Abstract
Within-canopy variation in leaf structural and photosynthetic characteristics is a major means by which whole canopy photosynthesis is maximized at given total canopy nitrogen. As key acclimatory modifications, leaf nitrogen content (NA) and photosynthetic capacity (AA) per unit area increase with increasing light availability in the canopy and these increases are associated with increases in leaf dry mass per unit area (MA) and/or nitrogen content per dry mass and/or allocation. However, leaf functional characteristics change with increasing leaf age during leaf development and aging, but the importance of these alterations for within-canopy trait gradients is unknown. I conducted a meta-analysis based on 71 canopies that were sampled at different time periods or, in evergreens, included measurements for different-aged leaves to understand how within-canopy variations in leaf traits (trait plasticity) depend on leaf age. The analysis demonstrated that in evergreen woody species, MA and NA plasticity decreased with increasing leaf age, but the change in AA plasticity was less suggesting a certain re-acclimation of AA to altered light. In deciduous woody species, MA and NA gradients in flush-type species increased during leaf development and were almost invariable through the rest of the season, while in continuously leaf-forming species, trait gradients increased constantly with increasing leaf age. In forbs, NA plasticity increased, while in grasses, NA plasticity decreased with increasing leaf age, reflecting life form differences in age-dependent changes in light availability and in nitrogen resorption for growth of generative organs. Although more work is needed to improve the coverage of age-dependent plasticity changes in some plant life forms, I argue that the age-dependent variation in trait plasticity uncovered in this study is large enough to warrant incorporation in simulations of canopy photosynthesis through the growing period.
Keywords: dry mass per unit area, light availability, nitrogen content, ontogeny, photosynthetic capacity, plasticity
Introduction
Plant canopies, either simple monotypic or more complex multi-species canopies, are characterized by major reductions in light availability from canopy top to bottom (Goudriaan 2016; Hikosaka 2005, 2014; Kitajima et al. 2005; Niinemets and Anten 2009). The strong within-canopy light gradients are further associated with conspicuous within-canopy variations in foliage structural, chemical and physiological traits. In particular, foliage nitrogen content per area (NA) and foliage photosynthetic capacity per area (AA) increase with increasing long-term light availability in the canopy in all plant functional types (Niinemets et al. 2015 for a review). Such a positive scaling of AA with long-term light availability importantly enhances whole canopy (whole plant) photosynthesis compared with a canopy consisting of leaves with invariable AA (Anten 2016; Anten and Bastiaans 2016; Field 1983; Gutschick and Wiegel 1988; Hikosaka et al. 2016; Niinemets 2016).
While the positive scaling of NA and AA with increasing light availability in the canopy is a uniform response, plant life forms differ in the extent to which variations in NA, the product of leaf dry mass per unit area (MA) and nitrogen content per dry mass (NM), and in AA, the product of MA and photosynthetic capacity per dry mass (AM) are driven by light-dependent changes in morphological (MA), and chemical and physiological (NM and AM) traits. To gain insight into the controls of NA and AA, its is important to consider that species strongly differ in the rates of leaf turnover and canopy expansion. In fast-growing continuously expanding canopies, younger leaves at the top of the canopy shade the older leaves. This is an inherent feature of herbaceous canopies (Hikosaka 2005; Kikuzawa 2003; Oikawa et al. 2006), but several woody species in aseasonal and seasonal climates can also form leaves continuously through the growing season either due to indeterminate growth of first flush shoots, multiple flushing and sylleptic shoot formation (Cline and Harrington 2007; Scarascia-Mugnozza et al. 1999). Given the continuous extension of existing shoots and formation of sylleptic shoots preferably in higher light (Pieters et al. 1999; Powell and Vescio 1986; Weih 2009; Wu and Hinckley 2001), lower canopy leaves are older than upper canopy leaves in such woody canopies (Dickmann et al. 1990; Kikuzawa 2003; Vapaavuori et al. 1989; Vapaavuori and Vuorinen 1989). In contrast, in flush-type deciduous woody species all foliage, and in flush-type evergreen species all current year foliage is formed simultaneously.
Major changes occur in leaf structure, chemistry and physiological activity during foliage development and aging (Miyazawa et al. 1998; Miyazawa and Terashima 2001; Niinemets et al. 2012), while light availability, in turn, can modify the rate of leaf development and senescence (Frantz et al. 2000; Ono et al. 2001; Vos and van der Putten 2001; Yasumura et al. 2007). Thus, presence of or lack of interactions of leaf age and light availability within the canopy can drive differences in the mechanisms of within-canopy photosynthetic acclimation. In continuously expanding canopies, most foliage has developed in a similar light environment, and the within-canopy gradients in NA and AA primarily reflect gradients in leaf age and re-acclimation to shade or leaf senescence in shade (Niinemets et al. 2015; Pons 2016). Thus, leaves at different positions in the canopy are structurally similar with only moderate within-canopy variations in MA if at all, while there are major changes in NM and AM, reflecting nitrogen reallocation from older leaves to younger leaves to the expanding lateral and axial canopy positions (Anten et al. 1998; Hikosaka 1996; Niinemets et al. 2015; Pons 2016; Pons and Pearcy 1994; Vapaavuori et al. 1989; Vapaavuori and Vuorinen 1989; Weih 2009). In contrast, in flush-type species, variation in MA mainly drives changes in NA and AA, and NM and AM change little or are invariable within the canopy, indicating that the within-canopy variation in NA and AA in these species reflects foliage adaptation to growth light environment (Niinemets et al. 2015).
Despite the basic mechanisms of within-canopy adaptation have been highlighted, past studies have mainly investigated within-canopy variations in leaf traits at some fixed moment of time with a few exceptions (Bertheloot et al. 2008; DeJong and Doyle 1985; Drouet and Bonhomme 1999; Niinemets et al. 2004a). Yet, leaf age structure of canopies varies through the growing period. In flush type species, leaf age continuously increases from budburst until leaf senescence. Despite continuous leaf turnover in fast-growing canopies, the average leaf age also increases with increasing the canopy age and time from the start of the growing season. In herbaceous canopies, the formation of new leaves stops after the onset of generative phase of development (Benbi 1994; Evers et al. 2010; Sadras et al. 2000). Analogously, in fast-growing woody canopies in seasonal climates, shoot growth typically slows down before the end of the growing season. This is relevant as leaves at different positions in the canopy can be exposed to vastly different light availabilities through their life-span due to seasonal changes in light availability, and shading that can occur due to early canopy expansion in flush-type deciduous and evergreen species, growth of new leaf flushes in evergreen flush-type species and continued canopy expansion in fast-growing herbaceous and woody species.
The key question is what is the capacity of foliage of different age to acclimate to prevailing light conditions during their development and what is the capacity of fully-developed and aging leaves to re-acclimate to changed light environments. There is some evidence that within-canopy gradients in leaf traits in young developing and weakly differentiated leaves are less steep than the trait gradients in mature canopies (Kull et al. 1998; Niinemets et al. 2004a; Noormets et al. 1996). On the other hand, once the leaves are anatomically fully-differentiated, anatomical constraints such as limited cell wall exposed surface area for spacing of additional chloroplasts can strongly constrain photosynthetic re-acclimation to modified light environment (Oguchi et al. 2005; Oguchi et al. 2006; Yamashita et al. 2002). In addition, initiation of leaf senescence leads to coordinated dismantling of foliage enzymatic apparatus, and retranslocation of foliage nitrogen, ultimately limiting the capacity for acclimation to changed light conditions (Hikosaka et al. 1993; Pons and Jordi 1998). Furthermore, leaf senescence can be triggered at different times in different canopy positions in dependence on species ecological strategy and rate of leaf turnover (Koike 1990; Koike et al. 1992). This evidence collectively suggests that leaf trait vs. light relationships vary through the canopy development and with leaf aging from budburst until senescence, with stronger effects expected for more dynamic fast-growing canopies.
Here a meta-analysis of within-canopy long-term light vs. leaf trait relationships across canopies of different age was carried out to gain insight into the dynamics of within-canopy trait variations in different plant life forms. The key aims of this analysis were: (1) to study how stable are leaf trait vs. light relationships in canopies with different leaf age, (2) to characterize how leaf age effects on within-canopy variation in leaf traits differ among plant life forms with different rate of leaf turnover, and (3) to understand what developmental, structural and physiological factors control the age-dependent variations in leaf trait vs. light relationships in different plant life forms. It was hypothesized that the light-dependent plasticity (trait variation for a given variation in light availability within the canopy) of leaf traits first increases with increasing leaf age due to foliage morphological and physiological differentiation and thereafter decreases due to structural constraints on re-acclimation and induction of senescence, and that these changes are more profound in species with greater leaf turnover. Overall, this analysis highlights significant changes in within-canopy leaf trait responses in dependence on leaf age and plant life form and emphasizes that the dynamics of canopy development needs consideration in simulating whole canopy carbon gain. Definition of all symbols used is provided in Table 1.
Tabel 1.
List of acronyms used with definitions and corresponding units
| Symbol | Definition | Unit |
|---|---|---|
| AA | leaf light-saturated net assimilation rate (photosynthetic capacity) per area | μmol m-2 s-1 |
| AM | leaf light-saturated net assimilation rate per dry mass | μmol g-1 s-1 |
| MA | leaf dry mass per unit area | g m-2 |
| NA | leaf nitrogen content per area | g m-2 |
| NM | leaf nitrogen content per dry mass | % |
| nL | number of leaves in the canopy (Eq. 3) | - |
| nS | leaf number per shoot | - |
| PL | relative light-dependent plasticity (Eq. 1) | m2 d mol-1 |
| PL,l | PL for the Qint range l (Eq. 1), e.g. PL,6-12 refers to the Qint range 6-12 mol m-2 d-1 | m2 d mol-1 |
| PL,l,j | PL,l for the leaf age class j | m2 d mol-1 |
| Qint | daily integrated quantum flux density averaged for specified number of days. In this study, Qint denotes Qint,b50 except when noted | mol m-2 d-1 |
| Qint,b30 | Qint averaged for 30 days since the start of leaf development or for the actual number of days since the onset of leaf development for leaves younger than 30 days | mol m-2 d-1 |
| Qint,b50 | as Qint,b30, but averaged for 50 days since the start of leaf development | mol m-2 d-1 |
| Qint,b60 | as Qint,b30, but averaged for 60 days since the start of leaf development | mol m-2 d-1 |
| Qint,min | minimum Qint value observed for the given canopy | mol m-2 d-1 |
| Qint,p50 | Qint averaged for 50 days preceding leaf sampling or actual number of days when leaves were younger than 50 days | mol m-2 d-1 |
| Qint,Rb50 | Qint,b50 value corresponding to the year of formation of the most recent leaf flush estimated for older leaf classes in evergreens | mol m-2 d-1 |
| Sj | standardize relative trait plasticity for the leaf age class j (Eq. 2) | - |
| Sj,l | Sj for the Qint range l, e.g. Sj,6-12 refers to the Qint range 6-12 mol m-2 d-1 | - |
| S̄j | average Sj for all light ranges available | - |
| ᾱ | average leaf age in the canopy | yr |
| ᾱF | ᾱ at the time of completion of leaf formation in the canopy | yr |
| αC | canopy age | yr |
| ϕ | phyllochron, time elapsed between the formation of subsequent leaves on the main stem | yr |
| λ | leaf longevity | yr |
| λ̄ | species-specific average leaf longevity | yr |
Material and Methods
Dataset
The study consisted of a subset of the global database of within-canopy variation in leaf traits (Niinemets et al. 2015). In particular, the data analyzed here come from studies where within-canopy gradients have been investigated for the same canopies repeatedly at different time points, or in the case of evergreens, from studies where multiple leaf age classes had been studied at the same time. Altogether 71 canopies that provided data for 238 within-canopy gradients were included in the analysis (Table 2). These 71 individual canopies corresponded to canopies of different species, different environmental and spacing treatments for given species (fertilization, ozone, drought and sowing density treatments), and replicates in time (studies conducted with herbaceous or deciduous species in different years; Table 2). In the case of multi-species canopies, each species was considered individually (Table 2). For studies with treatments, the treatment effect per se was not analyzed here, but given that treatments typically resulted in significant differences in overall light penetration and light availability at different height levels in the canopy, each treatment is considered as an individual canopy replicate (Table 2).
Table 2.
