Abstract
Sense organs that monitor forces in legs can contribute to activation of muscles as synergist groups. Previous studies in cockroaches and stick insects showed that campaniform sensilla, receptors that encode forces via exoskeletal strains, enhance muscle synergies in substrate grip. However synergist activation was mediated by different groups of receptors in cockroaches (trochanteral sensilla) and stick insects (femoral sensilla). The factors underlying the differential effects are unclear as the responses of femoral campaniform sensilla have not previously been characterized. The present study characterized the structure and response properties (via extracellular recording) of the femoral sensilla in both insects. The cockroach trochantero-femoral (TrF) joint is mobile and the joint membrane acts as an elastic antagonist to the reductor muscle. Cockroach femoral campaniform sensilla show weak discharges to forces in the coxo-trochanteral (CTr) joint plane (in which forces are generated by coxal muscles) but instead encode forces directed posteriorly (TrF joint plane). In stick insects, the TrF joint is fused and femoral campaniform sensilla discharge both to forces directed posteriorly and forces in the CTr joint plane. These findings support the idea that receptors that enhance synergies encode forces in the plane of action of leg muscles used in support and propulsion.
Keywords: Force, Synergies, Insect, Campaniform, Response, Sensitivity
1. Introduction
In standing and walking, leg and body muscles are activated as modular, synergistic groups (Chvatal and Ting, 2013; Safavynia and Ting, 2013). However, the specific mechanisms underlying the organization of muscle synergies are unclear (Gopalakrishnan et al., 2014; Laine et al., 2015). A number of experiments have shown that sense organs that monitor forces have widespread effects in motor neurons to leg muscles (Eccles et al., 1957; Harrison et al., 1983). These effects could function to enhance or reinforce synergies in posture and walking (Duysens et al., 2000, 2013; Hagio and Kouzaki, 2014). However, it is not known if all force detecting sense organs have the same effects on muscle synergies (Nichols, 1999; Nichols and Ross, 2009). Experiments in cats demonstrated that Golgi tendon organs of different leg muscles (ankle or knee extensors) had diverse effects in activation of groups of leg muscles during treadmill walking (Ross and Nichols, 2009). It was not determined if the effects on muscle synergies were correlated with the specific forces encoded by the receptors.
In insects, grasping the substrate, which is initiated at the start of the stance phase of walking, is achieved by activation of muscle synergies (Bassler et al., 1991). Substrate adhesion is an active process that requires contraction of a number of leg muscles at different intrinsic joints, including muscles acting at the foot (tarsus) and muscles of proximal leg segments (flexor, depressor) (Wile et al., 2008; Zill et al., 2014). The combined action of these muscles ensures that adequate adhesion is rapidly established and maintained after leg contact (Gorb, 2008). Our previous study in cockroaches and stick insects showed that leg campaniform sensilla, that encode forces as cuticular strains, can aid in activation of the synergist muscles that generate substrate grip (Zill et al., 2004, 2015a, b). The receptors of the leg act as an ensemble as campaniform sensilla at different locations reinforce the same muscle synergies with proximally located receptors affecting distal leg muscles. However, the specific groups of sensilla that produced effects on muscle synergies were species-specific: in cockroaches, synergist activation was mediated by trochanteral sensilla but this effect was associated with femoral campaniform sensilla in stick insects (Zill et al., 2015a, b).
The factors underlying the difference in effects of specific groups of force receptors upon muscle synergies are not known. Recordings of stick insect trochanteral campaniform sensilla suggested that the motor effects of individual groups of receptors depend upon their sensitivity to forces in the plane of action of the main coxal muscles (coxo-trochanteral (CTr) joint plane) that generate forces in support and propulsion (Cruse and Bartling, 1995; Zill et al., 2012; Dallmann et al., 2016). Although many groups of campaniform sensilla have previously been characterized, the specific structure, responses and directional sensitivities of the femoral groups are poorly understood (Pringle, 1938; Schmitz et al., 1991; Akay et al., 2001). In all insects, the femoral campaniform sensilla are located on the proximal end of the femur, adjacent to the trochanter femur joint (TrF) (Petryszak and Fudalewicz-Niemczyk, 1994). Unlike most leg joints which are similar in structure, the joint between the trochanter and femur is variable and species-specific in the range of movement it allows (Frantsevich and Wang, 2009). In most insects, this joint also represents the point at which autotomy (loss of distal leg segments) occurs (Carde, 2009). While the TrF joint has been shown to function adaptively in some insects (Watson et al., 2002; Bender et al., 2010), it has also been postulated to function primarily as a spring or shock absorber (Frantsevich and Wang, 2009).
In the present study, we examined the structure of the TrF joint in cockroaches and stick insects. We also characterized the response properties of the femoral campaniform sensilla, which have not previously been determined. These results demonstrate that the femoral groups differ in their directional sensitivity to imposed forces and to forces mimicking muscle contractions. The same group of receptors, therefore, shows different responses in different insect species. Our data also support the hypothesis that receptors that affect muscle synergies encode forces in the plane of action of major intrinsic leg muscles used in support and propulsion. Similar correlation of response specificity in force detection and activation of muscle synergies may occur in other animals, including vertebrates.
2. Methods
Experiments were performed on adult, female stick insects (Carausius morosus) raised in animal colonies at the University of Bielefeld or the University of Köln and adult, male cockroaches (Periplaneta americana) obtained commercially (Carolina Biological Supply).
2.1. Morphological studies
Legs were removed from animals anesthetized with carbon dioxide. To study the structure of the TrF joint in histological section, the trochanter and femur were isolated and immersed in Karnovsky’s fixative, dehydrated in ethanol, embedded in Spur’s resin and sectioned using an MT2 microtome (method of Moran et al., 1971). Sections were stained with toluidine blue (an indicator of potential elasticity in cuticular structures; Weis-Fogh, 1960; Wong et al., 2012). For whole mount preparations, the trochanter and femur were bisected, cuticle containing the femoral campaniform was further isolated and treated with 1 M potassium hydroxide for at least 1 h. To image joint membranes, preparations were cleared in Conray (an aqueous clearing agent, iothalamate meglumine, Mallinckrodt), viewed under UV illumination in an Olympus microscope and photographed using a Spot Camera (Diagnostic Imaging, Inc.; Zill et al., 2011). To view the caps of campaniform sensilla, specimens were fixed in 4% formalin prior to clearing in Conray. For confocal microscopy, specimens were imaged using a Leica TCS SP5 II microscope at the Marshall University Microscopy facility (methods of Zill et al., 2011).
For scanning electron microscopy, middle and hind legs of newly molted stick insects were isolated and partially dissected, then desiccated and sputter coated (Zill et al., 2011). The cuticle was imaged with an Hitachi S450 scanning electron microscope. Digital images of the cuticular caps were measured in ImageJ software (NIH, USA). Data from the middle and hind legs were pooled for analysis as measurements of the sizes and locations of the femoral sensilla indicated no significant difference between the groups in the serially homologous legs.
2.2. Physiological studies
Physiological studies were performed on the left middle legs of stick insects and the left hind legs of cockroaches. These specific legs were utilized to extend findings of previously published studies (Zill et al., 2012; 2015a, b). While the legs are of different body segments, the distribution, number and responses of campaniform sensilla are quite similar in middle and hind legs in both species (Hofmann and Bässler, 1982, 1986; Keller et al., 2007; Zill et al., 2009). Studies of the functions and forces generated by the legs also indicate that they serve similar functions in support and propulsion, although the hind legs are normally the major source of propulsive forces in the cockroach escape reaction (Hughes, 1952; Dallmann et al., 2016). In addition, recordings were found to be most viable in the cockroach hind legs due to their large size.
Animals (intact or after sensory ablation) were first securely restrained and the coxa of the leg was firmly fixed with cyanoacrylate adhesive to small staples placed above and below the segment. The distal leg was amputated in the distal femur, proximal to the femorotibial joint and a mixture of Vaseline and paraffin oil placed over the end of the femur to prevent desiccation. The proximal leg segments were not dissected and remained attached to the thorax to insure normal ventilation through the animal’s tracheal system.
Recordings of sensory activities were taken, in stick insects, from the main leg nerve using custom oil-hook electrodes (Schmitz et al., 1988) or, in cockroaches, through 50 μ silver wires (Goodfellow Ltd, AG005825) that were insulated to their tip and inserted in the distal coxa (Zill et al., 1999). The tibial flexor muscle was recorded myographically from the femur using a pair of the same type of wires (methods of Zill et al., 2015a, b).
2.3. Ablation of sense organs
To limit recordings to activities of the femoral campaniform sensilla, all groups of trochanteral receptors (Groups 1–4) were ablated with a sharp etched pin (Schmitz, 1993). The cell bodies of the femoral chordotonal organ were ablated by inserting the pin through the cuticle of the anterior side of the proximal femur.
2.4. Mechanical stimulation
The head of a minuten pin (firmly attached to a motor) was inserted into the cuticle distal to the attachment of the depressor muscle tendon. In both stick insects and cockroaches the proximal, ventral part of the trochanter is reinforced by an internal cuticular buttress that creates a small compartment distal to the muscle insertion (described in Zill et al., 2000, 2012). The tip of the pin was inserted through ventral cuticle into this compartment. The pin was also used to resist loads applied to the femur in different directions. Forces were applied to the femur via a probe with strain gauges mounted on a piezoelectric crystal (Zill et al., 2011). Voltages were generated using a CED laboratory interface (power 1401mkII, CED Ltd., Cambridge, UK). Half sine and ramp and hold waveforms were applied through pre-recorded templates in Spike 2 software (Cambridge Electronics, England). Forces were first applied dorsally, in the plane of movement of the coxo-trochanteral joint (which has been shown to activate the Group 3 trochanteral campaniform sensilla in both cockroaches and stick insects). The force probe was then rotated 90° to apply forces in a posterior direction, perpendicular to the joint plane (Zill et al., 2012). Posterior forces were usually applied with the TrF joint fully retracted (no movement) but could also be tested with the joint partially retracted to test responses to joint movements (Fig. 5C). Responses were also tested to forces applied at the depressor muscle insertion (via the motor) using the same waveforms with the CTr positioned at approximately 90°.
