Abstract
Amitriptyline is an old drug but is still prevalently used as the first-line treatment for a variety of common diseases. Surprisingly, knowledge of sexual risks with amitriptyline comes from only one clinical trial and several case reports from three decades ago. In the current study, a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) related to amitriptyline and sexual dysfunction (SD) was performed. The frequency, gender-difference, types, disease-specificity and time course of SD, and the relationship between SD and nonsexual adversity were studied. A total of 14 publications, including 8 qualified randomized clinical trials, were eligible. The frequency of SD in overall, male and female patients was 5.7, 11.9 and 1.7%, respectively. SD was six-fold higher in men than women. The frequency of SD was 6.9% in depressive patients compared with 0.8% in non-depressive patients (p = .008), and gradually decreased at 8 weeks after treatment (p = .02). Amitriptyline impacted arousal and libido more than orgasm and ejaculation in male patients but mainly libido in female patients. SD was significantly correlated with insomnia linearly whereas somnolence and nausea dually. Therefore, amitriptyline-associated SD mainly occurs in depressive and male patients, disturbs each phase of the sexual response cycle in men but mainly libido in women, gradually decreases under long-term treatment, and can be predicted by the co-existence of insomnia, somnolence or nausea during treatment. Clinicians should caution and tailor the gender and disease vulnerability of amitriptyline in their practice.
Keywords: amitriptyline, sexual dysfunction, impotence, orgasm, libido, ejaculation
Sexual pharmacotoxicity is common and harmful for reproductive and sexual health but is avoidable through sufficient safety data. Amitriptyline (C20H23N) was introduced more than half a century ago but is still prevalently used worldwide because of its high effectiveness and economic benefit (Leong et al., 2016). In the United States, the annual prescription of amitriptyline was more than 12 million and it was one-third of the leading antidepressant, citalopram, in 2011-2012 (Statistica, 2017). In the United Kingdom, 12 million and 14 million amitriptyline and citalopram prescriptions were dispensed, respectively, in 2015 (Health and Social Care Information Centre, 2016). Although amitriptyline has been mostly replaced by a newer generation of antidepressants (NGAs) for depression therapy, it is still recommended and widely prescribed as the first-line drug treatment for a variety of non-depressive disorders, especially migraines, fibromyalgia, neuropathic pain, post-herpetic neuralgia, chronic tension-type headache, central pain and interstitial cystitis (Moore, Derry, Aldington, Cole, & Wiffen, 2015; Pringsheim et al., 2012; Wong et al., 2017), which frequently occur in people within a sexually active age.
Similar to other antidepressants and antipsychotics, amitriptyline has been circulating as a high risk for sexual dysfunction (SD). Surprisingly, the source of data comes from only a few sporadic case reports of depressive patients (Nininger, 1978; Simpson, Blair, & Amuso, 1965) and one case-control study (Reimherr et al., 1990) published three decades ago in that SD was reported to occur in 7.7% of amitriptyline-treated male depressive patients. No systematic review of subsequent clinical trials was done. The shortage of persuasive evidence of the frequency and types of SD in both genders and between depressive and non-depressive conditions under amitriptyline use is insufficient to meet current standard of practice.
Methods
The aim of the current study was to elucidate the relationship between amitriptyline and sexual function in humans. The authors followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to complete the literature review. Any outcomes that reported the negative impact of sexual function or SD under amitriptyline use were included.
Types of SD
SD was any of the sexual disorders listed in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) or related either to changes in the sexual response cycle or impairments of the genitalia or reproductive organs that interfere with sexual activity. Isolated cases of sexual dissatisfaction that did not have associated psychiatric or biological disturbances were not included.
Amitriptyline
After the patent expired, many generic amitriptyline medications were marketed under different commercial names worldwide. To minimize incomplete coverage, the authors searched the brand names, chemical name, and commercial names of amitriptyline that were listed by the United States Food and Drug Administration, the Taiwan Food and Drug Administration, the Medicines and Healthcare Products Regulatory Agency of the United Kingdom, the European Medicines Agency, and Medindia (http://www.medindia.net/index.asp).
Literature Search
To obtain a large pool of data, any studies in literature evaluating the sexual effects of amitriptyline were enrolled. All papers, books, proceedings, and abstracts that were published before June 2016 were considered in this study if they were full-text manuscripts, book chapters, and abstracts that included any SD and had English or Chinese titles.
A computer-based search of the literature was performed using a catch of amitriptyline and a list of keywords in scientific databases. These keywords were “desire,” “libido,” “arousal,” “orgasm,” “anorgasmia,” “ejaculation,” “impotence,” “erectile dysfunction,” “vaginismus,” “hyposexuality,” “hypersexuality,” “paraphilia,” “sexual impulse control,” “sexual misconduct,” “priapism,” “sexual dysfunction,” “sexual deviation,” “sexual behavior,” “sexual intention,” “sexual motivation,” “sexual dissatisfaction,” or “sexual function.” The English scientific databases included PROQUEST, PUBMED, SCOPUS, DOAJ, and Cochrane Database of Systematic Reviews. The Chinese scientific databases were the Taiwan Periodical Literature System (http://readopac.ncl.edu.tw/nclJournal) and the Airiti Library (http://www.airitilibrary.com). Additionally, the references of each manuscript, book chapter, and proceeding were further reviewed to ensure a full coverage of the literature.
Level of Evidence and Grade of Recommendation
The level of evidence (LOE) and grade of recommendation (GOR) were adopted from the Oxford Centre for Evidence-Based Medicine.
Statistical Analysis
The frequency of SD was estimated in randomized clinical trials (case-control or case-placebo study) that provided the number and gender of patients and controls. The types of SD were classified based on all the reported patients from all the eligible observational studies, case-control studies, and case reports. The categorical variables (frequency and time onset of SD) were statistically analyzed using the Chi-square test or Fisher’s exact test. A probability less than .05 was considered statistical significance.
