Abstract
Objective
To investigate whether self-efficacy moderates the association between self-rated memory and depressive symptoms in a large sample of older adults. The influence of self-efficacy and depressive symptoms on memory performance was also examined in a subsample of individuals who reported poor memory.
Methods
Non-demented participants (n=3766) were selected from the 2012 wave of the Health and Retirement Study. Depressive symptomatology was assessed with the 8-item Center for Epidemiologic Studies Depression Scale. A modified version of the Midlife Developmental Inventory Questionnaire was used as the measure of self-efficacy. Participants were asked to rate their memory presently on a five-point scale from Excellent (1) to Poor (5). Immediate memory and delayed memory (after a 5-min interval) were measured by the number of correct words recalled from a 10-item word list.
Results
Multiple regression analyses revealed that negative ratings of memory were significantly associated with greater levels of depressive symptoms, with this effect being greatest in those with low levels of self-efficacy. Additionally, greater self-efficacy was associated with optimal objective memory performances but only when depressive symptoms were low in individuals who reported poor memory function (n=1196).
Conclusion
Self-efficacy moderates the relationship between self-rated memory function and depressive symptoms. Higher self-efficacy may buffer against the impact of subjective memory difficulty on one’s mood and thereby mitigating the effect of depressive symptoms on memory. Interventions should focus on increasing perceived self-efficacy in older adults reporting poor memory function to potentially minimize memory impairment.
Keywords: self-efficacy, self-rated memory, late-life depressive symptoms, memory performance
Introduction
The association between self-rated memory and depressive symptom severity in older adults is well established (Kahn et al., 1975; Grut et al., 1993; Collins and Abeles, 1996; Jonker et al., 1996; Derouesne et al., 1999; Clarnette et al., 2001; Pearman and Storandt, 2004; Minett et al., 2005). Given the equally well-established link between depressive symptoms and cognition in older adults, (e.g., Burt et al., 1995; Koenig et al., 2015; Luciano et al., 2015; O’Shea et al., 2015), it is surprising that the association between subjective memory and objective memory performance is less clearly delineated in the literature. While some studies report an association (Gagnon et al., 1994; Jonker et al., 1996), other studies have not (Stewart et al., 2001; Jungwirth et al., 2004; Rickenbach et al., 2015). Memory complaints are common in the elderly. In addition, the clinical diagnosis of memory impairment includes memory complaints as part of the criteria (American Psychiatric Association, 2013), it is, therefore, important to understand why and/or how the association between subjective memory and memory performance may vary. The mixed findings in the research literature may, in part, be explained by methodological factors, including sample sizes, study design, and testing procedures. However, it is equally possible that such inconsistencies may be the result of some moderating factors which attenuate the association between self-rated memory and objective memory performance.
Depressive symptoms have been hypothesized to be a modality through which poor subjective memory is negatively associated with objective memory performance in cognitively normal older adults, that is, in the cases where this association is found (Zandi, 2004; Hülür et al., 2014). In the study by Hülür et al., the authors reported that subjective memory ratings in individuals with functional limitations depended not only on memory performance but depressive symptom severity. The authors suggest that this finding may be indicative that these adults may monitor their state more closely. In addition, the authors reported a moderating effect of education on the association between subjective memory and memory performance. They found that individuals with higher levels of education tended to report more accurate depictions of their memory function which is also consistent with the findings reported by Zelinski et al. (2001). However, it is not clear whether depressive symptoms were also greater in those who reported poor memory ability and had high levels of education.
One important hypothesis as to why depressive symptoms may be associated with memory performance is that depressive symptoms may be a reaction to a decline in memory ability (Bassuk et al., 1998). Further support for this possibility are findings which show that depressive symptoms precede memory decline and not vice versa (Zahodne et al., 2014). While Zahodne et al. consider that depressive symptoms may be a result of underlying neural degeneration, it is also possible that depressive symptoms may have resulted from an awareness of change in cognitive ability. If depressive symptoms can be viewed as a response to perceived poor memory function or decline as some evidence suggests (Bassuk et al., 1998; Wilson et al., 2002; Zahodne et al., 2014), it is also possible that there will be some variation in how individuals respond emotionally, i.e., some people will experience greater levels of depressive symptoms than others.