Summary of the database of studies considering variation in within-canopy leaf plasticity as dependent on leaf age: list of species, number of ages studied, age range considered and availability of traits
| Species (replicate/treatment/site)1 | Life form2 | Number of ages | Age range (yr) | Traits available3 | References |
|---|---|---|---|---|---|
| Abies amabilis | t,en | 4 | 0.25 - 7.76 | NA, AA | (Brooks et al. 1996) |
| A. veitchii | t,en | 2 | 0.29 - 1.29 | MA | (Tadaki 1970; Tadaki et al. 1970) |
| Acer campestre | t,wdb | 2 | 0.24 - 0.41 | MA | (Eliásh 1979a; Eliásh 1979b; Huzulák and Eliásh 1975) |
| A. saccharum (control) | t,wdb | 3 | 0.23 - 0.40 | AA | (Tjoelker et al. 1995) |
| A. saccharum (ozone) | t,wdb | 3 | 0.23 - 0.40 | AA | (Tjoelker et al. 1995) |
| Avena fatua | g,a | 2 | 0.05 - 0.07 | AA | (Barnes et al. 1990; Beyschlag et al. 1990) |
| Betula platyphylla (1) | t,wdb | 4 | 0.16 - 0.40 | MA | (Araki 1972) |
| B. platyphylla (2) | t,wdb | 5 | 0.10 - 0.52 | MA | (Araki 1973) |
| B. platyphylla (3) | t,wdb | 5 | 0.10 - 0.52 | MA | (Araki 1973) |
| Carex acutiformis | g,p | 3 | 0.10 - 0.31 | MA, NA, NM | (Hirose et al. 1989) |
| Carpinus betulus | t,wdb | 2 | 0.26 - 0.43 | MA | (Eliásh 1979a; Eliásh 1979b; Huzulák and Eliásh 1975) |
| Cynara cardunculus cv. Biango avorio | f,p | 3 | 0.09 - 0.14 | MA, NA, NM | (Archontoulis et al. 2011) |
| Fagus crenata | t,wdb | 2 | 0.12 - 0.30 | MA, NA, NM | (Yamasaki 2003; Yamasaki and Kikuzawa 2003) |
| F. sylvatica (1) | t,wdb | 3 | 0.14 - 0.30 | AA | (Aranda et al. 2000) |
| F. sylvatica (2) | t,wdb | 2 | 0.13 - 0.26 | AA | (Aranda et al. 2000) |
| Glycine max cv. CNS | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| G. max cv. Coker 156 | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| G. max cv. Coker 368 | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| G. max cv. Jackson | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| G. max cv. Roanoke | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| G. max cv. SCE82-222 | f,a | 2 | 0.07 - 0.11 | NA | (Shiraiwa and Sinclair 1993) |
| Gossypium hirsutum cv. Sicala V-2i (control) | f,p | 2 | 0.09 - 0.17 | NA | (Milroy et al. 2001) |
| G. hirsutum cv. Sicala V-2i (fertilized) | f,p | 2 | 0.11 - 0.17 | NA | (Milroy et al. 2001) |
| Helianthus annuus cv. Panter (1 / control) | f,a | 5 | 0.06 - 0.13 | NA | (Archontoulis et al. 2011) |
| H. annuus cv. Panter (1 / drought) | f,a | 4 | 0.08 - 0.13 | NA | (Archontoulis et al. 2011) |
| H. annuus cv. Panter (2 / control) | f,a | 2 | 0.10 - 0.12 | NA | (Archontoulis et al. 2011) |
| H. annuus cv. Panter (2 / drought) | f,a | 4 | 0.07 - 0.14 | NA | (Archontoulis et al. 2011) |
| H. annuus cv. Prosol (control/lower density) | f,a | 5 | 0.08 - 0.12 | NM | (Sadras et al. 1993) |
| H. annuus cv. Prosol (fertilized/lower density) | f,a | 5 | 0.08 - 0.12 | NM | (Sadras et al. 1993) |
| H. annuus cv. Prosol (control/higher density) | f,a | 5 | 0.08 - 0.12 | NM | (Sadras et al. 1993) |
| H. annuus cv. Prosol (fertilized/higher density) | f,a | 5 | 0.08 - 0.12 | NM | (Sadras et al. 1993) |
| Hibiscus cannabinus cv. Everglades 41 | f,b | 4 | 0.12 - 0.2 | MA, NA, NM | (Archontoulis et al. 2011) |
| Juglans regia | t,wdb | 2 | 0.29 - 0.39 | MA, NA, NM | (Le Roux et al. 1999) |
| Mangifera indica cv. Lirfa | t,eb | 4 | 0.13 - 1.54 | MA, NA, NM, AA | (Urban et al. 2003) |
| Oryza sativa cv. Norin #18 | g,a | 5 | 0.07 - 0.14 | AA | (Takeda and Yajima 1975a, b) |
| O. sativa cv. Toyonishiki | g,a | 2 | 0.11 - 0.15 | AA | (Sato and Kim 1980a, b, c) |
| Picea abies | t,en | 6 | 0.21 - 5.21 | MA | (Koppel and Frey 1984) |
| P. abies | t,en | 3 | 0.34 - 2.34 | MA, NA, NM | (Niinemets 1997a; Niinemets 1997b; Niinemets 1997c) |
| Picea sitchensis | t,en | 2 | 0.31 - 1.31 | MA | (Lewandowska and Jarvis 1977) |
| P. sitchensis | t,en | 3 | 0.35 - 2.35 | MA | (Norman and Jarvis 1974) |
| Pinus contorta var. latifolia | t,en | 3 | 2.09 - 11.1 | NA, AA | (Schoettle and Smith 1999) |
| P. densiflora | t,en | 5 | 1.41 - 2.07 | NA, AA | (Han et al. 2004) |
| P. paustris | t,en | 3 | 0.25 - 0.92 | MA, NA, NM | (Niinemets et al. 2002b) |
| P. sylvestris (infertile site) | t,en | 3 | 0.14 - 2.12 | MA, NA, NM | (Niinemets et al. 2002a; Niinemets et al. 2001; Niinemets and Lukjanova 2003) |
| P. sylvestris (fertile site) | t,en | 3 | 0.32 - 2.34 | MA, NA, NM | (Niinemets et al. 2002a; Niinemets et al. 2001; Niinemets and Lukjanova 2003) |
| P. sylvestris | t,en | 2 | 0.12 - 0.26 | MA | (Hansen et al. 2002) |
| P. taeda | t,en | 3 | 0.25 - 1.08 | MA, NA, NM | (Niinemets et al. 2002b) |
| P. virginiana | t,en | 3 | 0.25 - 0.75 | MA, NA, NM | (Niinemets et al. 2002b) |
| Populus tremula | t,wdb | 8 | 0.01 - 0.38 | MA, NA, NM | (Niinemets et al. 2004a) |
| Prunus persica cv. O'Henry | t,wdb | 4 | 0.11 - 0.32 | NA | (DeJong and Doyle 1985) |
| P. persica cv. Springcrest | t,wdb | 4 | 0.12 - 0.27 | NA | (DeJong and Doyle 1985) |
| P. persica cv. Alexandra | t,wdb | 2 | 0.10 - 0.18 | MA, NA, NM, AA | (Walcroft et al. 2002) |
| Quercus glauca | t,eb | 2 | 0.29 - 0.95 | NA | (Miyazawa et al. 2004) |
| Q. ilex subsp. ilex | t,eb | 4 | 0.46 - 3.46 | MA, NA, NM, AA | (Niinemets et al. 2006a) |
| Q. robur | t,wdb | 2 | 0.13 - 0.27 | MA | (Hansen et al. 2002; Hansen et al. 2003) |
| Q. robur | t,wdb | 2 | 0.13 - 0.27 | MA | (Hansen et al. 2002; Hansen et al. 2003) |
| Rhizophora stylosa | t,eb | 12 | 0.12 - 1.04 | AA | (Suwa and Hagihara 2008) |
| Tilia cordata | t,wdb | 8 | 0.01 - 0.38 | MA, NA, NM | (Niinemets et al. 2004a) |
| Triticum aestivum cv. Apache | g,a | 6 | 0.07 - 0.12 | NA | (Bertheloot et al. 2008) |
| T. aestivum cv. Isengrain | g,a | 6 | 0.07 - 0.12 | NA | (Bertheloot et al. 2008) |
| T. aestivum cv. Bannock | g,a | 2 | 0.05 - 0.07 | AA | (Barnes et al. 1990; Beyschlag et al. 1990) |
| T. aestivum cv. Minaret (control/higher density) | g,a | 2 | 0.06 - 0.15 | NA | (Dreccer et al. 2000) |
| T. aestivum cv. Minaret (fertilized/higher density) | g,a | 3 | 0.06 - 0.15 | NA | (Dreccer et al. 2000) |
| T. aestivum cv. Minaret (control/lower density) | g,a | 3 | 0.06 - 0.15 | NA | (Dreccer et al. 2000) |
| T. aestivum cv. Minaret (fertilized/lower density) | g,a | 3 | 0.06 - 0.15 | NA | (Dreccer et al. 2000) |
| Vicia faba var. minuta cv. Tina (higher density) | f,a | 2 | 0.10 - 0.12 | NA | (Del Pozo and Dennett 1999) |
| V. faba var. minuta cv. Tina (lower density) | f,a | 2 | 0.10 - 0.12 | NA | (Del Pozo and Dennett 1999) |
| Xanthium strumarium var. canadense | f,a | 3 | 0.09 - 0.14 | NA | (Hikosaka et al. 1999) |
| Zea mays cv. hybrid DEA (lower density) | g,a | 3 | 0.07 - 0.10 | NA | (Drouet and Bonhomme 1999) |
| Z. mays cv. hybrid DEA (higher density) | g,a | 3 | 0.07 - 0.10 | NA | (Drouet and Bonhomme 1999) |
Species names follow the GRIN taxonomy for plants (USDA, ARS, National Genetic Resources Program, Germplasm Resources Information Network - (GRIN), National Germplasm Resources Laboratory, Beltsville, Maryland, http://www.ars-grin.gov/cgi-bin/npgs/html/index.pl?language=en). Replicate studies in time (different year of study) are noted with numeric codes 1-3, and application of treatments (ozonation, fertilization, adjustment of planting density) and presence of replicate sites are also noted. Within-canopy variation in leaf traits was studied in field-grown plants except for the study of Dreccer et al. (2000) where growth-chamber cultivated plants were used. For further details of database construction see Niinemets et al. (2015).
g,a - annual grass; g,b - perennial grass; f,a - annual forb; f,b - biennial forb; f,p - perennial forb; t,wdb - winter-deciduous broad-leaved tree; t,eb - evergreen broad-leaved tree; t,en - evergreen needle-leaved tree (conifer tree)
MA - leaf dry mass per unit area; NA - leaf nitrogen content per unit area; NM - leaf nitrogen content per unit dry mass; AA - leaf photosynthetic capacity (light-saturated net assimilation rate) per unit area
In the previous study, within-canopy variation in 12 key leaf traits was analyzed (Niinemets et al. 2015). Due to limited number of observations for several traits, here I analyze age effects on within-canopy variation in leaf dry mass per unit area (MA, available for 28 canopies), leaf nitrogen content per area (NA, 45 canopies), leaf nitrogen content per dry mass (NM, 20 canopies) and photosynthetic capacity (AA, light-saturated net assimilation rate at ambient CO2 concentration, 15 canopies).
Based on the number of gradients available, the species were grouped according to life form as needle-leaved evergreen trees (all conifer species), broad-leaved evergreen, broad-leaved winter deciduous trees, forbs and grasses. As explained below, winter-deciduous species were further divided between flush-type and continuous-type species based on the pattern of canopy development. In the case of grasses, all species were graminoid grasses, except Carex acutiformis that is a cyperoid grass with different pattern of leaf development. Graminoid grasses included in this analysis had a pronounced stem with apical growth where the youngest leaves are at the top of the stem. In the cyperoid perennial rhizomatous grass C. acutiformis, the bulk of the plants in natural stands are vegetative and do not form a stem. Thus, long leaves extend from the base of the plant such that the upper leaf parts are the oldest. These differences in canopy formation were associated with contrasting age-dependent changes in trait plasticity as explained in the Results. To save space, data for most traits are plotted on the same figure panels for conifers and evergreen broad-leaved species and for forbs and grasses (herbs), but still maintaining the life form grouping.
Long-term integrated light availability
Given the evidence that within-canopy acclimation of leaf traits is driven by total integrated light rather than by relative light or instantaneous light (Chabot et al. 1979; Niinemets and Keenan 2012), this analysis is based on integrated quantum flux density (Qint) that was estimated for each study in a consistent manner as explained in detail in Niinemets et al. (2015). In that study, Qint was defined as a standardized estimate of light availability that corresponds to daily integrated quantum flux density averaged for 50 days after the start of foliage development, or to daily integrated quantum flux density averaged for the actual number of days since the start of foliage development when leaves were either younger than 50 days or when the average leaf life-span for the given species was less than 50 days (Qint,b50; (Niinemets et al. 2015). This time period reflects the evidence from past studies indicating that foliage trait vs. integrated average light dependencies stabilize 30-60 d after leaf formation in canopies of both woody and herbaceous species (Niinemets and Keenan 2012; Niinemets et al. 2004a). However, given that canopies of widely varying leaf age were considered in this analysis, and that there can be a certain re-acclimation to current light availability once the leaves have fully matured, several additional estimates of long-term light availability were estimated according to the methodology of Niinemets and Keenan (2012): Qint averaged for 30 days since the start of foliage development or for the actual number of days since the onset of leaf development when leaves were younger than 30 days (Qint,b30), Qint averaged analogously for 60 (Qint,b60) days since the start of foliage development, and Qint averaged for 50 days preceding leaf sampling or actual number of days when leaves were younger than 50 days (Qint,p50).
