infinitely richer than simply an overall tally of breaks of beams (e.g., Van Abeelen, 1963). A rodent’s set of activities may include, for example, rearing, rotations, jumps, center region entries, margin area activity, and repetitive actions (e.g. stereotypy). Moreover, the extent of such activities may be influenced by the animals’ degree of familiarity (i.e. novel vs. familiar) with the test apparatus. In most of the motor studies detailed below of the effects of MDPV and α-PVP, only total beam breaks for a test session were recorded and, thus, many different kinds of action may (or may not) have been compiled into a single score. A typical study consisted of an animal placed in an unfamiliar (novel) or familiar (habituated) enclosure, surrounded by one set (for floor-plane movement) of beams. However, Marusich and colleagues (2012, 2014) have provided more detailed effects of MDPV and α-PVP, in comparison to those of cocaine and S(+)methamphetamine, with their adaptation of the Functional Observation Battery (FOB) used by the U.S. Environmental Protection Agency (EPA) to detect potential safety concerns of chemical substances. The FOB emphasizes visual observation of animals to categorize a range of behaviors that includes ataxia, exploration, convulsions, circling, hyperactivity, salivation, head weaving, head circling, compulsive (stereotypic) behavior and movements, and stimulation. These authors’ approach is particularly relevant because most stereotypic behavior can only be recorded by direct experimenter observation. For example, most automated monitoring devices can identify and record certain repetitive behaviors such as turning and retraced movements but they cannot account for stereotypic behavior that includes, for example, head bobbing and/or continual biting/licking of the test cage. In addition, it should be emphasized that so-called stereotypic actions are not outside the animals’ normal repertoire of behaviors. They are only considered as stereotyped when they occur at a frequency that is several times the normal frequency. In this review, the effects of MDPV and α-PVP on motor activity are divided into four sections: studies that tested the effects of the drugs in animals that were (a) not habituated (novel environment) to their test apparatus, (b) habituated (familiar envirnoment) to their test device, (c) studied by radio-telemetry in home cages and (d) evaluated under the FOB protocol.
Novel Environment
An early study by Fuwa et al., (2007) compared the motor activity (distance travelled and number of movements) effects of MDPV (20 mg/kg, p.o.) to those of S(+)methamphetamine (2 mg/kg) and MDMA (20 mg/kg) in mice that were naïve to test apparatus and reported that S(+)methamphetamine and MDMA increased these measures over 3 hours but MDPV increased them for only the first 90 minutes before a precipitous drop in activity (near control levels) occurred over the next 90 minutes. In other studies, Marusich et al., (2012) tested mice and compared the effects on motor activity (i.e. beam breaks) of MDPV (1, 3, 10 and 17 mg/kg, i.p.) to those of cocaine (10, 17, 30 and 42 mg/kg) and S(+)methamphetamine (1, 3 and 5.6 mg/kg). Mice significantly increased their beam breaks at all doses of each drug but the lack of no effect doses precluded the possibility of potency comparisons. Gatch et al., (2013) tested mice and compared the motor activity (horizontal beam breaks) effects of MDPV (0.03, 1, 3, 10 or 30 mg/kg, i.p.) to those of S(+)methamphetamine (0.25, 0.5, 1, 2 or 4 mg/kg), and cocaine (5, 10, 20 or 40 mg/kg). MDPV displayed significant motor stimulant effects between doses of 1 – 10 mg/kg and a significant decrease in activity at 30 mg/kg. In comparison, S(+)methamphetamine (0.5 – 2 mg/kg) and cocaine (10 – 40 mg/kg) produced significant increases in activity; however, 4 mg/kg of S(+)methamphetamine significantly decreased activity. Marusich et al., (2014) tested mice and compared motor activity (beam breaks) effects of α-PVP (1.0, 3.0 and 10.0 mg/kg; i.p.) and MDPV (0.3, 1.0 and 3.0 mg/kg); MDPV (1 and 3 mg/kg) and α-PVP (3 and 10 mg/kg) produced significant increased activity. In antagonism tests, locomotor stimulation induced by α-PVP (3 mg/kg) or MDPV (1 mg/kg) was significantly blocked by the dopamine D1 receptor antagonist SCH 23390 (0.03 mg/kg, s.c.). Lastly, Gatch et al., (2015) tested mice and evaluated the motor activity (horizontal beam breaks) effects of α-PVP (1, 2.5, 5, 10 and 25 mg/kg, i.p.) over 8 hours. α-PVP doses of 2.5 to 10 mg/kg significantly increased horizontal counts within 10 minutes post-injection and this effect lasted between 4 – 5 hours. In comparison, α-PVP at 25 mg/kg produced a delayed stimulant effect that did not occur until 60 minutes post-injection and this effect lasted about 4.5 – 5 hours.
Familiar Environment
Baumann et al., (2013) pre-habituated (to minimize effect of novelty) rats for 1 hour each weekday for 7 days before they were administered MDPV (0.1, 0.3, 1.0, or 3.0 mg/kg, s.c.) or cocaine (3.0, 10, or 17 mg/kg). Measurements included distance traveled and stereotypy (repeated interruption of the same photocell beam within 2 sec). MDPV (0.3 – 3 mg/kg) produced significant increases in distance travelled and stereotypy that were at least 10 times more potent than cocaine, which produced significant increases in distance travelled (10 and 17 mg/kg) and stereotypy (3 – 17 mg/kg). Kaizaki et al., (2014) tested mice that were allowed to become familiar to motor activity devices (with video tracking system for travel distance) for 10 minutes to “calm” themselves prior to injection of α-PVP (25 mg/kg, p.o.) or racemic methamphetamine (5 mg/kg). Both drugs significantly increased motor actions. In antagonism tests, α-PVP-induced locomotor stimulation was significantly blocked by the dopamine D1 receptor antagonist SCH 23390 (0.05 mg/kg, i.p.) or the dopamine D2 receptor antagonist sulpiride (50 mg/kg, i.m.); unfortunately, combination studies of both antagonists were not undertaken in attempts to completely block the effects of α-PVP. Wakabayashi et al., (2015) tested rats that were implanted with i.v. catheters and habituated to motor activity equipment for 6 hours/day for 3 consecutive days. Thereafter, animals received 4 injections over 20 seconds of either MDPV (0.1 mg/kg) or cocaine (1 mg/kg), which produced comparable increased motor activity (beam breaks). Lastly, Kiyatkin et al., (2015) tested rats and measured the effects of MDPV (0.1, 0.3 and 1.0 mg/kg, s.c.) on locomotor activity (photobeam breaks) and reported that the two higher doses produced significant increased activity within a 2.5 hour time period.