What then are the factors that may be associated with this variation in emotional response to negatively perceived memory function? We draw on the personal resource model in an attempt to answer this question. Much aging research literature has shown the buffering effects of personal resources (e.g., social support, perceived financial security, and self-efficacy) on outcomes related to health (Lachman and Weaver, 1998), physical function (Kurlowicz, 1998) and wellbeing (Kurlowicz, 1998), as well as cognitive ability (Zunzunegui et al., 2003; Windsor and Anstey, 2008; Zahodne et al., 2014) and rate of cognitive decline (Seeman et al., 2001), independent of demographic factors. In the present study, we explore one facet of personal resources that we consider to be most relevant to the current topic: an individual’s sense of self-efficacy.
Self-efficacy can be conceptualized as the belief that one can attain a desired goal and overcome challenges that may impede attaining the goal. Bandura (1989) was one of the first to propose the possible mechanisms through which self-efficacy may influence intervening processes related to memory performance. In this seminal work, Bandura theorized that greater self-efficacy is likely to lead to greater control and regulation of affective, cognitive, and/or motivational processes related to self-appraisal of memory function either by being associated with more positive appraisals of memory or by minimizing the effect of negative emotional responses on memory performance (Bandura, 1989). More recent empirical research has demonstrated that greater perceived self-efficacy is associated with a decrease in depressive symptoms in older adults (Robinson-Smith et al., 2000). Therefore, greater self-efficacy may enhance memory performances, even when subjective memory ratings are low by minimizing the influence of depressive symptoms on memory.
Overall, the extant body of literature indicates that higher perceived self-efficacy imparts a positive influence on a range of outcomes. However, to the best of our knowledge, no study has explored whether self-efficacy moderates the association between self-rated memory and depressive symptoms and memory performance in older adults. Similarly, no study has examined whether greater self-efficacy moderates the association between depressive symptoms and memory performance in older adults with poorly rated memory function. Thus, the aim of the present study was twofold. First, we aimed to examine (i) whether self-rated memory was associated with objective memory and (ii) whether levels of self-efficacy moderated the association between self-rated memory function and depressive symptoms in a large sample of older adults. Second, as we were interested in the potentially buffering effect of high self-efficacy in individuals who report poor memory function we examined whether the influence of depressive symptoms on memory performance varied as a function of self-efficacy. We considered that together these findings would help shed light on the complex association between self-rated memory, objective memory performance, and depressive symptoms.
Methods
Participants
Data for the present study were selected from the Health and Retirement Study (HRS), a biennial longitudinal panel study of the health, economic, social, and cognitive status of adults aged 50 years or older living in the United States. The HRS is sponsored by the National Institute on Aging and conducted by researchers at the University of Michigan, Ann Arbor. In brief, participants come from an ethnically diverse population (i.e., Hispanic, African American, and non-Hispanic White). Participants have been assessed at two-year intervals since the time at which the study began in 1992. Further details on the study design and sampling procedures have been described elsewhere (Juster and Suzman, 1995).
Of the 20 554 respondents interviewed in the 2012 wave of the study, we included only those 65 years or older as our age group of interest (n=10, 735). Participants were also excluded if they reported that they had ever been diagnosed with dementia or Alzheimer’s disease (n=819). This left us 9916 participants. However, just a subsample of participants had been administered a self-efficacy questionnaire. Thus, our final analyses included 3766 adults based on our inclusion/exclusion criteria. Demographic characteristics for these individuals are presented in Table 1. We also examined whether self-efficacy moderated the association between depressive symptoms and objective memory performance. in a subsample of the participants (N=1196) who had responded as ‘fair’ or ‘poor’ on a self-rated memory assessment.
Table 1.
