Abstract
Background
In the present study, the role and efficiency of strain elastography (SE) were evaluated in diagnosis and staging of acute appendicitis in pediatric patients.
Material/Methods
We enrolled 225 pediatric patients with suspected clinical and laboratory findings of acute appendicitis. Gray-scale sonographic findings were recorded and staging was made by the colorization method of SE imaging. Appendectomy was performed in all patients and the results of the surgical pathology were compared with the imaging findings. The sensitivity, specificity, and accuracy of SE imaging were determined in terms of evaluating the “acute appendicitis”.
Results
Sonographic evaluation revealed acute appendicitis in 100 patients. Regarding the SE analysis, cases with appendicitis were classified into 3 groups as: mild (n=17), moderate (n=39), and severe (n=44). The pathological evaluation revealed 95 different stages of appendicitis and normal appendix in 5 cases: acute focal (n=10), acute suppurative (n=46), phlegmonous (n=27), and perforated (n=12), regarding the results of surgical pathology. Five patients with pathologically proven “normal” appendix were noted as “mild stage appendicitis” based on gray scale and SE analysis. In total, when gray-scale and SE results were compared with pathology results regardless of the stage of appendicitis, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rates were 96%, 96%, 95%, 96.8%, and 96%, respectively. No statistically significant difference was detected between other groups (P<0.05).
Conclusions
In acute appendicitis, the use of SE imaging as a supportive method for the clinical approach can be useful in diagnosis, and its results are closely correlated with the histopathologic stage of appendix inflammation.
MeSH Keywords: Appendicitis, Elasticity Imaging Techniques, Pediatrics
Background
Acute appendicitis is the most frequent cause of acute pain in the lower right abdomen [1]. When diagnosis is based only on the clinical signs, the rate of false-positivity varies between 8% and 30%. Hence, clinical diagnosis alone leads to many inappropriate surgical interventions, but delay in diagnosis or a conservative approach may lead to progression of the disease [2]. Therefore, visualization methods are important in balancing these 2 approaches. The use of sonography and/or computed tomography (CT) in diagnosis markedly decreases the negative appendectomies [3]. Results of many studies have indicated high rates of accuracy of sonography and CT in the diagnosis of acute appendicitis [4–6]. However, there still exist some problems in diagnosis made by both sonography and CT. Normal appendix, periappendicular inflammation, and tip of the appendix cannot be detected in 15% of the patients [7,8]. In a study by Pickuth et al., rates of sensitivity and specificity were reported to be 87% and 74% for sonography, and 95% and 89% for CT, respectively, and this is why CT has been preferred for the detection of acute appendicitis [9]. Despite such preferences, sonography is still the primary visualization method in the evaluation of acute abdominal pain [10].
The morphological properties of the investigated tissue or lesion and its characteristics of contrast trapping can be determined by the conventional imaging techniques used in radiology. Elastography is a visualization method that measures the response of a tissue against an applied force, and thus the elasticity and stiffness of the tissue [2]. SE is a semi-static and semi-quantitative method. Hard tissues can be compressed as a whole, and they are therefore less deformable and less displaced compared to the soft tissues. The strain values of hard tissues are therefore low. The rate of displacement during the compression and decompression phase is called strain value and is indicated in the elastogram [11]. In general, hard tissues are observed in blue color and soft tissues in red color, while tissues with intermediate stiffness are observed in green color. Strain index (SI) is the ratio of the strain value of structures at the periphery of the investigated tissue to the strain value of the investigated tissue itself. The SI values of hard tissues are high because they are generally less compressible and less deformable compared to the surrounding tissues. SE is a novel visualization method, and it increases the accuracy of sonography in various challenging diagnostic situations.
We have assessed the efficacy of SE in the diagnosis and staging of inflammation in patients suspected of having appendicitis.
