Abstract
Background
Medulloblastoma in adult patients is rare, with 0.6 cases per million. Prognosis depends on clinical factors and medulloblastoma entity. No prospective data on the feasibility of radiochemotherapy exist. The German Neuro-Oncology Working Group (NOA) performed a prospective descriptive multicenter single-arm phase II trial to evaluate feasibility and toxicity of radio-polychemotherapy.
Methods
The NOA-07 trial combined craniospinal irradiation with vincristine, followed by 8 cycles of cisplatin, lomustine, and vincristine. Adverse events, imaging and progression patterns, histological and genetic markers, health-related quality of life (HRQoL), and cognition were evaluated. Primary endpoint was the rate of toxicity-related treatment terminations after 4 chemotherapy cycles, and the toxicity profile. The feasibility goal was reached if at least 45% of patients received at least 4 cycles of maintenance chemotherapy.
Results
Thirty patients were evaluable. Each 50% showed classic and desmoplastic/nodular histology. Sixty-seven percent were classified into the sonic hedgehog (SHH) subgroup without TP53 alterations, 13% in wingless (WNT), and 17% in non-WNT/non-SHH. Four cycles of chemotherapy were feasible in the majority (n = 21; 70.0%). Hematological side effects and polyneuropathy were prevalent toxicities. During the active treatment period, HRQoL and verbal fluency improved significantly. The 3-year event-free survival rate was 66.6% at the time of databank lock.
Conclusions
Radio-polychemotherapy did lead to considerable toxicity and a high amount of dose reductions throughout the first 4 chemotherapy cycles that may affect efficacy. Thus, we propose frequent patient surveillance using this regimen. Modifications of the regimen may increase feasibility of radio-polychemotherapy of adult patients with medulloblastoma.
Keywords: cognition, imaging, health-related quality of life, medulloblastoma, radiochemotherapy
Importance of the study
NOA-07 showed that combined radiochemotherapy followed by maintenance chemotherapy with cisplatin, lomustine, and vincristine is more toxic in adults than in children. This prospective descriptive trial constitutes a unique dataset that perpetuates the development of effective treatments in this curable population. The results have potential to influence or even change clinical practice. The NOA-07 protocol can be translated to the recommendation that adults with medulloblastoma should receive an attenuated radio-polychemotherapy regimen that considers the decreased feasibility of radio-polychemotherapy in adults in comparison to children. In addition, an attenuated NOA-07 regimen may serve as a basis for the standard arm in a subsequent trial. Current knowledge makes it likely that such a trial will ask a molecular question in its experimental arm and will lead into a molecularly stratified approach for adults with medulloblastoma.
Medulloblastoma is rare in adults. About 70% of cases occur in patients younger than 15 years.1 The US Surveillance, Epidemiology, and End Results database reports 0.6 cases per million in adults per year.2 Five-year overall survival (OS) depends on clinical prognostic factors3 and on histological entity and genetic subgroup4 and reaches 40%–90%. Long-term sequelae interfere with functioning in daily life.5
Four genetic subgroups (wingless [WNT], sonic hedgehog [SHH], Group 3, and Group 4) defined by expression patterns and epigenetic signatures allow a reliable prognostication.6 The 2016 World Health Organization (WHO) classification7 developed this further to a combination of histological criteria with classic (60%–70% in adults), desmoplastic/nodular (25%–40%), extensive nodular (<5%), and large cell/anaplastic (10%–25%) entities and genetically defined groups. These consist of WNT-activated, SHH-activated, and TP53 wildtype, SHH-activated, and TP53 altered, and non-WNT/non-SHH (Groups 3 and 4). Patients with MYC/MYCN amplification6,8 and p53 mutation9 have an inferior prognosis. In adults, Group 3 does not exist.
In children, the introduction of chemotherapy combined with craniospinal radiotherapy improved survival10–12—however, it was associated with significant unwanted effects.13 The same regimen was retrospectively evaluated in a nonrandomized adult subcohort.14 Radiochemotherapy compared with radiotherapy alone improved event-free survival (EFS) (4-y EFS, 74% vs 47%; P = 0.027) and OS (4-y OS, 94% vs 81%; P = 0.035). Retrospective data and small prospective trials also suggested an impact of chemotherapy in adult patients.15–17
NOA-07 prospectively evaluated toxicity-related treatment terminations after 4 cycles of adjuvant chemotherapy, as well as the toxicity profile in the first-line treatment of adult patients with medulloblastoma. Results of the active treatment period are presented here.
Materials and Methods
Treatment and Ethics
NOA-07 enrolled patients at 15 sites in Germany. The trial was approved by the ethics committee of the University of Regensburg (08-112-0058; substantial amendment of July 1, 2016). The trial was registered at ClinicalTrials.gov (NCT01614132).
All patients with age above 21 and Chang stage T1-4 and M0 or M1 as diagnosed locally at the trial sites were included. Treatment consisted of photon craniospinal irradiation (1.6 Gy/35.2 Gy, posterior fossa boost 1.8 Gy/55 Gy) in combination with vincristine (1.5 mg/m2 weekly, cap at 2.0 mg), followed by a maximum of 8 six-weekly cycles of cisplatin (70.0 mg/m2, day 1), lomustine (75.0 mg/m2, day 1), and vincristine (1.5 g/m2, cap at 2.0 mg, days 1, 8, 15). Treatment per protocol was at least one cycle of adjuvant chemotherapy (Supplementary Figure S1).