Radio-Telemetry
Fantegrossi et al., (2013) tested doses of MDPV (1, 3, 10 and 30 mg/kg, i.p.) in mice that were surgically implanted with radio-telemetry probes to monitor motor responses in their home cages under two ambient temperatures (20 °C vs. 28 °C). MDPV was shown to be a motor stimulant at all doses tested but that (a) its profiles of dose response functions are different at the two temperatures, with an inverted U-shaped dose response function at 28 °C and relatively flat dose response curve at 20 °C and (b) its effects, at least at 10 mg/kg, are significantly increased by a warm (28 °C) ambient temperature. In addition, MDPV at 30 mg/kg produced marked stereotypy (e.g., self-injurious skin-picking and chest biting behavior) at the 28 °C but not the 20 °C environment. In comparison, a single dose of 10 mg/kg of MDMA also produced motor stimulant effects but the two temperatures did not differentially influence the increased activity. This same research group has recently tested mice under the same test conditions and evaluated (±)MDPV (1, 3, 10 and 30 mg/kg, i.p.), S(+)MDPV (0.3, 1, 3 and 10 mg/kg) and R(−)MDPV (10, 30 and 100 mg/kg). When data were averaged over the test period of 6 hr, MDPV and its optical isomers did not alter motor activity at the 20 °C temperature. In contrast, MDPV produced increased motor activity at the 28 °C environment in a stereospecific manner. That is, only S(+)MDPV (3 and 10 mg/kg) and (±)MDPV (3, 10 and 30 mg/kg) significantly increased motor activity (Gannon et al., 2016). Aarde et al., (2013) assessed the effects of 0.5 – 5.6 mg/kg (s.c.) of MDPV vs. 0.5 – 5.6 mg/kg methamphetamine in rats implanted with radio-telemetry transmitters and tested in familiar activity cages. Motor measurement was counts per minute of changes in signal strength from radio-telemetry transmitter relative to baseline 30 min pre-injection. In addition, stereotypy (repetitive licking, biting or sniffing) was evaluated for MDPV under both response contingent self-administration and non-contingent administration of MDPV (0.5 – 5.6 mg/kg, s.c.). Both MDPV and S(+)methamphetamine produced inverted dose response functions; both drugs increased motor activity at lower doses (0.5 – 1.0 mg/kg) and decreased activity at the highest dose (5.6 mg/kg). In a follow-up study, Aarde et al., (2015) compared motor activity readings in rats that received α-PVP (1.0, 5.6 and 10 mg/kg, i.p.) to those of MDPV (1.0, 5.6 and 10 mg/kg). Both drugs, at 1.0 mg/kg, produced peak increased locomotor activity responses that lasted about 120 minutes; however, higher doses of each drug induced minimal effects on motor activity except for a transient increased locomotor activity effect at 5.6 mg/kg of α-PVP at 120 minutes that lasted no more than 15 minutes and at 10.0 mg/kg of MDPV at 105 minutes that lasted no more than 15 minutes.
Functional Observation Battery (FOB)
Marusich et al., (2012) reported that the FOB profile in mice indicated significant and similar stimulant-like increases in observational measures related to MDPV (1 – 30 mg/kg, i.p.), cocaine (10 – 42 mg/kg) and S(+)methamphetamine (1 – 10 mg/kg) but with some marked differences in their effects; MDPV (1 – 30 mg/kg) produced significant increased exploration whereas the other two drugs did not; S(+)methamphetamine (10 mg/kg) produced significant increased salivation whereas the other two drugs did not; and MDPV (3 – 10 mg/kg) and S(+)methamphetamine (3 – 5.6 mg/kg) produced significant increased compulsive (stereotyped) movements whereas cocaine did not. In their follow-up study, Marusich et al., (2014) reported that the FOB (also in mice) indicated significant and similar stimulant-like increases in observational measures related to MDPV (1 – 10 mg/kg), α-PVP (3 – 17 mg/kg), cocaine (10 – 42 mg/kg) and S(+)methamphetamine (1 – 10 mg/kg) but also with significant differences in their effects; MDPV (3 mg/kg) caused significant increased ataxia whereas the other three drugs did not; MDPV (1 – 10 mg/kg) and α-PVP (3 – 17 mg/kg) produced significant increased exploration but cocaine and S(+)methamphetamine did not; MDPV (1 – 3 mg/kg) produced significant “bizarre” behavior but the other three drugs did not; α-PVP (3 – 17 mg/kg) produced significant increased flattened body posture but the other three drugs did not; and MDPV did not induce stereotyped head weaving but α-PVP (3 – 17 mg/kg), cocaine (10 – 17 mg/kg) and S(+)methamphetamine (1 – 10 mg/kg) did.
b. Wheel-Running
For over a century now, animals have been observed and documented to engage in voluntary wheel-running and many studies have utilized this activity to model the effects of exercise on health (for review, see Sherwin 1998). However, animals’ wheel-running does not appear to coincide or be analogous with any of their other behaviors that occur naturally. Moreover, no consensus of opinion exists as to why this behavior occurs or what function it serves (Collier et al., 1990). For example, wheel-running has been suggested to reflect self-reinforcement, exploration, general activity, escape behavior, stereotypic activity, play behavior, adrenal gland activity, and body weight maintenance (Mason 1991). Sherwin (1998) has argued that wheel-running and general locomotor activity are not analogous and that several aspects of its occurrence indicate that it may be a unique behavior that is not amenable to characterization by models of “normal” animal behavior.