Material and Methods
Our study includes a total of 225 pediatric patients with ages from 1 to 18 (mean age of 13±3.8 years) who were admitted with pain in the lower right abdomen between March 2013 and May 2015. Of these patients, 103 were female and 122 were male. The teams of clinicians and radiologists were blind to the design of the study group. All participants were evaluated for their demographic data, clinical symptoms, visualization data, surgical data, and pathological reports. Written consent was obtained from the parents or from the first-degree relatives of all patients participating in the study. The study protocol of the research was reviewed and confirmed by the local ethics committee. Radiological evaluations were performed in unison and in consensus by 2 radiologists with 10- and 5-year experiences in the use of US. All analyses were performed using Hitachi (HI Vision Preirus, EZU-MT28-S1) device.
Routine gray-scale sonographic investigation was performed using a 5-mhz convex transducer, which was followed by investigation of the lower right abdomen with gradual compression technique by using a 13-mhz linear transducer. The presence of appendicolith in appendix, the widest outer diameter, wall thickness, compression state, increased echogenicities in periappendicular fluid, and the surrounding adipose tissue were recorded in all patients. In gray-scale analysis, an outer diameter of 6 mm and a wall thickness greater than 3 mm, non-compressible appendix, appendicoliths, increased periappendicular echogenicities, and associated fluid were evaluated as pathological. Following sonography, a real-time SE with gentle compression was performed to explore the increased stiffness in the appendix and surrounding tissue. All SE images were investigated in sections for the quality factor (QF) of the strain maps. Only images with QFs greater than 30 were selected for evaluation. Normal appendix was visualized as compressed or oval-shaped and without any increased stiffness in its wall (Figure 1); while inflamed appendix was visualized as a round structure with areas of increased stiffness in the appendiceal wall observed as blue regions in the strain maps (Figure 2). Periappendicular inflammation was visualized as blue regions. Stages of the inflammation regions were defined as mild, moderate, or severe according to the color-scoring system based on the distance starting from the outer appendiceal wall and including the area with abnormal stiffness. Increased stiffness was mostly observed only in the appendiceal wall and immediately in the juxta-appendicular region in mild inflammation. These are observed in regions up to 2 cm from the outer appendiceal wall in moderate inflammation. In marked inflammation, these areas are observed in regions further than 2 cm from the outer appendiceal wall (Figures 3–5). All patients fulfilling the criteria for acute appendicitis based on their gray-scale and ES analyses underwent surgery. Surgical pathology results were accepted as the criterion standard. During the surgery, the size of the appendix, inflammation-hyperemia in the wall, periappendicular inflammation/adhesion, states of the omentum and mesoappendix, and local fluid presence were recorded. The results of SE were compared with those of the surgery and pathology.
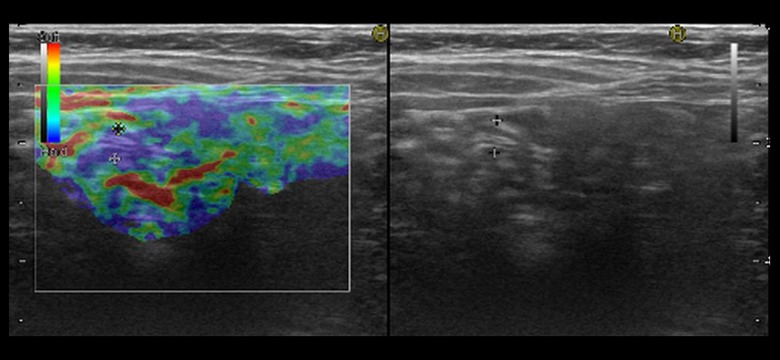
Figure 1.
Normal appendix. Normal appendix in the SE (diameter of the appendix 5.1 mm).
Figure 2.
Acute focal appendicitis. Lumen with a diameter of 5.6 mm, and a limited blue color-coding in the wall in SE.
Figure 3.
Phlegmonous appendicitis. Coding in the SE, with blue color in the central, and with green and blue colors in the periphery.
Figure 4.
Suppurative appendicitis. A markedly distended appendix in the SE, and a blue color-coding not exceeding 2 cm in the adjacent fatty tissues.
Figure 5.
Perforated appendicitis. A distended appendix with irregular margins in the SE, and a blue color-coding exceeding 2 cm in the surrounding fatty structures.
