Abstract
Malignancy is fuelled by distinct subsets of stem-like cells which persist under treatment and provoke drug-resistant recurrence. Eradication of these cancer stem cells has therefore become a prime objective for the development and design of novel classes of anti-cancer therapeutics with improved clinical efficacy. Here, we portray potentially clinically-relevant hallmarks of cancer stem cells and focus on their recently appreciated properties of cell variability and plasticity, both of which make them elusive targets for cancer therapies. We reason that this ‘disguise in heterogeneity’ has fundamental implications for clinical management and elaborate on rational strategies to combat this diversity and target a broad range of tumorigenic cells. We propose exploitation of cancer stem cell niche dependence as a promising approach to interfere with various, rather than few, cancer stem cell subsets and suggest cancer-associated fibroblasts as a prime microenvironmental target for tumor stemness-depleting intervention.
Keywords: Cancer stem cell, tumor heterogeneity, tumor microenvironment, stem cell niche, cancer-associated fibroblast, cancer treatment
1. Introduction
The perception on how tumors develop and are propagated in vivo has changed dramatically over the past decade. In particular, the original clonal models of cancer evolution have been abandoned and tumors are now appreciated to be tremendously complex comprising genetic and epigenetic heterogeneity within single site lesions. Moreover, comparative investigations of primary- versus secondary site tumor beds have revealed strong subclonal diversification of clinical metastases that might at least in part be responsible for the failure of many systemic therapies to control or eradicate metastatic disease.
One aspect of intratumoral heterogeneity is reflected by the pyramid-like structure of tumors with functionally-defined cancer stem cells (CSCs) at the apex of the malignant hierarchy. Conserved in most tumor entities, CSCs, or cancer-initiating cells, are endowed with unique functional properties and dictate the whole course of tumor evolution including cancer initiation, metastatic progression, and disease recurrence after clinical remission. Thus, these cells have emerged as a highly attractive target population for anti-cancer treatment, and strategies to eliminate these cells are being heavily explored. However, recent evidence has suggested that aside from dormancy and detoxification, CSC targeting approaches are faced with additional challenges including low immunogenicity of CSCs, cellular heterogeneity of CSC pools, and a general plasticity of stemness phenotypes. In this review, we summarize the latest advances in our understanding of CSC biology and function, and highlight potential implications of tumor cell variability for the conceptual design of CSC-directed therapies. We propose CSC heterogeneity as yet another example for Darwinian selection during tumor progression and suggest that microenvironment-targeted strategies will guide the development of anti-CSC treatments in the future, based on the inherent niche dependence of CSC populations.
2. The Cancer Stem Cell Concept
Organ development –and homeostasis depends on small populations of dedicated stem cells, which maintain tissues by continuous replacement and also secure demand-adapted regeneration in case of emergencies, such as injury [1]. Functionally, stem cells are characterized by their selective ability for self-renewal and differentiation, which allows them to generate all cell lineages within a given tissue [1]. Furthermore, stem cells exhibit a high degree of evolutionary fitness conferred, amongst others, by sophisticated mechanisms of detoxification [2, 3] and residence in protective microenvironments (i.e., stem cell niches) [4, 5].
Starting with the seminal article of Al-Hajj and co-workers in 2003 [6], the principles of stem cell biology have been increasingly used to explain basic biological and clinico-pathological features of cancer, even though the first connection between stem cells and malignancies were already proposed in the mid-20th century [7, 8]. In particular, it is now appreciated that cancer arises from the malignant transformation of a stem/progenitor cell or, alternatively, from a non-stem cell that has regained stemness potential by a dedifferentiation process [9–11]. This paradigm is corroborated by the remarkable convergence of stem cells and CSCs in terms of preferentially activated signalling cascades, as well as their overlapping expression of certain markers. As an example, both stem cells and CSCs show activation of the self-renewal-associated pathways Wnt/β-catenin, Bmi-1, sonic hedgehog Notch and PTEN [12], and both populations express tissue-specific stem cell markers, such as CD34 (blood) [13, 14] and Lgr5 (colon) [15, 16]. Importantly, this concordant molecular profile is reflected in several key aspects of CSC biology including longevity, dormancy/quiescence, niche dependence, and the potential for asymmetric cell division [17–20]. Accordingly, CSCs are selectively required for cancer initiation and subsequent propagation, properties that have led to the designation of CSCs as the ‘beating heart’ of malignant growth [18], and to their declaration as prime therapeutic targets [21]. Methodologically, CSCs can be purified from biological samples using flow cytometry/FACS employing phenotypic markers such as CD44 and CD133, or functional characteristics such as dye extrusion and enzymatic activity [22]. On the functional level, bona fide CSCs show tumor-initiating potential in vivo, are capable of anchorage-independent growth in vitro and are notably resistant to cytotoxic and targeted anti-cancer drugs as well as radiotherapy [18–20]. However, it has to be stressed that the frequency and identity as well as other hallmarks of CSCs vary substantially among tumor entities (Table 1). In addition, methodological factors such as the particular experimental conditions used can impact the detection of CSCs. As an example, tumor engraftment in more severely immune-compromised mice increases the detectable frequency of tumorigenic cells by several orders of magnitude [23], demonstrating the challenges in implementing a universal definition of CSCs.
Table 1. Phenotypic Identity and Estimated Frequency of CSCs in Various Tumor Entities.
Generally, CSCs from hematological malignancies are better characterized which is likely the result of the decade-long research in the field of hematopoiesis as a paradigm for stem cell biology. Hence, the molecular signature of these cells is well-characterized and strategies to re-sensitize them to treatment are already being explored [186, 187]. Conversely, CSCs from solid tumors remain much more elusive, entailing ambiguous phenotypic identities and significant challenges for drug development in most tumor types. Nevertheless, CSCs from both hematological malignancies and solid tumors share many characteristic properties including clonogenicity, tumor-initiating potential, asymmetric cell division, activation of (embryonic) stem cell pathways, detoxification/drug resistance, niche dependence, and their implication in disease recurrence. Note that this table is intended to exemplify the differences and similarities between various CSC populations and also depict their elusive nature and the difficulties in defining them. Accordingly, this table does not claim completeness. ‘Very low’, ‘low’ and ‘int to high’ represent CSC frequencies of <1%, 1-10% and >10%, respectively. CSC frequencies are not directly comparable between studies owing to differences in methodology and/or sampled material. ALDH, aldehyde dehydrogenase; CSC, cancer stem cell; SP, side population.
| Tumor Entity | Marker Signature of CSCs | Frequency | Reference |
|---|---|---|---|
| Blood Cancers | |||
| Acute lymphoid leukemia | CD34+/CD38-/CD19+ | low | [188] |
| Acute myeloid leukemia | CD34+/CD38- | low | [107] |
| Chronic myeloid leukemia | CD34+ | ―― | [14] |
| CD34+/CD38-/CD90+ | ―― | [189] | |
| CD34+/CD38-/CD26+ | very low | [190] | |
| Hodgkin lymphoma | CD27+/ALDH+/(CD19+/CD20+) | very low | [191] |
| Multiple myeloma | CD138-/CD34-/(CD19+/CD20+) | low | [192] |
| Myelodysplastic syndrome | CD34+/CD38-/CD90+ | very low | [193] |
| Solid Tumors | |||
| Bone sarcoma | Stro-1+/CD105+/CD44+ | ―― | [194] |
| CD133+ | low | [195] | |
| SP+ | low | [196] | |
| Breast cancer | CD44+/CD24- | low | [6] |
| Colorectal cancer | CD133+ | low | [133] |
| Lgr5+ | low | [135] | |
| Wnt+ | ―― | [134] | |
| CD44+ | low | [132] | |
| Krt19+/Lgr5- | ―― | [131] | |
| ALDH+ | low | [44] | |
| Glioblastoma | CD133+ | int to high | [197] |
| Hepatocellular carcinoma | CD133+/CD44+ | low | [198] |
| CD90+/CD44+ | low | [199] | |
| EpCAM+ | low | [200] | |
| Lung cancer | CD133+ | int to high | [125] |
| ALDH+ | low | [127] | |
| CD44+ | ―― | [128] | |
| CD117+ | ―― | [129] | |
| CD90+ | very low | [130] | |
| SP+ | low | [126] | |
| Medulloblastoma | CD133+ | int to high | [197] |
| Melanoma | ABCB5+ | int to high | [139] |
| CD271+ | int to high | [201] | |
| CD20+ | low | [202] | |
| Ovarian cancer | CD44+/CD24+ | very low | [118] |
| CD44+/CD24- | ―― | [119] | |
| CD44+/CD117+ | very low | [120] | |
| CD24+ | ―― | [121] | |
| ALDH+/CD133+ | very low | [123] | |
| ALDH+ | int to high | [124] | |
| SP+ | low | [37] | |
| Pancreatic cancer | CD44+/CD24+ | very low | [203] |
| Prostate cancer | CD44+/CD24- | low | [204] |
| SP+ | very low | [205] | |
| Renal cell carcinoma | CD105+ | low | [206] |
Several landmark studies have established that the transition from single site tumor growth to life-threatening metastatic disease is mechanistically enabled by CSCs, which seem to have particular resistance to the rate-limiting steps of the metastasis cascade including anoikis, extravasation, and re-settlement/survival in ‘unnatural’ environments [24–26]. Accordingly, metastatic cancer cells are enriched in stemness-associated gene signatures and also show functional stem cell properties [27]. One missing link between stem cell traits and metastasis could be the recent appreciation that CSCs exhibit a distinct transcriptional program otherwise found during developmental tissue remodelling and commonly referred to as epithelial-to-mesenchymal transition (EMT) [28, 29]. This could at least in part explain the increased migratory potential of these cells, as well as their poor response to treatment. Importantly, the relationship between EMT and CSCs seems to be causal, because if cells are forced to undergo an EMT (e.g., by treatment with TGF-β, knockdown of E-cadherin, or ectopic expression of the EMT transcription factors TWIST or Snail), they simultaneously acquire phenotypic and functional properties of stem cells [30, 31].