In evergreens supporting foliage formed in different years, the estimates Qint,b30, Qint,b50 and Qint,b60 refer to the year of formation of foliage, but estimation of actual light availability during formation of older leaves is difficult due to time-dependent increases in self-shading (Brooks et al. 1994; Brooks et al. 1996; Niinemets et al. 2006a). For studies with evergreens where multiple leaf ages were studied at the same time, Qint,b50 values corresponding to the formation of the most recent leaf flush (Qint,Rb50) were also estimated for older leaf classes. These latter estimates rather than Qint,b50 have been conventionally used in studies looking at within-canopy trait variations in evergreens (e.g., Warren and Adams 2001; Wright et al. 2006). They do not reflect the light availability during foliage formation, but are also not biased by possible underestimation of growth light environment due to enhanced self-shading during new foliage growth. Across all the data, the above-canopy estimates, Qint,b30, Qint,b50 and Qint,b60 were strongly correlated (for linear regressions, r2 = 0.87-0.97, P < 0.001 for all), but the correlations among Qint,b50 and Qint,Rb50 (r2 =0.48, P < 0.001) and Qint,b50 and Qint,p50 (r2 = 0.25, P < 0.001) were weaker. In the case of Qint,b50 and Qint,Rb50, this mainly reflected a strong variation in Qint,b50 values among different leaf flushes (spring, summer vs. autumn) in three subtropical conifers, Pinus palustris, P. taeda and P. virginiana (Table 2). In the case of Qint,b50 and Qint,p50, differences in light estimates underscored the seasonal variation in solar radiation through canopy development. Despite this variability, leaf plasticity estimates (see next section) and plasticity vs. leaf age responses were similar for different light estimates. Thus, in the final analysis, the relationships based on only Qint,b50 are demonstrated. When pertinent, quantitative and qualitative differences with other light estimates are highlighted in the appropriate sections of the Results.
Although some leaf traits might even respond to average light availability shorter than 5-10 days (e.g., Blanch et al. 2011; Logan et al. 1998; Niinemets et al. 1998), Qint values for very short time periods, less than 5-10 days cannot be sufficiently precisely estimated for a global analysis due to uncertainties with dates of the onset of leaf development and uncertainties in global radiation databases (Niinemets and Keenan 2012). Nevertheless, provided leaf phenological characteristics could be reliably worked out from the data (s. below for leaf age estimation), adequate periods of integration were used for leaves younger than 50 days as well.
Data fitting and estimation of relative trait plasticity and standardized relative plasticity
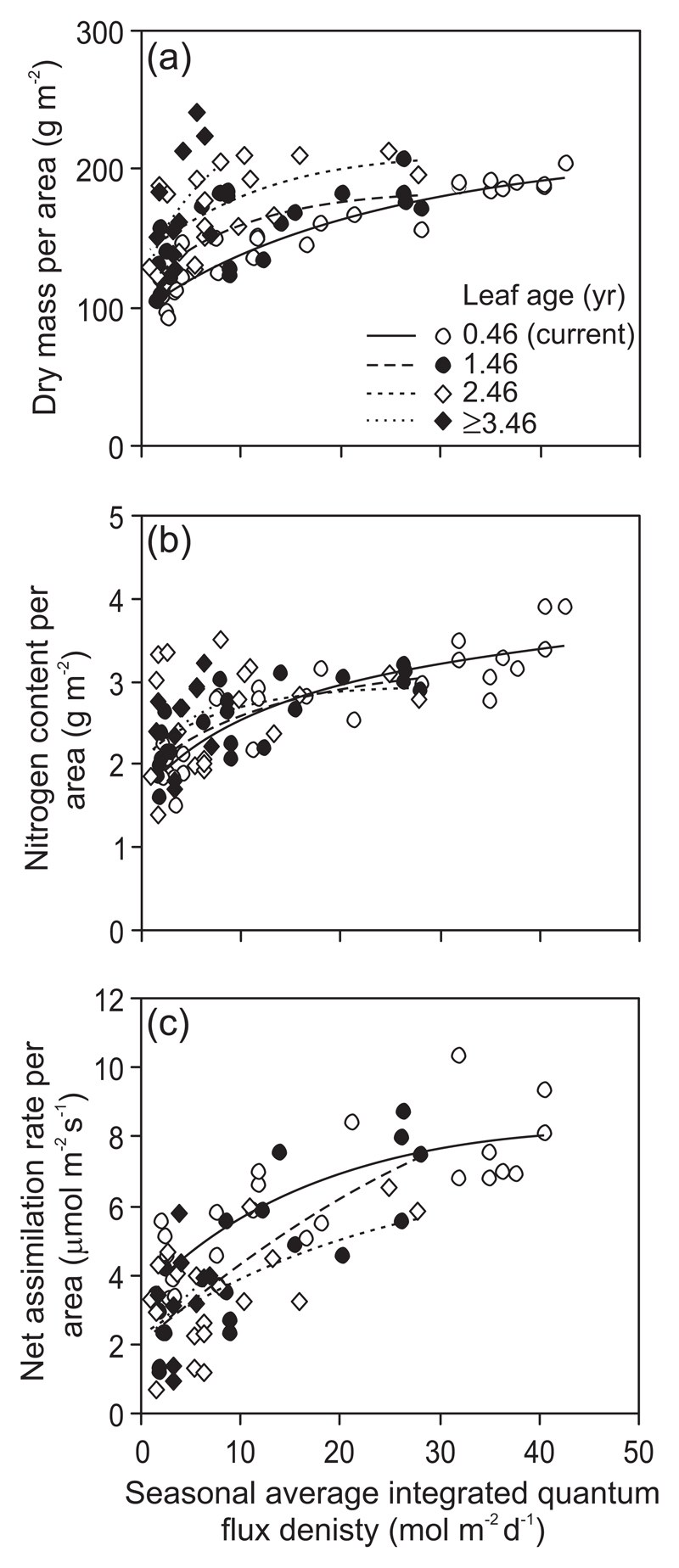
Qint vs. leaf trait data were fitted in all cases with three parameter non-linear equations (monomolecular equation for positive relationships and a modified monomolecular equation for negative relationships, Niinemets et al. 2015) that provided in all cases good fits to data (Fig. 1 for sample data fits for different-aged leaves of Quercus ilex) as explained in detail in Niinemets et al. (2015). For each leaf age class, the minimum Qint value present (Qint,min) was also estimated to characterize the age-dependent changes in light availability under specific conditions of the study.
Fig. 1.
Illustration of age-dependent changes in leaf dry mass per unit area (a), nitrogen content per unit area (b) and light-saturated net assimilation rate per unit area (c) for four leaf age classes in the Mediterranean evergreen broad-leaved species Quercus ilex (data of Niinemets et al. 2006a). Leaf age of 0.46 yr denotes current-year leaves. The data were fitted by a monomolecular asymptotic function in the form y = ymax(1-ed-fx), where ymax, d and f are empirical coefficients. The three-parameter monomolecular function provides a better fit to the data (greater r2) than two-parameter logarithmic or power functions typically used in fitting non-linear asymptotic biological relationships (Niinemets and Keenan 2012; Niinemets et al. 2015).
There are two major difficulties in directly comparing Qint vs. leaf trait relationships among different traits and among different plant life forms. First, different functional traits have numerically different values and functional traits vary among plant functional types (Niinemets et al. 2015; Wright et al. 2004), implying that the absolute values of trait change inherently differ for different traits and different plant functional types. Second, Qint vs. trait relationships typically are non-linear (Niinemets and Keenan 2012; Poorter et al. 2009), implying that the change in a trait value at a given change in Qint (trait plasticity) varies over the Qint gradient. Thus, conclusive comparisons of trait plasticity can be only made over the same light range. Yet, Qint ranges available varied among the studies due to several constraints, e.g., in multi-species canopies, shade-intolerant species often do not support leaves at low light in multi-species canopies, while understory species may not have been exposed to high Qint values, limiting the gradient range (Niinemets 2016; Niinemets et al. 2015). Both of these limitations were addressed by calculating the relative light-dependent plasticity (PL) of a given trait over the given Qint range:
| (1) |
where vi is the trait estimate corresponding to the seasonal average quantum flux density Qint,i and vi+x is the trait estimate corresponding to the second higher Qint value (Qint,i+x), and ΔQin is given as Qint,i+x - Qint,i, and the subscript l denotes the light range. Normalization of the trait plasticity with respect to the average trait value over the given Qint range, (vi+x +vi)/2 allows for direct comparison of plasticity of traits with different absolute values.
As defined here, the trait plasticity characterizes the trait variation corresponding to the given long-term variation in within-canopy light availability. This definition adheres to the traditional definition of phenotypic plasticity in studies investigating plant response to long-term light gradients (Portsmuth and Niinemets 2007; Valladares 2003; Valladares et al. 2007). Some plant traits can also respond to rapid changes in light availability within and among days (e.g., Niinemets 2016), but such rapid responses, trait changeability, cannot be singled out within the current dataset.
Given that leaf trait vs. Qint relationships are non-linear, it would be mathematically more correct to describe the plasticity as the standardized slope at the specified Qint, but such an estimate can be overly sensitive to small uncertainties at the edges of available Qint range where the non-linear fits become most uncertain (Niinemets et al. 2015). Although assuming a local linear response, the plasticity estimated by Eq. 1 is less sensitive to such uncertainties and over a sufficiently narrow Qint range approximates well the true plasticity. Here, the relative light-dependent plasticity was calculated over the Qint ranges 3-6, 6-12, 12-20, 20-30, and 30-40 mol m-2 d-1. In these calculations, the trait values, vi, were estimated from the parameterized trait vs. Qint responses using the extrapolation constraints specified in Niinemets et al. (2015). As the ranking of PL,l values for different traits and for different plant functional traits seldom changed for different light ranges used, and the light range 6-12 mol m-2 d-1 was available for the largest number of cases (Niinemets et al. 2015), this range was used to compare age-dependent changes in relative plasticity.
PL,l values for different traits typically vary among species and plant functional types and can be also affected by experimental treatment effects (e.g., Dreccer et al. 2000; Hirose et al. 1988). Such confounding effects can partly blur the leaf age effects on trait plasticity. To nail down the age effects per se, standardized relative plasticity was calculated as:
| (2) |
where PL,l,j is the relative trait plasticity for the leaf age class j and n is the number of leaf age classes present. Average Sj,l across all ages available for the given canopy is equal to 1. Sj,l allows for analyses of broad age-dependent trends in the given trait through all the data without confounding effects of inherent plasticity differences among species and plant functional types, and it also allows for comparison of age-dependent trends among different traits. However, Sj,l could be overly sensitive if PL,l values for the given canopy are small, indicating low plasticity. As PL,l values for NM in most tree species were very low, Sj,l estimates were not calculated for NM in trees.
Although the Qint range 6-12 mol m-2 d-1 was the most frequently present, it was still missing for some canopy gradients. Because PL,l depend on the light range l, averaging PL,l values across light ranges is not meaningful. Differently from PL,l, Sj,l values are not dependent on light range and thus, Sj,l values can be averaged across light ranges even if some ranges are missing. Thus, in addition to Sj,l values for the Qint range 6-12 mol m-2 d-1, average Sj,l values for all available light classes were calculated (S̄j). This made it possible to consider all light gradients in the ultimate analysis of age effects on within-canopy trait plasticity.
Estimation of average leaf age
In the current study, the tree species available originated mainly from temperate and Mediterranean climates, while subtropics was represented by three conifers (Pinus palustris, P. taeda, P. virginiana) and the broad-leaved species Rhizophora stylosa and tropics by the broad-leaved species Mangifera indica. In the case of most deciduous and evergreen trees in seasonal climates with pronounced cold period during winter, budburst occurs in spring at almost the same time through the canopy. Thus, all foliage in deciduous species, and foliage corresponding to the current leaf flush in evergreens has the same age. Typically, only one leaf flush is formed in the given year in cool temperate climates, while multiple leaf flushes through the growing season can be produced in warmer climates (Niinemets 2014). In the case of evergreens in our study, multiple annual leaf flushes were present in three subtropical pines (P. palustris, P. taeda and P. virginiana; Table 2), and in broad-leaved evergreens Mangifera indica (Bally 2006) and Rhizophora stylosa (Sharma et al. 2010), but not in the other species. The Mediterranean evergreen species Quercus ilex can have multiple leaf flushes, but in this analysis, a northernmost population was included that only supports one annual leaf flush (Niinemets et al. 2006a). In the case of single-flush deciduous and evergreen and multiple-flush evergreen species, leaf age was estimated considering the date of leaf sampling and estimated budburst date as explained in Niinemets et al. (2015).
Differently from flush-type species, an almost continuous- or semi-continuous-type of leaf formation in seasonal climates can occur in fast-growing early-successional forest species such as Salix spp. or young Alnus spp., Betula spp., and Populus spp. (Cline and Harrington 2007), but also in several fast-growing woody crops such as some cultured Prunus spp. (e.g., Gordon et al. 2006; Kervella et al. 1995). Among the winter-deciduous woody species in our study, only the young canopies of Betula platyphylla (Hoshika et al. 2013; Terazawa and Kikuzawa 1994) and canopies of Prunus persica (Gordon et al. 2006; Kervella et al. 1995) were characterized by continuous- to semi-continuous-type of leaf formation. In contrast, in annual and perennial herbs forming each year a new canopy, there is always a gradient in leaf age from canopy top to bottom. Furthermore, in grasses with basipetal leaf development, there is a significant gradient of leaf age within single leaves. This inherent developmental gradient is characteristic of all fast-growing canopies. For multiple sampling events, the question is however, how does average leaf age (ᾱ) change with increasing canopy age (αC). The age of individual leaves in different positions in the canopy was commonly not reported, but αC was available or could be derived based on the dates of canopy development and foliage harvesting. Due to the within-canopy leaf developmental gradient, canopy age significantly overestimates ᾱ of the canopy. This is especially relevant if αC exceeds the leaf life span, implying that leaves in the lower canopy have started to die off with the expansion of the canopy from the top. Assuming that the phyllochron (ϕ, time elapsed between the formation of subsequent leaves on the main stem) is constant during canopy development until the completion of leaf formation (e.g., Davidson et al. 2015), ᾱ for a young mature canopy that has just completed leaf formation is given as:
| (3) |
where nL is the total number of leaves in the canopy. Average leaf age of a canopy after elapsing a time of Δt, ᾱF+Δt, would become ᾱF+Δt. However, after full canopy expansion, there is a gradual loss of leaves from the lower canopy (e.g., Archontoulis et al. 2011; Hirose and Oikawa 2012; Milroy et al. 2001; Miyaji 1984). Thus, in determining changes in canopy average leaf age, it is important to consider that the longevity of the oldest (commonly lowermost) leaves cannot exceed their potential longevity. Therefore, in the current study, ᾱ corresponding to different canopy ages was found on the basis of the following considerations. Whenever ϕ was available or reported in the literature (e.g., Virdis et al. 2009; Yin and Kropff 1996), this estimate was used to determine ᾱ. In most cases, ϕ was not available, and ϕ for fully-developed young canopies was estimated as αC/nL. Although ϕ may vary somewhat through canopy development and depend also on environmental conditions, the curvilinearity of leaf number vs. time relationship is generally moderate (Virdis et al. 2009; Yin and Kropff 1996). Thus, this estimate can be considered as a good approximation of ϕ for the canopy. For older canopies with age αC+Δt, the number of leaves younger than the leaf longevity λ, nL+Δt, was estimated first and then ᾱF+Δt as:
| (4) |
If original papers reported presence of senescent leaves, presence of senescent leaves was taken as an estimate of λ for the given species in the given study. However, this was seldom the case, and an extensive literature search was carried out to obtain estimates of λ, and ultimately, average values of λ were calculated across all studies providing estimates of λ for the given species. However, average leaf longevity is not constant through the canopy, tending to be greater at the bottom of the canopy than in the top (Miyaji 1984). Given that only species-specific average values of leaf longevity, λ̄, were available, λ was approximated as 1.3λ̄. This approximation assured that ᾱ of the oldest senescent canopies was close to λ̄.