Demographic characteristics of the samples
| Starting sample (n = 3766) |
Sub-sample (n = 1196) |
|
|---|---|---|
|
|
||
| Mean (SD) | Mean (SD) | |
| Age | 75.36 (6.7) | 75.75 (6.5) |
| Gender | ||
| Male, n(%) | 1602 (41.6) | 529/44.2% |
| Female, n(%) | 2251 (58.4) | 667/55.8% |
| Ethnicity | ||
| White (n/%) | 3243/84.2% | 943/78.8% |
| African American (n/%) | 470/12.2% | 200/16.7% |
| Other (n/%) | 137/3.6% | 51/4.3% |
| Self-efficacy | 37 (8.41) | 35.0 (8.5) |
| Years of education | 12.63 (2.9) | 11.69 (3.28) |
| Range = 0–17 years | Range = 0–20 years | |
| Self-rated memory | 3.0 (0.89) | ---- ----- ------ |
| CES-D score | 1.24 (1.78) | 1.8 (2.1) |
| range = 0–8 | range = 0–8 | |
| Immediate memory | 5.0 (1.63) | 4.5 (1.57) |
| Delayed memory | 3.92 (1.93) | 3.5 (1.8) |
Note: There are some missing values for some variables in the analyses so they do not always add up to n = 3766 (starting sample) or n = 1188 (subsample). CES-D= Center for Epidemiologic Studies Depression Scale.
Measures
Depressive symptoms
The 8-item version of the Center for Epidemiologic Studies—Depression Scale (CES-D; Andresen et al., 1994) was used to assess number of depressive symptoms. Participants responded either ‘yes’ or ‘no’ to each of the questions.
Self-rated memory
Participants were asked to rate their memory at the present time on a five-point scale as ‘excellent (1)’, ‘very good (2)’, ‘good (3)’, ‘fair (4)’, or ‘poor (5)’. This question was asked before being administered the objective memory tests.
Objective memory
Memory performance was assessed using tests of immediate and delayed free recall. First, a list of 10 words was presented to participants. They were then asked to recall as many words back as possible immediately following the presentation (immediate memory) and then again after 5 min (delayed memory). Correctly recalled words were used as the measure of immediate and delayed memory performance, with higher scores indicating better performance.
Self-efficacy assessment
Data for self-efficacy ratings were drawn from a modified version of the Midlife Developmental Inventory; sense of control scale (MIDI) (Pearlin and Schooler, 1978; Lachman and Weaver, 1998) as part of the Psychosocial Leave Behind Survey. There were a total of 10 items measuring mastery and constraint. Responses ranged from strongly disagree (1) to strongly agree (6). Higher values indicate higher mastery and lower constraint (19). The constraint variables were recoded in such a way to be consistent with the mastery variables so that higher scores indicated greater self-efficacy.
Covariates
Age, gender, race (i.e., Caucasian, African American, Other), and years of education were all included in the analyses as covariates.
Ethics statement
The HRS was approved by the Behavioral Science Committee institutional review board.
The data collected for this study are publically available.
Statistical analysis
Using the Sense of Control scale provided in HRS 2012 dataset, we wanted to extract a unidimensional construct from the current bi-factor scaled construct of mastery and constraint, to ensure we were measuring autonomy/self-efficacy. Judge et al. (2002) indicate that that psychological constructs such as locus of control, self-esteem, and self-efficacy form a unidimensional construct, as opposed to separate domains. To test this we employed item response theory methodology, specifically, Mokken scaling. We used the public domain software R for Mokken scaling analysis (Mokken, 1971). The Mokken model of monotone homogeneity (MH) is based on the assumptions of unidimensionality, local stochastic independence, and monotonicity in the latent attribute (Watson et al., 2012). The model produces a ‘scalability’ diagnostic (Loevinger’s H coefficient) method to assess these assumptions (Van der Heijden et al., 2003). When interpreting H, the following guidelines are common: 0.3–0.4=weak scale, 0.4–0.5=medium scale, and >0.5=strong scale (Sijtsma and Molenaar, 2002). The items do in fact adhere to a continuum, which could be referred to as self-efficacy. However, the Mokken procedure indicated that the scale was of medium strength (Total H coefficient of .41). In order to produce ‘strong’ scalability, it was necessary to remove three items: I often feel helpless in dealing with the problems of life (constraint), Other people determine most of what I can and cannot do (constraint), and What happens in my life is often beyond my control (constraint). The final scale resulted in an overall H coefficient of .52 and included the following items: I have little control over the things that happen to me (constraint), There is really no way I can solve the problems I have (constraint), I can do just about anything I really set my mind to (mastery), When I really want to do something, I usually find a way to succeed (mastery), Whether or not I am able to get what I want is in my own hands (mastery). What happens to me in the future mostly depends on me (mastery) and I can do the things that I want to do (mastery). Reliability was also good, with a value of 0.88. To test for multicollinearity between this new scale and the CES-D scale we ran a simple bivariate correlation analyses, which revealed only a small to modest correlation between these two variables, r=0.300, thus, supporting the hypotheses that these are two distinct constructs.