2.1. Cancer Stem Cells are Therapy-Resistant and Mediate Disease Recurrence
Clinically, the relevance of CSCs is largely seen in their intrinsic resistance to various cytotoxic and targeted anti-cancer drugs, which secures their persistence during treatment and predisposes the patient to relapse [2]. This is in line with studies showing that states of remission or minimal residual disease (MRD), which often escape clinical detection, are established and sustained specifically by CSCs [32–35]. Indeed, CSCs are selected during in vivo chemotherapy, and recurrent tumors are enriched in CSCs or CSC-related gene signatures [36, 37]. Along similar lines, expression of surrogate CSC markers correlates with reduced survival in different tumor entities and also predicts poor response to therapeutic intervention [38–40].
Several non-overlapping mechanisms of protection contribute to the treatment refractoriness of CSCs. For instance, their inherent tendency to remain quiescent over extended periods of time substantially reduces their sensitivity to anti-proliferative drugs such as classical cytostatics and tyrosine kinase inhibitors (TKIs) [20, 41]. To overcome this limitation, a two-step strategy with cell cycle-triggering, dormancy-breaking agents (e.g., arsenic trioxide, G-CSF, IFN-α) was proposed to activate CSCs for re-sensitization to subsequent treatment [42]. Another possibility is the use of compounds whose mode of action is independent of cell cycle progression and in fact, the feasibility of this strategy has been demonstrated for hematological cancers where an epigenetic-modulating agent (i.e., HDACi) could synergize with classical TKI-based therapy to eradicate dormant leukemic stem cells (LSCs) [43]. These data also argue for a prominent role of epigenetic cues in mediating drug resistance of CSCs.
CSCs have also evolved – or adopted from their cell of origin – several mechanisms of detoxification, which protect them from both xenobiotics and harmful metabolic by-products. For instance, members of the ATP-binding cassette (ABC) family of drug transporters efflux a broad spectrum of cytotoxic or targeted anti-cancer drugs [2] and aldehyde dehydrogenase (ALDH) protects the CSCs from naturally occurring or therapy-induced reactive aldehydes [44, 45]. Moreover, CSCs, particularly those found in brain tumors, utilize O(6)-methylguanine-DNA-methyltransferase (MGMT) to counteract the cytotoxic effects of DNA-alkylating agents such as temozolomide [46], and in fact, MGMT promoter methylation is an established predictive marker for temozolomide treatment response [47]. Altogether, neutralization of CSC detoxification mechanisms is considered an attractive therapeutic concept reinforced by proof-of-concept studies [48, 49]; however, clinical benefit including prevention of disease recurrence remains to be shown in large trials.
Specific compounds may not be neutralized by either mechanism and manage to damage CSCs. Similarly, CSCs are not principally shielded from radiotherapy. However, even in the case of significant DNA damage, CSCs are able to evade cell death due to marked proficiency in DNA repair mediated at least in part by prompt and sustained activation of DNA damage response pathways [50] including the MRE11-RAD51-NBS1 [51] and ATR/Chk1 hubs [52].
Cancer immunotherapy pursues the goal to target malignant cells exploiting the natural specificity and adaptability of the immune system [53], having fuelled high hopes for curative intervention especially after primary cytoreduction using surgical or systemic regimens. Moreover, the discovery that certain chemotherapeutic agents as well as radiotherapy induce a specific type of cell death perceptible by the immune system (‘immunogenic cell death’) [54] suggests that treatment combinations of classical and immunotherapeutic modalities are promising and might even achieve long-term protective immunity. However, it was shown that CSCs evade immune recognition due to low expression of MHC class I and down-modulation of tumor-specific (or -associated) antigens [55, 56]. Furthermore, CSCs can express immune checkpoint molecules thereby preventing effective tumor antigen recognition and/or blocking cytotoxic anti-tumoral T cell responses [56, 57]. Finally, CSC niches often constitute an immunosuppressive environment hence limiting immunological accessibility. Thus, it must be deduced that CSCs are less visible for the immune system and escape immune surveillance, even though a single report [58] found that vaccination with CSC-derived antigens results in substantial anti-tumor immunity superior to that achieved with unselected ‘bulk’ tumor antigens.
Collectively, therapeutic CSC eradication is a highly attractive concept holding the potential to revolutionize cancer treatment through inhibition of metastasis and prevention of disease recurrence [59]. However, several lines of protection are operative in CSCs, which poses significant challenges especially for agents that directly act on the cancer cells. Tumor cell variability, including diversification within CSC subpopulations, further complicates the issue, hence asking for novel treatment concepts to target and eradicate these cells.
3. The Cancer Stem Cell – Heterogeneity Cycle
The traditional view on cancer development bases on a non-hierarchical model in which a malignant ancestor cell clonally expands to finally constitute the whole tumor (disregarding de novo mutations provoked by genomic instability). Generally, hematological malignancies have retained at least some aspects of mono- or oligoclonal evolution and a prime example here is Philadelphia+ chronic myelogenous leukemia which can be efficiently and sustainably (but often not curatively) targeted by agents that inhibit the causal fusion protein BCR-Abl [60, 61]. In contrast, other hematological cancers [62] and, in particular, solid tumors [63] have jettisoned more or less all features of oligoclonal growth, being composed of a plethora of phenotypically and functionally different populations that trigger further subclonal diversification, similarly as occurring in a viral quasi-species [64]. Most importantly, the extent of clonal diversity may even be an essential determinant in the pathogenesis of some tumors [65].
The description of tumor heterogeneity in the current detail only became possible owing to the compelling advances made in high-throughput and -content methods in recent years [66–70]. These techniques have allowed for in-depth characterization of the huge genetic, epigenetic and phenotypic variability of malignancies, thereby deepening our understanding of the basic principles of tumor evolution as well as the underlying mechanisms that drive metastasis, drug resistance and recurrence. Several landmark studies have applied multi-region sequencing to delineate spatial diversity within the tumor genome [71–73]. Using phylogenetic reconstruction, these studies found evidence for branched evolution among different regions of the tumor (including metastases), with only a limited number of overlapping mutations being detectable in all regions sampled. Importantly, this mutational variability was also reflected in differential DNA contents of the respective lesions (‘ploidy heterogeneity’) and furthermore involved functional aspects, depending on the type and location of the particular mutation. As an example, spatial heterogeneity of renal carcinoma for a missense mutation in an auto-inhibitory domain of the mTOR kinase was shown to entail constitutive activity of the enzyme in vitro as well as increased phosphorylation of downstream targets in vivo [72]. Thus, single biopsies are often not representative of the whole tumor mass, suggesting that clinical decisions are frequently based on incomplete information. Temporal genetic heterogeneity is also a characteristic hallmark of cancer that depicts the longitudinal evolution of tumor cells along the Darwinian selection line [71, 74]. However, in contrast to spatial heterogeneity where different cancer clones eventually co-exist, temporal heterogeneity can at least in part be explained by out-competition of weaker clones by populations that have increased proliferative capacity and/or a lowered apoptotic threshold. Moreover, provided that representative biopsies are repeatedly taken, temporal genetic heterogeneity can principally inform rational treatment decisions based on a precision medicine approach [75, 76], and it is expected that technical advances in minimally-invasive diagnostic procedures such as liquid biopsies of circulating tumor cells and/or circulating cell-free tumor DNA [77] will provide data for more precise decisions. Liquid biopsies are also promising because tumor-derived circulating material might comprise the fingerprints of several anatomical sites, hence such samples hold the potential to be representative of targetable traits shared by the whole tumor mass. Although not finally proven, it is likely that the principles of spatio-temporal tumor heterogeneity also apply to the epigenome [78]. However, whether genetic and epigenetic tumor heterogeneity are jointly regulated based on co-dependencies [79] or whether they exhibit distinct patterns and dynamics during tumor evolution [80] remains to be determined.