As the values of nL for herbaceous canopies with multiple sampling events were rarely available in the original studies, species-specific estimates were derived from the literature. For canopies with multiple sampling events, the following values of nL were used: Cynara cardunculus – 57 (Virdis et al. 2009); Glycine max – 34 (Munger et al. 1987); Gossypium hirsutum – 17 (Alarcon and Sassenrath 2011; Patterson 1988); Helianthus annuus – 25 (Hernández 2010); Hibiscus cannabinus – 60 (Abdul-Hamid et al. 2009); Oryza sativa – 12 (Yin and Kropff 1996); Triticum aestivum – 22 (Bos and Neuteboom 1998); Vicia faba – 19 (Chbouki et al. 2005); Xanthium strumarium var. canadense – 15 (Hirose and Oikawa 2012; Regnier and Harrison 1993); Zea mays – 14 (Drouet and Bonhomme 1999; Ford et al. 2008; Padilla and Otegui 2005). In the case of two woody species with continuous to semi-continuous leaf formation, Betula platyphylla and Prunus persica, leaf number per shoot (nS) is the equivalent of nL in herbaceous species. In B. platyphylla, values of nS = 15 and ϕ = of 6 d were used (Hoshika et al. 2013; Terazawa and Kikuzawa 1994). In P. persica, values of nS = 50 and ϕ = 4 d were used (Kervella et al. 1995).
Given that across all the data, canopy age and average leaf age were significantly correlated (r2 = 0.64, P < 0.001) and the statistical relationships among trait plasticity and αC were weaker than with ᾱ, only the relationships with ᾱ are demonstrated.
Data analysis
Linear and second order polynomial regression analyses were carried out to analyze the statistical relationships between leaf age and relative (Eq. 1) and standardized (Eq. 2-3) plasticity estimates and among plasticity estimates for different traits, and relationships of plasticity estimates with minimum quantum flux density using R version 3.1.1 (R Development Core Team 2012). The regression analyses were conducted separately within plant life forms and through all data pooled. To improve normality of data and regression residuals, leaf age was log-transformed in these analyses. All relationships were considered significant at P < 0.05.
Results
Changes in the minimum light availability through canopy development
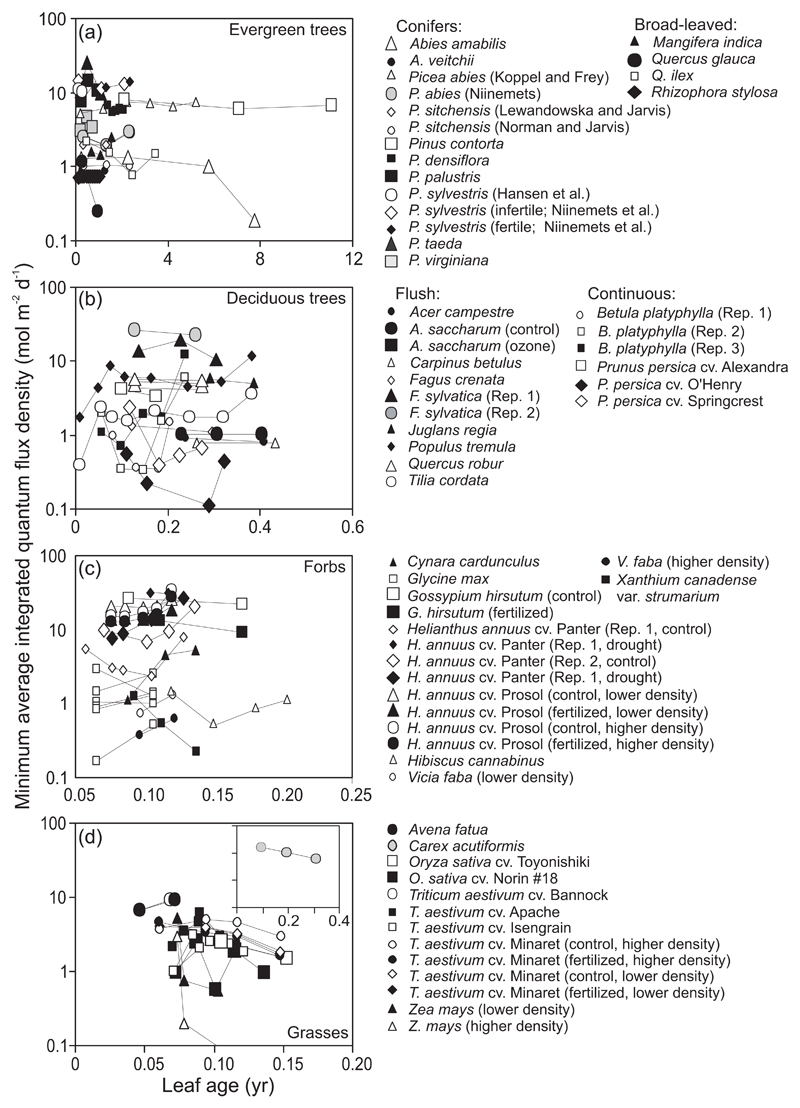
As noted in the introduction, in evergreen species, older leaves are expected to be overtopped by younger leaves, and thus, it is predicted that the light availability incident to older leaves decreases with increasing leaf age. Although the light availability for leaves developed at higher light decreases, the other question is whether the older leaves could be supported at lower light availabilities than those available for the younger leaves, i.e. whether there is a reduction in minimum light availability (Qint,min) with increasing leaf age. Indeed, there was evidence of a reduction of Qint,min with increasing leaf age in several conifer (e.g., A. amabilis, P. densiflora, Fig. 2a) and broad-leaved (Quercus glauca, Q. ilex) species. However, this was not always the case, and Qint,min even increased in Mangifera indica (Fig. 2a).
Fig. 2.
Changes in the minimum light availability incident to the leaves (Qint,min) with increasing leaf age for evergreen conifers and evergreen broad-leaved trees (a), winter-deciduous trees (b), forbs (c) and grasses (d) for all canopies included in the analysis (Table 2 for details). The lines connect successive values of Qint,min to better demonstrate time-dependent trends for the canopies included in the analysis. Light availability was characterized by daily integrated quantum flux density averaged for 50 days since leaf formation or for actual number of days when leaf age was less than 50 days (Qint, Material and methods and Table 1 for further information). In the case of deciduous species, herbs and forbs and in the evergreen broad-leaved species Rhizophora stylosa (Suwa and Hagihara 2008) and Mangifera indica (Urban et al. 2003) and in the evergreen conifer Pinus sylvestris in the study of Hansen et al. (2002), the same canopies were sampled at different moments of time. In the case of all other evergreen species, different-aged leaves were studied at the same time. Winter-deciduous species are separated among flush-type (all leaves formed at the same time in the beginning of the growing season) and continuous- to semi-continuous-type (leaves formed continuously or in multiple flushes on proleptic shoots, and on sylleptic shoots initiated through the growing season) species.
In winter-deciduous species, Qint,min initially increased with increasing leaf age in developing canopies (Populus tremula and Tilia cordata that had the youngest leaves present in this dataset, Fig. 2b), reflecting overall low integrated incident quantum flux densities in the spring when the days will still short. In flush-type species, Qint,min was constant in mature non-senescent canopies until leaf senescence (Fig. 2b). In contrast, canopies with continuous- to semi-continuous-type of leaf formation that produced leaves through the growing season had the minimum Qint,min values in mid-season, and Qint,min increased later in the season after the initiation of senescence of leaves formed in the beginning of the growing season (Fig. 2b).
In herbaceous species with continuous expansion of canopy until the onset of the generative phase of development, variation of Qint,min with average leaf age differed among forbs and grasses (Fig. 2c, d). In forbs where senescent leaves are abscised, an initial reduction in Qint,min observed in several species (e.g., Helianthus annuus and Xanthium strumarium var. canadense, Hibiscus cannabinus; Fig. 2c) was followed by an increase in Qint,min in the bulk of the species, although the increase in Qint,min started at different times in different species (Fig. 2c). In contrast, in grasses where senescent leaves remain attached to the plant and can potentially shade physiologically-active neighboring leaves, Qint,min decreased with increasing leaf age (Fig. 2d).
Age-dependent changes in leaf dry mass per unit area (MA) and its light-dependent plasticity
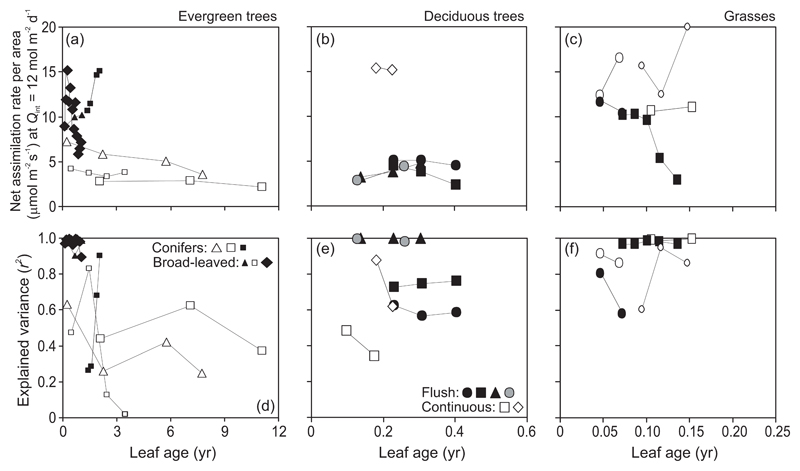
In evergreens, MA at a constant Qint increased with increasing leaf age, except for the subtropical conifers Pinus palustris, P. taeda, and P. virginiana that produce multiple annual leaf flushes (Fig. 3a). In deciduous tree species, MA initially increased with increasing leaf age until completion of leaf structural differentiation and was constant or moderately increased or decreased with further increases in leaf age similarly in both flush- and continuous-type species (Fig. 3b). Limited data available for herbaceous species indicated an increase of MA with increasing leaf age (Fig. 3c). In the cyperoid grass Carex acutiformis, MA values stabilized with further increases in leaf age.
Fig. 3.
Age-dependent changes in leaf dry mass per unit area (MA) at a constant moderately high average daily integrated quantum flux density (Qint) of 12 mol m-2 d-1 (a-c) and the degree of explained variance (r2) of Qint vs. MA relationships fitted by the monomolecular function (d-f) in different plant life forms. Succeeding trait values for given canopies are connected by lines to highlight the temporal trends. Values of MA at the given Qint were derived from fitted Qint vs. MA relationships (Fig. 1a for sample data fits). Symbols and definition of Qint as in Fig. 2.
In evergreens, the explained variance of MA vs. Qint relationships (Fig. 1a for sample relationships) typically decreased with increasing leaf age with the exception of Abies veitchii, Picea abies (study of Koppel and Frey 1984), Picea sitchensis (both studies available), P. taeda, and Pinus virginiana (Fig. 3d). In most deciduous species, r2 values initially increased until full leaf development and were thereafter constant for the rest of the growing season or somewhat decreased in the middle of the growing season (Fig. 3e). In the three forb species available, there was a negative age-dependent trend in r2, but not in the grass C. acutiformis (Fig. 3f). Despite the moderate age-dependent variation in r2 in deciduous trees and in herbs, r2 values were maintained at a high level with a few exceptions (Fig. 3e, f).