Z-scores were calculated based on the current sample for immediate and delayed memory performances. Using multiple regression analyses we first tested whether there was an association between self-rated memory and objective memory performance, controlling for depressive symptoms and demographic factors (i.e., age, education, race, and gender).We placed the covariates in the first step and self-rated memory in the second step. We then conducted a three-step hierarchical multiple regression analyses to assess whether there was an interaction between self-rated memory and self-efficacy to predict depressive symptom severity. Covariates were included in the first step, the main effects for self-rated memory and self-efficacy ratings (centered) variables were tested in the second step, and the interaction between self-rated memory and self-efficacy (centered) was tested in the final step.
In order to gain a better understanding of the role of self-efficacy and depressive symptoms on the association between subjective memory and objective memory we then examined the independent and interactive contribution of depressive symptoms and perceived self-efficacy in predicting objective memory in participants who responded with less than a ‘good’ rating of their memory (i.e., a response of 4 or 5 on the self-rated memory scale). Two separate three-step linear regression analyses were performed to test for these associations. The covariates were placed in the first step, CES-D scores and the self-efficacy variable in the second step, and the interaction term CESD × Self-efficacy in the final step, with either immediate or delayed memory performances as the outcome. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0.
Results
Self-rated memory reports were negatively associated with immediate (B=−0.108, SE (0.016) t(3747) =−6.698, p<0.001) and delayed memory (B=−0.096, SE (0.016), t(3747)=−6.000, p<0.001) performances. The main effect of depressive symptom severity was also negatively and independently associated with immediate (B=−0.056, SE (0.008), t (3747)=−6.698, p<0.001) and delayed memory performances (B=−0.049, SE (0.008), t(3747)=−6.066, p<0.001).
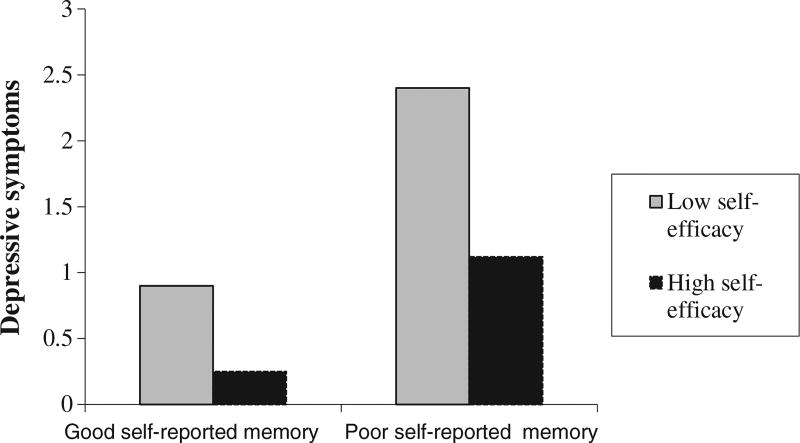
There was a significant and negative main effect of self-efficacy on depressive symptoms. The interaction between self-rated memory and self-efficacy was also a significant predictor of depressive symptom severity. Model summary results for the interaction between self-rated memory and self-efficacy on depressive symptoms are displayed Table 2. The interaction revealed that negatively rated memory scores had a stronger association with depressive symptom severity in individuals with low self-efficacy, while this association was minimal in individuals with high self-efficacy (Figure 1). There was a significant and negative main effect for depressive symptoms on immediate and delayed memory. The main effect of self-efficacy was positive and significant only for delayed memory performance.
Table 2.
Summary of hierarchical regression analysis showing interaction between self-rated memory and depressive symptoms (N = 3766)
| Predictor | B | SE (B) | β |
|---|---|---|---|
| Age | −0.005 | 0.004 | −0.018 |
| Gender | 0.326 | 0.054 | 0.090*** |
| Education | −0.077 | 0.009 | −0.129*** |
| Race | 0.048 | 0.024 | 0.030* |
| Self-rated memory | 0.246 | 0.031 | 0.124 |
| Self-efficacy | −0.062 | 0.003 | 0.291*** |
| Self-rated memory × self-efficacy | −0.009 | 0.004 | −0.037* |
| R2 | 0.16 |
Note: CES-D = Center for Epidemiologic Studies Depression Scale.
p <0.05;
p <0.001.