3.1. Crossroads of Metastasis, Cancer Stem Cells and Heterogeneity
A prime focus in oncology has been to understand and dissect the deadliest aspect of cancer, metastasis, and the mechanisms underlying the poor treatment response of metastatic lesions are now being uncovered. Even though specific microenvironments contribute to chemo-protection of metastatic cells [81], heterogeneity and dedifferentiation are the major reasons why metastases are hard to treat and, in fact, often exhibit therapy-refractoriness [27, 82–84]. In humans, the heterogeneous nature of metastasis was first comprehensively demonstrated by Campbell and co-workers who applied paired-end sequencing of multiple cancer lesions to show a different genetic make-up of secondary sites as well as ongoing, partially convergent, clonal evolution among metastases [82]. Importantly, they found that clonal rearrangements within the primary tumor occur early in development and often propagate into secondary sites, implying that metastasis-initiating cells are heterogeneous from the start. Formally, this concept was corroborated in a recent study making use of a genetically-engineered, tamoxifen-inducible pancreatic cancer model combined with the cell labelling system ‘Confetti’ [85]. Cre expression in PDX1-positive (pancreatic) cells activates oncogenic KRASG12D and deletes one allele of p53, and further leads to stochastic expression of one of four fluorescent proteins in the transformed cells. Using this elegant system, the authors modelled subclonal diversification and demonstrated polyclonality of both primary tumors and secondary sites including peritoneal, lung and diaphragm metastases. Thus, strong evidence suggests that cancer growth involves subclonal evolution processes early on, leading to intratumoral heterogeneity and ongoing diversification particularly in metastatic sites.
It is also well-established that dedifferentiation and (embryonic) stem cell signatures both correlate with poor prognosis in cancer patients [86]. This would argue for a stem cell program operative in metastasis-initiating cells, which would also produce cellular heterogeneity based on asymmetric cell division [87]. Hence, it is not surprising that a recent study, employing a highly sensitive flow cytometric capture assay to purify and analyze single metastatic breast cancer cells from patient-derived xenografts, found enrichment of stem cell-linked signatures in these cells, which furthermore correlated with a lower proliferative- but a superior tumor-initiating capacity [27]. Importantly, the authors also detected stem cell programs in rare cells of the primary tumor (~1%) that overlapped significantly with those of the metastatic cells. Thus, CSCs, or cancer cells that exhibit stemness, are clearly overrepresented in metastatic sites, and one could assume a stem cell origin of cancer metastasis [87]. In turn, this implies that stem cell properties are not primarily adopted in the new microenvironment of a respective secondary site, but that rare leader cells at the invasive front of the primary tumor (the metastasis-initiating cells) already possess these characteristics.
3.2. Dissecting the Heterogeneity of Ovarian Cancer Stem Cells
A hallmark of CSCs is their potential to undergo asymmetric cell division and produce differentiated progeny, resulting in phenotypic and/or functional tumor heterogeneity [9,19, 88–91]. Certainly, this is one reason why CSC-rich metastases are highly heterogeneous and clinically hard to treat. In contrast, the possibility that a given CSC pool is heterogeneous itself, has only been considered recently, potentially because it is more intuitive to think of a rare cell subset as a homogeneous population. Ovarian cancer is the most lethal gynecological tumor entity and is characterized by high rates of recurrence (>60%) as well as acquisition of drug resistance in the relapsed setting [92, 93]. Moreover, the semi-solid metastatic colonization of peritoneal surfaces by hundreds of independent tumor nodules further highlights the stem cell-driven nature of this tumor type [94]. To investigate the degree of cell variability present in ovarian CSC populations, we recently employed multicolour flow cytometry to sub-characterize the side population (SP), an established marker of ovarian [95] and other CSCs [96, 97] molecularly based on functional ABC drug transporter activity [98–100]. Interestingly, we found that the stem-like SP accounting for typically less than 1% of cells was highly heterogeneous, with a 7-marker panel identifying at least 5 but up to 19 subsets in various cell lines (7 markers theoretically define 27 = 128 cell populations, but only populations exceeding the cut-off of 0.1% are referred to here) [89]. As such, the cellular heterogeneity was comparable between the CSC subset and the bulk of non-CSC (non-SP), which was a surprising and novel finding, even though the link between CSCs and heterogeneity was drawn before [90, 101]. Moreover, we found that the CSC subpopulations were stable because at least under in vitro conditions and in the absence of selective pressure they could be resolved repeatedly over a period of 9 weeks [89]. We also believe that the degree of observed heterogeneity in CSC populations heavily depends on the number and quality of the investigated markers, with additional biphasic expressions increasing the number of subsets exponentially. Thus, novel single cell-based technologies, such as single cell DNA/RNA sequencing [69, 70, 102], mass cytometry (CyTOF) [67], next generation fluorescence flow cytometry [103] and the recent ‘imaging mass cytometry’ platform adding spatial resolution within tissue context [68], will dissect the heterogeneity of CSC communities in ever more detail [66], and their combination with single cell functional analyses will reveal corresponding functional correlates [104]. The question remains what will be the translational relevance of these high-content data, i.e., which aspects of them can be utilized to improve cancer therapies? At least from what is known now, the possibility must be considered that, if enough parameters are measured, every single CSC is unique. In addition, even genetically-defined cancer subclones can exhibit functional heterogeneity [105] and likewise, different subclones can functionally converge to cooperate for tumor maintenance [106].
3.3. Three (Non-Mutually-Exclusive) Models for Cancer Stem Cell Heterogeneity
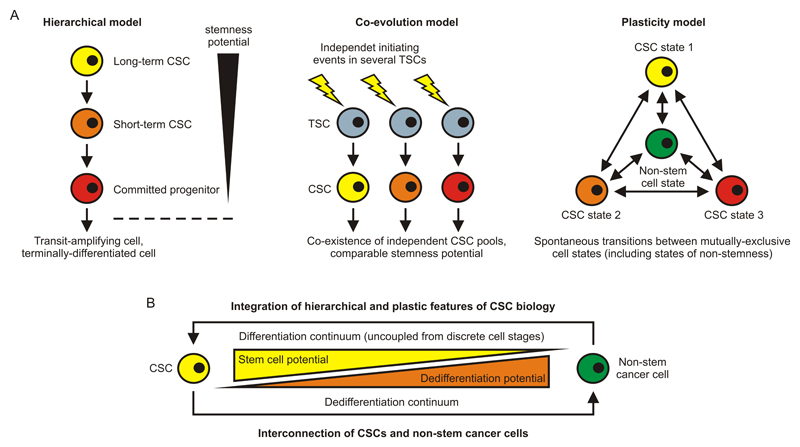
Conceptually, the heterogeneity of CSC populations might be depicted in three different, but complementary, models (Figure 1A). In the hierarchical model that largely reflects the hematopoietic paradigm of stem cell biology, a multi-potent long-term CSC sits at the apex and gives rise to all the more committed and differentiated cell types of the tumor system, including short-term CSCs, committed progenitor cells, transit-amplifying cells, and terminally-differentiated cells [107, 108]. The heterogeneity of the CSC pool would thereby originate from the fact that both short-term CSCs and committed progenitor cells have retained certain characteristics of long-term CSCs that grant them with remaining stemness, while the phenotypic identities differ. In the co-evolution model, different CSC populations have independently, or successively, arisen due to unique initiating events in individual stem cells [20, 109]. This model thus poses that independent CSC pools co-exist in vivo, which implies that the fitness of the individually transformed stem cells is roughly the same and/or that interclonal cooperation prevails over out-competition. However, the rarity of stem cells as well as the rather low frequency of initiator mutations precludes a substantial contribution of co-evolution to CSC heterogeneity in vivo. Finally, the plasticity model predicts that stochastic transitions between cell states [90, 110] can generate dynamic populations of CSCs with mixed phenotype and/or function. Hence, this model complies with the possibilities that (i) CSCs arise de novo from non-CSCs and that (ii) they reversibly adopt states of stemness that are biologically dissimilar. Most likely, the hierarchical and the plasticity model are predominantly governed by epigenetic regulation, whereas genetic initiating events may play the major role in the co-evolution model. However, in either case, microenvironmental cues are operative decisive for CSC behaviour and fate, and all models are subject to Darwinian selection processes occurring within the tumoral ecosystem.
Figure 1. Routes to Cancer Stem Cell Heterogeneity.
(A) In the hierarchical model of CSC biology, a long-term CSC gives rise to a short-term CSC, which in turn produces a committed progenitor cell. The stemness potential is thereby progressively reduced, but it is not completely lost until more downstream levels of the hierarchy are reached, such as the transit-amplifying- and the terminally-differentiated cell stages. This model is largely reflective of established paradigms of stem cell biology and adult tissue organization (e.g., hematopoiesis and colonic crypt homeostasis). In the co-evolution model, different CSC populations are produced simultaneously or successively by unique initiating events in independent TSCs. Alternatively, they emerge as a result of additional genetic or epigenetic hits that occur in pre-existing CSCs (scenario not depicted). Finally, the plasticity model foretells that CSCs, due to spontaneous and stochastic transitions, can switch between distinct cell states including states of stemness and states of non-stemness. This model would thus comply with the possibility that CSCs can arise de novo from non-stem cells and are dynamically regulated in the TME. (B) Model integrating hierarchical and plastic features of CSC biology. Both differentiation and dedifferentiation define a continuum of cell stages, and the potential to differentiate is inversely proportional to the potential to dedifferentiate. Thus, the most primitive (the CSC) and the most mature (terminally-differentiated) cell stages are directly connected. CSC, cancer stem cell; TSC, tissue stem cell.
