Abstract
Patient: Male, 61
Final Diagnosis: Semantic dementia
Symptoms: Primary progressive aphasia
Medication: —
Clinical Procedure: Neuroimaging
Specialty: Nuclear Medicine
Objective:
Rare co-existance of disease or pathology
Background:
Semantic dementia (SD) is a type of primary progressive aphasia with prominent language dysfunction, mostly within the spectrum of frontotemporal lobar degeneration (FTLD). Although there is an overlap in clinical manifestations of SD attributable to FTLD and neuropathologically proven Alzheimer disease (AD), clinical diagnostic clues are not readily available. We present a characteristic finding based on a single-photon emission computed tomography (SPECT)-based regional cerebral blood flow study and its statistical imaging analysis for a rare case of SD with AD-like pathology.
Case Report:
A 61-year-old male was referred to our hospital due to difficulties in self-management and impaired comprehension of word meaning suggestive of SD. Although his brain MRI revealed mild frontal lobe atrophy, his SPECT with three-dimensional stereotactic surface projections (3D-SSP) analysis showed left-sided hypo-perfusion that was more prominent in the inferior temporal gyrus and the inferior parietal lobule, with bilateral frontal lobe hypo-perfusion. The SPECT scan also showed involvement of the right inferior parietal area and, in medial aspects, the posterior cingulate cortex and adjacent precuneus; these finding were compatible with early hypo-perfused areas seen in AD. The lumbar cerebrospinal fluid biomarker findings seemed to fit SD in association with probable AD pathology.
Conclusions:
This is the first reported case to use SPECT with 3D-SSP statistical analysis as a potential, useful imaging modality for the diagnosis of SD with probable AD pathology.
MeSH Keywords: Alzheimer Disease; Aphasia, Primary Progressive; Biological Markers; Cerebrospinal Fluid; Frontotemporal Dementia; Tomography, Emission-Computed, Single-Photon
Background
Semantic dementia (SD) is a type of primary progressive aphasia (PPA) with prominent language dysfunction. Clinical criteria for PPA are categorized as SD, progressive non-fluent aphasia (PNFA), and logopenic progressive aphasia (LPA) [1,2]. All PPA variants reveal impaired language networks, usually located in the left hemisphere suggestive of frontotemporal lobar degeneration (FTLD), but there have been only a few case reports that have described the involvement of SD with Alzheimer disease (AD) pathology [3,4] and its differentiation from SD due to FTLD. For a clinical diagnosis of SD to distinguish an underlying pathology between FTLD and AD, many neuropsychological assessments (e.g., behavioral disturbance, superior letter and category fluency, and Repeat and Point test) have been attempted and failed [4].
Here, we present a rare case of SD with probable AD pathology, which was diagnosed early during disease progression using a single-photon emission computed tomography (SPECT) study of regional cerebral blood flow (CBF) and statistical imaging analysis that focused on the hypo-perfused areas around the posterior associative and/or posterior cingulate cortex.
Case Report
A 61-year-old, right-handed male with 18 years of formal education experienced difficulty in using public transportation a year before admission to our clinic. Six months prior to admission, he became unable to use e-mail and to manage his schedule. He was suspected to have a mood disorder and consulted a psychiatrist; however, he could not take prescribed medicine properly and his symptom did not improve. The psychiatrist suspected cognitive decline and referred the patient to our hospital. At his presentation at our hospital, the patient seemed to have depressive facial expression and impairment in expressive language. A review of his speech and language examinations found that he had difficulties in phonemic and semantic cues during fluent but empty speech, presenting with both word findings and impaired comprehension of word meaning (two-way anomia). His single word repetition was intact. His memory was relatively preserved, although his insight was poor. There was no significant medical, psychiatric, or family history of dementia. His physical examination and laboratory data, including vitamin B12 and thyroid hormones, were within normal limits.
His Mini-Mental State Examination score was 10/30 and his Clock Drawing Test score was 3/5, suggesting possible visuo-spatial deficits and/or profound impairment in comprehension. On the Western Aphasia Battery, his speech was fluent but lacked content. Auditory comprehension of sequential commands and sentence repetition were preserved, but confrontation and generative naming were impaired. Reading aloud, matching, and copy writing was preserved but reading comprehension and spontaneous writing impaired. He could not complete the Wechsler Memory Scale-Revised because of difficulties in comprehension.