Huang et al., (2012) compared the effects of MDPV (0.0, 0.5, 1.0 and 5.6 mg/kg, s.c.) to those of S(+)methamphetamine (0.0, 0.56, 1.0 and 5.6 mg/kg) and MDMA (0.0, 1.0, 5.6 and 7.5 mg/kg) on wheel-running in rats. MDPV and S(+)methamphetamine produced potent and inverted U-shaped functions: lower doses of MDPV (0.5 mg/kg) and S(+)methamphetamine (0.5 and 1.0 mg/kg) produced increased counts of wheel-running and a higher dose (5.6 mg/kg) of each drug produced decreased counts. Moreover, stereotypy (e.g., repetitive sniffing, licking and/or circular head motion) was scored as “present” in all of the rats at the end of the session following the highest dose of S(+)methamphetamine or MDPV but was scored “absent” after all other sessions. In contrast to those actions, MDMA produced only dose-related decreased wheel running and an absence of stereotypy.
c. Rotorod
The rotorod procedure is used to assess neuromuscular coordination or balance. Typically, rodents are trained to maintain themselves for a specified period of time on a rotating rod (rotorod) moving at a constant (e.g., 4 or 6 rpm) or gradually accelerating (0 to 10 rpm) speed. In order to stay on the rod, the animal must walk in a direction opposite to the direction of rotation. Groups of animals that have demonstrated the ability to negotiate the rod for a specified period of time (e.g., 1 min) would then be treated with either vehicle or dose of test drug and retested to determine their ability to maintain rotorod performance at various periods of time post-drug administration. Subjects that failed to stay on the rotorod for the specified test duration would be considered to have suffered loss of coordination or have neuromuscular impairment (ataxia).
Marusich et al., (2012) employed standard rodent rotorod devices and trained mice to successfully navigate the rotorod as it accelerated to the maximum 10 revolutions/min within 30 seconds for trials that lasted 150 seconds. The effects of MDPV (3, 10 and 30 mg/kg, i.p.) were compared to those of cocaine (10, 17 and 30 mg/kg) and S(+)methamphetamine (1, 3 and 10 mg/kg) on rotorod tests to evaluate motor coordination/balance/neurotoxicity. None of the drugs affected the animals’ performance.
d. Thermoregulation
An organism’s thermoregulatory mechanisms are designed to keep body temperature maintained within certain limits, an important aspect of homeostasis. Stimulant use or abuse can lead to a condition of hyperthermia in which body temperature increases significantly above normal; severe or “malignant hyperthermia” can lead to serious injury or death.
Aarde et al., (2013) obtained temperature readings from implanted radio-telemetry transmitters in rats that received MDPV (0.5 – 5.6 mg/kg, s.c.) or methamphetamine (0.5 – 5.6 mg/kg) for body temperature. MDPV produced negligible effects on temperature in comparison to the hyperthermia produced by methamphetamine. In addition, the effects on body temperature of MDPV (0.5 – 5.6 mg/kg, s.c.) under 23 °C room temperature vs. S(+)methamphetamine (1.0 or 5.6 mg/kg) under 20 °C and/or 25 °C room temperature were assessed. MDPV had negligible effects on body temperature compared to S(+)methamphetamine-induced hyperthermia. In contrast, Fantegrossi et al., (2013) assessed body temperature effects of MDPV (1.0 – 30 mg/kg, i.p.) in mice with radio-telemetry and reported the actions of MDPV at different environmental temperatures (28 °C vs. 20 °C). In the 20 °C environment, 10 mg/kg of MDPV increased the animals’ temperature by ~1.5 °C and in the 28 °C environment, 10 mg/kg of MDPV raised the animals’ temperature by ~2 °C. This same research group recently tested mice under the same test conditions and evaluated (±)MDPV (1, 3, 10 and 30 mg/kg, i.p.), S(+)MDPV (0.3, 1, 3 and 10 mg/kg) and R(−)MDPV (10, 30 and 100 mg/kg). When data were averaged over the test period of 6 hr, MDPV and its optical isomers had negligible effects on the animals’ body temperature in the 20 °C environment. In comparison, MDPV and its enantiomers produced slight but not statistically significant (except at 30 mg/kg of (±)MDPV) increases in the animals’ body temperature in the 28 °C environment. In other studies, King et al., (2014) monitored body temperature via implanted probes in Fischer (F344) and Lewis (LEW) rats and evaluated the effects of MDPV (1, 1.8, and 3.2 mg/kg, i.p.). MDPV produced marked hyperthermia in both strains of rats although more variability in temperature response was noted in LEW than in F344 animals. Merluzzi et al., (2014) measured thermoregulation via transponders and examined the effects of MDPV (1.0, 1.8 and 3.2 mg/kg, i.p.) in adolescent [defined as 28 to ~40 post natal days (PND) old] versus adult [defined as 77 to ~90 post natal days (PND) old] rats. MDPV generally decreased the body temperature of adolescent rats and increased the body temperature of adult rats. Kiyatkin et al., (2015) examined the effects of MDPV (0.1, 0.3 and 1.0 mg/kg, s.c.) on brain and body temperature of rats via implanted miniature thermocouple probes in the nucleus accumbens, temporal muscle and facial skin. In addition, animals were tested under typical laboratory environmental conditions (single-housed at 22 °C) and under simulated human drug use conditions (doubled housed con-specific intruder procedure at 29 °C). MDPV induced dose dependent increased brain temperature and hyperthermia under the lower ambient/housing condition but had minimal effects at the warmer/housing condition. Lastly, Aarde et al., (2015b) obtained temperature readings from implanted radio-telemetry transmitters in rats that received α-PVP (1.0, 5.6 and 10 mg/kg, i.p.) or MDPV (1.0, 5.6 and 10 mg/kg). Both drugs produced definitive trends toward decreased body temperature (i.e. hypothermia) at 5.6 and 10.0 mg/kg of α-PVP or MDPV.
e. Self Administration
Drug self-administration procedures are designed to assess the reinforcement (reward) properties of a drug and the most frequently used method employs animals that are implanted with a venous catheter and then trained to perform an operant response, usually presses of a lever, to obtain injections of drug. Over the years, such tests in non-human animals have been shown to be valid and reliable indicators of abuse liability of drugs in humans; animals will self-administer most drugs that are abused by humans (e.g., Griffiths & Ator, 1980; Panlilio & Goldberg, 2007). These assays usually measure number of drug infusions and/or rate of lever press per time period as a function of dose of drug received (infused) per injection. Typically, the data will reveal an inverted U-shaped dose-effect curve after completion of tests that thoroughly investigate an appropriate range of drug doses; increased drug infusions and/or response rate are interpreted as an indication of reinforcement effects and doses that result in decreased drug infusions and/or response rate have been associated with excessive drug consumption or adverse effects.