It is hard to tell which model applies best to the actual situation in a patient. Contribution from all of them is conceivable [90], even though the co-evolution model is less likely to play a dominant role due to probability issues. In contrast, the cell transition model has been fuelled by several recent studies demonstrating considerable plasticity of CSC populations. As an example, Chaffer and colleagues, in two independent studies on breast cancer [111, 112], found that CD44lo non-CSCs can spontaneously convert to a state of stemness (CD44hi), a process that is at least partially dependent on the EMT transcription factor ZEB1 and inducible by TGF-β. Similarly, Roesch and co-workers identified a subpopulation of label-retaining JARID1B-positive melanoma stem cells, which were required for continuous tumor growth indeed, but dynamically regulated and partially replenished from JARID1B-negative cells [113]. Finally, Chen and colleagues established that lung cancer cells can re-acquire stem cell properties through IGF-II-induced Nanog expression and dedifferentiation, challenging as well a strictly hierarchical organization of tumor stemness [114]. Overall, CSC plasticity by reversible cancer cell (de-)differentiation seems to play a major role in tumor maintenance and progression, suggesting that a tumor’s proclivity to generate de novo CSC, rather than its existing CSC content, determines the malignant potential (‘aggressiveness’). Importantly, therapeutic intervention by adoptive cell transfer can also induce reversible cancer cell dedifferentiation, demonstrating the plasticity of the phenotype and fate of cancer cells under treatment [115].
Of note, a plastic model of tumor stemness is in line with the recent concept that CSC characteristics [69] and differentiation [67] define a continuum of cellular states and are uncoupled from discrete developmental stages. Thus, the states exhibiting the most and the least stemness (e.g., the CSC and a terminally-differentiated cancer cell, respectively) are directly connected, or interdependent, suggesting relative ease to cross the continuum and lose or re-acquire stem cell properties (Figure 1B). In turn, such a model could also explain why the CSC phenotype is unstable (or reversible) [116] and underlies tremendous variation across and within tumor types, as well as in individual patients [117]. Treatment modalities that target a broad range of CSC populations are therefore of critical importance to allow an increasing rate of long-term cures. However, it is important to note that despite significant plasticity, not all cells of a tumor will adopt CSC properties at a given time, which is a prerequisite for the concept of targeting CSCs as such. Amongst others, this may be secured by a declining dedifferentiation potential as progenitor characteristics are re-acquired, which makes it extremely unlikely that a significant proportion of cells concurrently present as CSCs (Figure 1B).
4. Therapeutic Implications of Cancer Stem Cell Heterogeneity
The lack of a consensus marker (set) for CSC populations even within defined tumor entities is one consequence of the plasticity and variability of this cell pool. As an example, ovarian CSCs were reported to reside in a variety of cell fractions including CD44+/CD24+ [118], CD44+/CD24- [119], CD44+/CD117+ [120], CD24+ [121], ALDH+/CD133+ [122, 123], ALDH+ [124] and SP+ [89, 95]. Similarly, at least six different marker combinations have been used to define lung [125–130] and colon [44, 131–135] CSCs, respectively, and this ambiguity extends to most tumor entities (Table 1) [136]. Phenotypic instability also makes it possible that tumor-initiating capacity can stem from mutually-exclusive cell subsets [137] and in line with this notion, we observed in our studies that the designated non-CSC fraction (non-SP) still bore residual stem cell activity that supported low-level clonogenicity and engraftment [89]. We deduce from these data that direct targeting of CSCs based on exploitation of ‘specific’ (surface) markers is inefficient and probably foredoomed, even though the general feasibility of this approach has been proven in conceptual studies [138, 139]. It is likely that for similar reasons, other strategies for CSC eradication such as vaccination [58], administration of ‘CSC-selective’ [30] or cell cycle-activating agents [140], and epigenetic modulation [43], will also lack efficiency and/or broad applicability. As an example, we recently demonstrated that the ionophore antibiotics salinomycin and nigericin, both shown to bear CSC-selective toxicity in various tumor models and settings [30, 141–143], did not eliminate SP+ ovarian CSC due to drug transporter-mediated detoxification [48, 144]. Obviously, the differential expression/activity of the protective transporter among the different CSC populations (=CSC heterogeneity) was responsible for this discrepancy. Nonetheless, several CSC-targeting drugs, including inhibitors of the Notch, Wnt and hedgehog pathways, are currently evaluated in clinical trials that cover a broad spectrum of tumor entities [145]. Some of these agents showed promising clinical activity and/or preliminary signs of efficacy (e.g., the γ–secretase inhibitor PF-03084014 and the anti-DLL4 mAb enoticumab/REGN421); however, adverse effects have also been noted [145]. Common side effects of γ–secretase inhibitors included secretory diarrhoea as well as cutaneous rash, both of which will need to be limited. In contrast, enoticumab showed a more favorable safety profile, with nausea, fatigue, headache and hypertension being the most common severe adverse effects. It is also important to note that the definition of suitable endpoints is a particular challenge in clinical trials involving CSC-targeting drugs. This is because the used agent is directed against a minority population of cells whose eradication may not instantly translate into detectable clinical benefit but rather protect in the long run (i.e., prevention of disease recurrence) [145]. Hence, adequate surrogate markers for CSC treatment response are required in such trials, even though final conclusions can only be drawn from long-term follow-up studies. Moreover, many compounds have not been tested in an adjuvant clinical setting where persisting CSCs may most adequately be targeted, which also limits interpretation of the results available so far.
A link between tumor heterogeneity and drug resistance has been proposed since almost 40 years now [146, 147]. In a broader sense, this Darwinian selection process is very much reflective of what happens with bacterial cell cultures exposed to antibiotics: Even though (long-term) selective pressure can produce de novo resistance mutations, most of the resistance is thought to stem from rare pre-existing subclones, especially in case of short-term exposure to very high doses of antibiotics [148, 149]. Molecularly, the spontaneous emergence of drug-resistant subclones can be explained by copying errors occurring during DNA synthesis, which introduces mutations thereby generating new variants. In eukaryotes, elaborate proofreading and repair systems secure a low endogenous mutation rate [150], but cancer cells, including CSCs, are still prone to produce genetically dissimilar daughter cells because they innately exhibit genomic instability. Hence, it does not come as a surprise that longitudinal tracking of (medulloblastoma) cancer clones in mice and men revealed that the dominant clone at recurrence arose from a pre-existing clone that accounted only for a minor subset at the time of diagnosis [151].
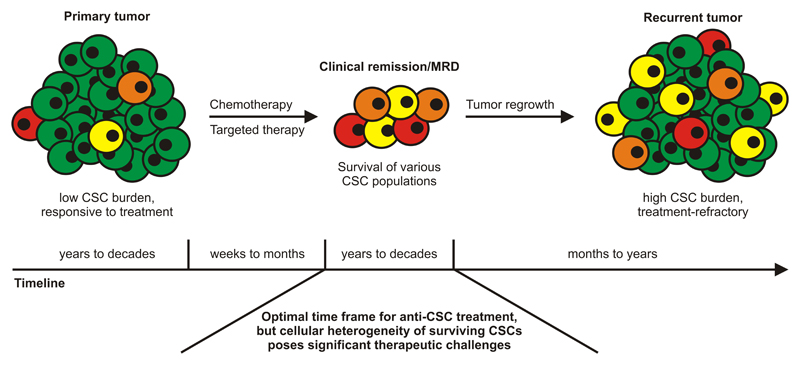
Clinically, the heterogeneity of CSCs promotes treatment failure and relapse by providing an additional source of drug-resistant and/or immune-privileged disease clones [89]. Thus, remission and MRD not only represent states of low tumor but high CSC burden, but also states of significant heterogeneity of the surviving (stem) cell fraction (Figure 2). Ultimately, this heterogeneity fuels the manifestation of clinically-apparent recurrent disease that is then stem cell-enriched and poorly responsive to both cytotoxic and targeted drugs. The question remains how can heterogeneous CSC populations be efficiently targeted prior to relapse? In the stem cell field, there is common sense that it is difficult and not necessarily constructive to focus primary therapies solely on CSC eradication. Clearly, this approach might not be feasible if high tumor loads need to be quickly reduced which is best accomplished using classical debulking regimens such as anti-proliferative chemotherapy, irradiation and surgery. Moreover, targeting CSCs in non-cytoreduced tumors might be a bit like looking for a needle in a haystack, e.g., because of ‘physical’ constraints such as drug accessibility. Based on these considerations, we deduce that the cytoreduced setting, including the different levels of remission and MRD, is extremely well-suited for CSC-directed treatment because the target cells, even though heterogeneous, are maximally exposed. However, some patients are also cured by the currently employed treatment modalities [61, 152] and the fate of the CSCs in these individuals is particularly interesting. Since the tumor at one time was formed (or ‘initiated’), it is unlikely that these patients’ tumors never harboured CSCs. Instead, they might have been eradicated during primary treatment or they might have survived the therapeutic intervention but subsequently never managed to initiate secondary tumors. Indeed, CSCs can be principally eliminated by chemotherapeutic drugs, provided that the dosing is sufficiently high [48]. On the other hand, surviving CSCs in cured patients might be contained by immunological means, or they might lack proper support from their new sparse environment. In either case, they cannot provoke local or distant recurrence and may sooner or later undergo cell death.
Figure 2. Multiple Cancer Stem Cell Subsets Confer Tumor Sustenance during Remission and Provoke Treatment-Refractory Recurrence.