MR Imaging Classification
Craniospinal imaging patterns (MRI with T1-weighted [w] images, T1-w post gadolinium [Gd]-containing contrast agent; T2-w, fluid attenuated inversion recovery) were evaluated prospectively. Inclusion was based on the local (neuro)radiology report. All imaging was reviewed for localization, extent of resection, and progression.18
Contrast enhancing and non-enhancing tumor volumes were calculated from voxel size x3 multiplied by the number of target voxels using the IntelliSpace Portal (Philips Healthcare). Extent of resection was evaluated for the enhancing tumor volume on early postsurgical MRI (24 to 72 h postoperative). Tumor progression was assessed on follow-up craniospinal MRIs after radiotherapy, before starting adjuvant chemotherapy and in 12-weekly time intervals over 3 years, and 6-monthly intervals thereafter.
Chang Classification
Despite the fact that the Chang3 classification was initially based on surgical reports and CT scans and that its prognostic value is doubtable, patients were classified according to Chang using MRI (condensed T1-w plus Gd and T2-w) criteria and results from lumbar punctures, with the aim to morphologically describe the study population.
Histological and Molecular Classification and CSF Assessment
All specimens were diagnosed locally and referenced by at least 2 experienced neuropathologists.7 After introduction of the 2016 WHO classification amendment,7 all specimens were reevaluated and an integrated diagnosis was reported.
Immunohistochemistry was performed as described.19 Genetic entities were defined using immunohistochemistry with antibodies against ß-catenin, Yap1, p75 nerve growth factor receptor, Otx2, and p53. DNA was extracted and direct sequencing (Sanger) of exon 3 of catenin beta-1 was performed.20 Genome-wide copy number estimation and analysis of allelic distribution were performed by molecular inversion profiling21 and analyzed by Nexus Copy Number 7.0 Discovery Edition software (BioDiscovery). If minimal DNA was available, a multiplex ligation-dependent probe amplification was done.22
Global DNA methylation profiling using the Illumina Human Methylation 450 Bead Chip array was performed. Medulloblastoma subgrouping was done as described.19,23 Genome-wide copy number profiles were generated using the “conumee” package (https://www.bioconductor.org/packages/release/bioc/html/conumee.html) in the R programming environment.
As meningeosis in adults with medulloblastoma is not well described, CSF punctures were done in all patients at the time of diagnosis and recommended every 3 months thereafter or if clinical symptoms occurred. CSF was referenced centrally.
Feasibility and Toxicity
Prospectively defined toxicity-related treatment terminations after 4 cycles of adjuvant chemotherapy and the toxicity profile were primary endpoints and prospectively documented.
Adverse events (using Common Toxicity Criteria v3.024) were evaluated and graded at every visit, and causality of treatment was assessed. Dose de-escalation rules are detailed in Supplementary Table S1. Severe adverse events were handled by a safety board following international guidelines. An external data safety monitoring board supervised the trial.
An arbitrary cutoff of 45 years was used in a post-hoc analysis to compare toxicity in younger versus older patients.
Efficacy
EFS, progression-free survival (PFS), and OS at 3 and 5 years were secondary endpoints and recorded at each site visit. EFS was defined as time from study inclusion to disease progression, death, or discontinuation of treatment. PFS was a combined imaging and clinical endpoint adapted to the Response Assessment in Neuro-Oncology criteria25 and defined as time from study inclusion to disease progression or death. OS was calculated from study inclusion to death.
Health-Related Quality of Life Evaluation
Health-related quality of life (HRQoL) was prospectively evaluated before radiochemotherapy, after radiochemotherapy, and each 3 months thereafter using the European Organisation for Research and Treatment of Cancer 30-item core quality of life questionnaire (QLQ-C30) and the 20-item QLQ for brain neoplasm (QLQ-BN20).26 Role, cognitive, and social functioning were defined for the primary analyses to reduce errors from multiple testing. The other scales were analyzed on an exploratory basis. A difference in the mean value of HRQoL parameters of ≥10 points was set to be “clinically meaningful.”27
Cognitive Testing
Cognition was prospectively evaluated at baseline before radiochemotherapy, after radiochemotherapy, and each 3 months thereafter. The Controlled Oral Word Association Test (COWA)28 was used to measure lexical verbal fluency. Semantic verbal fluency was measured by the Regensburger Wortflüssigkeitstest.29 Verbal working memory was measured with the digit span forward and backward from the German version of the Wechsler Adult Intelligence Scale–Revised.30 Trail Making Tests (Parts A and B) were used to measure complex visual perceptual tracking, planning, and flexibility.31 Raw scores were transformed into adjusted z-scores, and a mean z-score was calculated, indicating group performance. Performance not reaching a z-score of −1 was described as impaired. A healthy control population matched for age, gender, and education was used.
