Abstract
Background
Epidemiological evidence on the association between ambient air pollution and brain tumor risk is sparse and inconsistent.
Methods
In 12 cohorts from 6 European countries, individual estimates of annual mean air pollution levels at the baseline residence were estimated by standardized land-use regression models developed within the ESCAPE and TRANSPHORM projects: particulate matter (PM) ≤2.5, ≤10, and 2.5–10 μm in diameter (PM2.5, PM10, and PMcoarse), PM2.5 absorbance, nitrogen oxides (NO2 and NOx) and elemental composition of PM. We estimated cohort-specific associations of air pollutant concentrations and traffic intensity with total, malignant, and nonmalignant brain tumor, in separate Cox regression models, adjusting for risk factors, and pooled cohort-specific estimates using random-effects meta-analyses.
Results
Of 282194 subjects from 12 cohorts, 466 developed malignant brain tumors during 12 years of follow-up. Six of the cohorts also had data on nonmalignant brain tumor, where among 106786 subjects, 366 developed brain tumor: 176 nonmalignant and 190 malignant. We found a positive, statistically nonsignificant association between malignant brain tumor and PM2.5 absorbance (hazard ratio and 95% CI: 1.67; 0.89–3.14 per 10–5/m3), and weak positive or null associations with the other pollutants. Hazard ratio for PM2.5 absorbance (1.01; 0.38–2.71 per 10–5/m3) and all other pollutants were lower for nonmalignant than for malignant brain tumors.
Conclusion
We found suggestive evidence of an association between long-term exposure to PM2.5 absorbance indicating traffic-related air pollution and malignant brain tumors, and no association with overall or nonmalignant brain tumors.
Keywords: air pollution, brain cancer, brain tumor, traffic
Importance of the study
Increasing brain tumor incidence generated interest in environmental exposures. Traffic-related air pollution was declared carcinogenic to humans and linked to lung cancer. Experimental studies illustrated that particles can reach the brain causing inflammation and oxidative stress, but the current epidemiological evidence on air pollution and brain tumor risk is sparse and inconclusive. Within the framework of the European Study of Cohorts for Air Pollution Effects (ESCAPE), we examined the association between air pollution and brain tumor risk in 282194 subjects from 12 cohorts in 6 countries, who developed 466 malignant brain tumors during 12 years. Air pollution levels at the residence were estimated by standardized land-use regression models for PM ≤2.5, ≤10, 2.5–10 μm in diameter (PM2.5, PM10, PMcoarse), PM2.5 absorbance, and nitrogen oxides (NO2, NOx). We found suggestive evidence of an association between the traffic-related metric PM2.5 absorbance and malignant brain tumors, and no association with overall or nonmalignant brain tumors. The strength of the study was availability of data on nonmalignant brain tumors.
The average incidence of primary adult brain tumors (nonmalignant or malignant) in Europe in 2012 was 6.6 (7.8 in men and 5.6 in women) per 100000 people and approximately half of these were malignant (brain cancers).1 Incidence of brain tumors has been increasing in the industrialized countries, which is, in part, explained by improvements in diagnoses and high-resolution neuroimaging and an aging population, but occupational and environmental exposures have been suspected to play a role.2 Established brain tumor risk factors include age, ionizing radiation to the head, and inherited genetic risk,3 while infectious agents, high income, white race, exogenous hormone exposure (for nonmalignant tumors in women), occupations in agriculture4 and the petrochemical industry, and exposure to landfill pollution have been identified as potential risk factors.3 Outdoor and traffic-related sources of air pollution, such as gasoline and diesel engine exhaust, have been classified as carcinogenic to humans by the International Agency for Research on Cancer5,6 and are established risk factors for lung cancer and cardiovascular and respiratory diseases.7–9 Still, epidemiological evidence relating traffic-related air pollution to brain diseases is just emerging. Air pollution was recently linked to stroke,10 and the biological mechanism relevant for stroke was also suspected of being relevant for neurodegenerative diseases, such as Alzheimer’s, Parkinson’s diseases, and dementia.11 Experimental evidence in animals showed how particles,11 and most recently mineral magnetite,12 can reach the brain, via inhalation or directly through the nose and olfactory nerve, and cause neuroinflammation, oxidative stress, and neurodegeneration.11,13–17 Inflammation is suggested to be important in the pathogenesis of brain cancer.18 Furthermore, gene expression patterns similar to that seen in human brain tumors were found in rats after exposure to concentrated particles, in particular the coarse fraction.19 However, epidemiological evidence is sparse, consisting of 2 ecological studies,20,21 3 cohort studies,22–24 and a case-control study.25 A US study found association between airborne toxicant volatile organic compound emissions at a county level and the incidence of brain cancer,20 while a recent nationwide study found no association of brain cancer incidence and mortality at country level with any of 30 different hazardous air pollutants examined.21 The earliest cohort study, from 2009, based on the US Cancer Prevention Study cohort, found no associations between brain cancer mortality (n = 1284) and residential exposure to particulate matter (PM) with diameter <2.5 and 10 µg/m3 (PM2.5 and PM10) or nitrogen dioxide (NO2).22 A study from 2011 detected a strong association between long-term exposure to nitrogen oxides (NOx) and brain cancer incidence (n = 95) (hazard ratio and 95% CI: 2.28; 1.24–4.17 per 100 µg/m3) in the Danish Diet, Cancer and Health cohort,23 which was, however, not reproduced in a nationwide Danish case-control study with 4183 brain tumor cases.25 Finally, the recent study in the Danish Nurse Cohort (n = 121) found no association between brain tumor incidence (malignant or nonmalignant) and PM2.5, PM10, or NO2.24 With air pollution established as carcinogenic to humans, suggestive experimental evidence on the biological plausibility, and sparse and inconclusive epidemiological evidence, the rationale of this study was to examine association between air pollution and brain tumor in a large study combining information from several European cohorts.
In this study, by using the 12 European cohorts within the framework of the European Study of Cohorts for Air Pollution Effects (ESCAPE; http://www.escapeproject.eu/),26,27 we aim to examine the association between long-term exposure to ambient air pollution and incidence of brain tumor in total and separately for malignant and nonmalignant tumors.
Materials and Methods
Study Population
We invited 22 cohorts that contributed to earlier analyses within the ESCAPE framework on the association of air pollution with lung cancer.26 Of these, we included 12 cohorts from 6 European countries (Supplementary Figure S1) which had information on brain tumor incidence, at least 20 cases of brain tumor per cohort, and had the resources (statistical analyst available) for participation.
The 12 included cohorts were as follows (Table 1, Supplementary Fig. S1):
Table 1.