Definitive diagnosis of acute appendicitis is made by the pathological investigation of surgically extracted appendix material. In a macroscopically well-developed inflammation of the appendix, the layer of serosa has a fibrinous or purulent appearance, and the vessels become clearly apparent. Luminal obstruction by fecalith or some other agents are seen in approximately 1/3 of the cases. Microscopically, the appendiceal wall can have changes ranging from minimal focal inflammation to total necrosis. The degrees of abnormalities partially depend on the duration between the onset of symptoms and surgery. Acute appendicitis has 4 pathological stages: acute focal, acute suppurative, gangrenous (phlegmonous), and perforative [12].
Descriptive statistical data for the continuous variables are presented as the mean, standard deviation, and minimum and maximum values, while the number of cases and percentage values were used for the categorical variables. The relationships between the categorical variables were determined using the chi-square test. Statistical methods for diagnostic tests (sensitivity, specificity, accuracy) were also applied for determining the performances of the new tests. Statistical significance level was considered as 5%, and SPSS (version 20) statistical software was used for all statistical computations.
Results
In gray-scale analysis, normal appendix was visualized in a compressed state, with an outer diameter of less than 6 mm, and in SE analysis it was compressed and had mild yellow and green colors. In 125 cases, the initial physical and radiological examinations did not reveal acute appendicitis in 10 patients in whom even the appendix could not be visualized by US. At clinical follow-up, 10 of these 125 cases suggested appendicitis and surgery was then planned. In cases with acute appendicitis, the wall of the appendix was thick and non-compressed, and its diameter in most cases was greater than 6 mm. In 100 cases with suspected appendicitis, the diameter was greater than 6 mm. There were 7 cases with a diameter of 5–6 mm and normal according to the gray-scale analysis, but mild according to the color scoring in SE (Figure 1). In addition, periappendicular free fluid, increased mesenteric echogenicity, and formations of abscess were correlated with the severity of inflammation. Histopathological analysis was confirmed by the specific diagnosis of the resected tissue. In our study, appendicitis cases were classified into 3 groups based on the results of SE analysis: mild (n=17), moderate (n=39), and severe (n=44); and into 4 groups based on the surgical pathology results: acute focal (n=10), acute suppurative (n=46), phlegmonous (n=27), and perforated (n=12). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rates were 22.2%, 93.7%, 23.5%, 93.3%, and 88%, respectively, between mild appendicitis and acute focal appendicitis; 27.3%, 97.2%, 70.6%, 84.3%, and 83.3%, respectively, between mild appendicitis and acute suppurative appendicitis; 55.6%, 86%, 25.6%, 95.7%, and 83.6%, respectively, between moderate appendicitis and acute focal appendicitis; 54.5%, 91.5%, 61.5%, 89%, and 84.2%, respectively, between moderate appendicitis and acute suppurative appendicitis; 96%, 89.8%, 54.5%, 99.4%, and 90.5%, respectively, between severe appendicitis and phlegmonous appendicitis; and 100%, 84.7%, 27.3%, 100%, and 85.5%, respectively, between severe appendicitis and perforated appendicitis. No statistically significant difference was detected between other groups (Table 1). When gray-scale and ES results were compared with pathology results regardless of the stage of appendicitis, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rates were 96%, 96%, 95%, 96.8%, and 96%, respectively (Table 2).
Table 1.
Table of comparison based on SE and surgical-pathological stages.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Rate of accuracy | |
|---|---|---|---|---|---|
| HD-AFA | 22.2 | 93.7 | 23.5 | 93.3 | 88.0 |
| HD-ASA | 27.3 | 97.2 | 70.6 | 84.3 | 83.3 |
| OD-AFA | 55.6 | 86.0 | 25.6 | 95.7 | 83.6 |
| OD-ASA | 54.5 | 91.5 | 61.5 | 89.0 | 84.2 |
| AD-FA | 96.0 | 89.8 | 54.5 | 99.4 | 90.5 |
| AD-PA | 100.0 | 84.7 | 27.3 | 100.0 | 85.5 |
HD – mild; OD – moderate; AD – severe; AFA – acute focal appendicitis; ASA – acute suppurative appendicitis; FA – phlegmonous appendicitis; PA – perforated appendicitis.
Table 2.
