Key Points
Question
What is the effect of oral semaglutide on glycemic control in patients with type 2 diabetes?
Finding
In this randomized clinical trial of 632 patients with type 2 diabetes followed up for 31 weeks, oral semaglutide significantly reduced hemoglobin A1c level by up to 1.9% vs placebo (0.3%).
Meaning
Oral semaglutide resulted in better glycemic control than placebo over 26 weeks. Phase 3 studies are warranted to assess longer-term and clinical outcomes, as well as safety.
Abstract
Importance
Glucagon-like peptide-1 (GLP-1) receptor agonists are effective therapies for the treatment of type 2 diabetes and are all currently available as an injection.
Objectives
To compare the effects of oral semaglutide with placebo (primary) and open-label subcutaneous semaglutide (secondary) on glycemic control in patients with type 2 diabetes.
Design, Setting, and Patients
Phase 2, randomized, parallel-group, dosage-finding, 26-week trial with 5-week follow-up at 100 sites (hospital clinics, general practices, and clinical research centers) in 14 countries conducted between December 2013 and December 2014. Of 1106 participants assessed, 632 with type 2 diabetes and insufficient glycemic control using diet and exercise alone or a stable dose of metformin were randomized. Randomization was stratified by metformin use.
Interventions
Once-daily oral semaglutide of 2.5 mg (n = 70), 5 mg (n = 70), 10 mg (n = 70), 20 mg (n = 70), 40-mg 4-week dose escalation (standard escalation; n = 71), 40-mg 8-week dose escalation (slow escalation; n = 70), 40-mg 2-week dose escalation (fast escalation, n = 70), oral placebo (n = 71; double-blind) or once-weekly subcutaneous semaglutide of 1.0 mg (n = 70) for 26 weeks.
Main Outcomes and Measures
The primary end point was change in hemoglobin A1c (HbA1c) from baseline to week 26. Secondary end points included change from baseline in body weight and adverse events.
Results
Baseline characteristics were comparable across treatment groups. Of the 632 randomized patients (mean age, 57.1 years [SD, 10.6]; men, 395 (62.7%); diabetes duration, 6.3 years [SD, 5.2]; body weight, 92.3 kg [SD, 16.8]; BMI, 31.7 [SD, 4.3]), 583 (92%) completed the trial. Mean change in HbA1c level from baseline to week 26 decreased with oral semaglutide (dosage-dependent range, −0.7% to −1.9%) and subcutaneous semaglutide (−1.9%) and placebo (−0.3%); oral semaglutide reductions were significant vs placebo (dosage-dependent estimated treatment difference [ETD] range for oral semaglutide vs placebo, –0.4% to –1.6%; P = .01 for 2.5 mg, <.001 for all other dosages). Reductions in body weight were greater with oral semaglutide (dosage-dependent range, −2.1 kg to −6.9 kg) and subcutaneous semaglutide (−6.4 kg) vs placebo (−1.2 kg), and significant for oral semaglutide dosages of 10 mg or more vs placebo (dosage-dependent ETD range, –0.9 to –5.7 kg; P < .001). Adverse events were reported by 63% to 86% (371 of 490 patients) in the oral semaglutide groups, 81% (56 of 69 patients) in the subcutaneous semaglutide group, and 68% (48 of 71 patients) in the placebo group; mild to moderate gastrointestinal events were most common.
Conclusions and Relevance
Among patients with type 2 diabetes, oral semaglutide resulted in better glycemic control than placebo over 26 weeks. These findings support phase 3 studies to assess longer-term and clinical outcomes, as well as safety.
Trial Registration
clinicaltrials.gov Identifier: NCT01923181
This phase 2 randomized trial compared the effects on hemoglobin A1c levels of varying doses of oral once-daily semaglutide vs placebo vs subcutaneous semaglutide in patients with type 2 diabetes.
Introduction
Recombinant human proteins and peptides, such as glucagon-like peptide-1 (GLP-1) receptor agonists and insulin analogs, have expanded the range of diabetes treatment options. For peptide- or protein-based drugs, proteolytic degradation in the gastrointestinal tract poses a significant challenge for developing oral formulations.
The first oral GLP-1 analog, using semaglutide in a tablet co-formulated with the absorption enhancer sodium N-[8 (2-hydroxylbenzoyl) amino] caprylate (SNAC), is in clinical development for the treatment of type 2 diabetes. Semaglutide tablets are absorbed in the stomach, where SNAC causes a localized increase in pH, leading to higher solubility and protection against proteolytic degradation. Semaglutide is believed to be absorbed via the transcellular route.
Although several type 2 diabetes treatments are available, therapy selection involves consideration of the risks of adverse effects such as hypoglycemia or weight gain and complexity of treatment. GLP-1 receptor agonists reduce hyperglycemia by increasing insulin and decreasing glucagon secretion in a glucose-dependent manner, with a low risk of hypoglycemia. GLP-1 receptor agonists also provide significant weight loss by reducing appetite and energy intake, and 2 GLP-1 analogues, subcutaneous once-weekly semaglutide and liraglutide, have been shown to significantly improve cardiovascular outcomes. The most common adverse effects with this drug class are gastrointestinal, although events tend to be mild to moderate and transient. The oral formulation of semaglutide may improve acceptance and adherence for some patients compared with the injectable formulation of GLP-1 receptor agonists.
The objectives of this trial were to assess among patients with type 2 diabetes the dosage-response relationship of 5 dosages of oral semaglutide compared with placebo as well as open-label once-weekly subcutaneous semaglutide for glycemic control.
Methods
The trial was approved by local ethics committees and conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and the US Food and Drug Association Code of Federal Regulations (title 21, section 312.120). Participants provided written informed consent before trial-related activities commenced.
Trial Design
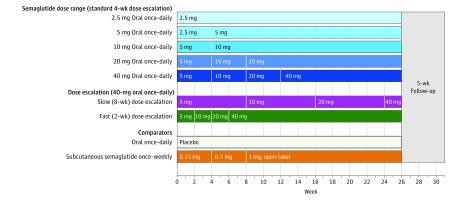
This 26-week, randomized, parallel-group, phase 2, dosage-finding trial (Figure 1), conducted between December 2013 and December 2014, assessed the dosage-response relationship on glycemic control (mean change in hemoglobin A1c [HbA1c]) level of 5 dosages (2.5, 5, 10, 20, and 40 mg) of once-daily oral semaglutide compared with placebo in a double-blind design (primary end point) and open-label, once-weekly subcutaneous semaglutide (secondary end point) in patients with type 2 diabetes. Oral semaglutide and placebo doses were blinded from both the investigator and the patient. In addition to a 4-week interval dose escalation (standard escalation), in which oral semaglutide or placebo doses were doubled every 4 weeks until the trial maintenance dose was achieved, the efficacy and safety of an 8-week interval (slow escalation) and a 2-week interval (fast escalation) dose escalation regimen for the highest dose (40 mg) of oral semaglutide were explored. The 26-week treatment period was followed by a 5-week follow-up period and visit at week 31. The trial protocol and statistical analysis plan are available in Supplement 1.
