Abstract
Firemaster 550 (FM 550) is a flame retardant (FR) mixture that has become one of the most commonly used FRs in foam-based furniture and baby products. Human exposure to this commercial mixture, composed of brominated and organophosphate components, is widespread. We have repeatedly shown that developmental exposure can lead to sex-specific behavioral effects in rats. Accruing evidence of endocrine disruption and potential neurotoxicity has raised concerns regarding the neurodevelopmental effects of FM 550 exposure, but the specific mechanisms of action remains unclear. Additionally, we observed significant, and in some cases sex-specific, accumulation of FM 550 in placental tissue following gestational exposure. Because the placenta is an important source of hormones and neurotransmitters for the developing brain, it may be a critical target of toxicity to consider in the context of developmental neurotoxicity. Using a mixture of targeted and exploratory approaches, the goal of the present study was to identify possible mechanisms of action in the developing forebrain and placenta. Wistar rat dams were orally exposed to FM 550 (0, 300 or 1000 µg/day) for 10 days during gestation and placenta and fetal forebrain tissue collected for analysis. In placenta, evidence of endocrine, inflammatory and neurotransmitter signaling pathway disruption was identified. Notably, 5-HT turnover was reduced in placental tissue and fetal forebrains indicating that 5-HT signaling between the placenta and the embryonic brain may be disrupted. These findings demonstrate that environmental contaminants, like FM 550, have the potential to impact the developing brain by disrupting normal placental functions.
Key Words: endocrine disruptors, flame retardants, behavior, serotonin, sexually dimorphic, EDC, brain
Introduction
Chemical flame retardants (FRs) are commonly applied to foam-based furniture and infant products to meet commercial flammability standards. Many FRs are additive rather than reactive, and therefore able to migrate, resulting in widespread human exposure (1). Previously, the most commonly used chemical FRs were mixtures of polybrominated diphenyl ethers (PBDEs). Due to toxicity concerns, including associations between PBDE exposure and behavioral and cognitive impairments in humans (2, 3, 4, 5, 6, 7), this class has largely been phased out, resulting in increased use of replacement FRs. These include alternative brominated and organophosphate ester (OPE) FRs (8), the toxicity of which are largely unknown. Structural similarities with known neurotoxicants and endocrine-disrupting chemicals (EDCs), including PBDEs (9) and organophosphate pesticides (10), have raised concerns regarding possible health impacts, especially on the developing brain. Because we have found evidence of behavioral effects and significant placental bioaccumulation in developmentally exposed rats (11, 12), we hypothesized that gestational exposure to replacement FRs can alter forebrain neurodevelopment and that the mode of action may involve altered signaling between placenta and the developing brain.
Throughout gestation, the placenta plays a critical role in regulating the fetal environment (13) including which chemicals are transferred to the fetus. The placenta also serves as a crucial and sometimes primary source of hormones, monoamines and other signaling factors for the developing brain (13). For example, the fetal side of the placenta is the sole source of 5-HT for the developing forebrain from GD 10–15 (5, 6). Placental function, including monoamine synthesis, can be disrupted by environmental stress, resulting in sex-specific effects on neurodevelopment and behavior. Notably, maternal inflammation has been shown to disrupt forebrain neurodevelopment via increased placental 5-HT output to the fetal brain (14). Similarly, an elegant series of rodent studies has shown that maternal stress induces male-specific effects on anxiety and other stress-related behaviors in the offspring via placental inflammation (15, 16). These examples support the hypothesis that disruption of placental output could be a mechanism by which environmental chemicals, such as FRs, influence developmental programming.
Firemaster 550 (FM 550) has become one of the most commonly used FRs in baby products and residential furniture (1, 8). This commercial mixture includes two brominated compounds, bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH, also known as BEH-TEBP) and 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB, also known as EH-TBB), and two OPEs including triphenyl phosphate (TPHP, also known as TPP) and a mixture of isopropylated triarylphosphate isomers (ITPs) (17, 18, 19, 20, 21). Human exposure is predominantly through inhalation and inadvertent ingestion of dust (22). Use is so extensive that FM 550 components have reached PBDE levels in US house dust (17) and the brominated components have been detected in dust samples globally, including the United Kingdom, Belgium, Iraq and New Zealand (23, 24, 25), as well as a variety of human tissues including breast milk, blood, hair, fingernails and urine (26, 27, 28, 29). The OPE components of FM 550, particularly TPHP which is a high production volume chemical (10–50 million lbs/year), have widely been used as plasticizers in products such as polyvinyl chloride (PVC) and circuit boards for many years, suggesting there may be multiple routes and sources of human exposure. TPHP is also present in nail polish, with elevated concentrations of its metabolite detectable in urine following application (26). Accumulating evidence of widespread exposure, endocrine-disrupting properties (11, 30, 31, 32), and potential neurotoxicity (33, 34), highlights the importance of investigating the neurodevelopmental effects of replacement FRs, including FM 550.
We have previously shown that perinatal FM 550 exposure can sex specifically disrupt anxiety-like behaviors in juvenile and adult rats (11, 12), at concentrations (100, 300 or 1000 μg/kg/day) well below the purported no observed adverse effect level (NOAEL). This NOAEL of 50 mg/kg/day was established for CN-2065/BZ-54, a mixture containing only the brominated components (TBB and TBPH). Studies in zebrafish, cell-based assays and other model systems have also revealed potential developmental neurotoxic and behavioral effects comparable to PBDEs (34, 35). Using Wistar rats, we recently showed that three of the FM 550 components, TBB, TBPH and TPHP, dose dependently accumulate in placenta, with higher concentrations of TBPH and TPHP in male-associated placentas (12). These findings are consistent with what has been seen for other brominated FRs, including some PBDEs, in human placental tissue, with male-associated placentas showing greater accumulation (36). The brominated components were also dose dependently detected in the fetuses (37). We hypothesized that sex-biased placental bioaccumulation might underlie sex-specific neurodevelopmental outcomes.
Mechanism of action data remains limited but indicate that FM 550 likely impacts neurodevelopment by multiple mechanisms including disruption of thyroid signaling and neurotransmitter activity (11, 38, 39, 40). Additionally, FM 550 and its components have been shown to interact with nuclear receptors for sex steroid hormones (41), which play a critical role in brain organization, as well as peroxisome proliferator-activated receptor gamma (PPARγ) (32, 42), an important regulator of adipogenesis. To probe these and other possible modes of action including disruption of 5-HT production and placental inflammation, we employed a diverse range of hypothesis-generating (RNA sequencing and untargeted metabolomics) and targeted (quantitative real-time PCR and targeted neurotransmitter analysis) approaches to examine fetal Wistar rat brain and placenta following gestational exposure to 300 or 1000 μg FM550/kg/day. Because we hypothesized that sexually dimorphic placental FR exposure could be a route by which sex-specific effects on brain and behavior arise, we incorporated sex as a biological variable.
Materials and methods
Animals
Animal care, maintenance and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services ‘Guide for the Care and use of Laboratory Animals’ and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee (IACUC). A supervising veterinarian approved and monitored all procedures throughout the duration of the project. For each aim, Wistar rats were obtained from Charles River (Raleigh, NC) and/or bred in house as indicated in humidity- and temperature-controlled rooms, each with 12-h:12-h light:darkness cycles at 25°C and 45–60% average humidity in the AAALAC approved Biological Resource Facility at NCSU. As in our prior studies (43, 44), and in accordance with recommended practices for EDC research (45, 46, 47), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles with metal sippers, soy-free diet, woodchip bedding and thoroughly washed polysulfone caging.
Dosing prep
All animals were orally dosed using a concentrated ethanol-based solution prepared in the Stapleton lab and then coded and transferred to the Patisaul lab so the dosing and subsequent testing could be performed in a blinded manner. A commercial mixture of FM 550 was obtained from Great Lakes Chemical (West Lafayette, IN, USA) and each dosing solution (0, 20 and 60 mg/mL) was prepared by weighing the appropriate amount of FM550 and diluting it in 100% ethanol with stirring for 6 h, and then stored in amber bottles at 4°C until use.
Animal husbandry and exposure
Detailed methods regarding dosing and internal FM 550 exposure levels were published in prior studies using the same animals (37). Briefly, adult Wistar rats (n = 48 females and 26 males) were maintained on Teklad 2020 (phytoestrogen-free) diet, and then paired and monitored for the presence of a sperm plug, designated gestational day (GD) 0. The males were then removed and the dams were housed individually. All paired females except one were successfully impregnated and a total of 24 were used for the gestational exposure. Exposure was once per day for 10 days across GDs 9–18. At the time of dosing, 20 µL of each dosing solution was pipetted onto ¼ of a soy-free food treat pellet (chocolate flavored AIN-76A Rodent Diet Test Tabs, Test Diet, Richmond, IN, USA) as we have done previously (48) resulting in the following exposure groups (animals randomly assigned): 0 µg FM 550 (vehicle) (49), 300 µg FM 550 (low) and 1000 µg FM 550 (high). The food treat was dispensed once the solution was completely dry and consumption was monitored to ensure that the dam ate the entire treat. Dams were not dosed by individual weight but rather by an average colony (all female) weight of 300 g at conception producing exposures of approximately 0, 1 and 3.3 mg/kg bw per day FM 550 (relative exposure likely decreased slightly across the 10-day window as body weight increased across pregnancy). This method reduced handling and, consequently, possible confounding by prenatal stress (50). Thyroid hormone assessments were made in a different set of animals and also used for a prior study (Supplementary Materials and methods, see section on supplementary data given at the end of this article) (37).
Tissue collection
Animals were euthanized on GD 18, four hours after final dosing (12:00 h ± 60 min) to control for time post exposure and time of collection. All dams and fetuses were weighed and euthanized by CO2 asphyxiation and rapid decapitation. A single paw was collected from each fetus to determine sex via PCR as previously described (12, 51). Although all individuals were collected, only one fetus per sex per litter, and its associated placenta, was used for each analysis (selected at random). Placentas were collected and flash frozen on powdered dry ice, and then homogenized in liquid nitrogen using a mortar and pestle. Two aliquots were prepared with approximately 25 or 200 mg of homogenized placenta and stored at −80°C to use for RNA extractions for RNA sequencing (n = 2 per sex per group) and qRT-PCR (n = 7 per sex per group) or metabolomics (control: n = 6 per sex, high: n = 4 per sex, low: n = 4 per sex) and targeted neurotransmitter analysis (control: n = 6 per sex, high: n = 4 per sex, low: n = 4 per sex), respectively.