Description of the 282194 participants from 12 European cohorts included in the study
| Tumor by Malignancy | Tumor by Location | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment | Original N | Final N | % Total Cohort | Mean Age, y | Person- years at Risk | Mean Follow-up Time, y | N Total Brain Tumor |
N Nonmalignant Brain Tumor |
N Malignant Brain Tumor |
N Tumor in Meninges | N Tumor in Brain | |
| EPIC-Umeå, Sweden | 1992–96 | 25 600 | 24 997 | 97 | 45.9 | 335 293 | 14 | – | – | 63 | 21 | 34 |
| HUBRO, Norway | 2000–01 | 21 363 | 18 974 | 89 | 48.2 | 161 377 | 9 | 39 | 19 | 20 | 12 | 21 |
| CEANS, Swedena | 1992–2002 | 22 036 | 19 224 | 87 | 56.5 | 199 113 | 11 | – | – | 37 | 21 | 16 |
| DCH, Denmark | 1993–97 | 38 064 | 37 250 | 98 | 56.8 | 552 776 | 16 | 200 | 106 | 94 | 73 | 108 |
| EPIC-NL, Netherlandsb | 1993–97 | 36 505 | 31 826 | 87 | 50.3 | 375 875 | 12 | 64 | 23 | 41 | 18 | 43 |
| VHM&PP, Austria | 1985–2005 | 131 907 | 131 187 | 99 | 41.3 | 2 332 547 | 19 | – | – | 176 | – | 182 |
| EPIC-Varese, Italy | 1993–97 | 11 893 | 10 571 | 89 | 51.6 | 113 976 | 12 | 34 | 20 | 15 | – | – |
| EPIC-Turin, Italy | 1993–2008 | 8774 | 8165 | 93 | 50.3 | 115 519 | 14 | 28 | 8 | 20 | 12 | 16 |
EPIC, European Prospective Investigation into Cancer and Nutrition; HUBRO, Oslo Health Study; CEANS, Cardiovascular Effects of Air Pollution and Noise in Stockholm; DCH, Danish Diet, Health and Cancer cohort; VHM&PP, Vorarlberg Health Monitoring and Prevention Programme. aPooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP. bPooled data from 2 Dutch cohorts: EPIC-MORGEN and EPIC-PROSPECT.
Five Swedish cohorts: European Prospective Investi gation into Cancer and Nutrition (EPIC)–Umeå, Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), Stockholm Screening Across the Lifespan Twin study and TwinGene (SALT/Twin gene), Stockholm 60 years old and IMPROVE study (60 y/IMPROVE), and Stockholm Diabetes Prevention Program (SDPP);
One Norwegian cohort: Oslo Health Study (HUBRO);
One Danish cohort: Diet, Cancer and Health (DCH) study, with only Copenhagen included;
Two Dutch cohorts: EPIC-Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (EPIC-MORGEN and EPIC-PROSPECT);
One Austrian cohort: Vorarlberg Health Monitoring and Prevention Programme (VHM&PP); and
Two Italian cohorts: EPIC-Varese and EPIC-Turin.
The majority of cohorts recruited participants from large cities and the surrounding suburban or rural communities, while few covered large regions of the country, such as EPIC-MORGEN in the Netherlands and the VHM&PP cohort in Austria. For DCH and VHM&PP, exposure to air pollution was assessed for the Copenhagen (DCH) and population in the valley (VHM&PP) part of the original cohort. Data from the 4 Swedish cohorts from Stockholm (SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP) as well as the 2 Dutch cohorts (EPIC-MORGEN and EPIC-PROSPECT) were pooled by the local analyst, and analyzed as single cohorts, named Cardiovascular Effects of Air Pollution and Noise in Stockholm (CEANS) and EPIC Netherlands (EPIC-NL), respectively. The use of cohort data in ESCAPE was approved by the local ethical and data protection authorities. Each cohort study followed the rules for ethics and data protection set up in the country in which it was based.
Brain Tumor Definition
Cohort members were followed for brain tumor incidence via linkage to national or regional cancer registries among cohort members who did not have cancer before cohort baseline (excluding nonmelanoma skin cancers). Our main outcome was incident primary tumor of the brain, meninges, and cranial nerves, defined according to codes of the International Classification of Diseases, 10th revision (ICD10): C70.0, C71.0–C71.9, C72.2–C72.5, D32.0, D33.0–D33.2, D33.3, D42.0, D43.0–D43.2, D43.3. We aimed to consider the following 5 outcomes: the total of all tumors combined; subtypes by malignancy of the tumors: malignant (brain cancer) (C70.0, C71.0–C71.9, C72.2–C72.5) and nonmalignant (benign) tumors (D32.0, D33.0–D33.2, D33.3, D42.0, D43.0–D43.2, D43.3); and subtypes by the location of the tumor: tumors of the brain (ICD10: C71.0–C71.9, D33.0–D332, D43.0–D43.2) or tumors of meninges (C70.0, D32.0, and D42.0).
Exposure Assessment
We estimated annual average concentrations of air pollution at baseline residence for each cohort participant by standardized area-specific land-use regression (LUR) models developed within the ESCAPE study, described in detail elsewhere.28,29 In brief, the LUR models are based on measurements of NO2 and NOx in all 12 cohorts, and PM2.5, PM10, and PM2.5 absorbance in 10 study areas (due to budgetary reasons) during one year between October 2008 and May 2011.30,31 The concentration of PMcoarse was calculated as the difference between PM10 and PM2.5. Subsequently LUR models were developed for each pollutant in each study area to predict air pollution concentration at the baseline residence of the cohort participants. Data from the nearest routine monitoring stations were used to back-extrapolate the LUR estimates to the baseline year in 14 of the 15 study areas using the ratio method. We also used traffic intensity (annual average number of motor vehicles per day) on the nearest road to the exact residential address at the cohort baseline for each participant, as an indicator of exposure to traffic-related air pollution.
Furthermore, we used estimated annual concentrations of 8 elements in PM2.5 and PM10 (copper [Cu], iron [Fe], zinc [Zn], sulfur [S], nickel [Ni], vanadium [V], silicon [Si], and potassium [K]) with area-specific LUR models developed within the framework of the European study of Transport-Related Air Pollution and Health Impacts—Integrated Methodologies for Assessing Particulate Matter (TRANSPHORM; www.transphorm.eu/)32,33 (see Supplementary material).
Statistical Analyses
We have used a 2-step approach by first estimating the association between different air pollutants and brain tumor in each cohort, and then combining the estimates from each cohort, for each pollutant and each brain tumor subtype, by meta-analyses. Pooling of the cohort data was not possible due to data transfer and privacy issues.
