Abstract
Eclampsia (together with epilepsy) being the first disease ever written down since the beginning of writings in mankind 5000 years ago, we will make a brief presentation of the different major steps in comprehension of Pre-eclampsia. 1) 1840. Rayer, description of proteinuria in eclampsia, 2) 1897 Vaquez, discovery of gestational hypertension in eclamptic women, 3) In the 1970’s, description of the “double” trophoblastic invasion existing only in humans (Brosens & Pijnenborg,), 4) between the 1970’s and the 1990’s, description of preeclampsia being a couple disease. The “paternity problem” (and therefore irruption of immunology), 5) at the end of the 1980’s, a major step forward: Preeclampsia being a global endothelial cell disease (glomeruloendotheliosis, hepatic or cerebral endotheliosis, HELLP, eclampsia), inflammation (J. Roberts. C Redman, R Taylor), 6) End of the 1990’s: Consensus for a distinction between early onset preeclampsia EOP and late onset LOP (34 weeks gestation), EOP being rather a problem of implantation of the trophoblast (and the placenta), LOP being rather a pre-existing maternal problem (obesity, diabetes, coagulopathies etc…). LOP is predominant everywhere on this planet, but enormously predominant in developed countries: 90% of cases. This feature is very different in countries where women have their first child very young (88% of world births), where the fatal EOP (early onset) occurs in more than 30% of cases. 7) What could be the common factor which could explain the maternal global endotheliosis in EOP and LOP? Discussion about the inositol phospho glycans P type.
Keywords: Preeclampsia, Immunology
Preeclampsia is characterized by the new onset of hypertension and proteinuria, or any other organ manifestation (including fetal growth restriction), after 20 weeks of gestation in a previously normotensive woman. Eclampsia is the occurrence of grand mal seizures in a woman with gestational hypertension or preeclampsia (Ghulmiyyah and Sibai, 2012).
It has been exactly one century (Zweifel, 1916) that preeclampsia has been first described as “a disease of theories”, a statement confirmed by Williams et al. (2007) and Higgins and Brennecke (1998) at the end of the 20th century. Where are we now? This paper wishes to historically describe the major steps in its description, definition, discoveries and comprehension of this strange human-specific reproductive disease and –hopefully, make a convincing case- that indeed giant leaps have been achieved to date to reach the ‘top of the mountain’. We think that we are probably closer than ever to reach a full comprehension of this disease, and that any reader can envisage to see the solution of the preeclamptic syndrome during his/her life time.
At first, preeclampsia (in contrast to eclampsia, curse of human reproduction) is a very young disease: a 20th-century’s description. Also, we have to constantly keep in mind the central tenet of this disease: all its acute harmful maternal consequences are completely reversible with the termination of pregnancy. To date, it is well known that the only definitive cure of preeclampsia is yet to deliver the baby and more specifically in fact the placenta (Roberts and Bell, 2013).
We propose in this analysis nine major steps:
1) Eclampsia the “eternal” disease
As far as writings had existed (5000 years ago, 3000 B.C.), we have reports coming from all continents (Lindheimer, 1999): Atharta Veda/ Sushruta (India), Wang Dui Me (China), Egyptian Papyrus (Africa), Hippocrates- Celsus-Galen (Europe). Epilepsy is such a spectacular event that it has been reported since the beginning of writings (the witness-writer being not a physician of course). Since then, birth-associated convulsions in mothers have constantly followed mankind as a possible eventuality in every pregnancy. Epilepsy-seizures-convulsions have highly frightened our ancestors, and, logically so (muscle contractions, visual disturbances, unusual head or eye movements, mouth alteration, loss of consciousness, and moreover a complete amnesia after the event), have been interpreted in all civilizations as a possession by a bad spirit and/or evil or that somebody had casted a spell on that pregnant woman. Eclampsia has terrorized our ancestors, namely because it happened during the fundamental event of human pregnancy, and also in very young, not to say adolescent, women (almost always during first pregnancies), with also a risk of maternal death in about 1/3 of cases. Mauriceau, at the end of the 17th century (1694) noticed that “primiparous women are at far most risk to develop convulsions than multiparous ones”. He did not use the term of “eclampsia” since the term has apparently been proposed by Bossier de Sauvage only in the mid-18th century (1739) distinguishing this maternal event from epilepsy (Bell, 2010; Chesley, 1984). We have recently proposed (Robillard et al., 2017a) that a grave dated of 28,000 years B.P. (“the human most ancient mother” ever found by paleoanthropologists, a 20 years old woman buried with her baby in her womb estimated at 8 months gestation) might well have died of eclampsia (23,000 years before the historic period, i.e. the invention of writings).