Whole fetal heads were collected, flash frozen and then cryosectioned (Leica CM 1900) from the rostral end in order to isolate forebrain via microdissection. Anatomical landmarks were identified with the assistance of a developing mouse brain atlas, with all forebrain tissue from plates 48–57 obtained via microdissection (52), placed in an Eppendorf tube and stored at −80°C to use for RNA extractions for RNA sequencing (n = 3 per sex per group) and qRT-PCR (n = 6 per sex per group) or targeted neurotransmitter analysis (control: n = 6 per sex, high: n = 6 per sex). Because fetal forebrain tissue was so small, we could not use samples from the same animals to run all types of analyses. Therefore, siblings from the same litters that were used for RNA extractions were also used for targeted neurotransmitter analysis. Thus, for the purposes of these experiments, same-sex litter mates were considered equivalent representatives of that litter. All subsequent analyses were performed by personnel blinded to exposure group.
RNA sequencing
As an initial hypothesis-generating approach, placentas (2 per sex per group from vehicle and high dose) and fetal brains (3 per sex per group including vehicle and high dose) underwent RNA sequencing (RNA-seq). The experimental design and analysis were developed in consultation with the NCSU Genomic Sciences Laboratory (GSL). RNA extraction was performed with the Qiagen RNEasy Miniprep kit according to the manufacturer protocol (Qiagen, Cat. 74134). RNA quality was determined using an Agilent 2100 Bioanalyzer and all samples, for both tissue types, were found to have an RNA integrity number (RIN) ≥ 10. Sequencing libraries were prepared as described previously (53), using NEBNext Ultra Directional RNA Library Prep kit and NEBNext Poly(A) mRNA Magnetic Isolation Module (catalogs E7420 and E7490; New England Biolabs, Ipswich, MA, USA), to be compatible with Illumina sequencing. Isolation, heat fragmentation and priming were performed according to manufacturer’s instructions. cDNA synthesis was followed by purification and size selection. Finally, library clean-up was performed used AMPure XP beads (Beckman Coulter Genomics, Brea, CA, USA; Cat. A63881) and quality was assessed using the Agilent 2100 Bioanalyzer. For each tissue, library sequencing was performed using a 125-bp single-end protocol on a single lane of an Illumina HiSeq2500 sequencer. Approximately, 27.4 million uniquely mapped reads were generated per placental library, and 23.7 million reads per fetal forebrain library. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE108965 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE108965).
RNA-seq data processing
Data analysis for placenta RNA-seq was performed by the Bioinformatics Core at the University of Virginia, on a fee for service basis as described previously (53). Fetal forebrain data analysis was conducted using a nearly identical analytical pipeline in consultation with the Bioinformatics Core of the NCSU Center for Human Health and Environment. Briefly, quality control of read data was evaluated with FastQC. Alignment was performed using STAR short read aligner (54) to Rattus norvegicus (rn6) reference genome.
For placental analysis, the number of reads mapped to GENCODE was determined using featureCounts software (55), and count data were normalized for sequencing depth and distortion. Finally, dispersion was estimated using DESeq2 Bioconductor (56, 57) package in the R statistical computing environment (58) to normalize count data, estimate dispersion and fit a negative binomial model for each gene, using an extended model matrix considering the main effects of both sex and treatment as well as the interaction term. For fetal forebrain analysis, the number of reads mapped to genome features was determined using htseq-count script from HTSeq (59) python package, and count data were imported to R statistical computing environment for further analysis.
Initially, genes that had no counts in more than 2/3 of the replicate samples were excluded from the analysis. Data normalization based on dispersion and differential analysis was conducted using DESeq2 (57) package. We fitted a generalized linear model (~Gender + Dose) between the expression count, Gender (M, F) and Dose (Control, High). Finally, differential expression analysis was preformed between female vs male and high vs control dose. For both placenta and fetal forebrain samples, the overall mean of the normalized counts, the log2 (fold-change), the P value and the adjusted P value (padj), adjusted for multiple testing using the Benjamini–Hochberg False Discovery Rate, were calculated.
Quantitative real-time PCR
Analysis was performed on placenta and fetal brain in all three treatment groups (vehicle, low and high dose); n = 7 per sex per group (one per sex per litter). Genes assessed using qRT-PCR were selected based on canonical pathways identified by IPA analysis, such as acute phase signaling, which spurred us to follow-up on genes related to inflammatory response (Cxcl10, Ccl5 and Ccr7) and farnesoid x receptor (FXR)/retinoid X receptor (RXR) signaling (Abcc2). A set of pre-identified genes of interest were also selected based on previous studies highlighting metabolic (Lepr, Igf1 and Pparg), thyroid (Ttr) and sex steroid signaling (Esr1 and Ar) pathways as potential targets of FM 550 and/or identification by RNA-seq with P value ≤ 0.01 as a cutoff. Additional genes of interest validated by qRT-PCR following neurotransmitter analysis included those related to 5-HT signaling and metabolism in placenta (Htr2A, Tdo2, Ido1, Ido2) and fetal forebrain (Htr1B and Maoa). RNA extractions were performed as described earlier for RNA sequencing. cDNA synthesis was performed using high-capacity RNA-to-cDNA kits (Applied Biosystems, Cat. 4387406) according to the manufacturer instructions. Incubation for reverse transcriptase reactions was 60 min at 37°C, 5 min at 95°C and the cDNA was stored at −20°C until use. An ABI StepOnePlus Real-Time PCR System and TaqMan probes were used for quantitative real-time PCR (qRT-PCR) as previously detailed (53). Triplicate reactions were run as well as negative controls (no template present) for each TaqMan assay. A house keeping gene (18s rRNA) was used to normalize CT values for differences in starting concentrations of cDNA. Relative changes in expression were determined using the Livak ΔΔ CT method (60).
GC-TOF-MS metabolomics
Aliquots of approximately 50 mg of placenta tissue were placed in a 2 mL Magna Lyser tube and mixed with 1000 µL of degassed 3:3:2 acetonitrile: isopropanol: water solution, pulse sample 2 × 30 s @ 2000 in Magna Lyser. Samples are vortexed, shaken for 5 min and then centrifuged at 4°C for 4 min at 13,100 g. Samples were completely dried by vacuum centrifuge, reconstituted with 450 µL 1:1 acetonitrile:water solution, centrifuged for 4 min at 14,000rcf and dried by vacuum in new tubes. Study samples were randomized, and quality control pool samples (prepared under identical conditions) were interspersed.
Samples were derivatized using a two-step method with acquisition parameters similar to methods described in detail (61, 62, 63). To summarize (as described previously (63)), a 0.5 µL volume was injected into an Agilent 6890 gas chromatograph (Santa Clara, CA, USA) with a 30 m long, 0.25 mm i.d. Rxi5Sil-MS column with 0.25 µm film thickness (Restek, Bellefonte PA, USA), using a 250°C injector temperature in splitless mode with 25 s splitless time, at a constant flow of 1 mL/min. The oven temperature was ramped (20°C/min ramp) from 50°C to 330°C. Data were acquired using a Leco Pegasus 4D TOF-MS with a 280°C transfer line temperature, electron ionization at −70 V and an ion source temperature of 250°C. Spectra were acquired from m/z 50 to 750 at 20 spectra/s and 1850 V detector voltage.
GC-TOF-MS data analysis
GC-TOF-MS data files were deconvoluted using the software ChromatTOF (Leco, St. Joseph, MI, USA) and processed in BinBase (64) for peak retention index calculations, spectral identification and generation of a table of peak identifications and intensities. Multivariate data analysis was conducted using SIMCA 13.0 (Umetrics, Umeå, Sweden) for data normalized to the sum of intensities. Mean centered and pareto scaled were analyzed by Principal Component Analysis (PCA) and Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA). Peaks with Variable Influence on Projections (VIP) ≥ 1.0 with a jack-knife confidence interval that did not include 0 were deemed important for differentiating the study groups. A 7-fold cross-validation was used to assess the predictive variation of the model (Q2) (65).
Neurotransmitters
Rat placenta samples (approximately 100 mg each) were weighed directly into Magna Lyser tubes and extracted for 2 × 30 s pulses in phosphate-citric acid tissue buffer solution containing 200 ng/mL of DHBA (internal standard) at 3:1 volume (µL):tissue (mg). Extracts were vortexed followed by centrifugation to precipitate solids. Supernatants were transferred by pipette to glass HPLC vials containing 300 µL target vials for analysis by UHPLC-ECD. Rat fetal forebrains were placed in 2 mL tubes with ceramic beads along with 20 µL perchloric acid solution spiked with 200 ng/mL 2, 3-dihydrobenzoic acid (DHBA – internal standard) per milligram of tissue. Samples were vortexed and subjected to three 10-s pulses on the Magna Lyser. Samples were centrifuged for 10 min at 4°C and at 14000 rcf. Extract supernatants were transferred to HPLC vials with 300 µL glass inserts and capped. Samples were randomized prior to analysis.
Sample extracts were analyzed on a Thermo Scientific Dionex 3000 series U-HPLC with Chromeleon v.6.80 software. Compound separation was achieved using a Thermo Scientific Hypersil Gold, 200 × 2.1 mm × 1.9 µm particle size column with an isocratic flow rate of 0.400 milliliter per minute with the column temperature set at 30°. Simultaneous detection of neurotransmitters was achieved on the TS 5600A 16 channel CoulArray electrochemical detector (ECD) with ESA software v.3.80. Coulometric cell potentials were set at −150, 0, 200, 400, 450, 600, 800, 850 and 900 mV with the cell chamber set at 30°C. Acquisition run time was 60 min.
Statistical analyses
Statistical analyses were performed using GraphPad Prism, version 6 (La Jolla, CA, USA). Placental and fetal forebrain neurotransmitter and metabolomics data were first analyzed using a 2-tailed exact Mann–Whitney U test with males and females binned together, to compare exposure groups to controls. The data were then separated by sex and a 2-tailed exact Mann–Whitney U test used to identify sex differences between male and female controls, and then possible exposure effects within sex. For the purposes of this exploratory study, metabolites and neurotransmitters that had either a P value <0.10 or a magnitude of fold-change ≥2 were determined to be important for differentiating study groups, and P values were not adjusted for multiple testing to minimize risk of type-2 error (66). For all other data, statistical significance was set at α ≤ 0.05. As was done for the metabolomics and neurotransmitter data, a 2-tailed exact Mann–Whitney U test was used in order to first assess effects of exposure with males and females combined, and then with males and females separated.