In the first study of the effects of MDPV in a self-administration paradigm, Watterson et al., (2012) trained four groups of rats to self administer (i.v.) either 0.05, 0.10, 0.20 mg/kg/infusion of MDPV or 0.05 mg/kg/infusion of S(+)methamphetamine and reported that MDPV maintained self-administration across all doses tested, as did the single dose of S(+)methamphetamine. Later, Aarde et al. (2013) trained separate groups of rats to self-administer (i.v.) MDPV (0.05 mg/kg/infusion) or S(+)methamphetamine (0.05 mg/kg/infusion) and found that both drugs were consistently self-administered within 10 training sessions. Dose response studies indicated that MDPV (0.01 – 0.50 mg/kg/infusion) was more potent than S(+)methamphetamine (0.01 – 0.25 mg/kg/infusion); in particular, for the per-infusion dose of 0.01 mg/kg, the infusion rate was (statistically) significantly higher when MDPV was available than when S(+) methamphetamine was available. In a follow-up study, Aarde and colleagues (2015a) successfully trained separate groups of rats to self-administer (i.v.) α-PVP (0.05 mg/kg/infusion or 0.1 mg/kg/infusion) or MDPV (0.05 mg/kg/infusion). Rats trained to 0.1 mg/kg/infusion of α-PVP received dose-response substitution tests with 0.0 (control), 0.025 mg, 0.05 mg, 0.1 mg, or 0.25 mg/kg/infusion of α-PVP, which determined that drug versus control infusions (a) increased significantly at the 0.025 mg/kg/infusion dose, (b) showed no difference at the 0.05 mg/kg/infusion dose and (c) decreased significantly at the 0.10 and 0.25 mg/kg/infusion doses. Also, Aarde et al (2015b) trained separate groups of rats to self-administer (i.v.) 0.05 mg/kg/infusion of MDPV to determine if drug intake could be modified when access to wheel running was either locked or unlocked. Rats’ intake of MDPV steadily increased across the study’s 20-session acquisition interval with no differences in consumption reported between the two access groups; however, the unlocked wheel groups’ wheel rotations declined significantly across the acquisition interval. The interpretation of these results was that MDPV can devalue “favorable” access to wheel running activity. Lastly, Schindler et al., (2015) trained rats to self-administer (i.v.) a dose of MDPV (0.03 mg/kg/injection) that was more than15 times more potent than a dose of cocaine (0.5 mg/kg/injection). Subsequent dose-response tests indicated that both drugs displayed an inverted U-shaped dose effect function for self-administered injections per session. MDPV doses of 0.003 mg/kg/injection and 0.01 mg/kg/injection increased infusions (0.01 mg/kg/injection also produced peak infusions) and doses of 0.03 mg/kg/injection and 0.10 mg/kg/injection decreased infusions. In comparison, peak infusions of 0.1 mg/kg/injection (smallest dose tested) of cocaine produced peak infusions and doses of 0.3, 0.5 and 1 mg/kg/injection decreased infusions. Taken together, these studies suggest that the order of potency for the drugs appears to be MDPV ≥ α-PVP > S(+) methamphetamine > cocaine.
f. ICSS
Animals will readily perform a response, such as a lever-press or nose-poke, to activate electrical stimulation to an electrode that has been surgically implanted in a specific (“pleasure”) area of their brain such as the medial forebrain bundle. Because the subject has direct control of the response and is able to self-administer the brain stimulation, the response in these situations is generally referred to as intracranial self-stimulation (ICSS). The rationale of this procedure to study drugs of abuse is that their mechanism of action involves, at least in part, the initiation or facilitation of neurochemical events at these brain sites that produce “reward” or “pleasure”. Consequently, the administration of known or purported drugs of abuse will either increase the rate at which the animal self-stimulates and/or lower the threshold for ICSS such that a smaller amount of electrical current is required to sustain the same self-stimulation behavior (for review, see Vlachou and Markou, 2011).
Watterson et al., (2012) studied the effects of MDPV (0.1, 0.5, 1 and 2 mg/kg, i.p.) on nose-poke response ICSS thresholds of rats that were unilaterally implanted with a bipolar electrode into the medial forebrain bundle and found that thresholds were substantially lowered following MDPV administration. In a later study, Watterson et al., (2014) compared the effects of α-PVP (0.1, 0.3, 1.0, 3.0 and 5 mg/kg, i.p.) to S(+)methamphetamine (0.1, 0.3, 1.0 and 3 mg/kg, i.p.) on ICSS thresholds of rats with electrodes in the medial forebrain bundle. α-PVP produced significant ICSS threshold reductions that were comparable to S(+)methamphetamine; i.e. both drugs produced similar and significant ICSS threshold reductions at 0.3 and 1.0 mg/kg. However, 5 mg/kg of α-PVP or 3 mg/kg of S(+)methamphetamine produced increased (“aversive”?) thresholds of ICSS. In other studies, Bonano et al., (2014a) tested rats that had electrodes implanted into the left medial forebrain bundle and responded (lever-pressed) for a continuum of low to high frequencies of brain stimulation. MDPV (0.32 – 3.2 mg/kg, i.p.) produced dose response and time course effects that indicated increases in low rates of ICSS maintained by low brain stimulation frequencies, but also produced abuse-limiting depression (“adverse”?) of high ICSS rates maintained by high brain stimulation frequencies; additionally, 3.2 mg/kg of MDPV produced a relatively long duration of effect (≥ 5 hr). In a follow-up study, this research group used the same ICSS procedures and evaluated the effects of optical isomers of MDPV: S(+)MDPV (0.032 – 1 mg/kg, i.p.) and R(−)MDPV (0.32 – 10 mg/kg). S(+)MDPV (at doses ≥ 0.1 mg/kg) produced dose related facilitation of ICSS, which was about three times more potent than (±)MDPV (i.e. at doses of ≥ 0.32 mg/kg). In contrast, R(−)MDPV failed to modify ICSS at doses up to 100 times greater than that of the lowest statistically significant effective dose of S(+)MDPV. Lastly, Bonano et al., (2014b) evaluated the effects of the neuropeptide S (NPS) receptor antagonist RTI-118 (3-oxo-1,1-diphenyl-N-(2-(piperidin-1-yl)ethyl)tetrahydro-1H-oxazolo[3,4-a]pyrazine-7(3H)-carboxamide) on abuse-related facilitation of ICSS produced by MDPV and cocaine in rats. RTI-118 alone produced negligible effects on ICSS, dose dependently blocked cocaine-induced facilitation of ICSS, but failed to block MDPV-induced facilitation of ICSS. Such results strongly suggest some differential mechanisms of action for MDPV in comparison to cocaine in the facilitation of ICSS in the medial forebrain bundle.