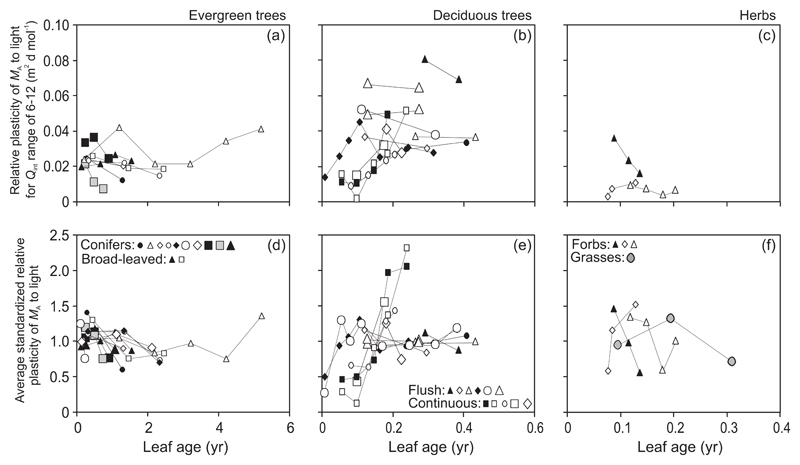
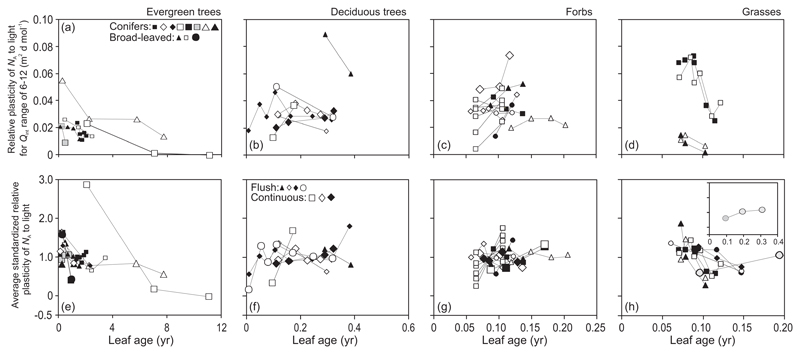
In general, the relative light-dependent plasticity of MA (Eq. 1) over the Qint range of 6-12 mol m-2 d-1 (PL,6-12) decreased with increasing leaf age in evergreens with the exception of the conifer Picea abies (study of Koppel and Frey 1984) and the broad-leaved evergreen Mangifera indica (Fig. 4a). Further analysis of the standardized relative plasticity (Sj) over the same light range (Sj,6-12) indicated that even after standardization, a certain species-specific variability was evident (Supplementary Information, Fig. S1a). Calculation of the average standardized relative plasticity values over all available light ranges (S̄j), allowed for inclusion of all canopies with available MA vs. Qint responses. Examination of S̄j values further underscored the broad trend of decreasing plasticity with increasing leaf age (Fig. 4d). Both Sj,6-12 and S̄j values indicated that age-dependent changes in MA plasticity were relatively moderate, within ca. ±0.5 relative units from the average of all leaf ages measured in the given canopy (Fig. 4d, Supplementary Information, Fig. S1a).
Fig. 4.
Relative light-dependent plasticity (Eq. 1) of leaf dry mass per unit area (MA) over the integrated quantum flux density (Qint) range of 6-12 mol m-2 d-1 (a-c) and average standardized plasticity (Eq. 2) calculated over all available light ranges [(d-)(f); Material and methods for the light ranges used] in relation to leaf age in different plant life forms. The relative plasticity is the relative light-dependent change in the trait value normalized with respect to the light range. Thus, it allows for comparison of plasticities of given trait among species and plant functional types with different trait values at the given light level (Fig. 3a-c), and comparison of light-dependent changes in different traits for given species (Niinemets et al. 2015). The standardized relative plasticity further normalizes for the variation of relative plasticity among species, among plant functional types and among Qint ranges. Symbols as in Fig. 2 and data presentation as in Fig. 3. Figure S1a-c shows standardized relative plasticity estimates corresponding to the Qint range 6-12 mol m-2 d-1.
In deciduous species, significant differences were observed in age-dependent changes in MA plasticity among flush- and continuous-type species. In flush-type species, the plasticity estimates increased in the youngest leaves and were thereafter almost invariable during the rest of the growing season (Fig. 4b, e). In continuous-type species, the plasticity estimates monotonically increased from leaf development until senescence (Fig. 4b, e). Comparison of standardized plasticity estimates Sj,6-12 (Supplementary Information, Fig. S1b) and S̄j (Fig. 4e) demonstrated that the age effects on MA plasticity were stronger in continuous- than in flush-type species.
In the case of herbaceous species with overall low MA plasticity, the age-dependent variation in PL,6-12 was also low in most cases (Fig. 4c). Further examination of Sj,6-12 and S̄j responses indicated a reduction of plasticity in three species and an increase in Cynara cardunculus (Fig. 4f, Supplementary Information, Fig. S1c).
Variations in leaf nitrogen contents per area (NA) and dry mass (NM) and their light-dependent plasticity
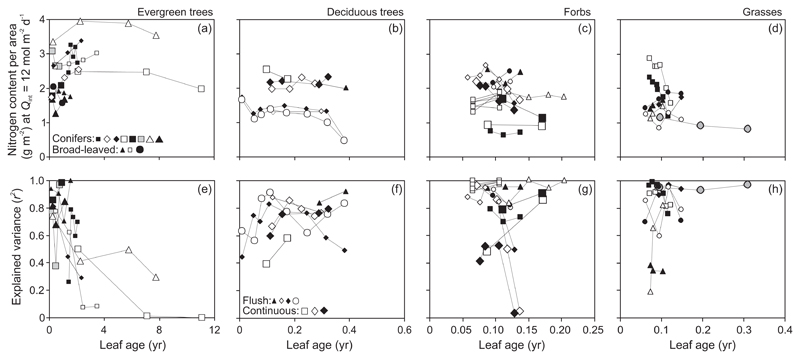
In evergreen species, NA at a given Qint was relatively constant or even somewhat increased (broad-leaved species Q. ilex, conifers P. densiflora and P. sylvestris) or declined (broad-leaved Quercus glauca and Mangifera indica and conifer P. virginiana) with increasing leaf age (Fig. 5a). The explained variance of NA vs. Qint relationships generally decreased with increasing leaf age (Fig. 5e).
Fig. 5.
Age-dependent modifications in leaf nitrogen content per unit area (NA) at a constant average daily integrated quantum flux density (Qint) of 12 mol m-2 d-1 (a-d) and the degree of explained variance (r2) of Qint vs. NA relationships (e-h) in different plant life forms. Figure 1b demonstrates sample data fits. Symbols are defined in Fig. 2 and data presentation follows Fig. 3.
In deciduous species, NA slightly decreased in the beginning of the growing season, and was constant over most of the season and declined at the end of the season in senescent leaves (Fig. 5b). The explained variance of NA vs. Qint relationships initially increased with leaf maturation and was thereafter almost constant through the rest of the growing season or somewhat declined in the middle of the season and for senescing leaves in some species (Fig. 5f).
In forbs, NA generally decreased moderately after an initial increase, although in some species NA somewhat increased (C. cardunculus) or was invariable (Hibiscus cannabinus) during the experimental period (Fig. 5c). The trend of an age-dependent decline in NA was similar in grasses (Fig. 5d) with the exception of Zea mays and fertilized treatments of Triticum aestivum (Fig. 5d). No clear time-dependent trend in r2 values of NA vs. Qint relationships was evident in herbaceous species with r2 values sometimes increasing with leaf development (forb Gossypium hirsutum in Fig. 5g and grasses Z. mays, higher density treatment, and T. aestivum fertilized treatments, Fig. 5h), and decreasing in older leaves (two Helianthus annuus gradients in Fig. 5g and T. aestivum in Fig. 5h), but in most cases, the age-dependent variation was small (Fig. 5g, h).
In evergreens, all NA plasticity estimates generally decreased with increasing leaf age (Fig. 6a, e, Supplementary Information, Fig. S2a) with some deviation from the general pattern only observed for S̄j in subtropical conifers (Fig. 6e). In deciduous species, the plasticity estimates increased in the growing leaves, and they were constant or only moderately changed during the rest of the growing season (Fig. 6b, f, Supplementary Information, Fig. S2b). Due to limited number of observations, no clearcut differences were evident among flush- and continuous-type species (Fig. 6b, f). In forbs, NA plasticity estimates generally increased with increasing leaf age. Only H. cannabinus with a moderate change through leaf aging and some H. annuus gradients with reduced S̄j in older leaves constituted exceptions to the general trend (Fig. 6c, g, Supplementary Information, Fig. S2c). In contrast, NA plasticity estimates decreased in all graminoid grasses (Fig. 6d, h, Supplementary Information, Fig. S2d), and were moderately affected by leaf age in the cyperoid grass C. acutiformis (Fig. 6h).
Fig. 6.
Light-dependent plasticity (Eq. 1) of leaf nitrogen content per unit area (NA) over the integrated quantum flux density (Qint) range of 6-12 mol m-2 d-1 (a-d) and average standardized plasticity (Eq. 2) calculated over all available light ranges (e-h) in dependence on leaf age in different plant life forms. Symbols as in Fig. 2, data presentation as in Fig. 3 and definition of plasticity estimates as in Fig. 4. Figure S2 demonstrates changes in standardized relative plasticity (Eq. 2) over the Qint range of 6-12 mol m-2 d-1.
As NA is the product of nitrogen content per dry mass (NM) and MA, examination of age-dependent changes in NM allows for gaining a mechanistic insight into differences in age-dependent changes in NA and MA and their light-dependent plasticity. In evergreens, NM at a given Qint moderately decreased or was almost constant (Fig. 7a). In deciduous species, NM was very high right after the budburst and declined rapidly to an almost constant value that was maintained until the onset of leaf senescence (Fig. 7b). In herbaceous species, NM decreased monotonically with increasing leaf age in all cases, but the response was less in C. acutiformis that was the only grass species with available NM data (Fig. 7c). The light-dependent variation in NM in woody species was low (see Niinemets et al. 2015), and the age-dependent changes in plasticity estimates were scattered (data not shown). In forbs, NM plasticity increased with increasing leaf age in all cases except for H. cannabinus (Fig. 7d for S̄j, responses were analogous for PL,6-12 and Sj,6-12). NM plasticity for the cyperoid grass C. acutiformis did not change with leaf aging (Fig. 7d).
Fig. 7.
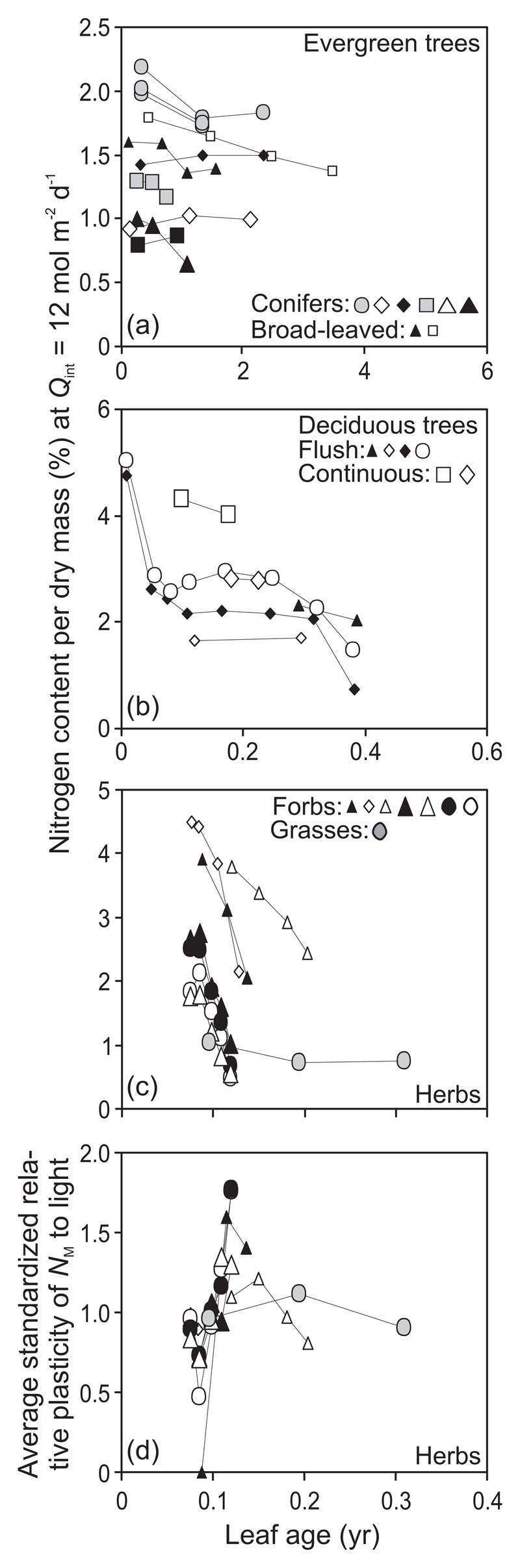
Leaf age effects on leaf nitrogen content per dry mass (NM) at a constant average daily integrated quantum flux density (Qint) of 12 mol m-2 d-1 in different plant life forms (a-c), and average standardized plasticity to light (Eq. 2, Fig. 4 for plasticity definition) in herbs (d). Plasticity of NM is only demonstrated for herbs because NM was weakly related to Qint in most tree species studied here in accordance with a global within-canopy plasticity dataset (Niinemets et al. 2015). Symbol definitions as in Fig. 2 and data presentation as in Fig. 3.
Changes in photosynthetic capacity per area (AA) and its light-dependent plasticity
In evergreens, AA at a given Qint tended to decrease with increasing leaf age with the exception of the conifer P. densiflora and the broad-leaved evergreen M. indica (Fig. 8a). Explained variance of AA vs. Qint relationships was higher in younger than in older leaves, except for Q. ilex where r2 for current year leaves was less than that for one-year-old leaves, and in P. densiflora where r2 strongly increased over a time period from autumn to spring (Fig. 8b).