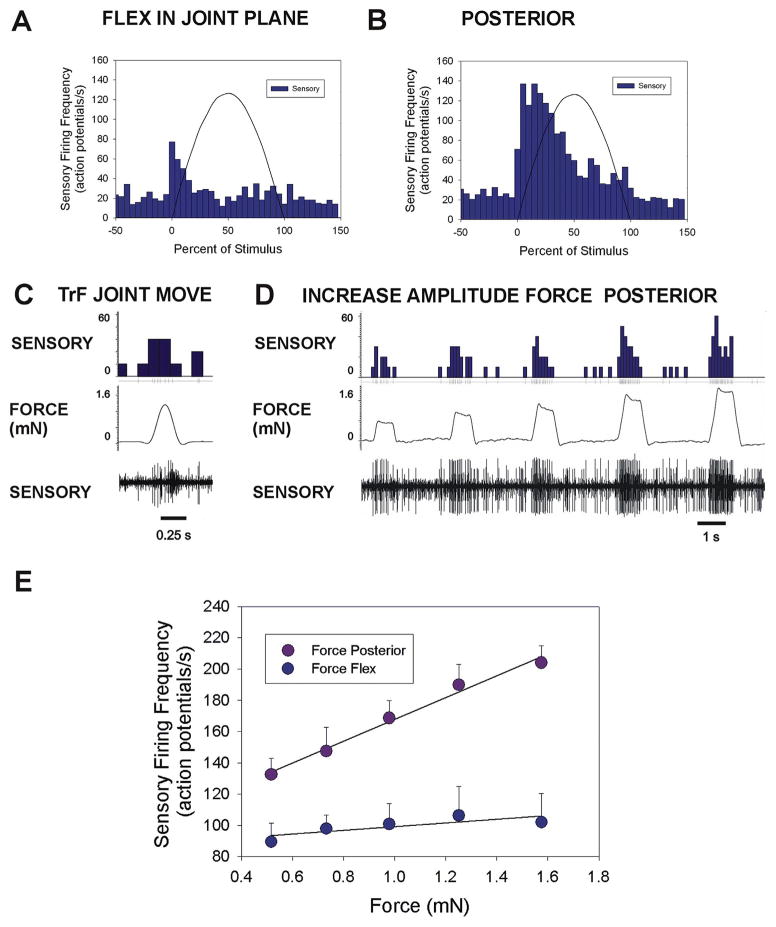
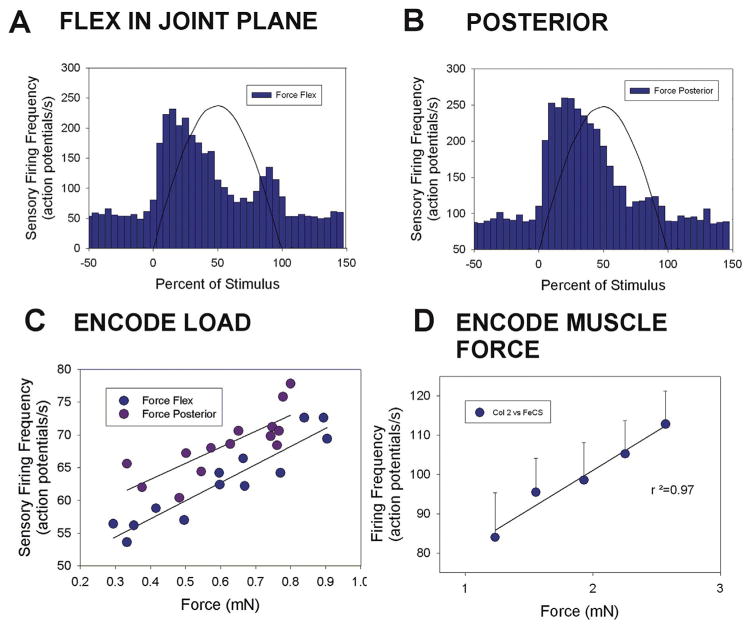
Fig. 5. Plots of responses and encoding of force magnitude in cockroach femoral campaniform sensilla.
A. and B. Cumulative plots of sensory discharges to forces applied in the plane of the CTr joint in the direction of joint flexion (A. n = 75 tests, N = 3 animals) and in a posterior direction, perpendicular to the joint plane (B. n = 85 tests, N = same 3 animals). Minimal sensory discharges occurred in the plane while intense firing was elicited in a posterior direction. C. Movement resisted by joint stiffness – Brief sensory discharges could be elicited when the TrF joint was not fully engaged. D. Amplitude series – Forces were applied in a posterior direction at varying amplitudes but the same rate of rise and decline with the TrF joint fully engaged E. Encoding of force magnitude – Plot of the mean sensory discharges (largest units) to forces applied as ramp and hold functions to forces in the joint plane (flexion) and posteriorly, perpendicular to the plane (flexion n = 8 repetitions of 5 levels; posterior n = 11 repetitions of 5 levels, N = 1). The femoral sensilla effectively encode force magnitude in a posterior direction but discharges were minimal in the plane of the coxo-trochanteral joint. (Sensory histograms in C, D: action potentials/second).
2.5. Cap stimulation
The caps of single sensilla were mechanically stimulated using a fine tungsten wire that was mounted to a separate piezo-electric crystal (method of Chapman et al., 1973). The cuticular caps were ablated using an etched minuten pin.
2.5.1. Data storage and analysis
Neurophysiological and force data were recorded using the CED interface and analyzed using custom scripts in Spike 2 software scripts. Spike sorting (in Spike 2) was used to differentiate sensory activities according to potential amplitude in extracellular recordings of sensory activities in cockroaches The response properties of stick insect femoral campaniform sensilla were tested at moderate levels of force as potential height varied due to the larger number of sensilla active in multiunit recordings. Although all recordings showed multiunit activities, plots of the firing frequency of receptors reflected the amplitude of forces applied as sustained stimuli and rectification/integration of signals was not used in analysis of the data. Data were plotted in Sigma plot.
3. Results
3.1. Anatomy and specializations of the trochantero-femoral joint
We examined the structure of the of trochanter-femur articulations in cockroaches and stick insects to gain insight into how forces are transmitted as strains at the joints. Most intrinsic joints in insect legs are comparable in design (hinge: coxo-trochanteral, femorotibial joints; ball and socket: tibio-tarsal joint, condylar joints: tarsal segments) but the joint between the trochanter and femur varies in structure and range of movement in different species (Frantsevich and Wang, 2009).
3.1.1. Cockroach trochanter-femur (TrF) joint
In cockroaches, the TrF articulation is a mobile joint (Fig. 1A; Pringle, 1938; Bender et al., 2010). Condyles on the anterior surface of the trochanter and femur form the joint hinges (Fig 1B top). These condyles appear dark due to heavy sclerotization (particularly the dorsal condyle) and represent the major points of force transmission between the trochanter and femur (Kaliyamoorthy et al., 2006). In contrast, the posterior surface of the joint has no condyles but is spanned by a joint membrane (Fig. 1B bottom). The membrane is not readily visible externally as the femur extends inside the posterior margin of the trochanter. A portion of membrane can be seen at the joint above (dorsal to) the femur and this region shows blue fluorescence in UV illumination (Fig. 1B bottom).
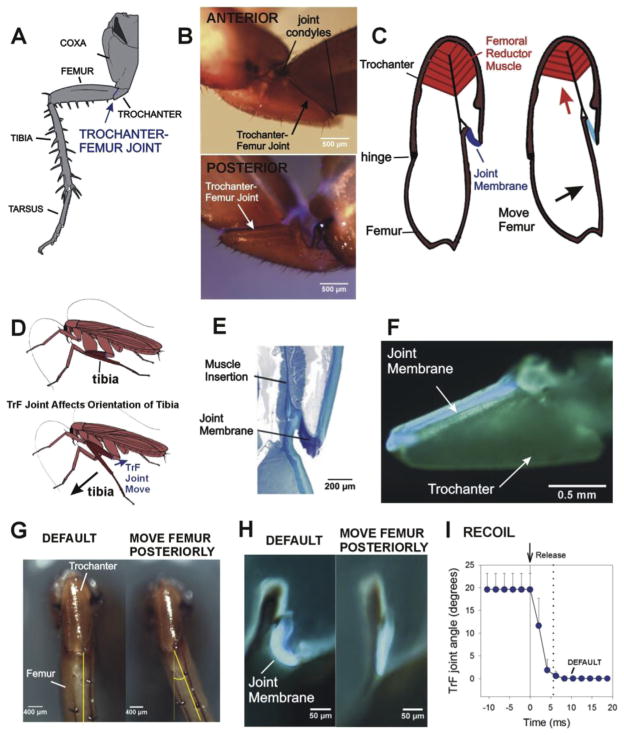
Fig. 1. Mobility and specializations of the cockroach trochantero-femoral joint.
A. Cockroach middle leg. The trochanter is a small segment upon which large muscles of the coxa and body wall insert. The trochanter articulates with the femur at the trochantero-femoral (TrF) joint. B. Whole mounts of cockroach trochanter – The joint between the trochanteral and femoral segments is a movable hinged articulation, with joint condyles on the anterior side (top). The posterior surface has no condyles and the joint membrane shows blue fluorescence in combined light/UV illumination. C. Left – Diagram of longitudinal section (parallel to the long axis of the trochanter) through trochanter and femur. The reductor femoris muscle is located in the trochanter and inserts onto the femur and the joint membrane. Contraction of the muscle pulls the femur posteriorly and stretches the joint membrane. D. Action of trochanter-femur joint on the distal leg – Posterior movement at the TrF brings the tibia to a more vertical position relative to the substrate. E. Histological section of TrF joint (same plane as 1C) – The joint membrane is thickened and stains intensely with toluidine blue at the insertion of the muscle. F. Fluorescence image of hind leg trochanter split longitudinally and viewed on its inner surface. The membrane shows blue fluorescence in UV illumination. G. Movement at TrF joint – Ventral view of trochanter and femur of cockroach hind leg at rest (default position, left) and after maximum posterior displacement of the TrF joint (right). H. Fluorescence images of TrF joint with the upper half of the trochanter and femur removed in a severed leg (orientation similar to 1E). The joint membrane is curved in the default (rest) position but stretched when the femur is moved posteriorly. I. Elastic recoil – The joint showed rapid recoil to the default position when it was released after posterior displacement.