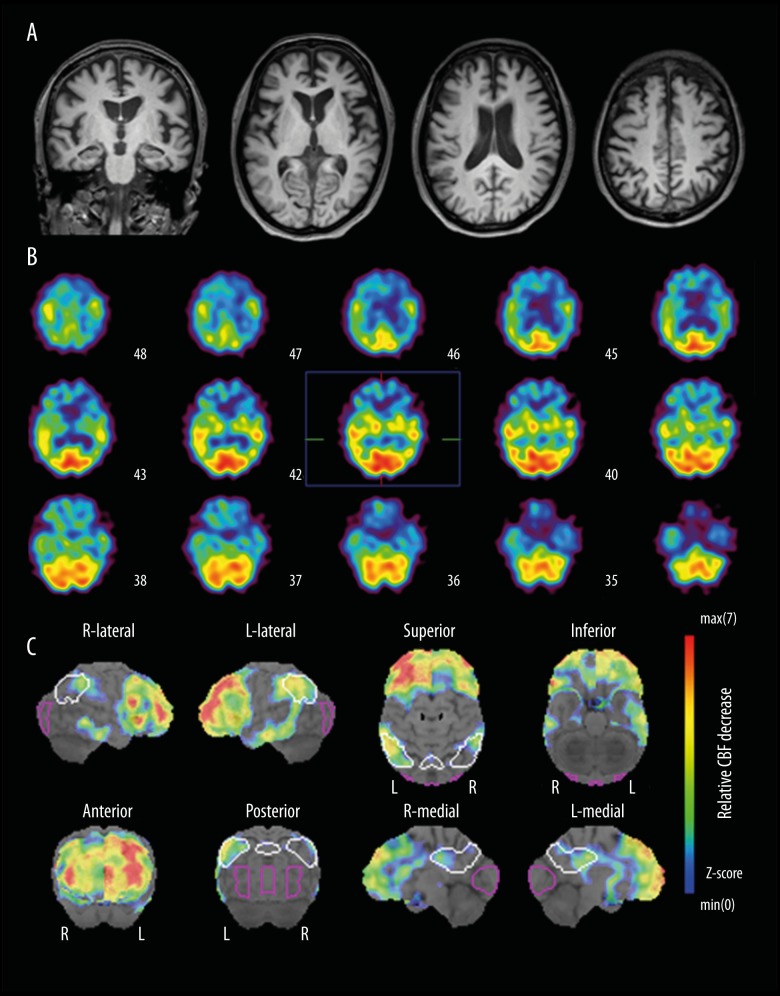
His brain MRI revealed mild atrophy in the frontal lobe, with no specific asymmetry (Figure 1A). N-isopropyl(123I) iodoamphetamine (IMP)-SPECT with three-dimensional stereotactic surface projections (3D-SSP) analysis [5,6] showed left-sided hypo-perfusion that was more prominent in the inferior temporal gyrus and the inferior parietal lobule, in conjunction with bilateral frontal lobe hypo-perfusion (Figure 1B). The 3D-SSP imaging further demonstrated an involvement of the right inferior parietal area and, in medial aspects, the posterior cingulate cortex and adjacent precuneus; these findings were compatible with early hypo-perfused areas in AD (Figure 1C). Lumbar cerebrospinal fluid (CSF) biomarker tests were performed after obtaining written informed consent for CSF sampling from the patient and his family. The concentrations of amyloid-β: 1–42 (Aβ42), total-Tau (t-Tau), and phosphorylated-Tau (p-Tau) in CSF was 369 pg/mL (normal reference value of ≥444 pg/mL), 567 pg/mL (reference value of <200 pg/mL), and 57.6 pg/mL (reference value of <50 pg/mL), respectively. Based on the patient’s early onset age and initial presentation with word-finding difficulty with fluent speech, in conjunction with neuroimaging and CSF biomarker findings, the diagnosis seemed to fit SD in association with probable AD pathology.
Figure 1.
(A) Brain MRI and (B) IMP-SPECT with (C) 3D-SSP analysis. Note that AD-like hypo-perfusion areas (posterior cingulate cortex and adjacent precuneus, in addition to relatively left-lateralized bilateral inferior parietal lobules) were detected by 3D-SSP analysis.