Figure 1. Trial Design.
The trial was conducted among 632 patients with type 2 diabetes who were 18 years or older receiving stable treatment with diet and exercise or stable treatment with metformin for 30 days prior to screening. Patients had a hemoglobin A1c level of 7.0% to 9.5% and an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or more. There was no dose escalation in the oral semaglutide 2.5-mg and placebo groups. In the other oral semaglutide groups, the dose was doubled from a starting dose of 2.5 mg or 5 mg every 4 weeks until the trial maintenance dose of the group (5-40 mg) was achieved (blue shades). The subcutaneous semaglutide dose was doubled every 4 weeks from a starting dose of 0.25 mg until a 1 mg trial maintenance dose was achieved. In addition, a slow dose escalation (purple) to 40 mg of oral semaglutide at 8-week intervals and a fast dose escalation (green) to 40 mg of oral semaglutide at 2-week intervals were included. Subcutaneous semaglutide was supplied as a 1.34-mg/mL solution in a 1.5-mL prefilled PDS290 pen injector (FlexTouch, Novo Nordisk A/S), and was administered in the abdomen, thigh, or upper arm on the same day of the week.
Patient Population
Patients (18 years or older) with type 2 diabetes and insufficient glycemic control (HbA1c level range, 7.0%-9.5%) on diet and exercise alone or with a stable dose (at least 30 days) of metformin were enrolled at 100 sites in 14 countries (eBox 1 in Supplement 2). Additional eligibility criteria were HbA1c level of 7.0% to 9.5% and a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 25 to 40 (for key exclusion criteria, see eBox 2 in Supplement 2). Because the trial was conducted in Europe, North America, and single countries in Africa, Asia, and the Middle East, race and ethnicity were recorded for completeness of data, according to local regulations. Race and ethnicity were self-reported by participants from categories predefined in the study protocol (race: American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or other Pacific Islander, white or other; ethnicity: Hispanic or Latino, or not Hispanic or Latino).
Drug Administration
An open-label design was chosen for once-weekly subcutaneous semaglutide to limit unnecessary injections. Patients were randomized using an interactive voice and web response system with equal ratio to 1 of 9 treatment groups, stratified according to history of treatment (metformin at screening, yes or no). Treatment groups included 5 oral semaglutide dosage groups (2.5, 5, 10, 20, and 40 mg) and an oral placebo group (these groups received a once-daily dose with 4-week interval dose escalation), and a 1-mg subcutaneous semaglutide group (receiving a once-weekly dose). Two additional 40-mg dosages were included to evaluate 8-week (slow) and 2-week (fast) dose escalation. Trial products were supplied by Novo Nordisk A/S. Oral semaglutide tablets (but not placebo) included 300 mg of SNAC (based on the Eligen Carrier concept, Emisphere Technologies). Patients administered oral semaglutide or placebo in the morning after at least 6 hours of fasting, and abstained from food or fluid intake for at least 30 minutes thereafter. If fasting plasma glucose exceeded 270 mg/dL/15 mmol/L (week 1-5), 240 mg/dL/13.3 mmol/L (week 6-11), or 200 mg/dL/11.1 mmol/L (week 12 to trial end), rescue medication was to be offered.
Study End Points and Assessments
The primary efficacy end point was change from baseline in HbA1c level at week 26. Secondary efficacy end points at week 26 included the proportion of patients achieving HbA1c level target of less than 7.0%; change from baseline in fasting plasma glucose and body weight; proportion of patients achieving weight loss of 5% or more and 10% or more; change from baseline in fasting insulin, fasting glucagon, fasting C-peptide, insulin resistance, and beta-cell function (homeostasis model assessment); fasting lipid profile; patient-reported outcomes (Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36; score range, 0-100, higher scores indicate better quality of life]; waist circumference; and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). In addition, a post hoc analysis investigated the proportion of patients achieving an HbA1c level target of 6.5% or less.
Safety end points included the number of treatment-emergent adverse events and severe (American Diabetes Association criteria) or blood glucose–confirmed (plasma glucose value of 70 mg/dL [to convert to mmol/L, multiply by 0.0555] or lower with symptoms) hypoglycemic episodes recorded from baseline until week 31. Change in vital signs, electrocardiogram, physical examination, and laboratory safety parameters were assessed after 26 weeks. Adverse events relevant to the GLP-1 drug class were given specific attention. eBox 3 in Supplement 2 lists the 8 predefined medical events of special interest that were adjudicated in a blinded fashion by an external, independent event-adjudication committee.
Statistical Analysis
For patients who completed the trial, the estimated treatment difference (ETD) between the pooled 40-mg oral semaglutide standard escalation and fast escalation groups and the placebo group was expected to be at least 0.64%, whereas for patients who discontinued treatment prematurely, the detectable mean difference was set conservatively to 0.32%, thus leading to a detectable mean difference of 0.58%. With these assumptions, enrollment of 134 patients in the pooled 40-mg standard and fast escalation groups and the 67 patients in the placebo group provided 90% power to demonstrate superiority of those pooled oral semaglutide dosage groups vs placebo for the primary end point at a 5% significance level, assuming a standard deviation of 1.2% and a premature treatment discontinuation rate of 20%. Therefore, enrollment of 67 patients in each of the 9 treatment groups was planned.
Statistical analyses of efficacy end points were based on data collected from all randomized patients during the treatment period who did not receive rescue medication (based on a modified intention-to-treat principle). Adverse events that occurred during the 26-week trial period from all exposed patients, with onset on or after the first day of treatment (including 5-week follow-up plus a visit window of 5 days), with or without rescue medication, are reported.