Figure 1.
The interaction between self-rated memory and self-efficacy on depressive symptoms. CES-D Center for Epidemiologic Studies—Depression. The moderator values (i.e. self-efficacy) are the sample mean/plus minus one standard deviation from the mean based on raw values.
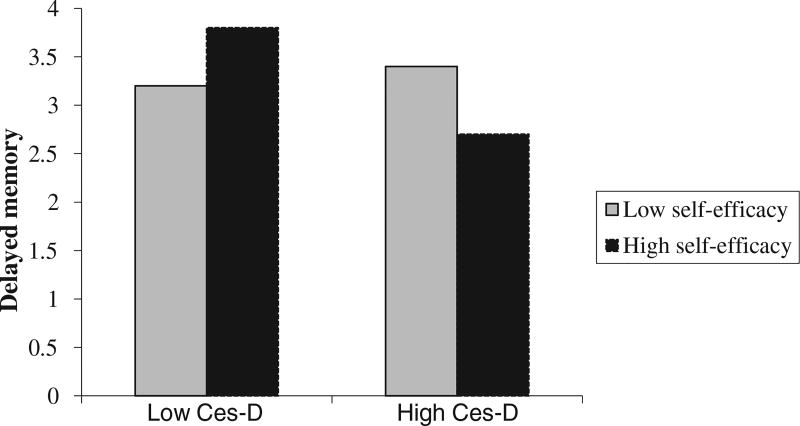
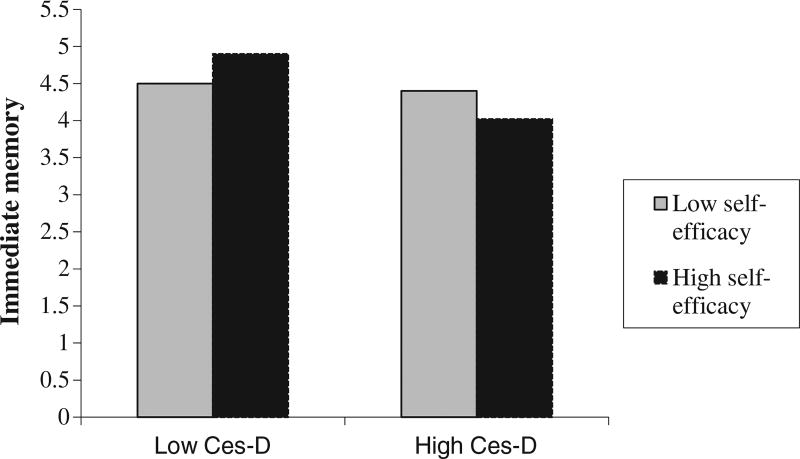
The results of the regression analyses performed on the subsample of participants are presented in Table 3. Overall, this sub-sample had higher depressive symptom scores and lower memory performances than the full sample. However, participants did not differ in age or gender ratios, as shown in Table 1. Model 1 and Model 2 show the significant interaction between depressive symptoms and self-efficacy on delayed and immediate memory performances, respectively. The interaction revealed that those high on self-efficacy and low on depressive symptoms had the greatest immediate and delayed performances as illustrated in Figures 2 and 3.
Table 3.
Summary of regression analyses for variables predicting immediate and delayed memory performances in the sub-sample (N= 1196)
| Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|
| Delayed memory | Immediate memory | |||||
| Predictor | B | SE (B) | β | B | SE (B) | β |
| CES-D score | −0.037 | 0.014 | 0.088** | −0.044 | 0.014 | −0.103 *** |
| Self-efficacy | 0.009 | 0.003 | 0.076** | 0.006 | 0.003 | 0.049 |
| Self-efficacy × depressive symptoms | −0.005 | 0.001 | −0.118 *** | −0.004 | 0.001 | −0.088** |
| R2 | 0.17 | 0.18 | ||||
Note: CES-D = Center for Epidemiologic Studies Depression Scale.
p< 0.05;
p< 0.01;
p< 0.001.