Results
Impact of prenatal FM 550 exposure on placental gene expression
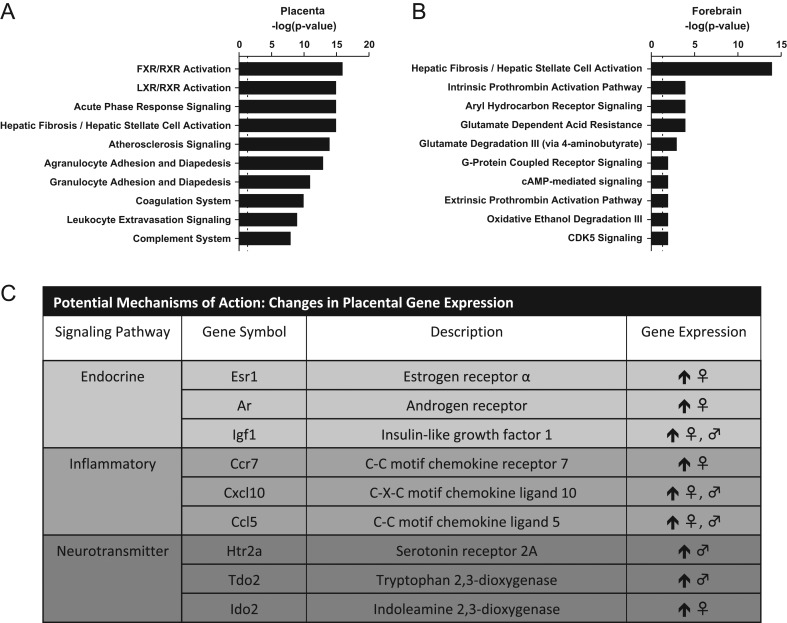
RNA-seq of the placental transcriptome was performed and analyzed as a hypothesis-generating approach to identify possible modes of action. Unfortunately, one pair of placentas, one male- and one female-associated placenta, from the same dam in the high-dose group had to be removed from the analysis for technical reasons. Unsupervised PCA of the remaining samples revealed reasonable separation of the exposure groups (Supplementary Fig. 1). RNA-seq results were not analyzed by sex due to very small sample sizes, (n = 2 exposed and n = 4 control) and the exploratory nature of the approach, but follow-up analyses on genes of interest by qRT-PCR, to validate RNA-seq findings, used a larger sample size (n = 7 per sex per group) and considered sex as a biological variable (Fig. 1C). Differential expression of over 2600 genes, at a P adjusted value of 0.05, was found in placental tissue from dams exposed to the highest dose of FM 550. Ingenuity Pathway Analysis (IPA, QIAGEN) software was used to identify potential pathways perturbed by FM 550 exposure. Several different iterations were employed using the full gene list with a P adjusted value of 0.05 as a cutoff. Additional parameters included fold-changes from 1 to 3 and, because we were specifically interested in how changes in placental function may impact the brain, filters to include only annotations were made in neural tissues and stem cells. The top two canonical pathways that were most impacted regardless of the analytical parameters used were the farnesoid x receptor FXR/RXR, and the liver x receptor (LXR)/RXR. Acute phase signaling was also strongly identified using the full gene list with a fold-change of 1 as the cutoff (Fig. 1A).
Figure 1.
Top 10 canonical pathways identified by IPA analysis, in placenta (A) and fetal forebrain (B) and changes in placental gene expression verified by qRT-PCR.
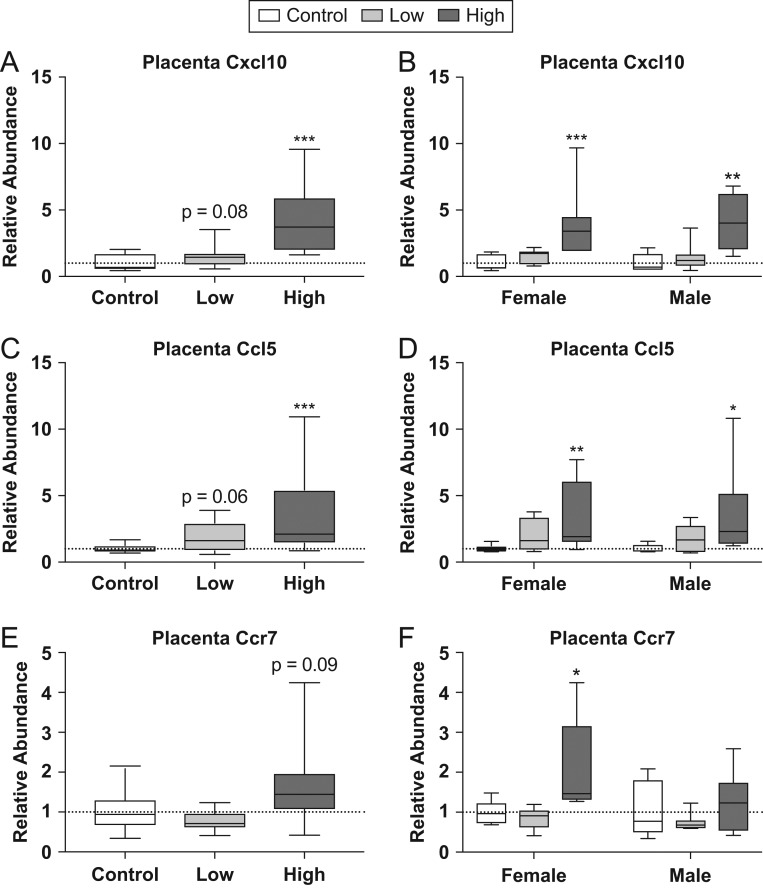
Following the identification of these canonical pathways, we followed up on several genes of interest using qRT-PCR. ATP-Binding Cassette Subfamily C Member 2 (Abcc2), which was identified in the FXR/RXR pathway, was found to be upregulated in RNA-seq data. However, this finding was not confirmed by qRT-PCR (Supplementary Fig. 3). We also explored genes related to inflammatory processes due to the identification of acute phase signaling as an enriched canonical pathway as well as the identification of multiple genes previously identified in an elegant series of studies by another group as upregulated in male placentas via maternal stress (15, 67). These genes included C-X-C motif chemokine ligand 10 (Cxcl10), C-C motif chemokine ligand 5 (Ccl5) and C-C motif chemokine receptor 7 (Ccr7) (Fig. 2). An increase in relative abundance was observed in the high dose compared to controls for both Cxcl10 (U = 7, P ≤ 0.001) and Ccl5 (U = 17, P ≤ 0.001). A suggestive increase in expression was also observed for Cxcl10 (U = 59, P = 0.08) and Ccl5 (U = 56, P = 0.06) in the mid dose groups compared to controls (Fig. 2A and C). When analyzed by sex, high-dose males and females were found to have a significant increase in expression of Cxcl10 (U = 3, P ≤ 0.004; U = 0, P ≤ 0.001) and Ccl5 (U = 5, P ≤ 0.01; U = 4, P ≤ 0.007) (Fig. 2B and D). Although the combined analysis for Ccr7 did not show a significant increase in expression in the high-dose group (U = 61, P = 0.09) (Fig. 2E), when separated by sex, high-dose females were found to have a significant increase in relative abundance compared to same-sex controls (U = 6, P ≤ 0.02) (Fig. 2F).
Figure 2.
Effects of prenatal FM 550 exposure on the relative abundance of genes related to inflammatory signaling in placental tissue. Significant upregulation of Cxcl10 and Ccl5 was observed at the highest dose when males and females were group for analysis (A and C). Similarly, significant upregulation was observed at the highest dose for Cxcl10 and Ccl5 in males and females when analyzed by sex (B and D). No significant effect of exposure was observed for Ccr7 when combined for analysis (E), however, when analyzed by sex, high-dose females showed a significant increase in expression (F). For each dose n = 7/sex. Graphs depict median with whiskers from minimum to maximum (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
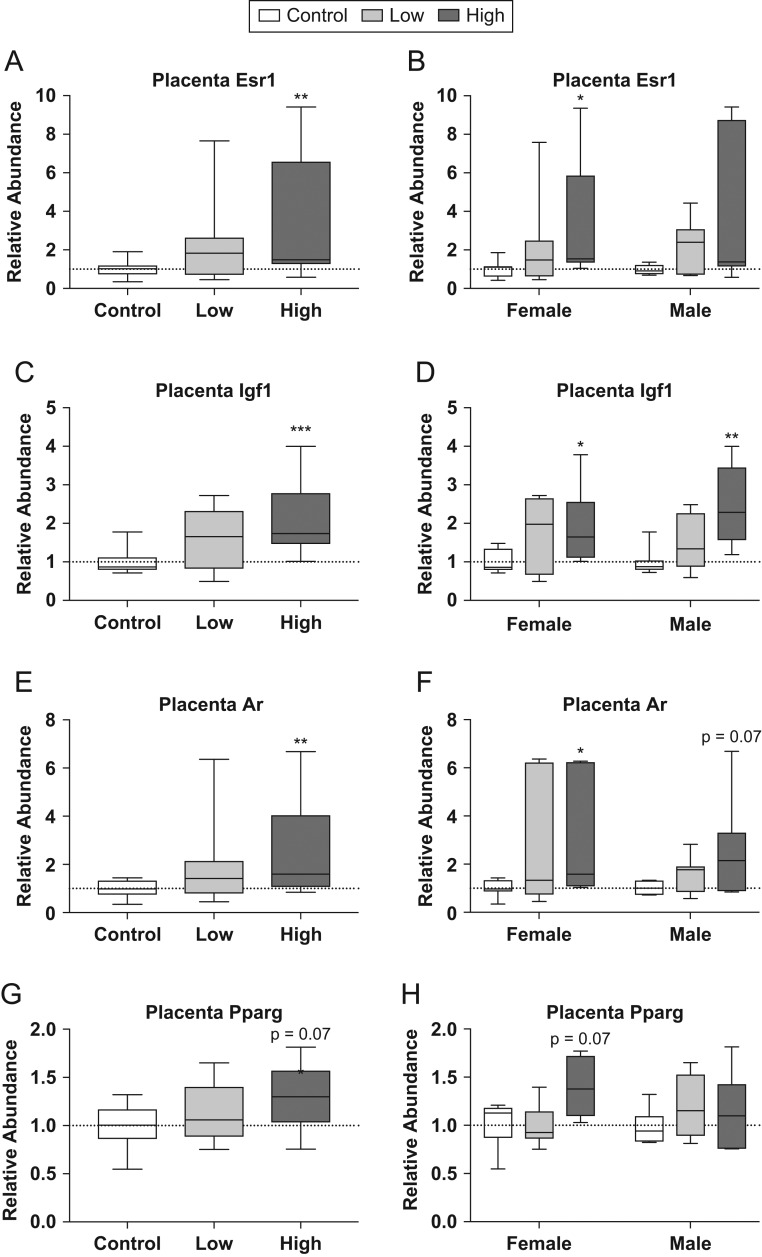
The RNA-seq results were also inspected for pre-identified genes of interest to see if they were differentially expressed. These included estrogen receptor α (Esr1), insulin-like growth factor 1 (Igf1), androgen receptor (Ar) and peroxisome proliferator-activated receptor gamma (Pparg) (Fig. 3). qRT-PCR confirmed higher relative abundance for Esr1 (U = 36, P ≤ 0.004), Igf1 (U = 16, P ≤ 0.001) and Ar (U = 36, P ≤ 0.004) in the high dose compared to controls (Fig. 3A, B, C, D, E and F). A similar trend was found for Pparg; however, it did not reach statistical significance (U = 53, P = 0.07) and the directionality did not reflect the change in expression observed in the RNA-seq data. Additional pre-identified genes of interest included leptin receptor (Lepr) and transthyretin (Ttr); however, exposure-related differences in Lepr and Ttr were not confirmed (Supplementary Fig. 3). Esr1, Igf1, Ar and Pparg were then analyzed by sex (Fig. 3E, F, G and H). For Esr1, high-dose females had significantly greater relative abundance than controls (U = 7, P ≤ 0.03; Fig. 3B), while both high-dose males and females showed significantly higher levels of Igf1 compared to their respective, same-sex controls (U = 5, P ≤ 0.01; U = 3, P ≤ 0.004; Fig. 3D). Ar showed significantly higher relative abundance in high-dose females compared to controls (U = 6, P ≤ 0.02; Fig. 3F), while high-dose males showed a similar trend, but did not reach statistical significance (U = 10, P = 0.07; Fig. 3F).