Statistical Analysis
In this prospective descriptive trial, a total of 30 patients was considered to be the minimum population to evaluate feasibility. The incidence of treatment terminations within the first 4 cycles of chemotherapy was determined as the primary endpoint to measure feasibility.32 Based on published data,33 we hypothesized that if the study population consisted of 30 patients and that fewer than 18 patients (60%) or maximally 17 patients (56.7%) would have to terminate treatment due to toxicity, the primary endpoint would be met (NQuery 4.0, Statistical Solutions).
All primary endpoint data are presented descriptively. The 3-year EFS/PFS rate was estimated by using binary proportions. PFS and OS analyses were performed using the Kaplan–Meier method. Correlations between data groups were evaluated by Spearman rank analysis. Comparative statistical analyses for rates and proportions were performed using chi-square analysis. For 2 group comparisons, Wilcoxon rank-sum tests were computed. The significance level was defined as P < 0.05 (Stata 14).
Results
Patient Characteristics
From 2009 to 2014, thirty-three patients were included at 15 German centers. Thirty patients were evaluable, and 25 of them (83.3%) were treated per protocol (Supplementary Figure S1). Median age was 37 (range, 21–53), the median KPS at inclusion was 90 (range, 50–100), and 77.0% of patients had a KPS of 90 or above at baseline (Table 1).
Table 1.
Baseline patient characteristics
| Characteristic | Mean ± SD, range / n / percent of total |
|---|---|
| Age at diagnosis | 37.2 ± 10 y |
| range: 21.7–53.7 y | |
| Gender | |
| Male | 19 (63.3%) |
| Female | 11 (36.7%) |
| KPS (at inclusion) | |
| 100 | 8 (26.7%) |
| 90 | 15 (50.0%) |
| 80 | 2 (6.7%) |
| 70 | 3 (10.0%) |
| 60 | 1 (3.3%) |
| 50 | 1 (3.3%) |
| 40-0 | 0 (0.0%) |
| Localization | |
| Lateral/hemispheric | 11 (36.7%) |
| Midline | 8 (26.7%) |
| With involvement of cerebellar peduncle | 6 (20.0%) |
| Midline and peduncular | 1 (3.3%) |
| Not available | 4 (13.3%) |
| Leptomeningeal spread in MRI | 9 (30.0%) |
| Leptomeningeal spread in lumbar puncture | 3 (10.0%) |
| Histological entity (n = 30) | |
| Classic | 15 (50%) |
| Desmoplastic/nodular | 15 (50%) |
| Anaplastic | 0 (0%) |
| Other | 0 (0%) |
| Genetic entity (n = 29) | |
| SHH | 20 (66.7%) |
| WNT | 4 (13.3%) |
| Group 4 | 5 (16.7%) |
| Not available | 1 (3.3%) |
MR Imaging Classification
Most tumors were lateral/hemispheric (Table 1), and 6.7% of patients showed signs of leptomeningeal spread (Tables 1 and 2). M2 and M3 disease were only diagnosed during MRI reference evaluation. These patients were included in the study based on local evaluation.
Table 2.
Modified Chang classification [3] evaluated from imaging and CSF patterns
| T1, n | T2, n | T3a, n | T3b, n | T4, n | Total, n (%) | |
|---|---|---|---|---|---|---|
| M0, n | 2 | 3 | 8 | 1 | 1 | 15 (50.0) |
| M1, n | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| M2, n | 0 | 0 | 8 | 0 | 1 | 9 (30.0) |
| M3, n | 0 | 0 | 1 | 0 | 0 | 1 (3.3) |
| M4, n | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Total, n (%) | 2 (6.7) | 3 (10.0) | 17 (56.7) | 1 (3.3) | 2 (6.6) | 26 (86.7)* |
Note. Condensed contrast-enhanced T1-w post Gd and T2-w imaging, lumbar puncture results, and additional staging were included. T stands for primary tumor, M for metastasis. T1 is tumor less than 3 cm in diameter and limited to midline, roof of the fourth ventricle, and cerebellar hemispheres. T2 is tumor more than 3 cm in diameter, invading one adjacent structure or partially filling the fourth ventricle. T4 is tumor spreading through the aqueduct of Sylvius, or tumor extending to the upper cervical cord. M0 stands for no evidence of metastasis. M1 is presence of microscopic tumor cells in CSF, M2 is gross nodular seeding demonstrated in the subarachnoid space or ventricles. M3 is gross nodular seeding in the spinal subarachnoid space; and M4 is extraneural metastasis. *Missing data due to lack of source imaging data.
Enhancing tumor was completely resected in 15 patients (Supplementary Table 2). After combined radiochemotherapy, 73.3% of patients had a complete response (Supplementary Table S2). No patient progressed before starting adjuvant chemotherapy.
Chang Classification
Twenty-six of 30 tumors were classifiable, showing a mixed pattern (Table 2). If evaluations were based on local readings, the incidence of M2 and M3 disease was much lower (6.7% of patients; n = 2).