All analyses were controlled for age, education, race, and gender.
Figure 2.
The interaction between depressive symptoms and self-efficacy on delayed memory. The moderator values (i.e. self-efficacy) are the sample mean plus/minus one standard deviation from the mean based on raw values.
Figure 3.
The interaction between depressive symptoms and self-efficacy on immediate memory. The moderator values (i.e. self-efficacy) are the sample mean plus/minus one standard deviation from the mean based on raw values.
Discussion
The current cross-sectional study explored the associations between self-rated memory reports, depressive symptoms, and objective memory and whether self-efficacy moderated some of these relationships in a large, ethnically diverse sample of older adults. The relationship between self-rated memory and objective memory was first examined. We then investigated whether levels of self-efficacy moderated the association between self-rated memory and depressive symptoms. In a follow-up analysis, we tested the interaction between depressive symptoms and self-efficacy on objective memory performance in a subsample of participants who reported ‘poor’ or ‘fair’ memory function. The results showed that lower self-rated memory reports were significantly and negatively associated with immediate and delayed memory performances. Given the mixed findings in the literature on the association between subjective memory and objective memory, it was not clear if we would find this association. However, we were more interested in understanding the moderating role of self-efficacy and depressive symptoms in participants with memory ratings reported low self-rated memory. We found that even when self-rated memory was poor, higher-self efficacy was associated with less depressive symptoms compared to when self-efficacy was low. The follow-up analyses also revealed that when depressive symptoms were low, greater self-efficacy was associated with better objective memory compared to when self-efficacy was low. This finding extends current research that explores the relationship between self-rated memory and objective memory performance and considers the role of depressive symptoms as modality by which both factors are linked. If the strength and influence of depressive symptoms is attenuated by individual differences in self-efficacy, as we showed in the present study, this may well explain some of the negative findings between self-rated and objective memory in other studies.
Our finding also highlights the role of psychological resources as influential and moderating factors in depressive symptom-related memory deficits and should be considered in future research examining this topic. While individuals who report high self-efficacy are not exempt from a negatively perceived view of memory function, how they respond emotionally (i.e., with less depressive symptoms) may buffer them from the independent effects of depressive symptoms on memory performance. Our study provides support for this hypothesis.
While the direction of the association between depressive symptoms and self-rated memory cannot be ascertained from the present findings, i.e. is not clear whether depression is a response to poorly perceived memory function or vice versa, our findings do seem to be consistent with Bandura’s social cognitive theory (Bandura, 1989). This theory asserts that those with higher levels of self-efficacy are better able to regulate their emotional, cognitive, and motivational processes to enhance their performance on a given task. It may be that having less depressive symptoms alone could benefit their performance or perhaps individuals with higher self-efficacy maintain some greater level of motivation to perform optimally even under the influence of depressive symptoms. However, the interaction between self-efficacy and depressive symptoms suggests that this latter explanation is not the case in the present study. Those who had high self-efficacy and high depressive symptoms showed lower performances than those with high self-efficacy and low depression.
Limitations and future directions
There are limitations to the study that should be considered when interpreting our findings. The cross-sectional design is an obvious limitation to understanding both the directionality and mechanisms involved in the interactive relationships between our variables of interest. However, given the limited research which considers the interactive relationship between these variables, we aimed to first demonstrate and explore whether these variables did indeed interact. Longitudinal analyses are likely the next step to further break apart and understand how the underlying mechanisms interact over time. However, we consider our study an important first step in demonstrating how the interaction between self-rated memory and self-efficacy influences depressive symptoms in older adults and memory performance at any given time. Our findings are also of relevance to clinical assessment strategies focused on identifying contributors and moderators of depressive symptoms in older-adults. Our study has shown that psychological resilience factors, namely self-efficacy, may modify self-rated memory related depressive symptoms and, thus, should be given more weight in assessment settings.