Cohort-Specific Statistical Analyses
We used Cox proportional hazards (PH) models for the cohort-specific analyses, with age as the underlying timescale, and censoring at the time of any other cancer diagnosis (except nonmelanoma skin cancer), death, emigration (to another country), or end of follow-up, whichever came first. We ran a model for total brain tumors, and separate models for tumors by malignancy (malignant and nonmalignant brain tumors) and by location (tumors in the brain and meninges). We analyzed all air pollutants and traffic intensity as linear variables in a separate single-pollutant model. The potential confounders were available from questionnaires at baseline. We specified 3 confounder models a priori: Model 1, adjusted for age (time axis), sex, and calendar time (years of enrollment); Model 2, additionally adjusted for educational level (low, medium, or high) and occupation in the petrochemical or chemical industry (yes, no); and Model 3, adjusted additionally for area-level socioeconomic status variables using random effects of the spatial area units in each cohort to check for spatial clustering of residuals of the models. Various definitions of area-level socioeconomic status were used, including unemployment rate at the municipality (EPIC-Umeå, HUBRO, CEANS, and EPIC-Varese), mean income in the municipality (DCH, VHM&PP), percentage of people with low income in the neighborhood (EPIC-NL), and area deprivation index at the census block (EPIC-Turin). We a priori chose Model 3 as the main confounder model. All cohorts except VHM&PP had information on education, and only CEANS, DCH, EPIC-Varese, and EPIC-Turin had information on occupation in the petrochemical or chemical industry. Only participants with no missing information in any of the exposures and confounders in Model 3 were included in all analyses. Individual cohorts adjusted with the maximum possible confounders in Model 3. We performed a number of sensitivity analyses within each cohort: We restricted analyses to participants who were long-term residents (lived at least 10 years at the baseline address); we restricted analyses to long-term residents who did not move between the baseline and the end of follow-up; we added the indicator of rural areas to adjust for different degrees of urbanization within the study area; we used diagnostic tools to check the PH assumption for the categorical predictors in Model 3, and stratified the Cox model for the predictors that did not meet the PH assumption. We examined the shape of the association between each pollutant and brain tumor by inputting the exposure term as a natural cubic spline with 2 inner knots (ie, 3 degrees of freedom) and by comparing the model fit of the linear and spline models by a likelihood ratio test. All cohort-specific analyses were performed in Stata v10–12 using a common script, except for models with random effects, for which we used R software v2.11–2.15.
Meta-Analyses
We performed meta-analyses of cohort-specific effect estimates with the DerSimonian-Laird method with random effects.34 As main analyses, we performed separate meta-analyses for each of 7 pollutants for malignant brain tumors in 12 cohorts, and for nonmalignant and total brain tumors in 6 cohorts. Additionally, as presented in the Supplementary material, we performed meta-analyses for elemental components of PM2.5 and PM10 and malignant brain tumors, and a number of sensitivity analyses. We calculated hazard ratios (HRs) and 95% CIs for fixed increments that were chosen to cover the range in concentrations within the different cohorts and to keep increments broadly comparable between pollutants. We evaluated the heterogeneity between cohort-specific results by applying the chi-square test from Cochran’s Q statistic, which was quantified by the I2 statistic.35 We tested effect modification with a meta-analysis of the pooled estimates from the different strata and by computing the χ2 test of heterogeneity. We considered cohort-specific estimates to be significantly heterogeneous when I2 was >50% or the P-value of the chi-square test was <0.05. We investigated the robustness of the results for malignant brain tumors by examining the effect of all pollutants after excluding the VHM&PP from the meta-analyses, since this was the largest and most influential cohort which lacked the data on tumor in the meninges, and had the smallest number of confounders available in Model 3. We used Stata v12.1 for all meta-analyses.
Results
Study Population
All 12 cohorts had data on malignant brain tumors (Table 1), where 466 primary malignant brain tumors were diagnosed in a total of 282194 men and women during a mean follow-up of 13.3 years or 4186476 person-years. Six of these cohorts (5 from Sweden: EPIC-Umeå and CEANS [consisting of 4 cohorts] and the Austrian VHM&PP) did not have information on nonmalignant tumors. Of 106786 men and women from 6 cohorts with data on both nonmalignant and malignant tumors (HUBRO, DCH, EPIC-NL [consisting of 2 cohorts], EPIC-Varese, and EPIC-Turin), 366 in total developed primary brain tumors during a mean follow-up of 12.6 years (1319523 person-years), of which 176 (48%) were nonmalignant and 190 (52%) malignant. Of these 6 cohorts, EPIC-Varese did not have information on tumor subtype by location (meninges or brain). Thus, analyses of the subtypes of brain tumors by location were based on 5 cohorts with complete data on total brain tumors (malignant and nonmalignant) and on brain tumor location (meninges or brain): HUBRO, DCH, EPIC-NL (consisting of 2 cohorts), and EPIC-Turin. Among the 96215 subjects from these 5 cohorts, 303 developed brain tumor in total during a mean follow-up of 12.7 years or 1205547 person-years. Of these 303 brain tumors, 115 (38%) were in the meninges and 188 (62%) in the brain.
Mean age at the time of enrollment ranged from 41.3 years in VHM&PP to 56.8 years in DCH (Table 1). The proportion of women ranged from 43.9% in EPIC-Turin to 78.9% in EPIC-Varese, while the proportion of highly educated ranged from 7.1% in EPIC-Varese to 45.2% in HUBRO. VHM&PP did not have data on education (see Supplementary Table S1).
Air Pollution Exposure
The air pollution levels at residence varied substantially within and between study areas, with increasing levels from Northern to Southern study areas (Table 2). EPIC-Umeå and EPIC-Varese did not have data on PM, and EPIC-Varese had none on traffic intensity. The mean concentration of PM2.5 ranged from 7.1 µg/m3 in CEANS (Sweden) to 30.2 µg/m3 in EPIC-Turin (Italy). NO2 ranged from 5.3 µg/m3 in EPIC-Umeå (Sweden) to 53.0 µg/m3 in EPIC-Turin. Average traffic intensity on the nearest road was lowest in EPIC-Umeå (849 vehicles/day) and highest in EPIC-Turin (4044 vehicles/day). Mean levels of PM2.5 and PM10 elements also varied substantially between study areas (Supplementary Table S2). The estimates in this study were given per fixed increments (10 µg/m3 for PM10 and NO2, 5 µg/m3 for PM2.5, etc), which were selected a priori by a protocol for the entire ESCAPE project, as they reflect broadly comparable contrasts in exposure for the different pollutants and are most commonly used in literature and in meta-analyses. These contrasts/increments should be considered in the context of the mean air pollution concentrations in different areas. For example, 10 µg/m3 represents 58% of the average PM10 concentration in Copenhagen, Denmark and only 21% of average PM10 concentration in Turin, Italy.
Table 2.
