Abstract
Polyethylene glycol (PEG) and related polymers are often used in the solubilization and noncovalent functionalization of carbon nanomaterials by sonication. For example, carbon nanotubes are frequently sonicated with PEG-containing surfactants of the Pluronic® series or phospholipid-PEG polymers to noncovalently functionalize the nanotubes. However, PEG is very sensitive to degradation upon sonication and the degradation products can be toxic to mammalian cells and to organisms such as zebrafish embryos. It is therefore useful to have a simple and inexpensive method to determine the extent of potential PEG sonolysis, as described in this chapter. Intact PEG polymers and degraded fragments are resolved on sodium dodecyl sulfate polyacrylamide gels by electrophoresis and visualized by staining with barium iodine (BaI2). Digitized images of gels are acquired using a flatbed photo scanner and the intensities of BaI2-stained PEG bands are quantified using ImageJ software. Degradation of PEG polymers after sonication is readily detected by the reduction of band intensities in gels compared to those of non-sonicated, intact PEG polymers. In addition, the approach can be used to rapidly screen various sonication conditions to identify those that might minimize PEG degradation to acceptable levels.
Keywords: Polyethylene glycol, Pluronic®, Phospholipid-PEG, Sonication, SDS-PAGE, Carbon nanotubes, Functionalization, Nanotoxicity
1 Introduction
Polyethylene glycol (PEG) and related polymers are often used as dispersants in the preparation of carbon nanotubes and other nanomaterials (NMs) for use in aqueous environments. Various PEG constructs are also used in the covalent or noncovalent functionalization of NMs, often to provide functionalities for appending specific ligands to the NMs for biomedical applications. It is well known that ultrasound sonication involved in the dispersion and functionalization processes generates cavitation bubbles that collapse, producing high local heat, pressures, and shear forces [1–3]. In addition, sonication in aqueous media is capable of splitting water into hydrogen atoms and hydroxyl radicals that combine to produce H2O2 and free radicals. Both sonolytic damage and free radical attacks on polymers have been proposed to contribute to the degradation of PEG [4–6], a lipid–PEG conjugate [7], and PEG-containing block copolymers of the Pluronic® series (also known as poloxamers) [8, 9]. Results of our recent studies [10, 11] further demonstrate that the sonolysis of PEG and PEG-containing polymers, including Pluronic® F-68 and F-127 (also known as poloxamer 188 and 407) and phospholipid-PEG (PL-PEG) constructs, is greatly affected by the ultrasound power, frequencies, and sonication times. Moreover, sonolysis of Pluronic® surfactants generates degradation products that are highly toxic to cultured cells and zebrafish embryos after probe or bath sonication [10, 12]. Thus, it is crucial to monitor polymer integrity after sonication under the specific experimental conditions used to determine whether structural alterations in polymer structure have occurred and whether potentially toxic byproducts have been generated. Described here is a simple and inexpensive approach to rapidly detect sonolytic damage to PEG polymers that does not require expensive instrumentation such as a mass spectrometer. The first step in the method outlined here is to prepare a sample of sonicated PEG under conditions anticipated to degrade the polymer. The sonicated PEG provides a positive control of degraded material while non-sonicated PEG provides a negative control of intact material. The second step involves sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) that is modified to resolve the intact PEG material and its degraded fragments in the gel [13]. After electrophoresis, PEG bands in the gel are stained with BaI2 [14]. An inexpensive flat-bed photo scanner capable of producing uncompressed image files with high resolution is sufficient to convert a SDS-PAGE gel with BaI2-stained PEG bands into a digital image file. Finally, the relative amounts of intact or degraded PEG in the gel are quantified using the free ImageJ software. The SDS-PAGE plus BaI2 staining method is now routinely embedded in our studies of nanotoxicology and cancer therapy research where sonication of various types of PEG-containing polymers is used [10–12].
2 Materials
PEG solution: 0.2 mM PEG8000 in water. Weigh 0.16 g polyethylene glycol (average molecular weight 8000) and dissolve in 80 mL MilliQ water. Add water to 100 mL, filter through 0.22 μM membrane and store at 4 °C.
Resolving gel buffer: 1.5 M Tris–HCl, pH 8.8. Weigh 18.16 g Tris base and dissolve in 75 mL MilliQ water. Adjust pH to 8.8 with 1 M HCl and add water to 100 mL. Store at room temperature for up to a year.
Stacking gel buffer: 0.5 M Tris–HCl, pH 6.8. Weigh 7.88 g Tris–HCl and dissolve in 75 mL MilliQ water. Adjust pH to 6.8 with 1 M NaOH and add water to 100 mL. Store at 4 °C.
40 % acrylamide: 40 % (w/v) acrylamide–bis-acrylamide (mix ratio 29:1). Store at 4 °C (see Note 1).
10 % (w/v) sodium dodecyl sulfate (SDS) in water. Weigh 10 g SDS (see Note 2) and dissolve in 80 mL MilliQ water. Add water to 100 mL. Store at room temperature.
10 % (w/v) ammonium persulfate (APS) in water. Weigh 1 g APS and dissolve in 8 mL MilliQ water. Add water to 10 mL. Store at 4 °C
TEMED: N,N,N,N′-tetramethyl-ethylenediamine. Store at 4 °C.