Depicted is the typical clinical course of various malignancies with an overall timeline that covers decades. CSC-poor primary tumors show a favorable response to the initial therapeutic intervention and the patients enter clinical remission or MRD. However, heterogeneous CSC fractions persist which eventually causes treatment-refractory recurrence rich in CSCs and tumor heterogeneity. Conceptually, the cytoreduced setting of remission/MRD is very well-suited to initiate targeted anti-CSC therapy, but the heterogeneity of the surviving cells poses considerable therapeutic challenges. Finding vulnerabilities common to most CSC populations is therefore of critical importance to enable broad eradication of these cells and prevent life-threatening recurrence. CSC, cancer stem cell; MRD, minimal residual disease.
4.1. Niche Dependence of Cancer Stem Cells
The search for broadly applicable and efficient CSC-targeting approaches asks for common vulnerabilities and leads to the very nature of this cell population. In order to be maintained in long-term, CSCs, similarly as physiological tissue stem cells (TSCs), must permanently prevent their differentiation into committed and terminal phenotypes [21, 153]. Thus, therapeutic induction of differentiation should deplete, or exhaust, CSCs and in fact, the feasibility of this approach has been proven in elegant studies. For example, CD105-expressing renal CSCs could be triggered to enter the epithelial differentiation program using treatment with the kidney homeostatic regulator interleukin-15 [154]. The resulting cells were non-tumorigenic and further exhibited sensitivity to chemotherapeutic drugs. Similarly, Ordóñez-Morán and co-workers recently demonstrated that retinoid-induced expression of the Wnt antagonist HOXA5 interferes with the maintenance of colon CSCs by triggering their differentiation [155]. In genetically-determined colon cancer models, this switch of fate was associated with tumor regression and inhibition of metastasis. However, although providing fascinating proof-of-principle, these studies involved direct targeting of CSCs, therefore potentially sparing at least some of the heterogeneous CSC fractions. An alternative approach holding much potential especially for multi-CSC targeting purpose is to deprive them of their microenvironmental niche on which they critically depend [17, 156]. Biologically, this niche represents a meshwork of highly specialized cells that concertedly ensure CSC maintenance by providing various factors that promote stemness while preventing differentiation, such as CXCL7 [157], PGE2 [158], HGF [134], and Jagged-1 [159]. Moreover, this niche might also indirectly protect CSCs based on local immune privilege, strictly regulated cell cycle entrance and progression, and favorable physico-chemical conditions such as limited accessibility for xenotoxins and low oxygen partial pressure. It might therefore not be coincidence that bone-metastatic prostate cancer cells, including CD133+/CD44+ putative prostate CSCs, target the endosteal hematopoietic stem cell niche to establish footholds in the marrow [160]. More importantly, in this study, experimental reduction of the niche size inhibited metastatic bone involvement whereas increasing it fostered the dissemination to this body site. In another elegant study, an activating monoclonal antibody against the adhesion molecule CD44 was demonstrated to eradicate LSC, an effect that was at least in part mediated by interference with the mandatory interaction with stem cell-supportive niches [138]. Finally, it was also shown that disseminating CD90+/CD24+ breast CSCs depend on stromal cell help to be maintained in secondary sites and subsequently be able to engraft metastases [161]. Thus, accumulating evidence suggests that CSC maintenance, and therefore ultimately tumor growth and progression, critically rely on a supportive microenvironment, rendering tumor stromal cells a rational therapeutic target for a new form of cancer treatment. Importantly, because tumors of different origin, genotype and histology share common microenvironmental elements, stroma-directed therapies also promise to be broadly applicable [162]. In addition, mutational adaptation and acquisition of drug resistance should be preventable to great extent, owing to the genetically stable nature of the targeted cells [163].
5. Stromal Cells as Prime Target for Cancer Stem Cell-Directed Therapy
The tumor microenvironment (TME) is a complex cellular meshwork composed of lymphocytes, myeloid cells, vascular and lymphatic endothelial cells, and fibroblasts [163, 164]. In addition, mesothelial cells and adipocytes are sometimes present, for instance when ovarian cancer cells colonize the omentum during peritoneal dissemination [94, 165]. While the importance of lymphocytes and vascular endothelial cells has long been appreciated and is already therapeutically harnessed (e.g., by checkpoint inhibition and anti-angiogenesis), the great impact of fibroblasts on tumorigenesis has only been recently uncovered. This is paradoxical because fibroblasts constitute a major component of the tumor stroma, particularly in breast cancer, lung cancer, melanoma, and hematological malignancies (bone marrow niche). Moreover, because cancer-associated fibroblasts (CAFs) represent non-transformed cell types exhibiting genomic integrity, their targeting holds the potential for long-term responses, based on prevention of quick mutational adaptation [163].
5.1. Targeting Cancer-Associated Fibroblasts to Deplete Tumoral Stemness
Although the origin and phenotype of CAFs might be diverse, they functionally converge to accelerate malignant growth in various ways. For instance, CAFs secrete a battery of growth and survival factors (e.g., IGF, HGF, VEGF) that act on neighbouring tumor- and endothelial cells to foster tumor cell proliferation and angiogenesis, respectively [166, 167]. They also lay into the extracellular space a dedicated matrix rich in collagens, fibronectin and other proteins such as tenascin C, thereby supplying the tumor cells with nutrients and structural support and further providing oncogenic signals [166, 167]. Other prominent CAF-secreted factors include those that prime the tumor cells to undergo an EMT and adopt migratory potential (e.g., TGF-β) [166], and those that exert their effects indirectly through modulation of the anti-cancer immune response (e.g., IL-6, IL-10, IDO) [168]. Apart from that, the tumor-promoting activity of CAFs is thought to arise mainly from their expression of stem cell ligands and –factors, which they provide to the tumor cells in both juxtacrine and paracrine manner.
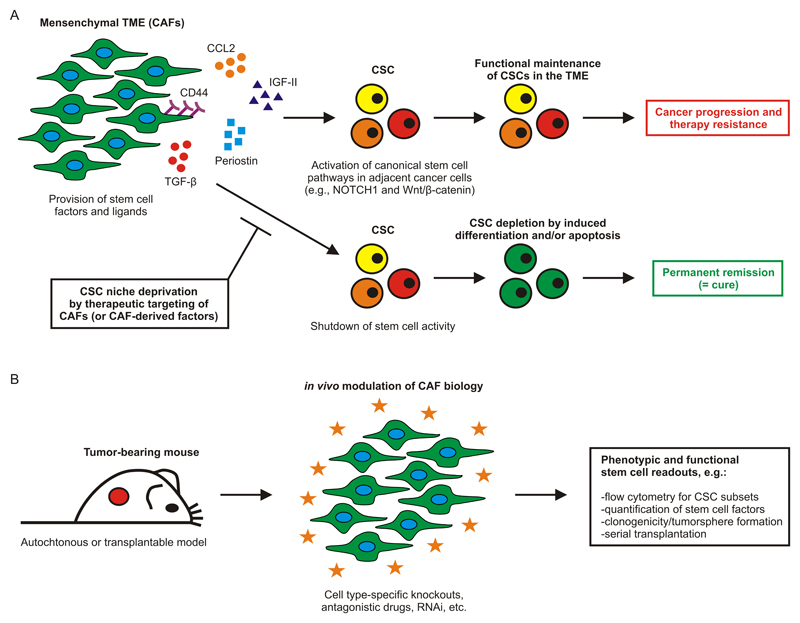
For example, it was shown that CAF-secreted CCL2 induces expression of NOTCH1 in breast cancer cells, thereby stimulating their CSC phenotype and granting them with self-renewal potential [169]. Likewise, CD90+ CAFs were shown to express IGF-II and engage with lung tumor cells in a paracrine network. Ligation of the IGFR1 receptor on the tumor cells caused Nanog induction and led to dedifferentiation and re-acquisition of stem cell properties [114]. CAF-specific expression of the extracellular matrix protein periostin [161] and the cell surface glycoprotein CD44 [170] were also shown to be required for functional sustenance of CSC populations in the TME. In the former case, the effect mechanistically based on periostin-mediated recruitment of Wnt ligands, which subsequently activated Wnt/β-catenin signalling in the adjacent tumor cells. Other CAF-produced factors that induce cancer cell dedifferentiation and/or facilitate CSC maintenance include TGF-β [171], annexin A1 [172] and, possibly, matrix metalloproteinases [173]. Altogether, there is a body of evidence suggesting that CSCs rely on particular signals provided by the fibroblastic tumor stroma, rendering CAFs a highly interesting target for therapeutic interference with cancer stemness (Figure 3A). However, the phenotypic identity of CAFs is still elusive and functional targeting strategies need to be elaborated.
Figure 3. Exploiting Niche Dependence to Broadly Target Cancer Stem Cells.
(A) The mesenchymal TME consisting mainly of CAFs forms a specialized niche allowing CSC maintenance and fuelling tumor progression. This is accomplished by provision of various paracrine and juxtacrine factors that concertedly activate and sustain canonical stem cell pathways in the adjacent CSCs. Hence, targeting this niche represents an attractive therapeutic concept promising to be effective against a broad range of heterogeneous CSC populations. (B) Experimental approach to identify novel stem cell regulators on tumor stromal cells for improved cancer therapies in the future. Murine cancer models allow in-depth characterization of CAFs and further facilitate mechanistic target dissection using cell- and molecular-biological techniques. Established stem cell readouts can reveal whether the mode of action of a particular intervention is indeed based on exhaustion of the tumoral stemness potential, or whether other mechanisms are also involved. CAF, cancer-associated fibroblast; CSC, cancer stem cell; TME, tumor microenvironment.