Discussion
SD with probable AD pathology is rare, as noted by several previous studies. In addition, studies using 11C-Pittsburgh compound B (11C-PIB)-PET imaging, which visualizes cerebral amyloid deposition in vivo, have reported only 1 of 9 SD cases (11%) as positive [7]. A systematic review that including 122 amyloid PET positive patients with PPA showed positivity in only 6 of 47 patients (12.8%) for the SD variant [8]. Qualitatively, the pattern of PIB binding in positive cases with SD variants has been reported to be similar to the pattern seen in AD [9] suggesting underlying AD pathology. However, PIB-PET is not easily available for routine clinical examination, and its use is limited to cases with a special research interest and at specific research facilities. Although many neuropsychological assessments (e.g., behavioral disturbance, superior letter and category fluency, and Repeat and Point test) have been attempted in patients with a clinical diagnosis of SD to distinguish between FTLD and AD, currently there are no standard indicators that contribute to the neuropathologic diagnosis of AD [4].
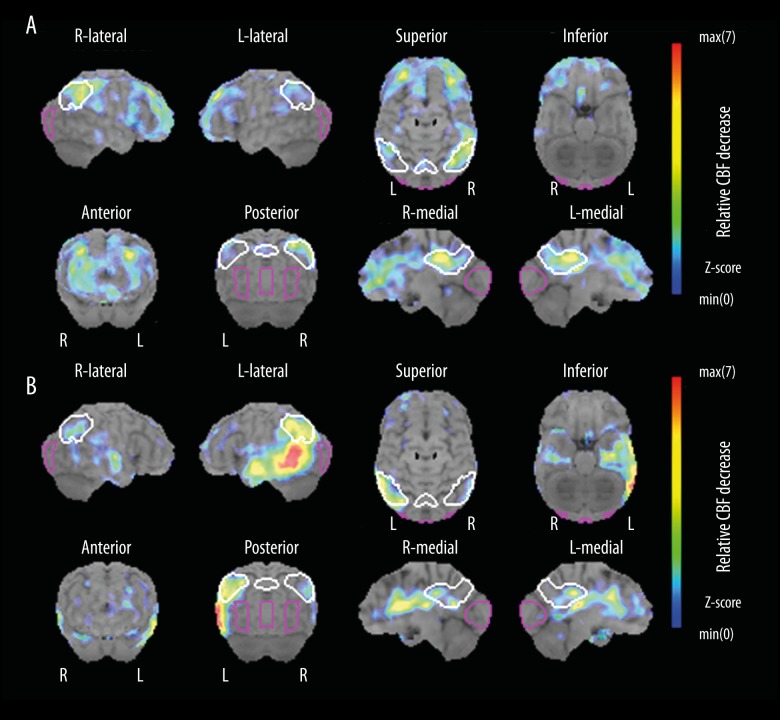
Brain SPECT imaging and 3D-SSP CBF analysis can distinguish AD converters from non-converters with high diagnostic performance in amnestic-mild cognitive impairment (aMCI) patients, and the presence of AD-like hypo-perfusion in the posterior associative (i.e., inferior parietal lobules) and/or posterior cingulate cortex of aMCI patients was shown to be predictive of conversion to AD within one to three years of follow-up [6]. Of note is the finding in our study that 3D-SSP showed relative hypo-perfused areas involved in the posteromedial areas of the parietal lobes in our patient with SD. The posterior cingulate cortex and adjacent precuneus might be an imaging marker for early stage of AD (Figure 2A), since impaired functional connectivity in the default mode network is supposedly involved in AD progression [10]. It is interesting that AD pathology has frequently been detected in patients with LPA [11]. In this study, we present a case of AD-like SPECT CBF (Figure 2B) and CSF biomarker findings. Differentiating underlying pathology of AD from FTLD is challenging in routine clinical practice without CSF studies or amyloid PETs on patients with SD. However, the characteristic SPECT-CBF pattern concomitance with the presence of AD-like hypo-perfusion in the posterior associative and/or posterior cingulate cortex, and the typical SD-like hypo-perfusion in the left-inferior temporal gyrus with bilateral frontal lobe as seen in this case could be considered a useful indicator for AD pathology.
Figure 2.
Representative 3D-SSP statistical images of AD-like SPECT patterns in (A) early AD in a 69-year-old female; and (B) LPA in a 78-year-old male). (A) Severe relative hypo-perfusion was demonstrated in the bilateral frontal lobes. (B) Relative hypoperfusion mainly in the bilateral temporo-parietal areas with CSF biomarker findings (Aβ42: 290 pg/mL, t-Tau: 803 pg/mL, and p-Tau: 57.8 pg/mL). (A, B) Relative hypo-perfused areas in posterior cingulate cortex and adjacent precuneus were also analyzed.