Isobutanol: water-saturated isobutanol. Measure 80 mL isobutanol into a bottle, add 20 mL MilliQ water. Mix vigorously, and then allow the two layers to separate without further disturbance (see Note 3).
1.5 M Tris–HCl, pH 6.8, dissolved in water. Weigh 23.64 g Tris–HCl and dissolve in 75 mL MilliQ water. Adjust pH to 6.8 with 1 M NaOH, add water to 100 mL, and store at 4 °C.
Electrophoresis buffer: 0.025 M Tris base, 0.192 M glycine, 0.01 % (w/v) SDS in MilliQ water. Make 1 L of 10× concentrated stock buffer by dissolving 30 g Tris base, 144 g glycine, and 10 g SDS in 800 mL MilliQ water, and then add water to 1 L. Store at room temperature. Dilute 100 mL 10× stock buffer with 900 mL MilliQ water to make 1 L working buffer before use.
0.1 % (w/v) bromophenol blue (BPB) dye solution: Dissolve 0.1 g BPB in 100 mL water and store at 4 °C.
SDS sample loading buffer (2×): 0.125 M Tris–HCl (pH 6.8), 4 % (w/v) SDS, 10 % (v/v) β-mercaptoethanol, 0.01 % (w/v) BPB, 20 % (v/v) glycerol. To make 10 mL add 0.83 mL of 1.5 M Tris–HCl (pH 6.8), 0.4 g SDS, 1 mL β-mercaptoethanol, 1 mL of 0.1 % (w/v) BPB, and 2 mL glycerol to 5 mL MilliQ water. Mix well and store in 1 mL aliquots at 4 °C.
Gel fixing solution: 50 % methanol, 10 % acetic acid in MilliQ water. To make 1 L, measure 100 mL acetic acid into a 1 L bottle, add 500 mL methanol and 400 mL MilliQ water, mix well and store at room temperature.
BaCl2 solution: 5 % (w/v) barium chloride in water. Weigh 5 g BaCl2 and dissolve in 80 mL MilliQ water. Add water to 100 mL and store at room temperature in an air tight bottle (see Note 4).
Iodine solution: 0.05 M (10 % v/v) I2 in water. Measure 10 mL iodine stock solution (as received at 0.5 M concentration) and add to 90 mL MilliQ water in a glass bottle. Wrap the bottle with aluminum foil to protect it from light and store at room temperature.
ImageJ 1.50b (available as of September 2015, see Note 5) on a Windows 7 computer with Java 1.6.0_20 (64-bit) installed.
3 Methods
3.1 Preparation of PEG Sonolysis Positive Control Samples
The following procedures describe sonication conditions using a bath sonicator in a 4 °C cold room.
Rinse a glass vial and cap (27.25 × 57 mm screw thread vial with rubber lined cap) three times with MilliQ water, place in a metal autoclave basket covered loosely with aluminum foil and bake at 200 °C for 2 h to destroy potential endotoxin contaminants (see Note 6). Allow the glass vial and cap to cool down to room temperature before use.
Fill the glass vial halfway with 10 mL of 0.2 mM PEG solution, and chill to 4 °C prior to sonication (see Note 7).
Prechill the bath sonicator and the cooling coil that connects to a refrigerated water bath circulator to 4 °C in a cold room (see Note 8).
Fill the bath sonicator tank with 1400 mL of 4 °C distilled water, just below the maximum water level marked inside the tank. Hang the cooling coil into the tank, on the inner sidewall, such that the coil is immersed in the water bath.
Secure the glass vial on a rack that hangs over the sonicator tank such that the vial is suspended in a central position in the sonication bath and the PEG solution in the vial is below the water bath level (see Note 9). Use only one vial at a time and place it in the same position each time, as changing location can change the power delivered to the vial.
Operate the sonicator at 37 kHz frequency (see Note 10) and 100 % power output level that corresponds to 120 W specified by the manufacturer (see Note 11).
Collect a 100 μL aliquot of PEG sample from the vial at 15, 30, 45, 60, and 90 min of sonication. Terminate the sonication at 120 min.
Store all sonicated and non-sonicated PEG samples at 4 °C for later use.
3.2 Resolving PEG by SDS-PAGE
Skip to step 9 if using pre-cast SDS-PAGE gels. The procedures described below in steps 1–9 are for casting multiple standard 4 % stacking, 15 % resolving SDS-PAGE mini-gels using a commercial gel caster system.
A gel sandwich consists of a single glass plate, a single alumina plate, and the gel between them. Assemble a stack of multiple gel sandwiches using two 1.5 mm spacers flanked between one 8 cm × 10 cm glass plate and one alumina plate of the same size for each gel (see Note 12). Place gel sandwiches in gel caster (see Note 13), ensure the glass plate of each sandwich faces the front. Secure caster faceplate onto the caster using clamps on both sides (see Note 14). Insert a gel comb into the first gel sandwich facing the front; make a mark at 1 cm below the bottom of the comb teeth on the caster faceplate to help judge how much acrylamide solution to pour into the gel later, and then remove the comb.
