ABSTRACT
Globular (G)-actin, the actin monomer, assembles into polarized filaments that form networks that can provide structural support, generate force and organize the cell. Many of these structures are highly dynamic and to maintain them, the cell relies on a large reserve of monomers. Classically, the G-actin pool has been thought of as homogenous. However, recent work has shown that actin monomers can exist in distinct groups that can be targeted to specific networks, where they drive and modify filament assembly in ways that can have profound effects on cellular behavior. This Review focuses on the potential factors that could create functionally distinct pools of actin monomers in the cell, including differences between the actin isoforms and the regulation of G-actin by monomer binding proteins, such as profilin and thymosin β4. Owing to difficulties in studying and visualizing G-actin, our knowledge over the precise role that specific actin monomer pools play in regulating cellular actin dynamics remains incomplete. Here, we discuss some of these unanswered questions and also provide a summary of the methodologies currently available for the imaging of G-actin.
KEY WORDS: G-actin, Profilin, Thymosin β 4, β-actin, γ-actin
Summary: This Review provides an overview of how the cell regulates its actin monomer pool, focusing on factors that could create distinct groups of G-actin, and proposes an active role for G-actin in cytoskeletal dynamics.
Introduction
The ability of actin to contribute to essential cellular functions such as motility, cell division and vesicle trafficking is dependent on the precise spatiotemporal control of its polymerization into actin filament architectures, and the disassembly of these structures back into the monomer pool. These processes can occur on short time scales, allowing the cell to rapidly respond to internal or external stimuli. Filamentous (F)-actin networks such as the lamellipodia are highly dynamic structures, with individual filament lifetimes as short as ten seconds (Watanabe and Mitchison, 2002) and complete network turnover in minutes (Lai et al., 2008; Theriot and Mitchison, 1991; Yamashiro et al., 2014), although this does vary depending on cell type and structure, as actin networks can also comprise metastable filaments that can last for days, as seen in myofibrils (Clark and Zak, 1981; Zak et al., 1977). The cell relies on a large reserve of monomers [globular (G)-actin] to maintain the dynamic nature of the actin cytoskeleton, with cellular concentrations reaching up to 1500 times the critical concentration of 0.1 μM at which monomers spontaneously polymerize into filaments (Gordon et al., 1977; Tilney, 1976; Vinson et al., 1998). In solution, G-actin concentration determines the polymerization rate (Pollard, 1986). In cells, the polymerization rates can be dramatically lower than the local monomer concentration (Koestler et al., 2009), indicating that polymerization is gated by monomer-binding proteins, such as the profilins (Carlsson et al., 1977; Ozaki and Hatano, 1984), thymosin β4 (Tβ4) (Safer et al., 1991) and actin assembly factors. Monomer levels are also regulated by filament turnover, for example, by inhibiting polymerization through capping proteins (Isenberg et al., 1980) or by stimulating filament disassembly through the actin-severing proteins of the ADF/cofilin family (Andrianantoandro and Pollard, 2006; Kiuchi et al., 2007; Vitriol et al., 2013).
Historically, the actin monomer pool has been thought of as homogenous. In this model, actin monomers supplied to networks such as those in the lamellipodia at the leading edge are controlled by their cellular concentration (Kiuchi et al., 2007; Pollard, 1986) and factors that influence diffusion, such as the viscosity of the cytoplasm (Drenckhahn and Pollard, 1986). This is supported by the observation that G-actin pools are depleted in response to different cellular stimuli that induce actin polymerization (Higashida et al., 2013; Kiuchi et al., 2011; Lomakin et al., 2015) or during polymerization-intensive processes, for example, cell migration (Cramer et al., 2002). Additionally, it has been shown that G-actin concentrations are relatively similar throughout the cell (Kiuchi et al., 2011), except in extreme examples of cells with polarized regions of assembly and disassembly, such as a motile keratocyte (Novak et al., 2008). However, even in this latter example, distribution of G-actin is not sensitive to fluctuations of actin assembly or cytoplasmic flow (Novak et al., 2008).
Recent work has caused the field to expand upon the idea of a single homogeneous G-actin pool to include distinct groups of monomers that drive and modify filament formation. Several lines of evidence showing non-random monomer distribution support this model: G-actin has been observed to directionally translocate to the leading edge (Fan et al., 2012b; Zicha et al., 2003), actin-encoding mRNAs localize to and are locally translated at regions of active polymerization (Hoock et al., 1991; Leung et al., 2006; Wang et al., 2016; Yao et al., 2006), and actin monomers have been shown to localize to actin-based structures, including the leading edge of migrating cells (Cao et al., 1993; Lee et al., 2013; Vitriol et al., 2015), neuronal growth cones (Lee et al., 2013; Wang et al., 2016) and dendritic spines (Lei et al., 2017). An argument against the significance of these events is that diffusion will rapidly dampen small gradients of G-actin that arise from localization or localized translation. However, these monomers can be functionally heterogeneous, bias specific types of actin assembly and have substantial influence over cellular behavior (Lee et al., 2013; Rotty et al., 2015; Suarez et al., 2015; Vitriol et al., 2015; Yao et al., 2006). Additionally, mathematical modeling has been used to demonstrate that competition between F-actin networks from a limited pool of G-actin is not sufficient to explain differential network growth (Mohapatra et al., 2017). Therefore the existence of heterogeneous pools of G-actin provides a means for the cell to modulate different actin networks in a spatially and temporally regulated manner.
Although the influence of different pools of G-actin most likely represents an additional layer of regulation and is not necessarily the predominant force driving polymerization, it is an important parameter for how actin networks dynamically behave that can have significant cellular consequences. However, the incorporation of this new information into existing hypothesized models of actin dynamics thus far has been slow. While the literature on actin dynamics continues to build an increasingly complex picture, many of these studies focus on F-actin and the competition of differential F-actin networks for a homogeneous monomeric pool (Burke et al., 2014; Lomakin et al., 2015). In this Review, we present a compilation of the evidence that G-actin is an active participant in the dynamic assembly and regulation of actin networks.
β- and γ-actin – small differences with potentially large consequences
The most obvious source of distinct pools of actin monomers is the presence of the various actin isoforms, which are known to have subtly different cellular functions (Perrin and Ervasti, 2010). Although there are six actin isoforms in total, only β-actin (encoded by ACTB) and γ-actin (encoded by ACTG1) are expressed in most mammalian cell types. β-actin is the predominantly expressed isoform, often found at twofold higher levels than to γ-actin (Erba et al., 1988; Furness et al., 2005; Kapustina et al., 2016; Khaitlina, 2001). Both isoforms were originally thought to have overlapping functions due to their close similarity, differing by only four amino acids, and their ubiquitous presence in most cell types. Additionally, it is difficult to purify β-actin without γ-actin, or vice-versa, causing most biochemical studies that investigate isoform differences to focus on muscle versus non-muscle actin. However, there is evidence that β- and γ-actin have distinct roles in cellular physiology and organism development.
Owing to the aforementioned difficulty in obtaining pure β-actin or γ-actin, there is little evidence of differential behavior at the biochemical level. However, one study using a baculovirus expression system to obtain individual isoforms determined that, in the presence of divalent Ca2+, β-actin has increased rates of both polymerization and depolymerization (Bergeron et al., 2010). The differences were much more subtle for actin bound to the divalent ion Mg2+, with only a slight difference detected in the nucleotide exchange rate between the two isoforms. The physiological relevance of these findings is not clear, since Mg2+–actin is the major form of actin in the cell (Herz et al., 1969) and the cation exchange on actin is slow (Estes et al., 1987), implying that Ca2+-bound actin only exists if there is a persistently high concentration of Ca2+. However, if actin was locally translated in a microdomain of high Ca2+ (Wei et al., 2009), or in a region of the cell with a sufficiently high Ca2+ gradient to ensure the binding of Ca2+ instead of Mg2+ (Beerman et al., 2015; Bergeron et al., 2010), then it may be possible to generate a small pool of Ca2+–actin. Furthermore, the ion-dependent polymerization differences also demonstrate that β- and γ-actin are not biochemically identical and may have other distinctive traits that are yet to be determined.
Non-redundancy between β- or γ-actin at the cellular and organismal level has been shown through loss-of-function and overexpression experiments. Deletion of β-actin in mice results in embryonic lethality, whereas γ-actin knockouts can survive into adulthood, although they show increased incidence of hearing loss and higher morbidity compared to wild-type mice (Belyantseva et al., 2009; Bunnell et al., 2011). In fibroblasts, knockdown and over-expression of β-actin reduces and enhances cell motility (Bunnell et al., 2011; Peckham et al., 2001), respectively, whereas γ-actin is not required for migration (Bunnell and Ervasti, 2010). In motor neurons, knockdown of β-actin reduces growth cone size and motility, whereas γ-actin-depleted neurons have normal axonal outgrowth, but display a reduction in axonal filopodia dynamics (Moradi et al., 2017). β-actin has also been found to be the more prevalent isoform in the G-actin pool (Kapustina et al., 2016), and β-actin knockouts in mouse embryonic fibroblasts have been shown to lead to decreased ratios of G- to F-actin (Bunnell et al., 2011). Differential isoform function can also affect cell adhesion properties. γ-actin is necessary for the formation of tight junctions (Baranwal et al., 2012), whereas β-actin has been shown to be dispensable at tight junctional complexes, but is required for operational adherens junctions (Baranwal et al., 2012). However, it is important to stress that the observed functional distinctions between the isoforms are general trends (Flamholz et al., 2014) and, to some degree, the actin isoforms can compensate for each other. This is evidenced in experiments showing an upregulation of α- and γ-actin in response to β-actin depletion (Moradi et al., 2017), or the downregulation of endogenous β- and γ-actin genes in response to exogenously expressed γ-actin (Lloyd et al., 1992) to maintain overall cellular actin levels.