In this patient case, our CSF analysis also supported probable AD pathology. Studies have found that among AD patients, Aβ42 in CSF decreases and both t-Tau and p-Tau increases. Although Aβ42 decreases and t-Tau increases are also seen in FTLD patients, the degree of alteration is smaller than in AD patients and p-Tau increases have not been reported [12]. Bien et al. [13] reported the CSF t-Tau/Aβ42 ratio (cutoff: 1.06) was useful in discriminating FTLD and AD. In our patient case, the CSF t-Tau/Aβ42 was 1.54, suggesting AD rather than FTLD. However, a final diagnosis can only be made by autopsy. Considering the frequency of FTLD pathology in SD, the possibility of FTLD pathology was thus not eliminated. According to previous reports, a p-Tau/t-Tau ratio less than 0.37 suggests FTLD-TDP rather than FTLD-Tau and AD [14]. Therefore, AD, as well as FTLD-TDP, were possible underlying pathologies suggested by the CSF analysis in our patient case.
In a previous study, cholinesterase inhibitors were administered to four patients with SD pathologically who were diagnosed with AD (mean age of 63 years (range 56–70 year), mean duration 5-years (range, 1–10 years) after initial onset of symptoms) based on the possibility that they had atypical AD [4]; however, all the patients failed to improve their clinical symptoms. Given that SD with AD pathology usually has prolonged survival after diagnosis as compared with the reported 6–7-year survival rate for FLTD [15], further investigation regarding the risk factors and/or biomarkers for progression to typical AD (e.g., family history of late onset dementia, APOEɛ4 genotype, and Amyloid PET may in part help determine if a patient with SD would benefit from interventions for AD.
Conclusions
Although the underlying pathology of AD in clinical SD is difficult to diagnose, our patient case established that 3D-SSP statistical analysis is a potential, useful imaging modality for the diagnosis of SD with probable AD pathology.
Footnotes
Conflicts of interest
None.
References:
- 1.Rohrer JD, Rossor MN, Warren JD. Alzheimer’s pathology in primary progressive aphasia. Neurobiol Aging. 2012;33(4):744–52. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies RR, Hodges JR, Kril JJ, et al. The pathological basis of semantic dementia. Brain. 2005;128(Pt 9):1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- 4.Chow TW, Varpetian A, Moss T, et al. Alzheimer’s disease neuropathologic changes in semantic dementia. Neurocase. 2010;16(1):15–22. doi: 10.1080/13554790903193174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutoh T, Totsune T, Takenaka S, et al. Reduced cbf recovery detected by longitudinal 3d-ssp spect analyses predicts outcome of postoperative patients after subarachnoid hemorrhage. Clin Exp Pharmacol Physiol. 2017 doi: 10.1111/1440-1681.12867. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Mori E, Fukuyama H, et al. Prediction of outcomes in mci with (123)iimp-cbf spect: A multicenter prospective cohort study. Ann Nucl Med. 2013;27(10):898–906. doi: 10.1007/s12149-013-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leyton CE, Villemagne VL, Savage S, et al. Subtypes of progressive aphasia: Application of the international consensus criteria and validation using B-amyloid imaging. Brain. 2011;134:3030–43. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 8.Villarejo-Galende A, Llamas-Velasco S, Gómez-Grande A, et al. Amyloid pet in primary progressive aphasia: Case series and systematic review of the literature. J Neurol. 2017;264(1):121–30. doi: 10.1007/s00415-016-8324-8. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu E, Liao Z, Mao D, et al. Directed functional connectivity of posterior cingulate cortex and whole brain in Alzheimer’s disease and mild cognitive impairment. Curr Alzheimer Res. 2017;14(6):628–35. doi: 10.2174/1567205013666161201201000. [DOI] [PubMed] [Google Scholar]
- 11.Kirshner HS. Primary progressive aphasia and Alzheimer’s disease: Brief history, recent evidence. Curr Neurol Neurosci Rep. 2012;12(6):709–14. doi: 10.1007/s11910-012-0307-2. [DOI] [PubMed] [Google Scholar]
- 12.Schoonenboom NS, Reesink FE, Verwey NA, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78(1):47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 13.Bian H, Van Swieten JC, Leight S, et al. Csf biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70(19 Pt 2):1827–35. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu WT, Watts K, Grossman M, et al. Reduced csf p-tau181 to tau ratio is a biomarker for ftld-tdp. Neurology. 2013;81(22):1945–52. doi: 10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal dementia progresses to death faster than alzheimer disease. Neurology. 2005;65(5):719–25. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]