Abstract
A chemical library comprised of nineteen synthesized pyridyl disulfides that emulate the chemical reactivity of allicin (garlic) was evaluated for antimicrobial activity against a panel of pathogenic bacteria. Gram-positive species including vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus (VISA, VRSA) demonstrated the highest level of susceptibility toward analogs with S-alkyl chains of 7–9 carbons in length. Further biological studies revealed that the disulfides display synergy with vancomycin against VRSA, cause dispersal of S. aureus biofilms, exhibit low cytotoxicity, and decelerate S. aureus metabolism. In final analysis, pyridyl disulfides represent a novel class of mechanism-based antibacterial agents that have a potential application as antibiotic adjuvants in combination therapy of S. aureus infections with reduced vancomycin susceptibility.
Keywords: Antibiotics, Disulfides, Narrow-spectrum, Staphylococcus aureus, Allicin, VRSA, MRSA, VISA
1. Introduction
Staphylococcus aureus is a bacterial pathogen of great clinical significance to public health [1]. Antibiotic-resistant forms including methicillin (MRSA), vancomycin-intermediate (VISA), and vancomycin-resistant (VRSA) variants are associated with invasive diseases in healthcare settings [2]. Multidrug-resistant (MDR) MRSA possessing resistance factors for three or more antibiotic classes are further implicated in high rates of antimicrobial treatment failures and chronic infections [1,2]. Consequentially, long-course treatment with first-line vancomycin (VAN) has led to the emergence of VISA and VRSA as impending and formable threats to public health [3]. Alternative antibiotics and treatment strategies therefore need to be developed to contend with these superbug infections.
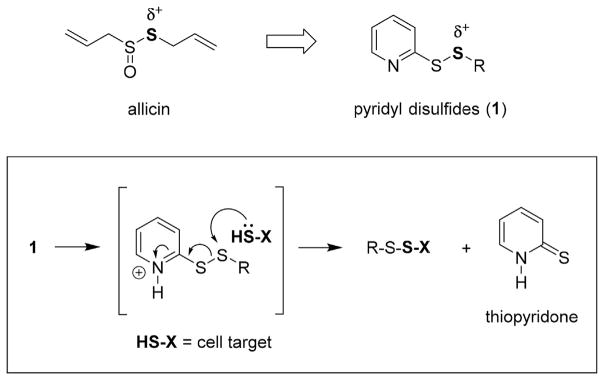
Allicin is a natural thiosulfinate in garlic (Allium sativum) that has been investigated for an extensive range of medicinal applications including the treatment of bacterial infections [4]. Select Gram-positive pathogens including MRSA and Bacillus anthracis (anthrax) exhibit susceptibility to allicin at clinically-relevant minimum inhibitory concentrations (MICs) [5]. In addition, synergism with VAN has been reported for VAN-resistant Enterococcus (VRE) [6]. By chemical nature, allicin is electrophilic (δ+) and reacts with thiophilic, sulfhydryl-baring molecules such as cysteine, glutathione (GSH) and coenzyme A (CoASH) [7]. Multiple mechanisms have been proposed for the antibacterial action of allicin [4]. Each mutually involves a thiol-disulfide exchange reaction between the S-allyl constituent of allicin and the terminal thiol of an intracellular metabolite, coenzymes, or enzyme [4]. Inhibition of fatty acid biosynthesis is thought to be a primary mechanism of growth inhibition in bacteria [8]. Despite exhibiting antimicrobial properties against S. aureus, the chemical and thermal instability of allicin [9] has led to efforts to identify analogs for therapeutic development.
Pyridyl disulfides (1, PySSRs) are electrophilic reagents in the synthesis of asymmetric (mixed) disulfides that mimic the chemical reactivity of allicin (Fig. 1) [10]. At physiological pH, protonation of the pyridyl ring increases reactivity with thiophilic substances to accelerate the thiol-disulfide exchange reaction. With a reaction profile reminiscent of allicin, we embarked on an exploratory investigation to define the antibacterial activity of the mechanism-based PySSRs as antibacterial agents. In this article, the structure activity relationship (SAR) and pharmacodynamic properties of S-(alkylthio)-2-pyridine disulfides are reported.
Fig. 1.
Mechanism of the thiol-disulfide exchange reaction for allicin-inspired pyridyl disulfides (1).
2. Results and discussion
2.1. Chemistry
2.1.1. Synthesis of S-(alkylthio)-2-pyridine disulfides
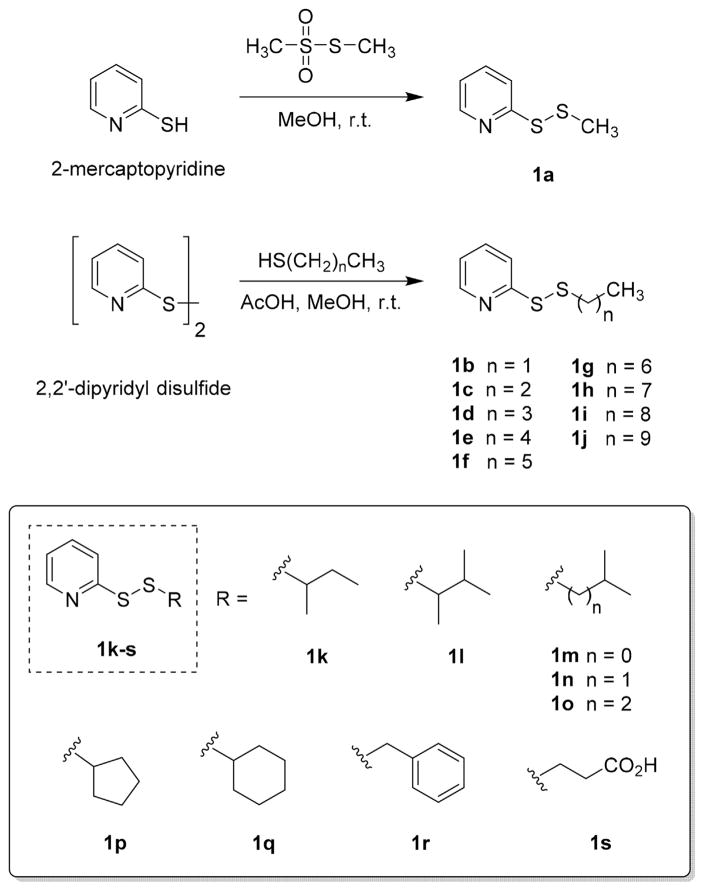
For the study, a focused chemical library consisting of nineteen PySSR members 1a–s (Scheme 1) derived from linear and branched thiols was prepared using classical organic chemistry. With exception of S-(methylthio) analog 1a, the compounds were synthesized by a thiol-disulfide exchange reaction from commercially available 2′2-dipyridyl disulfide and each respective thiol in MeOH [10]. Unlike synthetic allicin [9,11], pyridyl disulfides 1b–s were stable at r.t. following chromatographic purification. The only library member with which significant decomposition occurred after isolation was the S-methyl analog 1a.
Scheme 1.
Chemical library of synthesized pyridyl disulfides (1) used in the study.
2.2. Biological studies
2.2.1. Antibacterial spectrum of activity
Preliminary antibacterial testing was performed by the disc diffusion (Kirby-Bauer) assay [12] using VAN and cefotaxime as comparators. The compounds were evaluated at equimolar concentrations of 10 μM in DMSO against a ten species panel that included five S. aureus variants. The disulfides exhibited narrow spectrum activity against Gram-positive bacteria including MDR S. aureus (Table S1). Only Gram-negative A. baumannii, E. coli, and E. cloacae were weakly or partially inhibited by short chain analogs 1a–f. Significant growth inhibition was indiscriminate for the majority of the PySSR analogs against B. anthracis and S. epidermidis. Conversely, there was a striking correlation between the S-alkyl chain length and zone diameter for S. aureus. Unbranched analogs with chain lengths of 5–9 carbons (1e–i) exhibiting the highest degree of growth inhibition were carried forward for MIC susceptibility testing. Synergism with VAN was also observed in the form of a bridging effect between the neighboring zone of disulfide 1h (Fig. 2) and isobologram analysis was performed by the checkerboard titration assay.
Fig. 2.

Disc diffusion (Kirby-Bauer) assay revealed synergistic activity of pyridyl disulfide 1h and VAN against MRSA 43300.
2.2.2. Anti-MRSA activity
Susceptibility testing by the broth microdilution assay [12] indicated that PySSR derivatives 1g–i of 7–9 carbon chains in length were the most effective growth inhibitors of planktonic S. aureus (Table 1). The MIC range against a panel comprised of six S. aureus variants was 1.56–25 μM (0.8–6.8 μg/mL) compared to 0.39 to >50 μM (1.2–>150 μg/mL) for VAN. Reduced susceptibility was noted for linezolid-resistant S. aureus (LRSA) and heterogeneous VISA (hVISA), but not for VISA and VanA-type VRSA [13] for disulfides 1g and 1h. Additional tests revealed that the disulfides disrupt S. aureus biofilms and exhibited a bacteriostatic profile with MBC:MIC ratios >4, a metric used to delineate bacteriostatic agents [14]. By contrast, VAN with a MBC:MIC ≤2 was confirmed to be bactericidal. In the biofilm reduction (dispersal) assay [15], preformed S. aureus biofilms were treated with 1–5× MIC of disulfide 1h for 20 h and their densities were quantified by crystal violet (CV) staining. Fig. 3 shows that disulfide 1h disrupted staphylococcal biofilms in a dose-dependent manner comparable to VAN.
Table 1.
MIC and MBC data for select disulfides against S. aureus.
| Species | Strain | Test agenta | MICa | MBCa | ratiob |
|---|---|---|---|---|
| MSSA ATCC 25923 |
1e | 50 | – | – |
| 1f | 25 | – | – | |
| 1g | 12.5 | >50 | >4:1 | |
| 1h | 12.5 | >50 | >4:1 | |
| 1i | 12.5 | >50 | >4:1 | |
| VAN | 0.78 | 1.56 | 2:1 | |
|
| ||||
| MRSA ATCC 43300 |
1e | 50 | – | – |
| 1f | 50 | – | – | |
| 1g | 6.25 | >50 | >8:1 | |
| 1h | 6.25 | >50 | >8:1 | |
| 1i | 6.25 | >50 | >8:1 | |
| VAN | 0.39 | 0.78 | 2:1 | |
|
| ||||
| LRSA SA LinR#14 |
1e | >50 | – | – |
| 1f | 50 | – | – | |
| 1g | 25 | – | – | |
| 1h | 25 | – | – | |
| 1i | 25 | – | – | |
| VAN | 0.78 | 1.56 | 2:1 | |
|
| ||||
| hVISA Mu3 ATCC 700698 |
1e | 50 | – | – |
| 1f | 50 | – | – | |
| 1g | 12.5 | >50 | >4:1 | |
| 1h | 12.5 | >50 | >4:1 | |
| 1i | 6.25 | >50 | >8:1 | |
| VAN | 0.78 | 0.78 | 1:1 | |
|
| ||||
| VISA Mu50 ATCC 700699 |
1e | 50 | – | – |
| 1f | 25 | – | – | |
| 1g | 3.13 | 25 | 8:1 | |
| 1h | 3.13 | 25 | 8:1 | |
| 1i | 1.56 | 25 | 16:1 | |
| VAN | 3.13 | 3.13 | 1:1 | |
|
| ||||
| VRSA HIP11714 |
1e | >50 | – | – |
| 1f | 50 | – | – | |
| 1g | 6.25 | >50 | >8:1 | |
| 1h | 6.25 | >50 | >8:1 | |
| 1i | 12.5 | >50 | >4:1 | |
| VAN | >50 | – | – | |
Values reported in μM.
MBC:MIC.
Fig. 3.
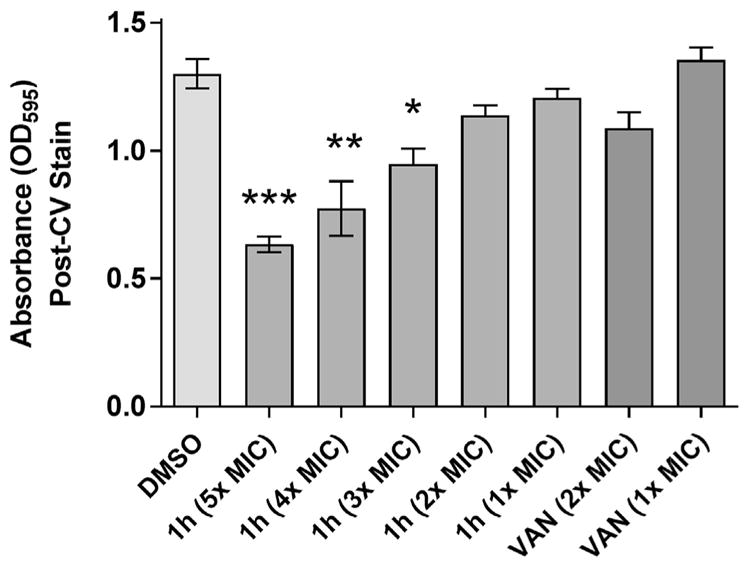
Effects of pyridyl disulfide 1h and VAN on S. aureus biofilm dispersal.
Susceptibility testing was expanded to include additional clinical isolates of VISA and VRSA. The MIC90s for both variants was 12.5 μM for PySSRs 1g–i (Table 2). The compounds notably retained their antibacterial effects against the majority of the VanA-type VRSA strains. Accordingly, synergy testing was performed by isobologram analysis using the classical checkerboard titration assay [16] in 96 well plate format to establish whether the disulfides are able to lower the MIC of VAN in VRSA. In combination with 6.25 μM 1h, susceptibility to VAN increased to MIC 1.56 μM from >50 μM (Fig. 4). The ΣFIC calculated from the MICs for each of the five lowest 1h–VAN combinations equated to ≤0.5, a metric used to define synergism [17].
Table 2.
MIC data for pyridyl disulfide 1e–i against S. aureus.
| Variant (no. strains) | Test agent | MIC50a | MIC90a | Rangea |
|---|---|---|---|---|
| VISA (12) | 1e | 50 | 50 | 50–>50 |
| 1f | 50 | 50 | 25–50 | |
| 1g | 12.5 | 12.5 | 3.13–25 | |
| 1h | 12.5 | 12.5 | 3.13–25 | |
| 1i | 6.25 | 12.5 | 1.56–25 | |
| VAN | 3.13 | 3.13 | 1.56–3.13 | |
|
| ||||
| VRSA (10) | 1e | 50 | 50 | 50–>50 |
| 1f | 50 | 50 | 25–50 | |
| 1g | 6.25 | 12.5 | 3.13–12.5 | |
| 1h | 6.25 | 12.5 | 3.13–12.5 | |
| 1i | 6.25 | 12.5 | 3.13–12.5 | |
| VAN | >50 | >50 | 12.5–>50 | |
Values reported in μM.
Fig. 4.
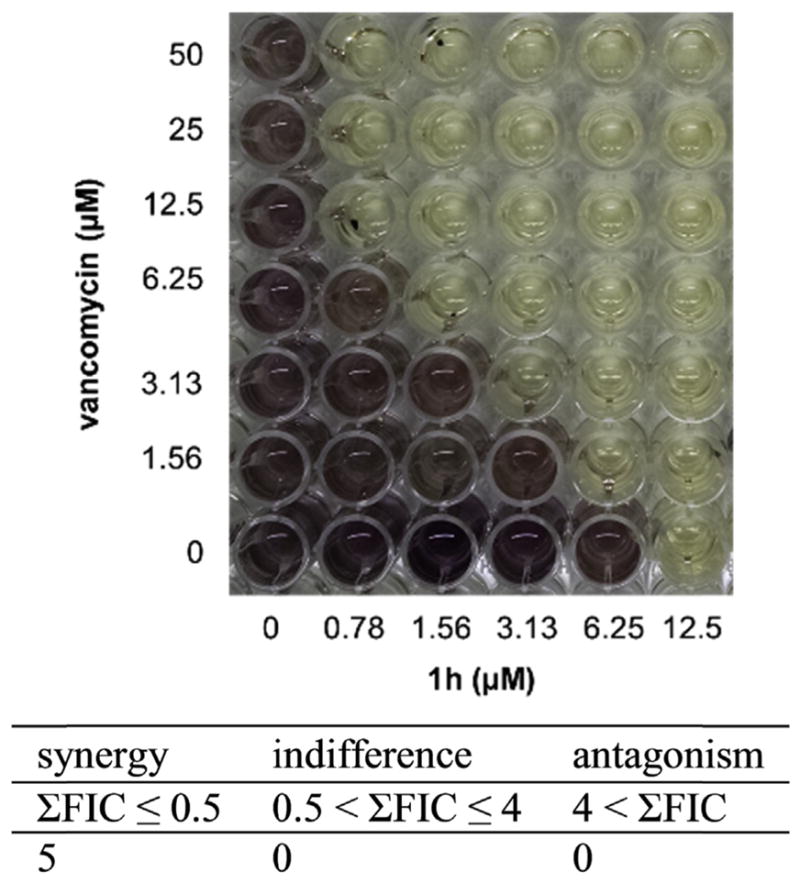
The checkerboard assay revealed synergism between vancomycin and disulfide 1h against VRSA HIP14300.
2.2.3. Resistance development studies
Development of antibiotic resistance in S. aureus is a frequent cause of treatment failure [2,3]. To compare the rates of resistance development in three variants of S. aureus (hVISA, VISA, and VRSA) to disulfide 1h, a serial passage study was performed to monitor the changes in susceptibility in cultures treated with 0.5× MIC of antibiotic for 20 h. Fig 5 shows that between passages 3 and 4, hVISA followed by VRSA and VISA exhibited decreased susceptibility to the disulfide. Between passages 5 and 7, the MIC of each test strain increased again by twofold or greater. Most notable was the resistance development in hVISA from 12.5 μM to >50 μM indicating that a subpopulation of resistant clones predominated after seven passages [18]. During the course of the study, we also monitored alterations in the MICs when disulfide 1h was combined with an equimolar amount of VAN. After seven passages, the only observed change was a twofold increase in the MIC with VRSA and hVISA between passages 2 and 4 (Fig. 5).
Fig. 5.
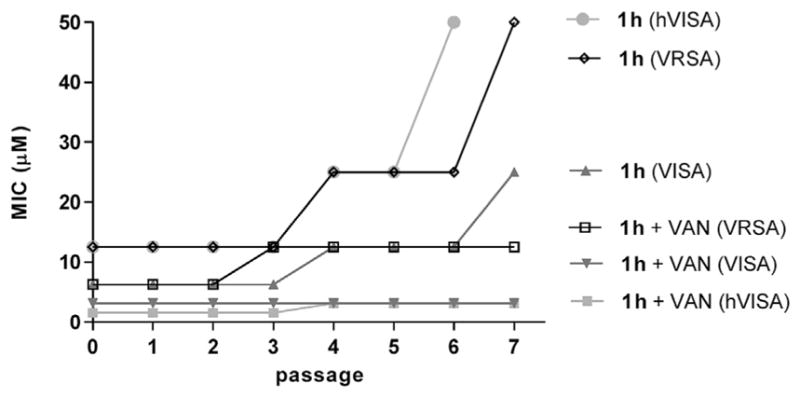
Development of resistance by serial passage for disulfide 1h ± vancomycin.
2.2.4. Effect of blood components studies
Due to their electrophilic and lipophilic properties, it was anticipated that the antibacterial activity of PySSRs may be altered by one or more components in bloods. Table 3 shows that media supplemented with GSH and 5–10% fetal bovine serum (FBS) increased the MICs of disulfides 1g–i, which was attributed to cleavage of the disulfide bond by GSH and serum protein binding, respectively. By comparison, the MICs of OXA and VAN were not affected by the additives. We similarly found that GSH and serum decreases the anti-MSSA activity of disulfiram (Antabuse™), a FDA-approved disulfide drug for the treatment of chronic alcoholism [19]. Based on these data, we concluded that in vivo dosing of PySSRs would have to compensate for blood components in a manner similar to disulfiram and other disulfide drugs (e.g., prosultiamine).
Table 3.
Effect of blood components on susceptibility of MSSA 25923.
| Additive | MIC (μM) | ||||
|---|---|---|---|---|---|
|
| |||||
| 1g | 1h | 1i | OXA | VAN | |
| null | 12.5 | 12.5 | 12.5 | 1.56 | 0.78 |
| glutathione, 100 μM | 50 | 50 | 50 | 1.56 | 0.78 |
| albumin, 0.05% vol/vol | 12.5 | 12.5 | 25 | 1.56 | 0.78 |
| serum, 5% vol/vol | 25 | 25 | 25 | 1.56 | 0.78 |
| serum, 10% vol/vol | 25 | 25 | 50 | 1.56 | 0.78 |
2.2.5. Cytotoxicity studies
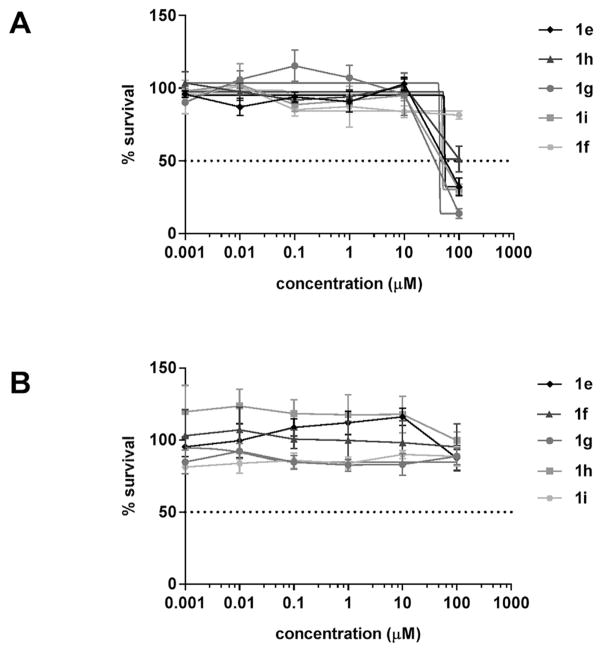
Disulfides 1e–i were evaluated for cytotoxicity using human breast MDA-MB-231 and liver HepG2 carcinoma cell lines. Fig. 6 shows that the half-maximal inhibitory concentrations (IC50s) are greater than the MIC90s of 12.5 μM for VISA and VRSA. For disulfide 1h, the IC50s were 74.9 and ≥ 100 μM for the respective cell lines. The attenuated cytotoxic activity was attributed to the GSH-rich content in mammalian cells. The thiophilic tripeptide has been shown to cancel out the antimicrobial activity of allicin in bacteria through thiol-disulfide exchange [7] and it is believed that GSH inactivates the PySSRs in mammalian cells. To this end, the Gram-positive species that exhibit susceptibility to the disulfides 1e–i have low intracellular GSH levels and alternatively use CoASH and bacillithiol in thiol-redox buffering [20]. A similar rationale is proposed for the lack of antimicrobial activity in Gram-negative bacteria that contain high levels of cytosolic GSH to maintain redox homeostasis [20,21].
Fig. 6.
Dose response curve of pyridyl disulfide 1e–i on breast (A) MDA-MB-231 and (B) liver HepG2 carcinoma cell lines.
2.2.6. Viability studies
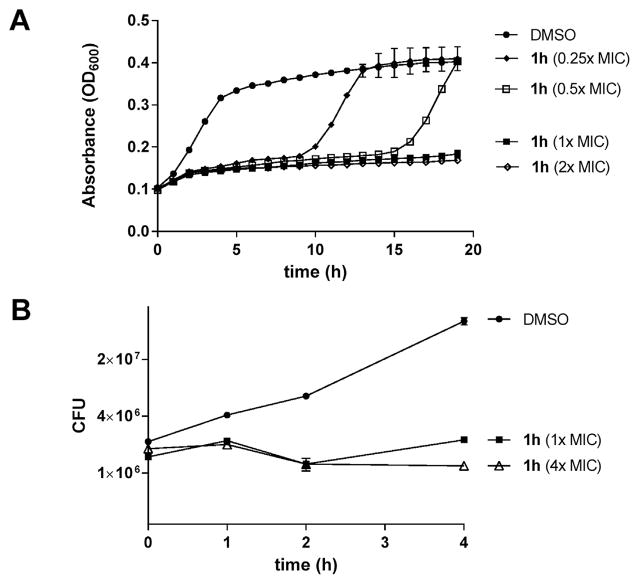
To further examine the effects of PySSR 1h on bacteria viability, we performed a series of growth and metabolic studies on S. aureus. Fig. 7A shows that treatment with 1–2× MIC suppressed growth over a 20 h period while exposure to 0.25 and 0.5× MIC of 1h delayed proliferation for 10 and 15 h, respectively. Likewise, treatment with 1 and 4× MIC attenuated the growth rate with little change in CFU numbers, which again suggested that the PySSRs are bacteriostatic in nature (Fig. 7B). In a follow-up study performed in HBSS with phenol red pH indicator, disulfide 1h was found to decelerate the metabolism of glucose to organic acids in a manner comparable to triclosan at a bacteriostatic test concentration (data not shown).
Fig. 7.
Effects of pyridyl disulfide 1h on growth in MSSA 25923.
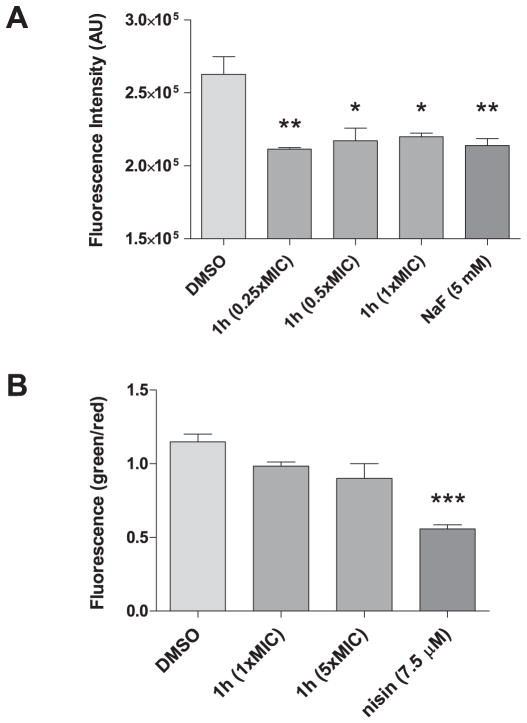
Bacteriostatic antibiotics have been previously shown to decelerate respiration and lower the cellular metabolic state in S. aureus [22]. By contrast, bactericidal agents were found to accelerate respiration and metabolism [23]. To further decipher the effect on metabolism, the C12-resazurin viability assay was used to detect for alterations in the redox activity of S. aureus treated with PySSR 1h. Fig. 8A shows that the disulfide decelerates metabolism in a similar manner to the positive control NaF. Although these results were again consistent with the bacteriostatic assessment, we further probed the effects on cell viability using the LIVE/DEAD BacLight™ assay. In the experiment, membrane permeability (damage) as a measure of cell viability is determined by comparing the green to red fluorescence ratio (λem 535/595 nm) of bacteria co-labeled with SYTO™ 9 and propidium iodide. Fig. 8B shows a negligible difference in membrane integrity between untreated and PySSR 1h treatment groups. Conversely, a significant increase in membrane permeability was observed for the pore-forming antibiotic nisin.
Fig. 8.
Effects pyridyl disulfide 1h on MSSA 25923 viability. Shown are the (A) C12-resazurin and (B) LIVE/DEAD BacLight™ assays for individual groups depicted as mean ± SEM (n = 3).
2.2.7. Plasma membrane studies
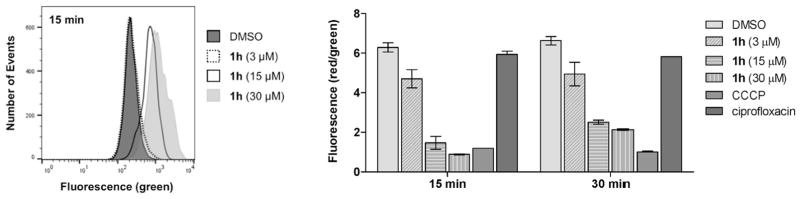
With compelling data that revealed the disulfides to be bacteriostatic agents that decelerate metabolism, we evaluated the effect of PySSRs on electron transport through differential measurements of the membrane potential. In the experiment, S. aureus was treated with either PySSR 1h, de-coupler CCCP (positive control), or ciprofloxacin (negative control). The cells were then labeled with DiOC2(3) and analyzed by flow cytometry at λex 488 nm. At 15 and 30 min intervals, the disulfide was found to dissipate the membrane potential (ΔΨ) in both a time- and concentration-dependent manner (Fig. 9). Rapid depolarization was observed within 15 min of treatment with 3–30 μM of the test drug. Between 15 and 30 min, moderate increases in membrane potentials were observed for the groups treated with 1h (15–30 μM), but not the CCCP control.
Fig. 9.
Comparative effects on membrane potential. Shown are green fluorescence (left; 530/30 filter) and the ratio of red (585/42 filter) to green fluorescence for individual groups, shown as mean ± SEM (right; n = 2).
2.2.8. Cellular redox studies
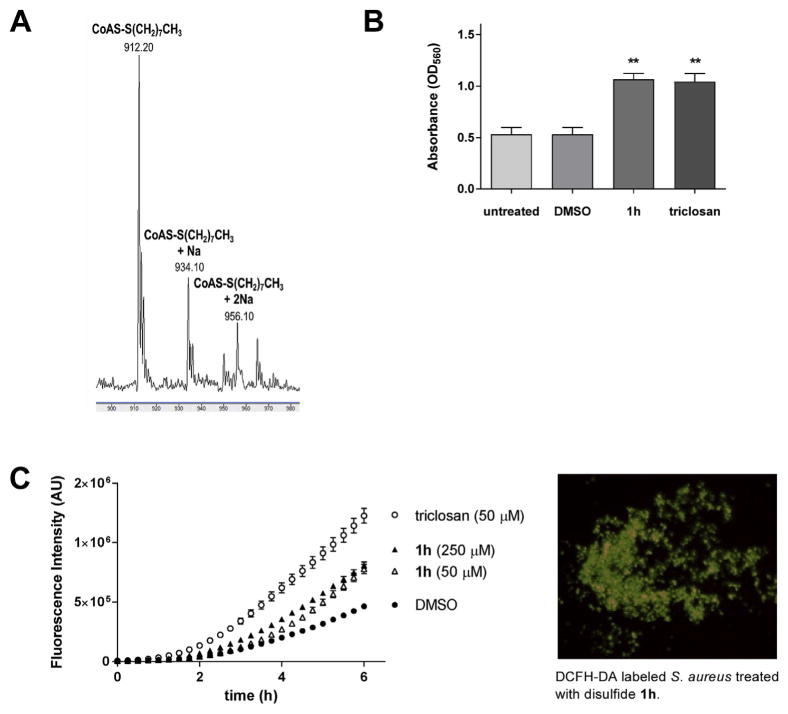
The antagonistic effects on the membrane potential and metabolism suggested that PySSRs could evoke a shift in the intracellular redox state. Within this context, S. aureus employs CoASH with bacillithiol in place of GSH as the major thiol involved in redox homeostasis [20,24]. Previous studies have shown that allicin reduces cellular thiol levels and alters both the metabolic and redox states of cells [25,26]. Based on these previous findings, we postulated that PySSR 1h mimics the antibacterial action of allicin by disrupting metabolism and thiol-redox buffering in S. aureus. To this end, growth inhibition by the disulfides was partly attributed to alterations in cytosolic thiol levels and the cellular redox state. To first establish whether PySSRs could decrease thiol levels through–SR exchange (Fig. 1), PySSR 1h was incubated with equimolar CoASH and mass spectroscopy was used to verify CoSSR formation (Fig. 10A).
Fig. 10.
Effects of pyridyl disulfides on CoASH and cellular redox state. (A) Mass spectroscopy revealed that S-(octylthio)-2-pyridine (1h) reacts with coenzyme A (CoASH) in situ by thiol-disulfide exchange. (B) Effect of disulfide 1h in S. aureus by nitroblue tetrazolium (NBT) staining. (C) Effect of disulfide 1h in S. aureus by dicholorodihydrofluorescin-diacetate (DCFH-DA) staining.
With confirmation of a thiol-disulfide exchange with CoASH, it was believed that perturbations in the cellular redox state is partly responsible for the antimicrobial effects of PySSRs. We explored this premise by labeling treated S. aureus cells with the oxidant-reactive probes nitroblue tetrazolium (NBT) [27] and 2′,7′-dicholorodihydrofluorescin-diacetate (DCFH-DA) [28]. Triclosan was included in the experiments as a generating positive control [29]. In the NBT assay, oxidation of the chromogenic probe was detected within 1 h of treatment with disulfide 1h and triclosan in S. aureus (Fig. 10B). Increased formation of oxidized DCFH [30] was also observed for PySSR 1h, but at a lower rate compared to triclosan (Fig. 10C). It should be noted that although these experiments revealed accelerated oxidation rates of the NBT and DCFH-DA probes, further studies are needed to establish whether the inhibitory effects of disulfide-based antibacterial drugs can be attributed to a shift in the cellular redox state in S. aureus [31].
3. Conclusions
S. aureus is an invasive, toxigenic pathogen that can inflict disease on every tissue, organ, and system in the human body [1,2]. MDR strains with reduced glycopeptide susceptibility are an emerging healthcare threat and new treatment strategies will be needed for patients with these resistant infections [3]. Thio-sulfinates such as allicin represent a unique mechanistic drug class whose activity spectrum is largely confined to Gram-positive species that includes S. aureus [5]. In this report, we have identified pyridyl disulfides as stable alternatives to allicin with a similar narrow spectrum profile. All twenty-five strains of S. aureus used in the study exhibited the highest susceptibility to PySSR derivatives bound with linear S-alkyl chains of 7–9 carbons in length. Quality control MRSA 43300 and VanA-type VRSA were equally susceptible to the disulfides; however, LRSA and hVISA were less sensitive for undetermined reasons. The latter was also found to have a higher rate of resistance development compared to VISA and VRSA variants (Fig. 5). Our serial passage experiment further revealed a lower rate of resistance selection when disulfide 1h was combined with equimolar VAN versus the disulfide alone.
In addition, the 8 carbon chain derivative 1h was identified in the disc diffusion assay as having synergy with VAN through observation of a bridging effect between the adjacent zones (Fig. 2) and further testing by isobologram analysis corroborated this relationship (Fig. 4). Although a basis for the synergistic relationship in VRSA could not be definitively established, we initiated preliminary investigative work to understand how PySSRs function as a growth inhibitor of S. aureus. Their bacteriostatic nature suggests that the pharmacodynamic effects are due to deceleration in cellular metabolism; however, PySSRs may also evoke a shift in the redox state that triggers perturbations of cell processes involved in growth [22]. Accordingly, an increase in the cellular oxidation state of bacteria has been shown to alter metabolic activity (e.g., respiration) [22] and gene expression [32]. Redox regulation in S. aureus is coordinated by low molecular weight thiols CoASH and bacillithiol rather than GSH [20]. Increased intracellular oxidant levels results in a decrease of the thiol:disulfide redox ratio and increased S-bacillithiolation of proteins, which is believed to protect against cytotoxic effects in S. aureus [24,33].
Within this context, mass spectroscopy revealed that thiol-disulfide exchange occurs between electrophilic PySSRs and thiophilic CoASH (Fig. 10A). Based on this finding, we hypothesize that the antimicrobial action of PySSR is partially attributed to pro-oxidant effects in S. aureus due to the thiol-disulfide exchange reactions in the cell. Increased oxidation of NBT and DCFH-DA was observed with disulfide treatment (Fig. 10B and C); however, further studies are needed to deduce the effects on the cellular redox state [31]. Another key finding of the investigation is that PySSR 1h decreases the membrane potential (Fig. 9) without damaging the plasma membrane (Fig. 8B). Based on these data, it is believed that the deceleration of metabolism by bacteriostatic PySSRs curbs respiration, de-energizing the cell membrane [22].
In final analysis, our data shows that PySSRs are bacteriostatic agents in Gram-positive pathogens including MDR variants of S. aureus. The disulfides possess a narrow activity spectrum and are thought to function as pro-oxidants in a manner reminiscent of allicin [4,25]. Our studies found that the mechanism-based PySSRs suppress metabolism presumably through interactions with thiophilic metabolites, coenzymes, and/or enzymes. These data further suggest that PySSRs have more than one mechanism of action responsible for the growth inhibition in S. aureus. Concomitantly, the onset of resistance would expect to be lower for an antibacterial agent that inhibits multiple cell processes; however, our serial passage experiments revealed the occurrence of resistance selection in S. aureus treated with 0.5× MIC PySSR 1h. With these findings, it is believed that the application of allicin-like drugs in antimicrobial therapy is limited to use as combination treatments for Gram-positive bacterial infections with reduced VAN susceptibility. Future investigations will focus on analyzing the potential place in therapy of disulfide-based drugs as adjuvant antibiotics for MDR S. aureus infections.
4. Materials and methods
4.1. Chemistry
Chemicals were purchased from commercial sources and used as received. Products were purified by flash chromatography on 60–100 mesh silica and visualized by UV on TLC plates (silica gel 60 F254). 1H and 13C NMRs were recorded on a 400 MHz NMR and referenced to residual CDCl3. Abbreviations used in the description of resonances are as follow: s (singlet); bs (broad singlet); d (doublet); t (triplet); q (quartet); qnt (quintet); m (multiplet); dt (doublet of triplets); ddd (doublet of doublets of doublets).
4.1.1. Synthesis of pyridyl disulfides 1; general procedure
Asymmetric S-(alkylthio)-2-pyridine disulfides were prepared by a modified procedure of previously reported methods [10]. To a stirring solution of 2′2-dipyridyl disulfide (0.75 mmol) and AcOH (25 μL) in 9 mL of MeOH was added the corresponding thiol (0.75 mmol) in 1 mL of MeOH at r.t. The mixture was stirred for 2–6 h and concentrated in vacuo. Flash silica gel chromatography with 0–25% EtOAc in hexanes afforded the pure asymmetric disulfides 1b–s as pale oils [10]. The S-methyl analog 1a was prepared from 2-mercaptopyridine (0.75 mmol) and S-methyl methanethiosulfonate (0.75 mmol) in 10 mL of MeOH. After stirring for 2 h at r.t., the solvent was evaporated and the product was isolated as a pale oil by flash silica gel chromatography with 3:1 hexanes:EtOAc. The spectroscopic data for disulfides 1e–i are provided.
4.1.1.1. S-(pentylthio)-2-pyridine (1e)
1H NMR (400 MHz, CDCl3) δ 8.45 (ddd, J = 4.9, 1.8, 0.9 Hz, 1H), 7.73 (dt, J = 8.1, 1.0 Hz, 1H), 7.69–7.59 (m, 1H), 7.07 (ddd, J = 7.4, 4.8, 1.1 Hz, 1H), 2.83–2.75 (m, 2H), 1.69 (q, J = 7.4 Hz, 2H), 1.43–1.24 (m, 5H), 0.88 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 160.7, 149.5, 136.9, 120.5, 119.5, 39.2, 30.7, 28.6, 22.3, 13.92; ESI–MS: m/z 214.1 [M+H]+
4.1.1.2. S-(hexylthio)-2-pyridine (1f)
1H NMR (400 MHz, CDCl3) δ 8.45 (ddd, J = 4.9, 1.9, 0.9 Hz, 1H), 7.73 (dt, J = 8.1, 1.1 Hz, 1H), 7.63 (ddd, J = 8.1, 7.4, 1.8 Hz, 1H), 7.07 (ddd, J = 7.3, 4.8, 1.1 Hz, 1H), 2.83–2.75 (m, 2H), 1.75–1.61 (m, 1H), 1.44–1.28 (m, 4H), 1.32–1.21 (m, 6H), 0.87 (t, J = 7.5 Hz, 2H).; 13C NMR (101 MHz, CDCl3) δ 160.7, 149.5, 136.9, 120.4, 119.5, 39, 31.4, 28.9, 28.2, 22.5, 14; ESI–MS: m/z 228.2 [M+H]+.
4.1.1.3. S-(heptylthio)-2-pyridine (1g)
1H NMR (400 MHz, CDCl3) δ 8.45 (ddd, J = 4.8, 1.8, 0.9 Hz, 1H), 7.73 (dt, J = 8.1, 1.0 Hz, 1H), 7.63 (ddd, J = 8.1, 7.4, 1.8 Hz, 1H), 7.07 (ddd, J = 7.4, 4.8, 1.1 Hz, 1H), 2.83–2.74 (m, 2H), 1.68 (q, J = 7.5 Hz, 2H), 1.43–1.28 (m, 3H), 1.25–1.40 (m, 8H), 0.91–0.82 (m, 3H).; 13C NMR (101 MHz, CDCl3) δ 160.7, 149.5, 136.9, 120.4, 119.5, 39, 31.7, 28.9, 28.4, 22.6, 14.1; ESI–MS: m/z 242.2 [M+H]+.
4.1.1.4. S-(octylthio)-2-pyridine (1h)
1H NMR (400 MHz, CDCl3) δ 8.44 (ddd, J = 4.8, 1.8, 0.9 Hz, 1H), 7.73 (dt, J = 8.1, 1.0 Hz, 1H), 7.63 (ddd, J = 8.1, 7.4, 1.9 Hz, 1H), 7.06 (ddd, J = 7.4, 4.8, 1.1 Hz, 1H), 2.83–2.74 (m, 2H), 1.68 (qnt, J = 7.3 Hz, 2H), 1.43–1.17 (m, 10H), 0.92–0.81 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 160.7, 149.5, 136.9, 120.5,119.5, 39, 31.8, 29.2 (2), 29, 28.5, 22.7,14.1; ESI–MS: m/z 256.2 [M+H]+.
4.1.1.5. S-(nonylthio)-2-pyridine (1i)
1H NMR (400 MHz, CDCl3) δ 8.44 (ddd, J = 4.8, 1.8, 0.9 Hz, 1H), 7.73 (dt, J = 8.1, 1.0 Hz, 1H), 7.63 (ddd, J = 8.1, 7.4, 1.9 Hz, 1H), 7.06 (ddd, J = 7.4, 4.8, 1.1 Hz, 1H), 2.85–2.67 (m, 2H), 1.68 (qnt, J = 7.3 Hz, 2H), 1.44–1.16 (m, 10H), 0.91–0.84 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 160.7, 149.5, 136.9, 120.4, 119.5, 38, 31.8, 29.4, 29.2, 29.2, 28.9, 28.5, 22.6, 14.1; ESI–MS: m/z 270.2 [M+H]+
4.2. Biological studies
Bacteria used in the study are listed in Tables S1 and S2 in the supplemental material. Bacteria were initially grown on Mueller-Hinton agar (MHA) from freezer stocks stored at −80 °C. Tryptic soy broth (TSB) and Luria-Bertani (LB) broth were used to grow starter cultures from single colonies for testing. Mammalian cell lines used in the study were propagated in Dulbecco’s Modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) from freezer stocks stored at −150 °C.
4.2.1. MIC and MBC determination
Minimal inhibitory concentrations (MICs) were determined by the broth microdilution method in 96-well flat bottom microplates [12]. A 0.5 McFarland standardized suspension prepared from an overnight culture was diluted to 1:100 in cation-adjusted Mueller Hinton broth (CAMHB) and treated with serial dilutions of test agents for 20 h at 37 °C. The lowest drug concentration that gave inhibition of visual growth was recorded as the MIC. The MIC50 and MIC90 values were defined as the lowest concentration by which 50 and 90%, respectively, of the strains were inhibited by the test agent. The minimal bactericidal concentrations (MBCs) were determined by spotting 5 μL aliquots from each well on agar media. Following overnight incubation, the lowest concentration to inhibit visual growth was recorded as the MBC.
4.2.2. Biofilm reduction assay
To assess for biofilm dispersal, biofilms were grown in a flat bottom 96-well microplate (Corning, Inc.) using 200 μL of a 1.5–2.0 × 105 inoculum of S. aureus 25293 in CAMHB [15]. After 30 h incubation at 37 °C, the media was removed by aspiration, the wells were washed with PBS, and the residual biofilms were treated with 1–5× MIC of test agent in 100 μL of CAMHB. Following 20 h incubation at 37 °C, the media was removed and the biofilms were treated for 0.5 h with 200 μL of aqueous 0.1% crystal violet. The staining solution was then discarded, the wells were washed with PBS, and 200 μL of 30% acetic acid (vol/vol) was added to solubilize the dye retained by the biofilms. Biofilm dispersal was assessed by comparing the optical absorption (OD595) of the acetic acid solutions from the treated vs. untreated wells.
4.2.3. Synergy studies
Isobologram analysis of PySSR-VAN treatment combinations was performed by the classical checkerboard titration assay [16]. Briefly, a 0.5 McFarland suspension of VRSA HIP14300 was diluted to 1:100 in CAMHB and 50 μL was used to inoculate a 96-well plate containing twofold serial dilutions of PySSR and VAN in 50 μL CAMHB. The plates were then sealed with adhesive film and incubated for 20 h at 37 °C. Growth inhibition was visualized by MTT staining using DMSO to solubilize the formazan product. The fractional inhibition (FIC) index was determined by the ratio of MIC of the combination treatment to the MIC of PySSR 1h or VAN alone. The ΣFIC (FIC1h + FICVAN) was interpreted according to the standard metrics: synergy ≤ 0.5; indifferent 0.5 < to ≤ 4; antagonism > 4 [17].
4.2.4. Growth studies
An overnight inoculum of S. aureus 25293 was grown to an OD600 of 0.1 and treated with 0, 0.25, 0.5, 1, and 2× MIC of test agents in a 96-well microplate. The plate was incubated at 37 °C in a Multiskan GO microplate reader (Thermo Fisher Scientific, Inc.) and the OD600 was measured at 1 h intervals following 30 s agitation. The results were plotted using GraphPad Prism. Time-kill studies were performed using a 1:100 dilution of 0.5 McFarland suspension of S. aureus 25293 treated with 0,1, and 4× MIC of test agent [34]. At time points 0, 1, 2, and 4 h, 10 μL samples were serially diluted in PBS and streaked onto MHA plates to enumerate the bacteria. Colony forming units (CFUs) were totaled after 30 h incubation and the data was plotted using GraphPad Prism.
4.2.5. Resistance studies
The serial passage assay [35] to determine resistance development was performed using hVISA Mu3, VISA AR-217, and VRSA AIS 1000505 treated with 0.5× MIC of PySSR 1h ± equimolar VAN. A 0.5 McFarland standardized suspension of each 0.5× MIC culture was diluted to 1:100 in CAMHB and treated with serial dilutions of the test agents. Following incubation for 20 h at 37 °C, the MICs were determined by visual inspection for each passage.
4.2.6. Cytotoxicity studies
The MTT assay was used to assess cellular viability by measuring the NAD(P)H-dependent cellular oxidoreductase enzyme activity. The yellow dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is converted to an insoluble blue formazan. Human breast adenocarcinoma cells MDA-MB-231 and human liver carcinoma cells HepG2 were seeded at 5000 cells per well in a 96-well plate, and allowed to adhere overnight. The next day, 0.001, 0.01, 0.1, 10, and 100 μM of test agents were added to the cells and incubated for 72 h. At the end of the experiment, the media was removed and MTT dye at 1 mg/mL in PBS pH 7.4 was added to each well and incubated for 2 h. The MTT solution was then removed and pure DMSO added to each well and incubated in orbital shaker for 30 min at 37 °C until the crystals were dissolved. The absorbance was measured at OD570 in a BioTek Synergy H plate reader (BioTek Instruments, Inc.).
4.2.7. Viability studies
The LIVE/DEAD BacLight™ assay (Molecular Probes, Inc.) to determine cell viability and membrane damage was performed on an overnight innocuum of S. aureus 25293 grown to an OD600 of 0.4 in LB broth. The cells were harvested by centrifugation (10,000 × g, 10 min, 4 °C), washed twice with PBS and resuspended in 100 mM phosphate buffer (KH2PO4/K2HPO4, pH 7.0) supplemented with 5 mM MgSO4. The bacteria were then energized with glucose (20 mM final concentration) for 10 min at 37 °C and treated with 1 or 5× MIC of test agent. Vehicle (DMSO; 5%) and 25 μg/mL nisin, a pore-forming antibiotic, were used as controls [36]. After 5 min incubation, 100 μL samples were combined with 100 μL of a 2×BacLight™ staining reagent in a black flat bottom 96-well microplate (Corning, Inc.) and mixed. The plate was covered and allow to stand at r.t. in the absence of light. After 15 min, the fluorescence emission of green (λex 485 nm, λem 535 nm) to red (λex 485 nm, λem 595 nm) ratio was measured on a Filter Max F3 fluorescent plate reader (Molecular Devices, Inc.). Membrane permeability (damage) as a measure of cell viability was determined by comparing the green to red fluorescence ratio (λem 535/595 nm) between treatment groups.
4.2.8. Metabolic activity assay
The Vybrant™Cell Metabolic assay (Invitrogen, Inc.) to measure metabolic (redox) activity of viable bacterial cells was performed on an overnight innocuum of S. aureus 25293 grown to an OD600 of 0.4 in LB broth. Cells were treated with 0.25, 0.5, or 1× MIC of test agent. Vehicle (DMSO; 5%) and NaF (5 mM), an inhibitor of glycolysis [36], were used as controls. After 15 min at 37 °C, aliquots of 200 μL were combined with 5 μM C12-resazurin in a black flat bottom 96-well microplate and incubated in the dark for 15 min. Metabolic activity was measured by the fluorescence intensity of the reduced metabolite C12-resorufin (λex 550 nm, λem 595 nm).
4.2.9. Glucose metabolism
Phenol red pH indicator was used to detect for glucose metabolism as a measure of increased acidity of the medium. A 90 μL innocuum of a OD600 1.0 suspension of S. aureus 25293 in Hank’s buffered salt solution (HBSS) was combined with 150 μL of 70 μM phenol red in water and 10 μL of either 2 mM disulfide, triclosan, vehicle (DMSO), or water in a 96-well plate. The plate was incubated at 37 °C in a Multiskan GO microplate reader for 15 h with OD560 readings at 0.5 h intervals.
4.2.10. Membrane potential assay
The BacLight™ Bacterial Membrane Potential kit (Molecular Probes, Inc.) was used to detect for drug-induced alterations in the proton gradient of S. aureus. An overnight culture was diluted 1:100 in LB, incubated at 37 °C with shaking for 2 h, and used to make a final 1:200 dilution for the experiment. Cells were treated with PySSR 1h (3–30 μM), vehicle, (DMSO; 2%) or the respiratory decoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP) and incubated at 37 °C with shaking. After 15 and 30 min, 200 μL samples were transferred to a fresh tube containing 2 μL of the fluorescent membrane potential indicator dye DiOC2(3). DiOC2(3) emits green fluorescence in all cells, but larger membrane potentials cause a shift towards red emission due to self-association of the dye at higher cytosolic concentrations [37]. After staining for 20 min, bacteria were analyzed on a BD Biosciences FACSAria with the following parameters: λex 488 nm, green fluorescence filter 530/30 and red fluorescence filter 585/42. The resulting data was processed using FlowJo v10.1 software (FlowJo, LLC).