The TrF joint is moved by a single muscle, the reductor femoris, which takes origin in the trochanter and inserts upon the dorsal side of the base of the femur (Fig. 1C, redrawn after Watson et al., 2002; Carbonell, 1947; Pringle, 1938). Contraction of the reductor pulls the femur posteriorly, perpendicular to the coxo-trochanteral joint. However, as a consequence of the angle of the TrF joint relative to the long axis of the femur (approximately 35° in the hind leg), reductor contraction acts to move the tibia into a more vertical position relative to the substrate (Fig. 1D). There is no muscular antagonist to the reductor femoris and the mechanism by which the femur is moved anteriorly at the joint has been unclear (Pringle, 1938), although it was thought to be associated with elasticity in the joint membrane (Watson et al., 2002).
We utilized several methods to study the structure and properties of the TrF joint membrane. Fig. 1E is a micrograph of a toluidine blue stained preparation showing the joint in transverse section (parallel to the base of the trochanter). The joint membrane appears as a very dark, curved region at the insertion of the femoral reductor muscle. The trochanter in Fig. 1F was split longitudinally and the posterior one-half was isolated with the joint membrane attached and viewed under UV illumination. A band of resilin-like blue fluorescence is continuous along the entire inner, posterior edge of the trochanter-femur joint (Neff et al., 2000). This is not visible in undissected legs as it is located internally. The intense toluidine blue staining and blue fluorescence are consistent with the presence of resilin in the joint membrane (Weis-Fogh, 1960; Wong et al., 2012). Due to its linkage to the insertion of the muscle apodeme, this region of the joint membrane should be stretched by contraction of the reductor muscle or posterior movement of the femur.
We studied the effects of manual (posterior) displacement of the joint in isolated hind legs (Fig. 1G–I). The maximum displacement of the TrF (before joint dislocation) was approximately 20–25° (Fig. 1G). We were able to directly visualize (under UV illumination) the posterior membrane during TrF joint displacement by removing the upper half of the trochanter and femur (Fig. 1H). The joint membrane appears curved in the default position (left) but is straightened and stretched during imposed joint remotion (right). We have also demonstrated that the joint rapidly recoils to its default position upon release from manual displacement in isolated legs with the TrF joint intact (Fig. 1I). Fig. 1I is a plot of the mean change in TrF joint angle (imaged at 480 frames/s) after the femur was released following posterior displacement (n = 7 tests in N = 4 animals). The duration of recoil was quite short (mean 5.6 ms ± 1.6 SD). The mean rate of change of joint angle was 3.54°/ms ± 1.13 SD, comparable to the rate of recoil measured in the cockroach distal tarsal joint (Frazier et al., 1999). These findings support the idea that the joint membrane act as a spring in parallel to the muscle apodeme.
3.1.2. Stick insect trochanter-femur joint
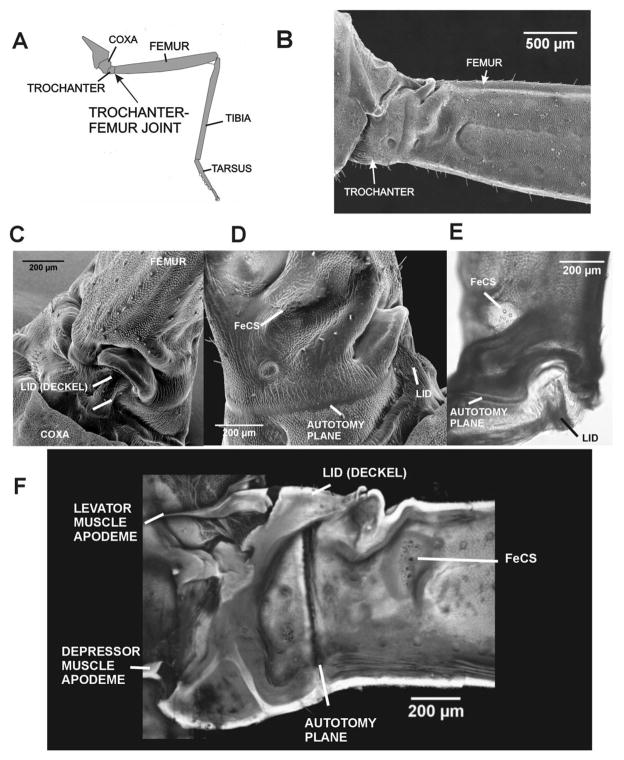
In stick insects, the TrF joint (Fig. 2A) is not moveable. The anterior and posterior surfaces of the joint appear fused and the junction of the trochanter and femur is only marked by a slight indentation (Fig. 2B) No joint condyles or membranes are apparent at this junction. Thus, there are apparently no specialized structures for force transmission across the TrF joint on its lateral surfaces. In contrast, the dorsal surface of the trochanter shows specialized projections and indentations (Fig. 2C; Schindler, 1979). In the center of the dorsal surface, the trochanter is thin walled and forms a projection termed the lid (lid, G. Deckel, Fig. 2C–E). Schindler (1979) considered elevation of the lid to be an initial stage in autotomy, in which the distal leg is self-amputated at the trochanter-femur joint. The lid extends toward and articulates with a crescent shaped indentation in the proximal margin of the femur (Fig. 2C and E). Study of whole mount preparations showed that the cuticle of the femur is thickened and sclerotized in this region, particularly in the areas lateral to the lid (Fig. 2E). These features could readily serve to transmit forces when contractions of the depressor muscle press the leg against the substrate. The femoral group of campaniform sensilla is located on the posterior surface of the femur in a thin-walled depression immediately distal to this region (Fig. 2D and E).
Fig. 2. Immobility and specializations of the trochanter-femur joint in stick insects.
A. Drawing of stick insect middle leg and trochanter-femur joint.. B. SEM of trochanter and femur in posterior view – The trochanter is functionally fused to the femur with no condyles or joint movement. C. SEM of upper (dorsal) surface of the trochanter – The dorsal surface of the trochanter forms a projection (termed the lid, G., Deckel) which interlocks with a depression on the proximal end of the femur. D. Posterior view (SEM) of proximal femur and lid – The femoral group of campaniform sensilla are located in a depression in the proximal femur, distal to the autotomy plane. E. Dorsal view of whole mount of TrF joint – the cuticle appears darkened and thickened in the region between the autotomy plane and femoral sensilla. F. Confocal projection image of inner (posterior) surface of a bisected coxa, trochanter and femur (the lid remained intact) – The apodeme (tendon) of the Levator Trochanteris muscle inserts upon the ‘lid’’ of the trochanter; the Depressor apodeme inserts upon the reinforced ventral end of the trochanter. The autotomy plane is clearly seen as a thin vertical line between the fused trochanter and femur.
We also examined the relationship of these structures to the insertion of the coxal muscles. Fig. 2F shows a confocal projection image of the inner side of the coxa, trochanter and femur in a specimen that was bisected. The lid and the sclerotized apodemes of the coxal muscles remained intact. The apodeme of the coxal levator muscle can clearly be seen to insert upon the central region of the lid while the depressor apodeme inserts on the opposite side of the trochanter. This relationship suggests that coxal levator muscle could play a role in autotomy (see Discussion).
3.2. Anatomy of femoral campaniform sensilla in cockroaches and stick insects
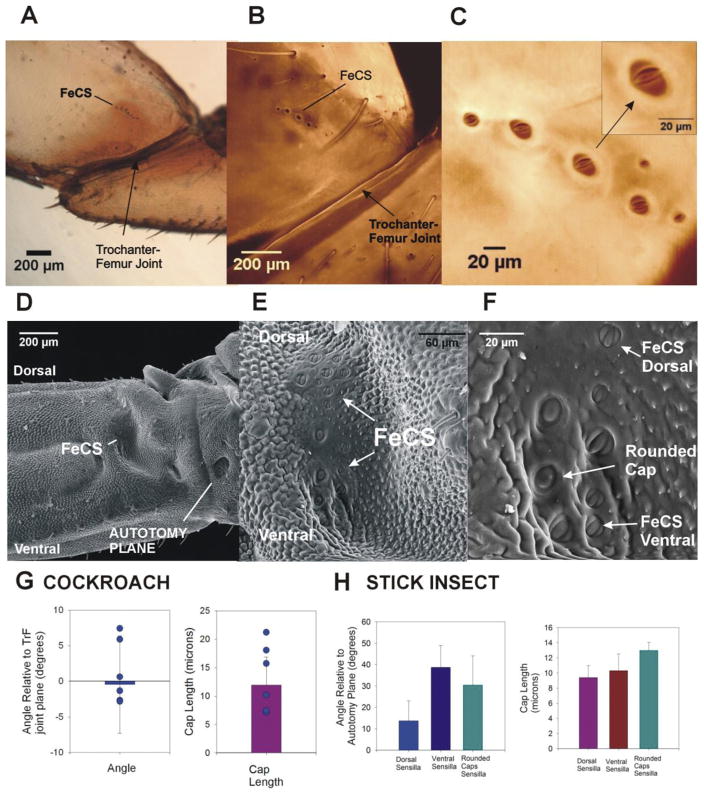
In both cockroaches and stick insects, the femoral group of campaniform sensilla is located on the dorsal surface at the proximal end of the femur. In cockroaches, the cuticle in this region shows no depressions or changes in thickness (Fig. 3A), as are found associated with the trochanteral campaniform sensilla (Zill et al., 2000). The femoral sensilla are readily identified as a row of cuticular caps (7–11) that extends ~200–500 microns distal to the trochanter femur joint (Fig. 3A and B) (although isolated receptors with small cuticular caps can occur adjacent to the main row). The caps are located in oval shaped cuticular collars (Fig. 3C). The cap long axes are oriented nearly parallel to the trochanter-femur joint (Fig. 3G left, mean – 0.45° ± 6.8 SD, n = 20, N = 3) but they vary considerably in size (Fig. 3G right mean long axis length = 11.9 microns ± 4.9, n = 20, N = 3). This orientation should make the group sensitive to forces on the distal end of the leg that are directed posteriorly and produce compressive strains on the dorsal surface of the femur.