Efficiency of US+SE in the diagnosis of acute appendicitis.
| Sensitivity | 96.0% |
| Specificity | 96.0% |
| False positivity | 4.0% |
| False negativity | 4.0% |
| Negative cut off value | 96.8% |
| Positive cut off value | 95.0% |
| Rate of accuracy | 96.0% |
Discussion
Acute appendicitis is the most common disorder that generally requires urgent abdominal surgery in the pediatric population. It is one of the major reasons of hospitalization among children. It is typically observed in older children and young adults. The incidence of acute appendicitis in children admitted to hospitals for ambulatory care with acute abdominal pain varies between 1% and 4%. Lifelong risk of acute appendicitis varies between 7–9%. Delayed diagnosis can result in perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death [13].
Due to the challenging nature of diagnosing appendicitis in the pediatric population, reported incidences of negative appendectomy are between 5% and 25%. The prevalence of appendix perforation in various pediatric series varies between 23% and 73%. The perforation rate is higher in infants than in preschoolers. Complications occur in more than 1/3 of the perforation cases. The mortality rate following acute appendicitis in the general population is approximately 1% and most of these cases are related to perforated appendicitis [14].
US is a fast and cost-effective visualization modality used in the diagnosis of acute appendicitis. When compared with the other visualization methods, US is practical, non-invasive, radiation-free, and requires minimal preparation. When compared with CT, it does not include ionizing radiation, it is more cost-effective, and enables dynamic evaluation of abdominal organs. Many studies show the high specificity and sensitivity of US in the diagnosis of acute appendicitis. Its sensitivity is reported as 85–100% and specificity as 89–98% [15].
CT provides high accuracy in the non-invasive evaluation of patients suspected of having appendicitis. The reported sensitivity rate is 88–100%, specificity is 91–99%, positive predictive value is 92–98%, negative predictive value is 95–100%, and accuracy is 94–98%. The most important advantage of CT is its ability to show the appendix, periappendicular tissue, and other intra-abdominal structures, even in extreme individuals. Therefore, when normal appendix is observed in CT, acute appendicitis is implicitly excluded by the radiologist, and when abnormal appendix is observed, the appendicitis diagnosis can be made. Its most important disadvantage is the risk of ionizing radiation, because pediatric patients are 10 times more sensitive to IR than are adults and elderly patients [16].
In a meta-analysis comparing US and CT in children and adults, the sensitivity of US in children was 88% and specificity was 94%, whereas the sensitivity of CT was 94% and specificity was 95%. Although CT has a greater sensitivity than US, the potential harm caused by radiation exposure in children should be kept in mind [17]. Exposure to ionizing radiation restrains the use of CT in pregnant women, young adults, and children [18].
Magnetic resonance (MR) is recommended as an alternative visualization method to CT in suspicion of acute appendicitis. Similar to US, MR does not involve ionizing radiation. In a study comparing non-contrast MR and US in children, the diagnostic performance of both methods in acute appendicitis was found to be close [19–21]. However, its high cost restricts the access of many patients. Magnetic resonance is costly and disadvantageous because it requires sedation.
Visualization methods revealing the inner structure of the tissues have become more frequently used in recent years. Real-time elastography has shown high sensitivity and specificity in the diagnosis of acute appendicitis through the qualitative evaluation of wall stiffness in inflamed appendicitis. Elastography is a visualization method that measures the response of a tissue against an applied force, and thus the elasticity and stiffness of the tissue. It is mostly used in combination with US due to its easy application, cost-effectiveness, time-effectiveness, and absence of harmful effects [22].
In a study by Kapoor et al. on 40 adult patients with pain in their lower right abdomen, using real-time SE, sensitivity and specificity of SE in the diagnosis of acute appendicitis were both found to be 100%, whereas sensitivity and specificity of US were found to be 88% and 100%, respectively [11]. In addition, areas of increased stiffness as a sign of periappendicular inflammation were classified into 3 stages. We based our study on the classification described by Kapoor et al. These findings were reported to be correlated with surgical findings, but pathological correlation was not studied. In our study, all US-SE findings were confirmed with surgical-pathological findings. When results of gray-scale US-SE and pathology were compared, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 96%, 96%, 95%, 96.8%, and 96%, respectively. To the best of our knowledge, our study is the first in the literature where SE findings are compared with surgical-pathological findings in acute appendicitis. More information on the ability of SE to reveal the severity and prevalence of inflammation can be obtained through studies using larger series.