Results
There were a total of 15 publications that explicitly recorded the frequency or types of sexual function change under amitriptyline use. They included one observational study (Couper-Smartt & Rodham, 1973), one review study (Montgomery, 1995), eight randomized case-control or case-placebo clinical trials (Bremner, 1995; Hekimian, Friedhoff, & Deever, 1978; Mathur, Sharma, Choudhary, & Jain, 2005; Reimherr et al., 1990; Sohn et al., 2012; Stahl, Zivkov, Reimitz, Panagides, & Hoff, 1997; van Ophoven, Pokupic, Heinecke, & Hertle, 2004; Zivkov & de Jongh, 1995), and five clinical case reports (Lucca, Ramesh, Ram, Kurian, & Mathew, 2016; Mitchell & Popkin, 1983; Nininger, 1978; Rao, Morriss, & Michael, 1998; Simpson et al., 1965). The review study, which included pooled clinical and preclinical trials of mirtazapine (Org 3770) in Europe and the United States before 1995, was discarded from this study as the demographic information was not fully described and part of the data has been published in another study (Montgomery, 1995). In the other 14 studies, there were a total of 706 amitriptyline-treated patients, of whom 575 were depressive and 131 were non-depressive (Table 1).
Table 1.
The Summary of Observational Study, Randomized Clinical Trials, and Case Reports of Amitriptyline and Sexual Dysfunction in Literature.
| Author(s) Year |
Disease | Region | Daily dose (mg) |
Patient number (male:female) |
Duration | Study control (Case Number) |
Methoda | Sexual dysfunction (Case Number)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | ||||||||
| 1. Observational Study | |||||||||||||||||||
| Couper-Smartt & Rodham (1973) c | Psychiatric diseases | United Kingdom | 75-150 | 5 | NR | - | Q | 2 | 1 | 1 | 1 | ||||||||
| 2. Randomized Clinical Trials | |||||||||||||||||||
| Sohn et al. (2012) | Irritable bowel syndrome | Korea | 10 | 106 (55:51) |
4 weeks | Tianeptine (122) | SR | 0 | 0 | 0 | 0 | ||||||||
| Mathur et al. (2005) | Depression | India | 150 | 20 (7:13) |
6 weeks | Citalopram (20) | HDRS, SR | 2 | 2 | 0 | 2 | ||||||||
| van Ophoven et al. (2004) | Interstitial cystitis | Germany | 25-100 (max) | 25 (3:22) |
4 months | Placebo (25) | SR | 1 | 1 | 0 | 1 | ||||||||
| Stahl et al. (1997) | Depression | United States | 40-280 | 193 (83:110) |
6 weeks | Org 3770 (194) Placebo (193) |
17-HDRS | 20 | 13 | 7 | 9 | 4 | 7 | ||||||
| Bremer et al. (1995)d | Depression | United States | 40-280 (max) | 50 (13:37) |
6 weeks | Org 3770 (50) Placebo (50) |
17-HDRS, SDS | 4 | 4 | 0 | 4 | ||||||||
| Zivokc & de Jongh et al. (1995)d | Depression | Yugoslavia | 75-225 | 111 (27:84) |
6 weeks | Org 3770 (113) | HDRS | 4 | 4 | 0 | 4 | ||||||||
| Reimherr et al. (1990) | Depression | United States | 150 mg | 149 (65:84) |
8 weeks | Sertraline (149) Placebo (150) |
HDRS, SCL-56 | 5 | 5 | 0 | 2 | 3 | |||||||
| Hekimian et al. (1978) | Depression | United States | 150 mg | 31 (16:15) |
4 weeks | Amoxapine (30) | HDRS, SDS, SES | 3 | 3 | 0 | 3 | ||||||||
| 3. Clinical Case Reports | |||||||||||||||||||
| Lucca et al. (2016); Rao et al. (1998); Mitchelle and Popkin (1983)e; Nininger (1978); and Simpson et al. (1965) | 16 (16:0) |
16 | 4 | 2 | 16 | 4 | 7 | 2 | 5 | ||||||||||
Note. aThe methods used for evaluating sexual adversity. 17-HDRS = 17-item Hamilton Depression Rating Scale; HDRS = 21-item Hamilton Depression Rating Scale; Q = Questionnaire designed by interviewers; SCL-56 = Symptom Checklist 56 items; SDS = Zung Self-Rating Depression Scale; Side Effects Scale.
Sexual dysfunction: A = overall of any sexual dysfunction; B = overall of libido disorder; C = overall of orgasm disorder; D = male any overall of any sexual dysfunction; E = male of libido disorder; F = impotence; G = male of orgasm disorder; H = ejaculatory disorder; I = female any overall of any sexual dysfunction; J = female of libido disorder; K = female of arousal disorder; L = female of orgasm disorder.
In five amitriptyline-treated patients, three out of them experienced any sexual dysfunction, including two men and one woman. (Couper-Smartt & Rodham, 1973)
A review of literature of clinical trials and preclinical human studies about mirtazapine (Org 3770) in Europe and United States before 1995, including Bremer et al. (1995) and Zivokc & de Jongh et al. (1995). The gender ratio was not further mentioned.
These patients are personal communication recorded in manuscript text.