Mean (standard deviation) of the air pollution and traffic intensity levels at the 282194 participants’ addresses in 12 European cohorts
| Cohort | PM2.5, µg/m3 |
PM2.5 absorbance, 10–5/m |
PM10, µg/m3 | PMcoarse, µg/m3 | NO2, µg/m3 |
NOx, µg/m3 |
Traffic Intensity on the Nearest Road (vehicles/day) |
|---|---|---|---|---|---|---|---|
| EPIC-Umeå, Sweden | – | – | – | – | 5.3 (2.5) | 8.8 (5.8) | 849 (1521) |
| HUBRO, Norway | 8.9 (1.3) | 1.2 (0.3) | 13.5 (3.1) | 4.0 (2.0) | 20.9 (8.0) | 38.2 (15.5) | 2509 (5098) |
| CEANS, Swedena | 7.1 (1.3) | 0.6 (0.2) | 14.7 (4.1) | 7.1 (3.1) | 10.8 (4.6) | 19.1 (10.2) | 1557 (4494) |
| DCH, Denmark | 11.3 (0.8) | 1.2 (0.2) | 17.2 (1.9) | 5.7 (1.0) | 16.5 (7.0) | 27.2 (18.5) | 3109 (7412) |
| EPIC-NL, Netherlandsb | 16.9 (0.6) | 1.4 (0.2) | 25.4 (1.5) | 8.5 (0.9) | 25.2 (6.2) | 37.9 (12.3) | 1290 (3797) |
| VHM&PP, Austria | 13.6 (1.2) | 1.7 (0.2) | 20.7 (2.4) | 6.7 (0.9) | 20.0 (5.5) | 40.1 (9.6) | 1718 (3647) |
| EPIC-Varese, Italy | – | – | – | – | 43.4 (17.3) | 85.9 (41.9) | – |
| EPIC-Turin, Italy | 30.2 (1.6) | 3.1 (0.4) | 46.6 (4.1) | 16.6 (2.7) | 53.0 (10.3) | 96.1 (20.3) | 4044 (9596) |
EPIC, European Prospective Investigation into Cancer and Nutrition; HUBRO, Oslo Health Study; CEANS, Cardiovascular Effects of Air Pollution and Noise in Stockholm; DCH, Danish Diet, Health and Cancer cohort; VHM&PP, Vorarlberg Health Monitoring and Prevention Programme. aPooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP. bPooled data from 2 Dutch cohorts: EPIC-MORGEN and EPIC-PROSPECT.
Associations Between Air Pollutants and Malignant Brain Tumor in 12 Cohorts
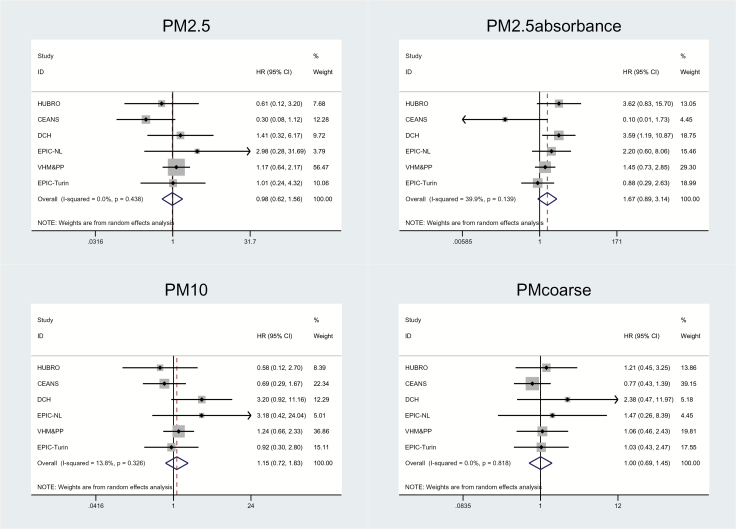
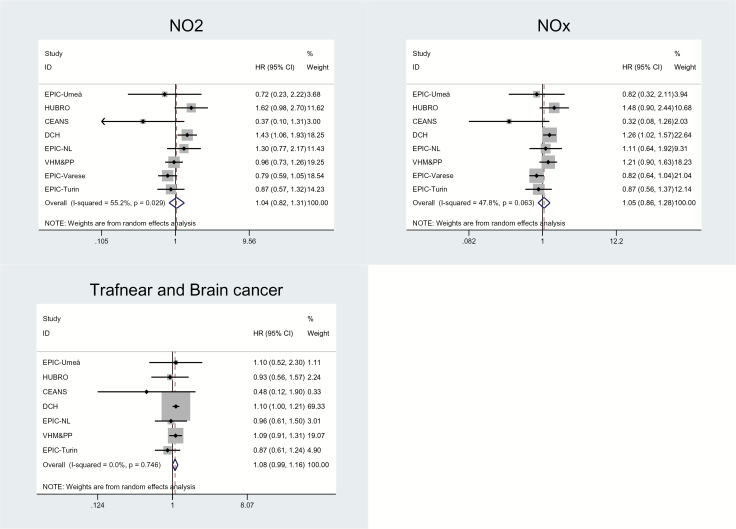
We found positive, statistically nonsignificant association between malignant brain tumor and PM2.5 absorbance (HR = 1.67; 95% CI: 0.89–3.14 per 10–5/m; P = 0.11) in the fully adjusted model (Model 3), with moderate heterogeneity in the individual cohort estimates (I2 = 39.9%) (Table 3, Fig. 1). Summary estimates for PM2.5 absorbance were enhanced or remained unchanged in the sensitivity analyses (see Supplementary Table S3). We found borderline significantly positive associations with traffic intensity on the nearest road (HR = 1.08; 95% CI: 0.99–1.16 per 5000 vehicles/day, P value = 0.07), but it is notable that 3 cohorts had HR above 1, and 4 cohorts had HR below 1 (Table 3, Fig. 2). We found weak positive associations with PM10, NO2, and NOx, and none with PM2.5 or PMcoarse. There was moderate or no heterogeneity in the summary estimates, except for NO2 and NOx, which showed substantial, statistically significant heterogeneity between individual cohort estimates.
Table 3.