Prepare 10 mL of 15 % acrylamide resolving gel solution for each gel by combining the following reagents in order in a 50-mL disposable plastic conical centrifuge tube: 3.75 mL MilliQ water, 2.5 mL of 1.5 M Tris–HCl (pH 8.8), 3.75 mL of 40 % acrylamide, and 100 μL of 10 % SDS. Mix by gentle inversions. Add 100 μL of 10 % ammonium persulfate and 5 μL of TEMED, mix by gentle inversion and immediately pour about 8 mL of the resolving gel mixture into a gel sandwich (the openings created by gaps between spacers) by draining along one side of the sandwich to avoid bubble formation. Fill the sandwich to the 1-cm mark drawn on the faceplate, gently rock the caster from side to side and tap the bottom of the caster against the bench top to allow air bubbles to rise up to the top, and gently overlay each gel with 400 μL of water-saturated isobutanol (see Note 15).
Let the caster stand until the resolving gel has completely polymerized, which usually takes about 30 min (see Note 16).
Decant the isobutanol, gently fill the caster with MilliQ water using a squirt bottle, then decant the water and invert the caster on a paper towel to drain out water (see Note 17).
Prepare 5 mL of 4 % acrylamide stacking gel solution for each gel by combining the following reagents in order in a 50-mL disposable plastic conical centrifuge tube: 3.15 mL water, 1.25 mL of 0.5 M Tris–HCl (pH 6.8), 0.5 mL of 40 % acrylamide, and 50 μL of 10 % SDS. Mix by gentle inversions. Add 50 μL of 10 % ammonium persulfate and 5 μL of TEMED, mix by gentle inversion as before and immediately pour onto the resolving gel, allowing excess stacking gel mixture to overfill the sandwich, scrape off bubbles on the top.
Quickly insert a 10-well gel comb (1.5 mm thick) into the gel sandwich, at a slight angle to prevent trapping air, and allow the comb sides to rest on the top of the spacers (see Note 18). Insert the remaining combs from back to front sandwiches in the same manner rather quickly before the gel polymerizes (see Note 19).
Let the stacking gel stand until polymerization is complete, which usually takes ~60 min.
Using a razor blade, detach the stack of gel sandwiches from the caster and separate them into individual gel sandwiches. Take care to keep the comb and spacers between the glass and alumina plates in place. Scrape excess gel from front and back of plates, spray with MilliQ water and wrap the gel sandwiches in plastic wrap, lay flat in a container with air-tight lid and store at 4 °C for later use (see Note 20).
Install a gel sandwich containing a 4 % stacking, 15 % resolving SDS polyacrylamide gel on a gel electrophoresis apparatus, glass plate facing the front, fill the reservoirs with electrophoresis buffer, and remove the comb (see Note 21).
Put 10 μL of sonicated or non-sonicated 0.2 mM PEG sample in a microcentrifuge tube. Add 10 μL of 2 × concentrated SDS sample loading buffer to each tube and mix the contents.
Heat the sample in a water bath or a heating block at 100 °C for 3 min.
Centrifuge the heated samples at 1000 × g for 10 s to bring down any condensate on the sides and caps of the tubes.
Load samples into the gel well (20 μL of heated sample per lane) by using a fine-tipped long gel-loading pipette tip to layer samples onto the well underneath the buffer. Electrophorese at a constant voltage of 100 V (see Note 22) until the blue dye front (from the BPB dye in the samples) has reached 0.5–1 cm from the bottom of the gel. This will take about 2 h (see Note 23).
Following electrophoresis, detach the gel sandwich from the electrophoresis apparatus, gently loosen and slide away both spacers, and pry the gel plates open with a small spatula. While the gel adheres to the alumina plate, use a razor blade to cut through the gel along the interface between the stacking and resolving gels, and discard the stacking gel. Carefully lift the resolving gel and immerse it into a tray of water (see Note 24).
3.3 BaI2 Staining of PEG Bands
Rinse the gel with distilled water and soak it in 100 mL of gel fixing solution for 5 min. Slosh the solution over the gel gently on a shaker.
Decant the fixing solution carefully; save it in a glass bottle and store at room temperature for reuse up to five times. Rinse the gel with distilled water, and soak it in 100 mL of BaCl2 solution for 10 min on a shaker.
Decant the BaCl2 solution carefully; save the BaCl2 solution for reuse up to five times. The color of the blue dye near the bottom of the gel will fade with staining and a horizontal strip of the gel near the dye front will turn white while the rest of the gel remains semi-transparent. Rinse the gel again with distilled water, and then soak it in 100 mL of 5 % iodine solution for 5 min or until dark brown bands appear in the gel (see Note 25). Longer exposure to iodine leads to darker background color.
Decant the iodine solution carefully back into a glass bottle (see Note 26); it can be reused multiple times until the color of the iodine solution becomes lighter. Remove excess iodine staining in the gel background by soaking the gel in distilled water while gently shaking, and replace with clean water when the water color turns brown. Repeat the de-staining with water multiple times until the PEG bands in the gel are readily visible and the rest of the gel is clear of any yellowish background stain (see Note 27). Cover the container in which the gel is soaking with a lid to protect the gel from incident light (see Note 28).
