Abstract
Cognitive decline is recognized as a prevalent and debilitating symptom of multiple sclerosis (MS), especially deficits in episodic memory and processing speed. The field aims to (1) incorporate cognitive assessment into standard clinical care and clinical trials, (2) utilize state-of-the-art neuroimaging to more thoroughly understand neural bases of cognitive deficits, and (3) develop effective, evidence-based, clinically feasible interventions to prevent or treat cognitive dysfunction, which are lacking. There are obstacles to these goals. Our group of MS researchers and clinicians with varied expertise took stock of the current state of the field, and we identify several important practical and theoretical challenges, including key knowledge gaps and methodologic limitations related to (1) understanding and measurement of cognitive deficits, (2) neuroimaging of neural bases and correlates of deficits, and (3) development of effective treatments. This is not a comprehensive review of the extensive literature, but instead a statement of guidelines and priorities for the field. For instance, we provide recommendations for improving the scientific basis and methodologic rigor for cognitive rehabilitation research. Toward this end, we call for multidisciplinary collaborations toward development of biologically based theoretical models of cognition capable of empirical validation and evidence-based refinement, providing the scientific context for effective treatment discovery.
It has been 140 years since Charcot described “marked enfeeblement of the memory” with “conceptions [that] are formed slowly” in persons with multiple sclerosis (MS).1 Such cognitive symptoms were overlooked during much of the 20th century before Rao et al.2 brought renewed attention to MS cognitive deficits in Neurology® about 25 years ago, beginning a quarter century of research on the prevalence, expression, and neural bases of MS cognitive dysfunction. Current work aims to incorporate cognitive assessment into MS clinics and clinical trials, utilize state-of-the-art neuroimaging to explicate neural bases of deficits, and develop effective symptomatic cognitive treatments. First, however, key knowledge gaps require attention and methodologic approaches need improvement to advance the field toward these goals, with the ultimate goal of effective, evidence-based, clinically feasible interventions to prevent or treat cognitive deficits. Academic articles typically emphasize what is known, but awareness of the unknown provides the catalyst for scientific discovery.e1 As such, our international team of MS experts identified critical gaps or flaws in our knowledge and makes recommendations for future work. This is not a thorough review, but a joint statement of critical research priorities.
Cognitive dysfunction due to MS
Cognitive profile
Slowed cognitive processing speed and episodic memory decline are the most common cognitive deficits in MS, with additional difficulties in executive function, verbal fluency, and visuospatial analysis.2–4 Anecdotally, patients often report difficulties with multitasking and word-finding, which are sorely underinvestigated. Cognitive decline often emerges early in disease,e2−e5 but impairment is more prevalente6 and may differ qualitatively (e.g., risk for working memory deficitse7) among persons with progressive vs relapsing disease. Although MS leads to deficits in multiple cognitive domains on the group level,2,3 we know little about variability in patient-level expression of cognitive deficits (e.g., patterns of isolated vs co-occurring deficits, discussed below). It is also unknown whether deficits in one cognitive domain (e.g., speed) contribute to dysfunction in other domains (e.g., memory). Although speed and memory are correlated in MS,e8 they are also robustly correlated in healthy personse9 (likely due to general abilitye10), so conclusions about direct links between decline in speed, memory, or any function independent of premorbid ability or disease-related mediators (e.g., cerebral atrophy) are premature and potentially misleading (i.e., may encourage unfounded expectations, e.g., that treatment of one function leads to improvement in correlated functions) (key priorities in table 1).
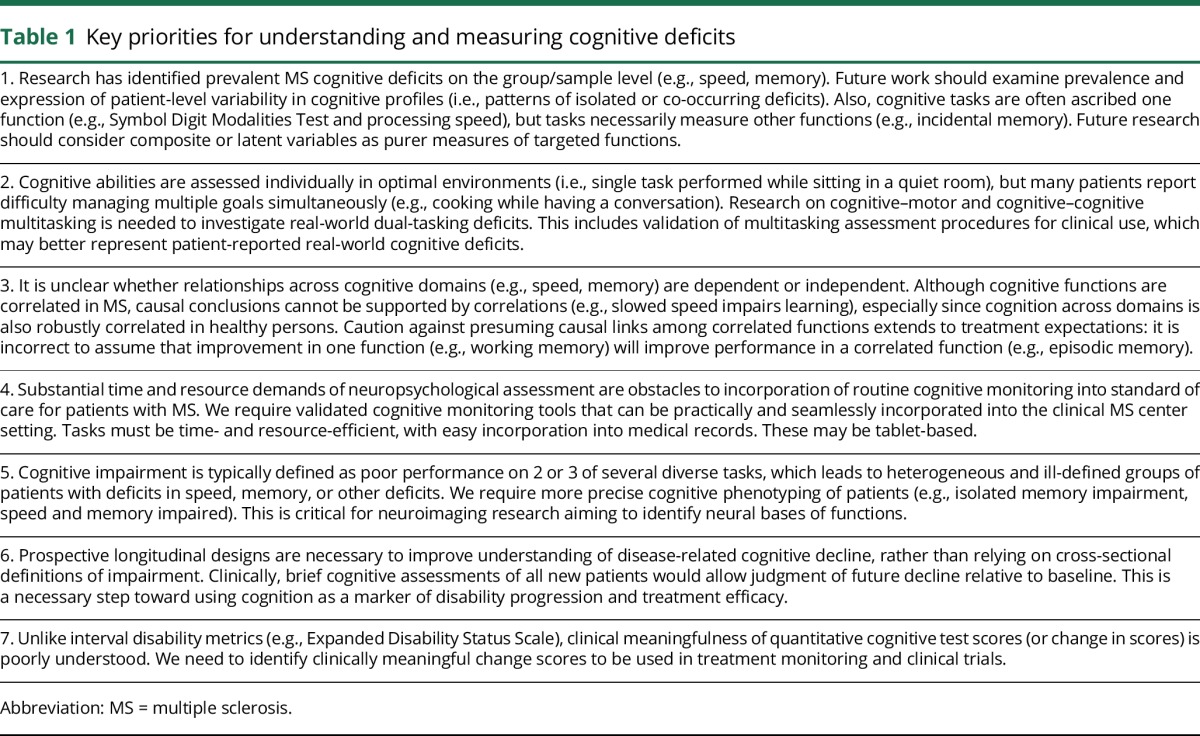
Table 1.