A standard repeated measures model analysis, with treatment, country, and stratification (metformin, yes or no) as fixed factors, and baseline value as a covariate, all nested within visit, was used for analysis of continuous end points, including the primary end point, body weight, BMI, waist circumference, and fasting plasma glucose. End points for patients attaining HbA1c level and weight loss targets were analyzed using a modified Poisson regression model with treatment, country, and stratification as fixed factors and baseline HbA1c level or body weight, respectively, as a covariate. Before analysis, missing data were imputed from a repeated measures model with treatment, stratum, country, and baseline value all nested within visit. This model was specified post hoc. Fasting insulin, glucagon, C-peptide, insulin resistance, beta-cell function and lipids were log-transformed at week 26 and analyzed by the standard repeated measures model analysis. Treatment-emergent adverse events were summarized descriptively. All analyses were performed using SAS (SAS Institute), version 9.3.
To investigate the efficacy of oral semaglutide without the risk of inflating a type I error, a confirmatory statistical analysis was carried out, whereby an initial comparison of the primary end point between the pooled 40-mg standard escalation and fast escalation groups vs placebo group was performed at week 26. The standard repeated measures model analysis was used to estimate the treatment difference and corresponding 2-sided P value at week 26. Efficacy of oral semaglutide was considered confirmed if the upper limit of the 95% CI for the ETD was less than 0, corresponding to a 2-sided P value of less than .05. If efficacy was confirmed, the comparisons between the 9 treatment groups and other secondary end points were evaluated with no adjustment for multiplicity and considered exploratory in nature.
Results
Patient Disposition and Baseline Characteristics
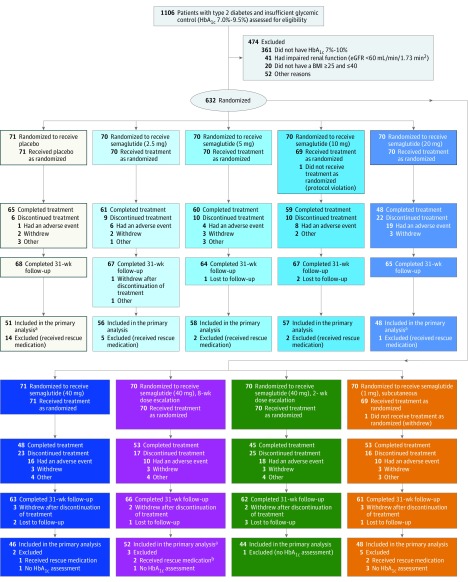
Of the 1106 patients screened, 632 were randomized and 630 exposed to trial medication (Figure 2). Baseline characteristics were similar in the 9 groups (mean age, 57.1 years (SD, 10.6); men, 395 of 630 patients (62.7%); mean HbA1c level, 7.9% (SD, 0.7%); diabetes duration, 6.3 years (SD, 5.2); body weight, 92.3 kg (SD, 16.8); BMI, 31.7 (SD, 4.3) (Table 1). Overall, 583 patients (92%) completed the trial (completed the 31-week follow-up visit), with 492 (78%) completing treatment (Figure 2). The proportion of patients completing the trial without rescue medication, and contributing to the analysis at week 26, was 64% to 83% in the dosage-dependent oral semaglutide groups, 73% in the subcutaneous semaglutide group, and 72% in the placebo group (Figure 2).
Figure 2. Flow of Patients Through the Trial of Semaglutide in Type 2 Diabetes.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c. “Completed treatment” refers to those patients who did not discontinue treatment prematurely (with or without the addition of rescue medication).
aParticipants included in the primary analysis had completed the week 26 assessment.
bDiscontinued treatment less than 1 week prior to week 26. The assessment at week 26 was still considered to be valid for the primary analysis due to the long half-life of semaglutide.
Table 1. Baseline Characteristics of Patients With Type 2 Diabetes and Insufficient Glycemic Controla.
| Placebo Group (n = 71) |
Oral Semaglutide Trial Maintenance Dosage Groupsb | 1-mg SC Semaglutide Group (n = 69) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 mg (n = 70) |
Standard Dose Escalationc | Slow Escalationc | Fast Escalationc | ||||||
| 5 mg (n = 70) |
10 mg (n = 69) |
20 mg (n = 70) |
40 mg (n = 71) |
40 mg (n = 70) |
40 mg (n = 70) |
||||
| Men, No. (%) | 40 (56.3) | 45 (64.3) | 47 (67.1) | 43 (62.3) | 44 (62.9) | 43 (60.6) | 41 (58.6) | 44 (62.9) | 48 (69.6) |
| Age, mean (SD), y | 58.9 (10.3) | 56.7 (9.9) | 55.7 (11.0) | 56.5 (10.1) | 58.3 (10.4) | 56.5 (10.2) | 57.1 (10.5) | 57.7 (10.8) | 56.8 (11.8) |
| Race, No. (%) | |||||||||
| White | 57 (80.3) | 57 (81.4) | 63 (90.0) | 57 (82.6) | 59 (84.3) | 63 (88.7) | 54 (77.1) | 59 (84.3) | 54 (78.3) |
| Black or African American | 6 (8.5) | 6 (8.6) | 2 (2.9) | 7 (10.1) | 4 (5.7) | 4 (5.6) | 7 (10.0) | 7 (10.0) | 4 (5.8) |
| Asian | 7 (9.9) | 7 (10.0) | 4 (5.7) | 4 (5.8) | 4 (5.7) | 3 (4.2) | 7 (10.0) | 4 (5.7) | 10 (14.5) |
| American Indian or Alaska Native | 1 (1.4) | 0 | 0 | 0 | 2 (2.9) | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 1 (1.4) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 2 (2.9) | 0 | 1 (1.4) |
| Ethnicity, No. (%) | |||||||||
| Hispanic or Latino | 9 (12.7) | 6 (8.6) | 7 (10.0) | 7 (10.1) | 7 (10.0) | 7 (9.9) | 9 (12.9) | 12 (17.1) | 7 (10.1) |