Fig. 8.
Age-dependent changes in leaf light-saturated net assimilation rate (photosynthetic capacity) per unit area (AA) at a constant average daily integrated quantum flux density (Qint) of 12 mol m-2 d-1 (a-c) and the degree of explained variance (r2) of Qint vs. AA dependencies (d-f) in different plant life forms. Sample fits of Qint vs. AA are shown in Fig. 1c. Symbols used are explained in Fig. 2, and data presentation follows Fig. 3.
In deciduous and herbaceous species, only limited data of age effects on AA light gradients were available. In deciduous species, AA initially increased as leaves matured, and declined in senescing leaves (Fig. 8b). In grasses, age-dependent responses in AA were variable, with AA declining in only one study with Oryza sativa (Fig. 8c). No clear pattern in r2 of AA vs. Qint relationships was evident for deciduous species and grasses (Fig. 8c, f).
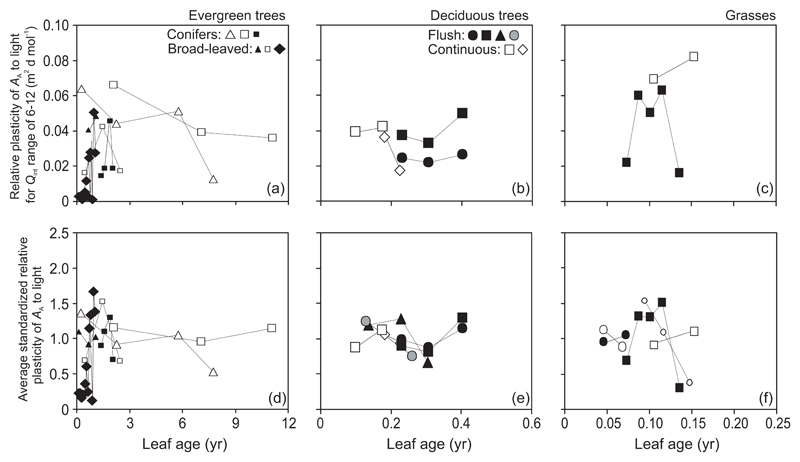
The plasticity of AA in evergreens initially tended to increase with increasing leaf age, and decline thereafter, or the plasticity monotonically decreased with increasing leaf age (Abies amabilis, Pinus contorta), although the variability was occasionally large (Fig. 9a, d, Supplementary Information, Fig. S1d). In deciduous species, the age-dependent change in plasticity was small, only ca. ±20% relative to the average plasticity, but the age range was also less than for the other traits. Due to limited data, age-dependent changes in AA plasticity could not be separated among flush- and continuous-type species (Fig. 9b, e, Supplementary Information, Fig. S1e). In grasses, the responses were variable (Fig. 9c, f, Supplementary Information, Fig. S1f), but S̄j data suggested an initial increase of plasticity followed by a reduction in senescing leaves (Fig. 9f).
Fig. 9.
Leaf age dependent changes in light-dependent plasticity (Eq. 1) of leaf photosynthetic capacity per unit area (AA) over the integrated quantum flux density (Qint) range of 6-12 mol m-2 d-1 (a-c) and average standardized plasticity (Eq. 2) calculated over all available light ranges (d-f) in different plant life forms. Symbols as in Fig. 2, presentation of data as in Fig. 3 and plasticity definitions as in Fig. 4. Age-dependent changes in standardized relative plasticity for Qint range of 6-12 mol m-2 d-1 are depicted in Fig. S2d-f.
Correlative relationships among light-driven plasticity and leaf age across plant life forms
As the age-dependent changes in all three different plasticity estimates, relative plasticity PL,6-12, its corresponding standardized plasticity Sj,6-12 and standardized plasticity averaged for all available light ranges, S̄j, were similar, and the largest number of observations was available for S̄j, here only the correlations with leaf age (log-transformed in all cases) are shown with this plasticity estimate except when pertinent. As before, the correlations refer to plasticity estimated with Qint,b50, and the responses with plasticity values estimated by other average integrated light measures are demonstrated when these alternative estimates provided additional insight into the patterns. In evergreens, MA plasticity data of Picea abies (Fig. 4a, d) and NA standardized plasticity for the youngest leaf age of Pinus contorta (Fig. 6e) were apparently outliers and the following analysis is conducted with and without these data. Analogously, the cyperoid grass C. acutiformis has a different growth pattern than all other grasses included in this analysis (see Methods), and thus, the plasticity relationships in grasses were analyzed with and without this species.
In evergreen conifers and broad-leaved species, the plasticity for MA was not significantly related to leaf age when these plant life forms were considered separately, but a negative correlation was observed when conifers and broad-leaved species were pooled (Table 3). Furthermore, when the data were analyzed without the outlying observation for P. abies (Fig. 4d), MA plasticity for conifers was negatively associated with leaf age (Table 3), and the relationships for all evergreens pooled was stronger (r = -0.49, P < 0.01). When MA plasticity for evergreens was calculated with Qint,b50 values corresponding to the formation of the most recent leaf flush (Qint,Rb50), the correlations with leaf age were weaker (data not shown).
Table 3.
Pearson correlations (r) of average standardized plasticity (S̄j) for leaf dry mass (MA) and nitrogen content (NA) with average leaf age (ᾱ) in different woody (evergreen conifers and evergreen broad-leaved species, deciduous broad-leaved species) and herbaceous (forbs and grasses) plant life forms
| Life form | S̄j for MA vs. Log(ᾱ) | S̄j for NA vs. Log(ᾱ) | ||||||
|---|---|---|---|---|---|---|---|---|
| r | P | n | Age range (yr) | r | P | n | Age range (yr) | |
| Evergreen conifers | -0.34& | 0.065 | 30 | 0.12-5.21 | -0.40# | 0.05 | 24 | 0.14-11.1 |
| Evergreen broad-leaved species | -0.36 | 0.43 | 7 | 0.13-2.46 | -0.62 | 0.08 | 9 | 0.13-2.46 |
| All evergreens | -0.35 | <0.04 | 37 | 0.12-5.21 | -0.42 | <0.02 | 33 | 0.13-11.1 |
| Deciduous broad-leaved (flush) | 0.55 | <0.005 | 28 | 0.01-0.43 | 0.51 | <0.03 | 19 | 0.01-0.39 |
| Deciduous broad-leaved (continuous) | 0.68 | <0.005 | 18 | 0.06-0.35 | 0.36 | 0.36 | 10 | 0.09-0.44 |
| All deciduous broad-leaved species | 0.42 | <0.005 | 46 | 0.01-0.43 | 0.44 | <0.02 | 29 | 0.01-0.44 |
| Forbs | -0.14 | 0.69 | 10 | 0.08-0.20 | 0.34 | <0.03 | 42 | 0.06-0.20 |
| Grasses | -0.35* | 0.07 | 29 | 0.06-0.31 | ||||
Standardized relative plasticity is defined by Eq. 2. Deciduous broad-leaved species are divided among flush-type species that form a single leaf flush in the beginning of the growing season and continuous-type species that form leaves continuously or in multiple flushes through the growing season
Due to limited data, S̄j for MA vs. Log(ᾱ) statistics for grasses could not be calculated.
r = -0.53, P < 0.005, without an outlying observation of Picea abies at ᾱ = 5.21 yr (Fig. 4d)
r = - 0.68, P < 0.001, without an outlying observation of Pinus contorta at ᾱ = 2.1 yr (Fig. 6e)
r = -0.63, P < 0.001 without data for cyperoid grass Carex acutiformis exhibiting an opposite response (Fig. 6h)
The plasticity for MA, was positively correlated with leaf age for all deciduous species pooled, and especially when flush- and continuous-type species were separately considered (Table 3). In continuous-type species, the response was even stronger (r = 0.79, P < 0.001) when the plasticity was estimated using Qint values averaged for 50 days preceding leaf sampling or actual number of days when leaves were younger than 50 days (Qint,p50). However, in the case of flush-type species, S̄j estimated with Qint,p50 was not correlated with leaf age (r = 0.27, P > 0.1). MA plasticity for available herb species analyzed together was not related to leaf age (Table 3).
The plasticity for NA decreased with increasing leaf age in conifers and for all evergreens analyzed together (Table 3). Without the outlying observation for P. contorta (Fig. 6e), the relationships were significantly stronger for conifers (Table 3) and all evergreens pooled (r = -0.67, P < 0.001). In fact, the non-standardized plasticity, PL,6-12 with all values included scaled strongly with leaf age (r = -0.62, P < 0.02 for conifers and r = -0.57, P < 0.01 for all evergreens pooled), indicating that the standardization (Eq. 2) was overly sensitive in this particular case of P. contorta. In deciduous species, NA plasticity increased with leaf age in flush-type species and when all deciduous species were pooled, but not in continuous-type species with limited data (Table 3). The relationship with flush-type species was statistically not significant when plasticity was calculated with Qint,p50 (P > 0.07).
NA plasticity increased with leaf age in forbs, and tended to decrease in grasses (Table 3), especially without the outlying observations for C. acutiformis (Fig. 6h). Both these relationships were stronger when S̄j was calculated with Qint,p50 (r = 0.39, P < 0.02 for forbs and r = -0.39, P < 0.04 for all grasses and r = -0.67, P < 0.001 without C. acutiformis) than with Qint,b50 (Table 3). In forbs and grasses, NA plasticity changes were positively correlated with age-dependent changes in minimum light availability, but not in other plant life forms (Supplementary Information, Fig. S3).
The plasticity for AA was positively associated with leaf age only in broad-leaved evergreens (r = 0.47, P < 0.05) and not in any other species group (P > 0.1). Analogously, PL,6-12 (r = 0.60, P < 0.02) and Sj,6-12 (r = 0.53, P < 0.05) were positively correlated with leaf age in broad-leaved evergreens.
Controls of age-dependent plasticity change across different plant life forms
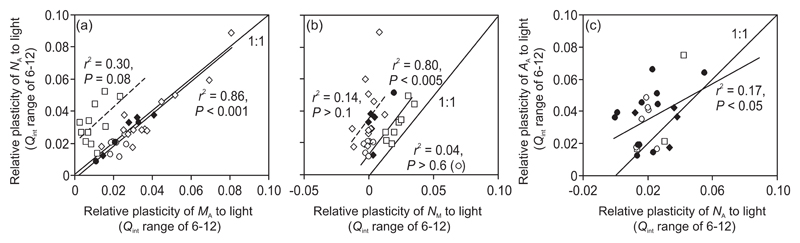
In woody species, PL,6-12 values for NA scaled positively with PL,6-12 values for MA across all leaf ages following a close to 1:1 relationship (Fig. 10a). In contrast, in herbaceous species, NA plasticity was more strongly associated with NM plasticity (Fig. 10b). Nevertheless, the data were positioned above the 1:1 line, indicating that MA plasticity also played a role in age-dependent changes in NA plasticity in herbaceous species (cf. Figs. 10a and 10b). Across all data pooled, AA plasticity was correlated with NA plasticity, but the relationship was weak (Fig. 10c).
Fig. 10.
Relative light-dependent plasticity (PL,l) of nitrogen content per area (NA) in relation to PL,l of leaf dry mass per unit area (MA; a) and PL,l of nitrogen content per dry mass (NM, b) and PL,l of leaf photosynthetic capacity in relation to PL,l of NA (c). In all cases, PL,l values (m2 d mol-1) are for the integrated quantum flux density range of 6-12 mol m-2 d-1. NA is the product of MA and NM, and thus, the relationships in (a) and (b) demonstrate the controls on NA plasticity by plasticities of underlying variables. Variation in plasticity estimates is driven both by age-dependent changes and plasticity differences among the canopies included in the analysis (Figs. 4, 6, 7, 9). Data were fitted by linear regressions. In (a), available data for tree species were fitted by a common regression. In (c), all data were fitted by a single regression. One-to-one lines are also demonstrated. Symbols as: ● - evergreen conifers; ○ - evergreen broad-leaved species; ◊ - flush-type deciduous broad-leaved species; ♦ - continuous-type deciduous broad-leaved species; ■ - grasses; □ - forbs.
Discussion
Why should light-dependent plasticity vary with canopy and leaf aging
A within-canopy gradient in leaf traits at some moment of time provides a snapshot of canopy functioning, and the key question is to what extent such a snapshot is representative for the entire growing season. As discussed in the introduction, leaf trait values at a certain within-canopy light availability can differ due to age-dependent changes in foliage structural and physiological differentiation as well as due to foliage re-acclimation to modified light conditions that can occur because of changes in light availability above the canopy during the season and within the canopy because of canopy expansion, resulting in shading of older leaves by newly developed leaves.
What is the evidence of dynamics in leaf age and light environment in canopies? Increases in average leaf age inevitably occur in flush-type woody species, but also in fast-growing continuously leaf-forming species due to cessation of canopy expansion and new leaf formation after the onset of generative phase of life cycle in herbs and changes in biomass allocation from production of new leaves to enhanced investment in woody tissues in fast-growing deciduous woody species. Indeed, significant changes in the minimum light availability (Qint,min) with leaf age were observed for a number of canopies (Fig. 2), although not always. As expected, Qint,min decreased with increasing leaf age in several evergreen canopies (Fig. 2a), but in some conifers, older leaves could not apparently be supported at increasingly low light intensities (Fig. 2a). This suggests a reduction of leaf survivorship once the shading by newer foliage becomes severe enough such that achieving a positive carbon balance becomes impossible (Hikosaka 2003; Hikosaka 2005; Niinemets 1997a). In deciduous woody species, Qint,min initially increased due to increasing the daily integrated solar radiation in spring, but decreased thereafter to a constant level at full canopy expansion in flush-type woody species, and it was strongly reduced due to canopy expansion until the cessation of formation of new leaves in continuous-type woody species (Fig. 2b). As expected, Qint,min typically decreased with increasing leaf age in grasses. However, in forbs (Fig. 2d), Qint,min was surprisingly constant in most cases or even increased sometimes, suggesting that upon reaching a certain Qint,min, older leaves senesced and were abscised, although some species could support leaves at increasingly lower Qint,min (Fig. 2d). This evidence altogether underscores that within-canopy light conditions do significantly vary through canopy development and leaf aging, providing a mechanistic basis for age-dependent changes in trait gradients.