Several markers are used to define CAFs, including αSMA and FAPα (both intracellular), and podoplanin and PDGFR-β (both on the cell surface) [164, 167, 174]. Using these markers, CAFs can be molecularly characterized both on the single cell level (flow cytometry, CyTOF) and within tissue context (e.g., confocal microscopy, imaging mass cytometry). From what is known now, CAFs generally express a panel of canonical mesenchymal markers (e.g., VCAM1, CD90, CD105, CD29, vimentin, desmin, endosialin, CXCL12) [164, 174], share properties with smooth-muscle/myofibroblastic cells [175], and show a characteristic localization in the peri-tumoral stroma [176–178], even though they can also be found in more central areas of the tumor, albeit at lower frequency. It is also clear that global gene expression profiling approaches, such as gene array and the more recent RNA-seq technology, will be key to identifying novel stem cell regulators on CAFs that might provide a leverage for therapeutic intervention. Ultimately, the identified targets will need to be mechanistically investigated using suitable in vivo models, to separate cause from correlation. This can be accomplished, amongst others, by CAF-specific target gene knockout using Cre/loxP recombination [179] and target-selective interference with antibodies, small compounds or RNAi. In either experimental setup possible readouts include, but or not limited to, flow cytometry for CSC subsets, quantification of stemness-associated genes/transcription factors, and functional assays such as clonogenicity and serial transplantation (both performed on ex vivo purified tumor cells) (Figure 3B) [20, 89]. Finally, time-controlled gene expression using tetracycline- [180] or tamoxifen-regulated [181] systems will be necessary to reveal whether CSCs depend on continuous provision of stem cell factors by CAFs, or whether short-term interruptions of these paracrine and juxtacrine signalling axes are already sufficient to gain therapeutic benefit.
Once promising targets on CAFs have been identified, rational drug design should be implemented. These efforts should focus on therapeutic/neutralizing antibodies (surface and secreted targets) as well as small molecule inhibitors (surface and intracellular targets). Moreover, therapeutic selectivity for CAFs needs to be established so that normal fibroblasts and mesenchymal cells, which form the niche for physiological TSCs, remain largely unaffected. It is proposed that concomitant profiling of healthy mesenchyme (e.g., dermal fibroblasts) can disclose factors that are preferentially expressed by CAFs. Along similar lines, basic research on the pathways that govern tumor-induced activation and reprogramming of fibroblasts into tumor-promoting CAFs [182–184] will pave the way for more selective anti-CAF treatments. Ultimately, the use of CSC interference by CAF modulation will have to be demonstrated in clinical trials involving recurrence-prone tumor entities (e.g., ovarian cancer) and long-term follow-ups (several years), but until here this new paradigm of cancer treatment seems absolutely promising.
6. Concluding Remarks
CSCs represent the apex population of the intratumoral hierarchy whose elimination is believed to permanently eliminate the disease. However, these cells are hard to be therapeutically targeted, because they are inherently resistant to cytotoxic and targeted drugs, and furthermore evade radiotherapy and immune surveillance. On top of that, recent data show that CSC populations underlie significant diversification and plasticity, so that direct targeting approaches with monotherapies are unlikely to succeed (‘disguise in heterogeneity’). We here propose another strategy for CSC targeting that exploits the intrinsic dependence of CSCs on productive interactions with microenvironmental cell types. CAFs express several stem cell factors and are highly abundant in the CSC niche. Blocking the relevant CAF-CSC signalling axes should thus deplete CSCs based on induced differentiation and/or apoptosis, leading to tumor regression.
It is important to consider the possibility of context-dependent CAF function and that specific CAF subpopulations might act to suppress, rather than support, tumor progression [185]. CAF-directed treatments must therefore be cleverly devised and specific for a population with clear and proven tumor-promoting activity. Lastly, the degree of niche dependence might vary among heterogeneous CSC fractions. Thus, on the path towards clinical application, combination therapies of microenvironment-targeted drugs with CSC-selective compounds should be envisaged to further limit potential persistence of CSCs during treatment.
Highlights.
-
-
CSCs are heterogeneous and underlie significant cell plasticity
-
-
CSC variability complicates their therapeutic targeting
-
-
Niche dependence is common to most CSC populations
-
-
Niche-forming CAFs emerge as prime targets for therapeutic CSC interference
Acknowledgment
Maximilian Boesch is supported by an Erwin Schroedinger Fellowship of the Austrian Science Fund (FWF). Dominik Wolf is supported by the Deutsche Forschungsgemeinschaft (DFG), the Austrian Society of Hematology and Oncology (OeGHO), and the Forschungsfonds of the Austrian National Bank (OeNB). The authors would like to apologize for not being able to cite all relevant literature due to space limitations.
Specific Abbreviations Used
- ABC
ATP-binding cassette
- ALDH
aldehyde dehydrogenase
- CAF
cancer-associated fibroblast
- CSC
cancer stem cell
- EMT
epithelial-to-mesenchymal transition
- FACS
Fluorescence-activated cell sorting
- LSC
leukemic stem cell
- mAb
Monoclonal antibody
- MGMT
O(6)-methylguanine-DNA-methyltransferase
- MRD
minimal residual disease
- SP
side population
- TKI
tyrosine kinase inhibitor
- TME
tumor microenvironment
- TSC
tissue stem cell
Author Contributions
Wrote the first draft of the manuscript: Maximilian Boesch, Sieghart Sopper, Dominik Wolf. Designed and assembled the figures: Maximilian Boesch. Wrote the final version of the manuscript: Maximilian Boesch, Sieghart Sopper, Alain G. Zeimet, Daniel Reimer, Guenther Gastl, Burkhard Ludewig, Dominik Wolf. Approved the manuscript for submission and publication: Maximilian Boesch, Sieghart Sopper, Alain G. Zeimet, Daniel Reimer, Guenther Gastl, Burkhard Ludewig, Dominik Wolf.
Disclosure of Potential Conflicts of Interest
There are no conflicts of interest to declare. There is also no non-author involvement in the preparation of the manuscript.
References
- [1].Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- [2].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- [3].Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, Smith C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121:1824–1838. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Furth JE, Kahn MC, Breedis C. The transmission of leukemia of mice with a single cell. American Journal of Cancer. 1937:276–282. [Google Scholar]
- [8].Makino S. Further evidence favoring the concept of the stem cell in ascites tumors of rats. Annals of the New York Academy of Sciences. 1956;63:818–830. doi: 10.1111/j.1749-6632.1956.tb50894.x. [DOI] [PubMed] [Google Scholar]
- [9].Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- [10].Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO reports. 2014;15:244–253. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- [12].Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- [13].Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. The Journal of clinical investigation. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- [15].Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- [16].Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells. 2012;30:2378–2386. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- [17].Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- [18].Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- [19].Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nature reviews. Drug discovery. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- [21].Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature reviews. Drug discovery. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- [22].Greve B, Kelsch R, Spaniol K, Eich HT, Gotte M. Flow cytometry in cancer stem cell analysis and separation. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2012;81:284–293. doi: 10.1002/cyto.a.22022. [DOI] [PubMed] [Google Scholar]
- [23].Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [25].Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107:18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- [27].Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ghiaur G, Gerber J, Jones RJ. Concise review: Cancer stem cells and minimal residual disease. Stem Cells. 2012;30:89–93. doi: 10.1002/stem.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, Mead AJ, Astrand-Grundstrom I, Strombeck B, Horvat A, Ferry H, Dhanda RS, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. The New England journal of medicine. 2010;363:1025–1037. doi: 10.1056/NEJMoa0912228. [DOI] [PubMed] [Google Scholar]
- [35].Vanner RJ, Remke M, Gallo M, Selvadurai HJ, Coutinho F, Lee L, Kushida M, Head R, Morrissy S, Zhu X, Aviv T, et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer cell. 2014;26:33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ayub TH, Keyver-Paik MD, Debald M, Rostamzadeh B, Thiesler T, Schroder L, Barchet W, Abramian A, Kaiser C, Kristiansen G, Kuhn W, et al. Accumulation of ALDH1-positive cells after neoadjuvant chemotherapy predicts treatment resistance and prognosticates poor outcome in ovarian cancer. Oncotarget. 2015;6:16437–16448. doi: 10.18632/oncotarget.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, Hudson DL, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Molecular cancer therapeutics. 2011;10:325–335. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, Canty AJ, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- [39].Liu JC, Voisin V, Bader GD, Deng T, Pusztai L, Symmans WF, Esteva FJ, Egan SE, Zacksenhaus E. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+:ERalpha- breast cancer. Proc Natl Acad Sci U S A. 2012;109:5832–5837. doi: 10.1073/pnas.1201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mimeault M, Batra SK. Molecular biomarkers of cancer stem/progenitor cells associated with progression, metastases, and treatment resistance of aggressive cancers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:234–254. doi: 10.1158/1055-9965.EPI-13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nature reviews. Immunology. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- [43].Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, Snyder DS, Huettner CS, Shultz L, Holyoake T, Bhatia R. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sato A, Sunayama J, Matsuda K, Seino S, Suzuki K, Watanabe E, Tachibana K, Tomiyama A, Kayama T, Kitanaka C. MEK-ERK signaling dictates DNA-repair gene MGMT expression and temozolomide resistance of stem-like glioblastoma cells via the MDM2-p53 axis. Stem Cells. 2011;29:1942–1951. doi: 10.1002/stem.753. [DOI] [PubMed] [Google Scholar]