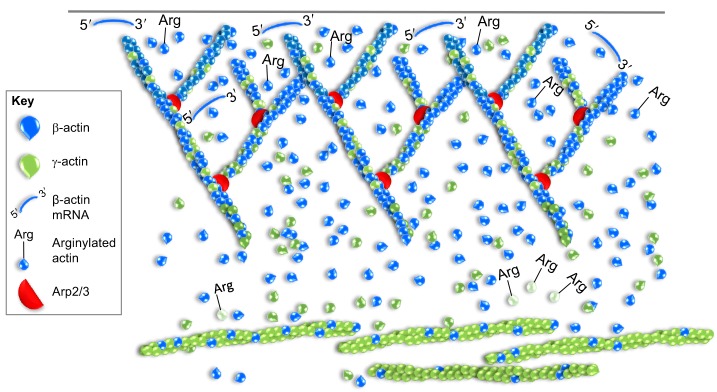
The functional difference between β-actin and γ-actin is also evidenced through their differential localization patterns (Fig. 1). Distinct separation of the two isoforms has been unambiguously shown in specialized cell types such as myoblasts and inner ear hair cells (Perrin and Ervasti, 2010), although in other cell types, the results are more indicative of an enrichment of each at particular networks. Several studies have found β-actin to preferentially localize to the lamellipodia at the leading edge (Gimona et al., 1994; Hill and Gunning, 1993; Hoock et al., 1991; Pasquier et al., 2015; Taneja and Singer, 1990), whereas γ-actin has been shown to be more enriched in filaments within the cytoplasm (Hill and Gunning, 1993; Otey et al., 1986; Pasquier et al., 2015; Shum et al., 2011). Other studies have shown that β-actin and γ-actin localize to different stress fiber subtypes (Shum et al., 2011) and that γ-actin is enriched in actin arcs behind the lamellipodia (Pasquier et al., 2015). However, there have been contradictory results depending on the cell type and staining methodology. For example, the use of a particular set of isoform-specific monoclonal antibodies in fibroblasts has shown that β-actin and γ-actin differentially localize to stress fibers and lamellipodia (Dugina et al., 2009), whereas the use of different antibodies against both these proteins suggests complete colocalization of the two isoforms in those structures (Bunnell et al., 2011). Because these antibodies are generated against nearly identical proteins, careful controls such as the use of isoform-depleted cells to demonstrate antibody specificity (Bunnell et al., 2011) are essential in order to properly interpret such results. It should also be noted that enrichment of an isoform at a particular structure should take into account relative expression levels. For example, if there was true equality of β- and γ-actin, their composition in every network of the cell should equal their relative expression. To date there have been no truly detailed, quantitative studies on β-actin and γ-actin localization that consider this, and most of the inferences made about their differences have been from subjective interpretation of images. As the resolution of light microcopy imaging increases, it will also be interesting to see whether there are isoform differences in the composition of sub-network structures, such pre-filopodial bundles within a lamellipodia, or even in individual filaments.
Fig. 1.
Incorporation of β-actin and γ-actin into distinct actin networks. Although they can overlap, β-actin and γ-actin isoforms have been shown to exhibit different cellular localizations. Illustrated here is a scenario where β-actin is enriched in the lamellipodia and γ-actin in actin arcs and/or stress fiber structures. β-actin-encoding mRNA is also localized and locally translated at the leading edge. Arginylation of β-actin (Arg) promotes localization and assembly in lamellipodia but targets γ-actin for degradation, as depicted by the pale color.
Spatial regulation of the actin isoforms also occurs at the mRNA level. The concentration of the G-actin pool can regulate actin mRNA synthesis, with mRNA levels decreasing in response to an increase in G-actin (Bershadsky et al., 1995; Lyubimova et al., 1997, 1999). The actin monomer-binding myocardin transcriptional coactivator (MAL, also known as MYOCD) and serum response factor (SRF) complex (MAL–SRF) is one such regulator of this feedback loop (Salvany et al., 2014). β-actin-encoding mRNA has been found at the leading edge of fibroblasts (Ross et al., 1997), and to localize to neuronal growth cones (Leung et al., 2006; Moradi et al., 2017; Zhang et al., 2001) and dendritic spines (Tiruchinapalli et al., 2003). The specific mRNA localization is regulated by an additional sequence in the 3′ untranslated region (UTR) of β-actin that interacts with zipcode-binding protein (ZBP1; also known as IGF2BP1), which controls the targeting and regulated translation of mRNA transcripts (Ross et al., 1997). Furthermore, ribosomal translation rates for γ-actin have been shown to be slower than for β-actin (Zhang et al., 2010). Localization of β-actin-encoding mRNA and its translation at the protrusions of moving cells can have profound effects on cell motility and axon guidance (Kislauskis et al., 1997; Vitriol and Zheng, 2012). The underlying mechanisms remain relatively unknown, but it may be that newly synthesized β-actin lacks a post-translational modification that is present in ‘older’ actin which hinders assembly (Wang et al., 2001), or that an actin chaperonin is biasing polymerization to specific actin populations (Brackley and Grantham, 2010; Saegusa et al., 2014). However, although de novo synthesis of actin has been shown to play an important role in the overall actin balance of a cell, it is not the predominant source of G-actin for actin polymerization. It has been estimated that up to 7% of F-actin in motile cells contains newly synthesized protein (Condeelis and Singer, 2005), though this may vary depending on cell type, suggesting that almost all F-actin is generated through polymerization of the existing monomer pool. Thus, localized translation of β-actin most likely affects actin dynamics in specialized situations. It should also be noted that local mRNA translation is functionally distinct from the localization of actin monomers at the leading edge, which occurs independently of protein translation (Lee et al., 2013) and at time scales that are faster than protein translation would allow for (Vitriol et al., 2015). However, both processes appear to positively regulate lamellipodia protrusions and cell movement.
Post-translational modifications of β-actin and γ-actin could also lead to their differential localization and incorporation into filament types. Arginylation of actin differentially affects the two isoforms (Karakozova et al., 2006; Zhang et al., 2010). While arginylation of γ-actin can target it for proteasomal degradation (Zhang et al., 2010), the same modification on β-actin positively affects its function and is required for both cell spreading and lamellipodia formation (Karakozova et al., 2006). Lack of arginylation in the β-actin isoform promotes a collapse of the leading edge in mouse embryonic fibroblasts (Karakozova et al., 2006). Other post-translational modifications of actin may also indirectly influence G-actin pools by altering filament stability in specific regions of the cell that are differentially populated by the β and γ isoforms. For example, the Mical family of redox enzymes promotes disassembly of F-actin (Hung et al., 2011), whereas methylation has been hypothesized to stabilize filaments (Nyman et al., 2002; Terman and Kashina, 2013). Localization of these processes to actin structures enriched in a specific isoform could potentially amplify the differences between β-actin and γ-actin monomer pools.
Little is known about how the cell recognizes or treats β-actin and γ-actin as separate entities. There have been a few studies showing a differential interaction of proteins with muscle and non-muscle actin; for instance, the increased cooperative binding of cofilin proteins to β- and/or γ-actin compared with α-actin (De La Cruz, 2005), although they did not discriminate between the β- and γ-isoforms. There are only a few known cases of proteins binding discretely to the β- or γ-isoforms; for instance, it has been shown that L-plastin binds to β-actin and not γ-actin (Namba et al., 1992), whereas annexin V has been shown to specifically bind γ-actin (Tzima et al., 2000). The actin N-terminus, which contains the four amino acids that differentiate β-actin and γ-actin, makes contacts with myosin and other actin-binding proteins (Vandekerckhove, 1990). However, there are no in-depth studies investigating the binding affinity of β-actin and γ-actin to important actin monomer-binding proteins, such as profilin-1 (hereafter referred to as profilin) or thymosin β4 (Tβ4; also known as TMSB4X). While profilin does not contact the N-terminus of actin (Schutt et al., 1993), Tβ4 may (Safer et al., 1997). Future studies could focus on the isoform-dependent differences in interactions with Tβ4 and other monomer binding proteins that make contact with the N-terminus of actin.
Profilin – the architect of the G-actin pool
Monomer-binding proteins, such as profilin and Tβ4, are required to maintain a reserve of free actin monomers at concentrations in excess of the critical concentration required for spontaneous actin polymerization. Profilin is functionally conserved throughout the eukaryotic kingdom (Blasco et al., 1991) and was originally identified as a protein that could bind to and sequester monomeric actin (Carlsson et al., 1977). However, studies showing the association of the profilin–actin complex with the barbed end of actin filaments in the presence of ATP-bound actin (Pantaloni and Carlier, 1993; Pollard and Cooper, 1984; Tilney et al., 1983), combined with its ability to accelerate exchange of ADP for ATP (Goldschmidt-Clermont et al., 1991b; Mockrin and Korn, 1980; Pantaloni and Carlier, 1993; Selden et al., 1999), defined a new role for profilin as an F-actin-promoting factor. Furthermore, it can interact with formins (Chang et al., 1996; Romero et al., 2004), Ena/VASP proteins (Kang et al., 1997; Reinhard et al., 1995), WAVE (also known as WASF) proteins (Miki et al., 1998), and WASp (also known as WAS) (Suetsugu et al., 1998) through poly-L-proline interactions to influence the assembly of specific types of filaments (Rotty et al., 2015; Suarez et al., 2015). Additionally, profilin can bind to phosphoinositides at the plasma membrane (Goldschmidt-Clermont et al., 1990; Machesky et al., 1990; Ostrander et al., 1995), which inhibits its ability to form a complex with G-actin (Goldschmidt-Clermont et al., 1991a; Lassing and Lindberg, 1985, 1988). Controlling phosphoinositides may thus provide a mechanism for modulating G-actin levels by regulating the availability of profilin. Post-translational modifications such as phosphorylation can also enhance the interaction between profilin and actin (Fan et al., 2012a). Its diversity in binding partners and ability to intersect with a number of signaling pathways allows profilin to serve as a key regulator of actin physiology.
Profilin has been found to drive the preferential assembly of distinct actin networks by biasing polymerization toward specific types of filaments. Profilin–actin (profilin-bound G-actin) is more likely to polymerize with formin- and Ena/VASP-mediated filaments than with filaments nucleated by Arp2/3 (Rotty et al., 2015; Suarez et al., 2015). While Arp2/3-mediated polymerization can occur in the presence of profilin (Machesky et al., 1999), profilin exhibits a concentration-dependent inhibitory effect on Arp2/3-dependent nucleation and branch formation (Rodal et al., 2003; Rotty et al., 2015; Suarez et al., 2015). This most likely occurs due to the ability of profilin to compete with the Arp2/3 activator WASp for actin monomer binding (Marchand et al., 2001; Suarez et al., 2015), although it can also bind directly to Arp2 and sterically hinder association with the WASp activator (Mullins et al., 1998; Suarez et al., 2015). Contrary to its effects on Arp2/3-mediated filaments, profilin accelerates polymerization of formin-bound filaments by as much as an order of magnitude (Kovar et al., 2006) and may also enhance VASP-mediated growth (Hansen and Mullins, 2010). Profilin inhibits the nucleation activity of formins (Kovar et al., 2003) but once the filaments are formed, their growth is accelerated. Thus, profilin-mediated actin polymerization favors the elongation of barbed ends that are associated with processive polymerases, such as formins and Ena/VASP, and hinders Arp2/3-mediated branching, which favors the growth of specific actin networks, assuming they are competing for the same monomeric pool. This competition is particularly apparent in the fission yeast Schizosaccharomyces pombe, where Arp2/3 and formin-based actin networks are easy to distinguish since they are spatially segregated. In fission yeast, ∼35–50% of polymerized actin is contained in Arp2/3-nucleated actin patches and ∼10–15% is in formin-mediated actin cables or at the cytokinetic ring (Burke et al., 2014; Sirotkin et al., 2010; Wu and Pollard, 2005). These networks are in a homeostasis; inhibiting one increases the presence of the other (Burke et al., 2014). As there is a tenfold difference in the concentration of Arp2/3 over that of formins (Burke et al., 2014), profilin may have a key role in regulating the balance between the two types of networks by favoring the growth of formin-mediated elongation and providing a braking mechanism for Arp2/3-nucleated networks. This hypothesis has been supported by experiments showing that increasing the profilin:actin ratio through overexpression will increase the formation of cables and decrease that of patches, and vice versa (Suarez et al., 2015).