Histological and Genetic Classification and CSF Evaluation
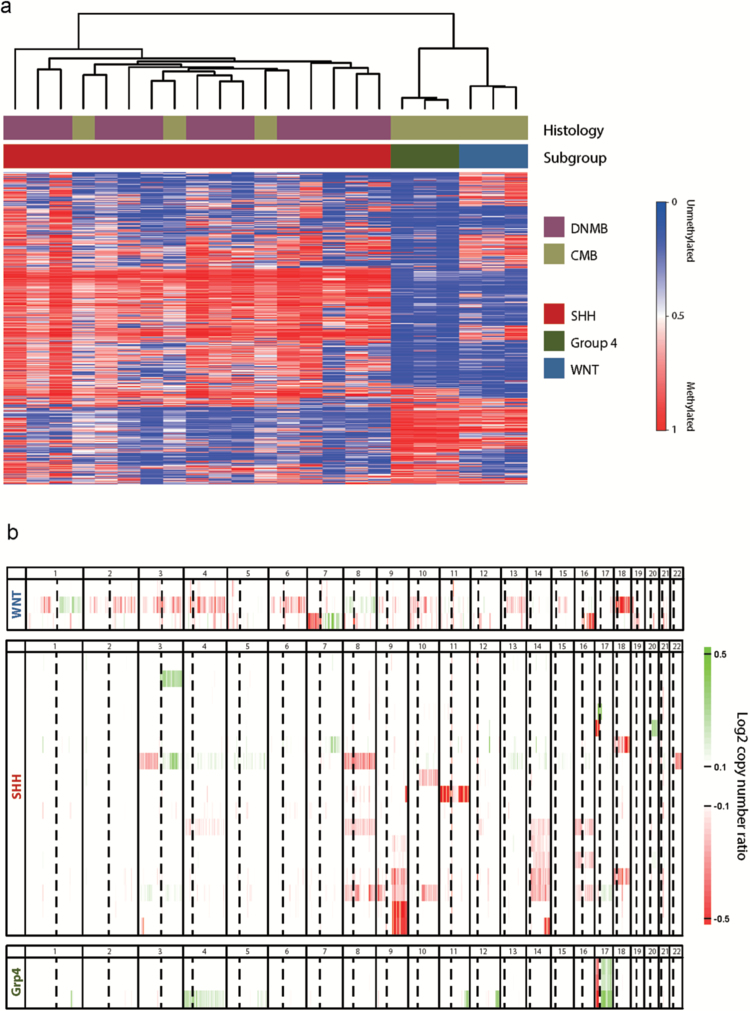
Each 50% of tumors were in the classic and desmoplastic/nodular entities (Table 1, Figure 1). Twenty-nine of 30 patients could be assigned to genetically defined entities by immunohistochemistry, and 23 of these were evaluable by 450k methylation classification34 with congruent results (Table 1). The catenin beta-1 exon 3 was mutated in all 4 WNT-activated tumors (D32V, S33F, S33P, S37Y). Whole genomic copy number and allelic distribution were analyzed without evidence for amplifications of MYC, MYCN, or GLI2. One SHH-activated tumor showed copy number losses 9q and chr14, typical for SHH-activated subgroups. However, 17p losses (TP53) were not found in SHH-activated cases, which is in line with the absence of TP53 accumulation (Figure 1).
Fig. 1.
Histological and genetic distribution in NOA-07. (A) Clustering of samples based on the 5000 most differentially methylated cytosine-phosphate-guanine probes (standard deviation), with DNA methylation values shown from unmethylated (blue) to methylated (red). SHH-activated, WNT-activated, and non-WNT/non-SHH (Group 4) tumors are clearly distinct. DNMB, desmoplastic/nodular medulloblastoma; CMB classic medulloblastoma. (B) Summary of copy number profiles per molecular subgroup. Log2 copy number ratios (tumor: normal) are displayed on a scale from loss (red) to gain (green). Notable changes include monosomy 6 in one WNT-activated sample, loss of 9q in a subset of SHH-activated tumors, and iso(17q) in all 3 non-WNT/non-SHH (Group 4) tumors.
Twenty-six of 30 patients (86.6%) had a lumbar puncture at diagnosis. Three patients were diagnosed with leptomeningeal spread on lumbar CSF evaluation, one of them with central review that verified the diagnosis (Table 1). Central review was performed only in the minority of cases (9/30 patients; 1 of them with discrepancy between local and central). Lumbar punctures after diagnosis were only performed if meningeosis was suspected, and no systematic data could be raised.
Feasibility and Toxicity
All patients received standard photon-based radiotherapy. Radiochemotherapy was completed in all patients (Table 3). Two patients terminated therapy before maintenance chemotherapy was started, one with tuberculosis and paralytic ileus, and one with pneumonia with sepsis. Two patients were lost because they withdrew informed consent, and one patient changed to a local institution after radiochemotherapy which was not registered as a study site. Seventy percent of patients (n = 21) tolerated at least 4 cycles of chemotherapy, all of them with dose modifications. Therefore, the prespecified feasibility goal of more than 60% of patients receiving at least 4 cycles of maintenance chemotherapy was met.
Table 3.