- [47].Everhard S, Kaloshi G, Criniere E, Benouaich-Amiel A, Lejeune J, Marie Y, Sanson M, Kujas M, Mokhtari K, Hoang-Xuan K, Delattre JY, et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Annals of neurology. 2006;60:740–743. doi: 10.1002/ana.21044. [DOI] [PubMed] [Google Scholar]
- [48].Boesch M, Zeimet AG, Rumpold H, Gastl G, Sopper S, Wolf D. Drug transporter-mediated protection of cancer stem cells from ionophore antibiotics. Stem Cells Translational Medicine. 2015;4 doi: 10.5966/sctm.2015-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Loebinger MR, Giangreco A, Groot KR, Prichard L, Allen K, Simpson C, Bazley L, Navani N, Tibrewal S, Davies D, Janes SM. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br J Cancer. 2008;98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maugeri-Sacca M, Bartucci M, De Maria R. DNA damage repair pathways in cancer stem cells. Molecular cancer therapeutics. 2012;11:1627–1636. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]
- [51].Cheng L, Wu Q, Huang Z, Guryanova OA, Huang Q, Shou W, Rich JN, Bao S. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. The EMBO journal. 2011;30:800–813. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gallmeier E, Hermann PC, Mueller MT, Machado JG, Ziesch A, De Toni EN, Palagyi A, Eisen C, Ellwart JW, Rivera J, Rubio-Viqueira B, et al. Inhibition of ataxia telangiectasia- and Rad3-related function abrogates the in vitro and in vivo tumorigenicity of human colon cancer cells through depletion of the CD133(+) tumor-initiating cell fraction. Stem Cells. 2011;29:418–429. doi: 10.1002/stem.595. [DOI] [PubMed] [Google Scholar]
- [53].Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- [55].Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, Martuza RL, Rabkin SD. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci U S A. 2013;110:12006–12011. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Parmiani G. Melanoma Cancer Stem Cells: Markers and Functions. Cancers. 2016;8 doi: 10.3390/cancers8030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, Prince ME, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, Nicolini FE, Muller-Tidow C, Bhatia R, Brunton VG, Koschmieder S, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. The Lancet. Oncology. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- [62].Mossner M, Jann JC, Wittig J, Nolte F, Fey S, Nowak V, Oblander J, Pressler J, Palme I, Xanthopoulos C, Boch T, et al. Mutational hierarchies in myelodysplastic syndromes dynamically adapt and evolve upon therapy response and failure. Blood. 2016 doi: 10.1182/blood-2015-11-679167. [DOI] [PubMed] [Google Scholar]
- [63].Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, Ji HP, Maley CC. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. 2016;22:105–113. doi: 10.1038/nm.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS pathogens. 2010;6:e1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, Paulson TG, Blount PL, Risques RA, Rabinovitch PS, Reid BJ. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nature genetics. 2006;38:468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- [66].Bendall SC, Nolan GP. From single cells to deep phenotypes in cancer. Nature biotechnology. 2012;30:639–647. doi: 10.1038/nbt.2283. [DOI] [PubMed] [Google Scholar]
- [67].Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schuffler PJ, Grolimund D, Buhmann JM, Brandt S, Varga Z, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nature methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- [69].Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Roth A, McPherson A, Laks E, Biele J, Yap D, Wan A, Smith MA, Nielsen CB, McAlpine JN, Aparicio S, Bouchard-Cote A, et al. Clonal genotype and population structure inference from single-cell tumor sequencing. Nature methods. 2016;13:573–576. doi: 10.1038/nmeth.3867. [DOI] [PubMed] [Google Scholar]
- [71].de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, Jamal-Hanjani M, Shafi S, Murugaesu N, Rowan AJ, Gronroos E, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346:251–256. doi: 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, Gold KA, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, Prandi D, Lorente D, Frenel JS, Pezaro C, Omlin A, et al. Tumor clone dynamics in lethal prostate cancer. Science translational medicine. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hiley C, de Bruin EC, McGranahan N, Swanton C. Deciphering intratumor heterogeneity and temporal acquisition of driver events to refine precision medicine. Genome biology. 2014;15:453. doi: 10.1186/s13059-014-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Swanton C. Cancer evolution: the final frontier of precision medicine? Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25:549–551. doi: 10.1093/annonc/mdu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer discovery. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- [78].Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Molecular cell. 2014;54:716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mazor T, Pankov A, Song JS, Costello JF. Intratumoral Heterogeneity of the Epigenome. Cancer cell. 2016;29:440–451. doi: 10.1016/j.ccell.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li S, Garrett-Bakelman FE, Chung SS, Sanders MA, Hricik T, Rapaport F, Patel J, Dillon R, Vijay P, Brown AL, Perl AE, et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016;22:792–799. doi: 10.1038/nm.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- [82].Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wu JM, Fackler MJ, Halushka MK, Molavi DW, Taylor ME, Teo WW, Griffin C, Fetting J, Davidson NE, De Marzo AM, Hicks JL, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Almendro V, Kim HJ, Cheng YK, Gonen M, Itzkovitz S, Argani P, van Oudenaarden A, Sukumar S, Michor F, Polyak K. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014;74:1338–1348. doi: 10.1158/0008-5472.CAN-13-2357-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maddipati R, Stanger BZ. Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer discovery. 2015;5:1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature genetics. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. The Lancet. Oncology. 2002;3:508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- [88].Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- [89].Boesch M, Zeimet AG, Reimer D, Schmidt S, Gastl G, Parson W, Spoeck F, Hatina J, Wolf D, Sopper S. The side population of ovarian cancer cells defines a heterogeneous compartment exhibiting stem cell characteristics. Oncotarget. 2014;5:7027–7039. doi: 10.18632/oncotarget.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [92].Cannistra SA. Cancer of the ovary. The New England journal of medicine. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- [93].Partridge EE, Barnes MN. Epithelial ovarian cancer: prevention, diagnosis, and treatment. CA: a cancer journal for clinicians. 1999;49:297–320. doi: 10.3322/canjclin.49.5.297. [DOI] [PubMed] [Google Scholar]
- [94].Zeimet AG, Reimer D, Sopper S, Boesch M, Martowicz A, Roessler J, Wiedemair AM, Rumpold H, Untergasser G, Concin N, Hofstetter G, et al. Ovarian cancer stem cells. Neoplasma. 2012;59:747–755. doi: 10.4149/neo_2012_094. [DOI] [PubMed] [Google Scholar]
- [95].Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [97].Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Boesch M, Reimer D, Rumpold H, Zeimet AG, Sopper S, Wolf D. DyeCycle Violet used for side population detection is a substrate of P-glycoprotein. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2012;81:517–522. doi: 10.1002/cyto.a.22038. [DOI] [PubMed] [Google Scholar]
- [99].Boesch M, Wolf D, Sopper S. Optimized Stem Cell Detection Using the DyeCycle-Triggered Side Population Phenotype. Stem cells international. 2016;2016 doi: 10.1155/2016/1652389. 1652389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- [101].Valent P, Bonnet D, Wohrer S, Andreeff M, Copland M, Chomienne C, Eaves C. Heterogeneity of neoplastic stem cells: theoretical, functional, and clinical implications. Cancer Res. 2013;73:1037–1045. doi: 10.1158/0008-5472.CAN-12-3678. [DOI] [PubMed] [Google Scholar]
- [102].Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, Gelmon K, Chia S, Mar C, Wan A, Laks E, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–426. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chattopadhyay P, Perfetto S, Gaylord B, Stall A, Duckett L, Hill J, Nguyen R, Ambroszak D, Balderas R, Roederer M. CYTO 2014. Fort Lauderdale: 2014. Toward 40+ parameter fluorescence flow cytometry; p. 215. [Google Scholar]
- [104].Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, Sanchez Castillo M, Oedekoven CA, Diamanti E, Schulte R, Ponting CP, et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015;16:712–724. doi: 10.1016/j.stem.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Klco JM, Spencer DH, Miller CA, Griffith M, Lamprecht TL, O'Laughlin M, Fronick C, Magrini V, Demeter RT, Fulton RS, Eades WC, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer cell. 2014;25:379–392. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508:113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- [108].Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. The Lancet Oncology. 2012;13:e83–89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- [109].Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- [110].Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- [111].Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Chen YJ, Lin CY, Pan SH, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nature communications. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- [115].Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wolfel T, Holzel M, Tuting T. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- [116].Dosch JS, Ziemke EK, Shettigar A, Rehemtulla A, Sebolt-Leopold JS. Cancer stem cell marker phenotypes are reversible and functionally homogeneous in a preclinical model of pancreatic cancer. Cancer Res. 2015;75:4582–4592. doi: 10.1158/0008-5472.CAN-14-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [118].Meirelles K, Benedict LA, Dombkowski D, Pepin D, Preffer FI, Teixeira J, Tanwar PS, Young RH, MacLaughlin DT, Donahoe PK, Wei X. Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance. Proc Natl Acad Sci U S A. 2012;109:2358–2363. doi: 10.1073/pnas.1120733109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clinical & experimental metastasis. 2012;29:939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- [120].Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- [122].Kryczek I, Liu S, Roh M, Vatan L, Szeliga W, Wei S, Banerjee M, Mao Y, Kotarski J, Wicha MS, Liu R, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. International journal of cancer. 2012;130:29–39. doi: 10.1002/ijc.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Molecular cancer therapeutics. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell death and differentiation. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- [126].Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- [127].Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Molecular cancer research : MCR. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PloS one. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Levina V, Marrangoni A, Wang T, Parikh S, Su Y, Herberman R, Lokshin A, Gorelik E. Elimination of human lung cancer stem cells through targeting of the stem cell factor-c-kit autocrine signaling loop. Cancer Res. 2010;70:338–346. doi: 10.1158/0008-5472.CAN-09-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yan X, Luo H, Zhou X, Zhu B, Wang Y, Bian X. Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncology reports. 2013;30:2733–2740. doi: 10.3892/or.2013.2784. [DOI] [PubMed] [Google Scholar]