Fig. 3. Structure of femoral campaniform sensilla in cockroaches and stick insects.
A. Posterior view of a whole mount of a cockroach trochanter and femur – The femoral group of campaniform sensilla is located on the posterior surface of the femur, distal to the trochanter-femur joint. B. and C. Confocal projection of images TrF joint – The cuticular caps of the femoral sensilla are arranged in a small, linear row on the proximal femur. At higher magnification (C), the caps of the sensilla are visible inside the cuticular collars. The orientation of the caps is consistent within the group and the cap long axis is approximately parallel to the TrF joint (see inset). D. SEM of posterior view of TrF joint in stick insects – The FeCS are located in a prominent depression of the posterior side of the femur. E and F. Higher magnification views of SEM. E. The caps of the FeCS are relatively dispersed in the distal wall of depression. F. Three subgroups of receptors can be distinguished based upon their position and cap orientation: dorsal sensilla (FeCS Dorsal), ventral sensilla (FeCS Ventral) and sensilla whose caps appear rounded rather than oval-shaped (Rounded Caps). G. Orientations and cap sizes of cockroach femoral campaniform sensilla – These graphs plot the mean orientation (left) and long axis length (right) of the caps of the femoral campaniform sensilla (points show range of values from one representative animal). The sensilla are generally oriented parallel to trochanter-femur joint and have a range of cap sizes (histogram at right). H. Orientations and cap sizes of stick insect femoral campaniform sensilla – Three types of receptors could be distinguished based upon their orientation (left) and cap shape. Rounded caps had the largest lengths (right).
In contrast, the femoral group in stick insects (Fig. 3D–F) is larger (mean 16 total receptors ± 1.58 SD, n = 80 caps measured in N = 5 animals [2 middle, 3 hind legs]) and the cuticular caps vary in shape and orientation. As viewed from the outer surface, the cuticular caps are located mainly in the distal wall of the prominent depression that is adjacent to the sclerotized region lateral and distal to the lid (Fig. 3E). The femoral sensilla can be considered as three subgroups based upon their location, structure and orientation of the cuticular caps (Fig. 3F and H). Receptors located dorsally (mean number of caps = 7.6 ± 0.9 SD) have cuticular caps that are oval shaped with long axes that are at a small angle relative to autotomy plane, which is approximately perpendicular to the long axis of the femur (Fig. 3H, mean cap orientation = 13.7° ± 9.3 SD; mean length = 9.5 microns ± 1.6 microns SD, N = 5 preparations in which all sensilla could be seen approximately orthogonal to the surface). Sensilla located ventrally (mean number = 5.0 ± 0.2 SD) have oval caps that are at a larger angle relative to the autotomy plane (Fig. 3H mean orientation 38.7° ± 10.2; mean length = 9.7 microns ± 2.2 SD, same data set). There is some overlap in these subgroups but the difference in angle between the dorsal and ventral groups is statistically significant (Student’s t-test, P < 0.01, same data set). The middle region contains large caps that are rounded in shape (mean number = 3.4 ± 0.5 SD) and have the largest cap length (mean length = 13.7 microns). These differences in shape and orientation imply that the subgroups of femoral campaniform sensilla could detect strains and forces in a number of directions.
3.3. Response properties of femoral campaniform sensilla in cockroaches
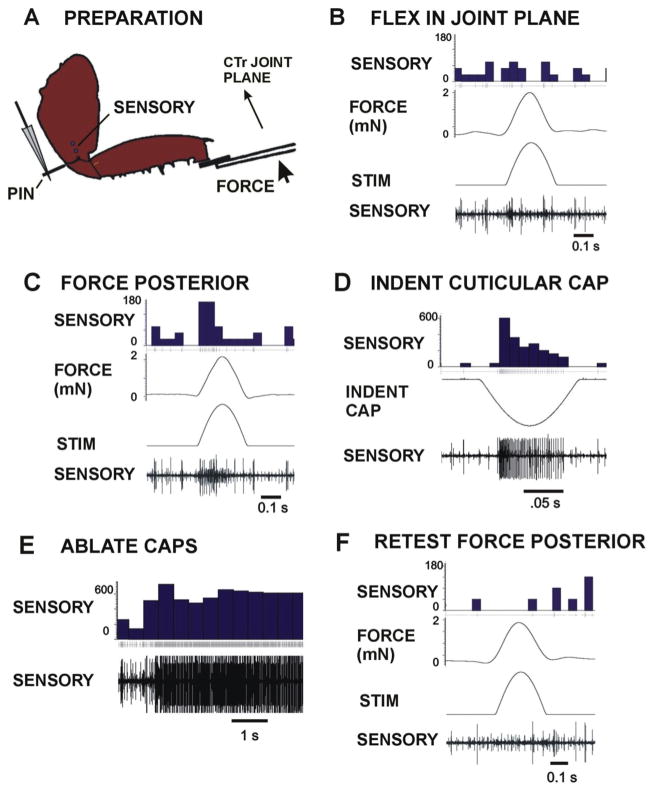
Responses of the femoral campaniform sensilla were characterized by applying forces to the femur while extracellular recordings were taken from the main leg nerve in preparations in which the trochanteral campaniform sensilla and other proprioceptive sense organs (trochanteral hair plate, femoral chordotonal organ) were ablated and the leg nerves cut in the distal femur and proximal coxa (Fig. 4A). Fig. 4B shows activities recorded to forces applied as half sine wave forms in the plane of movement of the CTr joint with forces resisted by the pin in the proximal trochanter. Only transient sensory discharges were obtained. However, intense firing of units with large and smaller amplitude occurred when the plane of force application was directed posteriorly, 90° perpendicular to the CTr joint plane (Fig. 4C). Stimulation of the caps of individual femoral campaniform sensilla produced unitary discharges that matched the extracellular amplitude of spikes recorded in tests of applied forces (Fig. 4D). Ablation of the caps (with a fine etched wire probe) produced intense injury discharges (Fig. 4E) and eliminated all responses in subsequent tests (Fig. 4F).
Fig. 4. Responses of cockroach femoral campaniform sensilla to imposed forces.
A. Preparation. The coxa of left hind leg of an intact cockroach was mounted via staples and the distal segments severed at the femoro tibial joint. All trochanter campaniform sensilla were ablated but the femoral group (FeCS) remained intact. Forces were applied to the femur in different directions with movement resisted by a pin (PIN) inserted into the proximal trochanter. Sensory activity was recorded with wires (SENSORY) in the distal coxa after severing the main leg nerve proximally. B. Flexion in the coxo-trochanteral joint plane – Forces applied in the joint plane produced only transient sensory discharges. C. Forces perpendicular (posterior) to the joint plane – Vigorous bursts were obtained to forces perpendicular to joint plane. D. and E. Indentation of the caps of single sensilla (D) and cap ablations (E) produced action potentials of amplitude equivalent to those seen in tests of responses to forces. F. No sensory discharges were seen when forces (posterior) were applied to the leg after cap ablation. (Sensory histograms in B–F: action potentials/second).
Fig. 5A and B shows cumulative histograms of the mean sensory discharges to forces applied (using half sine wave forms) in the cockroach CTr joint plane and perpendicular to the plane of movement. Very brief discharges, usually consisting of a few spikes, occurred at the onset of forces in the joint plane. In contrast, there was intense firing during the rising phase of the stimulus when forces were applied in a posterior direction. Tests of application of posterior forces when the TrF joint was not fully engaged (joint free to move and force resisted only by joint stiffness) could elicit brief discharges from the femoral campaniform sensilla and firing of other sensory neurons to the return movement to the default position (Fig. 5C). We also applied forces using ramp and hold functions to test the ability of the sensilla to encode sustained forces in a posterior direction with the TrF joint fully engaged. Fig. 5D shows a recording in which forces directed posteriorly were applied to femur at increasing levels. Firing of sensilla of both small and large extracellular amplitudes increased at higher magnitudes of force application. Fig. 5E is a cumulative plot of the discharges from a single preparation in which a full set of tests in forces were applied using ramp and hold functions at different levels both posteriorly and in the CTr joint plane. The firing frequency effectively encoded the amplitude of forces that were applied posterior to the plane of movement of the CTr joint (r2 = 0.99) but only weakly reflected the magnitude of forces in the joint plane.
3.4. Response properties of femoral campaniform sensilla in stick insects
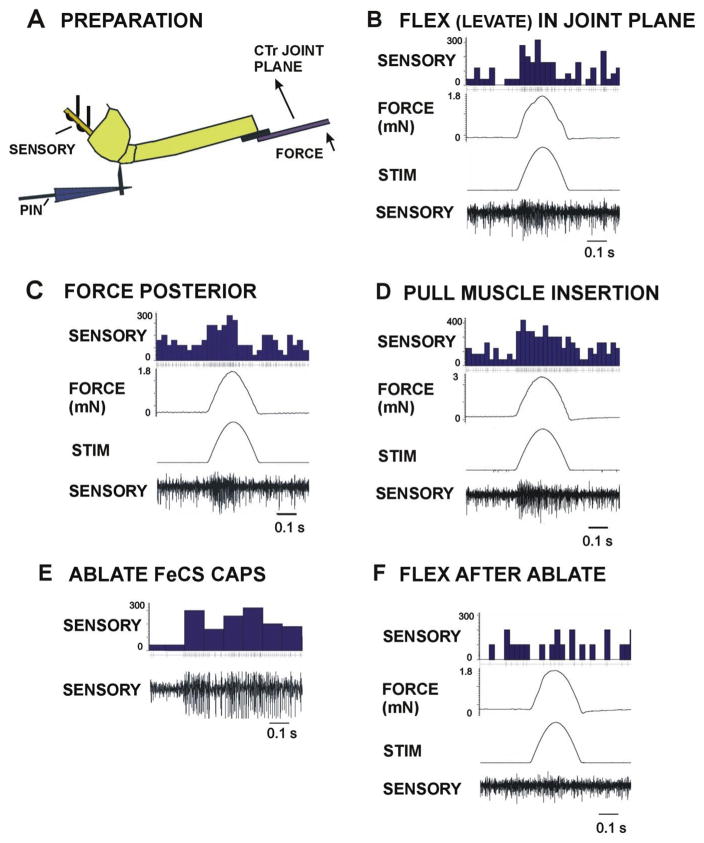
Similar tests were performed to characterize the responses of the femoral campaniform sensilla in stick insects (Fig. 6A). Vigorous multi-unit discharges occurred both to forces in the plane of movement of the CTr joint (Fig. 6B) and to forces applied in a posterior direction (Fig. 6C). In stick insects, we also mimicked the effects of contractions of the depressor muscle by applying forces to the motor that held the pin inserted into the depressor insertion (Zill et al., 2012). Intense discharges occurred to resisted muscle forces (Fig. 6D). We were able to confirm that the recordings were derived from the femoral campaniform sensilla by ablating the caps of the receptors (Fig. 6E) which eliminated all discharges to imposed forces (Fig. 6F).