3.4 Scanning BaI2-Stained SDS-PAGE Gels Using a Flat-Bed Photo Scanner
Configure the flat-bed scanner such that the gel is scanned using the highest pixel density (600 pixels per inch or greater), highest color-depth (a 16-bit Red Green Blue color palette or greater), and the image file is saved in an uncompressed TIFF (Tagged Image File Format) format (see Note 29). Also, a preview feature in the software is important to restrict scanning to only the area that contains the gel, otherwise the image files become substantially larger than a regular photo or document file.
Lay a clean and dry sheet of write-on transparency film on the scanner bed. Rotate and/or flip the gel in the water tray to the proper orientation before carefully lifting it out and laying it down slowly on the transparent sheet. Avoid trapping air bubbles underneath the gel. Do not push or pull the gel once it is laid flat on the sheet. Lift the sheet and rotate it slightly to position the gel straight on the scanner bed. Fill the sample loading wells with water to minimize shadows casted from the gel walls on the sides of the wells. Add a few drops of water on the gel, if necessary, to prevent dehydration. Gently lay another transparent sheet on top of the gel; from side-to-side to avoid trapping bubbles. Place a few sheets of crisp white paper over the transparent sheet and close the scanner lid before starting the scan.
At the preview step, crop the scan area to include only the gel or a portion of the gel of interest. Inspect the preview image to make sure air bubbles and water marks are absent. Also, rotate the preview image to match the correct orientation of the gel.
After scanning, preserve the gel by wrapping in plastic wrap (see Note 28), store flat at 4 °C protected from light, or discard it.
3.5 Quantifying PEG Band Intensity Using ImageJ Software
The following procedures outline only the essential steps required for quantifying BaI2-stained PEG band intensities, as shown in Fig. 1.
Fig. 1.
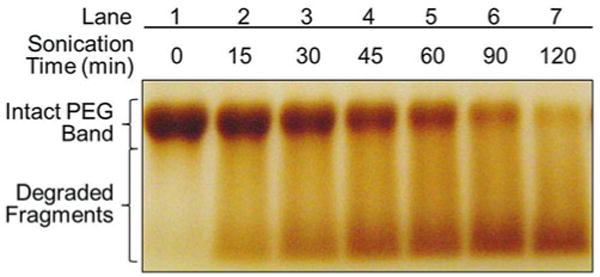
Representative sonolysis of polyethylene glycol as a function of bath sonication time resolved by SDS-PAGE with BaI2 stain. PEG 8000 at a concentration of 0.2 mM was sonicated using an ultrasonic bath operated at 37 kHz and 120 W. A representative gel was loaded with 10 μL of PEG samples acquired after bath sonication for different times. Intact PEG polymers and degradation products in the sonicated and non-sonicated PEG samples were resolved by SDS-PAGE followed by BaI2 staining
Launch ImageJ software (see Note 5).
Open Fig. 1 image file using File → Open in ImageJ.
- Crop the horizontal section of the gel marked ‘Intact PEG Band’ (see Note 30):
- Choose
 the Rectangular Selections Tool from the ImageJ toolbar.
the Rectangular Selections Tool from the ImageJ toolbar. - Draw a rectangle around the ‘Intact PEG Band’ section across the gel (see Note 31).
- Crop the selected area using Image → Crop (see Note 32).
- Save the cropped ‘Intact PEG Band’ gel section image using File → Save as → Tiff, and give it a File_name.tiff, then hit Save.
- The gel analysis routine requires the image to be a grayscale image.
- Convert the cropped RGB image to grayscale using Image → Type → 32-bit.
- Save the grayscale image using File → Save as Tiff, and give it a File_name.tiff, then hit Save.
If needed, the brightness and contrast of the grayscale image can be adjusted (see Note 33).
-
To acquire the total pixel intensity of the intact PEG bands before and after sonication, a rectangular area around the band is selected in each lane where the pixel intensity profile within a rectangle is plotted:
- Choose
 the Rectangular Selections Tool from the ImageJ toolbar.
the Rectangular Selections Tool from the ImageJ toolbar. - After drawing the rectangle over the band in first lane, press the “1” key on the keyboard or go to Analyze → Gels → Select First Lane to set the rectangle in place. The non-sonicated PEG band in lane #1 will now be highlighted and have a “1” marked in the middle of it (see Note 35).
- Use the mouse to click and hold in the middle of the “1” rectangle on the first lane and drag it over to the next lane on the right, where the 15-min sonicated sample was loaded. Center the rectangle over the lane left-to-right, but don’t worry about lining it up perfectly on the same vertical axis. ImageJ will automatically align the rectangle on the same vertical axis as the first rectangle.
- Press the “2” key on the keyboard or go to Analyze → Gels → Select Next Lane to set the current rectangle in place. The 15-min sonicated PEG band in lane #2 will now be highlighted and have a “2” marked in the middle of it, as shown in Fig. 2.
- Repeat these steps for the rest of bands in lanes #3 to #7, in order from left to right, on the gel. Make sure to press the “2” key each time (not to press 3, 4, 5 …) to set the rectangle in place (see Note 36).