Figure 3.
Effects of prenatal FM 550 exposure on the relative abundance of genes related to endocrine signaling in placental tissue. Significant upregulation of Esr1, Igf1 and Ar was observed at the highest dose when males and females were grouped for analysis (A, C, and E). Similarly, significant upregulation of Igf1 and Ar was in males and females exposed to the highest dose, while upregulation was found for Esr1 in high-dose females only (B, D, and E). No significant effect of exposure was observed for either Pparg in placental tissue (G and H). For each dose n = 7/sex. Graphs depict median with whiskers from minimum to maximum (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Impact of prenatal FM 550 exposure on placental neurotransmitters and metabolites
Untargeted GC-TOF-MS metabolomics analysis of second aliquot of placental tissue revealed significant changes (P ≤ 0.05) in 14 different metabolites or ratios of metabolites involved in processes such as glycolysis, amino acid metabolism and the citric acid cycle. These findings are summarized in Table 1 with their respective P values and directionality of change. Data have been uploaded to the NIH Common Fund metabolomics data repository at metabolomicsworkbench.org. Significance was tested for male-associated placenta, female-associated placenta and combined (not separated by sex), in comparison with the appropriate non-exposed control. Perturbations in benzoic acid, enolpyruvate, glutamic acid and the ratio of proline to glutamic acid were found when the placentas were not stratified by sex. Some sex differences were indicated for individual analytes. Only male-associated placentas showed a decrease in uracil, glycerol, the ratio of enolpyruvate/citric acid, guanosine, beta-hydroxybutyric acid, fructose and an increase in leucine and citric acid. Only female-associated placentas showed an increase in glucose 1 phosphate and proline and a decrease in salicylic acid, octadecanol and the ratio of enolpyruvate to glycerol. Overall, placenta tissues showed at least one metabolite that tested significant (P < 0.05) that is associated with glycolysis, amino acid metabolism, pyrimidine metabolism, fatty acid synthesis, the urea cycle and the TCA cycle.
Table 1.
Summary of untargeted metabolomics analysis in whole placenta.
| Pathway/metabolite species | Metabolite (placenta) | Female low (n = 4) vs control (n = 6) | Female high (n = 4) vs control (n = 6) | Male low (n = 4) vs control (n = 6) | Male high (n = 4) vs control (n = 6) | Combined low (n = 8) vs control (n = 12) | Combined high (n = 8) vs control (n = 12) |
|---|---|---|---|---|---|---|---|
| Glycolysis | Glucose 1 phosphate | 0.07 ↑ | |||||
| Glycolysis | Glycerol | 0.02 ↑ | 0.07 ↑ | 0.03 ↓ | |||
| Glycolysis | Glycine | 0.08 ↑ | |||||
| Glycolysis | Creatine major dehydrated | 0.06 ↑ | |||||
| Amino acid metabolism | Benzoic acid | 0.01 ↑ | 0.07 ↓ | 0.03 ↑ | |||
| Amino acid metabolism | Salicylic acid | 0.07 ↓ | |||||
| Glycolysis | Enolpyruvate | 0.04 ↓ | 0.07 ↓ | 0.002 ↓ | |||
| Glycolysis | Enolpyruvate/glycerol | 0.07 ↓ | 0.04 ↓ | ||||
| Amino acid | Leucine | 0.07 ↑ | |||||
| Ketogenesis | Beta-hydroxybutyic acid | 0.07 ↓ | 0.06 ↓ | ||||
| Pyrimidine metabolism | Uracil | 0.01 ↓ | 0.007 ↓ | ||||
| Fatty acid synthesis | Stearic acid | 0.06 ↑ | |||||
| Fatty acid synthesis | Octadecanol | 0.07 ↓ | |||||
| Citric acid cycle | Citric acid | 0.01 ↑ | 0.03 ↑ | ||||
| Glycolysis/citric acid cycle | Enolpyruvate/citric acid | 0.02 ↓ | 0.05 ↓ | 0.03 ↓ | |||
| Amino acid | Proline | 0.01 ↑ | 0.002 ↑ | ||||
| Amino acid | Proline/glutamic acid | <0.001 ↑ | 0.01 ↑ | ||||
| Amino acid | Glutamic acid | 0.07 ↓ | 0.04 ↓ | 0.02 ↓ | 0.04 ↓ | 0.001 ↓ | 0.001 ↓ |
| Urea cycle | Urea | 0.01 ↑ | 0.08 ↑ | ||||
| Urea cycle | Uric acid | 0.05 ↑ | |||||
| Purine metabolism | Guanosine | 0.04 ↓ | 0.02 ↓ | ||||
| Amino acid | Threonine | 0.06 ↑ | |||||
| Glycolysis | Fructose | 0.07 ↓ | 0.04 ↓ | ||||
| Amino acid | Hydroxy-proline | 0.098 ↓ |
All P values <1.0 are reported here with the direction of change. For the control group n = 6 per sex and for the low and high groups n = 4 per sex.
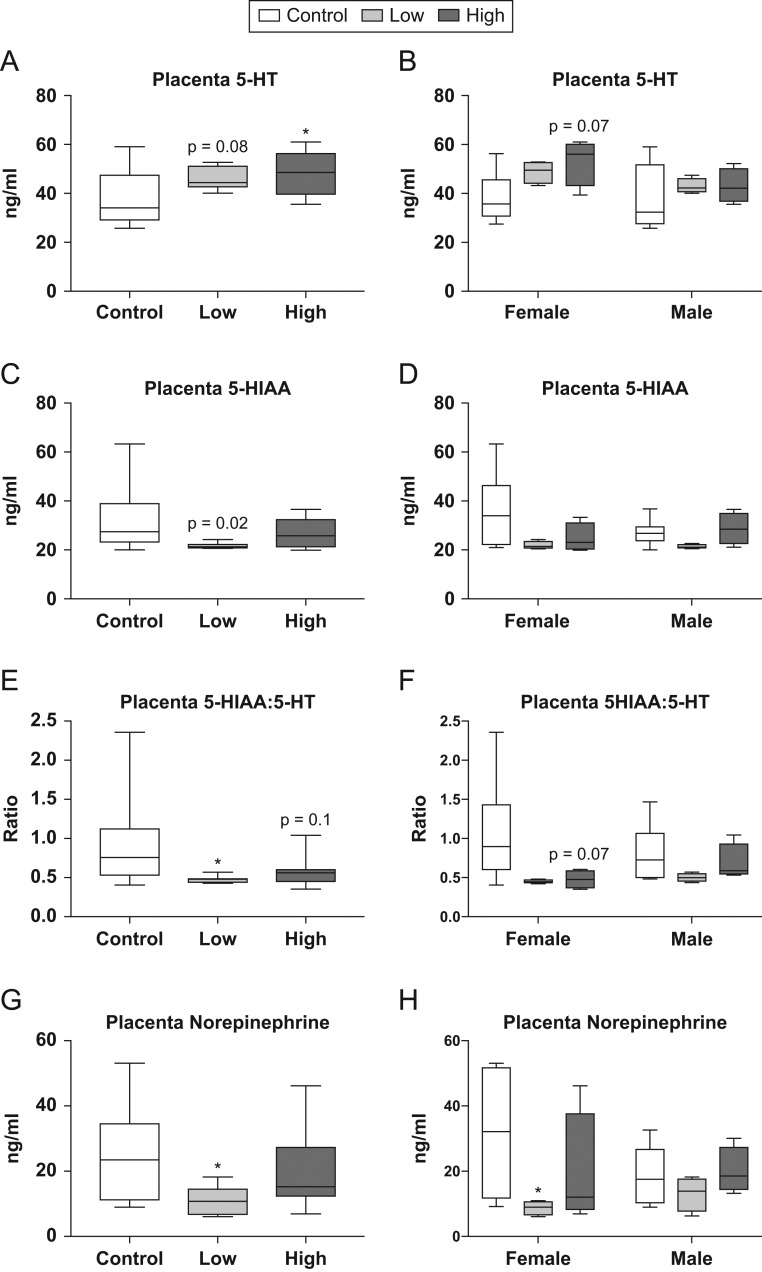
Targeted neurotransmitter analysis of the same placental tissue revealed dose-dependent changes in 5-HT and its primary metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (Fig. 4). An increase in 5-HT was suggested (U = 25, P = 0.08; Fig. 4A) in the low-dose group compared to controls, while 5-HT was significantly higher in placentas from high-dose animals compared to controls (U = 22, P = 0.047; Fig. 4A). Stratification by sex revealed that, although not quite statistically significant, female-associated placentas from the high-dose group appeared to have elevated levels of 5-HT compared to controls (U = 3, P = 0.07; Fig. 4B). 5-HIAA, 5-HTs primary metabolite, was found to be significantly reduced in placentas from the low-dose group compared to controls (U = 17, P ≤ 0.02; Fig. 4C). The ratio of 5-HIAA:5-HT was also assessed, as this is indicative of 5-HT turnover. A significant reduction in this ratio was found in placentas from the low-dose group compared to controls (U = 18, P ≤ 0.02; Fig. 4E); however, this relationship did not hold up when analyzed by sex. A suggestive reduction in 5-HIAA:5-HT was observed in placentas from the high-dose group compared to controls (U = 26, P = 0.10; Fig. 4E), as well as in high-dose placentas associated with female offspring (U = 3, P = 0.07; Fig. 4F). Neurotransmitter analysis also revealed a reduction in norepinephrine (NE) in response to the low-dose exposure (U = 18, P ≤ 0.02; Fig. 4G), with low-dose female-associated placentas having significantly less NE than controls (U = 2, P ≤ 0.04; Fig. 4H). No differences were identified for neurotransmitters measured in the male placentas.