Treatment compliance and duration
| Resection |
N Total Days, mean ± SD |
|---|---|
| Interval from diagnosis to resection | 10 ± 15.2 |
| Resection compliance | 30 (100%) |
| Grade of resection | |
| Complete | 15 (50.0%) |
| Partial | 8 (26.7%) |
| Biopsy | 0 (0.0%) |
| Data not available | 7 (23.3%) |
| Average percent of resection (in MRI) | 94.7% ± 12.4% |
| Radiochemotherapy | Number of patients; median days (% of total) |
| Interval from resection to radiochemotherapy | 53.0 ± 24 |
| Interval from radiochemotherapy to adjuvant chemotherapy | 48.0 ± 79 |
| Radiotherapy per protocol | 30 (100%) |
| Concomitant chemotherapy | |
| Less than 4 doses of vincristine (VCR) | 2 (6.6%) |
| 4–6 doses of VCR | 16 (53.3%) |
| 7–8 doses of VCR | 12 (40.0%) |
| Maintenance chemotherapy | Number of patients (% of total) |
| Interval from resection to adjuvant chemotherapy | 166 ± 76.5 |
| Dropout rate | |
| By cycle 1 | 5 (16.7%) |
| By cycle 2 | 5 (16.7%) |
| By cycle 3 | 9 (30.0%) |
| By cycle 4 | 9 (30.0%) |
| By cycle 5 | 9 (30.0%) |
| By cycle 6 | 11 (36.7%) |
| By cycle 7 | 17 (56.7%) |
| By cycle 8 | 20 (66.7%) |
| Cycles of chemotherapy given | 155 (100%) |
| Cycles given per protocol | 77 (49.7%) |
Note. Time to start of each treatment part was calculated from time of tumor resection to first day of the respective treatment. Extent of resection was calculated from postoperative MRI, and compliance to radiochemotherapy and maintenance chemotherapy was extracted. Data were calculated in the intent-to-treat population.
One patient progressed after the fifth adjuvant cycle. All other patients were discontinued due to treatment-related toxicity or withdrawal of informed consent (Table 3). Seventy-seven of 155 cycles were given per protocol (49.7%) (Table 3 and Supplementary Figure S2A and B).
Leukopenia was the major toxicity. Polyneuropathy and ototoxicity were the only grade 3 or 4 nonhematological toxicities (Table 4). Six patients had grade 1, four patients grade 2, and 4 patients grade 3 polyneuropathy.
Table 4.
Hematological and nonhematological toxicity grades 1/2 and 3/4*
| Toxicity Type | Radiochemotherapy (% of affected patients) | Radiochemotherapy (no. of events) | Adjuvant Chemotherapy (% of affected patients) | Adjuvant Chemotherapy (no. of events) | Active Treatment Phase
(% of affected patients) |
Active Treatment Phase (no. of events) |
|---|---|---|---|---|---|---|
| Grades 1/2 | ||||||
| Leukopenia | 53.3 | 16 | 66.7 | 62 | 76.7 | 78 |
| Thrombocytopenia | 56.7 | 17 | 50.0 | 46 | 76.7 | 63 |
| Anemia | 70.0 | 21 | 40.0 | 111 | 86.7 | 132 |
| Infection | 23.3 | 7 | 16.7 | 12 | 30.0 | 19 |
| Nausea | 66.7 | 20 | 46.7 | 39 | 73.3 | 59 |
| Emesis | 30.0 | 9 | 16.7 | 13 | 36.7 | 22 |
| Polyneuropathy | 30.0 | 9 | 63.3 | 81 | 70.0 | 81 |
| Ototoxicity | 10.0 | 3 | 23.3 | 33 | 23.3 | 36 |
| Grades 3/4 | ||||||
| Leukopenia | 36.7 | 11 | 66.7 | 68 | 56.7 | 79 |
| Thrombocytopenia | 3.3 | 1 | 36.7 | 33 | 40.0 | 34 |
| Anemia | 13.3 | 4 | 20.0 | 20 | 20.0 | 24 |
| Infection | 10.0 | 3 | 0.0 | 0 | 20.0 | 3 |
| Nausea | 6.7 | 2 | 0.0 | 0 | 13.3 | 2 |
| Emesis | 3.3 | 1 | 0.0 | 0 | 6.7 | 1 |
| Polyneuropathy | 16.7 | 5 | 20.0 | 12 | 26.7 | 12 |
| Ototoxicity | 0.0 | 0 | 20.0 | 1 | 3.3 | 1 |
*According to Common Terminology Criteria for Adverse Events v3.0.
Note. Toxicity was evaluated during radiochemotherapy and during maintenance chemotherapy. Percents were calculated in relation to patients under treatment at the respective time points.
Events were also calculated as events per cycle and showed an increase of toxicity over treatment time (Table 4, Supplementary Table S3, and Supplementary Figure S2B). Treatment was terminated or dose intensity was reduced in almost 60% of patients at cycle 4 due to side effects (Supplementary Figure S2B). Of note, vincristine was stopped early on in a large number of patients, whereas lomustine and cisplatin were typically stopped later in the course of treatment. The number of severe adverse events per patient was highly variable (range 0–23).