- Save the grayscale gel image with rectangles set around the bands, shown as Fig. 2, using File → Save as → Tiff, and give it a file name such as PEG_sonolysis_bands.tiff, then hit Save.
- After all the rectangles are set in place around every band, press the “3” key or go to Analyze → Gels → Plot Lanes to generate an intensity profile plot of each lane in the gel (see Note 37).
- Save the intensity profile plots of the gel, shown as Fig. 3, using File → Save as → Tiff, and give it a file name such as Plots_PEG_sonolysis_bands.tiff, then hit Save.
Each profile plot section in Fig. 3 represents the relative density of the contents of the band over each lane. The sections are arranged top to bottom on the profile plot, corresponding to the lanes from left to right in the gel. Because there were seven lanes selected in Fig. 2, there are seven sections in the profile plot shown in Fig. 3. Scroll the mouse wheel up-and-down to see the profile plot corresponding to each lane from left-to-right in the gel image.
The peaks in the profile plots correspond to the dark bands in the grayscale gel image. Higher peaks represent darker bands. Wider peaks represent bands that cover a wider size range (thicker bands) on the original gel.
- Images of real gels are likely to have some background signal, so the peaks don’t reach down to the baseline of the profile plot and appear to float above the baseline of the profile plot. It will be necessary to close off the peak before its size is measured:
- Choose
 the Straight Line selection Tool from the ImageJ toolbar.
the Straight Line selection Tool from the ImageJ toolbar. - For each peak you want to analyze in the profile plot, draw a line across the base of the peak to enclose the peak (see Note 38).
- After each peak has been closed off at the base, select
 the Wand Tool from the ImageJ toolbar.
the Wand Tool from the ImageJ toolbar. - Move up to the top profile plot section and click inside the peak with the
 Wand Tool; the border of the selected peak area is highlighted in yellow. A “Results” window will pop up showing the measurement of the peak area. The first column in the results table shows the order of the peaks clicked with the Wand, the second column (Area) shows the peak area measurement that indicates the overall pixel intensity of the peak.
Wand Tool; the border of the selected peak area is highlighted in yellow. A “Results” window will pop up showing the measurement of the peak area. The first column in the results table shows the order of the peaks clicked with the Wand, the second column (Area) shows the peak area measurement that indicates the overall pixel intensity of the peak. - Repeat this for each peak in order going down the profile plot (see Note 39).
-
When all of the peaks have been highlighted correctly, save the results by clicking
File → Save as in the Results window. Give it a file name and file type such as Results_PEG_sonolysis_peaks.txt, in a “Text Tab delimited” format that can be opened with Microsoft Excel for data analysis (see Note 40).
Set the pixel intensity value of a control non-sonicated PEG- containing polymer as 100 % and compare the values of samples subjected to sonication for increasing time; the relative levels of sonolytic degradation that has occurred in each sample can be readily assessed.
A representative application of the method described above is shown in Fig. 4 where the sonolytic degradation of distearoyl phosphatidylethanolamine-PEG-COOH (DSPE-PEG-COOH) was detected and quantified in a sample that also contained single-walled carbon nanotubes (SWNTs). In the absence of sonication, the PEG portion of DSPE-PEG-COOH was evident in a single band that was near the dye front after BaI2 staining. Within 15 min of sonication the intensity of the band had visibly declined due to PEG sonolysis and continued to decline until it was almost absent by 360 min.
Fig. 2.
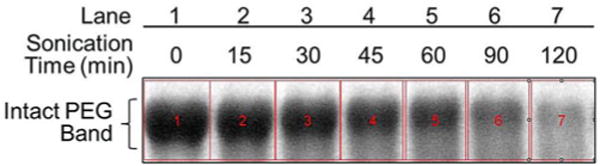
Representative grayscale gel image of intact polyethylene glycol bands with BaI2 stain. The section of the gel in Fig. 1 where intact PEG bands reside was cropped and converted to grayscale image using ImageJ software. The rectangles highlighted in red that enclose an intact, non-degraded PEG band in each lane define the areas where pixel intensity profile of the samples are analyzed and compared
Fig. 3.
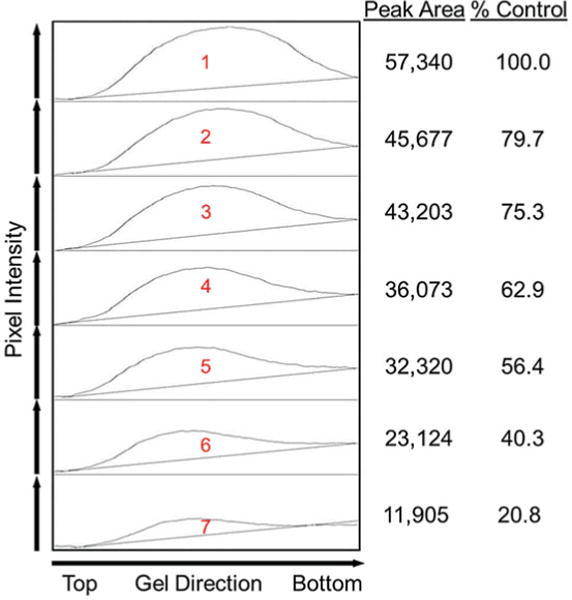
Quantifying intact PEG bands in non-sonicated and sonicated samples based on pixel intensity profile plots. On the left, the pixel intensities of the selected areas (shown as red rectangles in Fig. 2 that enclose the intact PEG band in each lane) are plotted against gel electrophoresis direction, from top to bottom. The pixel intensity of a band is quantified as the background-corrected area under the peak in the pixel intensity profile plot. In tabulated form on the right, the relative abundance of intact PEG in sonicated and non-sonicated samples is presented as peak area and as % of control where the amount of intact PEG in the non-sonicated sample in Lane 1 was set to 100 %
Fig. 4.