2) Tremendous leap: discovery of proteinuria. The emergence of the concepts: “toxemia” AND “pre”-eclampsia, 1840–43
In the era that midwives and physicians had a quite fatalistic approach concerning the inevitable, unpredictable (and incomprehensible) risk of developing eclampsia, the mid-19th century did see the fundamental discovery of proteinuria. We will refer also in this paragraph to the Mandy Bell’s (2010) and Leon Chesly’s papers (1984).
Coming back to the mid-19th century, eclampsia, with proteinuria, was therefore also linked to renal diseases (globally called “Bright disease”). Even while Demanet noted a connection between edematous women and eclampsia in 1797 (Chesley, 1978), it was Pierre Rayer, a Frenchman, in 1840 who described the presence of proteinuria in eclamptic women (Rayer, 1840). Because of the reversibility of the syndrome after delivery, in 1843 John Lever, an Englishman, and, one month later, JY Simpson, a Scotchman, specified that it was indeed a disease different from the Bright syndrome (Lever, 1843, Simpson, 1843). Nonetheless, this discovery widened the vision of eclampsia, as 2/3 of proteinuric women did not end in convulsions, but it also proposed an association with something else (“toxemia”, and, therefore appeared the concept of “pre-eclampsia”). It must be finally be brought to the credit of an Irishman, also in 1843, R Johns that he described the premonitory symptoms of eclampsia in women presenting proteinuria (Johns, 1843). These were exactly the same signs which we teach our students or midwives nowadays: headache, temporary loss of vision, severe pain in the stomach etc…. (Bell, 2010). It has been already well known since Mauriceau (1694) and De la Motte (1722) that delivery has a beneficial effect on convulsions (Chesley, 1984; Bell, 2010). You may imagine the reflections and apprehension of our masters at this time, when they had to manage a term pregnant woman with term proteinuria.
3) Giant leap: the discovery of hypertension, 1897–1903
It was only after Riva Rocci’s discovery, a young 31 year old Italian physician, of the inflatable arm-band in 1896 that blood pressure measurements entered in clinical practice. Vaquez in France in 1897 is credited with the discovery of eclamptic hypertension (Vaquez, 1897), followed few years later in 1903 by Cook and Briggs in the USA (Lindheimer, 1999). At that stage we realized that the “eternal eclampsia” was indeed only the tip of an iceberg: approximately 10% of human pregnancies present with gestational hypertension (therefore reversible), of which 3% evolve to preeclampsia (proteinuria), and, without medical intervention, around 1% suffer of eclampsia. The 20th century followed with a comprehensive exploration of the epidemiology of hypertensive disorders of pregnancy achieved by tremendous major textbooks all along the century (Robillard et al., 2017b), with the essential motto: preeclampsia, “disease of primigravidae”. Many examples have been analyzed in a preceding paper (Robillard et al., 1999).
4) Another giant leap: the discovery of the human-specific deep trophoblastic (haemochorial) implantation and its failure in pre-eclampsia, 1970’s.
In the early 1970’s, Brosens described that in humans, contrarily to other mammals (some 4300 different known species), the trophoblastic invasion was very deep, invading not only the decidua but also 1/3 of the uterine muscle (myometrium), but also that this deep trophoblast invasion was associated with a complete remodeling of the spiral arteries (Brosens et al., 1972). This very invasive process indeed lasts several weeks until the end of the first trimester (14th–16th week gestation), and in contrast with most other mammals (except perhaps the hominoid great apes, our cousins) where invasion occurs only in the first one or two weeks after implantation. Brosens and Pijnenborg proposed the concept of “double wave” implantation (Pijnenborg et al., 1980). They described that in preeclampsia, there was a defect of the second wave of this deep invasion in humans and that, for the rest of the gestation the trophoblast thus remained in a state they defined as “shallow implantation”. This first allowed to understand two facts: the explanation why this strange disease is essentially human (isolated reports of suspicion of eclampsia have been made for gorillas, but there was never a description of an epidemiological phenomenon as it is in humans, there is absolutely no teaching on eclampsia in veterinary schools). It also lead to the comprehension of why preeclamptic women presented hypertension: rising the maternal blood pressure could be a compensatory mechanism to try to bring enough nutrients to the human fetus across the placenta despite the shallow implantation (and even though, in many cases the fetus is in a state of growth restriction).
But the presence of this deep cytotrophoblast invasion in normal pregnancy raised also some fundamental questions. The haemochorial placenta in primates, and in particular this deep invasion in human represents a scenario where the human mother is facing a major immune challenge by what we may very roughly call the “fetal hemi-allograft paradox “. Moreover, besides the deep implantation, the deep trophoblastic implantation also results in a tremendous vascular remodeling with the maternal endothelial cells being replaced by trophoblastic cells inside the myometrium and being directly in contact with the maternal bloodstream, and hence the immune leucocytes. All these processes require direct cell to cell communication between maternal and placental (fetal) cell lineages. This intimate imbrication is less involved in epithelio or endotheliochorial placentae, where the maternal and fetal circulations are separated by at least four or five layers.