Feasibility appeared to be age dependent. Post-hoc analyses showed that 72.7% of patients below age 45 received 4 cycles of chemotherapy, but only 62.5% of patients older than 45. Testing for all 8 adjuvant cycles revealed that 45.5% of all patients younger than 45 years completed 8 cycles, whereas only 12.5% of patients over 45 years received all cycles. Severe adverse events were significantly more frequent in patients older than 45 years (P = 0.040). We observed no treatment-related deaths.
Health-Related Quality of Life
Compliance to HRQoL evaluation was >65% at most time points (Supplementary Table S4). Scoring was reduced (≥10 points) in role, cognitive, and social functioning directly postoperative, where role and social functioning were also significantly worse in comparison to a glioblastoma population35,36 (Supplementary Table S5). On a group level, role, cognitive, and social functioning improved over time (Figure 2). Similar results were found for the exploratory HRQoL items (Supplementary Figure S3).
Fig. 2.
HRQoL evaluation (main categories). Scores over time for the 3 preselected scales: (A) role functioning, (B) cognitive functioning, and (C) social functioning, with the number of patients with HRQoL data at each time point.
Cognition
Compliance to cognition testing was 44.5%. On a group level, scores for working memory remained within normal limits. Attention was impaired at all time points, and visual perception was impaired at the first and the second measurement but returned to normal in the third measurement with a mean performance above z = −1. Lexical verbal fluency (COWA, n = 7, M1 = −1.8, M3 = −0.6, P < 0.05) and semantic verbal fluency (food naming, n = 7, M1 = −1.92, M3 = −0.42, P < 0.05) were impaired before radiochemotherapy but reached an average level of z = −0.84 and z = −1.0 in the third measurement, indicating improvement to normal (Supplementary Figure S4).
Efficacy
At databank lock (June 1, 2016), median follow-up was 58.0 months, and a total of 7 patients had relapsed, among them 1 patient with M3 disease (classic medulloblastoma, genetic entity non-WNT/non-SHH; no MYC/MYCN amplification or TP53 mutation), 1 patient with non-WNT/non-SHH M0, and 5 patients with SHH-activated tumors, none of them with p53 mutation or MYCN amplification (Table 1). Three patients died in the treatment phase, all from tumor-related complications (epileptic seizure in the bathtub, aspiration pneumonia, and suicide).
The 3-year EFS rate was 66.6%, and the 3-year PFS and OS rates were 66.6% and 70.0%, respectively. With 83% of patients without progression and 90% of all patients still alive, median PFS and OS were not reached. Genetic subgroups were not prespecified to be explored for efficacy due to limited patient numbers.
Correlation Analysis
Only one of the non-WNT/non-SHH (Group 4) tumors was Chang M3 at diagnosis. Almost all tumors with a lateral localization in MRI were SHH activated (90.9%, P = 0.040). Non-WNT/non-SHH activated tumors were lateral in 20% of cases, and not a single WNT-activated tumor was lateral (P = 0.01). Both lateral location and SHH activation were associated with a higher proportion of complete resections (63.6% vs 36.8%, P = 0.018; and 45.3% vs 38.7%, P = 0.026). Accordingly, after volumetric evaluation of MRI, complete tumor resections were more prevalent in the SHH-activated tumors (97.5% vs 86.9%, P = 0.024).
Relapses were more prevalent in the SHH-activated tumors (80% of recurrent cases were SHH vs 64% of nonrecurrent cases; P = 0.091) (Supplementary Table S6).
Discussion
NOA-07 is a prospective descriptive trial to evaluate feasibility and toxicity of combined radio-polychemotherapy in adults with newly diagnosed medulloblastoma. The primary endpoint, the number of toxicity-related treatment terminations after 4 cycles of adjuvant chemotherapy, was justified retrospectively by upcoming data that suggest that a decreased number of treatment cycles is not decisive for PFS or OS in children with average-risk medulloblastoma.37 The regimen was feasible for at least 4 cycles of maintenance chemotherapy in 70.0% of patients. The prespecified study goal that more than 18 patients (60%) could be treated with at least 4 cycles of chemotherapy was therefore met. Considerable and increasing toxicity was observed, with polyneuropathy as the main non hematological toxicity occurring early within treatment, and leukopenia and thrombocytopenia as the most prevalent hematological toxicities. Sixty-seven percent of patients went off-study due to toxicity under maintenance chemotherapy. We therefore conclude that the regimen induces more severe toxicity than in comparable pediatric trials.
Recent publications suggest that vincristine, which induces a high rate of neurotoxicity, may be fully deleted without endangering efficacy38 and that alternative agents may possibly replace vincristine during concomitant radiochemotherapy.39 In addition, an attenuated maintenance regimen may decrease toxicity while sustaining efficacy.40
With adherence to strict de-escalation rules, toxicity was manageable. No unexpected severe adverse events were recorded. In a nonrandomized retrospectively evaluated cohort of young adults treated with the same regimen,13 28 of 47 patients (59.6%) received the full number of 8 maintenance chemotherapy cycles. Median age was 37.0 years in our trial and 28.5 years in the other cohort. This difference in age may indicate decreased feasibility in older patients. Accordingly, feasibility was age dependent in NOA-07, with a higher rate of adverse and a significantly higher rate of severe adverse events in patients above age 45.