Association between exposure to air pollution and malignant brain tumor incidence in 12a European cohorts
| Fixed Increase | N Cohorts | N | Model 1c HR (95% CI) |
Model 2d HR (95% CI) |
Model 3e HR (95% CI) |
P-value Model 3 |
I
2 (%) (P-value) |
|
|---|---|---|---|---|---|---|---|---|
| PM2.5 | 5 µg/m3 | 10b | 246 626 | 1.04 (0.66–1.63) | 1.01 (0.64–1.60) | 0.98 (0.62–1.56) | 0.94 | 0.0 (0.44) |
| PM2.5 absorbance | 10−5/m | 10b | 246 626 | 1.76 (0.90–3.43) | 1.72 (0.91–3.25) | 1.67 (0.89–3.14) | 0.11 | 39.9 (0.14) |
| PM10 | 10 µg/m3 | 10b | 246 626 | 1.17 (0.73–1.87) | 1.15 (0.74–1.78) | 1.15 (0.72–1.83) | 0.55 | 13.8 (0.33) |
| PMcoarse | 5 µg/m3 | 10b | 246 626 | 1.03 (0.72–1.47) | 1.02 (0.71–1.46) | 1.00 (0.69–1.45) | 0.99 | 0.0 (0.82) |
| NO2 | 10 µg/m3 | 12a | 282 194 | 1.04 (0.82–1.33) | 1.05 (0.83–1.32) | 1.04 (0.82–1.31) | 0.75 | 55.2 (0.03) |
| NOx | 20 µg/m3 | 12a | 282 194 | 1.06 (0.85–1.31) | 1.06 (0.86–1.30) | 1.05 (0.86–1.28) | 0.63 | 47.8 (0.06) |
| Traffic intensity | 5000 vehicles/day | 10b | 282 194 | 1.08 (1.00–1.16) | 1.07 (0.99–1.16) | 1.08 (0.99–1.16) | 0.07 | 0.0 (0.75) |
aEPIC-Umeå, CEANS (pooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP), HUBRO, DCH, EPIC-NL (pooled data from 2 cohorts: EPIC-MORGEN and EPIC-PROSPECT), VHM&PP, EPIC-Varese, and EPIC-Turin; bCEANS (pooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP), HUBRO, DCH, EPIC-NL (pooled data from 2 cohorts: EPIC-MORGEN and EPIC-PROSPECT), VHM&PP, and EPIC-Turin.
cAdjusted for age, sex, and year of enrollment. dModel 1 plus educational, and occupation in petrochemical industry; eModel 2 plus area-level socioeconomic status.
Fig. 1.
Adjusted associations between malignant brain tumor and PM2.5, PM2.5 absorbance, PM10, and PMcoarse (main Model 3) in 10 European cohorts (CEANS [pooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP], HUBRO, DCH, EPIC-NL [pooled data from 2 cohorts: EPIC-MORGEN and EPIC-PROSPECT], VHM&PP, and EPIC-Turin) result from cohort-specific analyses and random-effects analyses.
Fig. 2.
Adjusted associations between malignant brain tumor and NO2, NOx, and traffic intensity on the nearest road (main Model 3) in 12 European cohorts (aEPIC-Umeå, CEANS [pooled data from the 4 cohorts from Stockholm, Sweden: SNAC-K, SALT/Twin gene, 60 y/IMPROVE, and SDPP], HUBRO, DCH, EPIC-NL [pooled data from 2 cohorts: EPIC-MORGEN and EPIC-PROSPECT], VHM&PP, EPIC-Varese, and EPIC-Turin) result from cohort-specific analyses and random-effects analyses.
HRs for all pollutants were slightly attenuated after adjustment for individual-level confounders (Table 3). There was no evidence of deviation from linearity in associations between air pollutants and total brain tumor (results not shown).
Associations Between Elemental Components of PM and Malignant Brain Tumor
In the secondary analyses of the elemental components of PM2.5 and PM10, no statistically significant associations were detected for any component and malignant brain tumor. We detected the strongest association with the V component of PM2.5 (HR = 1.40; 95% CI: 0.75–2.60 per 2 ng/m3) and PM10 (HR = 1.31; 95% CI: 0.69–2.49 per 3 ng/m3) and the Ni component of PM2.5 (HR = 1.27; 95% CI: 0.44–3.64 per 1 ng/m3) and PM10 (HR = 1.28; 95% CI: 0.98–1.66 per 2 ng/m3), though with statistically significant heterogeneity between individual cohort estimates for the Ni component of PM2.5 (I2 = 76.3) (Supplementary Table S4 and Supplementary Figure S2). We also found a positive association with the copper component of PM2.5 and PM10, and the iron and sulfur components of PM2.5 (but not PM10).
Associations Between Air Pollutants and Total and Nonmalignant Brain Tumor in Six Cohorts
We found no association between any pollutant and nonmalignant brain tumor (Table 4), and even strong inverse association with PMcoarse and PM10 (Table 4, Supplementary Figures S3 and S4). We found positive but statistically nonsignificant associations between total brain tumor and PM2.5 absorbance (HR = 1.58; 95% CI: 0.73–3.40 per 10–5/m) and statistically significant association with traffic intensity on the nearest road (HR = 1.07; 95% CI: 1.00–1.14 per 5000 vehicles/day) (Table 4, Supplementary Figures S5 and S6). It is notable that for traffic intensity, only a single cohort had HR above 1, and the rest below 1.
Table 4.
Associationa between long-term exposure to air pollution and benign and total brain tumor incidence in 6a European cohorts
| Benign Brain Tumor | Total Brain Tumor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Increase | N Cohorts | N | HR (95% CI) | P-value |
I
2 (%) (P-value) |
HR (95% CI) | P-value |
I
2 (%) (P-value) |
|
| PM2.5 | 5 µg/m3 | 5b | 96 215 | 1.12 (0.46–2.73) | 0.81 | 0.0 (0.39) | 1.13 (0.61–2.09) | 0.69 | 0.0 (0.51) |
| PM2.5 absorbance | 10−5/m | 5b | 96 215 | 1.01 (0.38–2.71) | 0.98 | 49.8 (0.11) | 1.58 (0.73–3.40) | 0.25 | 63.6 (0.04) |
| PM10 | 10 µg/m3 | 5b | 96 215 | 0.54 (0.14–2.13) | 0.38 | 60.3 (0.05) | 0.97 (0.36–2.57) | 0.95 | 68.3 (0.02) |
| PMcoarse | 5 µg/m3 | 5b | 96 215 | 0.51 (0.24–1.10) | 0.08 | 0.0 (0.68) | 0.87 (0.55–1.38) | 0.56 | 0.0 (0.68) |
| NO2 | 10 µg/m3 | 6c | 106 786 | 0.95 (0.72–1.267 | 0.73 | 50.9 (0.09) | 1.04 (0.83–1.31) | 0.74 | 67.0 (0.02) |
| NOx | 20 µg/m3 | 6c | 106 786 | 0.97 (0.75–1.25) | 0.80 | 49.3 (0.10) | 1.01 (0.84–1.22) | 0.91 | 59.6 (0.04) |
| Traffic intensity | 5000 vehicles/ day | 5b | 96 215 | 1.07 (0.97–1.18) | 0.17 | 0.0 (0.82) | 1.07 (1.00–1.14) | 0.04 | 0.0 (0.46) |
P-value: for Model 3; aModel 3, adjusted for age, sex, year of enrollment, education, occupation in petrochemical industry, and area-level socioeconomic status; bHUBRO, DCH, EPIC-NL (which includes 2 cohorts: EPIC-MORGEN and EPIC-PROSPECT), and EPIC-Turin; cHUBRO, DCH, EPIC-NL (pooled data from 2 Dutch cohorts: EPIC-MORGEN and EPIC-PROSPECT), EPIC-Varese, and EPIC-Turin.