Quality of Publications
In the eight randomized clinical trials, the quality was considered good in four trials (Reimherr et al., 1990; Sohn et al., 2012; Stahl, Zivkov, Reimitz, Panagides, & Hoff, 1997; Zivkov & de Jongh, 1995) due to a prospective design, a high follow-up rate (> 80%), an adequate number of patients and controls (100-200 subjects in each group), a clear main outcome and a rational methodology for sexual and clinical assessment (LOE: IIb). In another four trials (Bremner, 1995; Hekimian et al., 1978; Mathur et al., 2005; van Ophoven et al., 2004), the design, follow-up rate, outcome and methodology were similar except for a modest number of patients and controls (less than 100 subjects in either group) (LOE: IIIb).
In the one observational study (Couper-Smartt & Rodham, 1973), SD was described in three out of five amitriptyline-treated patients (LOE: 4). In another case series report and four case reports (Lucca et al., 2016; Mitchell & Popkin, 1983; Nininger, 1978; Rao et al., 1998; Simpson et al., 1965), there were a total of 16 amitriptyline-treated patients (LOE: 4).
Methods of Sexual Dysfunction Evaluation
In the eight randomized clinical trials, SD was mainly evaluated by the 17-item or 21-item Hamilton Depression Rating Scale (HDRS) in six studies and self-reporting in another two studies. The SD item in the 17-item and 21-item HDRS is identical, that is, any genital symptom rating from 0 to 2 scores. However, other scales which assess sexual symptoms were also used together with HDRS in three out of those six studies, such as the Symptom Checklist 56 items (Reimherr et al., 1990), Zung Self-Rating Depression Scale (Bremner, 1995), and Side Effects Scale (Hekimian et al., 1978). In another study (Mathur et al., 2005), patients were requested to self-report sexual symptoms in addition to the HDRS (Table 1). The outcome of all scales or self-reporting was SD, which was described a disturbance of any sexual function in those studies. Therefore, the final SD data in this study represented a generalized concept of SD.
Frequency of SD
The 8 randomized clinical trials (Bremner, 1995; Hekimian et al., 1978; Mathur et al., 2005; Reimherr et al., 1990; Sohn et al., 2012; Stahl et al., 1997; van Ophoven et al., 2004; Zivkov & de Jongh, 1995) included 685 amitriptyline-treated patients (269 men and 416 women), 418 controls (164 men and 254 women), and 678 patients receiving other antipsychotic or antidepressant treatment (281 men and 397 women) (Table 2).
Table 2.
The Frequency of Any SD in Amitriptyline-Treated Patients, Patients Receiving Other Antidepressant or Antipsychotic Treatment, or Controls in Eight Randomized Clinical Trials.
| Gender and SD/population | Amitriptyline-treated | Other-treated | Control | ||||
|---|---|---|---|---|---|---|---|
| Overall | Any SD | 39, | 5.7% | 32, | 4.7% | 19, | 4.5% |
| No SD | 646, | 94.3% | 646, | 95.3% | 399, | 95.5% | |
| Total | 685 | 678 | 418 | ||||
| Male gender | Any SD | 32, | 11.9% | 32, | 11.4% | 8, | 4.9% |
| No SD | 237, | 88.1% | 249, | 88.6% | 156, | 95.1% | |
| Total | 269 | 281 | 164 | ||||
| Female gender | Any SD | 7, | 1.7% | 7, | 1.8% | 11, | 4.3% |
| No SD | 409, | 98.3% | 390, | 98.2% | 243, | 95.7% | |
| Total | 416 | 397 | 254 | ||||
Note. SD = sexual dysfunction; Other-treated = treated with antidepressant or antipsychotic other from amitriptyline.
Regarding the amitriptyline-treated patients, the SD frequency was 5.7% among the entire group, 11.9% among the male patients, and 1.7% among the female patients. The frequency of SD was six-fold higher in the men than in the women. Among the controls, the SD frequency was 4.5% for the entire group, 4.9% for the male controls, and 4.3% for the female controls. Among the patients who were receiving other antidepressants or antipsychotic treatment, the SD frequency was 4.7% for all patients, 11.4% for male patients, and 1.8% for female patients (Table 2 and Figure 1).
Figure 1.
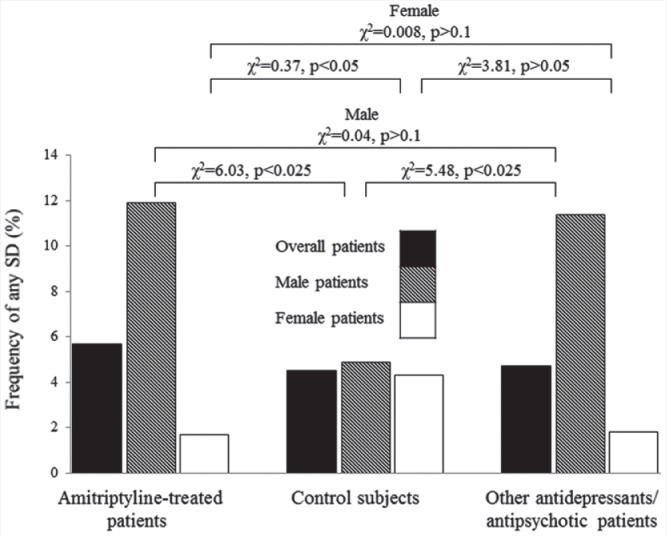
The frequency of any sexual dysfunction (SD) in amitriptyline-treated patients, control subjects and other antidepressant-treated patients. The SD frequency was higher in amitriptyline- or other antidepressant-treated male patients than male control subjects (p < .025), but was similar between amitriptyline- or other antidepressant-treated male patients. In regard to female gender, the SD frequency was lower in the amitriptyline-treated patients and other antidepressant-treated patients than controls.