This raises two questions: first why the “need” of such an aggressive trophoblastic invasion in humans? A “non-hemochorial mammal like” decidual implantation might have avoided the major risk of pre-eclampsia/eclampsia in human reproduction. Logically, in an evolutionary view, the answer seems to be: the human fetus needs higher nutritional exchanges with his mother compared with other hemochorial mammals.
One may pose the question: what are the nutritional differences between human fetuses and other hemochorial mammals (per body weight). Obviously, the main difference is the fetal brain development: in humans, in the last trimester of pregnancy 60% of nutritional supplies are only for the fetus brain (as compared with approximately 20% in most other mammals, Martin, 1996). Laurence Cole had already shown in the last decade that, especially in Primates, the different structures of the sugar side chains in hyperglycosylated chorionic gonadotrophin (H–HCG) where the degree of glycosylation correlates with the deepness of hemochorial placentation. Let us quote the paramount recent paper by Cole in Primatology, 2015 (open source) where the author proposes, that in the evolution of mammals (and primates), brain growth was initially blocked by the inefficiency of epitheliochorial placentation. To achieve hemochorial placentation, it was chorionic gonadotrophin (CG) and hyperglycosylated CG that promoted this different pathway for the implantation of the placenta. In this paper, he shows that the deepness of hemochorial implantation is correlated with the brain/body size ratio (BBR) in different Primate species: for example the lower Simian Primates having a 0.2% BBR have a 3N-2O (nitrogen, oxygen) sugar side chains; the Advanced Simian primates (Orangutans, Chimpanzees), 0.74% BBR, 3N-3O; Homo ergaster, Homo habilis, 1.0–1.2% BBR, 3N-4O; Homo erectus, Homo nean-derthalensis, and Homo sapiens, 2.1–2.7% BBR, 4N-4O (Cole, 2015). On this basis, we may speculate that the 4N-4O configuration may be the one which needs the higher maternal-fetal immunological compromises for a successful pregnancy, and therefore that Homo neanderthalensis and Homo erectus could have had also problems of eclampsia/pre-eclampsia (a for neanderthalensis it was suggested by Robillard and Chaline, 2003a; Chaline 2003).
5) New giant leap: everything which happens in the maternal disease (proteinuria, liver disease-HELLP, eclampsia-convulsions) has a global explanation: a major maternal inflammatory state (endothelial cells) in particularly affecting the endothelium (late 1980’s)
Over these decades it was discovered that preeclampsia is not only just hypertension during pregnancy, but that all its manifestations and organ involvement could be explained by one central theme ‘endothelial cell dysfunction’. In particularly the specialized endothelial cells appeared to be vulnerable for the ‘preeclamptic toxin’. Glomeruloendotheliosis in the kidney, Kupfer cells in the liver sinusoids (HELLP) and the endothelial cells involved in the blood –brain barrier (convulsions-eclampsia). As such the names of James Roberts, Robert Taylor, Ian Sargent and Christopher Redman will remain forever in history of science concerning the comprehension of preeclampsia (Roberts et al., 1989; Roberts and Redman, 1993; Redman et al., 1999). The corollary of this systemic endothelial cell dysfunction is the challenge to find the “factor X” explaining the maternal manifestations of this syndrome. According to Roberts (Roberts and Bell, 2013), to date this inquiry seems to have failed. This will be developed in point 8. From this discovery came also the concept of preeclampsia (or at least the early-onset preeclampsia associated with fetal growth restriction) as a “two-stage disease” (Redamn and Sargent, 2001) that Christopher Redman and Annetine Staff will develop in this issue: if the primum movens is the failure of the trophoblastic invasion at the beginning of pregnancy in first pregnancies, this phenomenon has nothing to do with the later phenomenon of endothelial cell dysfunction occurring typically in the last trimester of pregnancy.
6) A revolutionary (“revolvere”, to turn) leap: yes, preeclampsia may be predominantly a disease of first pregnancies, but at the level of a couple (“primipaternity concept”) and not only on the maternal side (primiparity), 1970’s–1990’s.