Associations Between Air Pollutants and Brain Tumor by Malignancy in Six Cohorts
In the analyses based on 6 cohorts with data on both malignant and nonmalignant brain tumors, HRs for all pollutants, except for traffic intensity on the nearest road, were higher for malignant brain tumors (Supplementary Table S5 and Supplementary Figures S7 and S8) than for nonmalignant brain tumors (Supplementary Figures S5 and S6), reaching statistical significance for malignant tumors and PM2.5 absorbance (HR = 2.03; 95% CI: 1.05–3.91 per 10–5/m).
Associations Between Air Pollutants and Brain Tumor by Location
In the analyses in 5 cohorts with data on all brain tumors and subtypes by location (brain or meninges), there was no clear pattern (Supplementary Table S6). HRs for the most pollutants were generally higher for tumors in the brain (Supplementary Figures S9 and S10) than in the meninges (Supplementary Figures S11 and S12), except for PM2.5, for which a very strong positive association was detected (HR = 2.53; 95% CI: 0.87–7.41; I2 = 0.0, per 5 µg/m3), and traffic intensity on the nearest road.
A schematic presentation of a number of analyses presented in the paper is given in Supplementary Figure S13.
Discussion
In this large, multicenter European study, we found suggestive evidence of an association between long-term exposure to the traffic-related marker PM2.5 absorbance and risk of malignant brain tumors, and no association with nonmalignant brain tumors.
Comparison with Previous Studies
Our results suggesting relevance of traffic-related PM air pollution, in terms of PM2.5 absorbance, for malignant brain tumor are novel. Lack of statistically significant associations between malignant brain tumor incidence and PM2.5, PM10, and NO2 in our study agrees with the findings by McKean-Cowdin et al from 2009, based on a US Cancer Prevention Study, of no association between brain cancer mortality (1284 cases) and residential exposure to PM2.5, PM10, or NO2 (all HRs below 1).22 McKean-Cowdin et al had data on only brain cancer mortality, which, in contrast to incidence, captures more aggressive types of malignant brain cancers. Our results agree with a study by Jørgensen et al24 of 28731 female nurses from a Danish Nurse Cohort (121 cases of total brain tumor) that found no association of PM2.5 or PM10 with malignant brain tumor incidence (HRs for all pollutants below 1), consistent with McKean-Cowdin et al.22 We found the strongest effects for PM2.5 absorbance, which captures a fraction of PM2.5 originating from incomplete combustion from motorized traffic, highly correlated to elemental carbon.36 PM2.5 absorbance may be a better proxy for traffic-related particles in the ultrafine-particle (UFP) size range (diameter <100 nm) than PM2.5. In Augsburg, Germany, average UFP level was highly correlated with PM2.5 absorbance (correlation coefficient [R] = 0.81), but the correlations with NO2 and NOx were even higher.37 In Amsterdam, average UFP was highly correlated with PM absorbance (R = 0.85) at 46 sites, and correlations with PM2.5 and PMcoarse were lower.38 UFPs are of particular concern with respect to brain, because experimental studies in animals indicate that inhaled UFPs can reach the brain by crossing the blood–brain barrier or directly via nasal passage and olfactory neurons, and accumulate in the brain,16,39,40 causing inflammation, oxidative stress, and DNA damage.13,41,42
Our results of weakly positive, statistically nonsignificant associations with NO2 and NOx disagree with a study from 2011 by Raaschou-Nielsen et al on malignant brain tumor incidence (95 cases) in 54304 participants from the DCH cohort which detected strong association with NOx (HR = 2.28; 95% CI: 1.25–4.19 per 100 µg/m3) and proximity to major street (<50 m) (HR = 1.89; 95% CI: 1.07–3.36).23 In the current analysis including half of the DCH cohort participants living in Copenhagen, association for NOx in the DCH cohort was also strongly positive and statistically significant (HR = 1.22; 95% CI: 1.05–1.42 per 20 µg/m3), as it was with traffic intensity (HR = 1.09; 95% CI: 1.02–1.17 per 5000 motor vehicles/day) (Fig. 2), showing consistency in the DCH cohort with 2 different estimation methods for NOx and 2 different proxies for traffic intensity. However, 2 recent Danish studies24,25 did not reproduce this strong association between NOx and brain tumor detected in DCH, in agreement with our findings. Poulsen et al in a Danish nationwide case-control study (2000–2009) with 4183 brain tumor cases in total, found only a weakly positive association with NOx (HR = 1.11; 95% CI: 0.84–1.46 per 100 µg/m3),25 in line with our findings, but detected a statistically significant positive association with non-glioma tumors (HR = 1.53; 95% CI: 1.02–2.29 per 100 µg/m3). Similarly, Jørgensen et al24 in 28731 female nurses from a Danish Nurse Cohort (121 brain tumor cases) found no association with NOx (HR = 1.02; 95% CI: 0.93–1.12 per 10.2 µg/m3), agreeing with the current study. However, neither Poulsen et al nor Jørgensen et al had data on the traffic intensity on the nearest road. While we detected the strongest associations with malignant tumors, both Poulsen et al25 and Jørgensen et al24 found slightly stronger effects for nonmalignant brain tumors. Overall, evidence from this study does not support association between PM2.5, PM10, NO2, and NOx with brain tumor development but suggests that PM originating from traffic, in terms of PM2.5 absorbance, may play a role in the development of malignant brain tumors. However, due to high heterogeneity in findings in the existing literature, it is premature to draw a conclusion on the causal relationship between air pollution and brain tumor. More studies with data from long-term exposure to PM2.5 absorbance, and preferably UFPs, are needed to confirm our novel findings.
Particle Composition Findings
We present a novel suggestive finding of the relevance of V and Ni components of PM2.5 and PM10 for malignant brain tumor development (Supplementary Table S5 and Supplementary Figure S2). Environmental exposure to V occurs in areas of persistent burning of fossil fuel. This metal is known to induce oxidative stress and oligodendrocyte damage and has been linked with carcinogenic, immunotoxic, and neurotoxic insults.43 Ni is a transitional heavy metal originating primarily from the combustion of fossil fuels,44 found to be carcinogenic to humans, causing cancers of the lung and of the nasal cavity.45 The Ni component of PM10 was the PM element that showed the strongest association with lung cancer incidence (HR = 1.59; 95% CI: 1.12–2.26 per 2 ng/m3) in a related study in 14 European cohorts.46 These novel results call for replication in other studies and should be taken with caution, especially for Ni component PM2.5, where a large heterogeneity between individual cohorts is observed.