Figure 4.
Effects of prenatal FM 550 exposure on neurotransmitter and neurotransmitter metabolite levels in whole placenta. A dose–response relationship was observed in placental 5-HT levels when sexes were grouped for analysis (A). No significant effect of either dose was observed for 5-HT levels when analyzed by sex; however, females showed a similar pattern compared to the grouped analysis (B). A significant reduction in 5-HIAA was observed at the low dose when sexes were grouped for analysis (C), and a consistent trend was observed for both sexes when analyzed by sex. Additionally, the ratio of 5-HIAA:5-HT was significantly reduced at the lowest dose in the grouped analysis (E) with a similar trend observed in the female analysis (F). Finally, a significant reduction in norepinephrine was observed at the lowest dose in both the grouped and female specific analysis (G and H). For the control group n = 6/sex while the low and the high dose n = 4/sex. Graphs depict median with whiskers from minimum to maximum (*P ≤ 0.05).
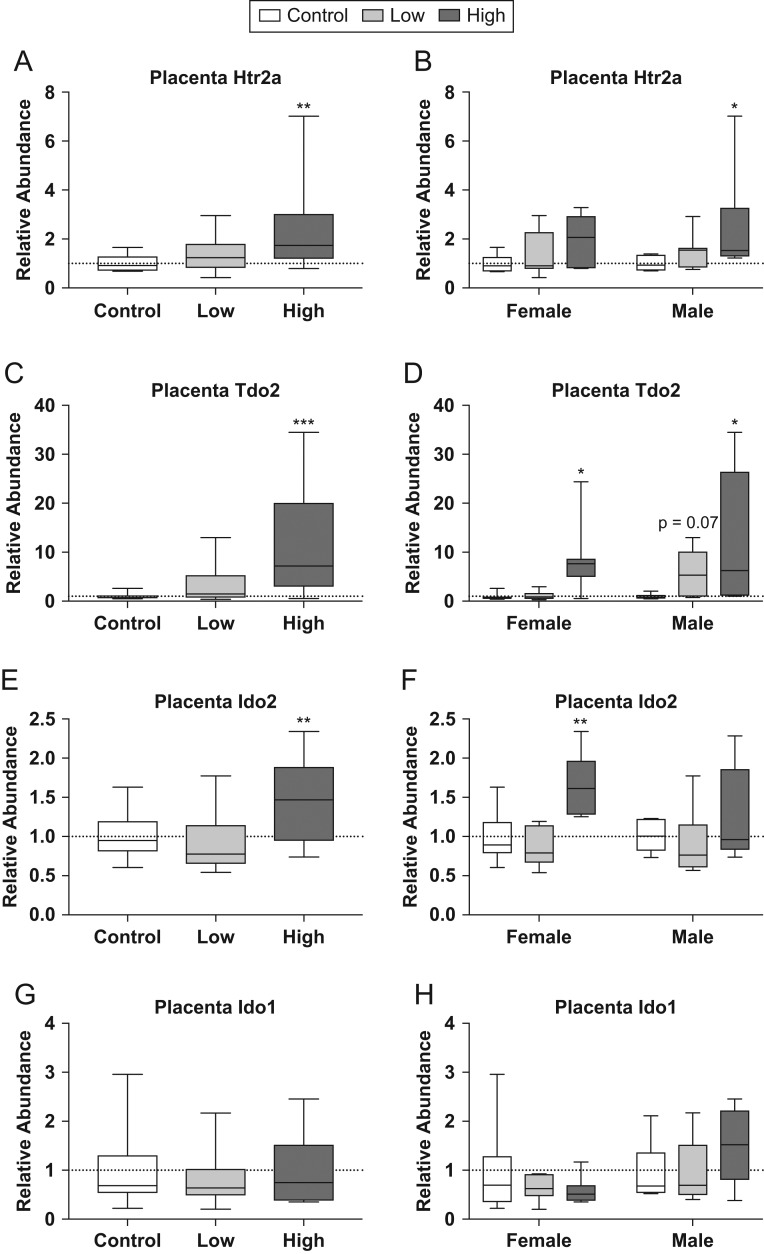
We then revisited our RNA-seq data to see if any genes related to 5-HT and NE signaling were impacted by exposure and identified two genes related to 5-HT signaling and metabolism differentially expressed between high-dose and control animals. These genes were 5-HT receptor 2A (Htr2A) and tryptophan 2,3-dioxygenase (Tdo2) (Fig. 5). qRT-PCR revealed significant changes in expression for both Htr2A (U = 36, P ≤ 0.004; Fig. 5A and B) and Tdo2 (U = 27, P ≤ 0.001; Fig. 5C and D). When analyzed by sex only high-dose males showed significantly greater relative abundance for Htr2A compared to same-sex controls (U = 6, P ≤ 0.02; Fig. 5B). However, high-dose females and high-dose males showed significantly greater expression of Tdo2 compared to same-sex controls (U = 7, P ≤ 0.03; U = 7, P ≤ 0.03; Fig. 5D). Indoleamine 2,3-dioxygenase 2 (Ido2) and indoleamine 2,3-dioxygenase 1 are similar in function to Tdo2 and, although not differentially expressed in the RNA-seq data set, were assessed using qRT-PCR (Fig. 5E, F, G and H). Significant change in expression of Ido2 (U = 44, P ≤ 0.01; Fig. 5E) was observed, with high-dose females showing increased expression compared to same-sex controls (U = 4, P ≤ 0.007; Fig. 5F). Ido1 was not significantly changed. Htr2A, Tdo2, Ido2 and Ido1 were then broken out by sex in order to assess whether or not there was a sex difference. No significant effect of sex was found for any of these genes.
Figure 5.
Effects of prenatal FM 550 exposure on the relative abundance of genes related to 5-HT signaling and metabolism in placental tissue. Significant upregulation of Htr2a, Tdo2 and Ido2 was observed at the highest dose in the grouped analysis (A, C, and E). When analyzed in a sex-specific manner, significant upregulation was observed at the highest dose in males for Htr2a (B), both sexes for Tdo2 (D), and in females only for Ido2 (D). No significant effect exposure was observed for Ido1 in placental tissue (G and H). For each dose n = 7/sex. Graphs depict median with whiskers from minimum to maximum (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Impact of prenatal FM 550 exposure on fetal forebrain gene expression
Unsupervised PCA analysis of the RNA-seq data on fetal forebrain only revealed exposure-related clustering for the males (Supplementary Fig. 2; data submitted to the GEO repository). Because the female data failed to meaningfully cluster, it was excluded from further analysis. 196 genes were differentially expressed between high-dose and control males at a P adjusted value of 0.05. As was done for the placental tissue, IPA was used to identify pathways that may be perturbed in the developing forebrain. Two of the top canonical pathways identified included aryl hydrocarbon signaling and glutamate degradation. The top canonical pathway was hepatic fibrosis/hepatic stellate cell activation and included Igf and a variety of collagen-related genes, all upregulated.
Impact of prenatal FM 550 exposure on fetal forebrain neurotransmitters
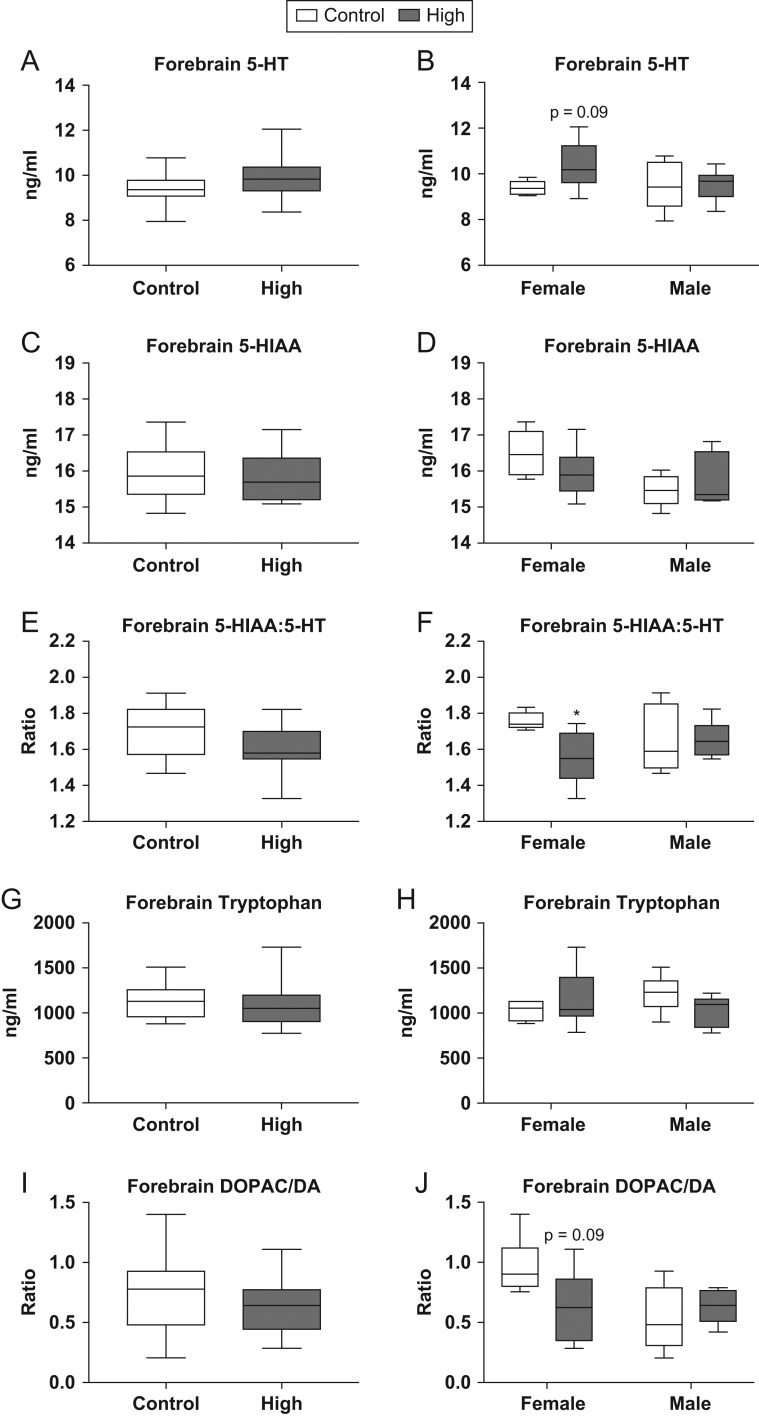
Targeted neurotransmitter analysis was also performed on fetal forebrain tissue from siblings in the same litters used for RNA-seq and qRT-PCR analysis (Fig. 6). No significant effect of exposure was found when males and females were combined for analysis (Fig. 6A, C, E, G and I). However, fetal forebrains from high-dose females showed a suggestive increase in 5-HT compared to controls (U = 7, P = 0.09; Fig. 6B). Additionally, high-dose females had a significantly lower 5-HIAA:5-HT ratio when compared to controls (U = 3.5, P ≤ 0.03; Fig. 6F). Other neurotransmitters measured in fetal forebrain included dopamine (DA), dihydroxyphenylacetic acid (DOPAC), HVA, NE, tryptophan and tyrosine. No significant effect of exposure was found for any of these neurotransmitters; however, the ratio of DOPAC:DA showed a suggestive reduction in high-dose females compared to controls (U = 7, P = 0.09; Fig. 6J). No significant effect of exposure was observed in male rats for any of the neurotransmitters analyzed.