Compared with the placebo controls, the odds ratio of amitriptyline-associated SD was 1.27 (χ2 = 0.69, p > .1) for the whole group of patients, 2.6 (χ2 = 6.03, p < .025) for male patients, and 0.37 (χ2 = 4.27, p < .05) for female patients. Amitriptyline significantly increased the risk of SD in male patients but decreased in female patients compared with controls. In comparison, the odds ratio of having any amitriptyline-associated SD was 1.21 (χ2 = 0.66, p > .1) for all patients, 1.05 (χ2 = 0.04, p > .1) for male patients, and 0.954 (χ2 = 0.008, p > .1) for female patients compared with those who received other antidepressants or antipsychotics. The risk of SD was similar between patients taking amitriptyline and those receiving other antidepressants or antipsychotics for both men and women. Finally, compared with the placebo controls, the odds ratio of SD associated with other antidepressants or antipsychotics was 1.03 (χ2 = 0.08, p > .1) for the whole group, 2.5 (χ2 = 5.48, p < .025) for males, and 0.40 (χ2 = 3.81, p > .05) for females. Other antidepressants or antipsychotics significantly increased the risk of SD in male patients compared with male controls, whereas it marginally decreased for female patients (Figure 1). Therefore, amitriptyline and other antidepressants or antipsychotics increased the risk of SD in male patients (GOR: B), but amitriptyline decreased the risk of SD in female patients (GOR: C). The risk of SD was similar for amitriptyline and other antidepressants or antipsychotics in both men and women (GOR: B).
Types of SD
In randomized clinical trials, the frequencies of amitriptyline-associated impotence, libido disorder, ejaculatory disorder and orgasmic disorder were 35.2, 35.2, 14.8, and 14.8%, respectively (Bremner, 1995; Hekimian et al., 1978; Mathur et al., 2005; Reimherr et al., 1990; Sohn et al., 2012; Stahl et al., 1997; van Ophoven et al., 2004; Zivkov & de Jongh, 1995). On the contrary, the types of SD in female patients or controls were reported in only one randomized clinical trial (Stahl et al., 1997), in which 6.4% of patients and 8.4% of control subjects had libido disorder at the end of study (χ2 = 2.60, p > .1). No other type of SD was mentioned.
In male patients, amitriptyline elicited a higher frequency of impotence and libido disorder than ejaculatory disorder or orgasm in the male patients (GOR: B). For the female patients, amitriptyline did not cause a higher risk of arousal or orgasm disorder whereas it showed an insignificant trend toward decreasing libido disorder (GOR: C). Of the 24 patients with libido disorder, hypolibidinism was described in 23 patients whereas hyperlibidinism was described in 1 female patient (Couper-Smartt & Rodham, 1973).
Relationship Between Time Course and SD
Of the five randomized clinical trials that included depressive patients, one was completed at 4 weeks (Hekimian et al., 1978), three were completed at 6 weeks (Bremner, 1995; Mathur et al., 2005; Stahl et al., 1997), and one was completed at 8 weeks (Reimherr et al., 1990). The frequency of any SD was 9.7% at 4 weeks, 9.9% at 6 weeks, and 3.4% at 8 weeks. When both depressive (Bremner, 1995; Hekimian et al., 1978; Mathur et al., 2005; Reimherr et al., 1990; Stahl et al., 1997; Zivkov & de Jongh, 1995) and non-depressive patients (Sohn et al., 2012; van Ophoven et al., 2004) were enrolled, the overall frequency of any SD was 4.1% at 4 weeks, 9.9% at 6 weeks, 3.4% at 8 weeks, and 4.0% at 12 weeks. Therefore, the frequency of any had SD decreased by 8 weeks in the depressive patients and the overall group of patients (6 weeks vs 8 weeks, Fisher’s exact test = 0.02) (GOR: B).
Relationship Between Dose and SD
In the eight randomized clinical trials, the dose of amitriptyline showed an inverse U-shape relationship with the frequency of any SD (Figure 2A). A higher frequency of SD was seen in patients who received between 100 and 150 mg per day treatment than other dose treatments.
Figure 2.
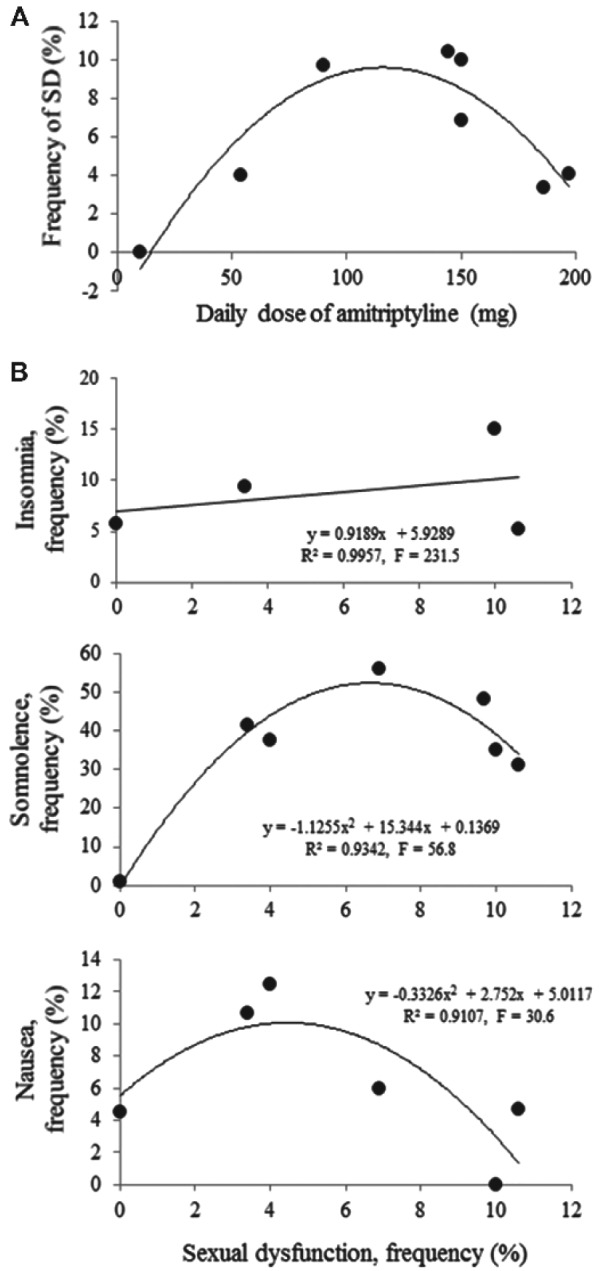
In randomized clinical trials, the dose of amitriptyline showed an inverse U-shape relationship with the frequency of any sexual dysfunction (SD) (A). The frequency of SD was higher in patients who received 100–150 mg/day treatment than another dose. The frequency of SD correlated linearly to the frequency of insomnia whereas dually to somnolence and nausea in amitriptyline-treated patients (B).