Strengths and Limitations
Our study benefited from a multicenter design and a large number of subjects recruited from general populations from around Europe, with large variation in air pollution levels, well-defined information on the brain tumor risk factors, and standardized definition of brain tumor from national and regional cancer registers. The major strength of our study is the standardized exposure assessment for a number of different air pollutants and PM elements, and standardized statistical analyses across all cohorts. The air pollution LUR models have been validated and were earlier linked to lung cancer.26 We adjusted the analyses for the most important risk factors but found little evidence of confounding in air pollution estimates. Finally, the strength of the study is that we had data on nonmalignant brain tumors.
Of 22 original ESCAPE cohorts, we included only the 12 cohorts which had data on brain tumor, at least 20 cases of brain tumor in total, and resources in terms of a statistical analyst to perform analyses. Of the cohorts not included in current analyses are the cohorts from Southern Europe, including those from Greece (EPIC-Athens), Italy (SIDRIA-Rome), and Spain (San Sebastian and Basque country), as well as those from the United Kingdom (UK) (EPIC-Oxford), Switzerland (SAPALDIA), France (E3N), and Germany (SALIA and KORA), while all the Nordic and Dutch cohorts (except Finish FINRISK) are included. Thus, some of the original 22 ESCAPE cohorts with highest levels of air pollution from Southern Europe are missing from current analyses, while there is an overrepresentation of cohorts from Northern countries, with lower air pollution levels.
Weakness of our study is lack of data on brain tumor subtypes in all 12 cohorts. We have detected significant positive association between PM2.5 absorbance and malignant brain tumor only in 6 cohorts which had information on all brain tumors (HR = 2.12; 95% CI: 1.06–4.25 per 10−5/m; see Supplementary Table S5), but not in a larger sample of a total of 12 cohorts with information on malignant tumors (HR = 1.67; 95% CI: 0.89–3.14 per 10−5/m). However, all sensitivity analyses of associations between PM2.5 absorbance and malignant brain tumor based on 6 cohorts showed enhanced HRs, ranging from 1.67 to 2.37 (see Supplementary Table S3), suggesting that the associations may be real and rather robust. Furthermore, we lacked information on brain tumor histology and morphology and could not study whether air pollution differentially affects more aggressive types of brain tumors, such as glioblastomas or anaplastic astrocytomas, opposed to more nonmalignant tumors such as meningiomas or low-grade gliomas. We lacked information on detailed occupational exposures to chemicals that may be related to brain tumor risk, apart from a crude definition of occupation in the petrochemical industry available in 8 out of 12 cohorts. We also lacked data on other potential risk factors, such as genetic predisposition, residential radon exposure, occupation in agriculture, and exposure to radiation to head and neck, but all of these are most likely not related to air pollution levels at residence. We used air pollution exposure estimated close to the time of the brain tumor diagnosis, and lack data on distant exposures during adulthood and early life, which may be more relevant for the development of brain tumor. We used an LUR model in this study developed on air pollution measurements between 2008 and 2011 but applied them to baseline addresses typically 10 to 15 years earlier, which likely resulted in some exposure misclassification. Several studies have documented stable spatial contrast of NO2 over study periods of 10–15 years.47–49 As motorized traffic is a major source of NO2, spatial contrasts likely have been stable for other traffic-related pollutants including PM2.5 absorbance. Another weakness of the study is that we used information on air pollution and confounders at the cohort baseline and did not have information on changes over time. Furthermore, we lacked information on participants’ activity patterns, time spent outdoors and away from home, commuting to work, etc. Exposure misclassification and lack of early-life exposures to air pollution may have biased our estimates toward zero, meaning that real associations would be even stronger than those observed here. Majority of the cohorts included in current analyses were recruited from urban areas, typically large cities and surrounding areas, except VHM&PP, which included primarily rural communities and several towns. However, there is still large variation in air pollution levels within the cohorts (Table 2), and especially between the cohorts with example of NO2 levels ranging from 8.8 µg/m3 in Umeå, Sweden to 96.1 in Turin, Italy.
Conclusion
In a large meta-analysis based on 12 European cohorts, on long-term exposure to ambient air pollution and brain tumor incidence, we found a suggestive evidence of an association between traffic-related PM2.5 absorbance and malignant brain tumors, and no association with overall or nonmalignant brain tumors.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the European Community’s Seventh Framework Programme (FP7/2007–2011) as part of the ESCAPE (grant 211250) and TRANSPHORM (grant 243406) projects. Zorana J. Andersen holds a grant from Novo Nordisk Foundation (NNF6935). Gudrun Weinmayr and Gabriele Nagel hold a grant from the German Cancer Aid (DKH ref.111010). Marie Pedersen holds a fellowship from the Danish Council for Independent Research (grant DFF-4004-00179). Financial support and mortality data for EPIC-MORGEN and EPIC-PROSPECT were received by the Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), and Statistics Netherlands (the Netherlands).
Conflict of interest statement. All authors declare that they have no actual or potential competing financial interest.