Fig. 6. Responses of stick insect femoral campaniform sensilla to imposed forces.
A. Preparation. The coxa of an intact stick insect left middle leg was mounted via staples and distal segments removed at the femoro-tibial joint. Forces (FORCE) were applied to the distal femur in different directions with movement is resisted by a pin (PIN) inserted into the proximal trochanter. The pin was attached to a motor to mimic contractions of the depressor muscle. Sensory activity was recorded from the main leg nerve with oil hook electrodes (SENSORY) proximal to the coxa (nerve crushed proximally). All trochanteral groups were ablated but the FeCS remained intact. B. Flexion (Levation) in the coxo-trochanteral joint plane – Forces applied to distal femur in the plane of movement of the CTr joint produced intense sensory discharges. C. Forces perpendicular (posterior) to the joint plane – Vigorous firing was also obtained to forces perpendicular to joint plane applied in a posterior direction. D. Pull on depressor insertion – Forces applied at the depressor insertion (via the pin and motor) also produced sensory discharges of similar amplitudes. E. Ablations of the caps of the femoral campaniform sensilla produced intense firing. F. No sensory responses were seen when forces were applied to the leg after cap ablation. (Sensory histograms in CD: potentials/second).
Fig. 7A and B are histograms of the responses of stick insect femoral campaniform sensilla to forces applied in the joint plane (Fig. 7A, n = 146 tests in N = 3 animals) and to forces directed posteriorly (Fig. 7B, n = 156 tests in the same preparations). Intense firing occurred during the rising phase of the stimulus to both directions of force. In some preparations, smaller discharges also occurred during the phase of decrease of forces in the joint plane (Fig. 7A). We also applied stimuli as ramp and hold functions to study encoding of force magnitude. Fig. 7C is a plot of the discharges that occurred to forces in the joint plane and in a posterior direction (n = 65 tests in plane, n = 70 tests posterior, each point is the mean value of 5 successive tests, N = 1). Forces of low amplitude were used to minimize the effects of spike summation in the multiunit recording. The discharges generally reflect the amplitude of applied force (r2 = 0.88 in plane, r2 = 0.65 posterior). Fig. 7D is a plot of the mean discharge that was elicited to forces applied to the depressor muscle insertion (each point is the mean of n = 46 tests from N = 3 preparations). The femoral sensilla thus encode both the muscle forces and load in the plane of joint movement, similar to the responses obtained from the trochanteral campaniform sensilla in stick insects and cockroaches (Zill et al., 2012).
Fig. 7. Plots of responses and encoding of load and muscle forces in stick insect femoral campaniform sensilla.
A. and B. Cumulative plots of sensory discharges to forces applied in the plane of the CTr joint in the direction of joint flexion (levation) and in a posterior direction perpendicular to the joint plane. Intense discharges were elicited both in a posterior direction and in the plane of the CTr joint. C. Encoding of load – Plot of the mean sensory firing frequencies to forces applied as ramp and hold functions to forces in the joint plane (flexion) and perpendicular to the plane. The femoral sensilla effectively encode force magnitude in the plane of the coxo-trochanteral joint and in a posterior direction perpendicular to the plane. D. Encoding of muscle force – Responses to depressor muscle forces were obtained by applying forces to the pin in the trochanter that were resisted in the distal femur by the force probe. The mean discharge frequency effectively encoded the muscle forces.
3.5. Force detection and effects upon muscle synergies
3.5.1. Cockroaches
Our previous study showed that forces applied in the plane of the CTr joint produced activation of the tibial flexor muscle (Zill et al., 2015a). Ablation experiments indicated that this effect was mediated by the trochanteral campaniform sensilla. In the present experiments, we recorded flexor activity when forces were first imposed in the joint plane (Fig. 8A) and then directed posteriorly (Fig. 8B) in the same preparations. While forces in the plane of the coxo-trochanteral joint produce intense firing little or no discharge was seen to forces applied in a posterior direction. This difference in flexor activation is also apparent in cumulative plots in Fig. 8C and D. A small transient discharge was present to posterior forces but the source is unclear as all groups of trochanteral sensilla and other receptors remained intact in these preparations. However, these findings support the idea that the femoral campaniform sensilla, which potently discharge to posterior forces, play little or no role in synergist activation in cockroaches.
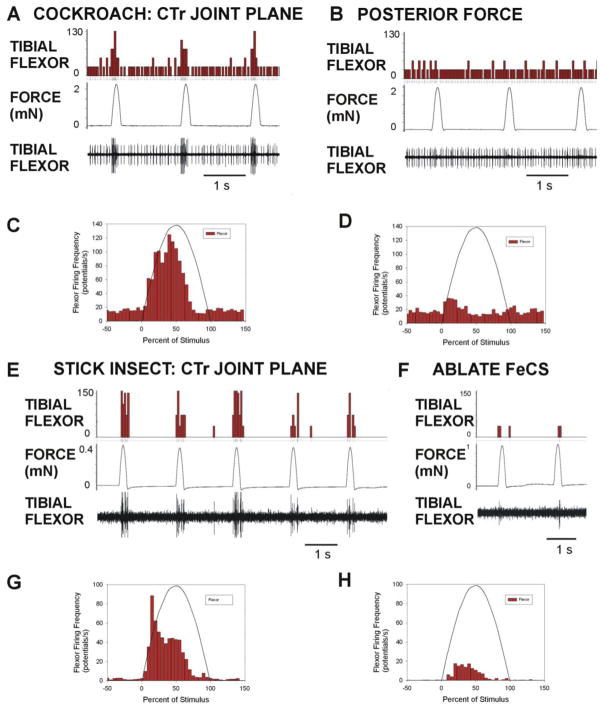
Fig. 8. Evaluation of contribution of femoral campaniform sensilla to synergist discharges in the tibial flexor muscle in cockroaches and stick insects.
Forces were applied to the femur in preparations in which the main leg nerve was not severed and motor innervation remained intact. A. and B. Forces applied in cockroaches in the plane of the CTr joint produced intense bursts in the tibial flexor muscle (A) but did not occur to forces directed posteriorly (B). C. and D. Histograms of mean responses in synergist muscle – Activation of synergist discharge in tibial flexor muscle was elicited by forces in the joint plane (C, n = 323 repetitions from N = 3 preparations) but only small transient firing was present in some preparations to posterior forces (D, n = 279 repetitions from the same animals). E. and F. Effects of ablation of femoral campaniform sensilla in stick insects – Forces applied in the CTr joint plane in stick insects produced bursts of activity in the flexor muscle in intact preparations (E). Only weak discharges occurred if the femoral campaniform sensilla had been ablated prior to the tests (F). G. and H. Histograms of flexor activation – Flexor activation to forces in the plane of the CTr joint (F) was greatly reduced but not entirely eliminated by ablation of the femoral campaniform sensilla (G, n = 174 tests in N = 3 animals; H, n = 190 tests in N = 3 animals). (Flexor muscle histograms in A, B, D, F: muscle potentials/second).
3.5.2. Stick insect groups that mediate activation of synergists
Previous studies by Akay et al. (2001) showed that application of forces (via a pen motor) that produced movements of the CTr joint toward levation produced flexor activation. Ablation experiments demonstrated that this effect was attributed to the femoral campaniform sensilla. Akay et al. (2001) also tested the effects of ablation of the femoral sensilla on activities of the flexor during stepping. Flexor bursts were initiated at a longer latency after ablation (see also Schmitz et al., 2015) but responses persists, implying that other sense organs or groups of campaniform sensilla could contribute to flexor activation. In the present study, we quantitatively compared the flexor activation produced by the motor in intact animals and in preparations in which the femoral campaniform sensilla had been ablated. As is reflected in both individual recordings (Fig. 8E and F) and pooled data (Fig. 8G and H), flexor activation was greatly reduced but not invariably eliminated by ablation of the femoral campaniform sensilla. These results confirm that the FeCS mediate flexor activation but indicate that other groups of campaniform sensilla may make a contribution to synergist recruitment in stick insects.
4. Discussion
These experiments characterized the structure and responses of the femoral campaniform sensilla in cockroaches and stick insects. Few previous studies have examined the response properties of the femoral sensilla (Akay et al., 2001; Akay, 2002), although they have been shown to be important in establishing interjoint coordination of muscle activities (Schmitz et al., 2015). The specific goal of the present studies was to test the hypothesis that the species-specific differences in effects of the femoral sensilla on muscles synergies were associated with differences in the forces detected by receptors (Akay et al., 2001; Zill et al., 2015a). We have shown that femoral campaniform sensilla of stick insects, which activate muscle synergies, effectively encode forces in the plane of action of the large proximal intrinsic leg muscles (CTr joint plane). In cockroaches, femoral receptors do not encode forces in the CTr joint plane and the effects on synergies are mediated, instead, by trochanteral campaniform sensilla. In the following, we will discuss 1) how specializations in leg structure and force transmission could contribute to differences in receptor responses and 2) how specificity in force detection could be a determining factor in activation of muscle synergies (Ross and Nichols, 2009).