g. Conditioned Place Preference
Conditioned place preference (CPP) experiments can provide data on the reinforcement or reward effects of known or potential drugs of abuse. In this procedure, animals receive a dose of drug or vehicle and are placed into 1 of 2 sides of a distinct environmental chamber; typically, chambers differ in visual (e.g., walls with different design patterns) and physical (e.g., barred or grid floors) characteristics. Drug is always paired with one of the chambers and vehicle with the other. Drug treatments and environmental conditions are continued over the course of several days, which creates associations between treatment and environment. Animals’ time spent in each chamber is the dependent measure. Subsequently, a test day is conducted on which no treatment is administered and animals are allowed access to both environments. The animals’ choice to spend more time in either environment is assumed to measure the conditioned reinforcement effect of the drug. If the drug has induced positive reinforcement or “reward”, then the animal will spend significantly more time in the drug-paired environment. Conversely, drugs might induce a “negative” effect in this procedure and animals will spend significantly less time in the drug-paired side, a result known as conditioned place aversion. The latter condition can also be studied in animals with conditioned taste aversion (CTA) procedures, where consumption of a drug-free liquid with a novel taste (usually saccharin) is paired with a drug treatment and aversive effects of the drug are inferred from animals’ subsequent avoidance of that fluid.
Karlsson et al., (2014) examined the effects of MDPV (0.5, 2, 5, 10 and 20 mg/kg, i.p.) versus racemic amphetamine (0.5, 2, 5, 10 and 20 mg/kg) in mice in a conditioned place preference procedure. MDPV induced increased preference score time (seconds) at all doses, whereas amphetamine produced increased preference time only at 10 and 20 mg/kg. King et al., (2015a) studied the effects of MDPV (1.0, 1.8 and 3.2 mg/kg, i.p.) in rats in a conditioned place preference (CPP) procedure and reported that MDPV, at all doses, produced the same percentage of (significant) increased time in the drug-paired chamber (i.e. place preference). In a follow-up study, King et al., (2015b) studied the effects of MDPV (1.0, 1.8 and 3.2 mg/kg, i.p.) in both male and female rats that underwent a combined CTA/CPP procedure in which drug was paired with both a novel saccharin solution and a novel environment and followed by an evaluation of changes in preferences for these stimuli. MDPV-induced CTA/CPP occurred in both sexes although taste aversion was somewhat weaker in females. Merluzzi et al., (2014) studied CTA in adolescent [defined as 28 to ~40 post natal days (PND) old] versus adult [defined as 77 to ~90 post natal days (PND) old] rats. MDPV (1.0, 1.8 and 3.2 mg/kg, i.p.) induced taste aversions in both age groups but these aversions were weaker and developed at a slower rate in adolescent animals. Also, King et al., (2014) examined the CTA effects of MDPV (1, 1.8, and 3.2 mg/kg, i.p.) in Fischer (F344) and Lewis (LEW) rat strains and reported that MDPV doses of 1.8 and 3.2 mg/kg induced CTA in both strains of animals. Lastly, Gatch et al., (2015) tested the effects of α-PVP (0.1, 0.3, 1.0, 3.0, 10 and 30 mg/kg, i.p.) in mice in a conditioned place preference test and reported that α-PVP produced an inverted U-shaped dose effect function with increased time spent on the drug-paired floor at doses of 0.3 to 10 mg/kg, but not at 0.1 or 30 mg/kg.
h. Drug Discrimination
Drug discrimination procedures are designed to evaluate the subjective effects of drugs in animals (Glennon and Young, 2011). In a typical experiment, subjects are trained to discriminate a dose of training drug from vehicle (usually saline) in a two-lever operant procedure. Once trained, stimulus generalization (substitution or recognition) tests can be conducted. Such tests are used to determine the similarity of the stimulus (i.e. internal) effects produced by a challenge drug (i.e. test drug) compared to those produced by the training drug. The challenge drug can be a different dose of the training drug (i.e. dose-response) or an entirely different drug. Stimulus generalization also may involve the administration of novel test or challenge drugs to the trained animals. For example, doses of a test drug can be administered to the trained animals to determine similarity of stimulus (internal) effects. Doses of the test drugs will cause animals to divide their responses between the training-drug appropriate lever (for responses to a drug that is “like” the training drug) and the vehicle-designated lever (for responses to an “inert” substance or a drug that is not training drug-like). If administration of a given dose of test drug results in the subjects making, typically, ≥ 80% of their responses on the training-drug-appropriate lever, then it is assumed that the test drug has produced a training-drug-like response; however, this does not necessarily mean that the two drugs produce their similar stimulus effects by identical mechanisms of action. If all doses of a test drug produce ≤ 20% drug-appropriate responding, then it is assumed that the test drug and the training drug produce dissimilar stimulus effects; however, this does not necessarily mean that the test drug is pharmacologically inactive but only that the stimulus effects of the two drugs are different. In some instances, administration of a test drug will result in partial generalization (≥ 20% but ≤ 80% drug-appropriate responding), which is the most difficult type of result to interpret. However, a training drug may partially generalize to a test drug because there is stimulus effects that are common to both the dose of training drug and the test drug but complete stimulus generalization will not occur because the overlap of pharmacological effects is incomplete.