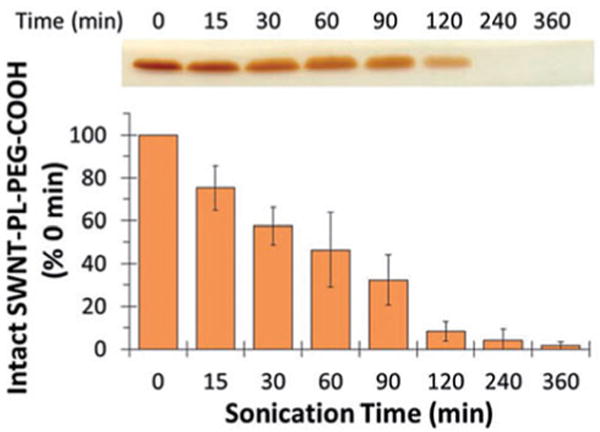
Sonolytic degradation of DSPE-PEG-COOH in the presence of SWNTs. 5 mg of SWNTs were sonicated in 10 mL of 0.35 mM DSPE-PEG-COOH for the indicated times. The samples were resolved by SDS-PAGE followed by BaI2 staining and the stained bands are shown at the top of the figure. The gels were then scanned and the band intensities quantified using ImageJ software. The data are presented as % density relative to 0 min as a function of sonication time, shown at the bottom of the figure. Reproduced from Murali et al. 2015 [11] with permission from SAGE Publications
Acknowledgments
The authors thank the University of Arizona SEMATECH/Semiconductor Research Corporation Engineering Research Center for Environmentally Benign Semiconductor Manufacturing (Grant ERC425-048), the National Cancer Institute (Grant R15-CA152917), and the National Institute for Environmental Health Sciences (Grant R15-ES023666) for supporting this work.
Footnotes
Unpolymerized acrylamide is a potent neurotoxin and is absorbed through the skin. The effects of acrylamide are cumulative. Wear gloves while working and avoid direct contact through bare skin.
SDS powder is harmful if inhaled and may cause respiratory irritation. Wear personal protective equipment, including gloves, clothing, and respiratory mask, to avoid breathing dust.
After the isobutanol and water layers settle, the upper layer will be isobutanol and the lower layer will be water; use the upper layer without disturbing the lower layer.
Barium chloride is one of the most common water-soluble barium salts. It is toxic if swallowed or inhaled. Avoid contact with skin, eyes, and clothing and avoid breathing vapors and dust.
The latest version of ImageJ software can be downloaded from the official ImageJ site at http://imagej.nih.gov/ij/, for initial installation or for upgrade. Extensive documentation related to ImageJ basic concepts, installation, tutorials and examples, macro language, and many more are all freely available [15]. In addition, ImageJ source code and extended plugins for specific applications are freely available in the public domain.
Lipopolysaccharides (LPS), also known as lipoglycans and endotoxin, are large lipid-polysaccharide molecules found in the outer membrane of gram-negative bacteria and elicit strong immune responses in animals. Inactivation of possible endotoxin contaminants is recommended when the final material is to be used with cultured cells or intact animals.
Sonolysis of PEG-containing polymers is concentration and temperature dependent.
The temperature of the sonication bath is kept below 18°C throughout the sonication period.
Sonication power level and ultrasound frequency are critical factors that influence sonolytic damage [2, 18]. There is an inverse relationship between frequency and the size of cavitation bubbles because larger bubbles generated at lower frequencies generally create more extreme heat and pressure when they implode [2, 3, 19]. Pluronic® polymers were readily degraded upon bath sonication at 37 kHz or probe sonication at 20 kHz frequency, but no apparent degradation was observed when subjected to bath sonication at 80 kHz frequency with similar power level and duration [10].
The power delivered to the sonicator bath can be determined by measuring the change in bath temperature (starting at 22°C) as a function of sonication time, at room temperature without a cooling coil [17]. This method uses the equation P = (dT/dt) × M × Cp, where P is the acoustic power (W), T is the temperature (K), t is time (s), M is the mass of water (1400 g for this bath sonicator), and Cp is the specific heat capacity of water at 298 K (4.18 J/g K). The power measured by this approach was ~80 W for this sonication system [11].
Alumina ceramic back plates have the advantage that they transfer heat 40 times more rapidly than glass plates, which facilitates cooling. However, regular glass plates can be used instead of alumina plates. Clean away any dust and gel pieces adhering to the plates from previous use. Spray a small amount of ethanol and wipe with a clean Kimwipes to remove any watermarks.