| Not Hispanic or Latino | 62 (87.3) | 64 (91.4) | 63 (90.0) | 62 (89.9) | 63 (90.0) | 64 (90.1) | 61 (87.1) | 58 (82.9) | 62 (89.9) |
| Body weight, mean (SD), kg | 93.8 (18.1) | 93.6 (15.6) | 93.1 (19.0) | 91.8 (14.0) | 93.8 (17.9) | 90.8 (16.5) | 93.3 (18.8) | 92.0 (15.4) | 88.8 (15.4) |
| BMI, mean (SD) | 32.6 (4.5) | 31.7 (4.1) | 31.6 (4.9) | 31.9 (4.4) | 32.0 (4.5) | 31.1 (4.1) | 32.3 (4.5) | 31.7 (3.8) | 30.7 (4.0) |
| HbA1c level, mean (SD), % | 8.0 (0.8) | 8.0 (0.7) | 7.8 (0.6) | 7.8 (0.7) | 7.9 (0.7) | 8.0 (0.7) | 8.0 (0.7) | 7.8 (0.8) | 7.8 (0.7) |
| FPG level, mean (SD), mg/dL | 171.7 (48.4) | 172.0 (40.2) | 172.6 (47.7) | 166.1 (36.9) | 165.1 (37.8) | 178.4 (49.1) | 172.8 (43.4) | 160.2 (30.7) | 172.8 (44.4) |
| Diabetes duration, mean (SD), y | 6.7 (5.1) | 6.1 (6.0) | 5.3 (4.7) | 5.8 (4.8) | 7.0 (5.3) | 7.7 (5.9) | 6.6 (4.9) | 5.6 (4.7) | 5.6 (5.0) |
| Metformin use, No. (%) | 58 (81.7) | 61 (87.1) | 60 (85.7) | 58 (84.1) | 59 (84.3) | 61 (85.9) | 60 (85.7) | 60 (85.7) | 58 (84.1) |
| SBP, mean (SD), mm Hg | 135.4 (15.5) | 132.3 (13.3) | 132.9 (13.8) | 134.1 (14.1) | 136.5 (15.8) | 134.0 (15.3) | 131.3 (14.6) | 135.0 (16.9) | 134.2 (14.5) |
| DBP, mean (SD), mm Hg | 80.6 (8.4) | 80.5 (8.0) | 81.5 (10.2) | 81.6 (10.0) | 80.7 (8.2) | 82.4 (8.4) | 82.4 (7.8) | 79.2 (9.1) | 80.9 (8.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; SBP, systolic blood pressure; SC, subcutaneous.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Full analysis set.
See Figure 1 for dosage regimen.
Standard escalation indicates 4-week intervals; slow escalation, 8-week intervals; fast escalation, 2-week intervals.
Glycemic Control
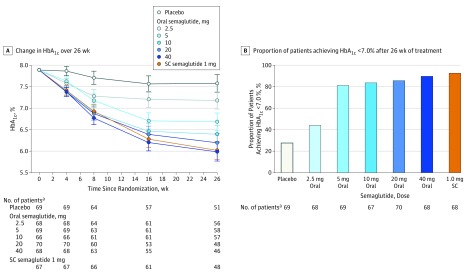
At week 26, pooled 40-mg oral semaglutide standard and fast escalation groups reduced mean HbA1c level by 1.8% compared with 0.3% with placebo (ETD, –1.5% [95% CI, –1.7% to –1.2%]; P < .001; confirmatory statistical analysis) thus achieving the primary end point.
All dosages of oral semaglutide reduced mean HbA1c level significantly more than placebo by week 26, in a dosage-dependent manner (Figure 3A). ETDs for dosage-dependent oral semaglutide vs placebo were −0.4% (95% CI, –0.7% to –0.1%] for the 2.5-mg group; –0.9% [95% CI, –1.2% to –0.6%] for the 5-mg group; –1.2% [95% CI, –1.5% to –0.9%] for the 10-mg group; –1.4% [95% CI, –1.7% to –1.1%] for the 20-mg group; and –1.6% [95% CI, –1.9% to –1.3%] for the 40-mg standard escalation group (P = .007 for the 2.5-mg group, <.001 for other dosages). The decrease in mean HbA1c level in the subcutaneous semaglutide group (1.9%) was also significantly greater than the placebo group (secondary analysis) (ETD, –1.6% [95% CI, –1.8% to –1.3%]; P < .001), and not significantly different from oral semaglutide dosages of 20 mg and 40 mg (standard escalation). The cumulative distribution of the HbA1c level reduction in the oral and subcutaneous semaglutide groups illustrates that with the exception of the 2.5-mg group, almost 100% of patients experienced a reduction in HbA1c level vs 74% in the placebo group (eFigure 1 in Supplement 2). In addition, a range of sensitivity analyses supported the primary comparisons with similar results in favor of oral semaglutide (eFigure 2 in Supplement 2).
Figure 3. Hemoglobin A1c (HbA1c) Efficacy Parameters From Baseline to Week 26 Among Patients With Type 2 Diabetes and Insufficient Glycemic Control.
RMM indicates repeated measures model; SC, subcutaneous. A, Data are estimated means from RMM with treatment, stratum, country, and baseline value all nested within visit. Error bars indicate 95% CIs. B, The proportion of patients achieving an HbA1c level of less than 7.0% after 26 weeks of treatment was significant for the oral semaglutide 2.5-mg group vs placebo (P = .01) and for all other oral semaglutide dosages and SC semaglutide (P < .001). Missing HbA1c values are imputed from RMM analysis before calculating the proportions of patients reaching the target.
aNo. of patients with an assessment (panel A) and imputed value (panel B).
For the oral and subcutaneous semaglutide groups, most patients reached the HbA1c level target of less than 7.0%: 44% in the 2.5-mg group (30 of 70 patients; estimated response rate ratio [RR], 1.6 [95% CI, 1.03 to 2.50]); 81% in the 5-mg group (56 of 70 patients; RR, 2.8 [95% CI, 1.9 to 4.2]); 84% in the 10-mg group (56 of 69 patients; RR, 2.9 [95% CI, 2.0 to 4.2]); 86% in the 20-mg group (60 of 70 patients; RR, 3.0 [95% CI, 2.1 to 4.4]); and 90% in the 40-mg standard escalation group (61 of 71 patients; RR, 3.3 [95% CI, 2.3 to 4.8]) (P = .04 for the 2.5-mg group, P < .001 for other dosages); vs 28% in the placebo group (19 of 71 patients) and 93% in the subcutaneous semaglutide group (63 of 69 patients; RR, 3.2 [95% CI, 2.2 to 4.7], P < .001) (Figure 3B).