After isolated reports in the 1970’s (isolated multiparae having preeclampsia while changing father), the first real epidemiological paper by Ikedife in Nigeria (1980) reported that in his practice among eclamptic women, many of them were not only multiparae, but more importantly also that 2/3 of them had a new partner. Interestingly, this is this same proportion of changing partner (2/3) that was described a decade later in Guadeloupe (French West Indies) by Robillard et al. (1994) in preeclamptic women. This “primipaternity” concept has been described elsewhere, this being extensively developed in different papers. At least, however, these observations challenged the established dogma: “preeclampsia is a disease of primiparae only”. But it predicted also that conceptions with oocyte donations, embryo donation (Wang et al., 2002) sperm from anonymous donors for example, already described by Need et al. in 1983, should be at higher risk of preeclampsia than the “normal” ones, whereas the well-known protective effect of any kind of pregnancies (including miscarriages and volunteer abortions) should disappear in case of a new paternity (Saftlas et al., 2003).
Further, preeclampsia being a disease of a couple-matching at first pregnancy raised obligatorily the concept that immunology had to be involved. At the very end of the 1990’s and the beginning of the first 2 decades of the 2000’s, tremendous advances have been made in the association between preeclampsia and immunology. Some major steps have been synthesized in Table 1. Gérard Chaouat will synthesize some aspects of the evolution of ideas in this topic in this issue.
Table 1.
Summary of major advances in immunology of reproduction these last 2 decades.
| Major advances in Immunology of Reproduction | Redman and Sargent, 2010 |
|---|---|
|
Incidentally, also, the Guadeloupean cohort (Robillard et al., 1994), because of linear decline of preeclampsia risk with time of sexual cohabitation before conception in first pregnancies raised the problem: how did the human species survived with this terrible risk while no effective contraception existed before the 20th century? The answer is that humans evolved to have the lowest fertility rate (25% only!) among the 4300 species of mammals: a young human couple needing in average 7 months to have a successful pregnancy is on the other hand relatively protected against the preeclampsia risk (Robillard et al., 2003a, 2003b).
7) A significant leap: the clear distinction between “early onset” and “late onset” preeclampsia: birth < or > 34 weeks gestation due to preeclampsia) the 34th week of gestation), or placental versus maternal preeclampsia, end of the 1990’s. The recognition that preeclampsia is a heterogeneous syndrome.
Again, this concept which arose at the end of the 20th century (Redman and Sargent, 2001) is developed in Roberts’ paper (2013): early onset PE (EOP, < 34 weeks gestation) and late onset (LOP, ≥ 34 weeks) have very different outcomes concerning essentially the terrible neonatal morbidity/mortality and rate of fetal growth restriction. While it needs to be recognized that a significant burden of maternal disease is by default associated with late disease. During the last decade of the 20th century, we arrived at a consensus between the “immunologists” and “vascularists”: late onset PE (LOP), i.e. primarily maternal pre-eclampsia might not be linked with a defective trophoblastic invasion in the first weeks of gestation but rather to a maternal predisposition for inflammation (notably, obesity, food inflammation, chronic hypertension) and/or vascular problems (thrombophilias, obesity, diabetes, antiphospholipid syndrome, dyslipidemia, insulin resistance etc…). This distinction between LOP and EOP also triggered the epidemiological observation that LOP is clearly the predominant phenotype: 90% of cases in developed countries, in contrast to only approximately 70% in developing areas (Robillard et al., 2016; Iacobelli et al., 2016). In other words, in developing areas this 30% incidence of early onset PE (EOP) pose a tremendous epidemiological challenge concerning maternal and neonatal morbidities. Schematically, we may propose that EOP is rather linked with the trophoblastic invasion phenotype (and therefore the “primipaternity concept”), and in particular fetal growth restriction (Kho et al., 2009), while LOP is rather “maternal phenotype” driven. This differentiates what Redman calls “maternal PE” and “placental preeclampsia”.
8) Current stage, which will be the 21th century’s giant leap: What is (are) the common factor(s) which may explain the maternal reversible endothelial cell dysfunction in early and late onset pre-eclampsia?
Schematically, then, we have three approaches of the problem 1) there are no mysterious toxin in the blood, 2) there are perhaps multiple toxins 3) still the hope to finally find THE factor X.
In a recent review James Roberts (2013) speaking of the search of the “holy grail” to find the linkage between inadequate placental perfusion and the maternal syndrome, concluded that “it is now conclusively established that there are no mysterious toxins in the blood”, after describing in details the candidate molecules such as antiangiogenic factors, cytokines, syncytiotrophoblast particles which have in common the oxidative stress. Indeed, there have been some deceptions concerning all these factors (which also do circulate in normal pregnancies), and James Roberts gives clearly his arguments.