Relationship Between Underlying Diseases and SD
Two studies enrolled exclusively non-depressive patients, including 112 male and 166 female patients with irritable bowel syndrome or interstitial cystitis (Sohn et al., 2012; van Ophoven et al., 2004). Only one male non-depressive patient developed SD under amitriptyline use (van Ophoven et al., 2004). No non-depressive patient who received the placebo developed SD. The frequency of amitriptyline-associated SD was only 0.8% in non-depressive patients, which was significantly lower than the 6.9% frequency in depressive patients (Fisher’s exact test = 0.008). The frequency of SD was 1.7% in non-depressive male patients, compared with 14.7% in depressive male patients (Fisher’s exact test = 0.005). Therefore, amitriptyline appears to be safer for non-depressive male patients than depressive male patients (GOR: B). The sample size was small for conclusions in female patients.
The daily mean dose of amitriptyline ranged from 10 to 54.2 mg in non-depressive patients to 90–196.9 mg in depressive patients. However, the frequency of SD in the non-depressive series of van Ophoven et al. was similar to or higher than in the depressive series of Reimherr et al. (1990) and Zovikc & De Jongh (1995), and of dry mouth than most of the depressive series.
Moreover, the study period was 4 weeks and 12 weeks, respectively in non-depressive patients whereas it ranged from 4 to 8 weeks in depressive patients. Therefore, a lower frequency of amitriptyline-associated SD in non-depressive patients is not simply related to the lower daily dose of amitriptyline.
Nonsexual Adverse Events
The frequency of amitriptyline-associated adverse events in randomized clinical trials were as follows, in order of frequency: dry mouth 60.3% (19.8–80.8%), somnolence 34.3% (0.9–56.0%), constipation 20.3% (3.1–32.2%), dizziness 16.2% (5.2–31.5%), fatigue 10.8% (2.1–23.5%), headache 9.7% (0–15.5%), agitation 9.6% (2.8–15.4%), tremor 9.2% (0.9–14.0%), blurred vision 8.6% (3.1–16.2%), nausea 6.6% (0–12.5%), and insomnia 6.4% (5.7-15.0%) (Bremner, 1995; Hekimian et al., 1978; Mathur et al., 2005; Reimherr et al., 1990; Sohn et al., 2012; Stahl et al., 1997; van Ophoven et al., 2004; Zivkov & de Jongh, 1995). The frequency of all of these events was higher than the 5.7% of SD.
SD showed three types of relationships with these adverse effects. The first type was a positive linear correlation with insomnia (r2 = .996, F = 231.5, p < .05). The second type was a biphasic correlation with somnolence (r2 = .9342, F = 56.8, p < .01) and nausea (r2 = .9107, F = 30.6, p < .05; Figure 2B). The third type was no statistical correlation, such as with dry mouth, constipation, tremor, and agitation.
Discussion
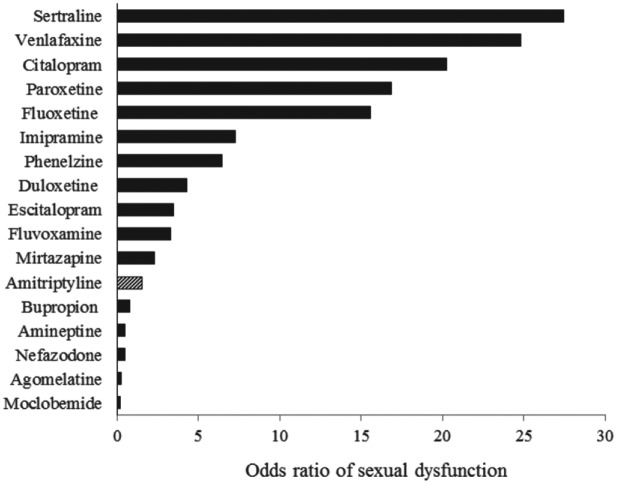
In the current study, the frequency of SD was 5.7% in amitriptyline-treated patients, ranging from 1.7% in women to 11.9% in men. The frequency of SD in male and female patients using amitriptyline was lower than that reported for the marketed NGAs (Reichenpfader et al., 2014; Serretti & Chiesa, 2009). Since the frequency of anticholinergic side effects, such as dry mouth, in index studies is similar to previous reports (Leucht, Huhn, & Leucht, 2012), as well as a similar duration of study period in index studies, the lower frequency of SD is unlikely to result from an inadequate pharmacological effect of amitriptyline. Accordingly, amitriptyline elicits a lower risk of SD than NGAs, imipramine, clomipramine and phenelzine (Figure 3) and shares a similar risk with amineptine, agomelatine, and moclobemide. Amitriptyline is a relatively safe replacement for other tricyclic/tetracyclic antidepressants or NGAs when drug-related SD is complicated and bothersome.