Figure 6.
Effects of prenatal FM 550 exposure on neurotransmitter and neurotransmitter metabolite levels in fetal forebrain. 5-HT levels were not significantly altered in fetal forebrain (A) although there was a trend for higher levels in females (B). No significant effect of exposure was observed for 5-HIAA (C); however, a significant difference between male and female controls was observed (D). A significant reduction in the ratio of 5-HIAA:5-HT was observed at the highest dose but only in females (F). No significant effect of exposure was found for tryptophan or DOPAC:DA (G, H, I and J). For each dose group n = 6/sex. Graphs depict median with whiskers from minimum to maximum (*P ≤ 0.05).
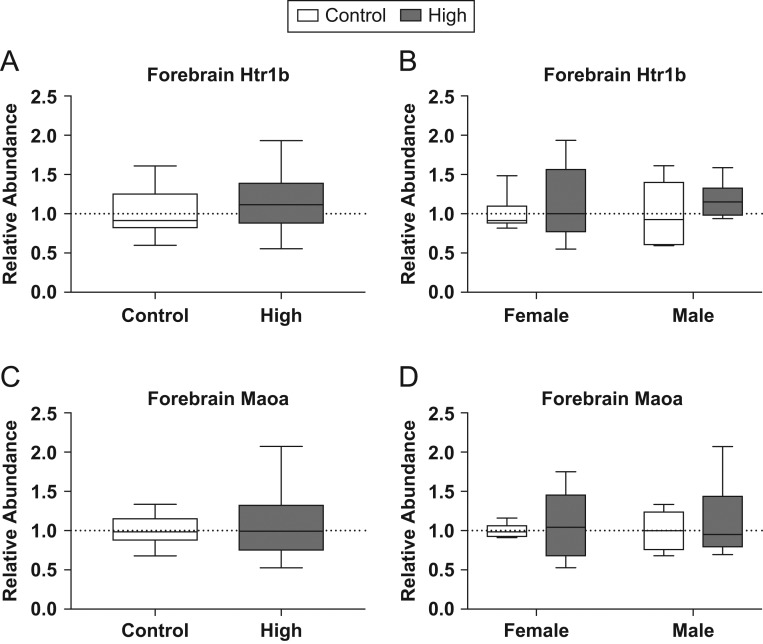
Due to interest in disruption of 5-HT signaling, two genes were assessed using qRT-PCR, 5-HT receptor 1B (Htr1b) and monoamine oxidase A (Maoa) (Fig. 7). Neither of these two genes were found to be significantly different in the high-dose group compared to controls when males and females were grouped for analysis or when they were broken out by sex.
Figure 7.
Effects of prenatal FM 550 exposure on the relative abundance of genes related to 5-HT signaling and metabolism in fetal forebrain. No significant effect of exposure was observed for Htr1b or Maoa in the fetal forebrain (A, B, C and D). For each dose n = 6/sex. Graphs depict median with whiskers from minimum to maximum.
Discussion
The present study identified multiple pathways by which gestational FM 550 exposure may impact the placenta and developing forebrain. Altered placental pathways included endocrine, inflammatory and neurotransmitter signaling, all of which play important roles in fetal growth and neurodevelopment. Multiple modes of action were not unexpected given that FM 550 is a mixture and many EDCs act via multiple mechanisms. Most significantly, 5-HT turnover was reduced in placental tissue and fetal forebrains indicating the novel possibility that 5-HT signaling between the placenta and the embryonic brain may be disrupted. Evidence of RXR signaling pathway disruption was also found in both tissues. By providing mechanistic insight, this study builds on our prior work showing that FM 550 components sex specifically accumulate in placental tissue, and developmental exposure can sex specifically alter activity and anxiety-related behaviors in exposed offspring (11, 12). Collectively, our data lead us to hypothesize that there may be causal and sex-specific links between FR-induced placental disruption and neurodevelopmental outcomes.
RNA-seq revealed numerous exposure-related expression changes in the placenta but comparatively few in fetal forebrain, with the latter indicating meaningful effects only in males. Transcriptional differences in brain are notoriously difficult to detect because expression is so low and the effect sizes of biologically relevant differences are typically small (under 2-fold) (68, 69, 70, 71, 72). This technical limitation may partially account for the comparatively low level of forebrain transcriptional targets detected. Thus, the two RNA-seq data sets were approached somewhat differently, with placental data analyzed in both sexes and forebrain data analyzed only in males.
In the placenta, the top two canonical pathways strongly identified as disrupted were the FXR/RXR and LXR/RXR pathways. RXR is a nuclear receptor (NR) that forms heterodimers with a variety of other NRs important for endocrine and metabolic regulation and fetal development (73, 74). These include FXR and LXR, which play a role in lipid and cholesterol homeostasis and inflammation (75, 76, 77). Differentially expressed genes in the FXR/RXR pathway included genes involved in the transport and metabolism of cholesterol and triglycerides, including high-density lipoprotein (Hdl), apolipoprotein A1 (Apoa1), hepatocyte nuclear factor 4 alpha (Hnf4α), lipoprotein lipase (Lpl) and Abcc2. Similarly, gene enrichment in the LXR/RXR pathway consisted of genes related to cholesterol metabolism and transport, including Lpl, Apoa1 and toll-like receptors 3 and 4 (Tlr3 and 4), as well as some inflammatory response genes, lipopolysaccharide-binding protein (Lbp) and interleukin 1 alpha (Il1α).
The third canonical pathway identified in at least one placental IPA analysis iteration was acute phase signaling, indicating a potential inflammatory response to FM 550 exposure. Immune activation has been recognized as a potential risk factor for neurodevelopmental and neurobehavioral deficits (67, 78, 79). Genes perturbed by FM 550 that were enriched in the acute phase signaling pathway included Lbp, C-reactive protein (Crp), Il1α and complements 2, 3 and 4 (C2, C3, C4), all of which are associated with an immune response. Additional pro-inflammatory response genes also differentially expressed in placental tissue, but not necessarily highlighted in the acute phase signaling pathway, included pro-inflammatory chemokines such as chemokine ligands (Cl2, 5, 6, 7, 12 and 21) and C-X-C motif chemokine ligands (Cxcl 9, 10, 11, 12, 16 and 17). Several genes positively regulated by inflammatory mediators were also significantly upregulated including prostaglandin-endoperoxide synthase 1 (Ptgs1), C-C motif chemokine receptor 7 (Ccr7), protein tyrosine phosphatase receptor type C (Ptprc) and selectin P (Selp). Similarly, placental upregulation of both 11-β-hydroxysteroid dehydrogenase 1 and 2 (Hsd11b1 and Hsd11b2), which are responsible for the inactivation of maternal cortisol (80), was observed in the high-dose group, providing further evidence that FM 550 potentially interacts with, and disrupts, the stress axis during gestation.
Many of these acute phase signaling genes, including Ccr7, Ptprc, Selp, Cxcl10 and Ccl 5, have previously been identified in other studies investigating the mechanisms by which prenatal stress alters neurodevelopment (67). A series of studies has shown that stress-induced upregulation of these placental inflammatory response genes is consistently male biased (15, 67) and can result in hyperactivity, higher anxiety and other effects indicative of a disrupted stress axis. These studies provide critical evidence of how placental disruption may be an origin of male-biased neurodevelopmental disorders (15, 16).
Pathway analysis of fetal forebrain also identified aryl hydrocarbon receptor (AHR) signaling and glutamate degradation as potentially disrupted in males. As in placenta, RXR was one of the genes altered by FM 550 in the AHR canonical pathway. Additionally, retinoic acid receptor (RAR), which forms heterodimers with RXR and control processes such as myelination (81), was also found to be differentially expressed in the AHR pathway, indicating that gestational exposure to FM 550 could be impacting brain development via RXR/RAR-mediated signaling in both brain and placenta. The top canonical pathway identified was hepatic fibrosis, primarily due to the upregulation of numerous collagen genes. Collagen plays a significant role in synaptic development, axonal guidance and lamination of the cerebral cortex; thus, it was interpreted that ‘hepatic fibrosis’ was a misclassification. Genes within the fibril-associated subfamily and the collagens that form filaments and networks were most enriched suggesting FM550 exposure may alter brain ultrastructure and patterning (82).
Most intriguingly, the combination of approaches revealed an unexpected but potentially critical mode of action worthy of follow-up investigation. In both the placenta and fetal forebrain the ratio of 5-HT to its primary metabolite 5-HIAA were reduced by exposure, particularly in females, suggesting decreased 5-HT availability and catabolism. The observed forebrain disruptions could be the result of direct toxicity to the developing brain, since we have previously demonstrated that the brominated components of FM 550 accumulate in fetal tissue (37) or a consequence of disrupted placental 5-HT synthesis. Significantly, the fetal side of the placenta is the sole source of 5-HT for the developing forebrain from GD 10 to 15, at which point serotonergic terminals begin to innervate the region, providing an additional supply (5, 6). Higher concentrations of 5-HT in the FM 550-exposed brains might signify efforts by the GD 18 brain to compensate for the lower placental supply. Higher 5-HT concentrations in the placenta are difficult to interpret because we used whole placenta, making it impossible to delineate on which side (fetal or maternal) 5-HT disruption occurred. Future studies will seek to identify which placental layers are most vulnerable to FM550, and the cellular location of 5-HT disruption particularly in the critical window of GD 10–15 when the placenta is the sole source of 5-HT. The observed heightened expression of Tdo2 suggests that altered tryptophan metabolism may be involved. Tryptophan is the precursor for not only 5-HT but also kynurenine via Tdo2 activity. Overexpression of placental Tdo2 could signify diversion of tryptophan from 5-HT synthesis, toward kynurenine and, possibly, increased production of its neurotoxic metabolites, such as quinolinic acid (83).