It is important to note that even in non-vertebrates, which lack Tβ4, where most of the polymerizable actin is bound to profilin (Kaiser et al., 1999; Tseng et al., 1984), profilin cannot maintain the monomer pool on its own; its activity is tightly coordinated with the depolymerization and polymerization of actin filaments. For this Review, we will focus on two predominant factors that regulate these processes as examples: ADF/cofilin family proteins and the F-actin capping protein (CP; a heterodimer formed of CAPZA1 or CAPZA2, and CAPZB). ADF/cofilin severs actin filaments to increase F-actin disassembly and the turnover of actin networks (Carlier et al., 1997; Elam et al., 2013) to create ADP-actin monomers. Upregulating ADF/cofilin activity will thus increase monomer concentration. Profilin will bind ADP-actin and, through its nucleotide exchange capacity (Goldschmidt-Clermont et al., 1991b), convert recently depolymerized actin into a polymerization-competent pool (Didry et al., 1998) (Fig. 2). Conversely, the monomer pool can also be regulated through the activity of CP, which can prevent profilin from promoting monomer addition at filament barbed ends (Kang et al., 1999; Pantaloni and Carlier, 1993). Profilin itself can bind to barbed ends and antagonize the binding of CP, although this has only been shown at concentrations that may not be physiologically relevant (Pernier et al., 2016). Since profilin inhibits nucleation of new filaments (Pollard and Cooper, 1984), capping of existing filaments can have a strong influence on the monomer pool by creating a system that is not at equilibrium, where profilin-bound actin is a kinetic phenomenon. Thus, a balance between filament assembly, disassembly and the regulatory activity of profilin is necessary to maintain the monomer pool and control the rate of filament polymerization.
Fig. 2.
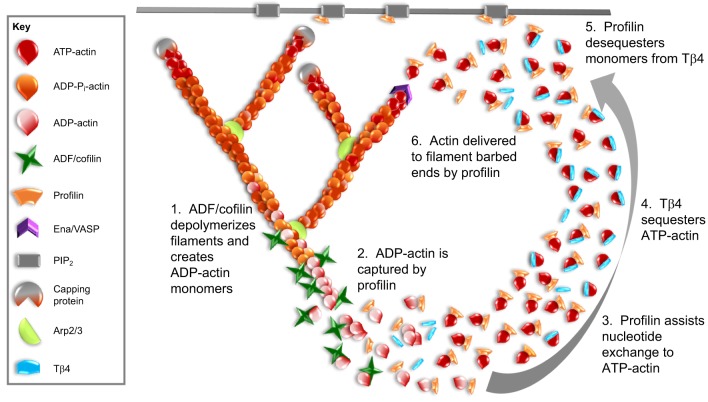
Profilin and Tβ4 regulate the monomer pool. This figure presents a model for how profilin and Tβ4 may work together to convert newly released actin monomers into a polymerization-competent pool that is then directed to re-polymerize into new filaments. After depolymerization of filaments by disassembly factors such as ADF/cofilin (1), profilin binds to the newly released monomers (2) due to its higher affinity for ADP-actin. Profilin induces nucleotide exchange (3), and the majority of monomers are transferred to Tβ4 (4), which has a 50-fold higher affinity for ATP-actin over ADP-actin and is present in the cell at higher concentrations than profilin. Tβ4 holds the monomers in a polymerization-competent pool. Owing to high rates of exchange, profilin de-sequesters monomers from Tβ4 (5) and delivers G-actin to barbed-end polymerases such as Ena/VASP (6). Profilin can also localize to the plasma membrane through an interaction with phosphatidylinositol 4,5-bisphosphate (PIP2). A profilin–Tβ4–G-actin ternary complex may assist in the transfer of G-actin from Tβ4 to profilin by providing an intermediate state, where the actin monomer does not dissociate from either protein, thereby preventing its spontaneous assembly into filaments.
Additionally, this coordination can bias which types of filaments are polymerized. First, profilin-bound actin will be preferentially incorporated into barbed ends associated with proteins that antagonize CP. Second, a balance between CP and profilin can be used to control nucleation-based versus elongation-based processes. CP is known to increase the Arp2/3-branched network density in biochemical assays by restricting filament length (Blanchoin et al., 2000), but it can also have more complex effects on network growth in the presence of profilin and barbed-end polymerases (Fig. 3). CP can stimulate ‘monomer funneling’ by reducing the concentration of free barbed ends and increasing G-actin concentration; this channels monomers to uncapped filament barbed ends, which are then able to grow at an accelerated rate (Carlier and Pantaloni, 1997). An alternative explanation of how CP can coordinate with profilin to alter network dynamics is through ‘monomer gating’, which suggests that capping of barbed ends would drive profilin-bound actin to coordinate with nucleators to create new filaments, rather than elongate existing ones (Akin and Mullins, 2008). The role of these two mechanisms in an in vivo setting, where the ratio of components cannot be carefully controlled and elongating formin or Ena/VASP filaments can be in close spatial proximity to Arp2/3 networks, remains to be determined. However, it is clear that the complex relationship between filament assembly and maintenance of the monomer pool must be carefully balanced to generate complex networks of actin that have defined roles in cell physiology.
Fig. 3.
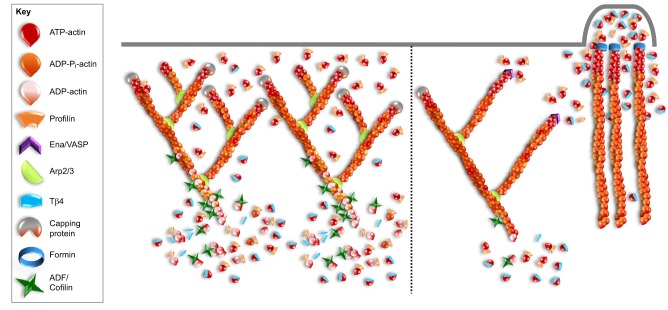
G-actin regulated assembly of actin networks. A scenario where profilin and Tβ4 regulate the assembly of specific actin networks. On the left, there is increased ADF/cofilin and capping protein activity; this leads to increased disassembly and barbed-end capping, thus resulting in an increased monomer pool. This creates a more densely branched Arp2/3-mediated actin network because nucleation, rather than elongation, becomes favored. Here, the role of profilin is predominantly to recycle the monomers back into a polymerization-competent pool. On the right, ADF/cofilin and capping protein activity is decreased, resulting in a smaller monomer pool. Consequently, competition for a limited supply of monomers enhances filament elongation by barbed-end polymerases, such as Ena/VASP and formins, and reduces Arp2/3-mediated branching due to a bias formed through profilin-bound actin. Additionally, Tβ4 prevents cytosolic actin monomers from polymerizing at barbed ends of Arp2/3-mediated filaments throughout the lamellipodia (right-hand side of this panel) and selectively releases G-actin near the plasma membrane, where it undergoes formin-mediated polymerization.
Tβ4 – more than a sequestering protein
Most vertebrates have additional help in regulating the actin monomer pool in the form of Tβ4 (Safer and Nachmias, 1994). Tβ4 is a small (4.9 kDa) protein that sequesters actin monomers (Low et al., 1981; Safer et al., 1991) and binds to G-actin in equimolar amounts (Goldschmidt-Clermont et al., 1992). It has largely been considered a negative regulator of filament assembly, because microinjection of the protein (Sanders et al., 1992), overexpression of the gene (Yu et al., 1994) or local photorelease of caged Tβ4 (Roy et al., 2001) all cause F-actin depolymerization. However, this relationship between increased Tβ4 and polymerization is not entirely straightforward, as Tβ4 has also been shown to promote polymerization and increased cell motility at higher concentrations (Carlier et al., 1996; Sun et al., 1996). Tβ4 can sequester G-actin by both sterically blocking its polymerization, inhibiting its nucleotide exchange, and allosterically altering the monomer conformation (Goldschmidt-Clermont et al., 1991b; Hertzog et al., 2004; Irobi et al., 2004; Sanders et al., 1992; Xue et al., 2014). Structural studies demonstrate Tβ4 behaves like an intrinsically unstructured protein (Zarbock et al., 1990) and becomes increasingly ordered upon binding to actin (Safer and Chowrashi, 1997; Safer et al., 1997). It is important to note that Tβ4 binds ATP-bound actin with a 50-fold higher affinity than does to ADP-actin (Carlier et al., 1993; Hertzog et al., 2004); thus its major function is to hold a polymerization-ready pool of monomers in reserve.
Tβ4 and profilin are thought to compete for binding to actin. The intracellular concentration of Tβ4 can be 10 times higher than that of profilin (Weber et al., 1992), while their affinities for ATP-actin are similar (Tβ4 KD ∼0.3 µM, Tβ4 and KD ∼0.1 µM, profilin) (Huff and Hannappel, 1997; Huff et al., 1995; Vinson et al., 1998; Weber et al., 1992; Yarmola and Bubb, 2004). Thus most G-actin in vertebrate cells is held ‘in reserve’ by Tβ4 rather than profilin owing to its higher concentration and comparable affinity for actin. However, profilin can de-sequester monomers from Tβ4 (Pantaloni and Carlier, 1993) due to the high exchange rate both proteins exhibit with actin (Goldschmidt-Clermont et al., 1992). In addition, similar to Tβ4 (Xue et al., 2014), profilin may allosterically alter the conformation of actin upon binding (Yarmola et al., 2001), which may further regulate competition dynamics. However, the relationship between Tβ4 and profilin cannot be described strictly by competition, as they can both simultaneously bind to G-actin to form a ternary complex under physiological conditions (>10 μM) (Xue et al., 2014; Yarmola et al., 2001). This allows a direct and regulated exchange of G-actin from a non-polymerizable, sequestered state to a polymerization-competent one (Fig. 2) without having to disassociate from a monomer-binding protein, thus reducing the risk of spontaneous polymerization (Xue et al., 2014). Moreover, this complex could help recruit Tβ4-bound G-actin to subcellular regions with a high profilin concentration through the ability of profilin to bind ATP-actin (Vitriol et al., 2015; Yarmola and Bubb, 2006; Yarmola et al., 2001). It should be noted that the profilin–Tβ4–G-actin complex has only been studied in bulk solution assays and has not yet been proven to exist in cells. Future in vivo experiments detailing Tβ4 and profilin localization dynamics at the molecular level will be required to determine the role of the tertiary complex in cellular actin physiology. It is also important to note that the relative concentrations of Tβ4 and profilin may be cell-type dependent, and most values come from older studies of highly differentiated cells such as platelets (Pollard et al., 2000). Adjusting the relative expression of either monomer-binding protein, or locally regulating their ability to interact with actin (Fan et al., 2012a), could have significant effects on how the G-actin pool is spatiotemporally controlled.