When less than the maximum number of gel sandwiches is needed, place an 8 cm × 10 cm plastic or glass space-filler behind the stack of gel sandwiches to fill up the caster completely.
The gel sandwich should not move after the caster is clamped. Add more space-filler plastic or glass plates if needed.
Oxygen inhibits polymerization of PAGE gels. Usually in preparing such gels a degassing step, using vacuum, is required; however for the small size gels used here, this step is not needed. An isobutanol layer is added on the top of each gel to keep the gel levels the same and to prevent contact of the gel with oxygen in the air. Deliver the isobutanol slowly along the spacer at the side of the sandwich. Make certain the caster is level using a bubble level.
Do NOT pour the excess acrylamide down the sink. The mixture will polymerize in the pipes and clog them. Wait until the mixture has polymerized in the 50-mL tube, then discard in hazardous trash. This excess material is also a good indicator whether the gel has polymerized properly and is ready for the next step.
The polymerized resolving gel should remain in place and inside the gel sandwiches even when the caster is upside-down.
Instead of the standard 10-well combs, 1.5-mm thick 15-well combs can also be used. More samples can be loaded in a single gel with 15 wells and the volume of each sample loaded per lane is also reduced by half (10 μL or less needed), which conserves valuable samples.
If acrylamide begins to ooze out when the first comb is inserted, it has already started to polymerize and there is no need to continue with the rest of the gels. Thus, practice quickly inserting the combs before making the stacking gel solution.
The gels will be good for up to a month if kept wet and refrigerated. Inspect the gel before use. If there are air bubbles in the gel or the gel has separated from the glass or alumina plate, discard the gel.
If possible, remove the comb after the reservoir is filled with electrophoresis buffer, then work the comb out slowly by gently rocking it side to side while pulling it out. Be cautious of collapsing wells when removing the comb. If a well(s) has collapsed, use a micropipette tip to gently lift the collapsed walls back to a standing position.
A constant current setting at 20 mA per gel is fine to use.
A pilot experiment should be performed to determine the migration of a PEG-containing polymer, intact and degraded, relative to the tracking blue dye after 1 h of electrophoresis.
Cut a small notch on one corner to mark the orientation of the resolving gel before staining.
Iodine is commonly used as a starch indicator to test for the presence of starch in foods, drugs, paper, textiles, and other products where iodine dissolved in an aqueous solution reacts with starch, resulting in an intensely purple-black colored complex.
Wrap the iodine solution bottle with foil to protect from light. Wear gloves and a lab coat to protect skin and cloth from iodine stains.
Speed up the de-staining step by using larger volumes and more frequent changes of water. Also, place the container on a shaker to agitate water over the gel, which helps to de-stain the gel more evenly.
The iodine stain will fade over time in water. The best way to preserve the stain is to wrap the gel in plastic wrap with no excess water, place it flat in a plastic bag or container together with a sheet of wet paper towel, and store at 4 °C protected from light. If the gel is left in water too long and bands becomes too faint, the gel can be re-stained with iodine until all bands are saturated with BaI2 stain again and then destain with water to remove background color.
The ability to store image data in a lossless format makes a TIFF file a useful image archive, because, unlike standard JPEG or PGN files, a TIFF file using lossless compression (or none) may be edited and re-saved without losing image quality.
The dominant bands in the gel shown in Fig. 1 correspond to the intact PEG band in lane 1 (the non-sonicated PEG sample on the far-left lane) and the sonicated bands in the other lanes (lanes 2–7) to the right.
To select the color of the rectangle go to Edit → Option → Colors, make a color selection from the list, and hit “OK”.
Instead of crop, a copy of the selected area can be created using Image → Duplicate. The copy is named in the “Title” box. Hit “OK” and a new window will open containing a copy of the selected area of the image.
- Choose Image → Adjust → Brightness/Contrast. A new window “B&C” will appear with a histogram of pixel intensity, displaying the range of pixels with the lowest intensity on the left and the highest on the right, and with corresponding values listed on the bottom of the graph. The current pixel intensity distribution of the image is shown in four sliders for the minimum, maximum, brightness, and contrast ranges.
- To make the bands look darker, by cutting off low-intensity pixels, slide the “Minimum” (top) slider to the right. This sets the bottom of the display range to a higher value than the previous display.
- To make the bands look brighter, by cutting off high-intensity pixels, slide the “Maximum” (second) slider to the left. This sets the top of the display range to a lower value than before.
- Adjusting the “Brightness” (third) slider changes both the top and the bottom intensity values displayed without changing the size of the range displayed.
- Adjusting the “Contrast” (fourth) slider changes both the top and bottom brightness values and the size of the display range.
- The “Auto” button chooses the best display range, based on a saturation of 0.35 % of the pixels in the image.
- The “Reset” button acts as an undo button and resets the image to its original (full) display range.
- The “Set” button allows the user to input values for the top and bottom of the display range with the option to propagate these values to all other images open in ImageJ.
- None of the adjustments made to the Brightness and Contrast of the image alter the histogram and the pixel intensity values of the image permanently until the “Apply” button is hit.
ImageJ assumes that the lanes run vertically (individual bands are horizontal), so the rectangle drawn should be tall and just narrow enough to enclose a single band.