Strain elastography indicates the stiffness in the tissues, defined as the change in length during compression divided by the length before compression. The stress in SE is usually applied externally either by manual compression with the transducer or by acoustic radiation force impulse (ARFI). The accuracy of strain elastography depends on the operator’s skill and experience; therefore, training and experience in acquiring strain elastograms is essential. Ultrasound elastography, using either SE or SWE, is a valid and useful tool used in addition to gray-scale and color Doppler US in thyroid evaluation, as evidenced by the literature and the EFSUMB guidelines. However, to achieve reliable SE, adequate training, suitable cutoff values for both strain and SWE, adequate equipment, and clinically appropriate examinations are necessary. Future technical developments to reduce inter-observer and intra-observer variability will be helpful. SWE appears to have the advantage of being lesser operator-dependent; in addition, the learning curve for strain elastography seems to be short [23] and has been improved significantly by the availability of real-time operator feedback. Some authors claim that SWS is an operator-independent and reproducible technique [24].
In a study by Göya et al. using acoustic radiation force impulse technique (ARFI) in the diagnosis of acute appendicitis, sensitivity and specificity of abdominal US were 83.3% and 80%, respectively, whereas these values were 100% and 98% for ARFI. Mean SW rates were 1.11 m/s in healthy appendix and 2.07 m/s in acutely inflamed appendix. ARFI imaging may be useful in guiding the clinical management of acute appendicitis by helping its diagnosis and determining the severity of appendix inflammation. When compared with our study, sensitivity and specificity values of ARFI in the diagnosis of acute appendicitis are slightly higher. This can be explained by the higher efficiency of the quantitative values obtained in ARFI than the visual classification in SE. With studies using larger series where SE and ARFI can be used in the diagnosis of acute appendicitis, correlation between sensitivity and specificity values and severity of inflammation can be better understood [25].
In a study of patients suspected of having acute appendicitis, using Shear Wave Elastography (SWE), elastic module values were statistically significantly higher in patients with acute appendicitis than in those who did not have acute appendicitis. Mean elastic module value in the appendicitis group was 25.0 kPa, in those who did not have appendicitis it was 10.4 kPa, and in healthy individuals it was 8.3 kPa. When an elastic module value ≥12.5 kPa is accepted, sensitivity and specificity of SWE in the diagnosis of acute appendicitis were 93% and 100%, respectively [26].
US cannot be used efficiently in cases with a diameter smaller than 6 mm, which constitute 15% of all appendicitis cases [14]. Appendix can be visualized in only 88% of healthy individuals. However, when the diameter is less than 6 mm, it is hard to distinguish an inflamed appendix from a healthy appendix [15]. There were 7 cases with a diameter of about 5–6 mm which could not be diagnosed in SE by US. As could be seen in our study, accurate diagnosis by color-coding method shows that appendix inflammation can be diagnosed by detecting the changes in wall stiffness using SE. This can be a useful additional criterion in confirming the clinical diagnosis of acute appendicitis, and can be even more useful in symptomatic patients with an appendix size less than 6 mm, and in cases with tip appendicitis.
SE can highly accurately and specifically diagnose acute appendicitis by qualitatively determining the wall stiffness of the inflamed appendix. SE is highly sensitive both in the diagnosis and in demonstration of the severity of inflammation [11]. In our study, we found that sensitivity of SE in surgical-pathological staging varied between 20% and 100% and was correlated with the severity of inflammation. These results suggest that staging with SE can be particularly effective in the surgical or medical approach of clinicians.
Inflammation of the periappendicular adipose tissue is seen as increased echogenicity in US analysis [17]. However, this evaluation is best performed by SE. Periappendicular inflammation is visualized as blue to dark-blue areas in SE based on the prevalence and severity of inflammation. The possibility to evaluate based on the severity increases the rate of early diagnosis and decreases the risk of complications in advanced cases. In this study, the real-time color change responses displayed by SE were used as a sensitive marker to evaluate different levels of inflammation by the peri-appendicial fat tissue. Even differentiations between acute focal appendicitis and mild appendicitis and between mild appendicitis and acute suppurative appendicitis could be made by SE, which was then confirmed by the pathological data. This means that when inflammation crosses the limits of the appendix, an evaluation closer to the pathological stage can be made.
As demonstrated in our study, the values of sensitivity and specificity increase proportionally with the severity of inflammation. It also plays an important role in the diagnosis of small-scale cases with inflamed appendix. Thus, SE visualization can be used in the evaluation of acute appendicitis in early stages as well as in advanced stages where the risk of perforation is at its highest. SE visualization can enable fast and accurate diagnosis of appendicitis in cases with low inflammation such as non-distended and tip appendicitis. In general, its diagnostic sensitivity markedly increases when used in combination with US visualization. Therefore, SE visualization enables rapid application of appropriate clinical treatment strategies.
Our study has various limitations. Some difficulties were experienced in the compliance of the pediatric patients. We did not have cases mimicking acute appendicitis such as diverticulitis, terminal ileitis, typhlitis, mesenteric lymph adenitis. In the medical center, surgeons did not prefer CT in appropriate patients and therefore comparisons could not be performed. The best advantage was that pediatric population had lower fat content than the adults and have a superficially located appendix, which enables us to make effective evaluation. Similar to standard US, elastography requires good patient cooperation.
Conclusions
SE has a high sensitivity in the diagnosis and staging of acute appendicitis, enables the staging to be correlated with the severity of pathological stage, and reveals the severity of the case to the clinician; therefore, it can contribute to shortening the treatment duration and decreasing the number of complicated advanced cases.
Footnotes
Source of support: Departmental sources
References
- 1.Birnbaum BA, Wilson SR. Appendicitis at the millennium. Radiology. 2000;215:337–48. doi: 10.1148/radiology.215.2.r00ma24337. [DOI] [PubMed] [Google Scholar]
- 2.Lane MJ, Liu DM, Huynh MD, et al. Suspected acute appendicitis: Nonenhanced helical CT in 300 consecutive patients. Radiology. 1999;213:341–46. doi: 10.1148/radiology.213.2.r99nv44341. [DOI] [PubMed] [Google Scholar]
- 3.Velanovich V, Satava R. Balancing the normal appendectomy rate with the perforated appendicitis rate: implications for quality assurance. Am Surg. 1992;58:264–69. [PubMed] [Google Scholar]
- 4.Rettenbacher T, Hollerweger A, Macheiner P, et al. Ovoid shape of the vermiform appendix: A criterion to exclude acute appendicitis – evaluation with US. Radiology. 2003;226:95–100. doi: 10.1148/radiol.2261011496. [DOI] [PubMed] [Google Scholar]
- 5.Puig S, Hormann M, Rebhandl W, et al. US as a primary diagnostic tool in relation to negative appendectomy: six years experience. Radiology. 2003;226:101–4. doi: 10.1148/radiol.2261011612. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser S, Frenckner B, Jorulf HK. Suspected appendicitis in children: US and CT – a prospective randomized study. Radiology. 2002;223:633–38. doi: 10.1148/radiol.2233011076. [DOI] [PubMed] [Google Scholar]
- 7.Lim HK, Lee WJ, Lee SJ, et al. Focal appendicitis confined to the tip: Diagnosis at US. Radiology. 1996;200:799–801. doi: 10.1148/radiology.200.3.8756934. [DOI] [PubMed] [Google Scholar]
- 8.Nikolaidis P, Hwang CM, Miller FH, Papanicolaou N. The nonvisualized appendix: Incidence of acute appendicitis when secondary inflammatory changes are absent. Am J Roentgenol. 2004;183:889–92. doi: 10.2214/ajr.183.4.1830889. [DOI] [PubMed] [Google Scholar]
- 9.Pickuth D, Heywang-Kobrunner SH, Spielmann RP. Suspected acute appendicitis: is ultrasonography or computed tomography the preferred imaging technique? Eur J Surg. 2000;166:315–19. doi: 10.1080/110241500750009177. [DOI] [PubMed] [Google Scholar]