Figure 3.
The odds ratio of marketed antidepressants (from Serretti & Chiesa, 2009) and amitriptyline for sexual dysfunction. Based on the eight randomized clinical trials in this study, the odds ratio was 1.5, which was higher than the bupropion, amineptine, nefazodone, agomelatine, and moclobemide but was much lower than most new generation antidepressants.
Another conspicuous finding of the study was a gender difference in amitriptyline-associated SD, that is, a six-fold higher risk of SD in men than in women. Generally, NGAs or tricyclic antidepressants other than amitriptyline elicit a modest higher frequency of SD in male depressive patients than female patients (Kornstein et al., 2000; Montejo, Llorca, Izquierdo, & Rico-Villademoros, 2001). However, amitriptyline associates with a deterioration of sexual function in male patients whereas a trend of improvement was identified in female patients in the current study. This difference appears to be responsible for the great disparity in SD frequency between men and women. The current results are in line with those of previous studies in that SD worsens in male patients taking amitriptyline but improves in female patients (Kornstein et al., 2000; Lee et al., 2013) without a parallel change in mood. Amitriptyline improves brain activity in erotic zones in female depressive patients (Yang et al., 2012) but displays no effect in male depressive patients (Graf, Walter, Metzger, & Abler, 2014). Other factors, such as gender differences in pharmacokinetics and pharmacodynamics or physiological sexual response, are unclear for the gender-difference of SD. Nevertheless, the current results clearly suggest that amitriptyline use is safer for SD occurrence in female patients than male patients.
The current findings also identify a gender-difference of SD type under amitriptyline use. NGAs consistently impact libido, arousal and orgasm in both genders but elicit a modest higher frequency of libido and orgasm disorder than arousal disorder in male patients compared with female patients (Clayton, Keller, & McGarvey, 2006; Reichenpfader et al., 2014; Serretti & Chiesa, 2009). The current study reports that amitriptyline complicates erectile and libido disorder more frequently than ejaculatory and orgasm disorder in male patients. In female patients, the risk of arousal or orgasm disorder did not increase and libido disorder trended to decrease. Amitriptyline elicits different SD types than NGAs and exerts the opposite effect for sexual response, especially the libido, between men and women. These differences may reflect a preexisting gender-difference of biological and social plasticity of the sexual response cycle (Lopez-Munoz & Alamo, 2011) and specific pharmacological properties of each drug. Accordingly, amitriptyline and NGAs elicit a gender- and type-difference of SD and that should be avoided in vulnerable individuals before treatment basing on the current findings.
The risk of amitriptyline-associated SD is rarely investigated in non-depressive subjects. The current findings first identify that amitriptyline elicits a significantly lower SD frequency in non-depressive than depressive patients. This result does not entirely relate to a lower daily dose of amitriptyline or the duration of the treatment period. In fact, the presence of a psychiatric disorder may increase the frequency of drug-associated sexual adversity (Chen et al., 2017). Clinically, a few other antidepressants and antipsychotics have been reported to elicit a lower SD frequency in non-depressive patients than depressive patients, such as a 22.6% and 3% for patients being treated with fluoxetine and desipramine for lumbago (Atkinson et al., 2007), and 29.8% with paroxetine for tinnitus (Baldo, Doree, Molin, McFerran, & Cecco, 2012). Sexual function did not change under treatment with bupropion or venlafaxine (Nuñez et al., 2013; Reed et al., 2014) and even improved with escitalopram compared with a placebo treatment for menopausal hot flashes in non-depressive women (Reed et al., 2012). Therefore, the sexual adversity of antidepressants, like amitriptyline, is drug and disease codependent, and amitriptyline is safer for non-depressive patients than for those with depression. This issue may relate to the phenotypic change of neurotransmitters and neuropeptides which becomes susceptible to SD under treatment with antidepressants or antipsychotics in depressive patients but not in non-depressive subjects. The amitriptyline-associated SD type is unknown in non-depressive patients as there is only one such case in all index studies.
The SD frequency was reported to decrease after 8 weeks of amitriptyline use in the current study. The time course of SD under amitriptyline use has not been mentioned before. Generally, the occurrence of amitriptyline-related adverse events depends on the dose and previous use of amitriptyline but not ethnicity. The time of onset and change of amitriptyline-associated non-SD adverse events are variable. Some adversities develop early and then decrease in frequency with longer use, such as dry mouth, daytime sleepiness, and headache, whereas others increase in frequency with longer use, such as tremor, blurred vision and dysuria (Bryant, Fisher, & Kluge, 1987). In patients with major depression, the overall amitriptyline-associated non-SD adversity began to decrease after 8 weeks of treatment, especially dry mouth, fatigue and postural hypotension (Mihajlović et al., 2010). Accordingly, long-term users of amitriptyline may suffer SD less frequently than short-term users by development of tolerance.
Dry mouth was the leading nonsexual adverse effect of amitriptyline use in the index studies, followed by somnolence, constipation, dizziness, fatigue, headache, agitation, tremor, blurred vision, nausea, and insomnia (Leucht et al., 2012). These adversities are mainly the result of the anticholinergic, serotoninergic, and adrenergic effect of amitriptyline. Interestingly, SD has been reported to show a linear correlation with insomnia and a dual relationship with somnolence and nausea in index studies. Somnolence results from central antagonistic action on histamine and acetylcholine, whereas nausea results from an excess of gastric serotoninergism. Because the neurobiological basis of SD is too complex to be explained by a single or even several pharmacological effects of amitriptyline, we did not attempt to speculate on the precise mechanism linking SD and insomnia, somnolence or nausea in the present study. However, their correlations suggest them as the potential predictors of SD when insomnia occurs or of a rebound of SD occurs when amitriptyline is tapered or withdrawn because of somnolence or nausea.