- [131].Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, Guha C, et al. Krt19(+)/Lgr5(-) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015;16:627–638. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- [133].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- [134].Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- [135].Hirsch D, Barker N, McNeil N, Hu Y, Camps J, McKinnon K, Clevers H, Ried T, Gaiser T. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis. 2014;35:849–858. doi: 10.1093/carcin/bgt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Medema JP. Cancer stem cells: the challenges ahead. Nature cell biology. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- [137].Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- [139].Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, Najima Y, Takagi S, Aoki Y, Wake A, Taniguchi S, Shultz LD, Ishikawa F. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nature biotechnology. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Chen T, Yi L, Li F, Hu R, Hu S, Yin Y, Lan C, Li Z, Fu C, Cao L, Chen Z, et al. Salinomycin inhibits the tumor growth of glioma stem cells by selectively suppressing glioma-initiating cells. Molecular medicine reports. 2015;11:2407–2412. doi: 10.3892/mmr.2014.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Dong TT, Zhou HM, Wang LL, Feng B, Lv B, Zheng MH. Salinomycin selectively targets 'CD133+' cell subpopulations and decreases malignant traits in colorectal cancer lines. Annals of surgical oncology. 2011;18:1797–1804. doi: 10.1245/s10434-011-1561-2. [DOI] [PubMed] [Google Scholar]
- [143].Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, Wang J. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer letters. 2011;311:113–121. doi: 10.1016/j.canlet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- [144].Boesch M, Sopper S, Wolf D. Ionophore antibiotics as cancer stem cell-selective drugs: open questions. The Oncologist. 2016;21 doi: 10.1634/theoncologist.2016-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews. Clinical oncology. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dexter DL, Leith JT. Tumor heterogeneity and drug resistance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1986;4:244–257. doi: 10.1200/JCO.1986.4.2.244. [DOI] [PubMed] [Google Scholar]
- [147].Heppner GH, Dexter DL, DeNucci T, Miller FR, Calabresi P. Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Res. 1978;38:3758–3763. [PubMed] [Google Scholar]
- [148].Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. The Lancet. Oncology. 2011;12:1169–1174. doi: 10.1016/S1470-2045(11)70123-1. [DOI] [PubMed] [Google Scholar]
- [149].Klein M, Kimmelman LJ. The Role of Spontaneous Variants in the Acquisition of Streptomycin Resistance by the Shigellae. Journal of bacteriology. 1946;52:471–479. doi: 10.1128/jb.52.4.471-479.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].McCulloch SD, Kunkel TA. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell research. 2008;18:148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, Remke M, Cavalli FM, Ramaswamy V, Lindsay PE, Jelveh S, et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016;529:351–357. doi: 10.1038/nature16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, Ishida Y, Kumagai T, Sato S, Ohashi K, Sakamaki H, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. The Lancet. Haematology. 2015;2:e528–535. doi: 10.1016/S2352-3026(15)00196-9. [DOI] [PubMed] [Google Scholar]
- [153].Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2006;17:1620–1624. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- [154].Azzi S, Bruno S, Giron-Michel J, Clay D, Devocelle A, Croce M, Ferrini S, Chouaib S, Vazquez A, Charpentier B, Camussi G, et al. Differentiation therapy: targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst. 2011;103:1884–1898. doi: 10.1093/jnci/djr451. [DOI] [PubMed] [Google Scholar]
- [155].Ordonez-Moran P, Dafflon C, Imajo M, Nishida E, Huelsken J. HOXA5 Counteracts Stem Cell Traits by Inhibiting Wnt Signaling in Colorectal Cancer. Cancer cell. 2015;28:815–829. doi: 10.1016/j.ccell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [156].Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer discovery. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of clinical investigation. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- [162].Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. Journal of cell science. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- [163].Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nature reviews. Immunology. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- [165].Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- [167].Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Seminars in cancer biology. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- [169].Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, Chen S, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kinugasa Y, Matsui T, Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells. 2014;32:145–156. doi: 10.1002/stem.1556. [DOI] [PubMed] [Google Scholar]
- [171].Hasegawa T, Yashiro M, Nishii T, Matsuoka J, Fuyuhiro Y, Morisaki T, Fukuoka T, Shimizu K, Shimizu T, Miwa A, Hirakawa K. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. International journal of cancer. 2014;134:1785–1795. doi: 10.1002/ijc.28520. [DOI] [PubMed] [Google Scholar]
- [172].Geary LA, Nash KA, Adisetiyo H, Liang M, Liao CP, Jeong JH, Zandi E, Roy-Burman P. CAF-secreted annexin A1 induces prostate cancer cells to gain stem cell-like features. Molecular cancer research : MCR. 2014;12:607–621. doi: 10.1158/1541-7786.MCR-13-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- [174].Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- [175].Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- [176].Horn LC, Schreiter C, Canzler A, Leonhardt K, Einenkel J, Hentschel B. CD34(low) and SMA(high) represent stromal signature in uterine cervical cancer and are markers for peritumoral stromal remodeling. Annals of diagnostic pathology. 2013;17:531–535. doi: 10.1016/j.anndiagpath.2013.05.009. [DOI] [PubMed] [Google Scholar]
- [177].Ryner L, Guan Y, Firestein R, Xiao Y, Choi Y, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, Fu L, et al. Upregulation of Periostin and Reactive Stroma Is Associated with Primary Chemoresistance and Predicts Clinical Outcomes in Epithelial Ovarian Cancer. Clin Cancer Res. 2015;21:2941–2951. doi: 10.1158/1078-0432.CCR-14-3111. [DOI] [PubMed] [Google Scholar]
- [178].Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, Bell RH., Jr Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–134. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- [179].Abremski K, Hoess R, Sternberg N. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell. 1983;32:1301–1311. doi: 10.1016/0092-8674(83)90311-2. [DOI] [PubMed] [Google Scholar]
- [180].Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, Philippe C, Herraiz Serrano C, Benamar S, Croce O, Sanz-Moreno V, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nature communications. 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- [184].Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer discovery. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, Van Etten RA, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. The Journal of clinical investigation. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Scott MT, Korfi K, Saffrey P, Hopcroft LE, Kinstrie R, Pellicano F, Guenther C, Gallipoli P, Cruz M, Dunn K, Jorgensen HG, et al. Epigenetic Reprogramming Sensitizes CML Stem Cells to Combined EZH2 and Tyrosine Kinase Inhibition. Cancer discovery. 2016 doi: 10.1158/2159-8290.CD-16-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K, Strombeck B, Garwicz S, Bekassy AN, Schmiegelow K, Lausen B, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- [189].Janssen JJ, Deenik W, Smolders KG, van Kuijk BJ, Pouwels W, Kelder A, Cornelissen JJ, Schuurhuis GJ, Ossenkoppele GJ. Residual normal stem cells can be detected in newly diagnosed chronic myeloid leukemia patients by a new flow cytometric approach and predict for optimal response to imatinib. Leukemia. 2012;26:977–984. doi: 10.1038/leu.2011.347. [DOI] [PubMed] [Google Scholar]
- [190].Herrmann H, Sadovnik I, Cerny-Reiterer S, Rulicke T, Stefanzl G, Willmann M, Hoermann G, Bilban M, Blatt K, Herndlhofer S, Mayerhofer M, et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951–3962. doi: 10.1182/blood-2013-10-536078. [DOI] [PubMed] [Google Scholar]
- [191].Jones RJ, Gocke CD, Kasamon YL, Miller CB, Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M, Lemas MV, et al. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Woll PS, Kjallquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S, Erlandsson R, Ngara M, Anderson K, Deng Q, Mead AJ, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer cell. 2014;25:794–808. doi: 10.1016/j.ccr.2014.03.036. [DOI] [PubMed] [Google Scholar]
- [194].Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- [196].Murase M, Kano M, Tsukahara T, Takahashi A, Torigoe T, Kawaguchi S, Kimura S, Wada T, Uchihashi Y, Kondo T, Yamashita T, et al. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Br J Cancer. 2009;101:1425–1432. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [198].Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. International journal of cancer. 2010;126:2067–2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- [199].Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- [200].Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- [203].Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- [204].Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Brown MD, Gilmore PE, Hart CA, Samuel JD, Ramani VA, George NJ, Clarke NW. Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. The Prostate. 2007;67:1384–1396. doi: 10.1002/pros.20620. [DOI] [PubMed] [Google Scholar]
- [206].Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]