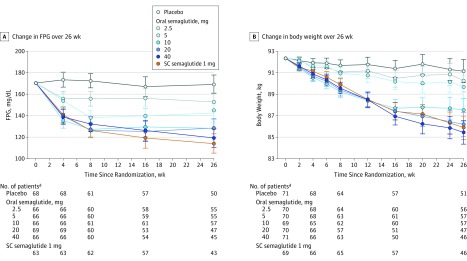
At week 26, significant dosage-dependent decreases in mean fasting plasma glucose level of up to 51 mg/dL from baseline were observed with oral semaglutide vs placebo (Figure 4A). Fasting plasma glucose level decreases with oral and subcutaneous semaglutide occurred mostly within the first 4 to 8 weeks (Figure 4A); at week 26, decreases observed with 40-mg standard escalation of oral semaglutide were not significantly different to those with subcutaneous semaglutide (Figure 4A). eTable 1 and eTable 2 in Supplement 2 show improvements in insulin, glucagon, and homeostasis model assessment insulin resistance and beta-cell function. No consistent pattern was observed with fasting C-peptide.
Figure 4. Fasting Plasma Glucose (FPG) Level and Body Weight Efficacy Parameters From Baseline to Week 26 Among Patients With Type 2 Diabetes and Insufficient Glycemic Control.
RMM indicates repeated measures model; SC, subcutaneous. Data are estimated means from the RMM with treatment, stratum, country, and baseline value all nested within visit. Error bars are 95% CIs.
aNo. of patients with an assessment.
Post Hoc Analysis
Patients reaching the HbA1c level target of 6.5% or less across the oral semaglutide dosages were 21% in the 2.5-mg group (14 of 70 patients; RR, 1.2 [95% CI, 0.6 to 2.3]); 52% in the 5-mg group (36 of 70 patients; RR, 2.8 [95% CI, 1.6 to 4.8]); 73% in the 10-mg group (49 of 69 patients; RR, 3.9 [95% CI, 2.3 to 6.5]); 77% in the 20-mg group (54 of 70 patients; RR, 4.3 [95% CI, 2.6 to 7.1]); and 82% in the 40-mg group (56 of 71 patients; RR, 4.7 [95% CI, 2.8 to 7.9]) (P = .60 for the 2.5-mg group, P < .001 for other dosages) vs 17% in the placebo group (12 of 71 patients) and 85% in the subcutaneous semaglutide group (58 of 69 patients; RR, 4.5 [95% CI, 2.7 to 7.6], P < .001).
Body Weight
Body weight decreased over time (Figure 4B). At week 26, the decrease from baseline in mean body weight in the oral semaglutide groups was dosage-dependent and significantly greater than placebo (–1.2 kg) (ETD: 2.5-mg group, –0.9 kg [95% CI, −2.4 to 0.6]; 5-mg group, −1.5 kg [95% CI, −3.0 to 0.0]; 10-mg group, –3.6 kg [95% CI, −5.1 to −2.1]; 20-mg group, −5.0 kg [95% CI, −6.5 to −3.4]; 40-mg standard escalation group, −5.7 kg [95% CI, −7.3 to −4.2]) (significant vs placebo in the ≥10-mg dosages [P < .001]) (Table 2). No significant difference was observed between 20 mg and 40-mg standard escalation groups of oral semaglutide and the subcutaneous semaglutide group. The proportion of patients achieving 5% weight loss was significantly greater for oral semaglutide dosage groups of 10-mg and higher (P < .001; 10-mg group: 38 of 69 patients [56%], RR, 4.1 [95% CI, 2.2 to 7.6]; 20-mg group: 45 of 70 patients [64%], RR, 5.2 [95% CI, 2.8 to 9.6]; 40-mg standard escalation group: 50 of 71 patients [71%], RR, 5.4 [95% CI, 9.2 to 9.9]) vs the placebo group (9 of 71 patients [13%]), and the subcutaneous semaglutide (45 of 69 patients [66%], RR, 5.2 [95% CI, 2.8 to 9.6], P < .001) (eTable 1 in Supplement 2).
Table 2. Hemoglobin A1c (HbA1c), Fasting Plasma Glucose, and Body Weight From Baseline to Week 26 Among Patients With Type 2 Diabetes and Insufficient Glycemic Controla.
| Placebo Group (n = 71) |
Oral Semaglutide Groups | 1-mg SC Semaglutide Group (n = 69) |
|||||
|---|---|---|---|---|---|---|---|
| 2.5 mg (n = 70) |
Standard Dose Escalationb | ||||||
| 5 mg (n = 70) |
10 mg (n = 69) |
20 mg (n = 70) |
40 mg (n = 71) |
||||
| Primary Analysis | |||||||
| HbA1c level at week 26, mean (95% CI), % | 7.6 (7.4 to 7.8) |
7.2 (7.0 to 7.4) |
6.7 (6.5 to 6.9) |
6.4 (6.2 to 6.6) |
6.2 (6.0 to 6.4) |
6.0 (5.8 to 6.2) |
6.0 (5.8 to 6.2) |
| Change from baseline in HbA1c level to week 26, mean (95% CI), % | –0.3 (–0.5 to –0.1) |
–0.7 (–0.9 to –0.5) |
–1.2 (–1.4 to –1.0) |
–1.5 (–1.7 to –1.3) |
–1.7 (–1.9 to –1.5) |
–1.9 (–2.1 to –1.7) |
–1.9 (–2.1 to –1.7) |
| ETD for comparator vs placebo for HbA1c level (95% CI), % | NA | –0.4 (–0.7 to –0.1) |
–0.9 (–1.2 to –0.6) |
–1.2 (–1.5 to –0.9) |
–1.4 (–1.7 to –1.0) |
–1.6 (–1.9 to –1.3) |
–1.6 (–1.8 to –1.3) |
| P value | NA | <.01 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Secondary Analyses | |||||||
| ETD for comparator vs SC semaglutide for HbA1c level (95% CI), % | NA | 1.2 (0.9 to 1.4) |
0.7 (0.4 to 1.0) |
0.4 (0.1 to 0.7) |
0.2 (–0.1 to 0.5) |
<–0.0 (–0.3 to 0.3) |
NA |
| P value | NA | <.001 | <.001 | .01 | .24 | .80 | NA |
| Change from baseline in fasting plasma glucose to week 26, mean (95% CI), mg/dL | –1.1 (–9.6 to –7.5) |
–17.3 (–25.6 to –9.1) |
–27.8 (–36.1 to –19.4) |
–42.1 (–50.4 to –33.9) |
–41.9 (–50.6 to –33.1) |
–51.2 (–60.0 to –42.4) |
–56.3 (–65.3 to –47.4) |
| ETD for comparator vs placebo for fasting plasma glucose (95% CI), mg/dL | NA | –16.3 (–28.2 to –4.3) |
–26.7 (–38.7 to –14.6) |
–41.0 (–52.8 to –29.2) |
–40.8 (–52.9 to –28.6) |
–50.1 (–62.4 to –37.8) |
–55.3 (–67.6 to –42.9) |
| P value | NA | <.01 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Change from baseline in body weight to week 26, mean (95% CI), kg | –1.2 (–2.3 to –0.1) |
–2.1 (–3.1 to –1.0) |
–2.7 (–3.7 to –1.6) |
–4.8 (–5.8 to –3.7) |
–6.1 (–7.3 to –5.0) |
–6.9 (–8.0 to –5.8) |
–6.4 (–7.5 to –5.3) |
| ETD for comparator vs placebo for body weight (95% CI), kg | NA | –0.9 (–2.4 to 0.6) |
–1.5 (–3.0 to 0.0) |
–3.6 (–5.1 to –2.1) |
–5.0 (–6.5 to –3.4) |
–5.7 (–7.3 to –4.2) |
–5.2 (–6.8 to –3.7) |
| P value | NA | .25 | .06 | <.001 | <.001 | <.001 | <.001 |
Abbreviations: ETD, estimated treatment difference; NA, not applicable; SC, subcutaneous.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Data are estimated means from repeated measures model analysis with treatment, stratum, and country as fixed factors and baseline value as covariate, all nested within visit.
Standard escalation indicates 4-week intervals.
Other Efficacy End Points
eTable 1 and eTable 2 show other efficacy end points and efficacy data for the oral semaglutide dose escalation groups. No clinically meaningful changes were observed in patient-reported outcomes, as measured by SF-36, version 2 (eTable 1 and eTable 2 in Supplement 2).
Adverse Events
Treatment-emergent adverse events for all treatment groups are shown in Table 3. There were no fatal events. The number of serious adverse events and patients reporting them were low (total of 31 events reported in 21 patients), with no grouping of events (eTable 3 in Supplement 2). The most common adverse events were gastrointestinal, which were mostly mild to moderate in severity with oral semaglutide (Table 3). The proportion of patients reporting gastrointestinal events was higher with oral semaglutide (31%-77%; 255 of 490 patients) and subcutaneous semaglutide (54%; 37 of 69 patients) than with placebo (28%; 20 of 71 patients). Overall, similar proportions of patients reported gastrointestinal-related adverse events in the three 40-mg dose escalation groups (2, 4, and 8 weeks). Fewer nausea events were reported when patients started on a lower dose (eg, 2.5 mg vs 5 mg) (eFigure 3 in Supplement 2). With continued therapy, nausea prevalence and severity decreased in most patients (eTable 4 in Supplement 2), partly explained by some patients discontinuing treatment prematurely because of these events.
Table 3. Treatment-Emergent Adverse Events Among Patients With Type 2 Diabetes and Insufficient Glycemic Controla.
| Placebo Group, No. of Patients (%) [No. of Events] (n = 71) | Oral Semaglutide Groups, No. of Patients (%) [No. of Events] | 1-mg SC Semaglutide Group, No. of Patients (%) [No. of Events] (n = 69) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 mg (n = 70) |
Standard Dose Escalationb | Slow Escalationb | Fast Escalationb | ||||||
| 5 mg (n = 70) |
10 mg (n = 69) |
20 mg (n = 70) |
40 mg (n = 71) |
40 mg (n = 70) |
40 mg (n = 70) |
||||
| Adverse events | 48 (68) [127] | 47 (67) [142] | 44 (63) [169] | 52 (75) [233] | 57 (81) [289] | 56 (79) [230] | 55 (79) [233] | 60 (86) [245] | 56 (81) [218] |
| Serious adverse eventsc | 5 (7) [8] | 1 (1) [1] | 2 (3) [2] | 2 (3) [5] | NR | 1 (1) [1] | 3 (4) [3] | 5 (7) [9] | 2 (3) [2] |
| Premature treatment discontinuation due to adverse events | 1 (1) [2] | 6 (9) [7] | 4 (6) [6] | 8 (12) [16] | 19 (27) [42] | 16 (23) [30] | 10 (14) [22] | 18 (26) [35] | 10 (14) [20] |
| Severityd | |||||||||
| Severe | 2 (3) [4] | 1 (1) [1] | 2 (3) [2] | 6 (9) [19] | 6 (9) [8] | 5 (7) [9] | 7 (10) [9] | 6 (9) [8] | 4 (6) [5] |
| Moderate | 17 (24) [25] | 23 (33) [35] | 18 (26) [41] | 26 (38) [64] | 32 (46) [79] | 32 (45) [83] | 28 (40) [72] | 31 (44) [70] | 29 (42) [65] |
| Mild | 46 (65) [98] | 36 (51) [106] | 39 (56) [126] | 44 (64) [150] | 48 (69) [202] | 47 (66) [138] | 47 (67) [152] | 49 (70) [167] | 46 (67) [148] |
| Gastrointestinal adverse events reported in ≥5% of patients | |||||||||
| All gastrointestinal adverse events | 20 (28) [32] | 22 (31) [44] | 22 (31) [49] | 37 (54) [101] | 39 (56) [127] | 43 (61) [128] | 38 (54) [116] | 54 (77) [111] | 37 (54) [86] |
| Nausea | 1 (1) [1] | 9 (13) [12] | 10 (14) [13] | 23 (33) [28] | 24 (34) [36] | 24 (34) [37] | 23 (33) [29] | 26 (37) [28] | 22 (32) [23] |
| Vomiting | 3 (4) [3] | 4 (6) [8] | 4 (6) [5] | 15 (22) [19] | 11 (16) [14] | 14 (20) [25] | 11 (16) [22] | 17 (24) [21] | 6 (9) [6] |
| Dyspepsia | 3 (4) [3] | 2 (3) [6] | 5 (7) [7] | 6 (9) [6] | 8 (11) [8] | 6 (8) [8] | 6 (9) [8] | 5 (7) [5] | 10 (14) [11] |
| Abdominal distension | 5 (7) [5] | NR | 3 (4) [3] | 1 (1) [1] | 2 (3) [2] | 6 (8) [6] | 1 (1) [1] | 3 (4) [3] | 3 (4) [4] |
| Abdominal pain | NR | 3 (4) [3] | 1 (1) [1] | 1 (1) [1] | 4 (6) [7] | 3 (4) [3] | 4 (6) [4] | 3 (4) [3] | 4 (6) [4] |
| Abdominal discomfort | NR | NR | 1 (1) [1] | 2 (3) [2] | 3 (4) [3] | 2 (3) [2] | 1 (1) [1] | 4 (6) [5] | 5 (7) [7] |
| Abdominal pain upper | NR | NR | 2 (3) [2] | 1 (1) [1] | 6 (9) [8] | 2 (3) [2] | 4 (6) [6] | 1 (1) [1] | NR |
| Eructation | NR | NR | NR | 1 (1) [1] | 2 (3) [2] | 5 (7) [5] | 1 (1) [1] | 2 (3) [2] | 2 (3) [2] |
| Diarrhea | 7 (10) [10] | 5 (7) [6] | 7 (10) [7] | 16 (23) [20] | 14 (20) [18] | 10 (14) [15] | 14 (20) [27] | 13 (19) [16] | 10 (14) [14] |
| Constipation | 4 (6) [5] | 4 (6) [4] | 4 (6) [4] | 6 (9) [8] | 5 (7) [8] | 9 (13) [11] | 7 (10) [7] | 8 (11) [9] | 7 (10) [7] |
| Gastroesophageal reflux disease | 1 (1) [1] | 2 (3) [2] | 2 (3) [2] | 4 (6) [4] | 5 (7) [7] | 4 (6) [4] | 4 (6) [4] | 4 (6) [4] | 1 (1) [1] |
Abbreviations: NR, none reported; SC, subcutaneous.