4.1. Specializations of the trochanter-femur (TrF) joint
A major difference in the design of the legs of cockroaches and stick insects is in the mobility of the TrF joint (Schindler, 1979; Watson et al., 2002). In cockroaches, the articulation is a mobile and asymmetric hinged joint, with cuticular linkage only through condyles on its anterior surface. In stick insects, the joint is immobile, permitting transmission of cuticular strains over a broader area of the articulation. The variations in structure and adaptive capabilities of the trochanter-femur joint in a number of insect species have been elegantly and extensively analyzed by Frantsevich and Wang (2009). They found that in most insects the TrF joint is moveable in a plane that is approximately orthogonal to the plane of the coxo-trochanteral (CTr) joint. The range of movement of the TrF joint is often small (<20°) in comparison to the extensive movements of the CTr or femoro-tibial (FT) joints (although in some species it is much larger, ex. wasp Ammophila, 50–60°). Frantsevich and Wang (2009) suggested that mobility of the TrF joint provides dynamic stability in legged locomotion by acting as a spring to dampen external forces.
4.1.1. Mobility and elasticity of the cockroach TrF joint
Movements of the cockroach TrF joint are generated by a single muscle, the femoral reductor muscle, which acts to draw the femur posteriorly (Pringle, 1938). Previous studies have suggested that elastic elements in the joint serve as an antagonist to the reductor (Watson et al., 2002). Our study has presented evidence that the TrF joint membrane is a elastic composite that can serve as a muscle antagonist (Neff et al., 2000): 1) the membrane is reversibly stretched during movements imposed in a posterior direction, 2) the joint shows rapid elastic recoil to the default position after displacement; 3) the joint membrane shows blue fluorescence and toluidine blue staining, consistent with the presence of resilin. Elastic structures that act as muscle antagonists have been identified in the legs of cockroaches (Frazier et al., 1999; Neff et al., 2000; Picker et al., 2012), stick insects (Bässler, 1983), beetles (Ichikawa et al., 2016; Nadein and Betz, 2016) and other insects (Burrows and Sutton, 2012; Michels and Gorb, 2012). These structures minimize inertia (Weis-Fogh, 1960) and can also potentially serve to dampen the effects of postural perturbations (Noah et al., 2004; Dudek and Full, 2009). The findings of the present study, therefore, support the hypothesis of Frantsevich and Wang (2009) that the elastic elements at the TrF joints can act as a spring or ‘shock absorber’.
Previous studies have also shown that, in cockroaches, the mobility of the TrF joint serves an important adaptive function in redirecting forces generated by the leg (Watson et al., 2002; Bender et al., 2010). The hind legs (and to a lesser extent the middle legs) in cockroaches are oriented at acute angles to the substrate which maximizes forces generated by the rear legs in propulsion for escape running (Hughes, 1952). In these legs, movements at the body coxa joint are limited, an adaptation that functionally provides a stable base for force generation by coxal muscles. However, this adaptation also restricts the extent to which force direction can be changed, as when the animal surmounts an obstacle (Harley et al., 2009). This limitation is overcome by relatively small movements at the TrF joint that produce significant changes in the orientation and plane of action of the distal leg segments (Watson et al., 2002). Similar effects are found in other insect appendages, in which contraction of relatively small basal muscles produces large changes in force direction (wings: Hedenström, 2014; front legs: Szczecinski et al., 2015).
4.1.2. Stick insect
In stick insects the TrF joint is functionally fused and lacks specialized condyles for force transmission. Instead, the articulation of the trochanter and femur interlocks the segments, particularly on its dorsal aspect (Schindler, 1979). These features should provide high stability, which may be essential as the body coxa joints in stick insects are highly mobile (Cruse and Bartling, 1995; Dallmann et al., 2016). This mobility permits the forces generated by legs to be directed in a number of orientations relative to the body. Recent studies, that have measured the ground reaction forces in freely moving stick insects, have shown that, unexpectedly, the largest torques generated in walking occur at the CTr joint (Dallmann et al., 2016). Extensive rotational forces also occur (pronation/supination) that could be damaging if the TrF joint were mobile. Stick insects are large, plant-dwelling insects and loading could readily occur in a number of orientations. Immobility of the TrF joint is also found in other large insects in legs in which the body-coxa joint shows great mobility (crickets: Hustert, 1982; locusts, Laurent and Richard, 1986).
However, the TrF joint is also the site of leg autotomy, in which distal segments of the leg are shed as a defensive response when the appendage is grasped by a predator (Cardé, 2009) or as consequence of leg damage in molting (Maginnis, 2008). The specific mechanisms underlying autotomy in insects are unclear (Schmidt and Grund, 2003). Schindler (1979) considered that autotomy was initiated by twisting of the leg, accompanied by contraction of small internal muscle fibers that produced elevation of the dorsal segment of the trochanter (lid). Our morphological studies showed the levator trochanteris muscle inserts upon the lid and suggest that the levator could also readily contribute to autotomy.
4.2. Force (strain) transmission and response properties of femoral campaniform sensilla
4.2.1. Cockroach
The cockroach FeCS are a relatively small group of receptors that are located in a region of cuticle that lacks the morphological specializations (thickenings, sclerotization) found associated with many other groups of campaniform sensilla (Zill et al., 2000, 2012). Because the condyles of the TrF joint are on the anterior side of the femur, the sensilla are relatively isolated from strains produced by the large coxal and body wall muscles which generate support and propulsion (Kaliyamoorthy et al., 2006).
We found that the cuticular caps of cockroach FeCS have a gradient of sizes but a single consistent orientation, with long axes approximately parallel to TrF joint axis. The position and orientation of the receptors should make them specifically able to monitor the forces generated at the TrF joint (Pringle, 1938). This was confirmed by recordings of the sensilla to imposed forces: the receptors vigorously and effectively encode forces applied posteriorly to the femur while only responding weakly to forces in the CTr joint plane. What are the functions of the cockroach femoral sensilla? Posterior forces could occur at the TrF joint during leg use in walking as the reductor femoris is reported to be activated near the initiation of the stance phase when the joint at an acute angle to the substrate (Bender et al., 2010). The sensilla may also be particularly important and adaptive when the TrF joint is fully engaged in climbing over obstacles (Watson et al., 2002; Harley et al., 2009). Signals from the campaniform sensilla could function adaptively both to resist perturbations and to prevent accidental autotomy. The latter hypothesis is supported by the observation that the cockroach FeCS have very large spikes (Fig. 4D) and, potentially, fast conducting axons. Thus, information from the femoral CS in cockroaches may contribute to the adaptation of leg use even though they do not encode the major forces generated in propulsion. It is also important to note that other receptors are present at the cockroach TrF joint that could signal position and movement (Guthrie, 1967).
4.2.2. Stick insect
In contrast to the cockroach FeCS, our morphological studies showed that extensive modifications of the cuticle surrounding the FeCS in stick insects that could enhance their sensitivity to forces developed by coxal muscles. The stick insect femoral campaniform sensilla are also larger in number and more diverse in shape and orientation of the cuticular caps. We identified subgroups of sensilla based upon their location and cap orientation: a dorsal group had caps at a small angle relative to autotomy plane; ventral group with cap long axes at greater angles (close to 45°) to the plane. In addition some sensilla located centrally had large, rounded caps. The locations of sensilla with round caps were somewhat variable and overlapped with the sensilla of other cap orientations at the center of the group (the curvature of the depression in the femur complicated quantification of the variability). However, the difference in orientations of the dorsal and ventral subgroups was consistent and most apparent in regions away from the center of the depression (Fig. 3E). Similar variation in cap orientation and shape has been reported in FeCS of other insects (moth: Kent and Griffin, 1990; flies: Gnatzy et al., 1987; Zill et al., 2015b).
The diversity in orientation of the receptors was also reflected in the responses of the stick insect femoral group to imposed forces: vigorous discharges were obtained both to forces in the plane of the CTr joint and to forces directed posterior to the long axis of the femur. Afferent discharges also occurred to forces which mimicked resisted contractions of the trochanteral depressor muscle. We were unable to determine whether these sensitivities were correlated with specific subgroups of receptors or whether the round sensilla contributed to both discharges. However, it is important to note that the orientation of the ventral subgroups of receptors (~45°) could make these sensilla sensitive to forces that produce twisting (rotation about the long axis) of the femur (Higdon et al., 1967). These types of forces may occur during supination/pronation movements (Dallmann et al., 2016) although this was not directly tested due to technical constraints.
The responses of stick insect FeCS in the CTr joint plane are consistent with previous observations that the receptors mediate, in part, activation of flexor motor neurons in response to levation movements of the femur (Akay et al., 2001; Akay, 2002). The sensitivity of the FeCS to forces applied at the depressor muscle insertion may also contribute to their effects in stepping. In walking, depressor muscle activity lowers the leg to the substrate during the swing phase (Rosenbaum et al., 2010). Activation of the tibial flexor muscle occurs after leg contact and resistance to depressor forces that should produce activation of the FeCS. This idea is supported by the finding that ablation of the FeCS produced a decrease in the magnitude of flexor muscle activity (Akay et al., 2001) and affected timing of the onset of flexor bursts (Schmitz et al., 2015).