Age-dependent changes in leaf structure and structural plasticity in different plant life forms
Structural leaf differentiation in developing leaves involves increases in overall leaf thickness, increases of individual mesophyll cells and thickness of cell walls (Hanba et al. 2001; Miyazawa et al. 1998; Miyazawa and Terashima 2001; Niinemets et al. 2012; Tosens et al. 2012), ultimately leading to increases in leaf dry mass per unit area (MA) as was also observed in this meta-analysis (Fig. 3). Furthermore, in evergreen species, MA of fully-developed leaves (Fig. 3a) changes with increasing leaf age due to secondary needle growth, i.e. growth of new xylem and phloem inside the needle central cylinder (Ewers 1982; Gilmore et al. 1995).
Despite similar increases in MA in different plant life forms, this meta-analysis highlighted profound differences in how MA light-dependent plasticity depended on leaf age in different plant life forms. In evergreens, the strength (r2) of average daily integrated light vs. Qint relationships generally decreased (Fig. 3d) and the relative trait plasticity (PL, Eq. 1, Fig. 4a) and standardized plasticity (Sj, Eq. 2; Fig. 4d and Table 3) decreased with increasing leaf age with the exception of the conifer Picea abies. Greater scatter of MA vs. light responses and lower plasticity of older leaves in evergreens indicates that leaves developed at higher light cannot morphologically acclimate to reduced light conditions upon shading. Different plasticity response in P. abies remains unclear. This contrasting response might reflect the circumstance that within the conifer shoot, there is a large self-shading of adjacent needles, especially for high-light grown shoots (Cescatti and Zorer 2003; Niinemets et al. 2006b) such that leaf morphology varies in dependence on needle axial location (lateral, upper, lower) within the shoot (Norman and Jarvis 1974). Given that MA (Fig. 3a) and Qint,min (Fig. 2a) values of P. abies were only moderately affected by leaf age, shading could have resulted in preferential abscission of needles less acclimated to the new lower light environment, leading to a virtual constancy of MA values and plasticity.
Differently from evergreens, MA plasticity increased with increasing leaf age in deciduous woody species (Fig. 4b, e and Table 3), whereas the increase was stronger for continuous- than for flush-type species. In fact, in flush-type species, the change in MA plasticity occurred in early season during leaf development and the within-canopy MA gradients were invariable for the rest of the growing season (Fig. 4b, e). In continuous-type species, the overall period for leaf development was longer due to new leaf formation, but clearly, as more leaves in the canopy reached the full maturity and became structurally differentiated, the structural gradient through the canopy increased. Greater capacity for changes in leaf structural characteristics in continuous-type species is also underscored by the stronger relationships when plasticity was calculated with Qint values estimated as an average of 50 days before foliage collection (Qint,P50) rather than with Qint values for 50 days since the budburst (Qint,b50).
A certain reduction in r2 of MA vs. Qint relationships in the middle of growing season observed in several deciduous woody species (Fig. 3e) is surprising, but it can reflect Qint-dependent differences in the accumulation of starch and soluble carbohydrates in leaves photosynthesizing most actively (Le Roux et al. 1999; Niinemets and Kull 1998). In the case of continuous-type deciduous woody species, it can also indicate formation of news leaves with initially weaker response to Qint (Fig. 2b, 4e).
Analogously to continuous-type deciduous woody species, one would expect that in herbs, MA plasticity increases with increasing the average leaf age as more leaves fully mature. However, herbs have a faster rate of leaf development than woody species (Miyazawa et al. 1998; Niinemets et al. 2012), implying that leaves developing under high light and becoming shaded with canopy expansion spend a shorter part of their whole developmental period in shade. This likely explains why MA plasticity in herbs is typically lower than in woody species (Niinemets et al. 2015) as was also observed in our study (Fig. 3c). The plasticity responses observed within this narrow range of variation were contradictory (Fig. 4c, f), calling for further studies on age-dependent changes in morphological plasticity of herbs.
Age-dependent variations in nitrogen gradients differ among plant life forms
Leaf age effects on nitrogen content per area (NA), the product of MA and nitrogen content per dry mass (NM) depend on variations in its components. Analogously, age effects on light-dependent plasticity of NA are driven by the age effects on the plasticities of MA and NM. In evergreens, NA was moderately affected by leaf age or even increased in some species (Fig. 5a), reflecting minor reductions in NM (Fig. 7a) and increases in MA (Fig. 3a) with increasing leaf age. A low age-dependent change in NA with leaf age in non-senescent leaves of evergreens is in agreement with past observations (Cordell et al. 2001; Escudero and Mediavilla 2003; Rapp et al. 1999). Differently from evergreens, in deciduous woody species, NA (Fig. 5b) decreased in the beginning of the growing season, was almost constant through most of the growing season and decreased after the onset of leaf senescence. Thus, even MA increased in the growing leaves (Fig. 3b), age-dependent reduction in NM was much stronger during early leaf development, while resorption of nitrogen from senescing leaves dominated the late-season reduction in NA (Fig. 7b). Although NM consistently decreased in forbs (Fig. 7c), NA changed less in most cases (Fig. 5c), suggesting a compensation due to increased MA (Fig. 3c). In the case of grasses, limited data were available for NM (Fig. 7c), but NA decreased across available data as expected with some exceptions in fertilized treatments (Fig. 5d).
Given that in woody species, the within-canopy gradients in NM were small (data not shown, see Niinemets et al. 2015), and variation in NA plasticity was strongly associated with variation in MA plasticity across species and leaf ages (Fig. 10a), the age effects on NA plasticity were expected to mirror changes in MA gradients. Indeed, in woody evergreens, both NA plasticity (Fig. 6a, e and Table 3), and r2 values of NA vs. Qint relationships (Fig. 5e) decreased with increasing leaf age similarly to corresponding MA traits (cf. Fig. 3d, Fig. 4a, d and Table 3). Analogously to MA plasticity, in deciduous woody species, NA plasticity initially increased with increasing leaf age, reflecting early foliage morphological differentiation, and the plasticity changed thereafter little during the rest of the growing season (Fig. 6b, f and Table 3). While clear differences among flush-type and continuous-type species were observed for MA plasticity, limited coverage of continuous-type species does not allow for conclusive comparisons of NA plasticity among canopies with different leaf turnover.
In herbs, surprising differences were observed in age-dependent changes in NA plasticity among forbs (Fig. 6c, g and Table 3) and grasses (Fig. 6d, h and Table 3). A moderate age-dependent increase in NA plasticity in forbs was associated with limited age-dependent changes in NA (Fig. 5c), major reductions in NM (Fig. 7c) and increased NM plasticity (Fig. 7d), and increased minimum light availability (Fig. 2c). In contrast, the age-dependent reduction in NA plasticity in grasses was associated with reduced minimum light availability (Fig. 2d), while little data were available for age effects on other drivers except for the cyperoid grass Carex acutiformis that was an outlier in the grass NA plasticity relationships (Fig. 6h). However, contrary to the graminoid grasses included in the analysis, the leaves of this species grow from the plant base such that the youngest foliage elements are at the bottom of the canopy, explaining the diverse response.
Despite incomplete evidence, one can still speculate about the possible mechanisms that is operative in age-dependent changes in NA plasticity in herbaceous species. In forbs, the gradient becomes stronger as plants loose older leaves at the bottom of the canopy and recycle nitrogen from remaining lower leaves to the upper leaves (Fig. 7c), while upper leaves mature increasing their MA and NA values similarly to continuous-type flush species. In fact, although NA plasticity across species and leaf ages in forbs was primarily driven by NM plasticity (Fig. 10b), the data did not fall on a 1:1 line, suggesting a role in MA plasticity as well (marginally significant in Fig. 10a). In grasses, much of the nitrogen seems to be resorbed from the upper canopy in the generative phase of leaf development for the growth of generative organs, while resorption from lower canopy leaves contributes less to generative growth. This suggestion is supported by evidence from classical studies on grasses, indicating that the uppermost flag leaves play a major role in providing nitrogen and carbohydrates to generative organs during grain filling (Araus et al. 1986; Evans 1983; Hunt 1985).
Changes in photosynthetic plasticity through leaf ages
Although the light-saturated net assimilation rate at current ambient CO2 concentration, the photosynthetic capacity, per unit leaf area (AA) typically scales with NA (Evans 1989), AA also depends on nitrogen use efficiency that characterizes the fraction of nitrogen invested in light-limiting components of photosynthesis machinery (e.g., Niinemets et al. 2015; Reich et al. 1995). In addition, in stressed leaves, photosynthetic capacity can be reduced due to reductions in stomatal conductance, inhibition of the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and photoinhibition (e.g., Flexas et al. 2004; Werner et al. 2001). In the current study, age-dependent changes in AA at a given Qint, a reduction with increasing leaf age in evergreens (Fig. 8a), and an increase in AA during leaf development and reduction in senescing leaves in deciduous woody (Fig. 8b) and herbaceous (Fig. 8c) species broadly agreed with past observations (Niinemets et al. 2012 for a review). However, there were several exceptions such as age-dependent increases in evergreens Mangifera indica and Pinus densiflora (Fig. 8a) and a certain variability in herbs (Fig. 8c). Such discrepancies can be indicative of other leaf-age independent effects such as different level of stress, including photoinhibition, sustained through the autumn to spring season (Slot et al. 2005) and resorption and refilling of N pools in older leaves during growth of new spring leaf flush (Weikert et al. 1989) in P. densiflora as well as changes in source-sink relationships through the fruiting period in M. indica that can affect the activity of rate-limiting photosynthetic enzymes (Crafts-Brandner et al. 1991; Urban et al. 2004) in addition to alterations in nitrogen remobilization (Weinbaum et al. 1994).
Provided the changes in NA drive within-canopy modifications in AA, age-dependent changes in AA plasticity should follow the age effects on NA plasticity. This suggestion is confirmed by a correlation, although scattered, among AA and NA plasticities across all data (Fig. 10c). However, the data on AA plasticity were limited, and within plant life forms, the trends in AA plasticity were somewhat contradictory to NA plasticity data. In fact, in broad-leaved Rhizophora stylosa (Fig. 9a, d), AA plasticity increased with increasing leaf age reflecting mainly the increase through canopy development, consistent with the delayed photosynthetic development in evergreens (Miyazawa et al. 1998; Miyazawa and Terashima 2001). Analogously, greater plasticity in one-year-old leaves in Quercus ilex and M. indica may indicate delayed developmental effects. Furthermore, the reduction in AA plasticity with leaf age in conifers was less than that in MA and NA plasticities (cf. Fig. 4a, d, Fig. 6a, e, Fig. 9a, d and Supplementary information Fig. S1 and S2). Despite the structural constraints on re-acclimation upon shading (s. Introduction), there is evidence of re-acclimation to modified light conditions in conifers and broad-leaved evergreens through changes in nitrogen partitioning such that the photosynthetic plasticity of older leaves might remain relatively high despite reductions in the plasticities of MA and NA (Brooks et al. 1994; Brooks et al. 1996; Niinemets et al. 2006a). Nevertheless, the AA vs. Qint relationships became more scattered (Fig. 8d) and ultimately plasticity decreased, suggesting that the re-acclimation is limited.
In broad-leaved species, the overall change in photosynthetic plasticity with leaf age was small (Fig. 9b, e), but the leaf age range was also less than for MA and NA, and obviously, the leaf early development and senescence periods were largely missing (cf. Fig. 4b, e and Fig. 6b, f). However, differently from NA plasticity, there was a certain reduction in AA plasticity in the middle of the growing season. Such a reduction might be indicative of a stronger photosynthetic reduction due to reduced stomatal conductance in the upper canopy where the evaporative demand is greater, especially under soil drought conditions (Aasamaa et al. 2004; Niinemets et al. 2004b). A greater degree of reduction of photosynthesis rate due to photoinhibition in the upper canopy (Niinemets and Kull 2001; Werner et al. 2001) can also explain such a decline.
As in evergreens, photosynthesis in some deciduous woody species can re-acclimate, although slowly, to modified light conditions by changes in the size and number of chloroplasts per leaf area (Oguchi et al. 2005; Oguchi et al. 2006). In particular in continuous-type species where light availability strongly decreased (Fig. 2b) and MA plasticity increased (Table 3) with increasing leaf age, AA plasticity is expected to be changed as well, but there are currently no data to test this hypothesis.
In grasses, the initial increase in AA plasticity during canopy development was followed by a reduction during grain filling period (Fig. 9c, f). This early increase suggests a somewhat longer developmental period for differentiation of photosynthetic apparatus, especially at higher light, than the developmental period for NA (cf. Fig. 6h). The reduction of AA plasticity later into the growing season is fully compatible with changes in NA plasticity as nitrogen is resorbed from upper canopy leaves during grain filling (cf. Fig. 6h).