We have made considerable progress in understanding the nature of the ‘toxin’ or toxins. It is not in the scope of this overview to present a comprehensive review of the importance of the imbalance between pro – and antiangiogenic factors, i.e. VEGG and PLGF vs sFlt-1 and sEndoglin. The landmark publications by Karumanchi and his group (Levine et al., 2004) represented a giant step forward. It is important to realize that even these factors are not the whole story; (1) the imbalance itself is ‘just ‘ a pivotal consequence of the underlying maternal systemic inflammatory state (Redman et al., 1999) and (2) there are probably multiple toxins (see excellent review, Hartley et al., 2015). It is currently unknown whether or not different toxins play a preferential role is different clinical phenotypes of the syndrome
The optimistic unicist concept, what James Roberts calls the “holy grail”. Here we clearly assume the philosophical bias of esthetics. Such as Marcel Dassault (the father of the Mirage and Rafale) who used to say: “a good plane must be an esthetic one”. Or, Albert Einstein who during all his life adopted the (unreasonable) axiom: “natural laws must be esthetic” (and even if he was wrong when he said “God does not play dice” concerning quantic physics). And also, because history of science teach us in physics, chemistry, medicine etc…, that when people give many origins to a problem (the famous word “multifactorial”), it may be that they have not found the solution yet.
Here we wish to discuss of one molecule that has been overlooked by many investigators: the inositol phosphoglycans P-type (IPG-P): It has been known for 17 years now (Kunjara et al., 2000) that these IPG-P are abundantly, and specifically, found in the urine of preeclamptic women and, more than this, these IPG-P molecules appear in these urine 2–4 weeks before the clinical onset of the disease in EOP as well as in LOP disease (Williams et al., 2007; Dawonauth et al., 2014). IPG-P (discovered only in 1986) is a peculiar molecule, as in fact it is a transmembraneous second messenger at the cellular level, and as such, has a fugacious existence and effects on both sides of cellular membranes. Like all second messengers (adenosine di-phosphate, ADP, or adenosine tri-phosphate, ATP, for example) it is centered on a phosphate (and also in the case of inositol phosphate glycans P type by a Manganese ion, Mn + +).
This IPG-P second messenger has nothing to do in plasma or blood circulation in mammals, as its fundamental role is to be the cornerstone of cellular carbohydrate metabolism in all kinds of eukaryote cells (plants, fungi, parasites, worms, snakes, insects, birds etc…, and in all mammal tissues. It is only the mammals who have further “chosen” insulin above IPG-P) only inside the cells. Its action at the mitochondrial level is to regulate the non-oxydative pathways of glycogen synthesis (70% of the carbohydrate metabolism in cells), (McLean et al., 2008).
In our knowledge, the unique and massive presence of this normally cellular messenger in the blood circulation is preeclampsia specific, and only preeclampsia (it does not show in diabetics for example). Because of their biochemical structure made of lipids (stearate, oleate, glycerol) and carbohydrate (inositol), connected by a phosphate, IPG’s are very difficult to measure in a blood sample, also they are not hydrophilic. Yet, finding large amounts of IPG-P in preeclamptic urines implies that these IPG-P previously transited in the maternal plasma. Yet, being not hydrosoluble, IPG-P molecules have no other alternative then to coagulate in long-chain structures mimicking exactly a bacterial endotoxin or a malaria toxin (Caro et al., 1996). Presence of IPG-P in maternal urines 2–4 weeks before the clinical onset of preeclampsia suggests that, for weeks, an endotoxin-like molecule circulated continuously in the maternal circulation and could affect in particular the kidneys, the liver, the brain for example. It is tempting to think that a constant endotoxin infusion in a circulation may induce some inflammatory state and endothelial cell dysfunction, very similar to the animal low-dose endotoxin preeclampsia originally published by Faas et al. (1994). Therefore, IPG-P may contribute to endothelial damage as proposed by Kunjara et al. (2016).
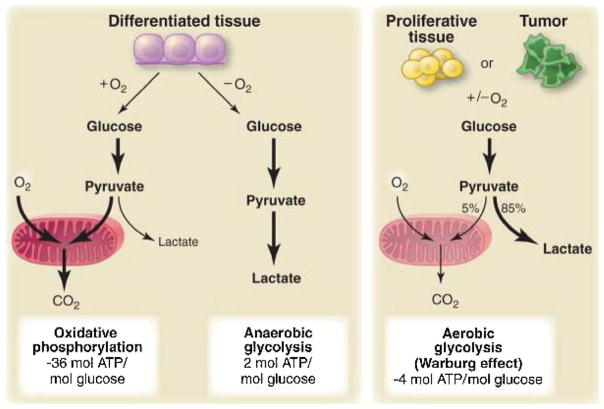
It is also noteworthy that IPG-P (also called D-chiro inositols in medical literature for readers interested in metabolism or diabetes) is clearly involved in the insulin resistance syndrome (Scioscia et al., 2015) encountered in preclampsia (EOP and LOP). Why there is a secretion or leakage of IPG-P in the maternal circulation (Rademacher et al., 2007) is still unclear (fetal starving signal?). Rademacher has proposed (Workshops 2014 & 2016) that its presence in preeclampsia may reflect a persisting state of Warburg metabolism (The common feature of this altered metabolism is the increased glucose uptake and fermentation of glucose to lactate, see Fig. 1). IPG-P for sure has to be a reliable and specific biomarker for preeclampsia in the near future (before and after the clinical onset of the disease, and as such should be included in the diagnosis of PE), but it may also be a significant part of the pathogenesis of the preeclamptic global endothelial damage. Studies made for years in Mauritius island by Lalita Dawonauth et al. are impressive (Dawonauth et al., 2014), proving also that this technology is accessible in emerging countries (a cheap procedure).