- 10.Mostbeck G, Adam EJ, Nielsen MB, et al. How to diagnose acute appendicitis: Ultrasound first. Insights Imaging. 2016;7:255–63. doi: 10.1007/s13244-016-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor A, Kapoor A, Mahajan G. Real-time elastography in acute appendicitis. J Ultrasound Med. 2010;29:871–77. doi: 10.7863/jum.2010.29.6.871. [DOI] [PubMed] [Google Scholar]
- 12.Rosai J. ALV Acute appendicitis: Rosai and Ackerman’s surgical pathology. 9th ed. Vol. 1. China: Elsevier-Mosby; 2004. pp. 871–77. [Google Scholar]
- 13.Sivit CJ, Siegel MJ, Applegate KE, Newman KD. When appendicitis is suspected in children. Radiographics. 2001;21:247–62. doi: 10.1148/radiographics.21.1.g01ja17247. questionnaire 288–94. [DOI] [PubMed] [Google Scholar]
- 14.Wiersma F, Sramek A, Holscher HC. US features of the normal appendix and surrounding area in children. Radiology. 2005;235:1018–22. doi: 10.1148/radiol.2353040086. [DOI] [PubMed] [Google Scholar]
- 15.Rettenbacher T, Hollerweger A, Macheiner P, et al. Outer diameter of the vermiform appendix as a sign of acute appendicitis: evaluation at US. Radiology. 2001;218:757–62. doi: 10.1148/radiology.218.3.r01fe20757. [DOI] [PubMed] [Google Scholar]
- 16.Pinto Leite N, Pereira JM, Cunha R, et al. CT evaluation of appendicitis and its complications: imaging techniques and key diagnostic findings. Am J Roentgenol. 2005;185:406–17. doi: 10.2214/ajr.185.2.01850406. [DOI] [PubMed] [Google Scholar]
- 17.Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83–94. doi: 10.1148/radiol.2411050913. [DOI] [PubMed] [Google Scholar]
- 18.Karabulut N, Kiroglu Y, Herek D, et al. Feasibility of low-dose unenhanced multi-detector CT in patients with suspected acute appendicitis: comparison with sonography. Clin Imaging. 2014;38:296–301. doi: 10.1016/j.clinimag.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Orth RC, Guillerman RP, Zhang W, et al. Prospective comparison of MR imaging and US for the diagnosis of pediatric appendicitis. Radiology. 2014;272:233–40. doi: 10.1148/radiol.14132206. [DOI] [PubMed] [Google Scholar]
- 20.Avcu S, Cetin FA, Arslan H, et al. The value of diffusion-weighted imaging and apparent diffusion coefficient quantification in the diagnosis of perforated and nonperforated appendicitis. Diagn Interv Radiol. 2013;19:106–10. doi: 10.4261/1305-3825.DIR.6070-12.1. [DOI] [PubMed] [Google Scholar]
- 21.Didier RA, Hopkins KL, Coakley FV, et al. Performance characteristics of magnetic resonance imaging without contrast agents or sedation in pediatric appendicitis. Pediatr Radiol. 2017 doi: 10.1007/s00247-017-3897-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–68. doi: 10.1097/ruq.0b013e31815b7ed6. [DOI] [PubMed] [Google Scholar]
- 23.Tatar IG, Kurt A, Yilmaz KB, et al. The learning curve of real time elastosonography: A preliminary study conducted for the assessment of malignancy risk in thyroid nodules. Med Ultrason. 2013;15:278–84. doi: 10.11152/mu.2013.2066.154.igt2. [DOI] [PubMed] [Google Scholar]
- 24.Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: A new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95:5281–88. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 25.Goya C, Hamidi C, Okur MH, et al. The utility of acoustic radiation force impulse imaging in diagnosing acute appendicitis and staging its severity. Diagn Interv Radiol. 2014;20:453–58. doi: 10.5152/dir.2014.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha SW, Kim IY, Kim YW. Quantitative measurement of elasticity of the appendix using shear wave elastography in patients with suspected acute appendicitis. PLoS One. 2014;9:e101292. doi: 10.1371/journal.pone.0101292. [DOI] [PMC free article] [PubMed] [Google Scholar]