4.3. Effects on muscle synergies are correlated with receptor response sensitivity
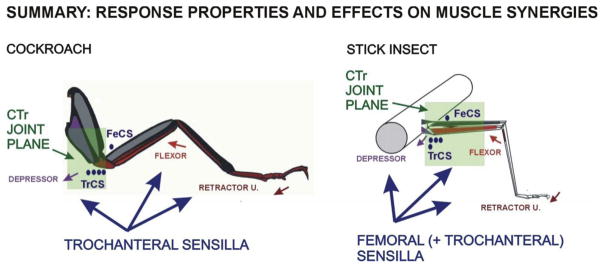
The present study has shown that the effects of campaniform sensilla upon muscle synergies are correlated with their sensitivity to forces in the coxo-trochanteral joint plane (Summary, Fig. 9). In both stick insect and cockroach legs, there is a proximal to distal gradient in the size of intrinsic leg muscles: muscles acting at the coxo-trochanteral joints produce the largest forces (torques) generated in support and propulsion (Pearson and Iles, 1971; Schmitz, 1986; Toth et al., 2013). In both species, groups of trochanteral campaniform (Groups 3, 4) detect the net forces at the CTr and more proximal body coxa joints (Zill et al., 1999, 2012). However, signals from the femoral sensilla are species-specific: the femoral CS in cockroaches signal forces only at the mobile TrF joint while stick insect femoral CS are also sensitive to forces in the CTr plane. In stick insects, the CTr and FT joints form a single leg plane, due to immobility of the TrF joint (Cruse and Bartling, 1995). In addition, caps of stick insects FeCS are oriented at a variety of angles (and include round caps) so they may also be sensitive to strains produced both by the depressor and levator muscles. The greater mobility of the stick insect body coxa joint may require the recruitment of synergist muscles for grip (flexor, retractor) when the animal is inverted and pulls (using the levator) instead of pushing (with the depressor muscles) with proximal leg muscles (Duch and Pflüger, 1995; Mendes et al., 2014; Zill et al., 2015b). This hypothesis is supported by preliminary experiments and will be tested in future studies.
Fig. 9. Summary diagram of force receptors and muscle synergies in stick insects and cockroaches.
Activation of muscle synergists is correlated with response sensitivity to forces in the CTr joint plane. See text for discussion.
These findings, more generally, show that the effects of force detecting sense organs on muscle synergists are not homogeneous but depend upon the specific forces that the sense organs encode. Some formulations of control of posture and walking model the effects of force receptors but do not specify the specific forces they encode (antigravity load, propulsion, grip, etc.; Schilder, 2016). That information may be essential in understanding how activation of muscles asgroups of synergists emerges from theinteraction ofbrain, intersegmental as well as local feedback (Ritzmann and Zill, 2017).
These results also warn against assuming that receptors at similar locations have the same effects in walking systems of different animals (Ayali et al., 2015; Isakov et al., 2016). Differences in the specific forces that are encoded by sense organs may also be important in evaluating the effects of receptors in vertebrates such as Golgi tendon organs (Nichols, 1999). Studies of the effects of force feedback in spontaneous locomotion of cats have shown that effects on muscle synergists are not monotonic but depend upon the specific muscle groups from which the force signals are derived, potentially in a proximal to distal gradient (Ross and Nichols, 2009). The determination of specificity of effects on muscle groups based upon sensory responsiveness may be utilized and simplify the activation of muscles as synergists in both vertebrates and invertebrates (Kargo and Giszter, 2000; Hart and Giszter, 2010; Kistemaker et al., 2013).
Acknowledgments
Funding
Support: EICCI from the Cluster of Excellence 227 (CITEC), DFG; DFG grant BU 857-14; NSF grant MRI 0959012. The authors thank one of the reviewers for helpful comments.
References
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol. 2001;85:594–604. doi: 10.1152/jn.2001.85.2.594. [DOI] [PubMed] [Google Scholar]
- Akay T. Ph D Thesis. University of Cologne; 2002. The Role of Sensory Signals for Interjoint Coordination in Stick Insect Legs (Carausius morosus and Cuniculina impigra) [Google Scholar]
- Ayali A, Borgmann A, Büschges A, Couzin-Fuchs E, Daun-Gruhn S, Holmes P. The comparative investigation of the stick insect and cockroach models in the study of insect locomotion. Curr Opin Insect Sci. 2015;12:1–10. [Google Scholar]
- Bässler U. Neural Basis of Elementary Behavior in Stick Insects. Springer; Berlin: 1983. [Google Scholar]
- Bässler U, Rohrbacher J, Karg G, Breutel G. Interruption of searching movements of partly restrained front legs of stick insects, a model situation for the start of a stance phase? Biol Cybern. 1991;65:507–514. [Google Scholar]
- Bender JA, Simpson EM, Ritzmann RE. Computer-assisted 3D kinematic analysis of all leg joints in walking insects. PLoS One. 2010;5(10):e13617. doi: 10.1371/journal.pone.0013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M, Sutton GP. Locusts use a composite of resilin and hard cuticle as an energy store for jumping and kicking. J Exp Biol. 2012;215:3501–3512. doi: 10.1242/jeb.071993. [DOI] [PubMed] [Google Scholar]
- Carbonell CS. The thoracic muscles of the cockroach Periplaneta americana (L.) Smithson Misc Coll. 1947;107:1–32. [Google Scholar]
- Cardé RT. Autotomy. In: Resh Vincent H, Cardé Ring T., editors. Encyclopedia of Insects. 2. Chapter 17. Academic Press; San Diego: 2009. [Google Scholar]
- Chapman KM, Duckrow RB, Moran DT. Form and role of deformation in excitation of an insect mechanoreceptor. Nature. 1973;244:453–454. doi: 10.1038/244453a0. [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci. 2013;7:48. doi: 10.3389/fncom.2013.00048. http://dx.doi.org/10.3389/fncom.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse H, Bartling Ch. Movement of joint angles in the legs of a walking insect, Carausius morosus. J Insect Physiol. 1995;41:761–771. [Google Scholar]
- Dallmann CJ, Dürr V, Schmitz J. Joint torques in a freely walking stick insect reveal distinct functions of leg joints in propulsion and postural control. Proc R Soc B. 2016 Jan 27;283:20151708. doi: 10.1098/rspb.2015.1708. http://dx.doi.org/10.1098/rspb.2015.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Pflüger HJ. Motor patterns for horizontal and upside-down walking and vertical climbing in the locust. J Exp Biol. 1995;198:1963–1976. doi: 10.1242/jeb.198.9.1963. [DOI] [PubMed] [Google Scholar]
- Dudek DM, Full RJ. An isolated leg’s passive recovery from dorso-ventral perturbations. J Exp Biol. 2009;210:3209–3217. doi: 10.1242/jeb.008367. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load regulating mechanisms in gait and posture, comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, De Groote F, Jonkers I. The flexion synergy, mother of all synergies and father of new models of gait. Front Comput Neurosci. 2013;7:14. doi: 10.3389/fncom.2013.00014. http://dx.doi.org/10.3389/fncom.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurons caused by impulses in Golgi tendon organ afferents. J Physiol. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantsevich L, Wang W. Gimbals in insect legs. Arthropod Struct Dev. 2009;38:16–30. doi: 10.1016/j.asd.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Frazier S, Larsen G, Neff D, Quimby L, Carney M, DiCaprio R, Zill S. Elasticity and movements of the cockroach tarsus in walking. J Comp Physiol A. 1999;185:157–172. [Google Scholar]
- Gnatzy W, Grünert U, Bender M. Campaniform sensilla of Calliphora vicina (Insecta, Diptera) I Topography Zoomorphol. 1987;106:312–319. [Google Scholar]
- Gopalakrishnan A, Modenese L, Phillips AT. A novel computational framework for deducing muscle synergies from experimental joint moments. Front Comput Neurosci. 2014;8:153. doi: 10.3389/fncom.2014.00153. http://dx.doi.org/10.3389/fncom.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorb SN. Biological attachment devices: exploring nature’s diversity for biomimetics. Philos Trans R Soc A. 2008;366:1557–1574. doi: 10.1098/rsta.2007.2172. [DOI] [PubMed] [Google Scholar]
- Guthrie DM. Multipolar stretch receptors and the insect leg reflex. J Insect Physiol. 1967;13:1637–1644. [Google Scholar]
- Hagio S, Kouzaki M. The flexible recruitment of muscle synergies depends on the required force-generating capability. J Neurophysiol. 2014;112:316–327. doi: 10.1152/jn.00109.2014. [DOI] [PubMed] [Google Scholar]
- Harley CM, English BA, Ritzmann RE. Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J Exp Biol. 2009;212:1463–1476. doi: 10.1242/jeb.028381. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Johannisson T. Shared reflex pathways of Group I affterents of different cat hind-limb muscles. J Physiol. 1983;338:113–127. doi: 10.1113/jphysiol.1983.sp014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci. 2010;30:1322–1336. doi: 10.1523/JNEUROSCI.5894-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenström A. How insect flight steering muscles work. PLoS Biol. 2014;12:e1001822. doi: 10.1371/journal.pbio.1001822. http://dx.doi.org/10.1371/journal.pbio.1001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon A, Ohlsen EH, Stiles WB, Weese JA. Mechanics of Materials. 2. Wiley; New York: 1967. [Google Scholar]
- Hofmann T, Bässler U. Anatomy and physiology of trochanteral campaniform sensilla in the stick insect, Cuniculina impigra. Physiol Entomol. 1982;7:413–426. [Google Scholar]
- Hofmann T, Bässler U. Response characteristics of single trochanteral campaniform sensillae in the stick insect, Cuniculina impigra. Physiol Entomol. 1986;11:17–21. [Google Scholar]
- Hughes GM. The coordination of insect movements. I The walking movements of insects. J Exp Biol. 1952;29:267–284. [Google Scholar]
- Hustert R. The proprioceptive function of a complex chordotonal organ associated with the mesothoracic coxa in locusts. J Comp Physiol. 1982;147:389–399. [Google Scholar]
- Ichikawa T, Toh Y, Sakamoto H. Structure and function of the elastic organ in the tibia of a tenebrionid beetle. Naturwissenschaften. 2016;103:41. doi: 10.1007/s00114-016-1363-2. [DOI] [PubMed] [Google Scholar]