Limitations
Although this study provides a new and evidence-based view of the relationship between sexual function and amitriptyline, there are still five limitations. First, sexual function was evaluated using questionnaires, self-reports, or both. Self-report may underestimate the negative impact in woman. Second, the severity of SD and subtype of SD, such as ejaculation disorder, were not clearly subclassified in some index studies. Therefore, the final data indicates a generalized SD. Third, most of the data came from depressive patients, who experience a more complex change in the sexual response cycle than non-depressive patients. Fourth, there were no sufficient data for populations outside North America. Lastly, the results may be criticized as most of the studies have been completed for more than 10 years. Fortunately, the quality of most publications is good for comparison.
Conclusions
A systematic review of the literature concerning amitriptyline and its sexual effects was completed. The results are noteworthy and practical. In contrast to the previous view that amitriptyline carries a high risk of SD, current data clearly reveal that the frequency of amitriptyline-associated SD is factually lower than the rate of SD associated with most NGAs and other tricyclic antidepressants. SD is more common in men with comorbid depression or men undergoing short-term treatment. Amitriptyline is safer for women than for men and for non-depressive than for depressive subjects. SD mainly presents as impotence and ejaculation disorder in men and as libido disorder in women. The most common adverse event is dry mouth. A linear or dual relationship was identified between SD and somnolence, insomnia and nausea, and these effects may predict for the risk of SD with amitriptyline treatment and the need for discontinuation.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: All the authors hereby declare to have no conflicts of interest, including consulting fees, paid expert testimony, employment, grants, honoraria, patents, royalties, stocks, or other financial or material gain, that may involve the subject matter of the this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material is available for this article online.
References
- Atkinson J. H., Slater M. A., Capparelli E. V., Wallace M. S., Zisook S., Abramson I., . . . Garfin S. R. (2007). Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain: A preliminary concentration-controlled trial. Journal of Clinical Psychopharmacology, 27, 135–142. [DOI] [PubMed] [Google Scholar]
- Baldo P., Doree C., Molin P., McFerran D., Cecco S. (2012). Antidepressants for patients with tinnitus. The Cochrane Database of System Reviews, 9, CD003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. D. (1995). A double-blind comparison of Org 3770, amitriptyline, and placebo in major depression. Journal of Clinical Psychiatry, 56, 519–525. [PubMed] [Google Scholar]
- Bryant S. G., Fisher S., Kluge R. M. (1987). Long-term versus short-term amitriptyline side effects as measured by a postmarketing surveillance system. Journal of Clinical Psychopharmacology, 7, 78–82. [PubMed] [Google Scholar]
- Chen L. W., Chen M. Y., Chen K. Y., Lin H. S., Chien C. C., Yin H. L. (2017). Topiramate-associated sexual dysfunction: A systematic review. Epilepsy & Behavior, 73, 10–17. [DOI] [PubMed] [Google Scholar]
- Clayton A., Keller A., McGarvey E. L. (2006). Burden of phase-specific sexual dysfunction with SSRIs. Journal of Affective Disorders, 91, 27–32. [DOI] [PubMed] [Google Scholar]
- Couper-Smartt J. D., Rodham R. (1973). A technique for surveying side-effects of tricyclic drugs with reference to reported sexual effects. The Journal of International Medical Research, 1, 473–476. [DOI] [PubMed] [Google Scholar]
- Graf H., Walter M., Metzger C. D., Abler B. (2014). Antidepressant-related sexual dysfunction - perspectives from neuroimaging. Pharmacology, Biochemistry & Behavior, 121, 138–145. [DOI] [PubMed] [Google Scholar]
- Health and Social Care Information Centre. (2016). Prescriptions dispensed in the community. Retrieved from http://content.digital.nhs.uk/catalogue/PUB20664/pres-disp-com-eng-2005-15-rep.pdf
- Hekimian L. J., Friedhoff A. J., Deever E. (1978). A comparison of the onset of action and therapeutic efficacy of amoxapine and amitriptyline. Journal of Clinical Psychiatry, 39, 633–637. [PubMed] [Google Scholar]
- Kornstein S. G., Schatzberg A. F., Thase M. E., Yonkers K. A., McCullough J. P., Keitner G. I., . . . Keller M. B. (2000). Gender differences in treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry, 157, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Lee T. I., Issac J., Lin S. H., Yeh T. L., Lee I. H., Chen P. S., . . . Yang Y. K. (2013). Gender difference in antidepressant-related sexual dysfunction in Taiwan. General Hospital Psychiatry, 35, 407–411. [DOI] [PubMed] [Google Scholar]
- Leong C., Enns M. W., Sareen J., Alessi-Severini S., Bolton J., Prior H. J., Chateau D. (2016). New antidepressant use in older adults: A Canadian population-based study (1997–2013). Aging & Mental Health, 21, 720–729. [DOI] [PubMed] [Google Scholar]
- Leucht C., Huhn M., Leucht S. (2012). Amitriptyline versus placebo for major depressive disorder. The Cochrane Database of System Reviews, 12, CD009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Munoz F., Alamo C. (2011). Frontiers in Neuroscience: Neurobiology of Depression. Boca Raton, FL: CRC Press. [Google Scholar]
- Lucca J. M., Ramesh M., Ram D., Kurian J., Mathew N. (2016). Psychotropic medication- induced sexual dysfunction and its interference with patient’s daily performance: A cross-sectional study. Egyptian Journal of Psychiatry, 37, 36–40. [Google Scholar]