Fantegrossi et al., (2013) trained mice to discriminate the stimulus effects of 0.3 mg/kg (i.p.) of MDPV from saline vehicle and reported that the stimulus was time-(duration of training dose effect was ~60 min) and dose-dependent. In tests of stimulus generalization, the MDPV stimulus (ED50 = 0.03 mg/kg) generalized and was found to be equipotent to MDMA (ED50 = 0.03 mg/kg) and about three times more potent than S(+)methamphetamine (ED50 = 0.08 mg/kg). In another study, Gatch et al., (2013) trained rats to discriminate the stimulus effects of either 10 mg/kg of cocaine (i.p.) or 1 mg/kg of S(+)methamphetamine from saline. In the cocaine-trained animals, stimulus generalization tests showed that MDPV produced a cocaine-like response in an all-or-none fashion; MDPV (ED50 = 0.68 mg/kg) was slightly more than four times more potent than cocaine (ED50 = 3.09 mg/kg). In the S(+)methamphetamine-trained rats, MDPV produced a methamphetamine-like response in a dose-related manner and MDPV (ED50 = 0.67 mg/kg) was about two times less potent than S(+)methamphetamine (ED50 = 0.37 mg/kg). Later, Gatch et al., (2015) reported that the cocaine and S(+)methamphetamine stimuli generalized in a dose related manner to α-PVP but, unfortunately, ED50 potency comparisons between the training drugs and α-PVP were not reported. Naylor et al., (2015) also trained rats to discriminate S(+)methamphetamine (1 mg/kg, i.p.) and reported that S(+)methamphetamine (ED50 = 0.3 mg/kg) stimulus generalization occurred to α-PVP (ED50 = 0.7 mg/kg) and cocaine (ED50 = 3.3 mg/kg). Thus, the order of potency to produce S(+)methamphetamine-like effects was S(+)methamphetamine > α-PVP > cocaine. In other studies, Harvey and Baker (2016) trained separate groups of rats to discriminate 1.5 mg/kg of MDMA from saline vehicle or a mixture of 1.5 mg/kg of MDMA and 0.5 mg/kg of S(+)amphetamine from saline vehicle; presumably, the latter combination of drugs would enhance, to some degree, the CNS stimulant component of MDMA. In tests of stimulus generalization, the MDMA-stimulus generalized partially to MDPV (maximum 50 – 60% MDMA-appropriate responding) over a fairly wide range of doses (i.e. 0.25 – 3 mg/kg of MDPV). In comparison, the stimulus mixture of MDMA + amphetamine generalized completely to MDPV but in an inverted U-shaped dose response function; MDPV, at doses between 0.125 mg/kg to 2 mg/kg, produced complete MDMA + amphetamine-like responding whereas MDPV at 3 mg/kg produced approximately 45% MDMA + amphetamine-like responding. Lastly, Gannon et al., (2016) trained mice to discriminate the stimulus effects of 10 mg/kg (i.p.) of cocaine (ED50 = 2.2 mg/kg) from saline and reported that cocaine-like effects of MDPV and its optical isomers occurred in a stereoselective manner: S(+)MDPV (ED50 = 0.1 mg/kg) was twice as potent as (±)MDPV (ED50 = 0.2 mg/kg) and about seventy-five times more potent than R(−)MDPV (ED50 = 7.3 mg/kg).
i. Behavioral Summary
The effects of MDPV and α-PVP on motor activity and wheel-running in rodents are dose-related and their most noticeable effect at relatively low doses is stimulation of behaviors and, at relatively large doses, repetitive motor sequences and other stereotyped behaviors characterized by licking, gnawing, sniffing and head bobbing. In some animals, stereotyped behavior included picking of the skin, sometimes to the degree of self-mutilation. Such results occurred with mice and rats injected with these drugs via different routes of administration and under novel or familiar environments. Importantly, MDPV- and α-PVP-induced stimulation of motor activities was shown to be susceptible to blockade by dopamine D1 and D2 receptor antagonists. On the other hand, these drugs produced no significant effects on coordinated movement (balance) in a rotorod task and their ability to increase animal body temperature, especially compared to that of S(+)methamphetamine or MDMA, seemed to be minimal.
MDPV and α-PVP produced effects in animals that clearly indicate abuse potential as evidenced by the fact that they support intravenous self-administration, lower thresholds for ICSS, and produce significant place preference effects that are similar to those of the well-known CNS stimulants (and drugs of abuse) S(+)methamphetamine and cocaine. This conclusion is also supported by drug discrimination studies that demonstrated stimulus generalization between MDPV and S(+)methamphetamine regardless of which drug was used as training agent. In addition, α-PVP and MDPV (and its optical isomers) were demonstrated to produce cocaine-like stimulus effects and α-PVP produced an S(+)methamphetamine-like stimulus effect; however, to date, no reports have surfaced of animals trained to discriminate the stimulus effects of α-PVP. Lastly, MDPV and MDMA produced the most interesting drug discrimination data and they might indicate an example of asymmetric stimulus generalization. That is, in animals trained to discriminate MDPV from vehicle, stimulus generalization occurred to MDMA but in animals trained to discriminate MDMA from vehicle, only partial generalization occurred to MDPV. These results suggest some similarity and some difference in stimulus effects and, consequently, mechanisms of action between the two drugs. As such, when MDPV is employed as training drug, its stimulus effects are predominately (if not solely) mediated by catecholamine-induced actions. When MDMA is administered as test drug, its catecholamine-mediated stimulant component to its mechanism of action is sufficient (at some dose) to achieve MDPV-like stimulus generalization. However, when MDMA is employed as training drug, its stimulus effects are mediated mainly by serotonin-induced and, to a lesser extent, catecholamine-induced actions. When MDPV is administered as test drug, only partial MDMA-like generalization occurs because MDPV has the catecholamine component but is mostly devoid of the serotonin component, which precludes the occurrence of complete stimulus generalization. However, when the stimulus effects of MDMA are made more stimulant-like (as in the Harvey and Baker, 2016 study), complete generalization (substitution) occurs to MDPV. Thus, while human traffickers of MDPV may try to promote it as a substitute for MDMA (Ecstasy), experienced users of MDMA will likely identify it as having some (stimulant-like) effects of MDMA but the “empathogenic” component of MDMA will not be experienced.