Treatment-emergent adverse events were defined as events that had an onset, or increase in severity, on or after the first day of exposure to the follow-up visit scheduled 5 weeks (plus 5-day visit window) after last trial product dose or the patient’s end-of-trial date, depending on which was encountered first. All adverse events were coded using the Medical Dictionary for Regulatory Activities, edition 17.1. No fatal events were reported.
Standard escalation indicates 4-week intervals; slow escalation, 8-week intervals; fast escalation, 2-week intervals.
A serious adverse event was defined as an experience that at any dose results in any of the following: death; a life-threatening experience; in-patient hospitalization or prolongation of existing hospitalization; a persistent or significant disability or incapacity; a congenital anomaly or birth defect; or a situation in which medical judgment deems that medical or surgical intervention is necessary to prevent 1 of the outcomes listed in this definition of serious adverse events.
Severity of adverse events was defined as follows: mild (transient symptoms, no interference with daily activities); moderate (marked symptoms, moderate interference with daily activities); severe (considerable interference with daily activities, unacceptable).
Premature treatment discontinuation due to adverse events was more frequent with oral (6%-27%; 81 of 490 patients) and subcutaneous (14%; 10 of 69 patients) semaglutide than with placebo (1%; 1 of 71 patients) (Table 3), and was mostly due to gastrointestinal adverse events (4%-21% [65 of 490 patients] with oral semaglutide vs 12% [8 of 69 patients] with subcutaneous semaglutide and none with placebo). The proportion of patients prematurely discontinuing treatment due to adverse events was slightly lower with 40-mg slow escalation of oral semaglutide from a starting dose of 5 mg up to 40 mg (14%; 10 of 70 patients) compared with the other 40-mg groups (40-mg standard escalation, 23% [16 of 71 patients]; 40-mg fast escalation, 26% [18 of 70 patients]) and the 20-mg group (27% [19 of 70 patients]).
The overall number of hypoglycemic episodes was low and similar for oral semaglutide, subcutaneous semaglutide, and placebo. The overall rate of severe or blood glucose–confirmed hypoglycemia was low, with only 2 episodes of severe hypoglycemia reported (subcutaneous semaglutide group, 1 patient; oral semaglutide 40-mg fast escalation group, 1 patient) (eTable 5 in Supplement 2).
Reductions in systolic and diastolic blood pressure occurred in all treatment groups; systolic blood pressure reductions were more pronounced with oral and subcutaneous semaglutide than with placebo (eTable 1 and eTable 2 in Supplement 2). Change in mean heart rate ranged from –1.7 to 3.0 beats/min with oral semaglutide vs 2.6 beats/min with subcutaneous semaglutide and –4.0 beats/min with placebo. At week 26, changes in heart rate were significantly greater with oral semaglutide 5 mg or higher and subcutaneous semaglutide compared with placebo (eTable 1 and eTable 2 in Supplement 2). Six cardiovascular events in 5 patients were confirmed by adjudication (oral semaglutide: 10-mg group, 1 patient; 40-mg slow escalation group, 2 patients; placebo: 2 patients) (eTable 6 in Supplement 2).
Three events of pancreatitis in 3 patients were confirmed by adjudication (subcutaneous semaglutide group, 1 patient; oral semaglutide: 20-mg group, 1 patient; 40-mg standard escalation group, 1 patient]) (eTable 7 in Supplement 2); the 3 events were mild to moderate in severity (eTable 7 in Supplement 2). Mean lipase levels increased from 42.0 U/L (to convert to μkat/L, multiply by 0.0167) at baseline in the dosage-dependent oral semaglutide groups by 9% to 55% (1.09-1.55) and in the subcutaneous semaglutide group by 36% (1.36) vs a reduction of 1% in the placebo group (0.99) (eFigure 5 in Supplement 2). Mean amylase levels increased from 56.8 U/L (to convert to μkat/L, multiply by 0.0167) at baseline by 7% to 25% (1.07-1.25) for the dosage-dependent oral semaglutide groups, 22% (1.22) for the subcutaneous semaglutide group vs 5% (1.05) for the placebo group (eFigure 5 in Supplement 2).
Three event adjudication committee (EAC)–confirmed neoplasm events were reported (oral semaglutide: 10-mg group, 1 basal cell carcinoma; 40-mg fast escalation group, 1 gastrointestinal tract adenoma [benign]; subcutaneous semaglutide group, 1 keratoacanthoma [benign]). No thyroid events were confirmed.
Discussion
Among patients with type 2 diabetes, oral semaglutide resulted in better glycemic control than placebo over 26 weeks (primary end point). From a mean baseline HbA1c level of 7.9%, between 44% (2.5-mg group) and 90% (40-mg standard escalation group) of patients receiving oral semaglutide achieved the target HbA1c level of less than 7.0%. Clinically relevant (5% or more) weight loss was achieved in up to 71% of patients receiving oral semaglutide. The magnitude of improvements with oral semaglutide at 20 mg and 40-mg standard escalation was not significantly different than subcutaneous semaglutide and was similar across the dosage escalation groups.