- Isakov A, Buchanan SM, Sullivan B, Ramachandran A, Chapman JK, Lu ES, Mahadevan L, de Bivort B. Recovery of locomotion after injury in Drosophila melanogaster depends on proprioception. J Exp Biol. 2016;219:1760–1771. doi: 10.1242/jeb.133652. [DOI] [PubMed] [Google Scholar]
- Kaliyamoorthy S, Zill SN, Quinn RD. Force sensors in hexapod locomotion. In: Kordic V, Laznica A, Merdan M, editors. Mobile Robotics: Moving Intelligence. Advanced Robotic Systems; Vienna, Austria: 2006. pp. 495–512. [Google Scholar]
- Kargo WJ, Giszter SF. Afferent roles in hind-limb wipe-reflex trajectories: free-limb kinematics and motor patterns. J Neurophysiol. 2000;83:1480–1501. doi: 10.1152/jn.2000.83.3.1480. [DOI] [PubMed] [Google Scholar]
- Keller BR, Duke EF, Amer AS, Zill SN. Tuning posture to body load: decreases in load produce discrete sensory signals in the legs of freely standing cockroaches. J Comp Physiol A. 2007;193:881–891. doi: 10.1007/s00359-007-0241-y. [DOI] [PubMed] [Google Scholar]
- Kistemaker DA, Knock Van Soest AJ, Wong JD, Kurtzer I, Gribble PL. Control of position and movement is simplifled by combine muscle spindle and Golgi tendon organ feedback. J Neurophysiol. 2013;109:1126–1139. doi: 10.1152/jn.00751.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent KS, Griffin LM. Sensory organs of the thoracic legs of the moth Manduca sexta. Cell Tiss Res. 1990;259:209–223. doi: 10.1007/BF00318442. [DOI] [PubMed] [Google Scholar]
- Laine CM, Martinez-Valdes E, Falla D, Mayer F, Farina D. Motor neuron pools of synergistic thigh muscles share most of their synaptic input. J Neurosci. 2015;35:12207–12216. doi: 10.1523/JNEUROSCI.0240-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Richard D. The organization and role during locomotion of the proximal musculature of the cricket foreleg. I Anatomy and innervation. J Exp Biol. 1986;123:255–283. [Google Scholar]
- Maginnis TM. Autotomy in a stick insect (Insecta: Phasmida): predation versus molting. Fla Entomol. 2008;91:126–127. [Google Scholar]
- Mendes CS, Rajendren SV, Bartos I, Márka S, Mann RS. Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PLoS One. 2014;9:e109204. doi: 10.1371/journal.pone.0109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels J, Gorb SN. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J Microsc. 2012;245:1–16. doi: 10.1111/j.1365-2818.2011.03523.x. [DOI] [PubMed] [Google Scholar]
- Moran DT, Chapman KM, Ellis RA. The fine structure of cockroach cam-paniform sensilla. J Cell Biol. 1971;48:155–173. doi: 10.1083/jcb.48.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadein K, Betz O. Jumping mechanisms and performance in beetles. I Flea beetles (Coleoptera: Chrysomelidae: Alticini) J Exp Biol. 2016;219:2015–2027. doi: 10.1242/jeb.140533. [DOI] [PubMed] [Google Scholar]
- Neff D, Frazier SF, Quimby L, Wang R, Zill S. Identification of resilin in the leg of the cockroach: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Struct Dev. 2000;29:75–83. doi: 10.1016/s1467-8039(00)00014-1. [DOI] [PubMed] [Google Scholar]
- Noah JA, Quimby L, Frazier SF, Zill SN. Sensing the effect of body load in legs: responses of tibial campaniform sensilla to forces applied to the thorax in freely standing cockroaches. J Comp Physiol A. 2004;190:201–215. doi: 10.1007/s00359-003-0487-y. [DOI] [PubMed] [Google Scholar]
- Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol. 1999;81:467–478. doi: 10.1152/jn.1999.81.2.467. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Ross KT. The implications of force feedback for the lambda model. Adv Exp Med Biol. 2009;629:663–679. doi: 10.1007/978-0-387-77064-2_36. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Iles JF. Innervation of coxal depressor muscles in the cockroach, Periplaneta americana. J Exp Biol. 1971;54:215–232. doi: 10.1242/jeb.54.1.215. [DOI] [PubMed] [Google Scholar]
- Petryszak A, Fudalewicz-Niemczyk A. External proprioceptors on the legs of insects of higher order. Acta Biol Cracoviensia Ser Zool. 1994;36:13–22. [Google Scholar]
- Picker M, Colville JF, Burrows M. A cockroach that jumps. Biol Lett. 2012;8:390–392. doi: 10.1098/rsbl.2011.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JWS. Proprioception in insects. II the action of the campaniform sensilla on the legs. J Exp Biol. 1938;15:114–131. [Google Scholar]
- Ritzmann RE, Zill SN. Control of locomotion in hexapods. In: Byrne John H., editor. The Oxford Handbook of Invertebrate Neurobiology. Oxford University Press; Oxford: 2017. pp. 1–28. http://dx.doi.org/10.1093/oxfordhb/9780190456757.013.20. [Google Scholar]
- Rosenbaum P, Wosnitza A, Büschges A, Gruhn M. Activity patterns and timing of muscle activity in the forward walking and backward walking stick insect Carausius morosus. J Neurophysiol. 2010;104:1681–1695. doi: 10.1152/jn.00362.2010. [DOI] [PubMed] [Google Scholar]
- Ross KT, Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol. 2009;101:184–197. doi: 10.1152/jn.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error. J Neurophysiol. 2013;110:1278–1290. doi: 10.1152/jn.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder RJ. How do animals know how much they weight? J Exp Biol. 2016;219:1275–1282. doi: 10.1242/jeb.120410. [DOI] [PubMed] [Google Scholar]
- Schindler G. Funktionsmorphologische Untersuchungen zur Autotomie der Stabheuschrecke Carausius morosus Br. (Insecta: Phasmida) Zool Anz. 1979;203:316–326. [Google Scholar]
- Schmidt J, Grund M. Rhythmic activity in a motor axon induced by axotomy. NeuroReport. 2003;14:1267–1271. doi: 10.1097/00001756-200307010-00016. [DOI] [PubMed] [Google Scholar]
- Schmitz J. The depressor trochanteris motoneurones and their role in the coxotrochanteral feedback loop in the stick insect Carausius morosus. Biol Cybern. 1986;55:25–34. [Google Scholar]
- Schmitz J. Load-compensating reactions in the proximal leg joints of stick insects during standing and walking. J Exp Biol. 1993;183:15–33. [Google Scholar]
- Schmitz J, Büschges A, Delcomyn F. An improved electrode design for en passant recording from small nerves. Comp Biochem Physiol. 1988;91:769–772. doi: 10.1016/0300-9629(88)90963-2. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Dean J, Kittmann R. Central projections of leg sense organs in Carausius morosus (Insecta, Phasmida) Zoomorphol. 1991;111:19–33. [Google Scholar]
- Schmitz J, Gruhn M, Büschges A. The role of leg touchdown for the control of locomotor activity in the walking stick insect. J Neurophysiol. 2015;113:2309–2320. doi: 10.1152/jn.00956.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczecinski NS, Martin JP, Bertsch D, Ritzmann RE, Quinn RD. Neuro-mechanical model of praying mantis explores the role of descending commands in pre-strike pivots. Bioinspiration Biomim. 2015;10:065005. doi: 10.1088/1748-3190/10/6/065005. http://dx.doi.org/10.1088/1748-3190/10/6/065005. [DOI] [PubMed] [Google Scholar]
- Toth TI, Schmidt J, Büschges A, Daun-Gruhn S. A neuro-mechanical model of a single leg joint highlighting the basic physiological role of fast and slow muscle fibres of an insect muscle system. PLoS One. 2013;8:e78247. doi: 10.1371/journal.pone.0078247. http://dx.doi.org/10.1371/journal.pone.0078247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE, Zill SN, Pollack AJ. Control of obstacle climbing in the cockroach, Blaberus discoidalis. I Kinematics J Comp Physiol A. 2002;188:39–53. doi: 10.1007/s00359-002-0277-y. [DOI] [PubMed] [Google Scholar]
- Weis-Fogh T. A rubber-like protein in insect cuticle. J Exp Biol. 1960;37:889–906. [Google Scholar]
- Wile GD, Daltorio KA, Diller ED, Palmer LR, Gorb SN, Ritzmann RE, Quinn RD. Screenbot: walking inverted using distributed inward gripping. Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS’08); Nice, France: IEEE/RSJ; 2008. pp. 1513–1518. [Google Scholar]
- Wong DCC, Pearson RD, Elvin CM, Merritt DJ. Expression of the rubber-like protein, resilin, in developing and functional insect cuticle determined using a Drosophila anti-rec 1 resilin antibody. Dev Dyn. 2012;241:333–339. doi: 10.1002/dvdy.23724. [DOI] [PubMed] [Google Scholar]
- Zill S, Büschges A, Schmitz J. Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A. 2011;197:851–867. doi: 10.1007/s00359-011-0647-4. [DOI] [PubMed] [Google Scholar]
- Zill S, Chaudhry S, Exter A, Büschges A, Schmitz J. Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev. 2014;43:441–455. doi: 10.1016/j.asd.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Zill S, Chaudhry S, Büschges A, Schmitz J. Force feedback reinforces muscle synergies in insect legs. Arthropod Struct Dev. 2015a;44:541–553. doi: 10.1016/j.asd.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Zill SN, Büschges A, Schmitz J, Neff D, Chaudhry S. Common Mechanisms and Specializations in Force Detection and Control in Cockroaches, Stick Insects and Drosophila. Society for Neuroscience. 2015b Abstracts 41 program no. 798.06. [Google Scholar]
- Zill SN, Frazier SF, Neff D, Quimby L, Carney M, DiCaprio R, Thuma J, Norton M. Three dimensional graphic reconstruction of the insect exoskeleton through confocal imaging of endogenous fluorescence. Microsc Res Tech. 2000;48:367–384. doi: 10.1002/(SICI)1097-0029(20000315)48:6<367::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Zill SN, Keller BR, Duke ER. Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking. J Neurophysiol. 2009;101:2297–2304. doi: 10.1152/jn.00056.2009. [DOI] [PubMed] [Google Scholar]
- Zill SN, Ridgel AL, DiCaprio RA, Frazier SF. Load signalling by cockroach trochanteral campaniform sensilla. Brain Res. 1999;822:271–275. doi: 10.1016/s0006-8993(99)01156-7. [DOI] [PubMed] [Google Scholar]
- Zill S, Schmitz J, Büschges A. Load sensing and control of posture and locomotion. Arthropod Struct Dev. 2004;33:273–286. doi: 10.1016/j.asd.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Zill S, Schmitz J, Chaudhry S, Büschges A. Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol. 2012;108:1453–1472. doi: 10.1152/jn.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]