Although sequestration of monomers was initially thought to be the only function of Tβ4, recent studies show that it may also help to allow actin monomers to move from the cell interior to the plasma membrane without polymerizing, thereby positively influencing actin assembly at the leading edge of migrating cells (Fan et al., 2012b, 2009; Lee et al., 2013; Vitriol et al., 2015). The mechanism for this localization is still unknown, although it may be due to an interaction with profilin (Lee et al., 2013), which may stimulate a local dissociation of Tβ4 and G-actin. In neuronal cells, Tβ4 is not required for lamellipodia formation, which predominantly assembles from actin recycled from its own disassembly (Vitriol et al., 2015), but it is required to create sustained lamellipodial protrusions needed for directed cell migration and axon guidance (Lee et al., 2013). Detailed spatiotemporal analysis revealed that the monomers localized to the leading edge by Tβ4 do not arrive until after a protrusion is initiated (Vitriol et al., 2015), further indicating that they are needed to maintain forward momentum of the lamellipodia, perhaps when local monomer reserves are depleted by an increase in assembly (Boujemaa-Paterski et al., 2017). Experiments and mathematical modeling have shown that diffusion is sufficient to explain the localization kinetics of Tβ4–G-actin (Vitriol et al., 2015). Tβ4 may also help to bias polymerization to specific actin networks. Using a photoactivatably labeled actin to follow movement from the cytoplasmic pool to the leading edge, it was determined that Tβ4 helps to target cytoplasmic actin monomers to formin-mediated filaments at the leading edge of lamellipodia by helping them to bypass Arp2/3-mediated barbed ends (Vitriol et al., 2015) (Fig. 3). While its specific mechanism of action has yet to be determined, what is now readily apparent is that Tβ4, in addition to its role in monomer sequestration, also plays an active role in the creation of actin networks.
Visualizing actin monomers in the cell
Most of what we know about G-actin comes from in vitro systems. This is largely because G-actin is difficult to image and thus is frequently ‘visualized’ through inference rather than direct detection. With the exception of specialized situations (Lee et al., 2013; Lei et al., 2017), G-actin is not localized and fills the entire cytoplasm, making direct detection difficult. When a small fraction of monomers are specifically localized, they may be difficult to detect against such a high background of non-localized protein. Additionally, unlike F-actin, for which highly useful probes have been developed for imaging of both fixed and live cells (Melak et al., 2017), there are only few reagents available that specifically label monomers. To label G-actin in fixed cells one can use DNAse I (Hitchcock, 1980), vitamin D-binding protein (Van Baelen et al., 1980) or the monomer-specific antibody JLA20 (Lin, 1981) (Fig. 4). DNAse I is commonly used to label G-actin, but is the least monomer-specific probe (Lee et al., 2013). Another concern in labeling actin monomers is that they can be extracted from the cell during membrane permeabilization, even after paraformaldehyde fixation (Lee et al., 2013). In our own laboratory, we have found that acetone is best for preserving the localization of G-actin at the leading edge, whereas Triton X-100 causes a complete extraction of this monomer pool. Additionally, because the background of non-localized G-actin is so high, it is useful to use a ratiometric imaging approach with a volume indicator to see discrete subcellular localizations of monomers, or to use an F-actin probe (such as fluorescently labeled phalloidin) to obtain the G- to F-actin ratio (Lee et al., 2013; Wang et al., 2016). The local G- to F-actin ratio can also highlight localized actin monomers in living cells. This is done using a fluorescent protein-labeled actin construct to visualize total actin and a second probe such as Lifeact, which is specific for F-actin (Vitriol et al., 2015; Kapustina et al., 2016; Lee et al., 2013; Wang et al., 2016). Please see the detailed discussion of fluorescently labeled actin probes for important caveats of using labeled actin in Box 1.
Fig. 4.
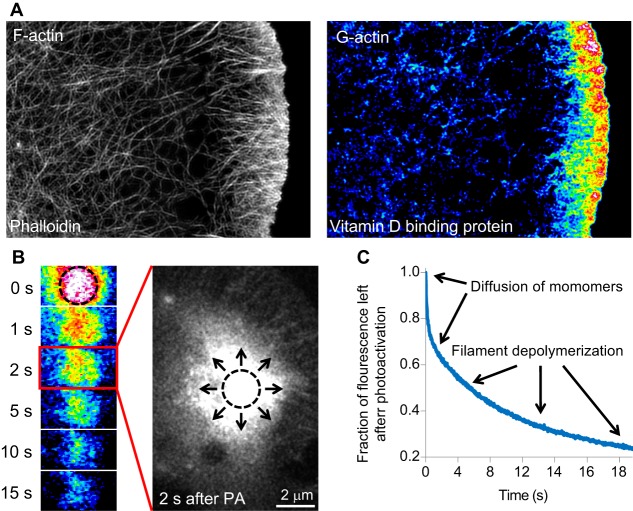
Methods for visualizing G-actin. (A) Actin monomers can be visualized in fixed cells with monomer-specific probes such as vitamin D-binding protein. Here, structured illumination super-resolution images show the same cell stained with both phalloidin, to visualize F-actin, and vitamin D-binding protein, to visualize actin monomers. The vitamin D-binding protein image is pseudocolored to emphasize changes in fluorescence, with warmer colors representing increased fluorescence. Actin monomers can be seen localized to the leading edge. (B) G-actin can be visualized in live cells by measuring rapid changes in fluorescently labeled actin. Here, a pulse-chase experiment is performed using actin labeled with photoactivatable GFP. After photoactivation (PA, occurring in the region marked by the dotted circle), the actin is highlighted and can be followed over time. The G-actin rapidly diffuses away from the PA region in seconds (represented by the arrows in the magnified image), which is emphasized by the middle panel showing a larger image of the cell two seconds after actin was photoactivated. (C) A representative graph from this type of experiment shown in B, highlighting which parts of the fluorescence decay curve are due to diffusion of monomers away from the photoactivated region and which parts arise from the disassembly of F-actin that occurs on a slower time scale. Images in A are similar to those published in Lee et al., 2013; images in B are similar to those published in Kapustina et al., 2016.
Box 1. Discussion of fluorescently labeled actin probes.
Actin can be visualized in living cells with purified protein labeled with a fluorescent dye or DNA encoding for a fluorescent protein–actin fusion. Both have their benefits and drawbacks. Dye-labeled actin is closer to the physiological state of endogenous actin because fluorescent dyes, such as rhodamine or the Alexa Fluor dyes, are ∼50 times smaller than a 27 kDa fluorescent protein. This allows the actin to be more readily incorporated into formin-mediated filaments (Yamashiro et al., 2014), which can exclude actin that has been labeled with tags larger than 2 kDa (Chen et al., 2012). For experiments in non-muscle cells, commercially available dye-labeled actin should be used with caution, since it is predominantly sourced from rabbit skeletal muscle. The differences in biochemical properties and affinity for actin-binding proteins between muscle and non-muscle actin can be significant (Herman, 1993) and introducing actin from a different species or an isoform that does not normally exist in the cell could potentially result in artefactual behavior (Tseng and Pollard, 1982). Non-muscle actin can be purified from other sources, such as brain or platelets, although it will always be present as a mix of β- and γ-actin (Schafer et al., 1998). To isolate a specific non-muscle isoform, it would need to be generated from either a knockout animal or a recombinant system (Bergeron et al., 2010). Purified actin also needs to be introduced by microinjection or electroporation, which is not amenable to all cell types, and the experimental window is limited to the half-life of the labeled actin.
Fluorescent protein (FP)-labeled actin has the advantage of being genetically encoded and can be stably expressed in cells. FP–actin can be made in a variety of colors, including photoactivatable and photoconvertible versions for pulse-chase experiments (Lippincott-Schwartz and Patterson, 2009). However, a concern of incorporating a large tag onto actin is that it may interfere with assembly or binding partner interactions. FP–actin can co-polymerize with unlabeled actin or form pure filaments with approximately the same critical concentration as non-labeled actin (Aizawa et al., 1997; Choidas et al., 1998; Liu et al., 2004; Westphal et al., 1997). However, in yeast, replacement of the actin gene with GFP–actin is lethal (Wu and Pollard, 2005), and to date there have been no reports of homozygous gene replacement with FP-labeled actin in higher eukaryotes. Heterozygous gene replacement of β-actin in human iPSCs does give viable cells, but they exhibit a significantly reduced expression of the FP–actin (Roberts et al., 2017). If filaments are composed of more than 30% FP–actin, they will bind to myosin, but exhibit movement defects in in vitro gliding assays (Aizawa et al., 1997; Westphal et al., 1997) caused by an interaction between the FP and myosin (Agbulut et al., 2007). In lower eukaryotes, such as fission yeast, Dictostelium and C. elegans, FP–actin will not localize to formin-based structures like the cytoplasmic ring (Carvalho et al., 2009; Chen et al., 2012; Wu and Pollard, 2005). However, in mammalian cells, FP–actin can label the cytokinetic ring (Murthy and Wadsworth, 2005; Zhou and Wang, 2008) and FP–actin monomers require formin activity for optimal translocation to the leading edge (Vitriol et al., 2015). Thus, the issue of formin exclusion of FP–actin appears to be most relevant for non-vertebrate model systems, although rigorous experiments have not been performed with vertebrate formins.
In summary, there is no perfect, one-size-fits-all approach to labeling actin for live-cell fluorescence imaging. Both techniques have been successfully used in a number of experiments. Dye-labeled actin is likely the best choice if microinjection or electroporation is an option. However, there are many cases where FP–actin would be adequate, and even preferred, if parameters such as isoform-type of the labeled actin or the need to pulse-label a specific actin population are essential. Importantly, findings generated with any type of exogenous actin should be validated with additional experiments.
Live imaging of non-localized G-actin is difficult due to its rapid diffusion (Lanni and Ware, 1984; Vitriol et al., 2015) (Fig. 4). This is exemplified in single-molecule imaging experiments, where even at imaging rates of 10 frames/s, actin molecules only become visible as speckles after their incorporation into the F-actin network (Yamashiro et al., 2014). Therefore, the presence of actin monomers must be inferred from the observed rapid changes in fluorescence. There are several methods that can effectively measure spatiotemporal fluorescence fluctuations, including fluorescence correlation spectroscopy (FCS) (Engelke et al., 2010) or pulse-chase techniques, such as fluorescence recovery after photobleaching (FRAP) or photoactivation/photoconversion (Lippincott-Schwartz et al., 2003). One issue inherent to FCS is that it relies on longer imaging times and is only sensitive to changes of a fluorescent molecule with a concentration in the nanomolar range (Kim et al., 2007); therefore, it will be difficult to measure spatiotemporal fluctuations of G-actin under physiological conditions with this method. However, FRAP and photoactivation experiments can be performed as rapidly as the imaging system will allow and can provide relevant information about G-actin concentration, diffusion and localization. Traditional analysis of FRAP and photoactivation curves yields parameters, such as the half time and the immobile fraction (Lippincott-Schwartz et al., 2003), which can be powerful indicators of the relative G-actin movement and concentration when they are compared between different experimental conditions.