To select the color and font of the labels go to Image → Overlay → Labels. A new window “Labels” will appear, in which color, font size, and other properties of the labels can be selected. Hit the “OK” button to accept the selections.
If an error has been made in setting the size or the order of the rectangles, reset the rectangles using Analyze → Gels → Reset, and start it over from the first lane.
If it is desirable to have two plots to compare side-by-side, go to Analyze → Gels → Re-plot Lanes to reset the rectangles.
This step requires some subjective judgment to decide where the peak ends and the background noise begins.
If a mistake has been made when clicking with the Wand tool, such as clicking the area outside a peak, the program still records the pixel intensity of that clicked region, and it will report that value as an entry in the Results window. A quick fix is to highlight the unwanted entry in the Results window, hit “Clear” under the “Edit” tab to erase it before clicking the next area with the Wand tool. To erase the whole set of measurements and reset the Results window counter, go to Analyze → Gel → Label Peaks, go back to the top of the profile plot, and begin clicking inside the peaks again, starting with the first peak in the first section. The Results window should clear and begin showing new values.
The values in the Results windows can be copied to a spreadsheet program, such as Microsoft Excel, by clicking Edit → Select All to highlight all the values, followed by Edit → Copy in the Results window. Go to an Excel spreadsheet, right-click and hit Paste, and the two columns of values will be transferred into Excel in the exact order.
References
- 1.Neppiras EA. Acoustic cavitation series: Part one: Acoustic cavitation: an introduction. Ultrasonics. 1984;22:25–28. [Google Scholar]
- 2.Suslick KS. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 3.Suslick KS, Flannigan DJ. Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu Rev Phys Chem. 2008;59:659–683. doi: 10.1146/annurev.physchem.59.032607.093739. [DOI] [PubMed] [Google Scholar]
- 4.Vijayalakshmi SP, Madras G. Effect of temperature on the ultrasonic degradation of polyacrylamide and poly(ethylene oxide) Polym Degrad Stab. 2004;84:341–344. [Google Scholar]
- 5.Vijayalakshmi SP, Madras G. Effect of initial molecular weight and solvents on the ultrasonic degradation of poly(ethylene oxide) Polym Degrad Stab. 2005;90:116–122. [Google Scholar]
- 6.Kawasaki H, Takeda Y, Arakawa R. Mass spectrometric analysis for high molecular weight synthetic polymers using ultrasonic degradation and the mechanism of degradation. Anal Chem. 2007;79:4182–4187. doi: 10.1021/ac062304v. [DOI] [PubMed] [Google Scholar]
- 7.Zeineldin R, Al-Haik M, Hudson LG. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9:751–757. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- 8.Koda S, Mori H, Matsumoto K, Nomura H. Ultrasonic degradation of water-soluble polymers. Polymer. 1994;35:30–33. [Google Scholar]
- 9.Watanabe T, Okabayashi M, Kurokawa D, Nishimoto Y, Ozawa T, Kawasaki H, Arakawa R. Determination of primary bond scissions by mass spectrometric analysis of ultrasonic degradation products of poly(ethylene oxide-block-propylene oxide) copolymers. J Mass Spectrom. 2010;45:799–805. doi: 10.1002/jms.1771. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Hughes T, Beck S, Vakil S, Li S, Pantano P, Draper RK. Generation of toxic degradation products by sonication of Pluronic® dispersants: implications for nanotoxicity testing. Nanotoxicology. 2013;7:1272–1281. doi: 10.3109/17435390.2012.736547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murali VS, Wang R, Mikoryak CA, Pantano P, Draper R. Rapid detection of polyethylene glycol sonolysis upon functionalization of carbon nanomaterials. Exp Biol Med (Maywood) 2015;240:1147–1151. doi: 10.1177/1535370214567615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Meredith AN, Lee MJ, Miadzvedskaya L, Braun E, Pantano P, Harper S, Draper R. Toxicity assessment and bioaccumulation in zebrafish embryos exposed to carbon nanotubes suspended in Pluronic® F-108. Nanotoxicology. 2016;10(6):689–698. doi: 10.3109/17435390.2015.1107147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Kurfürst MM. Detection and molecular weight determination of polyethylene glycol-modified hirudin by staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1992;200:244–248. doi: 10.1016/0003-2697(92)90460-o. [DOI] [PubMed] [Google Scholar]
- 15.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 16.Nascentes CC, Korn M, Sousa CS, Arruda MAZ. Use of ultrasonic baths for analytical applications: a new approach for optimisation conditions. J Braz Chem Soc. 2001;12:57–63. [Google Scholar]
- 17.Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment— issues and recommendations. Nanotoxicology. 2011;5:711–729. doi: 10.3109/17435390.2010.528846. [DOI] [PubMed] [Google Scholar]
- 18.Suslick KS, Price GJ. Applications of ultrasound to materials chemistry. Annu Rev Mater Sci. 1999;29:295–326. [Google Scholar]
- 19.Sostaric JZ, Riesz P. Adsorption of surfactants at the gas/solution Interface of cavitation bubbles: an ultrasound intensity-independent frequency effect in sonochemistry. J Phys Chem B. 2002;106:12537–12548. [Google Scholar]