4.2.11. NBT and DCFH-HA assays
The colorimetric nitroblue tetrazolium (NBT) assay [27] to detect for alterations in intracellular oxidation rates was performed using a OD600 1.5 suspension of S. aureus 25293 in HBSS. A 80 μL innocuum was combined with 150 μL aqueous solution of 1 mg/mL NBT and 10 μL of either 2 mM PySSR 1h, triclosan, vehicle (DMSO), or water in a 96-well microplate and incubated with shaking for 1 h at 37 °C. The reaction was stopped with 10 μL 0.1 M HCl, the plate was centrifuged at 1500 × g for 10 min at 0 °C, and the supernatant was removed. The pellets were then treated with 25 μL of DMSO, 10 μL 2 N KOH, and 50 μL of HBSS to solubilize the formazan product. Intracellular oxidant species generation was measured by the intensity of blue coloration at OD560.
The fluorometric 2′,7′–dichlorofluorescin diacetate (DCFH-HA) assay [28] was similarly performed using a OD600 1.5 suspension of S. aureus 25293 in HBSS. To 80 μL of inoculums in a black flat bottom 96-well microplate was added 5 μL of either 2 or 10 mM PySSR 1h, 10 mM triclosan, vehicle (DMSO), or water followed by 15 μL of 100 μM DCFH-HA in DMSO. Intracellular oxidation of deacylated DCFH was measured by the intensity of fluorescence (λex 485 nm, λem 535 nm).
4.3. Statistical analyses
Data are presented as ± standard error of the mean (SEM). Statistical significance was assessed by analysis of variance (ANOVA) with Tukey’s posttest for multiple comparisons using Prism 5.0 (GraphPad Software, Inc) software. P values of < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This research was supported by the Marshall University School of Pharmacy FRS Grant Program. The authors also thank Marshall University Department of Chemistry for use of the NMR facility and Dr. Mohammad F. Hossain for mass spectroscopy analyses. Bacterial strains were acquired from the American Type Culture Collection, FDA-CDC Antimicrobial Resistance Isolate Bank, and the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources, NIAID, NIH.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejmech.2017.10.018.
References
- 1.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 2.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, Peters G. New insights into methicillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012;39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Gould IM. Treatment of bacteremia: methicillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA) Int J Antimicrob Agents. 2013;42:S17–S21. doi: 10.1016/j.ijantimicag.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 6.Jonkers D, Sluimer J, Stobberingh E. Effect of garlic on vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1999;43:3045. doi: 10.1128/aac.43.12.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa H, Watanabe K, Suma K, Origuchi K, Matsufuji H, Seki T, Ariga T. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci Biotechnol Biochem. 2009;73:1948–1955. doi: 10.1271/bbb.90096. [DOI] [PubMed] [Google Scholar]
- 8.Focke M, Feld A, Lichtenthaler K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990;261:106–108. doi: 10.1016/0014-5793(90)80647-2. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa H, Suma K, Origuchi K, Kumagai H, Seki T, Ariga T. Biological and chemical stability of garlic-derived allicin. J Agric Food Chem. 2008;56:4229–4235. doi: 10.1021/jf8000907. [DOI] [PubMed] [Google Scholar]
- 10.Barton DHR, Hesse RH, O’Sullivan AC, Pechet MM. A new procedure for the conversion of thiols into reactive sulfenylating agents. J Org Chem. 1991;56:6697–6702. [Google Scholar]
- 11.Sheppard JG, Long TE. Allicin-inspired thiolated fluoroquinolones as antibacterials against ESKAPE pathogens. Bioorg Med Chem Lett. 2016;26:5545–5549. doi: 10.1016/j.bmcl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen JH, Turnidge JD. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. ASM Press; Washington, DC: 2007. pp. 1152–1172. [Google Scholar]
- 13.Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:4580–4587. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 15.Bauer J1, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2013;57:2726–2737. doi: 10.1128/AAC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody JA. Synergism testing: broth microdilution checkboard and broth macrodilution methods. In: Isenberg HD, editor. Clinical Procedures Handbook. ASM Press; Washington, DC: 1992. pp. 5.18.1–5.18.28. [Google Scholar]
- 17.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long TE. Repurposing thiram and disulfiram as antibacterial agents for multi-drug resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 2017;61:e00898–17. doi: 10.1128/AAC.00898-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loi VV, Rossius M, Antelmann H. Redox regulation by reversible protein S-thiolation in bacteria. Front Microbiol. 2015;6:187. doi: 10.3389/fmicb.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey RC, Brown WC, Adams WB, Worsham MB. Occurrence of glutathione in bacteria. J Bacteriol. 1978;133:1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. Antibiotic efficacy is linked to bacterial cellular respiration. Proc Natl Acad Sci U S A. 2015;112:8173–8180. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belenky P, Ye JD, Porter CB, Cohen NR, Lobritz MA, Ferrante T, Jain S, Korry BJ, Schwarz EG, Walker GC, Collins JJ. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015;13:968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi BK, Roberts AA, Huyen TT, Bäsell K, Becher D, Albrecht D, Hamilton CJ, Antelmann H. S-bacillithiolation protects conserved and essential proteins against hypochlorite stress in firmicutes bacteria. Antioxid Redox Signal. 2013;18:1273–1295. doi: 10.1089/ars.2012.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller A, Eller J, Albrecht F, Prochnow P, Kuhlmann K, Bandow JE, Slusarenko AJ, Leichert LI. Allicin induces thiol stress in bacteria through S-Allylmercapto modification of protein cysteines. J Biol Chem. 2016;291:11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruhlke MC, Portz D, Stitz M, Anwar A, Schneider T, Jacob C, Schlaich NL, Slusarenko AJ. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic Biol Med. 2010;49:1916–1924. doi: 10.1016/j.freeradbiomed.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Albesa I, Becerra MC, Battán PC, Páez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317:605–609. doi: 10.1016/j.bbrc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 28.Dridi B, Lupien A, Bergeron MG, Leprohon P, Ouellette M. Differences in antibiotic-induced oxidative stress responses between laboratory and clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2015;59:5420–5426. doi: 10.1128/AAC.00316-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacic P, Somanathan R. Triclosan (mechanism of bactericidal action and toxicity): metabolism, electron transfer and reactive oxygen species. In: Hepel M, Andreescu S, editors. Oxidative Stress: Diagnostics, Prevention, and Therapy, Vol. 2 ACS Symposium Series. American Chemical Society; Washington, DC: 2015. pp. 237–244. [Google Scholar]
- 30.Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014;111:E2100–E2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang W, Small DA, Toghrol F, Bentley WE. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J Bacteriol. 2006;188:1648–1659. doi: 10.1128/JB.188.4.1648-1659.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posada AC, Kolar SL, Dusi RG, Francois P, Roberts AA, Hamilton CJ, Liu GY, Cheung A. Importance of bacillithiol in the oxidative stress response of Staphylococcus aureus. Infect Immun. 2014;82:316–332. doi: 10.1128/IAI.01074-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma P. Methods for determining bactericidal activity and antimicrobial interactions: synergy testing, time-kill curves, and population analysis. In: Schwalbe R, Steele-Moore L, Goodwin AC, editors. Antimicrobial Susceptibility Testing Protocols. CRC Press; Boca Raton, FL: 2007. pp. 275–298. [Google Scholar]
- 35.Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother. 2007;1:1150–1154. doi: 10.1128/AAC.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swe PM, Cook GM, Tagg JR, Jack RW. Mode of action of dysgalacticin: a large heat-labile bacteriocin. J Antimicrob Chemother. 2009;63:679–686. doi: 10.1093/jac/dkn552. [DOI] [PubMed] [Google Scholar]
- 37.Novo D, Perlmutter NG, Hunt RH, Shapiro HM. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry. 1999;35:55–63. doi: 10.1002/(sici)1097-0320(19990101)35:1<55::aid-cyto8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.