6. CONCLUSION
MDPV and α-PVP have become notorious drugs of abuse in the past several years. Although both agents had been previously reported in the patent literature with valid therapeutic claims, for one reason or another they were never really exploited by pharma. However, these early findings were, seemingly, recently exploited by clandestine chemists. This led to a flurry of activity to better understand the actions and mechanism of action of what are now termed synthetic cathinones.
The nomenclature of the synthetic cathinones has presented a problem. Various chemical terms have been used to describe them both in the patent and scientific literature. Certain synthetic cathinones have roots that are 100 years old; however, the term cathinone only appeared 40 years ago and the term synthetic cathinone is even more recent (Glennon, 2014). Today, it is recognized that, depending on the substituents present in the molecule, synthetic cathinones can act either as releasing agents (i.e., substrates) or re-uptake inhibitors (i.e., blockers) at DAT, NET, and/or SERT. Current studies are aimed at understanding the structure-activity relationships required for these actions and selectivity.
Synthetic cathinones undergo a variety of routes of metabolism. Those agents possessing an extended α side chain (e.g. MDPV and α-PVP) or an amine moiety in the form of a pyrrolidine (e.g. MDPV and α-PVP) form a greater number of metabolites than agents with a shorter side chain or a simpler amine; the presence of aromatic substituents, such as the 3,4-methylenedioxy ring found in MDPV, results in additional metabolites. The contribution of metabolites of synthetic cathinones to their abuse-related actions or side effects remains largely unexplored.
The behavioral effects of certain synthetic cathinones have been extensively investigated; the behavioral actions of many others have not. Apart from methcathinone itself, two of the best investigated synthetic cathinones are MDPV and α-PVP. Overall, the effects of MDPV and α-PVP are not unlike those of the well-known stimulants S(+)methamphetamine and cocaine and, as such, the continuum of dose related behavioral effects associated with the latter agents may also have translational relevance to the human use/abuse of these newer drugs of abuse. Thus, low doses of these agents might tend to induce euphoria and increased attention and faster/improved performance on cognitive tasks, but increased doses and/or chronic administration may lead to symptoms of behavioral decay such as severe anxiety, obsessive thoughts, aggressive behavior, paranoia and eventual entry into stimulant-induced psychosis characterized by delusions/hallucinations. MDPV and α-PVP produce effects in animals that clearly indicate an abuse potential.
Acknowledgments
Work from the author’s laboratory was supported by U.S. Public Health Services grants DA-033930.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015a;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology. 2015b;232:1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman M. Flakka becoming more popular than cocaine in South Florida. The Fix, June 15, 2015. 2015 and online at: https://www.thefix.com/content/flakka-becoming-more-popular-cocaine-south-florida.
- Adorjan; Boehringer Ingelheim GmbH. α-Aminocétones comportant un groupe amino hétérocyclique. FR5502. French patent. 1967 Dec 4;
- Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA. Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects. Addict Biol. 2014 doi: 10.1111/adb.12201. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer Ingelheim GmbH. α-Substituted ketones and processes for their preparation. GB 1149366. British Patent. 1969 Apr 23;
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014a;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Runyon SP, Hassler C, Glennon RA, Negus SS. Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats. Eur J Pharmacol. 2014b;743:98–105. doi: 10.1016/j.ejphar.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Solis E, et al. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Abraham P, et al. Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for cocaine addiction. J Med Chem. 2009;52:6768–6781. doi: 10.1021/jm901189z. [DOI] [PubMed] [Google Scholar]
- Cho AK, Kumagai Y. Metabolism of amphetamine and arylisopropylamines. In: Cho AK, Segal DS, editors. Amphetamine and its Analogs. Psychopharmacology, Toxicology, and Abuse. Academic Press; San Diego: 1994. pp. 43–77. [Google Scholar]
- Collier GH, Johnson DF, CyBulski KA, McHale CA. Activity patterns in rats (Rattus norvegicus) as a function of the cost of access to four resources. J Comp Psychol. 1990;104:53–65. doi: 10.1037/0735-7036.104.1.53. [DOI] [PubMed] [Google Scholar]
- DEA (Drug Enforcement Administration) 3,4-Methylenedioxypyrovalaerone (MDPV) (Street Names: “bath salts,” “Ivory Wave,” “plant fertilizer, “ “Vanilla Sky,” “Energy-1”) 2013 May; [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, et al. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T, Fukumori N, Tanaka T, Kubo Y, Ogata A, Uehara S, Honda Y, Kodama T. Microdialysis study of drug effects on central nervous system. Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone Ann Rep Tokyo Metrop Inst Public Health. 2007;58:287–292. [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “Bath Salt” constituent 3,4-Methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther. 2016 doi: 10.1124/jpet.115.229500. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharmacol Exp Ther. 2015;354:103–110. doi: 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Adv Pharmacol. 2014;69:581–620. doi: 10.1016/B978-0-12-420118-7.00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. Wiley; Hoboken: 2011. [Google Scholar]
- Grapp M, Sauer C, Vidal C, Muller D. GC-MS analysis of the designer drug α-pyrrolidinovalerophenone and its metabolites in urine and blood in an acute poisoning case. Forensic Sci Int. 2016;259:e14–e19. doi: 10.1016/j.forsciint.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Ator NA. Benzodiazepine self-administration in animals and humans: a comprehensive literature review. NIDA Res Monogr. 1980;33:22–36. [PubMed] [Google Scholar]
- Harvey EL, Baker LE. Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine + MDMA mixture. Psychopharmacology. 2016;233:673–680. doi: 10.1007/s00213-015-4142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffe W. CH 395998. Dr A Wander A.G; Bern, Switzerland: Verfahren zur Herstellung von α-Pyrrolidino-valerophenonen. 1966 Jan 14;
- Heffe W. CH 401054. Dr A Wander A.G; Bern, Switzerland: Verfahren zur Herstellung von α-Pyrrolidino-valerophenonen. 1966 Apr 30;