To extract more quantitative information, such as estimates of absolute concentration or rate constants, mathematical modeling is required. A minimal model would consider the diffusion of G-actin and the polymerization/depolymerization of actin filaments (Kapustina et al., 2016; McGrath et al., 1998). Other factors, such as non-isotropic diffusion caused by different cell morphologies, movement of F-actin during the timecourse of the experiment, the fact that FRAP/photoactivation is non-instantaneous, photobleaching and the frame rate of the experiment, will either need to be accounted for or determined to not be important for the measurement. We have found that the three-dimensional cell morphology and the imaging frame rate are essential parameters to accurately interpret a photoactivation experiment (Kapustina et al., 2016). To assist in the analysis of actin photoactivation experiments, we have developed a method called ‘modeling assisted analysis of photoactivation’, which can provide simultaneous measurements of both actin monomers and filaments in a three-dimensional environment by fitting experimental data with curves from a simulated library (Kapustina et al., 2016). Another technique that employs mathematical modeling is ‘sequential fluorescence decay after photoactivation’ (sFDAP), which uses serial photoactivation combined with model-based curve fitting to obtain information about the local G-actin concentration and its changes upon extracellular stimuli (Kiuchi et al., 2011). Finally, another useful experiment to perform with photoactivatable/photoconvertible actin is to pulse-label a subset of actin within the cell and then measure its incorporation into other actin networks or its re-incorporation into the network from which it was derived (Vitriol et al., 2015). While not a direct observation of G-actin, it offers the ability to visualize the source of monomers that constitute F-actin structures (see Box 1 and Fig. 4). With these quantitative live-cell imaging tools at the ready, the onus is now on cell biologists to utilize them to their full potential to test theories of how actin monomers regulate cytoskeletal dynamics.
Concluding remarks
The cytoskeleton field has amassed a large body of knowledge about the biochemical properties of actin polymerization and how monomer-binding proteins influence these reactions. Many of these studies have inventoried the participants and regulatory proteins involved in actin dynamics, their localization, and the kinetics of these dynamics. Mathematical models have also begun to address how the cell maintains actin homeostasis and the changes it has to make in order to undergo specialized processes such as motility or cell division. The next challenge will be to incorporate these individual puzzle pieces into a single, comprehensive picture and, as this Review contends, one that takes into account the active contribution of the actin monomer. Mathematical models that incorporate actin monomers and their interactions with binding proteins have provided a promising means to start addressing some of these unresolved questions (Boujemaa-Paterski et al., 2017; Ditlev et al., 2013; Kapustina et al., 2016; Novak et al., 2008; Vitriol et al., 2015). However, many aspects still require more work. For example, it is clear that β- and γ-actin have distinct mechanistic roles in cellular actin physiology and organism development (Perrin and Ervasti, 2010), though it is still unclear as to why this is. Developing a map of β- and γ-actin localization with single or even sub-filament resolution to detect their preferentially assembly into different structures would help to better understand how they are recognized and used by the cell as different entities. It would be equally valuable to obtain a map of all localized profilin molecules, and analyze whether or not they are bound to actin monomers and/or Tβ4 to determine how the interplay and competition of these two factors controls the assembly of specific actin networks. It is also unknown exactly how much influence specific pools of actin monomers have on actin physiology; that is, if they have a general role in actin polymerization or if they are exclusively reserved for bursts of actin assembly into specialized structures. Finally, there is now strong evidence that actin can also diffuse through the cytoplasm as an oligomer (Raz-Ben Aroush et al., 2017; Smith et al., 2013). The extent to which oligomers exist in different cell types and the roles they play in cytoskeletal dynamics remain to be determined. Either way, we can no longer view G-actin as a passive factor in actin filament assembly and behavior of the actin network. It will be exciting to see the field define how actively G-actin regulates cytoskeletal dynamics in the upcoming years.
Acknowledgements
The authors would like to express appreciation to the three anonymous reviewers, who thoroughly read the manuscript and provided suggestions which improved the final version of this Review. The authors would also like to thank those involved in discussions held at the 2016 American Society for Cell Biology meeting for inspiring us to write the discussion of fluorescently labeled actin probes.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This project was supported by a Pathway to Independence Award from the National Institutes of Health (R00 NS087104) to E.A.V. Deposited in PMC for release after 12 months.
References
- Agbulut O., Huet A., Niederländer N., Puceat M., Menasché P. and Coirault C. (2007). Green fluorescent protein impairs actin-myosin interactions by binding to the actin-binding site of myosin. J. Biol. Chem. 282, 10465-10471. 10.1074/jbc.M610418200 [DOI] [PubMed] [Google Scholar]
- Aizawa H., Sameshima M. and Yahara I. (1997). A green fluorescent protein-actin fusion protein dominantly inhibits cytokinesis, cell spreading, and locomotion in Dictyostelium. Cell Struct. Funct. 22, 335-345. 10.1247/csf.22.335 [DOI] [PubMed] [Google Scholar]
- Akin O. and Mullins R. D. (2008). Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133, 841-851. 10.1016/j.cell.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianantoandro E. and Pollard T. D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13-23. 10.1016/j.molcel.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Baranwal S., Naydenov N. G., Harris G., Dugina V., Morgan K. G., Chaponnier C. and Ivanov A. I. (2012). Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol. Biol. Cell 23, 3542-3553. 10.1091/mbc.E12-02-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman R. W., Matty M. A., Au G. G., Looger L. L., Choudhury K. R., Keller P. J. and Tobin D. M. (2015). Direct in vivo manipulation and imaging of calcium transients in neutrophils identify a critical role for leading-edge calcium flux. Cell Rep. 13, 2107-2117. 10.1016/j.celrep.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva I. A., Perrin B. J., Sonnemann K. J., Zhu M., Stepanyan R., McGee J., Frolenkov G. I., Walsh E. J., Friderici K. H., Friedman T. B. et al. (2009). Gamma-actin is required for cytoskeletal maintenance but not development. Proc. Natl. Acad. Sci. USA 106, 9703-9708. 10.1073/pnas.0900221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron S. E., Zhu M., Thiem S. M., Friderici K. H. and Rubenstein P. A. (2010). Ion-dependent polymerization differences between mammalian beta- and gamma-nonmuscle actin isoforms. J. Biol. Chem. 285, 16087-16095. 10.1074/jbc.M110.110130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. D., Gluck U., Denisenko O. N., Sklyarova T. V., Spector I. and Ben-Ze'ev A. (1995). The state of actin assembly regulates actin and vinculin expression by a feedback loop. J. Cell Sci. 108, 1183-1193. [DOI] [PubMed] [Google Scholar]
- Blanchoin L., Amann K. J., Higgs H. N., Marchand J.-B., Kaiser D. A. and Pollard T. D. (2000). Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007-1011. 10.1038/35010008 [DOI] [PubMed] [Google Scholar]
- Blasco R., Cole N. B. and Moss B. (1991). Sequence analysis, expression, and deletion of a vaccinia virus gene encoding a homolog of profilin, a eukaryotic actin-binding protein. J. Virol. 65, 4598-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boujemaa-Paterski R., Suarez C., Klar T., Zhu J., Guérin C., Mogilner A., Théry M. and Blanchoin L. (2017). Network heterogeneity regulates steering in actin-based motility. Nat. Commun. 8, 655 10.1038/s41467-017-00455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley K. I. and Grantham J. (2010). Subunits of the chaperonin CCT interact with F-actin and influence cell shape and cytoskeletal assembly. Exp. Cell Res. 316, 543-553. 10.1016/j.yexcr.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Bunnell T. M. and Ervasti J. M. (2010). Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton (Hoboken) 67, 564-572. 10.1002/cm.20467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell T. M., Burbach B. J., Shimizu Y. and Ervasti J. M. (2011). beta-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 22, 4047-4058. 10.1091/mbc.E11-06-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. A., Christensen J. R., Barone E., Suarez C., Sirotkin V. and Kovar D. R. (2014). Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr. Biol. 24, 579-585. 10.1016/j.cub.2014.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. G., Fishkind D. J. and Wang Y. L. (1993). Localization and dynamics of nonfilamentous actin in cultured cells. J. Cell Biol. 123, 173-181. 10.1083/jcb.123.1.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.-F. and Pantaloni D. (1997). Control of actin dynamics in cell motility. J. Mol. Biol. 269, 459-467. 10.1006/jmbi.1997.1062 [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Jean C., Rieger K. J., Lenfant M. and Pantaloni D. (1993). Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: possible implication in the regulation of actin dynamics. Proc. Natl. Acad. Sci. USA 90, 5034-5038. 10.1073/pnas.90.11.5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.-F., Didry D., Erk I., Lepault J., Van Troys M. L., Vandekerckhove J., Perelroizen I., Yin H., Doi Y. and Pantaloni D. (1996). Tbeta 4 is not a simple G-actin sequestering protein and interacts with F-actin at high concentration. J. Biol. Chem. 271, 9231-9239. 10.1074/jbc.271.16.9231 [DOI] [PubMed] [Google Scholar]
- Carlier M.-F., Laurent V., Santolini J., Melki R., Didry D., Xia G.-X., Hong Y., Chua N.-H. and Pantaloni D. (1997). Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 136, 1307-1322. 10.1083/jcb.136.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L., Nyström L.-E., Sundkvist I., Markey F. and Lindberg U. (1977). Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 115, 465-483. 10.1016/0022-2836(77)90166-8 [DOI] [PubMed] [Google Scholar]
- Carvalho A., Desai A. and Oegema K. (2009). Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell 137, 926-937. 10.1016/j.cell.2009.03.021 [DOI] [PubMed] [Google Scholar]
- Chang F., Woollard A. and Nurse P. (1996). Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 109, 131-142. [DOI] [PubMed] [Google Scholar]