Another limitation of the study concerns the general measure of self-rated memory. A measure probing more specific aspects of individuals self-rated memory function in more depth may have yielded stronger effect sizes. Overall, the study used brief measures of depressive symptoms and memory function; this may well have influenced the precision of our measures and, therefore, lacked the strength to account for a larger amount of variance in the models. Nevertheless, our aim was to establish reliable associations between sets of variables and derive an understanding of these associations from the pattern of results that emerged. Future research would benefit from using more comprehensive and precise measures to more fully understand the degree of contribution that self-efficacy and depressives symptoms lend to the association between self-rated memory and objective memory function. Also, information on the participants self-rated memory performance was gathered as part of their participation in a larger study. Therefore, we also consider whether the current results would apply to individuals who seek out health professionals because of a memory complaint. Future research examining this question would be worthwhile. Regarding the self-efficacy scale that was used in the present study, some authors have referred to it as a ‘locus of control scale’ (Dzivakwe, 2011) as well as a scale measuring ‘control beliefs’ (Duan-Porter et al., 2015). However, the HRS documentation does report that ‘Sense of Control – Self-Efficacy – Agency – Mastery. Authors in the literature use a variety of terms for these constructs’ (Smith et al., 2013, p22). While we did conduct further analyses to develop a more unidimensional scale that we consider to be reflective of self-efficacy in line with Bandura’s (1989) concept, future research may benefit from using other measures of self-efficacy. Measures of specific aspects of self-efficacy including those relating to cognition, physical function, and health, may be of interest and value. Furthermore, additional objective memory measures assessing other types of memory, specifically working memory, may add greatly to our understanding of self-rated memory and objective memory performance in older adults. There is some precedence for this consideration given a recent study that found self-efficacy to be more closely tied to working memory than episodic memory performance (Zahodne et al., 2014). Additional research should explore how self-rated memory reports relate to differing aspects of memory in the context of individual differences in self-efficacy.
Conclusion
Together our findings suggest that greater self-efficacy is advantageous both emotionally and cognitively even when memory ratings are poor. Older adults with high levels of self-efficacy and low depressive symptoms who also report a poor memory function had the highest memory performances. Thus, interventions aimed at improving perceived self-efficacy may be particularly beneficial in older adults with poor subjective memory.
Key points.
Self-efficacy moderates the association between self-rated memory and depressive symptoms.
Older adults who report poor memory have optimal memory performances when self-efficacy is high and depressive symptoms are low.
Interventions should focus on increasing perceived self-efficacy in older adults with poor subjective memory to potentially buffer them from the influence of depressive symptoms on memory function.
Footnotes
Conflict of interest
None declared.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Association Pub; Arlington, VA: 2013. [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- Bandura A. Regulation of cognitive processes through perceived self-efficacy. Dev Psychol. 1989;25:729. [Google Scholar]
- Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55(12):1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Clarnette RM, Almeida OP, Forstl H, Paton A, Martins RN. Clinical characteristics of individuals with subjective memory loss in Western Australia: results from a cross-sectional survey. Int J Geriatr Psychiatry. 2001;16:168–174. doi: 10.1002/1099-1166(200102)16:2<168::aid-gps291>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Collins MW, Abeles N. Subjective memory complaints and depression in the able elderly. Clin Gerontol. 1996;16:29–54. [Google Scholar]
- Derouesne C, Lacomblez L, Thibault S, Leponcin M. Memory complaints in young and elderly subjects. Int J Geriatr Psychiatry. 1999;14:291–301. [PubMed] [Google Scholar]
- Duan-Porter W, Hastings SN, Neelon B, Van Houtven CH. Control beliefs and risk for death, stroke and myocardial infarction in middle-aged and older adults: an observational study. J Gen Intern Med. 2015;30:1156–1163. doi: 10.1007/s11606-015-3275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzivakwe VG. Doctoral dissertation. University of North Texas: 2011. Psychosocial determinants of diabetic control and satisfaction with diabetes care. [Google Scholar]