Fig. 1.
Schematic representation of the differences between oxidative phosphorylation, anaerobic glycolysis, and aerobic glycolysis (Warburg effect).
Last, but not least, IPG-P being centered by a Mn + + ion might be vulnerable to Magnesium sulfate (Mg + +), as demonstrated by incubation with Mg + + at moderate/high concentrations (laboratory data not published), giving an explanation to Horn’s idea in 1906: “why not trying magnesium sulfate in eclamptic seizures?” (Chesley, 1984, Bell, 2010).
9) (Near?) Future stage: finding a cure for the maternal disease (“second stage”).
Here we are not speaking of primary prevention (sperm induced partner specific tolerance?) or secondary prevention (aspirin), but we wish to discuss the treatment of the maternal global endotheliosis when it is established. One of the promising trail is to lower it by pravastatin prevention (based on the CO metabolism), Costantine et al., 2016. If we could gain by it seven, ten or twenty days in EOP before extracting the baby, it should be a tremendous leap for the safe survivals of extreme prematures. Guillermina Girardi will specifically speak of this aspect in this issue.
Coming back to the IPG-P molecule: if it can be demonstrated its involvement in the maternal endothelial disease, for sure, we should be able to find an “anti-IPG-P” treatment over the next decade.
1. Conclusion
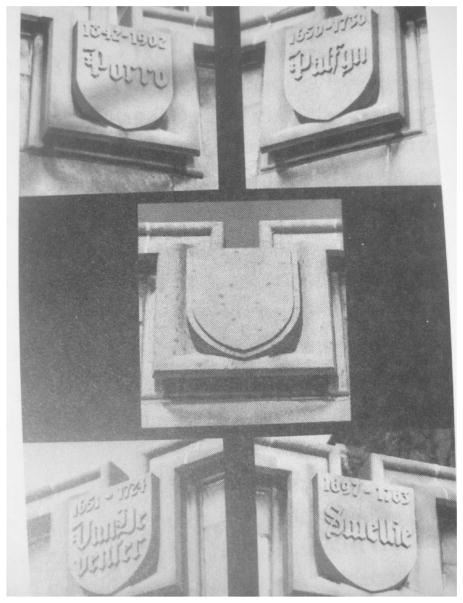
As stated in the opening section – we are optimists. Our understanding of this heterogeneous syndrome has dramatically improved. We do not know yet if the IPG-P issue is the “holy grail”, but, at least, it is evident that we must explore it. If confirmed, it should be credited to the Redman’s Oxford team a tremendous synthesis the last decades: inflammation, endotheliosis (Roberts et al., 1989; Redman et al., 1999; Redman and Sargent, 2010), immunology (Redman, 1981; Sargent, et al., 1982; Sargent et al., 2006; Redman and Sargent, 2010), and the discovery in the early 1990’s that preeclamptic placentae were abnormally full of glycogen, later explained by IPG-P overdose by the London Rademacher team (Kunjara et al., 2000, Williams et al., 2007, Dawonauth et al., 2014). We might to remind you that in the Lying in Hospital of Chicago university (89 Nobel prices all sciences confounded), there is an empty placard among medical discoveries in Obstetrical field waiting for “the one who will find the cause and/or the cure of preeclampsia », see Fig. 2. If the IPG-P “does not work”, the next generation should certainly find something. New exciting approaches are currently being researched targeting the fundamental molecular pathophysiology of the condition. One might also speculate that if we are to successfully tackle the preeclampsia conundrum, we will also have resolved placental fetal growth restriction and placental preterm birth.
Fig. 2.
University of Chicago (Lying-in Hospital). People who contributed to the field of Obstetrics. Empty Placard for the one who will find the cause and/or the cure of pre-eclampsia. Lindheimer et al., 1999, page 36 of the textbook: Chesley’s hypertensive disorders in pregnancy.