- Holliday AR, Morris RB, Sharpley RP. Compound 84/F 1983 compared with amphetamine and placebo in regard to effects on human performance. Psychopharmacologia. 1964;6:192–200. doi: 10.1007/BF00404009. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Numazawa S. New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci. 2014;39:1–6. doi: 10.2131/jts.39.1. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. 3,4-Methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol Biochem Behav. 2014;126:163–169. doi: 10.1016/j.pbb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 2015a;146:116–119. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL. Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav. 2015b;137:16–22. doi: 10.1016/j.pbb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacology. 2015;40:436–445. doi: 10.1038/npp.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Sakloth F, Jain AD, et al. Structural modification of the designer stimulant α-pyrrolidinovalerophenone (α-PVP) influences potency at dopamine transporters. ACS Chem Neurosci. 2015a;6:1726–1731. doi: 10.1021/acschemneuro.5b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci. 2015;6:771–777. doi: 10.1021/acschemneuro.5b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppe H. 1-(3′4′-Methylenedioxy-phenyl)-2-pyrrolidino-alkanones-(1) 3,478,050. U S Patent. 1969 Nov 11;
- Köppe H, Ludwig G, Zeile K. Verfahren zur Herstellung von α-Aminoketonen mit heterocyclischer Aminogruppe. DE1545591. German patent. 1969 Aug 7;
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough B, Partilla EJS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Matsuta S, Shima N, Kamata H, Kakehashi H, Nakano S, Sasaki K, Kamata T, Nishioka H, Miki A, Katagi M, Zaitsu K, Sato T, Tsuchihashi H, Suzuki K. Metabolism of the designer drug α-pyrrolidinobutiophenone (α-PBP) in humans: identification and quantification of the phase I metabolites in urine. Forensic Sci Int. 2015;249:181–188. doi: 10.1016/j.forsciint.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, et al. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL. Age dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev Psychobiol. 2014;56:943–954. doi: 10.1002/dev.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J Mass Spec. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- Michaelis W, Russel JH, Schindler O. Metabolism of pyrovalerone hydrochloride. J Med Chem. 1970;13:497–503. doi: 10.1021/jm00297a036. [DOI] [PubMed] [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL. Discriminative stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend. 2015;149:280–284. doi: 10.1016/j.drugalcdep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreira N, Erratico C, Kosjek T, van Nuijs AL, Heath E, Neels H, Covaci A. In vitro Phase I and Phase II metabolism of α-pyrrolidinovalerophenone (α-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal Bioanal Chem. 2015;407:5803–5816. doi: 10.1007/s00216-015-8763-6. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer C, Peters FT, Haas C, Meyer MR, Fritschi G, Maurer HH. New designer drug alpha-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom. 2009;44:952–964. doi: 10.1002/jms.1571. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylene-N-methylcathinone (methylone) in male rats. Psychopharmacology. 2015 doi: 10.1007/s00213-015-4057-0. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger E. Verfahren zur Herstellung von α-Pyrrolidinoketonen und deren Salzen. 1,161,274. German patent. 1964 Jan 16;
- Seeger E. US 3287217. Boehringer Ingelheim GmbH, Biberach an der Riss; Germany: Compositions and methods for stimulating the central nervous system and increasing the blood pressure. 1966 Nov 22;
- Seeger E. US 3314970. Boehringer Ingelheim GmbH, Biberach an der Riss; Germany: α-Pyrrolidino ketones. 1967 Apr 18;
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Shima N, Katagi M, Kamata H, Matsuta S, Sasaki K, Kamata T, Nishioka H, Miki A, Tatsuno M, et al. Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol. 2014;32:59–67. [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer D, Fritschi G, Maurer HH. Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry. J Chromatog B. 2003a;796:253–266. doi: 10.1016/j.jchromb.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Springer D, Fritschi G, Maurer HH. Metabolism and toxicological detection of the new designer drug 3,4-methylenedioxy-α-pyrrolidinopropiophenone studied in urine using gas chromatography-mass spectrometry. J Chromatog B. 2003b;793:377–388. doi: 10.1016/s1570-0232(03)00350-7. [DOI] [PubMed] [Google Scholar]
- Strano-Rossi S, Cadwallader AB, de la Torre X, Botrè F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/ quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spect. 2010;24:2706–2714. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- Stille H, Ackermann E, Eichenberger E, Lauener H. Vergleichende pharmakologische Untersuchung eines neuen zentralen Stimulans, 1-p-Tolyl-1-oxo-2-pyrrolidino-n-pentan-HC1. Arzneim Forsch Arzneimittel-Forsch. 1963;18:871–877. [PubMed] [Google Scholar]
- Storrs C. What is flakka (aka gravel) and why is it more dangerous than cocaine? Special to CNN May 26, 2015, and online. 2015 http://www.cnn.com/2015/05/26/health/flakka-gravel-illegal-drugs/
- Karl Thomae. GB 933507. Dr Karl Thomae GmbH, Biberach an der Riss; Germany: GmbH α-Pyrrolidino-ketones. 1963 Aug 8;
- Tyrkkö E, Pelander A, Ketola RA, Ojanperä I. In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem. 2013;405:6697–6709. doi: 10.1007/s00216-013-7137-1. [DOI] [PubMed] [Google Scholar]
- Uralets V, Rana S, Morgan S, Ross W. Testing for designer stimulants: metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol. 2014;38:233–241. doi: 10.1093/jat/bku021. [DOI] [PubMed] [Google Scholar]
- Van Abeelen JHF. Mouse mutants studied by means of ethological methods I. Ethogram. Genetica. 1963;34:79–94. [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation: Animal models of drug addiction. New York: Humana Press; 2011. [Google Scholar]
- Wander A., Dr GB 927475. Dr. A. Wander S.A; Bern, Switzerland: SA α-Pyrrolidino-valerophenones. 1963 May 29;
- Watterson LR, Burrows BT, Hernandez RD, Moore KN, Grabenauer M, Marusich JA, Olive MF. Effects of α-pyrrolidinopentiophenone and 4-methyl-N-ethylcathinone, two synthetic cathinones commonly found in second-generation “bath salts,” on intracranial self-stimulation thresholds in rats. Int J Neuropsychopharmacol. 2014;18:1–7. doi: 10.1093/ijnp/pyu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Anti-Doping Agency. List of Prohibited Substances and Methofs. Cathinone and its analogues (e.g. mephedrone, methedrone, α-pyrrolidinovalerophenone) 2016 http://list.wada-ama.org/list/s6-stimulants/cathinone-and-its-analogues-e-g-mephedrone-methedrone-%CE%B1-pyrrolidinovalerophenone/