Key priorities for understanding and measuring cognitive deficits
We administer isolated cognitive tasks in rooms designed to minimize distractions; however, monotasking under ideal conditions may not capture patient-reported real-world deficits, especially in multitasking: the ubiquitous demand of young and middle adulthood to effectively manage multiple simultaneous goals (e.g., preparing dinner while having a conversation). Indeed, evidence suggests that cognition is more negatively affected in patients with MS (relative to controls) when performing cognitive tasks while walking (cognitive–motor dual task),5 and in the context of environmental noise (distraction).6 In addition to existing neuropsychological tools, the field should develop, validate, and utilize cognitive–cognitive and cognitive–motor dual-task paradigms to better address patient-reported multitasking deficits, which may be more sensitive for identifying real-world functional deficits,e11,e12 and also for predicting future decline.
Cognitive assessment
Cognitive processing speed is typically assessed as the amount of work performed within a time limit (e.g., number of items completed). Episodic memory is assessed as the amount of information learned and recalled (e.g., words, visual stimuli). Cognitive batteries developed for MS3,7,e13 include tests of processing speed, memory, and other functions individually administered by trained professionals. We critically reviewed the most widely used tasks (table 2), and identified the Symbol Digit Modalities Test (SDMT), Brief Visuospatial Memory Test-Revised (BVMT-R), and Selective Reminding Test or California Verbal Learning Test–II (CVLT-II) as the most sensitive tasks currently available for cognitive monitoring in MS. The SDMT is most sensitive, likely because good performance depends on multiple functions affected by MS (mostly processing speed, but also memory and visual scanning).e14 Limitations of these tasks and recommended pathways for improvements are noted in table 1. Patients referred for specific clinical or research questions beyond monitoring often require more comprehensive evaluations.
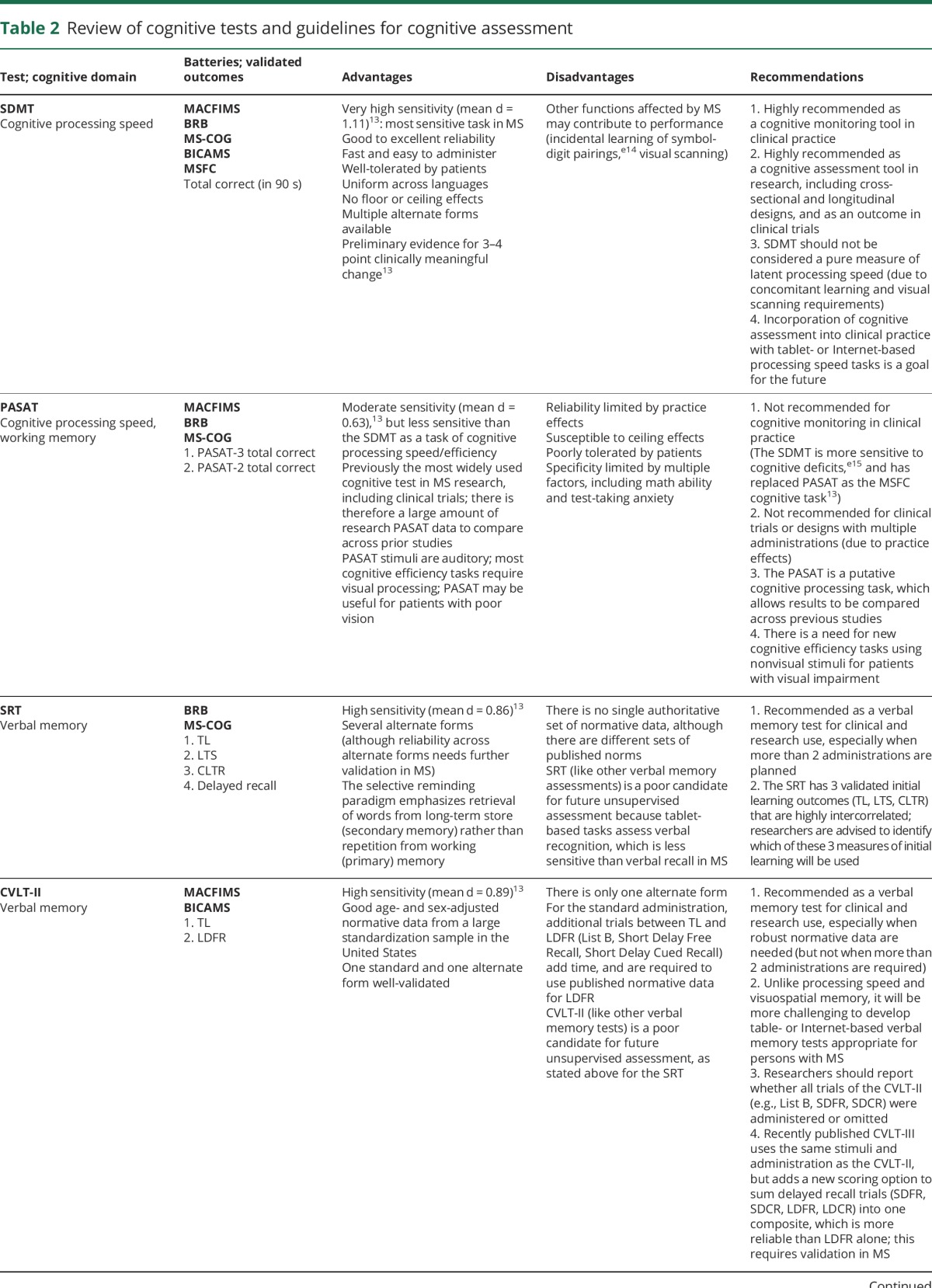
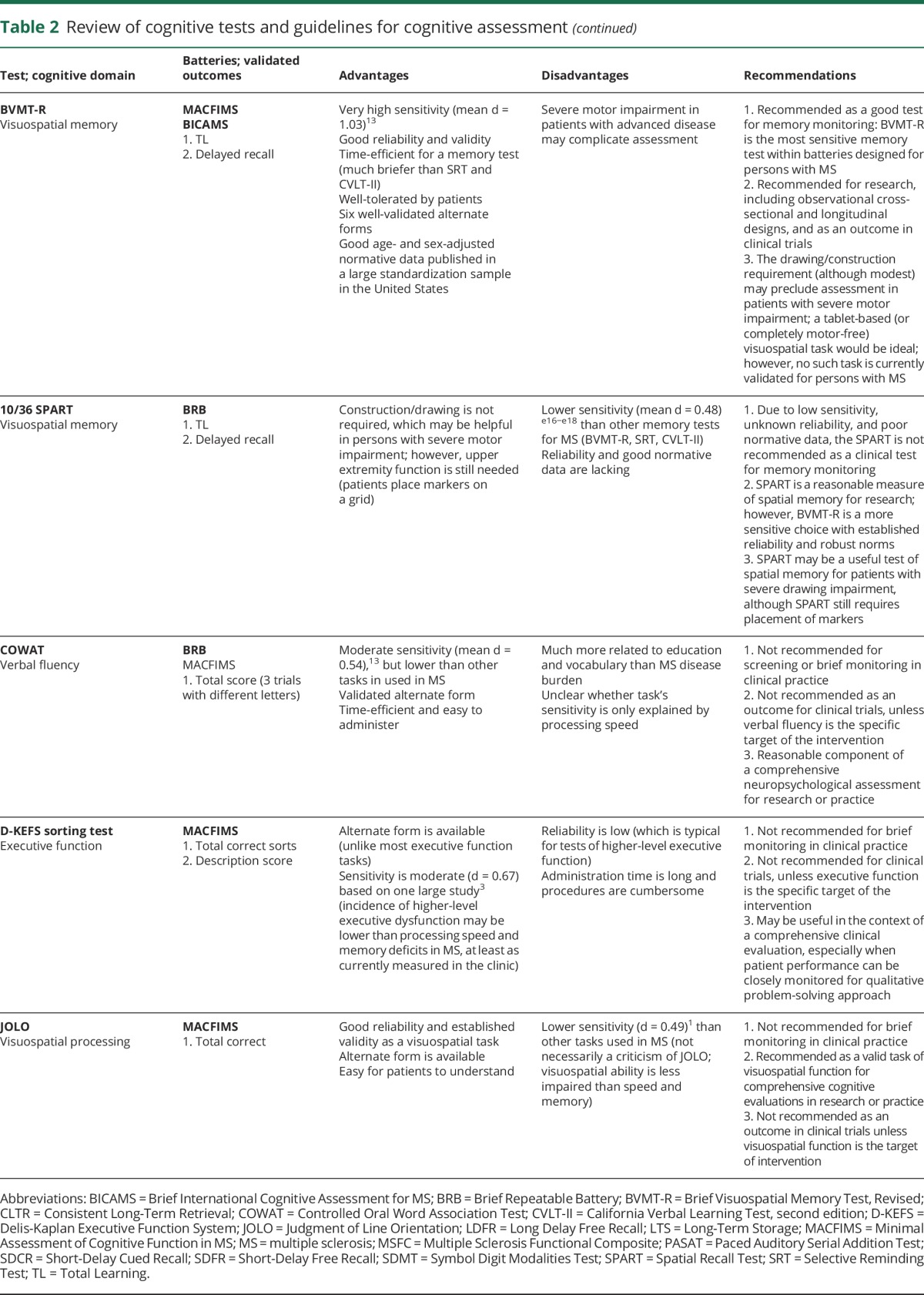
Table 2.
Review of cognitive tests and guidelines for cognitive assessment
Although MS batteries are brief by neuropsychological standards, the need for even 15 minutes7 of one-on-one testing for every patient is not practical, so cognitive monitoring is not currently part of MS standard care. Computerized testing may be a viable alternative to conventional paper-and-pencil assessment.e19 For instance, the Processing Speed Test (PST)8 is a tablet-based test modeled after the SDMT (and part of the MS Performance Teste20: tablet-based Multiple Sclerosis Functional Composite). Electronic data from tablet-based tasks may be integrated with electronic medical records to promote cognitive monitoring as standard care: a key innovation that would lead to (1) better detection of cognitive decline, (2) large datasets from representative samples to advance understanding of prevalence, time course, and risk factors for decline, and (3) greater feasibility of postmarket studies of disease-modifying therapy (DMT) effects on cognition.
Defining cognitive impairment
Cognitive tests yield quantitative values, but it is sometimes preferred to distill scores into classifications of “intact” or “impaired.” Impairment is typically defined as performance below a chosen threshold (e.g., 1.5 SD below normal2,3) but definitions of impairment have varied across studies,e21 affecting prevalence estimates of impairment. A large cross-sectional study of impaired performance on the Minimal Assessment of Cognitive Function in MS found that about 28%–52% of patients were impaired on tests of speed (Paced Auditory Serial Addition Test, SDMT) and 30%–55% on tests of memory (CVLT-II, BVMT-R).3 Rates reflect the percentage of patients impaired on each test at one time, but not the number of patients impaired (1) on composite (or latent) measures of each function (performance on single tasks is affected by multiple cognitive processes, which reduces specificity), (2) in multiple functions (deficit co-occurrence), or (3) at any point in life (lifetime prevalence). It is also unknown whether prevalence of cognitive impairment has changed quantitatively or qualitatively since the approval of newer DMT.
Studies often characterize patients as cognitively intact or impaired based on overall performance across several tests measuring different cognitive functions (e.g., failure on 3 of 11 testse22), but this threshold can be met by failing speed or memory tasks alone, or a mix of speed, memory, and other tasks. This leads to heterogeneous “impaired” groups of patients with different isolated or co-occurring cognitive deficits, making interpretation of results challenging, and comparisons across studies troublesome, especially for imaging studies aiming to identify neural correlates of impairment (which likely differ across specific cognitive domains). Future work should better characterize groups as impaired in isolated or combined deficits (phenotypes, e.g., memory impaired but speed intact; speed and memory impaired), and also utilize purer measures of each cognitive domain (e.g., latent variables or composite domain scores).
Cognitive decline
When a patient reports a cognitive problem, he or she is describing a change in function from a previous level; however, the majority of cognitive research studies and clinical evaluations are cross-sectional. Clinicians and researchers examining single time points may miss decline that does not cross a given threshold for “cognitive impairment.” As shown (figure), patients with previous function above the 50th percentile but declining 1.5 SDs (red arrows) are not categorized as impaired, although such patients likely notice and report cognitive decline. Conversely, patients meeting criteria for impairment may experience varying degrees of decline before impairment (yellow arrows), thereby adding to heterogeneity of impaired groups. Clinically, baseline cognitive assessment in newly diagnosed patients would support accurate judgment of decline from previous function, which would be important for monitoring cognitive disability progression and potentially evaluating treatment efficacy (see discussion of regression-based norms,9 links.lww.com/WNL/A185; links.lww.com/WNL/A186). Finally, the field requires large prospective studies with combined cognitive and MRI assessment following newly diagnosed patients (relative to controls) over many years to better understand how cognitive decline in each domain progresses. A small example of such a study was previously performed with 44 patients over 7 years.4
Figure. Cognitive decline from previous functioning.
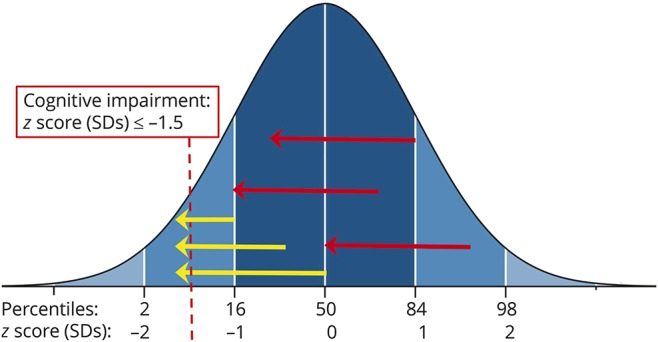
About half of persons with multiple sclerosis are considered cognitively impaired in prevalence studies, which is based on performance below a chosen threshold (yellow arrows crossing −1.5 SDs). As illustrated, however, patients may experience and report notable decline from previous function without crossing the threshold into impairment (red arrows), although such decline likely affects real-world functioning. For example, the uppermost red arrow represents a person with above average cognition prior to disease onset (84th percentile). Despite a decline of 1.5 SD, this person's current performance is within the average range (dark blue shaded area), and she or he would be categorized as cognitively intact in research studies. Clinically, this person may be told that he or she does not have impairment, which conflicts with his or her real experience of decline.
Clinically meaningful change
The Expanded Disability Status Scalee23 is an interval scale of physical disability in MS ranging from 0 (no disability) through 1 to 10 (0.5-point steps) reflecting disability milestones (e.g., 6.0 = unilateral gait assistance), with rubrics for clinically meaningful change.e24 There is no interval scale for cognition in MS, and it has been challenging to validate cognitive test score changes indicative of clinical meaningfulness10: an obstacle to interpretation of cognitive outcomes in prevention and treatment research. Based on work with timed ambulation metrics,e25 preliminary benchmarks of clinically meaningful cognitive test values11 or change in test values over time12 have been suggested (see reference 13 for SDMT), with employment status often used as an objective anchor. Ideally, we would also identify change in cognitive scores associated with cognitive difficulties in everyday life. One challenge is that patient-reported deficits and cognitive test performance are often discrepant,e26 which may be due in part to discrepancies between laboratory cognitive tasks and real-life cognitive demands. Note also that adequate test–retest reliability with repeated measurementse27,e28 is an essential and prerequisite step when validating meaningful change on cognitive tasks.
Cognitive monitoring holds promise as a useful tool for disease surveillance. Indeed, cognitive decline is associated with MRI markers of MS disease burden (see below),14 and cognition can be impaired even before (or without) physical disability.e29,e30 Emerging evidence also supports the notion of a “cognitive relapse” whereby cognitive changes may be the only behavioral indicator of disease activity (i.e., without sensorimotor symptoms).15,e31 As such, brief cognitive monitoring tools may identify disease activity that would otherwise go untreated, and early cognitive deficits may indicate a poor prognosis for later disability and cerebral atrophy.e32
Neuroimaging and cognitive function
Neuroimaging research on cognition
Given the essential role of MRI in MS diagnosis and disease surveillance, the field of MS is at the forefront of novel and innovative MRI technology, which provides multiple tools for investigating cognitive deficits due to MS.14 Cognitive deficits were linked to greater lesion load in early research,16 and subsequent work shows the importance of white matter lesion location,17 microstructural injury,18 gray matter lesions,19 cortical20 and subcortical21–23 gray matter brain atrophy, and discrepant patterns of cerebral activation with fMRI.24 Advances in ultra-high-field MRI,e33 myeline34 and molecular25 imaging, imaging of demyelination and remyelination,26 and nonconventional MRI techniques to assess microstructural cerebral changes27 will provide even more ways to investigate MS-related cognitive deficits. A challenging but essential next step is to integrate rich multimodality imaging data into testable and biologically informed models of disease-related cognitive deficits, utilizing unique strengths of each imaging approach. This aspirational but critical goal will require collaboration among experts in imaging modalities, neuroscience, and cognition. Such work will inform development of biologically plausible approaches to cognitive rehabilitation (key priorities in table 3).
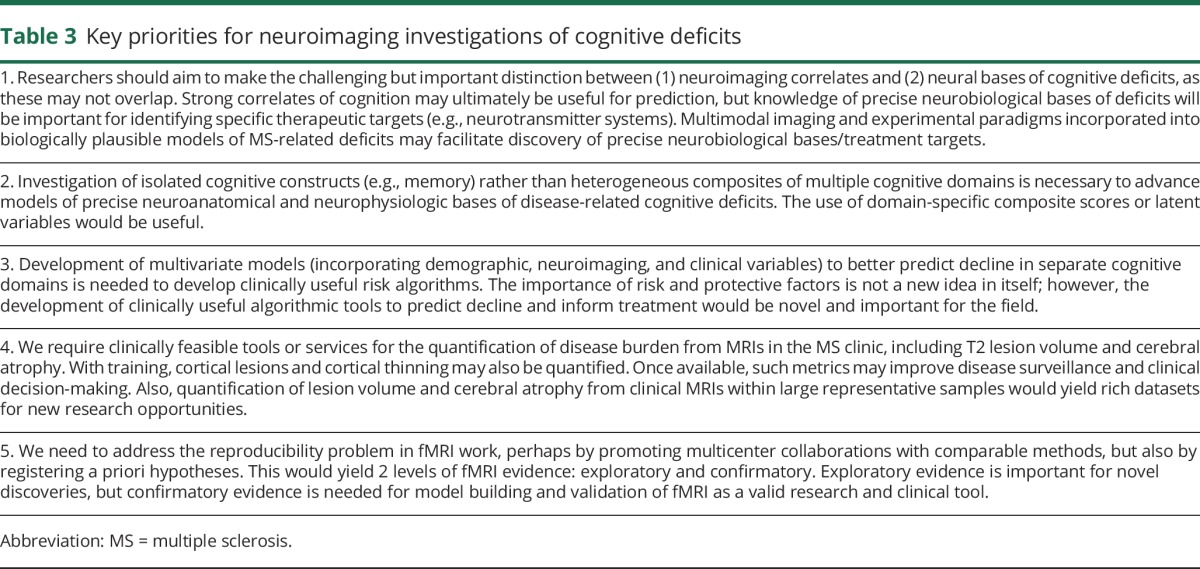
Table 3.
Key priorities for neuroimaging investigations of cognitive deficits
Neural bases of cognitive deficits
Neuroanatomical correlates of cognitive deficits exist (e.g., thalamus21–23), but it is unclear whether such correlates (1) directly underlie deficits, or (2) are reliable proxies of overall (or other) cerebral damage, which mediate links to cognition. For instance, the thalamus is highly susceptible to retrograde degeneration,e35 and has better scan-to-scan reliability than other structures,e36 perhaps making thalamic volume a good summary measure of disease burden across patients with variable CNS damage, even if thalamic change does not directly underlie a specific deficit (e.g., memory). Although reliable correlates of cognition may be useful for prediction (discussed below), knowledge of precise neural bases is important for identifying therapeutic targets (e.g., neurotransmitter systems) for treatment of specific deficits. We need large prospective longitudinal studies with multimodality neuroimaging to carefully document temporal correlations of specific emerging cognitive deficits with changes in specific brain structures and functions, thereby informing advanced models of disease-related deficits, which will help identify therapeutic targets. For instance, longitudinal work may help substantiate cross-sectional links between memory deficits and hippocampal changes: atrophy on MRI,28 lesions on double inversion recovery,29 glutamate concentration on magnetic resonance spectroscopy,30 abnormal activation and functional connectivity on fMRI,31 and demyelination and synaptic loss on histology.32 (See links.lww.com/WNL/A185 and links.lww.com/WNL/A186 for information how segmentation of hippocampal subfields28,33,34 and thalamic nuclei35 may advance understanding of cognitive deficits.)
The search for specific neural correlates of distinct cognitive functions is encumbered by the imprecision of (1) heterogeneously impaired groups and (2) single cognitive tasks with multiple processing demands. MS research may be informed by lifespan research using factor analytic techniques to derive purer latent measures of speed, memory, and other functions.36 This approach to behavioral assessment would complement recent factor analytic MRI analyses identifying nonrandom patterns of regional cortical thinning due to MS.20
Quantifying risk for future cognitive decline
Longitudinal studies have linked baseline MRI to risk for cognitive decline, including T2 lesion volume, cerebral atrophy, microstructural damage, and cortical lesions.4,19,37 Although labor-intensive and expensive, we need more prospective multimodality neuroimaging studies with large representative samples to create algorithms of risk for cognitive decline. Combined with demographic, reserve,38 and clinical variables, such algorithms should be assessed for specificity and sensitivity in confirmation samples, thereby evaluating clinical utility. Accurate algorithms may aid in early treatment decisions (e.g., aggressiveness of DMT), and advance research and practice of early cognitive intervention. Note that clinical feasibility of MRI-informed risk algorithms is currently limited by the feasibility of employing advanced scanning sequences during clinical MRIs, as well as access to the specialty skills needed to derive quantitative MRI metrics. Indeed, even relatively basic metrics of total cerebral atrophy and T2 lesion volume are rarely available to clinicians. Different groups are working to bridge this gap by providing cerebral atrophy analysis services to clinicians, with the goal of incorporating atrophy consideration into standard clinical care.e37
Functional neuroimaging
fMRI provides a proxy of brain function, which may help explain cognitive deficits due to MS.24,31,39,40 There has been growing concern, however, about poor reproducibility of fMRI results, including contradictory findings of cross-sectional MS studies (e.g., memory deficits linked to lower39 and higher31 functional connectivity), and a lack of sufficiently large longitudinal studies.41 Inconsistencies may be due in part to differences in data collection and analysis approaches, statistical power, and heterogeneity across patient samples. There may be ways to improve the approach and consequent value of fMRI. First, reproducibility should be established with large collaborative multicenter studies using uniform methods, with out-of-sample replication of results. Next, following a model of “registered reports” in cognitive neurosciencee38 and trial registration (clinicaltrials.gov), an online repository for posting specific study methods and hypotheses prior to data collection may be helpful. This would yield 2 levels of evidence: exploratory and confirmatory. Although exploratory research is important, certified confirmatory results would improve confidence in (1) individual study results, (2) the fMRI method in general, and (3) any future clinical utility of fMRI techniques.
Finally, it is integral that fMRI and behavioral findings be incorporated into working theoretical models of cognitive dysfunction, which will provide the scientific context for a priori hypothesis generation and testing, and model refinement. As discussed in the context of the functional reorganization hypothesis (see references 42 and e39), such models must move beyond overly simplistic views of large network changes as either maladaptive or compensatory, and instead create more dynamic, biologically plausible models informed by multimodality neuroimaging methods.
Treatment and prevention of cognitive impairment
Cognitive rehabilitation
Improved understanding of MS cognitive deficits will inform the nascent field of cognitive rehabilitation, which seeks to restore cognitive functioning (often through intensive cognitive training programs) or teach compensatory strategies to attenuate the deleterious effect of refractory cognitive deficits on quality of life. Efficacy for such interventions in MS is currently low, inconclusive, or preliminary, as concluded by (1) a Cochrane review of 20 randomized or quasi–randomized controlled trials (RCTs) of behavioral interventions to improve cognition in MS (data search up to July 2013),43 (2) a separate systematic review of 33 original intervention studies (including nonrandomized trials, search up to January 2014),44 and (3) a Cochrane review of 15 intervention trials specifically targeting memory (search up to June 2015).45 In addition to small sample sizes, quality of studies was limited by several methodologic flaws (e.g., poor blinding, unvalidated outcomes). Note also that a Cochrane review of symptomatic pharmaceutical treatment of memory deficits in MS revealed only 7 RCTs (data search up to June 2013),46 with no evidence for efficacy (key priorities in table 4).
Table 4.
Key priorities for treatment and prevention of cognitive deficits

We require a science of cognitive rehabilitation capable of yielding high levels of evidence. Toward this end, we must develop theoretical models of MS-related cognitive dysfunction and identify mechanisms of action to treat deficits, followed by large RCTs with validated outcomes. This rigorous pathway to high-level evidence is required by regulatory agencies before approving clinical use of new agents (e.g., DMTs), and perhaps similar regulation should be considered for cognitive rehabilitation. There is less industry funding to perform large labor-intensive RCTs of cognitive rehabilitation interventions; however, grant funding may be sought for multicenter collaborations, which may increase sample size and representativeness. Finally, standards for a priori reporting of methods must be upheld for cognitive rehabilitation RCTs, including greater transparency for outcomes (e.g., specific scores on specific tests registered on clinicaltrials.gov, rather than nonspecific references to “cognition” or “memory tests”). Cognitive rehabilitation researchers are directed to Simons and colleagues47 for a thorough discussion of essential guidelines for the conduct of high-quality cognitive intervention trials.
Theoretical models
As proposed for the field of rehabilitation generally,48 we must identify mechanisms of action and active ingredients of cognitive rehabilitation interventions. For instance, if we discover that a training program improves memory by strengthening hippocampal structure and function, we may also consider other interventions targeting hippocampal health; e.g., aerobic exercise,e40 intellectual enrichment,e41 glucose control,e42 and stress management.e43 Availability of alternative treatments is important when considering clinical feasibility, as time-consuming, expensive cognitive training programs may be feasible for some patients, whereas other patients will be more likely to engage in alternative approaches (e.g., exercise). A model should also distinguish different types of rehabilitation approaches and goals: (1) restorative interventions aiming to bolster underlying neurophysiologic bases of memory, vs (2) compensatory approaches aiming to improve memory through strategies (e.g., mnemonics) or aids (e.g., diaries; see discussione44,e45). This has implications for trial outcomes: e.g., given that compensatory strategies only work when patients use them (like a cane for walking), we should not assess treatment efficacy with standardized memory tests that prevent or encumber strategy usage (as standardized administrations may prevent compensatory strategy usage).
Secondary structural and functional neuroimaging outcomes may help identify mechanisms of action for interventions49 and identify markers of capacity to benefit from interventions (responders vs nonresponders). This may help hone treatments and inform new treatment development, and identify subgroups with residual capacity to respond to interventions. Like cognitive outcomes, specific neuroimaging outcomes should be stated (registered) prior to data collection, or otherwise be described as exploratory. Finally, the goal of enhancing brain structure and function in biologically plausible and lasting ways likely requires greater “doses” (duration and intensity) of interventions than is typically performed, and perhaps combined therapies with potentially synergistic effects (e.g., training plus neurostimulatione46).
Primary prevention
We should also promote primary prevention of cognitive decline, in part through interventions and healthy lifestyles that promote brain maintenance.50,51 Candidate modifiable lifestyle factors to build or maintain brain reserve include physical exercise,52 mentally active lifestyles (cognitive reserve),38,53 management of cardiovascular risk factors54 and other comorbidities,55 smoking cessation,56 and stress management.57 Research should strive to understand mechanisms of action for protective factors (e.g., moderating MS disease activity vs maintaining brain volume in a non-disease-specific way). Studies typically examine few risk or protective factors at a time, but we need larger studies of numerous factors in the same large cohort to understand (1) whether and to what extent each risk or protective factor makes independent contributions to an outcome, (2) mediating or interactive effects among different factors on an outcome, and (3) how (1) and (2) differ across outcomes (e.g., speed, memory). We also need to raise the level of evidence (e.g., RCTs) linking lifestyle factors to cognition, and explore additional variables (e.g., diete47,e48). (DMTs as protective factors are discussed in links.lww.com/WNL/A185 and links.lww.com/WNL/A186.)
Holistic approach to cognitive rehabilitation
Effective intervention will require patient understanding, motivation, and compliance, as well as willingness by clinicians to consider the unique circumstances of individual patients (e.g., family/social support, comorbidities, goals). Ideal holistic rehabilitation approaches consider cognitive, emotional, and psychosocial aspects of each patient's life.58 Patient education may promote metacognition and active participation. For instance, therapeutic feedback after neuropsychological assessments may support understanding of one's cognitive profile, and aid patients in finding ways to maximize cognitive strengths and minimize weaknesses in daily life. Education on cognitive deficits and factors affecting cognition (e.g., sleep, medications, mood, fatigue) may promote active participation and a positive sense of agency among patients.59 Indeed, preliminary results on structured metacognitive training with peer support are encouraging.e49 To advance this holistic approach, research is also needed to better understand shared neural bases for mood and cognitive dysfunction,60 which may yield new directions for cognitive treatments. Finally, treatment will likely be most effective when tailored to a patient's specific deficit in the context of his or her degree of spared cognition and cerebral reserve. For instance, a memory deficit due to diffuse white matter lesions may require a different treatment approach than a deficit secondary to a focal hippocampal lesion. Consideration of distinct subtypes of deficits requiring distinct treatment approaches (i.e., precision medicine) is a key challenge and opportunity for the future.
Discussion
The literature on cognition in persons with MS has grown exponentially over the last 25 years, and cognitive dysfunction is now recognized as a core symptom of MS. Herein we discussed obstacles and challenges for the field and made recommendations for moving research forward. The next 25 years will bring redoubled collaboration across centers and areas of expertise, and utilize advances in neuroimaging, genetics/epigenetics, and validation of cognitive endpoints. Collaborations and advanced methods are invaluable, but the real science of cognition and cognitive rehabilitation in MS will rely on multidisciplinary collaborations toward development of biologically based theoretical models of cognition capable of empirical validation and evidence-based refinement, providing the necessary context for effective treatment discovery.
Glossary
- BVMT-R
Brief Visuospatial Memory Test–Revised
- CVLT-II
California Verbal Learning Test–II
- DMT
disease-modifying therapy
- MS
multiple sclerosis
- PST
Processing Speed Test
- RCT
randomized controlled trial
- SDMT
Symbol Digit Modalities Test
Author contributions
J.F.S. conceptualized and initiated the project and coordinated efforts by coauthors. All authors made intellectual contributions, and read, revised, and approved the final manuscript.
Study funding
No targeted funding reported.
Disclosure
J. Sumowski reports no disclosures relevant to the manuscript. R. Benedict receives research support from Acorda, Novartis, Genzyme, Biogen, and Mallinckrodt Pharmaceuticals; is on the speakers' bureau for EMD Serono; consults for Biogen, Genentech, Genzyme, Novartis, AbbVie, Roche, and Sanofi; and receives royalties from Psychological Assessment Resources. C. Enzinger has received funding for travel, speaker honoraria, or research funding from Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Genzyme, and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis. He is serving on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis; and as academic editor for PLoS One. M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd.; has received funding for travel from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries Ltd.; serves as a consultant to Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Pepgen Corporation, and Teva Pharmaceutical Industries Ltd.; serves on speakers' bureaus for Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries Ltd.; and receives research support from Bayer Schering Pharma, Biogen Idec, Novartis, Merck Serono, Teva Pharmaceutical Industries Ltd., CurePSP, and the Jacques and Gloria Gossweiler Foundation. J. Geurts serves on the editorial boards of MS Journal, BMC Neurology, MS International, and Neurology®, the Scientific Advisory Board of the Dutch MS Research Foundation and MS Academia, Merck-Serono, and has served as a consultant for Merck- Serono, Biogen Idec, Novartis, Genzyme, and Teva Pharmaceuticals. P. Hamalainen reports no disclosures relevant to the manuscript. H. Hulst serves as a consultant for Genzyme, Merck-Serono, Teva Pharmaceuticals, and Novartis. M. Inglese has received research grants from Novartis and Teva Neuroscience. V. Leavitt reports no disclosures relevant to the manuscript. M. Rocca has received speakers honoraria from Biogen Idex, Excemed, and Novartis. E. Rosti-Otajarvi reports no disclosures relevant to the manuscript. S. Rao has received royalties from the Cleveland Clinic for licensing the tablet-based Processing Speed Test. He has received honoraria, royalties, consulting fees, or research funding from Biogen, Genzyme, Novartis, and the CHDI Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Charcot JM. Lectures on the Diseases of the Nervous System. Sigerson G, trans. London: New Sydenham Society; 1877. [Google Scholar]
- 2.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: I: frequency, patterns, and prediction. Neurology 1991;41:685–691. [DOI] [PubMed] [Google Scholar]
- 3.Benedict RH, Cookfair D, Gavett R, et al. Validity of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychol Soc 2006;12:549–558. [DOI] [PubMed] [Google Scholar]
- 4.Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 2011;76:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler 2009;15:1215–1227. [DOI] [PubMed] [Google Scholar]
- 6.Patel VP, Zambrana A, Walker LA, Herrmann N, Feinstein A. Distraction adds to the cognitive burden in multiple sclerosis. Mult Scler 2016;23:106–113. [DOI] [PubMed] [Google Scholar]
- 7.Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler 2012;18:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SM, Losinski G, Mourany L, et al. Processing speed test: validation of a self-administered, iPad-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler J 2017;23:1929–1937. [DOI] [PubMed] [Google Scholar]
- 9.Parmenter BA, Testa SM, Schretien DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis. J Int Neuropsychol Soc 2010;16:6–16. [DOI] [PubMed] [Google Scholar]
- 10.Benedict RH, Walton MK. Evaluating cognitive outcome measures for MS clinical trials: what is a clinically meaningful change? Mult Scler 2012;18:1673–1679. [DOI] [PubMed] [Google Scholar]
- 11.Benedict RH, Drake AS, Irwin LN, et al. Benchmarks of meaningful impairment on the MSFC and BICAMS. Mult Scler 2016;22:1874–1882. [DOI] [PubMed] [Google Scholar]
- 12.Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol 2010;24:1131–1145. [DOI] [PubMed] [Google Scholar]
- 13.Benedict RHB, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognitive performance outcome measure for multiple sclerosis. Mult Scler 2017;23:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 2015;14:302–317. [DOI] [PubMed] [Google Scholar]
- 15.Benedict RH, Morrow S, Rodgers J, et al. Characterizing cognitive function during relapse in multiple sclerosis. Mult Scler 2014;20:1745–1752. [DOI] [PubMed] [Google Scholar]
- 16.Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 1989;39:161–166. [DOI] [PubMed] [Google Scholar]
- 17.Kincses ZT, Ropele S, Jenkinson M, et al. Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler 2011;17:681–689. [DOI] [PubMed] [Google Scholar]
- 18.Roosendaal SD, Geurts JJ, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage 2009;44:1397–1403. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012;135:2952–2961. [DOI] [PubMed] [Google Scholar]
- 20.Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2016;139:115–126. [DOI] [PubMed] [Google Scholar]
- 21.Schoonheim MM, Popescu V, Rueda Lopes FC, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 2012;79:1754–1761. [DOI] [PubMed] [Google Scholar]
- 22.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007;69:1213–1223. [DOI] [PubMed] [Google Scholar]
- 23.Pinter D, Khalil M, Pichler A, et al. Predictive value of different conventional and non-conventional MRI-parameters for specific domains of cognitive function in multiple sclerosis. Neuroimage Clin 2015;7:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca MA, Valsasina P, Hulst HE, et al. Functional correlates of cognitive dysfunction in multiple sclerosis: a multicenter fMRI Study. Hum Brain Mapp 2014;35:5799–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petracca M, Vancea RO, Fleysher L, Jonkman LE, Oesingmann N, Inglese M. Brain intra- and extracellular sodium concentration in multiple sclerosis: a 7 T MRI study. Brain 2016;139:795–806. [DOI] [PubMed] [Google Scholar]
- 26.Bodini B, Veronese M, Garcia-Lorenzo D, et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol Epub 2016 Feb 18. [DOI] [PMC free article] [PubMed]
- 27.Enzinger C, Barkhof F, Ciccarelli O, et al. Nonconventional MRI and microstructural cerebral changes in multiple sclerosis. Nat Rev Neurol 2015;11:676–686. [DOI] [PubMed] [Google Scholar]
- 28.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain 2008;131:1134–1141. [DOI] [PubMed] [Google Scholar]
- 29.Roosendaal SD, Moraal B, Pouwels PJ, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 2009;15:708–714. [DOI] [PubMed] [Google Scholar]
- 30.Muhlert N, Atzori M, De Vita E, et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry 2014;85:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BM, Barkhof F, Geurts JJ. Memory impairment in multiple sclerosis: relevance of hippocampal activation and hippocampal connectivity. Mult Scler 2015;21:1705–1712. [DOI] [PubMed] [Google Scholar]
- 32.Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol 2011;69:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 2011;12:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca MA, Longoni G, Pagani E, et al. In vivo evidence of hippocampal dentate gyrus expansion in multiple sclerosis. Hum Brain Mapp 2015;36:4702–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisecco A, Rocca MA, Pagani E, et al. Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum Brain Mapp 2015;36:2809–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern Y, Habeck C, Steffener J, et al. The reference ability neural network study: motivation, design, and initial feasibility analyses. NeuroImage 2014;103:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology 2013;81:1759–1767. [DOI] [PubMed] [Google Scholar]
- 38.Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Mult Scler 2013;19:1122–1127. [DOI] [PubMed] [Google Scholar]
- 39.Leavitt VM, Paxton J, Sumowski JF. Default network connectivity is linked to memory status in multiple sclerosis. J Int Neuropsychol Soc 2014;20:937–944. [DOI] [PubMed] [Google Scholar]
- 40.Eijlers AJ, Meijer KA, Wassenaar TM, et al. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 2017;88:952–960. [DOI] [PubMed] [Google Scholar]
- 41.Enzinger C, Pinter D, Rocca MA, et al. Longitudinal fMRI studies: exploring brain plasticity and repair in MS. Mult Scler 2016;22:269–278. [DOI] [PubMed] [Google Scholar]
- 42.Schoonheim MM. Functional reorganization is a maladaptive response to injury–Commentary. Mult Scler 2017;23:194–196. [DOI] [PubMed] [Google Scholar]
- 43.Rosti-Otajarvi EM, Hamalainen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev 2014:CD009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitolo M, Venneri A, Wilkinson ID, Sharrack B. Cognitive rehabilitation in multiple sclerosis: a systematic review. J Neurol Sci 2015;354:1–9. [DOI] [PubMed] [Google Scholar]
- 45.das Nair R, Martin KJ, Lincoln NB. Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev 2016;3:CD008754. [DOI] [PubMed] [Google Scholar]
- 46.He D, Zhang Y, Dong S, Wang D, Gao X, Zhou H. Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev 2013;CD008876. [DOI] [PubMed] [Google Scholar]
- 47.Simons DJ, Boot WR, Charness N, et al. Do “brain training” programs work? Psychol Sci Public Interest 2016;17:103–186. [DOI] [PubMed] [Google Scholar]
- 48.Whyte J, Dijkers MP, Hart T, et al. Development of a theory-driven rehabilitation treatment taxonomy: conceptual issues. Arch Phys Med Rehabil 2014;95(1 suppl):S24–S32.e22. [DOI] [PubMed] [Google Scholar]
- 49.Prosperini L, Piattella MC, Gianni C, Pantano P. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural plasticity. 2015;2015:481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology 2014;82:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyberg L, Lövdén M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci 2012;16:292–305. [DOI] [PubMed] [Google Scholar]
- 52.Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 2012;8:487–497. [DOI] [PubMed] [Google Scholar]
- 53.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: what you've got and how you use it. Neurology 2013;80:2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:181–187. [DOI] [PubMed] [Google Scholar]
- 55.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol 2010;9:820–828. [DOI] [PubMed] [Google Scholar]
- 56.Manouchehrinia A, Tench CR, Maxted J, Bibani RH, Britton J, Constantinescu CS. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013;136:2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology 2012;79:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson BA. Neuropsychological rehabilitation. Annu Rev Clin Psychol 2008;4:141–162. [DOI] [PubMed] [Google Scholar]
- 59.Hämäläinen P, Rosti-Otajärvi E. Is neuropsychological rehabilitation effective in multiple sclerosis? Neurodegenerative Dis Manag 2014;4:147–154. [DOI] [PubMed] [Google Scholar]
- 60.Colasanti A, Guo Q, Giannetti P, et al. Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 2016;80:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