- Gagnon M, Dartigues JF, Mazaux J, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- Grut M, Jorm AF, Fratiglioni L, et al. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc. 1993;41:1295–1300. doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Hülür G, Hertzog C, Pearman A, Ram N, Gerstorf D. Longitudinal associations of subjective memory with memory performance and depressive symptoms: between-person and within-person perspectives. Psychol Aging. 2014;29:814. doi: 10.1037/a0037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. J Am Geriatr Soc. 1996;44:44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J Pers Soc Psychol. 2002;83:693. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Jungwirth S, Fischer P, Weissgram S, et al. Subjective memory complaints and objective memory impairment in the Vienna–Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30(suppl):135–145. [Google Scholar]
- Kahn RL, Zarit SH, Hilbert NM, Niederehe G. Memory complaint and impairment in the aged: the effect of depression and altered brain function. Arch Gen Psychiatry. 1975;32:1569–1573. doi: 10.1001/archpsyc.1975.01760300107009. [DOI] [PubMed] [Google Scholar]
- Koenig AM, Delozier IJ, Zmuda MD, et al. Neuropsychological functioning in the acute and remitted states of late-life depression. J Alzheimers Dis. 2015;45:175–185. doi: 10.3233/JAD-148006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlowicz LH. Perceived self-efficacy, functional ability, and depressive symptoms in older elective surgery patients. Nurs Res. 1998;47:219–226. doi: 10.1097/00006199-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. J Pers Soc Psychol. 1998;74:763. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- Luciano M, Pujals AM, Marioni RE, et al. Current versus lifetime depression, APOE variation, and their interaction on cognitive performance in younger and older adults. Psychosom Med. 2015;77:480–492. doi: 10.1097/PSY.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Minett TS, Dean JL, Firbank M, English P, O’brien JT. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13:665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- Mokken RJ. A theory and procedure of scale analysis: with applications in political research. Walter de Gruyter; 1971. [Google Scholar]
- O’shea DM, Fieo RA, Hamilton JL, et al. Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. Int J Geriatr Psychiatry. 2015;30:614–622. doi: 10.1002/gps.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- Pearman A, Storandt M. Predictors of subjective memory in older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59:P4–P6. doi: 10.1093/geronb/59.1.p4. [DOI] [PubMed] [Google Scholar]
- Rickenbach EH, Agrigoroaei S, Lachman ME. Awareness of memory ability and change:(in) accuracy of memory self-assessments in relation to performance. J Population Ageing. 2015;8:71–99. doi: 10.1007/s12062-014-9108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Smith G, Johnston MV, Allen J. Self-care self-efficacy, quality of life, and depression after stroke. Arch Phys Med Rehabil. 2000;81:460–464. doi: 10.1053/mr.2000.3863. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20:243. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Sijtsma K, Molenaar IW. Introduction to nonparametric item response theory. Sage; 2002. [Google Scholar]
- Smith J, Fisher G, Ryan L, et al. Psychosocial and lifestyle questionnaire, 2006–2010. Documentation Report Core Section LB. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- Stewart R, Russ C, Richards M, et al. Depression, APOE genotype and subjective memory impairment: a cross-sectional study in an African–Caribbean population. Psychol Med. 2001;31:431–440. [PubMed] [Google Scholar]
- Van Der Heijden P, Van Buuren S, Fekkes M, Radder J, Verrips E. Unidimensionality and reliability under Mokken scaling of the Dutch language version of the SF-36. Qual Life Res. 2003;12:189–198. doi: 10.1023/a:1022269315437. [DOI] [PubMed] [Google Scholar]
- Watson R, Van Der Ark LA, Lin LC, et al. Item response theory: how Mokken scaling can be used in clinical practice. J Clin Nurs. 2012;21:2736–2746. doi: 10.1111/j.1365-2702.2011.03893.x. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes L, De Leon CM, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- Windsor TD, Anstey KJ. A longitudinal investigation of perceived control and cognitive performance in young, midlife and older adults. Aging Neuropsychol Cogn. 2008;15:744–763. doi: 10.1080/13825580802348570. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Nowinski CJ, Gershon RC, Manly JJ. Which psychosocial factors best predict cognitive performance in older adults? J Int Neuropsychol Soc. 2014a;20:487–495. doi: 10.1017/S1355617714000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, Manly JJ. Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. Am J Geriatric Soc. 2014b;62(1):130–134. doi: 10.1111/jgs.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Demen. 2004;19:353–360. doi: 10.1177/153331750401900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Burnight KP, Lane CJ. The relationship between subjective and objective memory in the oldest old: comparisons of findings from a representative and a convenience sample. J Aging Health. 2001;13:248–266. doi: 10.1177/089826430101300205. [DOI] [PubMed] [Google Scholar]
- Zunzunegui M-V, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. J Gerontol B Psychol Sci Soc Sci. 2003;58:S93–S100. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]