Although effects were small and subtle, particularly in brain, further interrogation with a more highly powered study, particularly in regards to potential sex-specific vulnerabilities, is imperative because even small disruptions of 5-HT availability (increased or decreased) can have profound and life-long impacts on brain development and function (84, 85). For example, 5-HT disruption has been implicated in a variety of behavioral phenotypes including anxiety and hyperactivity (86); two behaviors we have previously linked with perinatal FM 550 exposure (11, 12). Disruption of 5-HT systems can impact multiple neurodevelopmental processes including synaptogenesis, cell proliferation and migration, myelination and differentiation (86, 87). That placental norepinephrine levels were reduced in low-dose females is also of interest given that gestational exposure to serotonin-norepinephrine reuptake inhibitors (SNRIs) can induce congenital heart defects via disruption of placental and fetal heart 5-HTsignaling (88). Disruption of placental neurotransmitter synthesis and secretion would represent a novel mode of chemical neurodevelopmental disruption.
We also followed up on specific genes of interest involved in steroid hormone signaling because prior studies by us and others have revealed that these pathways are susceptible to perturbation by FM 550 (11, 32, 35, 41, 89). In addition to thyroid hormone disruption, which has classically been linked to brominated FRs, especially PBDEs (90, 91), evidence of sex steroid disruption by FM 550 components has also been observed (41). For example, TPHP has been shown to increase plasma 17β-estradiol concentrations and upregulate Esr1 in the brain (92). Here, we observed upregulated Esr1 in placentas from the high-dose group (Fig. 1A and B), but very little evidence of thyroid hormone disruption. In a previously published pilot study, we observed increased serum thyroxine (T4) concentrations in FM 550-exposed dams (11). For the present study, we quantified serum T3 and T4 levels in the dams exposed during gestation, but also in dams exposed during lactation, and serum from their PND 21 lactationally exposed pups (Supplementary Materials and methods). Elevated concentrations of triiodothyronine (T3) in lactating dams from the low-dose group were the only significant finding (Supplementary Fig. 4). The RNA-seq data also did not reveal meaningful changes in thyroid hormone-related gene expression, including changes in receptor or deiodinase (activating/deactivating enzymes) expression in brain or placenta.
Gene expression related to metabolic pathways were also of primary interest because FM 550 and its constituents have been identified as possible obesogens (11). Mechanisms by which EDCs induce metabolic reprogramming and heighten risk of obesity or metabolic disorders are multifaceted and involve numerous hormones including leptin and insulin. During gestation, leptin is produced by the placenta and signals whether fetal fat stores are sufficient (93). Although RNA-seq analysis suggested reduced Lepr expression in high-dose placentas, qRT-PCR was not confirmatory. By contrast, Igf1, which plays a significant role in fetal and placental growth through mitogenic and metabolic processes (94, 95), was found to be upregulated by RNA-seq data and qRT-PCR (Fig. 3C and D) in high-dose placentas. Several studies have shown that environmental contaminants can disrupt the Igf axis, including FRs. For example, cord blood PBDE concentrations have been positively correlated with increased placental Igf1 expression in neonates and low birth weight (96). The metabolomics data are also supportive of sex-specific effects on endpoints related to fetal nutrient availability and growth. Overall, the collective data set support the hypothesis that FM 550 has the potential to disrupt placental genes related to fetal growth and development.
Conclusions
The placenta represents a novel, but potentially causal link between the toxic effects of environmental contaminants and the developing brain. Multiple lines of evidence have shown that placental dysfunction, resulting from environmental insult, can lead to significant alterations in brain development and behavior (16, 97). The data reported herein indicate that altered placental function, in the absence of overt pathology, resulting from FM 550 exposure, may be a route by which this commercial FR mixture can disrupt neurodevelopment and alter later in life behaviors. This study provides preliminary evidence that FM 550 can impact multiple placental pathways including endocrine, inflammatory and neurotransmitter signaling; disruption of which could potentially translate to impaired fetal growth and neurodevelopment. Because FM 550 is a mixture, that multiple possible modes of action were detected is not implausible. Independent examination of the individual components will be needed to identify specifically which compounds may be developmentally neurotoxic. Follow-up studies focusing on the specific subregions of the fetal and maternal placental compartments, particularly between GD10 and 15 when the placenta is the sole source of 5-HT for the developing brain, will also be needed to gain better anatomical resolution and mechanistic understanding. Finally, although some sex differences in vulnerability and response were observed in this exploratory study, we were underpowered to fully detect and account for them. Future investigations should be sufficiently powered to hone in on these possible differences. Our results provide novel evidence that gestational exposure to FM 550, at levels below the purported NOAEL of 50 mg/kg/day, can impact physiological endpoints in the rat placenta and fetal forebrain.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by R56 NIEHS R56ES022957 to H B P, NIEHS P30ES025128 to NCSU, NIEHS T32ES021432 to NCSU and NIH Common Fund U24 DK097193 to S S. A P and H M S were supported by NIEHS R01ES016099.
Acknowledgements
The authors appreciate the insight and input of Seth Kullman related to FXR/RXR signaling and Stephen Turner for performing the bioinformatics on the placental RNA-seq data set. They also appreciate the support of the Biological Resources Facility at NCSU for all of the animal care and husbandry.
References
- 1.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environmental Science and Technology 2009. 43 7490–7495. ( 10.1021/es9014019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurology 2014. 13 330–338. ( 10.1016/S1474-4422(13)70278-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current Problems in Pediatric and Adolescent Health Care 2014. 44 277–318. ( 10.1016/j.cppeds.2014.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environmental Health Perspectives 2012. 120 a258–a260. ( 10.1289/ehp.1104285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011. 197 1–7. ( 10.1016/j.neuroscience.2011.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature 2011. 472 347–350. ( 10.1038/nature09972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011. 32 261–267. ( 10.1016/j.neuro.2010.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental Science and Technology 2012. 46 13432–13439. ( 10.1021/es303471d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. Journal of Neuroscience 2003. 23 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naveau E, Pinson A, Gérard A, Nguyen L, Charlier C, Thomé J-P, Zoeller RT, Bourguignon J-P, Parent A-S. Alteration of rat fetal cerebral cortex development after prenatal exposure to polychlorinated biphenyls. PLoS ONE 2014. 9 e91903 ( 10.1371/journal.pone.0091903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster((R)) 550 in rats: an exploratory assessment. Journal of Biochemical and Molecular Toxicology 2013. 27 124–136. ( 10.1002/jbt.21439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB. Sex specific placental accumulation and behavioral effects of developmental firemaster® 550 exposure in Wistar rats. Scientific Reports 2017. 7 7118 ( 10.1038/s41598-017-07216-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konkel L. Lasting impact of an ephemeral organ: the role of the placenta in fetal programming. Environmental Health Perspectives 2016. 124 A124–A129. ( 10.1289/ehp.124-A230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, Bonnin A. Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. Journal of Neuroscience 2016. 36 6041–6049. ( 10.1523/JNEUROSCI.2534-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues in Clinical Neuroscience 2016. 18 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 2016. 41 207–218. ( 10.1038/npp.2015.231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environmental Science and Technology 2008. 42 6910–6916. ( 10.1021/es801070p) [DOI] [PubMed] [Google Scholar]
- 18.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. Journal of Chromatography A 2013. 1303 48–53. ( 10.1016/j.chroma.2013.06.042) [DOI] [PubMed] [Google Scholar]
- 19.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environmental Science and Technology 2010. 44 5627–5632. ( 10.1021/es100697q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, Webster TF. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environmental Health Perspectives 2011. 119 1247–1252. ( 10.1289/ehp.1003271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodier PM. Developing brain as a target of toxicity. Environmental Health Perspectives 1995. 103 (Supplement 6) 73–76. ( 10.1289/ehp.95103s673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Threadgill DW, Churchill GA. Ten years of the collaborative cross. Genetics 2012. 190 291–294. ( 10.1534/genetics.111.138032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Omrana LS, Harrada S. Polybrominated diphenyl ethers and ‘novel’ brominated flame retardants in floor and elevated surface house dust from Iraq: implications for human exposure assessment. Emerging Contaminants 2015. 2 7–13. ( 10.1016/j.emcon.2015.10.001) [DOI] [Google Scholar]
- 24.Ali N, Harrad S, Goosey E, Neels H, Covaci A. ‘Novel’ brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere 2011. 83 1360–1365. ( 10.1016/j.chemosphere.2011.02.078) [DOI] [PubMed] [Google Scholar]
- 25.Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H, ‘t Mannetje A, Coakley J, Douwes J, Covaci A. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere 2012. 88 1276–1282. ( 10.1016/j.chemosphere.2012.03.100) [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM. Nail polish as a source of exposure to triphenyl phosphate. Environment International 2016. 86 45–51. ( 10.1016/j.envint.2015.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology 2001. 22 619–624. ( 10.1016/S0161-813X(01)00033-X) [DOI] [PubMed] [Google Scholar]
- 28.Zhou SN, Buchar A, Siddique S, Takser L, Abdelouahab N, Zhu J. Measurements of selected brominated flame retardants in nursing women: implications for human exposure. Environmental Science and Technology 2014. 48 8873–8880. ( 10.1021/es5016839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM. Urinary Tetrabromobenzoic Acid (TBBA) as a biomarker of exposure to the flame retardant mixture firemaster 550. Environmental Health Perspectives 2014. 122 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson PI, Stapleton HM, Mukherjee B, Hauser R, Meeker JD. Associations between brominated flame retardants in house dust and hormone levels in men. Science of the Total Environment 2013. 445–446 177–184. ( 10.1016/j.scitotenv.2012.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environmental Health Perspectives 2010. 118 318–323. ( 10.1289/ehp.0901332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicology Letters 2014. 228 93–102. ( 10.1016/j.toxlet.2014.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schug TT, Abagyan R, Blumberg B, Collins TJ, Crews D, DeFur PL, Dickerson SM, Edwards TM, Gore AC, Guillette LJ, et al Designing endocrine disruption out of the next generation of chemicals. Green Chemistry 2013. 15 181–198. ( 10.1039/C2GC35055F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicology and Teratology 2015. 52 194–209. ( 10.1016/j.ntt.2015.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, 3rd, Jarema KA, Padilla S, et al Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicology and Teratology 2015. 52 181–193. ( 10.1016/j.ntt.2015.09.003) [DOI] [PubMed] [Google Scholar]