- Mathur A., Sharma D. K., Choudhary A., Jain M. (2005). Efficacy and safety of citalopram versus amitriptyline in the treatment of major depression. Indian Journal of Psychiatry, 47, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlović G., Djukić-Dejanović S., Jovanović-Mihajlović N., Janković S., Janjić V., Jovanović M., . . .Radmanović B. (2010). Comparison of safety between individualized and empiric dose regimen of amitriptyline in the treatment of major depressive episode. Psychiatria Danubina, 22, 354–357. [PubMed] [Google Scholar]
- Mitchell J., Popkin M. (1983). Antidepressant drug therapy and sexual dysfunction in men: a review. Journal of Clinical Psychopharmacology, 3, 76–79. [PubMed] [Google Scholar]
- Montejo A. L., Llorca G., Izquierdo J. A., Rico-Villademoros F. (2001). Incidence of sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic- Related Sexual Dysfunction. Journal of Clinical Psychiatry, 62(Suppl 3), 10–21. [PubMed] [Google Scholar]
- Montgomery S. A. (1995). Safety of mirtazapine: A review. International Journal of Clinical Psychopharmacoogy, 10(Suppl 4), 37–45. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Derry S., Aldington D., Cole P., Wiffen P. J. (2015). Amitriptyline for neuropathic pain in adults. The Cochrane Database of System Reviews, 7, CD008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nininger J. E. (1978). Inhibition of ejaculation by amitriptyline. American Journal of Psychiatry, 135, 750–751. [DOI] [PubMed] [Google Scholar]
- Nuñez G. R., Pinczowski H., Zanellato R., Tateyama L., Schindler F., Fonseca F., Del Giglio A. (2013). Bupropion for control of hot flashes in breast cancer survivors: A prospective, double-blind, randomized, crossover, pilot phase II trial. Journal of Pain and Symptom Management, 45, 969–979. [DOI] [PubMed] [Google Scholar]
- Pringsheim T., Davenport W., Mackie G., Worthington I., Aubé M., Christie S. N., . . . Becker W. J. (2012). Canadian Headache Society guideline for migraine prophylaxis. Canadian Journal of Neurological Sciences, 39(Suppl 2), S1–S59. [PubMed] [Google Scholar]
- Rao R., Morriss R. K., Michael A. (1998). Amitriptyline-induced anorgasmia reversed by nefazodone. Irish Journal of Psychological Medicine, 15, 76–77. [Google Scholar]
- Reed S. D., Guthrie K. A., Joffe H., Shifren J. L., Seguin R. A., Freeman E. W. (2012). Sexual function in nondepressed women using escitalopram for vasomotor symptoms: A randomized controlled trial. Obstetrics & Gynecology, 119, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. D., Mitchell C. M., Joffe H., Cohen L., Shifren J. L., Newton K. M., . . . Guthrie K. A. (2014). Sexual function in women on estradiol or venlafaxine for hot flushes: A randomized controlled trial. Obstetrics & Gynecology, 124, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenpfader U., Gartlehner G., Morgan L. C., Greenblatt A., Nussbaumer B., Hansen R. A., . . . Gaynes B. N. (2014). Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: Results from a systematic review with network meta-analysis. Drug Safety, 37, 19–31. [DOI] [PubMed] [Google Scholar]
- Reimherr F. W., Chouinard G., Cohn C. K., Cole J. O., Itil T. M., LaPierre Y. D., . . . Mendels J. (1990). Antidepressant efficacy of sertraline: A double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. Journal of Clinical Psychiatry, 51(Suppl B), 18–27. [PubMed] [Google Scholar]
- Serretti A., Chiesa A. (2009). Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. Journal of Clinical Psychopharmacology, 29, 259–266. [DOI] [PubMed] [Google Scholar]
- Simpson G. M., Blair J. H., Amuso D. (1965). Effects of anti-depressants on genito-urinary function. Diseases of the Nervous System, 26, 787–789. [PubMed] [Google Scholar]
- Sohn W., Lee O. Y., Kwon J. G., Park K. S., Lim Y. J., Kim T. H., . . . Kim J. I. (2012). Tianeptine vs amitriptyline for the treatment of irritable bowel syndrome with diarrhea: A multicenter, open-label, non-inferiority, randomized controlled study. Neurogastroenterology & Motility, 24, 860–e398. [DOI] [PubMed] [Google Scholar]
- Stahl S., Zivkov M., Reimitz P. E., Panagides J., Hoff W. (1997). Meta-analysis of randomized, double-blind, placebo-controlled, efficacy and safety studies of mirtazapine versus amitriptyline in major depression. Acta Psychiatrica Scandinavica Supplement, 391, 22–30. [DOI] [PubMed] [Google Scholar]
- Statistica. (2017). Top antidepressant drugs in the United States based on prescriptions dispensed in 2011-2012 (in 1,000s). Retrieved from https://www.statista.com/statistics/242651/top-depression-drugs-in-the-us-based-on-prescriptions-dispensed-2011-2012
- van Ophoven A., Pokupic S., Heinecke A., Hertle L. (2004). A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. Journal of Urology, 172, 533–536. [DOI] [PubMed] [Google Scholar]
- Wong J., Motulsky A., Abrahamowicz M., Eguale T., Buckeridge D. L., Tamblyn R. (2017). Off-label indications for antidepressants in primary care: Descriptive study of prescriptions from an indication based electronic prescribing system. British Medical Journal, 356, j603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. C., Park J. I., Kim G. W., Eun S. J., Lee M. S., Han K. L., Jeong G. W. (2013). Effects of antidepressant treatment on sexual arousal in depressed women: A preliminary FMRI study. Psychiatry Investigation, 9, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkov M., de Jongh G. D. (1995). Org 3770 versus amitriptyline: A 6-week randomized double-blind multicentre trial in hospitalized depressed patients. Human Psychopharmacology, 10, 173–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials