The Central Brain Tumor Registry of the United States (CBTRUS) was established to provide descriptive statistical data on all primary brain tumors. The following editorial describes some of the historical events that led to its establishment and its current role as the recognized “go-to” resource for researchers; clinicians and treatment facilities; federal and state agencies; manufacturers of medical drugs and devices; and organizations that provide education, emotional support, and guidance for brain tumor patients and their caregivers.
In March 1988, a group of neuropathologists met in Houston, Texas to discuss classification of brain tumors.1 In March 1988, a little boy died from a medulloblastoma. Who knew these two events would be linked at some future date!
In 1989, five people met at the American Brain Tumor Association headquarters. Their aim was to count every patient with a diagnosis of primary brain or other central nervous system (CNS) tumor. At that time, only brain tumors with a malignant behavior (International Coding of Diseases for Oncology second edition [ICD-O-2] behavior code /3) were mandated to be reported by cancer registries as per the National Cancer Act of 1971.2 During the course of their investigation, two committee members left: a glioblastoma patient to focus on his family, and the other to grieve for the loss of another family member to a brain tumor.
The determination and personal motivation of the three remaining on the committee—Gail Segal, Naomi Berkowitz, and Carol Kruchko—grew. They developed a Feasibility Study to investigate the formation of a central brain tumor registry for the United States. They hired Dr Faith Davis, an epidemiologist from the University of Illinois at Chicago (UIC) School of Public Health to fulfill the study’s aims. Dr Davis brought the expertise of Dr William Haenszel, who was responsible for developing the Mantel–Haenszel statistical test commonly used in epidemiology studies.3 Even though Dr Haenszel was elderly and had Parkinson’s disease, he came to UIC monthly and tutored Dr Davis’ research team, which now included Ms Kruchko, on cancer surveillance. He had developed the first national system to track cancer cases and their possible causes with the establishment of the Surveillance, Epidemiology, and End Results (SEER) program.3,4
Dr Haenszel’s stature in the cancer registry community provided the group with the connections needed to bring a brain tumor registry to fruition. Many of his former colleagues were still working at SEER. Dr Connie Percy was revising ICD-O-2, and in 2000, ICD-O-3 was published with a cover in her signature purple color. It continues to guide tumor registrars in coding the site (topography) and histology (morphology) of neoplasms.5 Dr John Young had left SEER after serving as director from 1978 to 1988. Dr Young had overlapping positions as Chief, California Cancer Registry and Adjunct Associate Professor, Department of Epidemiology and Biostatistics, School of Medicine, University of California at San Francisco. He was instrumental in forming the American Association of Central Cancer Registries (AACCR, later the North American Association of Central Cancer Registries [NAACCR]) in 1987 to develop and promote uniform data standards for cancer registration.6 It was during an AACCR meeting that Dr Davis and Ms Kruchko proposed including benign brain tumors in cancer collection to Drs Percy and Young and others.
Meanwhile, the Feasibility Study had demonstrated that it was possible to collect data on benign brain tumors through the cancer registry mechanism. The timing could not have been better for introducing this practice. There was already discontent with cancer registration, as only a sample of cases were being collected through SEER. It seemed plausible that every primary cancer case would be collected, including all cases of brain tumors, and that it was an appropriate time to add another change to cancer collection and include benign brain tumors. In 1992, the Cancer Registries Amendment Act, Public Law 102–515 was passed,7 and the Centers for Disease Control and Prevention (CDC) was charged with executing the new law as part of the National Program of Cancer Registries (NPCR). While every cancer case would now be collected, there was more work to be done before adding benign brain tumors to collection practices.
On July 28, 1992, CBTRUS was incorporated as a nonprofit 501(c)(3).8 The Board included Drs Darell Bigner, Steven Brem, Fred Hochberg, and Michael Walker, along with Bonnie Feldman, Donald Segal, Michael Traynor, and Ms Kruchko. Mr Traynor’s group, the Ride for Kids, continued to provide the primary funding, and Dr Davis moved forward with a pilot study. A poster was presented at the Tenth International Conference on Brain Tumour Research and Therapy in Stalheim, Norway in 1993 by Ms Kruchko with backup from Dr Roger McLendon, a neuropathologist who has remained a steadfast supporter of CBTRUS efforts. The subsequent manuscript, entitled “Descriptive Epidemiology of Primary Brain and CNS Tumors: Results from the Central Brain Tumor Registry of the United States, 1990–1994,” was published in the inaugural issue of Neuro-Oncology.9 Later that same year, CBTRUS assembled an advisory board of epidemiologists and population scientists, some of whom, such as Drs Margaret Wrensch, Melissa Bondy, and Hoda Anton-Culver, have continued to provide guidance.
While CBTRUS worked to demonstrate the importance of including benign brain tumors in population-based collection of brain tumors by self-publishing Statistical Reports, the international neuropathology community had continued the work of March 1988 to establish a single classification scheme, the 1993 World Health Organization (WHO) Histological Typing of Tumours of the Central Nervous System.10,11 In 1998, WHO published Histological Groups for Comparative Studies.12 Dr Kleihues was the Director of the International Agency for Research on Cancer at the time and was responsible for the easy “carry with you” design of the WHO classification books. The 1993 WHO classification influenced updates and revisions to the CBTRUS Histology Grouping Scheme which are still in use today.
In 1998, a petition for benign brain tumor collection to the National Coordinating Council on Cancer Surveillance resulted in four recommendations13:
(1) Adopt a site definition for collecting precise data for all primary intracranial and CNS tumors;
(2) Develop a standard definition for use across registries with input from surveillance stakeholders and pathologists;
(3) Mandate collection of the site definition by all registries, hospital- and population-based;
(4) Enlist appropriate government and professional organizations in development and implementation.
Recommendations 1 and 2 were adopted during the Consensus Conference hosted by CBTRUS during the Society for Neuro-Oncology (SNO) conference of 2000 and removed two obstacles to collection.14 Recommendations 3 and 4 required the passage of Public Law 107–260, the Benign Brain Tumor Cancer Registries Amendment.15 How this law came to be sponsored and passed by Congress is a story of dedication, passion, hard work, and luck. With its passage, CBTRUS found itself in the unique position of being the only surveillance organization with recognized reporting experience for all brain and other CNS tumors.
During 2004–2006, CBTRUS developed the Central Nervous System Tumor Implementation and Reporting Guide for NPCR under the leadership of Dr Bridget McCarthy.16 Later, Ms Kruchko entered into an agreement on behalf of CBTRUS with CDC for exclusive access to its data on all primary brain and other CNS tumors. Approval was reached for access to these data from all state cancer registries, also referred to as Central Cancer Registries in the United States, each of which had its own legislative rules. CBTRUS remains the only site-specific organization which has access to NPCR data, and in 2010, CBTRUS contributed to the Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring Tumors of the Brain and Other Nervous System.17
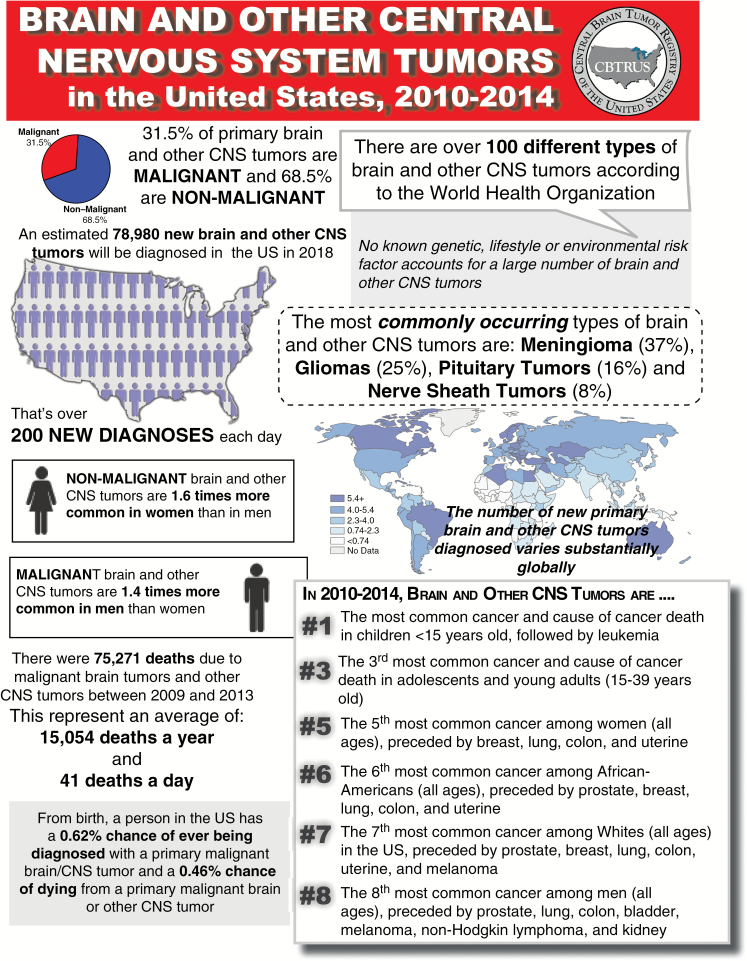
In 2011, CBTRUS received approval from the SNO Board to publish its Statistical Reports as Supplements to Neuro-Oncology. In November 2017, the Sixth Supplement was published online and in print (Figure 1).18
Figure 1.
Overview of key brain tumor statistics (CBTRUS 2017 Annual Statistical Report18).
There have been many milestones in the CBTRUS story. Since 2013, Dr Jill Barnholtz-Sloan, Professor at Case Comprehensive Cancer Center at Case Western Reserve University School of Medicine in Cleveland, Ohio, has been the Principal Scientific Investigator for CBTRUS. Her team has utilized the CBTRUS analytic files to publish over 20 manuscripts to date. In addition, the annual CBTRUS Statistical Report is among the most highly cited articles each year for Neuro-Oncology. CBTRUS has remained true to its commitment to keep its Histology Grouping correlated with the WHO classification. In 2017, it secured approval for the collection of biomarkers integrated in the 2016 WHO Classification of Tumours of the Central Nervous System19 in cancer registration practices beginning in 2018.
In 1988, two independent events occurred that influenced the missions of organizations and individuals aimed at improving the accuracy and completeness of primary brain tumor reporting. In 2018, the story continues.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 2016-M-9030, The Sontag Foundation, Genentech, Novocure, AbbVie, along with the Musella Foundation, the Children’s Brain Tumor Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily reflect the official views of the CDC.
Conflict of interest statement. There are no conflicts of interest to report.
References
- 1. Fields WS. Brain tumours: morphological aspects and classification. Brain Pathol. 1993;3(3):251–253. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Act of 1971, 92nd Congress § 1828. 1971; http://www.gpo.gov/fdsys/pkg/STATUTE-85/pdf/STATUTE-85-Pg778-3.pdf. [Google Scholar]
- 3. Levy PS, Haenszel W M.. Encyclopedia of Biostatistics. Chichester: John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 4. Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 5. International Agency for Research on Cancer. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). 2000. [Google Scholar]
- 6. Seiffert JE. Development and use of the North American Association of Central Cancer Registries standards for cancer registries. Top Health Inf Manage. 1997;17(3):35–44. [PubMed] [Google Scholar]
- 7. Cancer Registries Amendment Act, 102nd Cong. § 515 (1992). http://www.gpo.gov/fdsys/pkg/STATUTE-106/pdf/STATUTE-106-Pg3372.pdf. [Google Scholar]
- 8. Central Brain Tumor Registry of the United States. About CBTRUS 2017; http://cbtrus.org/aboutus/aboutus.html. [Google Scholar]
- 9. Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol. 1999;1(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3(3):255–268. [DOI] [PubMed] [Google Scholar]
- 11. Kleihues P, Burger PC, Scheithauer BW.. Histological Typing of Tumours of the Central Nervous System, 2nd ed Berlin, New York: Springer-Verlag; 1993. [Google Scholar]
- 12. Parkin D, Shanmugaratnam K, Sobin L, Ferlay J, Whelan S.. IARC Technical Report No. 31: Histological Groups for Comparative Studies. International Agency for Research on Cancer, World Health Organization; 1998. [Google Scholar]
- 13. Brain Tumor Progress Review Group. Report of the Brain Tumor Progress Review Group. Bethesda, MD, 2000. [Google Scholar]
- 14. McCarthy BJ, Surawicz T, Bruner JM, Kruchko C, Davis F. Consensus conference on brain tumor definition for registration. November 10, 2000. Neuro Oncol. 2002;4(2):134–145. http://www.ncbi.nlm.nih.gov/pubmed/11916506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benign Brain Tumor Cancer Registries Amendment Act, 107th Congress § 260. 2002; http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf. [Google Scholar]
- 16. Centers for Disease Control and Prevention. Data Collection of Primary Central Nervous System Tumors. National Program of Cancer Registries Training Materials. Atlanta, Georgia: Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 17. Kohler BA, Ward E, McCarthy BJ et al. . Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011; 103(9):714–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostrom QT, Gittleman H, Liao P et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(S5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]