Improvements in glycemic control and body weight with oral semaglutide were achieved with a low rate of hypoglycemia. No unexpected safety findings were identified. Gastrointestinal adverse events, with consequent premature treatment discontinuation, was observed in the oral semaglutide groups, consistent with the known adverse effects of GLP-1 receptor agonists. Fewer adverse events were reported when patients started on a low dose (eg, 2.5 mg) and the frequency of gastrointestinal adverse events was highest during the dose-escalation period and decreased over time in the oral semaglutide groups.
Three cases of acute pancreatitis were confirmed by adjudication in patients receiving both oral and subcutaneous semaglutide. Imbalances of pancreatitis have also been reported in the clinical development programs for other incretin-based therapies, although these imbalances were not found in the long-term studies of subcutaneous semaglutide and liraglutide. As observed with other incretin-based therapies, lipase levels increased with semaglutide (oral and subcutaneous groups) vs placebo.
Overall, a significant increase in heart rate was seen with semaglutide (oral and subcutaneous groups) compared with placebo, similar to observations with other long-acting GLP-1 receptor agonists. A cardiovascular benefit has been observed in long-term outcome trials with subcutaneous semaglutide and liraglutide.
This study has several limitations including duration. Longer-term data will provide more information about the safety and efficacy durability of oral semaglutide. A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication. Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents. There was no adjustment for multiplicity in the statistical analyses, which may contribute to a type I error.
The adverse event profile of oral semaglutide was comparable with subcutaneous semaglutide. The three 40-mg dose escalation groups (2, 4, and 8 weeks) provided similar treatment effects; however, the proportion of patients reporting adverse events during escalation appeared lower with a starting dose of 2.5 mg vs 5 mg.
Conclusions
Among patients with type 2 diabetes, oral semaglutide resulted in better glycemic control than placebo over 26 weeks. These findings support phase 3 studies to assess longer-term and clinical outcomes, as well as safety.
Trial Protocol and Statistical Analysis Plan
eFigure 1. Change in Mean HbA1c—Cumulative Distribution Function at Week 26
eFigure 2. Sensitivity Analyses
eFigure 3. Proportion of Patients With Nausea and Mean Number of Events Over Time
eFigure 4. Lipase and Amylase
eBox 1. Countries Involved in the Trial
eBox 2. Key Exclusion Criteria
eBox 3. Predefined Events Types That Were Sent for Blinded Adjudication by an Independent External Committee
eTable 1. Additional Efficacy and Safety End Points
eTable 2. Efficacy and Safety End Points for Oral Semaglutide Dose Escalation Arms at Week 26
eTable 3. Serious Adverse Events by System Organ Class
eTable 4. Nausea by Time and Severity
eTable 5. Hypoglycemic Events
eTable 6. Event Adjudication Committee-Confirmed Cardiovascular Events
eTable 7. Event Adjudication Committee-Confirmed Pancreatitis Events
eAppendix. List of Investigators
References
- 1.Alani AW, Robinson JR. Mechanistic understanding of oral drug absorption enhancement of cromolyn sodium by an amino acid derivative. Pharm Res. 2008;25(1):48-54. [DOI] [PubMed] [Google Scholar]
- 2.Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15(1):15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS One. 2015;10(6):e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secher A, Jelsing J, Baquero AF, et al. . The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. [DOI] [PubMed] [Google Scholar]
- 9.Cooke CE, Lee HY, Tong YP, Haines ST. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin. 2010;26(1):231-238. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency ICH Topic E6(R1): guideline for good clinical practice (CPMP/ICH/135/95). 2002.
- 12.US Government Publishing Office Electronic Code of Federal Regulations. 2015; https://www.ecfr.gov/cgi-bin/text-idx?SID=3ee286332416f26a91d9e6d786a604ab&mc=true&tpl=/ecfrbrowse/Title21/21tab_02.tpl. Accessed June 6, 2017.
- 13.Malkov D, Angelo R, Wang HZ, Flanders E, Tang H, Gomez-Orellana I. Oral delivery of insulin with the eligen technology: mechanistic studies. Curr Drug Deliv. 2005;2(2):191-197. [DOI] [PubMed] [Google Scholar]
- 14.Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical Dictionary for Regulatory Activities Introductory guide to MedDRA, version 17.1. 2014; https://www.meddra.org/sites/default/files/guidance/file/intguide_17_1_english.pdf. Accessed August 23, 2017.
- 16.Meier JJ, Rosenstock J. Therapy: gastrointestinal safety of incretin therapies: are we there yet? Nat Rev Gastroenterol Hepatol. 2016;13(11):630-632. [DOI] [PubMed] [Google Scholar]
- 17.Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract. 2012;18(4):472-477. [DOI] [PubMed] [Google Scholar]
- 18.AstraZeneca Bydureon (exenatide) extended release prescribing information. 2012; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/022200Orig1s008.pdf. Accessed June 6, 2017.
- 19.GlaxoSmithKline Tanzeum (albiglutide) extended release prescribing information. 2015; https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tanzeum/pdf/TANZEUM-PI-MG-IFU-COMBINED.PDF. Accessed June 6, 2017.
- 20.Eli Lilly Trulicity (dulaglutide) extended release prescribing information. 2014; http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed June 6, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure 1. Change in Mean HbA1c—Cumulative Distribution Function at Week 26
eFigure 2. Sensitivity Analyses
eFigure 3. Proportion of Patients With Nausea and Mean Number of Events Over Time
eFigure 4. Lipase and Amylase
eBox 1. Countries Involved in the Trial
eBox 2. Key Exclusion Criteria
eBox 3. Predefined Events Types That Were Sent for Blinded Adjudication by an Independent External Committee
eTable 1. Additional Efficacy and Safety End Points
eTable 2. Efficacy and Safety End Points for Oral Semaglutide Dose Escalation Arms at Week 26
eTable 3. Serious Adverse Events by System Organ Class
eTable 4. Nausea by Time and Severity
eTable 5. Hypoglycemic Events
eTable 6. Event Adjudication Committee-Confirmed Cardiovascular Events
eTable 7. Event Adjudication Committee-Confirmed Pancreatitis Events
eAppendix. List of Investigators