Long-term neurotoxicity is a major concern in radiochemotherapy regimens involving the brain.5,41,42 In a Canadian trial that focused on medulloblastoma patients in their second life decade, long-term ototoxicity and neurotoxicity of grade 2 or above occurred in 45.0% and 71.0% of patients.5 Long-term toxicity results of NOA-07 are lacking due to the short follow-up time of median 58.0 months and will be reported after all patients have been followed for 5 years.
HRQoL and cognition are important correlates of toxicity and long-term outcomes. During the active treatment phase of NOA-07, HRQoL and cognitive function improved. Long-term data will be supplemented by a social outcome analysis that will mainly focus on long-term social function.
Study results from Packer and coworkers indicate that radiotherapy plus concomitant and adjuvant chemotherapy is superior to radiotherapy alone in children.9,11,43 Patients in the Friedrich trial13 experienced a 4-year EFS of 68.0% and a 4-year OS of 89.0%, similar to premature data in NOA-07. A recent meta-analysis by Kocakaya et al showed that patients receiving chemotherapy first-line survived significantly longer (median OS: 108 mo, 95% CI: 68.6–148.0) than patients treated with radiotherapy alone (median OS: 57 mo, 95% CI: 39.6–74.4).44 Importantly, published data also show that the risk of recurrence appears to increase markedly with time.16 In conclusion, published evidence strongly indicates a role for combined radiochemotherapy for adults with medulloblastoma but also warrants long-term follow-up in this population. The NOA-07 protocol is the first trial that evaluated these questions prospectively.
We further analyzed MR imaging and histological and molecular patterns to detect unusual patterns on descriptive and correlative levels. Our results correspond well to published results.4,6,19,23,44 Of note, metastatic disease (Chang M1 to M3) was found in one third of patients during central review, but only 6.7% in local evaluations. This points to a strict central review strategy during diagnostic workup, as metastatic disease is connected to worse outcomes3 and may mandate adapted treatment strategies.
Reference analysis of medulloblastoma subgroups showed the expected histological and genetic distribution. The genetic pattern in WNT-activated adult patients in our dataset was different from that of children and might indicate a different biology and explain the worse prognosis.44 The SHH subgroup is highly overrepresented in adults, and SHH-activated tumors had a favorable outcome in infants and young children (5-y OS, 77.0%) compared with older children (5-y OS, 68.0%) and adults (5-y OS, 34.0%).6 In the NOA-07 trial, 5 of 7 early relapses were SHH activated. All SHH-activated tumors in our series represented the SHH-activated TP53 wildtype entity, none showed MYC/MYCN amplification, and the subgroup comprised a higher rate of complete resections and comparable dose intensities during radiochemotherapy.
Shortcomings of this trial are limited patient numbers, the distribution of patients to a large number of centers that may increase toxicity rates due to lower experience of the involved investigators, and the nonrandomized design. However, the trial was powered for feasibility and toxicity as its primary endpoint.
In summary, this prospective descriptive trial evaluated feasibility and toxicity of a radio-polychemotherapy regimen in a homogeneous cohort of intermediate prognostic adults with medulloblastoma, as well as imaging, histological and molecular parameters, HRQoL, cognition, and EFS, PFS, and OS outcomes. Long-term evaluations are ongoing. We conclude that combined radiochemotherapy is associated with considerable toxicity and mandates predefined tapering rules and dose modifications in the majority of patients. Modified regimen may increase feasibility of radio-polychemotherapy of adult patients with medulloblastoma.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by internal grants from the German Neuro-Oncology Society (NOA) and an unrestricted educational grant from medac GmbH.
The sponsors had no influence on study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. The Corresponding Author had full access to all the data in the study and had full responsibility for the decision to submit for publication.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
This work is dedicated to our esteemed colleague, Joachim Kühl, MD, deceased, who was one of the driving forces to initiate this trial. We cordially thank all investigators and study staff in the centers who did a tremendous job to compile all data.