- 36.Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environmental Health 2016. 15 113 ( 10.1186/s12940-016-0199-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM. Editor’s highlight: transplacental and lactational transfer of firemaster(R) 550 components in dosed Wistar rats. Toxicological Sciences 2016. 153 246–257. ( 10.1093/toxsci/kfw122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicological Sciences 2002. 66 105–116. ( 10.1093/toxsci/66.1.105) [DOI] [PubMed] [Google Scholar]
- 39.Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environmental Science and Technology 2013. 47 10012–10021. ( 10.1021/es402650x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environmental Health Perspectives 2011. 119 1454–1459. ( 10.1289/ehp.1003235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dishaw LV, Macaulay LJ, Roberts SC, Stapleton HM. Exposures, mechanisms, and impacts of endocrine-active flame retardants. Current Opinion in Pharmacology 2014. 19 125–133. ( 10.1016/j.coph.2014.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro. Environmental Health Perspectives 2014. 122 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 2009. 30 350–357. ( 10.1016/j.neuro.2009.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, Gore AC. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE 2012. 7 e43890 ( 10.1371/journal.pone.0043890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biology of Reproduction 2009. 81 807–813. ( 10.1095/biolreprod.109.077008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology 2007. 24 199–224. ( 10.1016/j.reprotox.2007.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology 2008. 29 504–519. ( 10.1016/j.neuro.2008.02.015) [DOI] [PubMed] [Google Scholar]
- 48.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. Journal of Biochemical and Molecular Toxicology 2013. 27 124–136. ( 10.1002/jbt.21439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Naarden Braun K, Pettygrove S, Daniels J, Miller L, Nicholas J, Baio J, Schieve L, Kirby RS, Washington A, Brocksen S, et al Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. MMWR Surveillance Summaries 2007. 56 29–40. [PubMed] [Google Scholar]
- 50.Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Research Reviews 2010. 65 56–79. ( 10.1016/j.brainresrev.2010.06.002) [DOI] [PubMed] [Google Scholar]
- 51.Poletti A, Celotti F, Rumio C, Rabuffetti M, Martini L. Identification of type 1 5alpha-reductase in myelin membranes of male and female rat brain. Molecular and Cellular Endocrinology 1997. 129 181–190. ( 10.1016/S0303-7207(97)04056-2) [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G. Atlas of the Developing Mouse Brain at E17.5, P0 and P6. 1st edn. Amsterdam, the Netherlands: Elsevier, 2007. [Google Scholar]
- 53.Puzianowska-Kuznicka M, Pietrzak M, Turowska O, Nauman A. Thyroid hormones and their receptors in the regulation of cell proliferation. Acta Biochimica Polonica 2006. 53 641–650. [PubMed] [Google Scholar]
- 54.Wise LM, Sadowski RN, Kim T, Willing J, Juraska JM. Long-term effects of adolescent exposure to bisphenol A on neuron and glia number in the rat prefrontal cortex: differences between the sexes and cell type. Neurotoxicology 2016. 53 186–192. ( 10.1016/j.neuro.2016.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013. 155 1596–1609. ( 10.1016/j.cell.2013.11.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology 2010. 11 R106 ( 10.1186/gb-2010-11-10-r106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron 2009. 64 61–78. ( 10.1016/j.neuron.2009.09.002) [DOI] [PubMed] [Google Scholar]
- 58.R Core and Team. R: A Language and Environment for Statistical Computing, 2015. (available at: http://www.R-project.org/) [Google Scholar]
- 59.Anders S, Pyl PT, Huber W. HTSeq – a Python framework to work with high-throughput sequencing data. Bioinformatics 2015. 31 166–169. ( 10.1093/bioinformatics/btu638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001. 25 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 61.Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S, Nikolau B. Quality control for plant metabolomics: reporting MSI-compliant studies. Plant Journal 2008. 53 691–704. ( 10.1111/j.1365-313X.2007.03387.x) [DOI] [PubMed] [Google Scholar]
- 62.Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, Fiehn O. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Analytical Chemistry 2009. 81 10038–10048. ( 10.1021/ac9019522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chou H, Pathmasiri W, Deese-Spruill J, Sumner S, Buchwalter DB. Metabolomics reveal physiological changes in mayfly larvae (Neocloeon triangulifer) at ecological upper thermal limits. Journal of Insect Physiology 2017. 101 107–112. ( 10.1016/j.jinsphys.2017.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skogerson K, Wohlgemuth G, Barupal DK, Fiehn O. The volatile compound BinBase mass spectral database. BMC Bioinformatics 2011. 12 321 ( 10.1186/1471-2105-12-321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eriksson LB, Byrne T, Johansson E, Trygg J, Vikström C. Multi- and Megavariate Data Analysis: Basic Principals and Applications. Umeå, Sweden: Umetrics AB, 2013. [Google Scholar]
- 66.Bender R, Lange S. Adjusting for multiple testing – when and how? Journal of Clinical Epidemiology 2001. 54 343–349. ( 10.1016/S0895-4356(00)00314-0) [DOI] [PubMed] [Google Scholar]
- 67.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 2014. 155 2635–2646. ( 10.1210/en.2014-1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. Impact of low dose oral exposure to bisphenol A (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: a CLARITY-BPA consortium study. Endocrinology 2016. 157 3856–3872. ( 10.1210/en.2016-1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology 2014. 155 3853–3866. ( 10.1210/en.2014-1253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanitz A, Gypas F, Gruber AJ, Gruber AR, Martin G, Zavolan M. Comparative assessment of methods for the computational inference of transcript isoform abundance from RNA-seq data. Genome Biology 2015. 16 150 ( 10.1186/s13059-015-0702-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Lin L, Jiang P, Wang D, Xing Y. A comparison of RNA-Seq and high-density exon array for detecting differential gene expression between closely related species. Nucleic Acids Research 2011. 39 578–588. ( 10.1093/nar/gkq817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. Journal of Neuroscience 2009. 29 12815–12823. ( 10.1523/JNEUROSCI.3331-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakanishi T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. Journal of Toxicological Sciences 2008. 33 269–276. ( 10.2131/jts.33.269) [DOI] [PubMed] [Google Scholar]
- 74.Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends in Endocrinology and Metabolism 2010. 21 676–683. ( 10.1016/j.tem.2010.06.009) [DOI] [PubMed] [Google Scholar]
- 75.Knabl J, Vattai A, Hüttenbrenner R, Hutter S, Karsten M, Jeschke U. RXRalpha is upregulated in first trimester endometrial glands of spontaneous abortions unlike LXR and PPARgamma. European Journal of Histochemistry 2016. 60 2665 ( 10.4081/ejh.2016.2665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xing Y, Saner-Amigh K, Nakamura Y, Hinshelwood MM, Carr BR, Mason JI, Rainey WE. The farnesoid X receptor regulates transcription of 3beta-hydroxysteroid dehydrogenase type 2 in human adrenal cells. Molecular and Cellular Endocrinology 2009. 299 153–162. ( 10.1016/j.mce.2008.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaik FB, Prasad DV, Narala VR. Role of farnesoid X receptor in inflammation and resolution. Inflammation Research 2015. 64 9–20. ( 10.1007/s00011-014-0780-y) [DOI] [PubMed] [Google Scholar]
- 78.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in Behavioral Neuroscience 2009. 3 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Frontiers in Neuroendocrinology 2012. 33 267–286. ( 10.1016/j.yfrne.2012.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS. Foetal and placental 11beta-HSD2: a hub for developmental programming. Acta Physiologica 2014. 210 288–295. ( 10.1111/apha.12187) [DOI] [PubMed] [Google Scholar]
- 81.Huang JK, Jarjour AA, Oumesmar BN, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, et al Retinoid X receptor gamma signaling accelerates CNS remyelination. Nature Neuroscience 2011. 14 45–53. ( 10.1038/nn.2702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hubert T, Grimal S, Carroll P, Fichard-Carroll A. Collagens in the developing and diseased nervous system. Cellular and Molecular Life Sciences 2009. 66 1223–1238. ( 10.1007/s00018-008-8561-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freese A, Swartz KJ, During MJ, Martin JB. Kynurenine metabolites of tryptophan: implications for neurologic diseases. Neurology 1990. 40 691–695. ( 10.1212/WNL.40.4.691) [DOI] [PubMed] [Google Scholar]
- 84.Booij L, Tremblay RE, Szyf M, Benkelfat C. Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. Journal of Psychiatry and Neuroscience 2015. 40 5–18. ( 10.1503/jpn.140099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.St-Pierre J, Laurent L, King S, Vaillancourt C. Effects of prenatal maternal stress on serotonin and fetal development. Placenta 2016. 48 (Supplement 1) S66–S71. ( 10.1016/j.placenta.2015.11.013) [DOI] [PubMed] [Google Scholar]
- 86.Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and alpha-synuclein by the pyrethroid insecticide permethrin. Toxicology and Applied Pharmacology 2003. 192 287–293. ( 10.1016/S0041-008X(03)00326-0) [DOI] [PubMed] [Google Scholar]
- 87.Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environmental Health Perspectives 2005. 113 123–136. ( 10.1289/ehp.7254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laurent L, Huang C, Ernest SR, Berard A, Vaillancourt C, Hales BF. In utero exposure to venlafaxine, a serotonin-norepinephrine reuptake inhibitor, increases cardiac anomalies and alters placental and heart serotonin signaling in the rat. Birth Defects Research Part A: Clinical and Molecular Teratology 2016. 106 1044–1055. ( 10.1002/bdra.23537) [DOI] [PubMed] [Google Scholar]
- 89.Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 2013. 314 76–83. ( 10.1016/j.tox.2013.09.004) [DOI] [PubMed] [Google Scholar]
- 90.Gascon M, Vrijheid M, Martínez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environment International 2011. 37 605–611. ( 10.1016/j.envint.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 91.Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 2012. 33 842–852. ( 10.1016/j.neuro.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 92.Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquatic Toxicology 2013. 134–135 104–111. ( 10.1016/j.aquatox.2013.03.013) [DOI] [PubMed] [Google Scholar]
- 93.Romano ME, Savitz DA, Braun JM. Challenges and future directions to evaluating the association between prenatal exposure to endocrine disrupting chemicals and childhood obesity. Current Epidemiology Reports 2014. 1 57–66. ( 10.1007/s40471-014-0007-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. Journal of Anatomy 2009. 215 60–68. ( 10.1111/j.1469-7580.2008.01035.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta 2003. 24 803–812. ( 10.1016/S0143-4004(03)00080-8) [DOI] [PubMed] [Google Scholar]
- 96.Xu X, Yekeen TA, Xiao Q, Wang Y, Lu F, Huo X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environmental Pollution 2013. 182 63–69. ( 10.1016/j.envpol.2013.07.005) [DOI] [PubMed] [Google Scholar]
- 97.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Developmental Neurobiology 2012. 72 1317–1326. ( 10.1002/dneu.22045) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a