Supplementary Material
Acknowledgments
The data collection for HUBRO, Norway was conducted as part of the Oslo Health Study 2000–2001 in collaboration with the Norwegian Institute of Public Health. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred. The authors would like to thank Jon Wickmann for his efforts to coordinate the Oslo group and to assure the quality of exposure assessment in the HUBRO cohort.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 2. Gomes J, Al Zayadi A, Guzman A. Occupational and environmental risk factors of adult primary brain cancers: a systematic review. Int J Occup Environ Med. 2011;2(2):82–111. [PubMed] [Google Scholar]
- 3. Bondy ML, Scheurer ME, Malmer B et al. ; Brain Tumor Epidemiology Consortium Brain tumor epidemiology: consensus from the brain tumor epidemiology consortium. Cancer. 2008;113(7 Suppl):1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt LS, Nielsen H, Schmiedel S, Johansen C. Social inequality and incidence of and survival from tumours of the central nervous system in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):2050–2057. [DOI] [PubMed] [Google Scholar]
- 5. Loomis D, Grosse Y, Lauby-Secretan B et al. ; International Agency for Research on Cancer Monograph Working Group IARC The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–1263. [DOI] [PubMed] [Google Scholar]
- 6. Benbrahim-Tallaa L, Baan RA, Grosse Y et al. ; International Agency for Research on Cancer Monograph Working Group Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13(7):663–664. [DOI] [PubMed] [Google Scholar]
- 7. Raaschou-Nielsen O, Andersen ZJ, Beelen R et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14(9):813–822. [DOI] [PubMed] [Google Scholar]
- 8. Franklin BA, Brook R, Arden Pope C 3rd. Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40(5):207–238. [DOI] [PubMed] [Google Scholar]
- 9. Newby DE, Mannucci PM, Tell GS et al. ; ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ljungman PL, Mittleman MA. Ambient air pollution and stroke. Stroke. 2014;45(12):3734–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heusinkveld HJ, Wahle T, Campbell A et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. [DOI] [PubMed] [Google Scholar]
- 12. Maher BA, Ahmed IA, Karloukovski V et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci U S A. 2016;113(39):10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calderón-Garcidueñas L, Calderón-Garcidueñas A, Torres-Jardón R, Avila-Ramírez J, Kulesza RJ, Angiulli AD. Air pollution and your brain: what do you need to know right now. Prim Health Care Res Dev. 2015;16(4):329–345. [DOI] [PubMed] [Google Scholar]
- 15. Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oberdörster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract—and beyond?Environ Health Perspect. 2002;110(8):A440–A441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreyling WG, Semmler-Behnke M, Seitz J et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21(Suppl 1):55–60. [DOI] [PubMed] [Google Scholar]
- 18. Sowers JL, Johnson KM, Conrad C, Patterson JT, Sowers LC. The role of inflammation in brain cancer. Adv Exp Med Biol. 2009;816:75–105. [DOI] [PubMed] [Google Scholar]
- 19. Ljubimova JY, Kleinman MT, Karabalin NM et al. Gene expression changes in rat brain after short and long exposures to particulate matter in Los Angeles basin air: comparison with human brain tumors. Exp Toxicol Pathol. 2013;65(7-8):1063–1071. [DOI] [PubMed] [Google Scholar]
- 20. Boeglin ML, Wessels D, Henshel D. An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ Res. 2006;100(2):242–254. [DOI] [PubMed] [Google Scholar]
- 21. Valberg PA, Long CM. Do brain cancer rates correlate with ambient exposure levels of criteria air pollutants or hazardous air pollutants (HAPs)?Air Qual Atmos Heal. 2012;5(1):115–123. [Google Scholar]
- 22. McKean-Cowdin R, Calle EE, Peters JM et al. Ambient air pollution and brain cancer mortality. Cancer Causes Control. 2009;20(9):1645–1651. [DOI] [PubMed] [Google Scholar]
- 23. Raaschou-Nielsen O, Andersen ZJ, Hvidberg M et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jørgensen JT, Johansen MS, Ravnskjær L et al. Long-term exposure to ambient air pollution and incidence of brain tumours: the Danish Nurse Cohort. Neurotoxicology. 2016;55:122–130. [DOI] [PubMed] [Google Scholar]
- 25. Poulsen AH, Sørensen M, Andersen ZJ, Ketzel M, Raaschou-Nielsen O. Air pollution from traffic and risk for brain tumors: a nationwide study in Denmark. Cancer Causes Control. 2016;27(4):473–480. [DOI] [PubMed] [Google Scholar]
- 26. Raaschou-Nielsen O, Andersen ZJ, Beelen R et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14(9):813–822. [DOI] [PubMed] [Google Scholar]
- 27. Beelen R, Raaschou-Nielsen O, Stafoggia M et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383(9919):785–795. [DOI] [PubMed] [Google Scholar]
- 28. Beelen R, Hoek G, Vienneau D et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—the ESCAPE project. Atmos Environ. 2013;72:10–23. [Google Scholar]
- 29. Eeftens M, Beelen R, de Hoogh K et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas: results of the ESCAPE project. Environ Sci Technol. 2012;46(20):11195–11205. [DOI] [PubMed] [Google Scholar]
- 30. Cyrys J, Eeftens M, Heinrich J et al. Variation of NO2 and NOx concentrations between and within 36 European study areas: results from the ESCAPE study. Atmos Environ. 2012;62:374–390. [Google Scholar]
- 31. Eeftens M, Tsai MY, Ampe C et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2—results of the ESCAPE project. Atmos Environ. 2012;62:303–317. [Google Scholar]
- 32. de Hoogh K, Wang M, Adam M et al. Development of land use regression models for particle composition in twenty study areas in Europe. Environ Sci Technol. 2013;47(11):5778–5786. [DOI] [PubMed] [Google Scholar]
- 33. Tsai MY, Hoek G, Eeftens M et al. Spatial variation of PM elemental composition between and within 20 European study areas—results of the ESCAPE project. Environ Int. 2015;84:181–192. [DOI] [PubMed] [Google Scholar]
- 34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 36. Janssen NA, de Hartog JJ, Hoek G et al. Personal exposure to fine particulate matter in elderly subjects: relation between personal, indoor, and outdoor concentrations. J Air Waste Manag Assoc. 2000;50(7):1133–1143 [DOI] [PubMed] [Google Scholar]
- 37. Wolf K, Cyrys J, Harciníková T et al. Land use regression modeling of ultrafine particles, ozone, nitrogen oxides and markers of particulate matter pollution in Augsburg, Germany. Sci Total Environ. 2017;579:1531–1540. [DOI] [PubMed] [Google Scholar]
- 38. Hoek G, Beelen R, Kos G et al. Land use regression model for ultrafine particles in Amsterdam. Environ Sci Technol. 2011;45(2):622–628. [DOI] [PubMed] [Google Scholar]
- 39. Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know?Adv Drug Deliv Rev. 2014;71:2–14. [DOI] [PubMed] [Google Scholar]
- 40. Nemmar A, Hoet PH, Vanquickenborne B et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–414. [DOI] [PubMed] [Google Scholar]
- 41. Calderón-Garcidueñas L, Maronpot RR, Torres-Jardon R et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31(5):524–538. [DOI] [PubMed] [Google Scholar]
- 42. Calderón-Garcidueñas L, Leray E, Heydarpour P, Torres-Jardón R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: the clinical impact on children and beyond. Rev Neurol (Paris). 2016;172(1):69–80. [DOI] [PubMed] [Google Scholar]
- 43. Zwolak I. Vanadium carcinogenic, immunotoxic and neurotoxic effects: a review of in vitro studies. Toxicol Mech Methods. 2014;24(1):1–12. [DOI] [PubMed] [Google Scholar]
- 44. Grandjean P. Human exposure to nickel. IARC Sci Publ. 1984;(53):469–485. [PubMed] [Google Scholar]
- 45. Straif K, Benbrahim-Tallaa L, Baan R et al. ; WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453–454. [DOI] [PubMed] [Google Scholar]
- 46. Raaschou-Nielsen O, Beelen R, Wang M et al. Particulate matter air pollution components and risk for lung cancer. Environ Int. 2016;87:66–73. [DOI] [PubMed] [Google Scholar]
- 47. Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. Stability of measured and modelled spatial contrasts in NO(2) over time. Occup Environ Med. 2011;68(10):765–770. [DOI] [PubMed] [Google Scholar]
- 48. Gulliver J, de Hoogh K, Hansell A, Vienneau D. Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain. Environ Sci Technol. 2013;47(14):7804–7811. [DOI] [PubMed] [Google Scholar]
- 49. Cesaroni G, Porta D, Badaloni C et al. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health. 2012;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.