References
- 1. Peris-Bonet R, Martinez-Garcia C, Lacour B et al. . Childhood central nervous system tumours—incidence and survival in Europe (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2064–2080. [DOI] [PubMed] [Google Scholar]
- 2. Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer. 2012;118(5):1313–1322. [DOI] [PubMed] [Google Scholar]
- 3. Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 4. Remke M, Hielscher T, Northcott PA et al. . Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–2723. [DOI] [PubMed] [Google Scholar]
- 5. Tabori U, Sung L, Hukin J et al. ; Canadian Pediatric Brain Tumor Consortium Medulloblastoma in the second decade of life: a specific group with respect to toxicity and management: a Canadian Pediatric Brain Tumor Consortium Study. Cancer. 2005;103(9):1874–1880. [DOI] [PubMed] [Google Scholar]
- 6. Kool M, Korshunov A, Remke M et al. . Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 8. Korshunov A, Remke M, Kool M et al. . Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathol. 2012;123(4):515–527. [DOI] [PubMed] [Google Scholar]
- 9. Zhukova N, Ramaswamy V, Remke M et al. . Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer RJ, Sutton LN, Elterman R et al. . Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81(5):690–698. [DOI] [PubMed] [Google Scholar]
- 11. Packer RJ, Goldwein J, Nicholson HS et al. . Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. [DOI] [PubMed] [Google Scholar]
- 12. Packer RJ, Gajjar A, Vezina G et al. . Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 13. Packer RJ, Gurney JG, Punyko JA et al. . Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 14. Friedrich C, von Bueren AO, von Hoff K et al. . Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: a prospective observational multicentre study. Eur J Cancer. 2013;49(4):893–903. [DOI] [PubMed] [Google Scholar]
- 15. von Bueren AO, Friedrich C, von Hoff K et al. . Metastatic medulloblastoma in adults: outcome of patients treated according to the HIT2000 protocol. Eur J Cancer. 2015;51(16):2434–2443. [DOI] [PubMed] [Google Scholar]
- 16. Kocakaya S, Beier CP, Beier D. Chemotherapy increases long-term survival in patients with adult medulloblastoma—a literature-based meta-analysis. Neuro Oncol. 2016;18(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110(9):2035–2041. [DOI] [PubMed] [Google Scholar]
- 18. Perreault S, Ramaswamy V, Achrol AS et al. . MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pietsch T, Schmidt R, Remke M et al. . Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goschzik T, Zur Mühlen A, Kristiansen G et al. . Molecular stratification of medulloblastoma: comparison of histological and genetic methods to detect Wnt activated tumours. Neuropathol Appl Neurobiol. 2015;41(2):135–144. [DOI] [PubMed] [Google Scholar]
- 21. Japp AS, Gessi M, Messing-Jünger M et al. . High-resolution genomic analysis does not qualify atypical plexus papilloma as a separate entity among choroid plexus tumors. J Neuropathol Exp Neurol. 2015;74(2):110–120. [DOI] [PubMed] [Google Scholar]
- 22. Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30(12):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hovestadt V, Remke M, Kool M et al. . Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trotti A, Colevas AD, Setser A et al. . CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 25. Galanis E, Wu W, Cloughesy T et al. . Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol. 2012;13(5):e196–e204. [DOI] [PubMed] [Google Scholar]
- 26. Aaronson NK, Ahmedzai S, Bergman B et al. . The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 27. King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5(6):555–567. [DOI] [PubMed] [Google Scholar]
- 28. Benton AL, Hamsher K.. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 29. Aschenbrenner S, Tucha O, Lange KW.. Regensburger Wortflüssigkeitstest RWT Handanweisung. Göttingen, Bern. Toronto, Seattle: Hogrefe; 2000. [Google Scholar]
- 30. Aster von M, Neubauer A, Horn R.. Wechsler Intelligenztest für Erwachsene WIE. 2. Auflage. Frankfurt/M: Pearson Assessment & Information GmbH; 2009. [Google Scholar]
- 31. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. [DOI] [PubMed] [Google Scholar]
- 32. Bowen DJ, Kreuter M, Spring B et al. . How we design feasibility studies. Am J Prev Med. 2009;36(5):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colevas D. Toxicity Monitoring: Why, What, When? New York: Demos Medical Publishing; 2010(Oncology Clinical Trials):151–162. [Google Scholar]
- 34. Hovestadt V, Jones DT, Picelli S et al. . Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature. 2014;510(7506):537–541. [DOI] [PubMed] [Google Scholar]
- 35. van de Poll-Franse LV, Mols F, Gundy CM et al. . Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. [DOI] [PubMed] [Google Scholar]
- 36. Taphoorn MJ, Henriksson R, Bottomley A et al. . Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. [DOI] [PubMed] [Google Scholar]
- 37. Nageswara Rao AA, Wallace DJ, Billups C, Boyett JM, Gajjar A, Packer RJ. Cumulative cisplatin dose is not associated with event-free or overall survival in children with newly diagnosed average-risk medulloblastoma treated with cisplatin based adjuvant chemotherapy: report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014;61(1):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarbell NJ, Friedman H, Polkinghorn WR et al. . High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013;31(23):2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Esbenshade AJ, Kocak M, Hershon L et al. . A phase II feasibility study of oral etoposide given concurrently with radiotherapy followed by dose intensive adjuvant chemotherapy for children with newly diagnosed high-risk medulloblastoma (protocol POG 9631): a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017;64(6): doi: 10.1002/pbc.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dagri JN, Evans A, Torkildson J et al. . Feasibility of an attenuated maintenance chemotherapy regimen directed at adolescents and young adults with newly diagnosed localized medulloblastoma and other central nervous system embryonal tumors. J Adolesc Young Adult Oncol. 2014;3(3):106–111. [Google Scholar]
- 41. Shan ZY, Liu JZ, Glass JO, Gajjar A, Li CS, Reddick WE. Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magn Reson Imaging. 2006;24(8):1015–1022. [DOI] [PubMed] [Google Scholar]
- 42. Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–1049. [DOI] [PubMed] [Google Scholar]
- 43. Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korshunov A, Remke M, Werft W et al. . Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28(18):3054–3060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.