- Chen Q., Nag S. and Pollard T. D. (2012). Formins filter modified actin subunits during processive elongation. J. Struct. Biol. 177, 32-39. 10.1016/j.jsb.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choidas A., Jungbluth A., Sechi A., Murphy J., Ullrich A. and Marriott G. (1998). The suitability and application of a GFP-actin fusion protein for long-term imaging of the organization and dynamics of the cytoskeleton in mammalian cells. Eur. J. Cell Biol. 77, 81-90. 10.1016/S0171-9335(98)80075-7 [DOI] [PubMed] [Google Scholar]
- Clark W. A. Jr. and Zak R. (1981). Assessment of fractional rates of protein synthesis in cardiac muscle cultures after equilibrium labeling. J. Biol. Chem. 256, 4863-4870. [PubMed] [Google Scholar]
- Condeelis J. and Singer R. H. (2005). How and why does beta-actin mRNA target? Biol. Cell 97, 97-110. 10.1042/BC20040063 [DOI] [PubMed] [Google Scholar]
- Cramer L. P., Briggs L. J. and Dawe H. R. (2002). Use of fluorescently labelled deoxyribonuclease I to spatially measure G-actin levels in migrating and non-migrating cells. Cell Motil. Cytoskeleton 51, 27-38. 10.1002/cm.10013 [DOI] [PubMed] [Google Scholar]
- De La Cruz E. M. (2005). Cofilin binding to muscle and non-muscle actin filaments: isoform-dependent cooperative interactions. J. Mol. Biol. 346, 557-564. 10.1016/j.jmb.2004.11.065 [DOI] [PubMed] [Google Scholar]
- Didry D., Carlier M.-F. and Pantaloni D. (1998). Synergy between actin depolymerizing factor/cofilin and profilin in increasing actin filament turnover. J. Biol. Chem. 273, 25602-25611. 10.1074/jbc.273.40.25602 [DOI] [PubMed] [Google Scholar]
- Ditlev J. A., Mayer B. J. and Loew L. M. (2013). There is more than one way to model an elephant. Experiment-driven modeling of the actin cytoskeleton. Biophys. J. 104, 520-532. 10.1016/j.bpj.2012.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D. and Pollard T. D. (1986). Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J. Biol. Chem. 261, 12754-12758. [PubMed] [Google Scholar]
- Dugina V., Zwaenepoel I., Gabbiani G., Clement S. and Chaponnier C. (2009). Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J. Cell Sci. 122, 2980-2988. 10.1242/jcs.041970 [DOI] [PubMed] [Google Scholar]
- Elam W. A., Kang H. and De La Cruz E. M. (2013). Biophysics of actin filament severing by cofilin. FEBS Lett. 587, 1215-1219. 10.1016/j.febslet.2013.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke H., Heinrich D. and Rädler J. O. (2010). Probing GFP-actin diffusion in living cells using fluorescence correlation spectroscopy. Phys. Biol. 7, 046014 10.1088/1478-3975/7/4/046014 [DOI] [PubMed] [Google Scholar]
- Erba H. P., Eddy R., Shows T., Kedes L. and Gunning P. (1988). Structure, chromosome location, and expression of the human gamma-actin gene: differential evolution, location, and expression of the cytoskeletal beta- and gamma-actin genes. Mol. Cell. Biol. 8, 1775-1789. 10.1128/MCB.8.4.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Selden L. A. and Gershman L. C. (1987). Tight binding of divalent cations to monomeric actin. Binding kinetics support a simplified model. J. Biol. Chem. 262, 4952-4957. [PubMed] [Google Scholar]
- Fan Y., Gong Y., Ghosh P. K., Graham L. M. and Fox P. L. (2009). Spatial coordination of actin polymerization and ILK-Akt2 activity during endothelial cell migration. Dev. Cell 16, 661-674. 10.1016/j.devcel.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Arif A., Gong Y., Jia J., Eswarappa S. M., Willard B., Horowitz A., Graham L. M., Penn M. S. and Fox P. L. (2012a). Stimulus-dependent phosphorylation of profilin-1 in angiogenesis. Nat. Cell Biol. 14, 1046-1056. 10.1038/ncb2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Eswarappa S. M., Hitomi M. and Fox P. L. (2012b). Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J. Cell Biol. 198, 47-55. 10.1083/jcb.201111088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamholz A., Phillips R. and Milo R. (2014). The quantified cell. Mol. Biol. Cell 25, 3497-3500. 10.1091/mbc.E14-09-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness D. N., Katori Y., Mahendrasingam S. and Hackney C. M. (2005). Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear. Res. 207, 22-34. 10.1016/j.heares.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Gimona M., Vandekerckhove J., Goethals M., Herzog M., Lando Z. and Small J. V. (1994). Beta-actin specific monoclonal antibody. Cell Motil. Cytoskeleton 27, 108-116. 10.1002/cm.970270203 [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J. and Pollard T. D. (1990). The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science 247, 1575-1578. 10.1126/science.2157283 [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G. and Pollard T. D. (1991a). Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science 251, 1231-1233. 10.1126/science.1848725 [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Doberstein S. K. and Pollard T. D. (1991b). Mechanism of the interaction of human platelet profilin with actin. J. Cell Biol. 113, 1081-1089. 10.1083/jcb.113.5.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Furman M. I., Wachsstock D., Safer D., Nachmias V. T. and Pollard T. D. (1992). The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol. Biol. Cell 3, 1015-1024. 10.1091/mbc.3.9.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Boyer J. L. and Korn E. D. (1977). Comparative biochemistry of non-muscle actins. J. Biol. Chem. 252, 8300-8309. [PubMed] [Google Scholar]
- Hansen S. D. and Mullins R. D. (2010). VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 191, 571-584. 10.1083/jcb.201003014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M. (1993). Actin isoforms. Curr. Opin. Cell Biol. 5, 48-55. 10.1016/S0955-0674(05)80007-9 [DOI] [PubMed] [Google Scholar]
- Hertzog M., van Heijenoort C., Didry D., Gaudier M., Coutant J., Gigant B., Didelot G., Préat T., Knossow M., Guittet E. et al. (2004). The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell 117, 611-623. 10.1016/S0092-8674(04)00403-9 [DOI] [PubMed] [Google Scholar]
- Herz R., Weber A. and Reiss I. (1969). Role of magnesium in the relaxation of myofibrils. Biochemistry 8, 2266-2271. 10.1021/bi00834a005 [DOI] [PubMed] [Google Scholar]
- Higashida C., Kiuchi T., Akiba Y., Mizuno H., Maruoka M., Narumiya S., Mizuno K. and Watanabe N. (2013). F- and G-actin homeostasis regulates mechanosensitive actin nucleation by formins. Nat. Cell Biol. 15, 395-405. 10.1038/ncb2693 [DOI] [PubMed] [Google Scholar]
- Hill M. A. and Gunning P. (1993). Beta and gamma actin mRNAs are differentially located within myoblasts. J. Cell Biol. 122, 825-832. 10.1083/jcb.122.4.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. (1980). Actin deoxyroboncuclease I interaction. Depolymerization and nucleotide exchange. J. Biol. Chem. 255, 5668-5673. [PubMed] [Google Scholar]
- Hoock T. C., Newcomb P. M. and Herman I. M. (1991). Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J. Cell Biol. 112, 653-664. 10.1083/jcb.112.4.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T. and Hannappel E. (1997). Oxidation and reduction of thymosin beta(4) and its influence on the interaction with G-actin studied by reverse-phase HPLC and post-column derivatization with fluorescamine. Anal. Chim. Acta 352, 249-255. 10.1016/S0003-2670(97)00132-3 [DOI] [Google Scholar]
- Huff T., Zerzawy D. and Hannappel E. (1995). Interactions of beta-thymosins, thymosin beta 4-sulfoxide, and N-terminally truncated thymosin beta 4 with actin studied by equilibrium centrifugation, chemical cross-linking and viscometry. Eur. J. Biochem. 230, 650-657. 10.1111/j.1432-1033.1995.tb20606.x [DOI] [PubMed] [Google Scholar]
- Hung R.-J., Pak C. W. and Terman J. R. (2011). Direct redox regulation of F-actin assembly and disassembly by Mical. Science 334, 1710-1713. 10.1126/science.1211956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobi E., Aguda A. H., Larsson M., Guerin C., Yin H. L., Burtnick L. D., Blanchoin L. and Robinson R. C. (2004). Structural basis of actin sequestration by thymosin-beta4: implications for WH2 proteins. EMBO J. 23, 3599-3608. 10.1038/sj.emboj.7600372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Aebi U. and Pollard T. D. (1980). An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature 288, 455-459. 10.1038/288455a0 [DOI] [PubMed] [Google Scholar]
- Kaiser D. A., Vinson V. K., Murphy D. B. and Pollard T. D. (1999). Profilin is predominantly associated with monomeric actin in Acanthamoeba. J. Cell Sci. 112, 3779-3790. [DOI] [PubMed] [Google Scholar]
- Kang F., Laine R. O., Bubb M. R., Southwick F. S. and Purich D. L. (1997). Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): implications for actin-based Listeria motility. Biochemistry 36, 8384-8392. 10.1021/bi970065n [DOI] [PubMed] [Google Scholar]
- Kang F., Purich D. L. and Southwick F. S. (1999). Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J. Biol. Chem. 274, 36963-36972. 10.1074/jbc.274.52.36963 [DOI] [PubMed] [Google Scholar]
- Kapustina M., Read T. A. and Vitriol E. A. (2016). Simultaneous quantification of actin monomer and filament dynamics with modeling-assisted analysis of photoactivation. J. Cell Sci. 129, 4633-4643. 10.1242/jcs.194670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakozova M., Kozak M., Wong C. C., Bailey A. O., Yates J. R. III, Mogilner A., Zebroski H. and Kashina A. (2006). Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science 313, 192-196. 10.1126/science.1129344 [DOI] [PubMed] [Google Scholar]
- Khaitlina S. Y. (2001). Functional specificity of actin isoforms. Int. Rev. Cytol. 202, 35-98. 10.1016/S0074-7696(01)02003-4 [DOI] [PubMed] [Google Scholar]
- Kim S. A., Heinze K. G. and Schwille P. (2007). Fluorescence correlation spectroscopy in living cells. Nat. Methods 4, 963-973. 10.1038/nmeth1104 [DOI] [PubMed] [Google Scholar]
- Kislauskis E. H., Zhu X. and Singer R. H. (1997). beta-Actin messenger RNA localization and protein synthesis augment cell motility. J. Cell Biol. 136, 1263-1270. 10.1083/jcb.136.6.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T., Ohashi K., Kurita S. and Mizuno K. (2007). Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J. Cell Biol. 177, 465-476. 10.1083/jcb.200610005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T., Nagai T., Ohashi K. and Mizuno K. (2011). Measurements of spatiotemporal changes in G-actin concentration reveal its effect on stimulus-induced actin assembly and lamellipodium extension. J. Cell Biol. 193, 365-380. 10.1083/jcb.201101035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler S. A., Rottner K., Lai F., Block J., Vinzenz M. and Small J. V. (2009). F- and G-actin concentrations in lamellipodia of moving cells. PLoS ONE 4, e4810 10.1371/journal.pone.0004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Kuhn J. R., Tichy A. L. and Pollard T. D. (2003). The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875-887. 10.1083/jcb.200211078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N. and Pollard T. D. (2006). Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423-435. 10.1016/j.cell.2005.11.038 [DOI] [PubMed] [Google Scholar]
- Lai F. P. L., Szczodrak M., Block J., Faix J., Breitsprecher D., Mannherz H. G., Stradal T. E. B., Dunn G. A., Small J. V. and Rottner K. (2008). Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 27, 982-992. 10.1038/emboj.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F. and Ware B. R. (1984). Detection and characterization of actin monomers, oligomers, and filaments in solution by measurement of fluorescence photobleaching recovery. Biophys. J. 46, 97-110. 10.1016/S0006-3495(84)84002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I. and Lindberg U. (1985). Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature 314, 472-474. 10.1038/314472a0 [DOI] [PubMed] [Google Scholar]
- Lassing I. and Lindberg U. (1988). Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J. Cell. Biochem. 37, 255-267. 10.1002/jcb.240370302 [DOI] [PubMed] [Google Scholar]
- Lee C. W., Vitriol E. A., Shim S., Wise A. L., Velayutham R. P. and Zheng J. Q. (2013). Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr. Biol. 23, 1046-1056. 10.1016/j.cub.2013.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W., Myers K. R., Rui Y., Hladyshau S., Tsygankov D. and Zheng J. Q. (2017). Phosphoinositide-dependent enrichment of actin monomers in dendritic spines regulates synapse development and plasticity. J. Cell Biol. 216, 2551-2564. 10.1083/jcb.201612042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.-M., van Horck F. P. G., Lin A. C., Allison R., Standart N. and Holt C. E. (2006). Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 9, 1247-1256. 10.1038/nn1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J. (1981). Monoclonal antibodies against myofibrillar components of rat skeletal muscle decorate the intermediate filaments of cultured cells. Proc. Natl. Acad. Sci. USA 78, 2335-2339. 10.1073/pnas.78.4.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. and Patterson G. H. (2009). Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol. 19, 555-565. 10.1016/j.tcb.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Altan-Bonnet N. and Patterson G. H. (2003). Photobleaching and photoactivation: following protein dynamics in living cells. Nat. Cell Biol. 5, S7-S14. 10.1038/ncb1032 [DOI] [PubMed] [Google Scholar]
- Liu A. X., Zhang S. B., Xu X. J., Ren D. T. and Liu G. Q. (2004). Soluble expression and characterization of a GFP-fused pea actin isoform (PEAc1). Cell Res. 14, 407-414. 10.1038/sj.cr.7290241 [DOI] [PubMed] [Google Scholar]
- Lloyd C., Schevzov G. and Gunning P. (1992). Transfection of nonmuscle beta- and gamma-actin genes into myoblasts elicits different feedback regulatory responses from endogenous actin genes. J. Cell Biol. 117, 787-797. 10.1083/jcb.117.4.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin A. J., Lee K.-C., Han S. J., Bui D. A., Davidson M., Mogilner A. and Danuser G. (2015). Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nat. Cell Biol. 17, 1435-1445. 10.1038/ncb3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Hu S. K. and Goldstein A. L. (1981). Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc. Natl. Acad. Sci. USA 78, 1162-1166. 10.1073/pnas.78.2.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimova A., Bershadsky A. D. and Ben-Ze'ev A. (1997). Autoregulation of actin synthesis responds to monomeric actin levels. J. Cell. Biochem. 65, 469-478. [DOI] [PubMed] [Google Scholar]
- Lyubimova A., Bershadsky A. D. and Ben-Ze'ev A. (1999). Autoregulation of actin synthesis requires the 3'-UTR of actin mRNA and protects cells from actin overproduction. J. Cell. Biochem. 76, 1-12. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Goldschmidt-Clermont P. J. and Pollard T. D. (1990). The affinities of human platelet and Acanthamoeba profilin isoforms for polyphosphoinositides account for their relative abilities to inhibit phospholipase C. Cell Regul. 1, 937-950. 10.1091/mbc.1.12.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Mullins R. D., Higgs H. N., Kaiser D. A., Blanchoin L., May R. C., Hall M. E. and Pollard T. D. (1999). Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA 96, 3739-3744. 10.1073/pnas.96.7.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J.-B., Kaiser D. A., Pollard T. D. and Higgs H. N. (2001). Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 3, 76-82. 10.1038/35050590 [DOI] [PubMed] [Google Scholar]
- McGrath J. L., Tardy Y., Dewey C. F. Jr, Meister J. J. and Hartwig J. H. (1998). Simultaneous measurements of actin filament turnover, filament fraction, and monomer diffusion in endothelial cells. Biophys. J. 75, 2070-2078. 10.1016/S0006-3495(98)77649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melak M., Plessner M. and Grosse R. (2017). Actin visualization at a glance. J. Cell Sci. 130, 525-530. 10.1242/jcs.189068 [DOI] [PubMed] [Google Scholar]
- Miki H., Suetsugu S. and Takenawa T. (1998). WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932-6941. 10.1093/emboj/17.23.6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockrin S. C. and Korn E. D. (1980). Acanthamoeba profilin interacts with G-actin to increase the rate of exchange of actin-bound adenosine 5'-triphosphate. Biochemistry 19, 5359-5362. 10.1021/bi00564a033 [DOI] [PubMed] [Google Scholar]
- Mohapatra L., Lagny T. J., Harbage D., Jelenkovic P. R. and Kondev J. (2017). The limiting-pool mechanism fails to control the size of multiple organelles. Cell Syst. 4, 559 10.1016/j.cels.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M., Sivadasan R., Saal L., Lüningschrör P., Dombert B., Rathod R. J., Dieterich D. C., Blum R. and Sendtner M. (2017). Differential roles of alpha-, beta-, and gamma-actin in axon growth and collateral branch formation in motoneurons. J. Cell Biol. 216, 793-814. 10.1083/jcb.201604117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R. D., Kelleher J. F., Xu J. and Pollard T. D. (1998). Arp2/3 complex from Acanthamoeba binds profilin and cross-links actin filaments. Mol. Biol. Cell 9, 841-852. 10.1091/mbc.9.4.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. and Wadsworth P. (2005). Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 15, 724-731. 10.1016/j.cub.2005.02.055 [DOI] [PubMed] [Google Scholar]
- Namba Y., Ito M., Zu Y., Shigesada K. and Maruyama K. (1992). Human T cell L-plastin bundles actin filaments in a calcium dependent manner. J. Biochem. 112, 503-507. 10.1093/oxfordjournals.jbchem.a123929 [DOI] [PubMed] [Google Scholar]
- Novak I. L., Slepchenko B. M. and Mogilner A. (2008). Quantitative analysis of G-actin transport in motile cells. Biophys. J. 95, 1627-1638. 10.1529/biophysj.108.130096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman T., Schüler H., Korenbaum E., Schutt C. E., Karlsson R. and Lindberg U. (2002). The role of MeH73 in actin polymerization and ATP hydrolysis. J. Mol. Biol. 317, 577-589. 10.1006/jmbi.2002.5436 [DOI] [PubMed] [Google Scholar]
- Ostrander D. B., Gorman J. A. and Carman G. M. (1995). Regulation of profilin localization in Saccharomyces cerevisiae by phosphoinositide metabolism. J. Biol. Chem. 270, 27045-27050. 10.1074/jbc.270.45.27045 [DOI] [PubMed] [Google Scholar]
- Otey C. A., Kalnoski M. H., Lessard J. L. and Bulinski J. C. (1986). Immunolocalization of the gamma isoform of nonmuscle actin in cultured cells. J. Cell Biol. 102, 1726-1737. 10.1083/jcb.102.5.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K. and Hatano S. (1984). Mechanism of regulation of actin polymerization by Physarum profilin. J. Cell Biol. 98, 1919-1925. 10.1083/jcb.98.6.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D. and Carlier M.-F. (1993). How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell 75, 1007-1014. 10.1016/0092-8674(93)90544-Z [DOI] [PubMed] [Google Scholar]
- Pasquier E., Tuset M.-P., Sinnappan S., Carnell M., Macmillan A. and Kavallaris M. (2015). gamma-Actin plays a key role in endothelial cell motility and neovessel maintenance. Vasc. Cell 7, 2 10.1186/s13221-014-0027-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M., Miller G., Wells C., Zicha D. and Dunn G. A. (2001). Specific changes to the mechanism of cell locomotion induced by overexpression of beta-actin. J. Cell Sci. 114, 1367-1377. [DOI] [PubMed] [Google Scholar]
- Pernier J., Shekhar S., Jegou A., Guichard B. and Carlier M.-F. (2016). Profilin interaction with actin filament barbed end controls dynamic instability, capping, branching, and motility. Dev. Cell 36, 201-214. 10.1016/j.devcel.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin B. J. and Ervasti J. M. (2010). The actin gene family: function follows isoform. Cytoskeleton 67, 630-634. 10.1002/cm.20475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. (1986). Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103, 2747-2754. 10.1083/jcb.103.6.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. and Cooper J. A. (1984). Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry 23, 6631-6641. 10.1021/bi00321a054 [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Blanchoin L. and Mullins R. D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545-576. 10.1146/annurev.biophys.29.1.545 [DOI] [PubMed] [Google Scholar]
- Raz-Ben Aroush D., Ofer N., Abu-Shah E., Allard J., Krichevsky O., Mogilner A. and Keren K. (2017). Actin turnover in lamellipodial fragments. Curr. Biol. 27, 2963-2973.e2914. 10.1016/j.cub.2017.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Giehl K., Abel K., Haffner C., Jarchau T., Hoppe V., Jockusch B. M. and Walter U. (1995). The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 14, 1583-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B., Haupt A., Tucker A., Grancharova T., Arakaki J., Fuqua M. A., Nelson A., Hookway C., Ludmann S. A., Mueller I. A. et al. (2017). Systematic gene tagging using CRISPR/Cas9 in human stem cells to illuminate cell organization. Mol. Biol. Cell 28, 2854-2874. 10.1091/mbc.E17-03-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal A. A., Manning A. L., Goode B. L. and Drubin D. G. (2003). Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 13, 1000-1008. 10.1016/S0960-9822(03)00383-X [DOI] [PubMed] [Google Scholar]
- Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D. and Carlier M.-F. (2004). Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419-429. 10.1016/j.cell.2004.09.039 [DOI] [PubMed] [Google Scholar]
- Ross A. F., Oleynikov Y., Kislauskis E. H., Taneja K. L. and Singer R. H. (1997). Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 17, 2158-2165. 10.1128/MCB.17.4.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty J. D., Wu C., Haynes E. M., Suarez C., Winkelman J. D., Johnson H. E., Haugh J. M., Kovar D. R. and Bear J. E. (2015). Profilin-1 serves as a gatekeeper for actin assembly by Arp2/3-dependent and -independent pathways. Dev. Cell 32, 54-67. 10.1016/j.devcel.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Rajfur Z., Jones D., Marriott G., Loew L. and Jacobson K. (2001). Local photorelease of caged thymosin beta4 in locomoting keratocytes causes cell turning. J. Cell Biol. 153, 1035-1048. 10.1083/jcb.153.5.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa K., Sato M., Sato K., Nakajima-Shimada J., Harada A. and Sato K. (2014). Caenorhabditis elegans chaperonin CCT/TRiC is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Mol. Biol. Cell 25, 3095-3104. 10.1091/mbc.E13-09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D. and Chowrashi P. K. (1997). Beta-thymosins from marine invertebrates: primary structure and interaction with actin. Cell Motil. Cytoskeleton 38, 163-171. [DOI] [PubMed] [Google Scholar]
- Safer D. and Nachmias V. T. (1994). Beta thymosins as actin binding peptides. BioEssays 16, 473-479. 10.1002/bies.950160706 [DOI] [PubMed] [Google Scholar]
- Safer D., Elzinga M. and Nachmias V. T. (1991). Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J. Biol. Chem. 266, 4029-4032. [PubMed] [Google Scholar]
- Safer D., Sosnick T. R. and Elzinga M. (1997). Thymosin beta 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry 36, 5806-5816. 10.1021/bi970185v [DOI] [PubMed] [Google Scholar]
- Salvany L., Muller J., Guccione E. and Rorth P. (2014). The core and conserved role of MAL is homeostatic regulation of actin levels. Genes Dev. 28, 1048-1053. 10.1101/gad.237743.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. C., Goldstein A. L. and Wang Y. L. (1992). Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc. Natl. Acad. Sci. USA 89, 4678-4682. 10.1073/pnas.89.10.4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Jennings P. B. and Cooper J. A. (1998). Rapid and efficient purification of actin from nonmuscle sources. Cell Motil. Cytoskeleton 39, 166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt C. E., Myslik J. C., Rozycki M. D., Goonesekere N. C. W. and Lindberg U. (1993). The structure of crystalline profilin-beta-actin. Nature 365, 810-816. 10.1038/365810a0 [DOI] [PubMed] [Google Scholar]
- Selden L. A., Kinosian H. J., Estes J. E. and Gershman L. C. (1999). Impact of profilin on actin-bound nucleotide exchange and actin polymerization dynamics. Biochemistry 38, 2769-2778. 10.1021/bi981543c [DOI] [PubMed] [Google Scholar]
- Shum M. S. Y., Pasquier E., Po'uha S. T., O'Neill G. M., Chaponnier C., Gunning P. W. and Kavallaris M. (2011). gamma-Actin regulates cell migration and modulates the ROCK signaling pathway. FASEB J. 25, 4423-4433. 10.1096/fj.11-185447 [DOI] [PubMed] [Google Scholar]
- Sirotkin V., Berro J., Macmillan K., Zhao L. and Pollard T. D. (2010). Quantitative analysis of the mechanism of endocytic actin patch assembly and disassembly in fission yeast. Mol. Biol. Cell 21, 2894-2904. 10.1091/mbc.E10-02-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. B., Kiuchi T., Watanabe N. and Vavylonis D. (2013). Distributed actin turnover in the lamellipodium and FRAP kinetics. Biophys. J. 104, 247-257. 10.1016/j.bpj.2012.11.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C., Carroll R. T., Burke T. A., Christensen J. R., Bestul A. J., Sees J. A., James M. L., Sirotkin V. and Kovar D. R. (2015). Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex. Dev. Cell 32, 43-53. 10.1016/j.devcel.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu S., Miki H. and Takenawa T. (1998). The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 17, 6516-6526. 10.1093/emboj/17.22.6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-Q., Kwiatkowska K. and Yin H. L. (1996). beta-Thymosins are not simple actin monomer buffering proteins. Insights from overexpression studies. J. Biol. Chem. 271, 9223-9230. 10.1074/jbc.271.16.9223 [DOI] [PubMed] [Google Scholar]
- Taneja K. L. and Singer R. H. (1990). Detection and localization of actin mRNA isoforms in chicken muscle cells by in situ hybridization using biotinated oligonucleotide probes. J. Cell. Biochem. 44, 241-252. 10.1002/jcb.240440406 [DOI] [PubMed] [Google Scholar]
- Terman J. R. and Kashina A. (2013). Post-translational modification and regulation of actin. Curr. Opin. Cell Biol. 25, 30-38. 10.1016/j.ceb.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A. and Mitchison T. J. (1991). Actin microfilament dynamics in locomoting cells. Nature 352, 126-131. 10.1038/352126a0 [DOI] [PubMed] [Google Scholar]
- Tilney L. G. (1976). The polymerization of actin. II. How nonfilamentous actin becomes nonrandomly distributed in sperm: evidence for the association of this actin with membranes. J. Cell Biol. 69, 51-72. 10.1083/jcb.69.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Bonder E. M., Coluccio L. M. and Mooseker M. S. (1983). Actin from Thyone sperm assembles on only one end of an actin filament: a behavior regulated by profilin. J. Cell Biol. 97, 112-124. 10.1083/jcb.97.1.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruchinapalli D. M., Oleynikov Y., Kelic S., Shenoy S. M., Hartley A., Stanton P. K., Singer R. H. and Bassell G. J. (2003). Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. 23, 3251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng P. C. and Pollard T. D. (1982). Mechanism of action of Acanthamoeba profilin: demonstration of actin species specificity and regulation by micromolar concentrations of MgCl2. J. Cell Biol. 94, 213-218. 10.1083/jcb.94.1.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng P. C., Runge M. S., Cooper J. A., Williams R. C. Jr and Pollard T. D. (1984). Physical, immunochemical, and functional properties of Acanthamoeba profilin. J. Cell Biol. 98, 214-221. 10.1083/jcb.98.1.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Trotter P. J., Orchard M. A. and Walker J. H. (2000). Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur. J. Biochem. 267, 4720-4730. 10.1046/j.1432-1327.2000.01525.x [DOI] [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R. and De Moor P. (1980). Vitamin D-binding protein (Gc-globulin) binds actin. J. Biol. Chem. 255, 2270-2272. [PubMed] [Google Scholar]
- Vandekerckhove J. (1990). Actin-binding proteins. Curr. Opin. Cell Biol. 2, 41-50. 10.1016/S0955-0674(05)80029-8 [DOI] [PubMed] [Google Scholar]
- Vinson V. K., De La Cruz E. M., Higgs H. N. and Pollard T. D. (1998). Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry 37, 10871-10880. 10.1021/bi980093l [DOI] [PubMed] [Google Scholar]
- Vitriol E. A. and Zheng J. Q. (2012). Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron 73, 1068-1081. 10.1016/j.neuron.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitriol E. A., Wise A. L., Berginski M. E., Bamburg J. R. and Zheng J. Q. (2013). Instantaneous inactivation of cofilin reveals its function of F-actin disassembly in lamellipodia. Mol. Biol. Cell 24, 2238-2247. 10.1091/mbc.E13-03-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitriol E. A., McMillen L. M., Kapustina M., Gomez S. M., Vavylonis D. and Zheng J. Q. (2015). Two functionally distinct sources of actin monomers supply the leading edge of lamellipodia. Cell Rep. 11, 433-445. 10.1016/j.celrep.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Boja E. S., Tan W., Tekle E., Fales H. M., English S., Mieyal J. J. and Chock P. B. (2001). Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 276, 47763-47766. 10.1074/jbc.C100415200 [DOI] [PubMed] [Google Scholar]
- Wang W., Rai A., Hur E.-M., Smilansky Z., Chang K. T. and Min K.-T. (2016). DSCR1 is required for both axonal growth cone extension and steering. J. Cell Biol. 213, 451-462. 10.1083/jcb.201510107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N. and Mitchison T. J. (2002). Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295, 1083-1086. 10.1126/science.1067470 [DOI] [PubMed] [Google Scholar]
- Weber A., Nachmias V. T., Pennise C. R., Pring M. and Safer D. (1992). Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry 31, 6179-6185. 10.1021/bi00142a002 [DOI] [PubMed] [Google Scholar]
- Wei C., Wang X., Chen M., Ouyang K., Song L.-S. and Cheng H. (2009). Calcium flickers steer cell migration. Nature 457, 901-905. 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M., Jungbluth A., Heidecker M., Mühlbauer B., Heizer C., Schwartz J.-M., Marriott G. and Gerisch G. (1997). Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr. Biol. 7, 176-183. 10.1016/S0960-9822(97)70088-5 [DOI] [PubMed] [Google Scholar]
- Wu J.-Q. and Pollard T. D. (2005). Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310-314. 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Xue B., Leyrat C., Grimes J. M. and Robinson R. C. (2014). Structural basis of thymosin-beta4/profilin exchange leading to actin filament polymerization. Proc. Natl. Acad. Sci. USA 111, E4596-E4605. 10.1073/pnas.1412271111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Mizuno H., Smith M. B., Ryan G. L., Kiuchi T., Vavylonis D. and Watanabe N. (2014). New single-molecule speckle microscopy reveals modification of the retrograde actin flow by focal adhesions at nanometer scales. Mol. Biol. Cell 25, 1010-1024. 10.1091/mbc.E13-03-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Sasaki Y., Wen Z., Bassell G. J. and Zheng J. Q. (2006). An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat. Neurosci. 9, 1265-1273. 10.1038/nn1773 [DOI] [PubMed] [Google Scholar]
- Yarmola E. G. and Bubb M. R. (2004). Effects of profilin and thymosin beta4 on the critical concentration of actin demonstrated in vitro and in cell extracts with a novel direct assay. J. Biol. Chem. 279, 33519-33527. 10.1074/jbc.M404392200 [DOI] [PubMed] [Google Scholar]
- Yarmola E. G. and Bubb M. R. (2006). Profilin: emerging concepts and lingering misconceptions. Trends Biochem. Sci. 31, 197-205. 10.1016/j.tibs.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Yarmola E. G., Parikh S. and Bubb M. R. (2001). Formation and implications of a ternary complex of profilin, thymosin beta 4, and actin. J. Biol. Chem. 276, 45555-45563. 10.1074/jbc.M105723200 [DOI] [PubMed] [Google Scholar]
- Yu F.-X., Yin H.-L., Morrison-Bogorad M. and Lin S.-C. (1994). Effects of thymosin beta 4 and thymosin beta 10 on actin structures in living cells. Cell Motil. Cytoskeleton 27, 13-25. 10.1002/cm.970270103 [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G. and Rabinowitz M. (1977). Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J. Biol. Chem. 252, 3430-3435. [PubMed] [Google Scholar]
- Zarbock J., Oschkinat H., Hannappel E., Kalbacher H., Voelter W. and Holak T. A. (1990). Solution conformation of thymosin beta 4: a nuclear magnetic resonance and simulated annealing study. Biochemistry 29, 7814-7821. 10.1021/bi00486a006 [DOI] [PubMed] [Google Scholar]
- Zhang H. L., Eom T., Oleynikov Y., Shenoy S. M., Liebelt D. A., Dictenberg J. B., Singer R. H. and Bassell G. J. (2001). Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 31, 261-275. 10.1016/S0896-6273(01)00357-9 [DOI] [PubMed] [Google Scholar]
- Zhang F., Saha S., Shabalina S. A. and Kashina A. (2010). Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science 329, 1534-1537. 10.1126/science.1191701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. and Wang Y.-L. (2008). Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol. Biol. Cell 19, 318-326. 10.1091/mbc.E07-08-0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha D., Dobbie I. M., Holt M. R., Monypenny J., Soong D. Y., Gray C. and Dunn G. A. (2003). Rapid actin transport during cell protrusion. Science 300, 142-145. 10.1126/science.1082026 [DOI] [PubMed] [Google Scholar]