Footnotes
Conflict of interest
None.
References
- Bell MJ. A historical overview of preeclampsia-eclampsia. J Obstet Gynecol Neonatal Nurs. 2010;39(5):510–518. doi: 10.1111/j.1552-6909.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- Caro HN, Sheikh J, Taverne JH, Playfair HL, Rademacher TW. Structural similarities among malaria toxins, insulin second messengers, and bacterial endotoxin. Infect Immun. 1996;64(8):3438–3441. doi: 10.1128/iai.64.8.3438-3441.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaline J. Increased cranial capacity in hominid evolution and preeclampsia. J Reprod Immunol. 2003;59(2):137–152. doi: 10.1016/s0165-0378(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Chesley LC. Epidemiology of preeclampsia-eclampsia. In: Chesley LC, editor. Hypertensive Disorders of Pregnancy. Appleton-Century-Crofts; New-York: 1978. pp. 35–55. [Google Scholar]
- Chesley LC. History and epidemiology of preeclampsia-eclampsia. Clin Obstet Gynecol. 1984;24(4):801–820. doi: 10.1097/00003081-198412000-00004. [DOI] [PubMed] [Google Scholar]
- Cole LA. The evolution of the Primate, Hominid and Human brain. J Primatol. 2015;4:124. [Google Scholar]
- Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214(6):e1–720. doi: 10.1016/j.ajog.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawonauth L, Rademacher L, L’Omelette AD, Jankee S, Lee Kwai Yan MY, Jeeawoody RB, Rademacher TW. Urinary inositol phosphoglycan-P type: near patient test to detect preeclampsia prior to clinical onset of the disease. A study on 416 pregnant Mauritian women. J Reprod Immunol. 2014;101–102:148–152. doi: 10.1016/j.jri.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;171(1):158–164. doi: 10.1016/0002-9378(94)90463-4. [DOI] [PubMed] [Google Scholar]
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Hartley JD, Fergusson BJ, Moffet A. The role of shed placental DNA in the systemic Inflammatory of preeclampsia. Am J Obstet Gynecol. 2015;213(3):268–277. doi: 10.1016/j.ajog.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Higgins JR, Brennecke SP. Pre-eclampsia still a disease of theories? O Curr Opin Obstet Gynecol. 1998;10:129–133. doi: 10.1097/00001703-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Iacobelli S, Bonsante F, Robillard PY. Pre-eclampsia and preterm birth in Reunion Island: a 13 years cohort-based study. Comparison with international data. J Matern Fetal Neonatal Med. 2016;29(18):3035–3040. doi: 10.3109/14767058.2015.1114081. [DOI] [PubMed] [Google Scholar]
- Ikedife D. Eclampsia in multiparae. Br Med J. 1980;280:985–986. doi: 10.1136/bmj.280.6219.985-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns R. Observations of puerperal convulsions. Dublin J Med Sci. 1843;24(1):101–115. [Google Scholar]
- Kho EM, McCowan LM, North RA, Roberts CT, Chan E, Black MA, Taylor RS, Dekker GA SCOPE Consortium. Duration of sexual relationship and its effect on preeclampsia and small For gestational age perinatal outcome. J Reprod Immunol. 2009;82(1):66–73. doi: 10.1016/j.jri.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kunjara S, Greenbaum AL, Wang DY, Caro HN, McLean P, Redman CW, Rademacher TW. Inositol phosphoglycans and signal transduction systems in pregnancy in preeclampsia and in diabetes: evidence for a significant regulatory role in preeclampsia at placental and systemic levels. Mol Genet Metab. 2000;69:144–158. doi: 10.1006/mgme.2000.2964. [DOI] [PubMed] [Google Scholar]
- Kunjara S, McLean P, Rademacher L, Rademacher TW, Fascilla F, Bettocchi S, Scioscia M. Putative key role of inositol messengers in endothelial cells in preeclampsia. Int J Endocrinol. 2016:7695648. doi: 10.1155/2016/7695648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever J. Cases of Puerperal Convulsions with Remarks 2. Guy’s Hospital Report; London: 1843. pp. 495–517. [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadahani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumantchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Lindheimer MD, Roberts JM, Cunnigham FG, Chesley L. Introduction, history, controversies and definitions. In: Lindheimer JM, Roberts FG, Cunningham FG, editors. Chesley’s Hypertensive Disorders in Pregnancy. Appleton and Lange; Stamford, Connecticut: 1999. pp. 3–41. [Google Scholar]
- Martin RD. Scaling of the mammalian brain: the maternal energy hypothesis. News Physiol Sci. 1996;11:149–156. [Google Scholar]
- Mauriceau F, editor. Traité Des Maladies Des Femmes Grosses Et De Celles Qui Sont Accouchées. D’Houry; Paris: [Google Scholar]
- McLean P, Kunjara S, Greenbaum AL, Gumaa K, López-Prados J, Martin-Lomas M, Rademacher TW. Reciprocal control of pyruvate dehydrogenase kinase and phosphatase by inositol phosphoglycans. Dynamic state set by push-pull system. J Biol Chem. 2008;283(48):33428–33436. doi: 10.1074/jbc.M801781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need JA, Bell B, Meffin E, Jones WR. Pre-eclampsia in pregnancies from donor inseminations. J Reprod Immunol. 1983;5:329–338. doi: 10.1016/0165-0378(83)90242-5. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human deciduas from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Gumaa K, Scioscia M. Preeclampsia insulin signaling and immunological dysfunction: a fetal, maternal or placental disorder? J Reprod Immunol. 2007;76(1–2):78–84. doi: 10.1016/j.jri.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Rayer PE, editor. Traité Des Maladies Des Reins Et Des Altérations De La sécrétion Urinaire. Baillières; Paris: 1840. pp. 1837–1841. [Google Scholar]
- Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29(7–8):518–522. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- Redman CW. Immunological factors in the pathogenesis of preeclampsia. Contrib Nephrol. 1981;25:120–127. doi: 10.1159/000396021. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven’t we cured the disease? J Reprod Immunol. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced-hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Hulsey TC, Périanin J, Janky E, Miri EH, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344:973–975. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Dekker GA, Hulsey TC. Revisiting the epidemiological standard of preeclampsia: primigravidity or primipaternity? Eur J Obstet Gynecol Reprod Biol. 1999;84:37–41. doi: 10.1016/s0301-2115(98)00250-4. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Hulsey TC, Dekker GA, Chaouat G. Preeclampsia and human reproduction. An essay of a long term reflection. J Reprod Immunol. 2003a;59(2):93–100. doi: 10.1016/s0165-0378(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Chaline J, Chaouat G, Hulsey TC. Eclampsia, Preeclampsia, and the evolution of the human brain. Curr Anthropol. 2003b;44:130–135. [Google Scholar]
- Robillard PY, Dekker G, Iacobelli S, Chaouat G. An essay of reflection: why does preeclampsia exist in humans, and why are there such huge geographical differences in epidemiology? J Reprod Immunol. 2016;114:44–47. doi: 10.1016/j.jri.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Scioscia M, Coppola D, Chaline J, Bonsante F, Iacobelli S. La Donna di Ostuni, a case of eclampsia 28,000 years ago. J Matern Fetal Neonatal Med. 2017a;24:1–4. doi: 10.1080/14767058.2017.1312333. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Dekker GA, Chaouat G, Le Bouteiller P, Scioscia M, Hulsey TC. Preeclampsia and the 20th century: le siècle des Lumières. J Matern Fetal Neonatal Med. 2017b doi: 10.1016/j.preghy.2018.05.013. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157(12):1108–1114. doi: 10.1093/aje/kwg101. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Redman CW, Stirrat GM. Maternal cell-mediated immunity in normal and preeclamptic pregnancy. Clin Exp Immunol. 1982;50(3):601–609. [PMC free article] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CWG. NK cells and human pregnancy- an inflammatory view. Trends Immunol. 2006;27(9):399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Scioscia M, Karumanchi SA, Goldman-Wohl D, Robillard PY. Endothelial dysfunction and metabolic syndrome in preeclampsia: an alternative viewpoint. J Reprod Immunol. 2015;108:42–47. doi: 10.1016/j.jri.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Simpson JY. Contributions tot he pathology and treatment of the uterus. London and Edinburgh Mon. J Med Sci. 1843;3:1009–1027. [Google Scholar]
- Vaquez N. De la pression artérielle dans l’éclampsie puerpérale. Bull Soc Med Hop Paris. 1897;119:14. [Google Scholar]
- Wang JX, Knottnerus AM, Schuit G, Norman RJ, Chan A, Dekker GA. Surgically obtained sperm, and risk of gestational hypertension and pre-eclampsia. Lancet. 2002;359(9307):673–674. doi: 10.1016/S0140-6736(02)07804-2. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Gumaa K, Scioscia M, Redman CW, Rademacher TW. Inositol phopsphoglycans P-Type in preeclampsia. A novel marker? Hypertension. 2007;49:84. doi: 10.1161/01.HYP.0000251301.12357.ba. [DOI] [PubMed] [Google Scholar]
- Zweifel P. Eklampsie. In: Dederlein A, editor. Handbuch Der Geburtshilfe. Bergman: Wiesbade; 1916. pp. 672–723. [Google Scholar]