Limitations and potentials of the current analysis and a conspectus for the future work
Current study summarizes the bulk of the information on age-dependent changes in within-canopy gradients in leaf traits. Rigorous normalization procedures (Eq. 1-2) were developed to remove the interfering variation due to differences in absolute trait values among species, plant life forms, treatments and growth conditions. In addition, different integrated light values were employed to test for the effects of growth and current leaf light environment on trait plasticity changes.
Despite the compiled set of data constitutes the most extensive database on temporal variations in within-canopy trait gradients, this analysis highlights several key limitations with the existing body of data. First, in most studies, the age range investigated did not cover the whole leaf and canopy development from leaf initiation until senescence. In particular, there is a lack of data for within-canopy gradients in early season in woody species and for young developing canopies in herbaceous species. Analogously, there is a lack of data for late-season gradients. While these periods have not been in the focus of researchers due to overall low leaf physiological activities during these periods, having data over the entire growing season is fundamentally important for conclusive understanding of canopy developmental processes. Furthermore, leaf growth and senescence periods comprise a significant period of leaf life span, and for accurate simulation of canopy photosynthesis over the entire growing period, these parts of leaf life cannot be left out. Fore example, as the result of limited age range, early developmental responses in plasticity of all traits remain largely unknown for herbaceous species.
The second limitations is that only a few studies have looked at a suite of relevant functional traits simultaneously. This complicates drawing conclusions about the structural, chemical and physiological coordination of canopy functioning through the leaf age sequence. Particularly limited were data on photosynthesis, which is the key trait characterizing foliage physiological activity, but clearly a trait that is more complicated to measure than MA and NA. Finally, uneven coverage of traits for different life forms, e.g. limited coverage of MA in herbs, and NA and AA for flush-type deciduous woody species, also complicates comparisons of age-dependent plasticity changes among plant life forms.
Lack of appropriate replication in several cases makes it difficult to distinguish among exceptional and characteristic responses, and thus, it limits the generality of some of the conclusions that can be drawn on the basis of these data. Exceptional, outlying responses, constitute an integral part of biological variability, and in the case of age effects on within-canopy plasticity of leaf traits, they might reflect multiple superimposed processes such as fruiting, and temporal variability in environmental and/or biological stresses. Future studies are needed to fill the outlined gaps in data coverage. Particular emphasis is needed on gaining a better insight into age-dependent variations in photosynthesis gradients through the canopies and on understanding the structural, chemical and physiological controls on development and re-acclimation of AA gradients and appropriate time-kinetics in different plant life forms. Thus, while trying to understand the controls on temporal variations in within-canopy gradients in photosynthesis, it is relevant to consider all key functional traits, MA, NA and AA simultaneously, and document the temporal variations in light environment above and within the canopy through the study period.
Conclusions and implications for modeling canopy photosynthesis
Major changes occur in leaf structure, chemistry and physiological activity during foliage development and aging, and as this study demonstrates, these age-dependent changes importantly modify the within-canopy variation in key foliage traits. The age effects differ among plant functional types and this diversity reflects fundamental differences in the rate of leaf turnover and in the duration of leaf developmental and senescence periods. In woody evergreens, plasticities in leaf dry mass (MA) and nitrogen content (NA) per area decrease with increasing leaf age, indicating limited re-acclimation capacity to modified light conditions, but there is a certain re-acclimation capacity in photosynthetic capacity per area (AA). In flush-type deciduous species, the plasticity changes mainly occur during early leaf development, while in continuous-type deciduous woody species and in herbaceous species, the plasticity changes occur through leaf life span. Significant differences, the increase in plasticity in continuous-type deciduous woody species and forbs and the reduction in grasses, are observed among species with fast leaf turnover as well, but the data coverage is not complete for all traits, limiting the generality of conclusions.
Currently, the age-dependent variation in foliage plasticity is largely ignored in simulations of canopy photosynthesis. In fact, in species with short-lived leaves, modeling studies currently typically use snapshots of canopy leaf trait gradients measured at a certain moment of time. In evergreens, studies either use current-year or one-year-old leaves, or some studies have pooled different leaf ages in developing NA vs. Qint and AA vs. Qint relationships (Medhurst and Beadle 2005; Warren and Adams 2001; Wright et al. 2006). Although more experimental work is needed to improve the coverage of periods of leaf development and senescence in several plant life forms, the data available indicated that the age-dependent plasticity changes were typically within ±50% of average plasticity for the given canopies, and greater changes, occasionally more than ±100% were observed for some traits in several plant life forms. I argue that these changes need to be included in quantitative simulations of canopy photosynthesis through the whole growing period with major improvements expected for evergreens and for early and late growing season for all other plant life forms.
Supplementary Material
For online publication only:
Supplementary information S1. Additional figures characterizing the age-dependent changes in relative trait plasticity (Fig. S1 and S2) and correlations among relative plasticity for nitrogen content with minimum canopy light availability (Fig. S3.)
Acknowledgements
The study has been prepared in frames of grants from the Estonian Ministry of Science and Education (institutional grant IUT-8-3), and the European Commission (European Research Council advanced grant 322603 SIP-VOL+).
References
- Aasamaa K, Sõber A, Hartung W, Niinemets Ü. Drought acclimation of two deciduous tree species of different layers in a temperate forest canopy. Trees. 2004;18:93–101. [Google Scholar]
- Abdul-Hamid H, Yusoff M-H, Ab-Shukor N-A, Zainal B, Musa M-H. Effects of different fertilizer application level on hrowth and physiology of Hibiscus cannabinus L. (kenaf) planted on BRIS soil. J Agr Sci. 2009;1:121–131. [Google Scholar]
- Alarcon VJ, Sassenrath GF. Modeling cotton (Gossypium spp.) leaves and canopy using computer aided geometric design (CAGD) Ecol Model. 2011;222:1951–1963. [Google Scholar]
- Anten NPR. Optimization and game theory in canopy models. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 355–377. [Google Scholar]
- Anten NPR, Bastiaans L. The use of canopy models to analyze light competition among plants. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 379–398. [Google Scholar]
- Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T. Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia. 1998;113:314–324. doi: 10.1007/s004420050382. [DOI] [PubMed] [Google Scholar]
- Araki M. The studies on the specific leaf area of forest trees.II. The effects of RLI, season and shading on the specific leaf area in young birch (Betula platyphylla Sukatchev var. Japonica (Miq.)) stand. J Jap For Soc. 1972;54:184–191. [Google Scholar]
- Araki M. The studies on the specific leaf area of forest trees. III. The effects of RLI, season and shading on the specific leaf area in young birch (Betula platyphylla Sukatchev var.japonica (Miq.)) stand (supplementary report) J Jap For Soc. 1973;55:227–233. [Google Scholar]
- Aranda I, Gil L, Pardos JA. Water relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe. Trees. 2000;14:344–352. [Google Scholar]
- Araus JL, Tapia L, Azcón-Bieto J, Caballero A. Photosynthesis, nitrogen levels, and dry matter accumulation in flag wheat leaves during grain filling. In: Marcelle R, Clijsters H, Van Poucke M, editors. Biological control of photosynthesis Proceedings of a conference held at the 'Limburgs Universitair centrum', Diepenbeek; Belgium. 26-30 August 1985; Dordrecht - Boston - Lancaster: Martinus Nijhoff Publishers; 1986. pp. 199–207. [Google Scholar]
- Archontoulis SV, Vos J, Yin X, Bastiaans L, Danalatos NG, Struik PC. Temporal dynamics of light and nitrogen vertical distributions in canopies of sunflower, kenaf and cynara. Field Crops Res. 2011;122:186–198. [Google Scholar]
- Bally ISE. Mangifera indica (mango) Permanent Agriculture Resources; Hōlualoa, Hawai‘i: 2006. [Google Scholar]
- Barnes PW, Beyschlag W, Ryel R, Flint SD, Caldwell MM. Plant competition for light analyzed with a multispecies canopy model. III. Influence of canopy structure in mixtures and monocultures of wheat and wild oat. Oecologia. 1990;82:560–566. doi: 10.1007/BF00319801. [DOI] [PubMed] [Google Scholar]
- Benbi DK. Prediction of leaf area indices and yields of wheat. J Agr Sci. 1994;122:13–20. [Google Scholar]
- Bertheloot J, Martre P, Andrieu B. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiol. 2008;148:1707–1720. doi: 10.1104/pp.108.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyschlag W, Barnes PW, Ryel R, Caldwell MM, Flint SD. Plant competition for light analyzed with a multispecies canopy model.II. Influence of photosynthetic characteristics on mixtures of wheat and wild oat. Oecologia. 1990;82:374–380. doi: 10.1007/BF00317486. [DOI] [PubMed] [Google Scholar]
- Blanch J-S, Llusià J, Niinemets Ü, Noe SM, Peñuelas J. Instantaneous and historical temperature effects on α-pinene emissions in Pinus halepensis and Quercus ilex. Journal of Environmental Biology. 2011;32:1–6. [PubMed] [Google Scholar]
- Bos HJ, Neuteboom JH. Morphological analysis of leaf and tiller number dynamics of wheat (Triticum aestivum L.): responses to temperature and light intensity. Ann Bot. 1998;81:131–139. [Google Scholar]
- Brooks JR, Hinckley TM, Sprugel DG. Acclimation responses of mature Abies amabilis sun foliage to shading. Oecologia. 1994;100:316–324. doi: 10.1007/BF00316960. [DOI] [PubMed] [Google Scholar]
- Brooks JR, Sprugel DG, Hinckley TM. The effects of light acclimation during and after foliage expansion on photosynthesis of Abies amabilis foliage within the canopy. Oecologia. 1996;107:21–32. doi: 10.1007/BF00582231. [DOI] [PubMed] [Google Scholar]
- Cescatti A, Zorer R. Structural acclimation and radiation regime of silver fir (Abies alba Mill.) shoots along a light gradient. Plant Cell Environ. 2003;26:429–442. [Google Scholar]
- Chabot BF, Jurik TW, Chabot JF. Influence of instantaneous and integrated light-flux density on leaf anatomy and photosynthesis. Amer J Bot. 1979;66:940–945. [Google Scholar]
- Chbouki S, Shipley B, Bamouh A. Path models for the abscission of reproductive structures in three contrasting cultivars of faba bean (Vicia faba) Can J Bot. 2005;83:264–271. [Google Scholar]
- Cline MG, Harrington CA. Apical dominanance and apical control in multiple flushing of temperate woody species. Can J For Res. 2007;37:74–83. [Google Scholar]
- Cordell S, Goldstein G, Meinzer FC, Vitousek PM. Morphological and physiological adjustment to N and P fertilization in nutrient-limited Metrosideros polymorpha canopy trees in Hawaii. Tree Physiol. 2001;21:43–50. doi: 10.1093/treephys/21.1.43. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME, Egli DB. Fruit removal in soybean induces the formation of an insoluble form of ribulose-1,5-bisphosphate carboxylase/oxygenase in leaf extracts. Planta. 1991;183:300–306. doi: 10.1007/BF00197802. [DOI] [PubMed] [Google Scholar]
- Davidson A, Da Silva D, Quintana B, DeJong TM. The phyllochron of Prunus persica shoots is relatively constant under controlled growth conditions but seasonally increases in the field in ways unrelated to patterns of temperature or radiation. Sci Hortic. 2015;184:106–113. [Google Scholar]
- DeJong TM, Doyle JF. Seasonal relationships between leaf nitrogen content (photosynthetic capacity) and leaf canopy light exposure in peach (Prunus persica) Plant Cell Environ. 1985;8:701–706. [Google Scholar]
- Del Pozo A, Dennett MD. Analysis of the distribution of light, leaf nitrogen, and photosynthesis within the canopy of Vicia faba L. at two contrasting plant densities. Australian Journal of Agricultural Research. 1999;50:183–189. [Google Scholar]
- Dickmann DI, Michael DA, Isebrands JG, Westin S. Effects of leaf display on light interception and apparent photosynthesis in two contrasting Populus cultivars during their second growing season. Tree Physiol. 1990;7:7–20. doi: 10.1093/treephys/7.1-2-3-4.7. [DOI] [PubMed] [Google Scholar]
- Dreccer MF, Van Oijen M, Schapendonk AHCM, Pot CS, Rabbinge R. Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy. Impact on canopy photosynthesis. Ann Bot. 2000;86:821–831. [Google Scholar]
- Drouet JL, Bonhomme R. Do variations in local leaf irradiance explain changes to leaf nitrogen within row maize canopies. Ann Bot. 1999;84:61–69. [Google Scholar]
- Eliásh P. Leaf diffusion resistance pattern in an oak-hornbeam forest. Biol Plant. 1979a;21:1–8. [Google Scholar]
- Eliásh P. Some ecophysiological features in leaves of plants in an oak-hornbeam forest. Folia Geobot Phytotax. 1979b;14:29–42. [Google Scholar]
- Escudero A, Mediavilla S. Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. The Journal of Ecology. 2003;91:880–889. [Google Scholar]
- Evans JR. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.) Plant Physiol. 1983;72:297–302. doi: 10.1104/pp.72.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Evers JB, Vos J, Yin X, Romero P, van der Putten PEL, Struik PC. Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation. J Exp Bot. 2010;61:2203–2216. doi: 10.1093/jxb/erq025. [DOI] [PubMed] [Google Scholar]
- Ewers FW. Secondary growth in needle leaves of Pinus longaeva (bristlecone pine) and other conifers: quantitative data. Amer J Bot. 1982;69:1552–1559. [Google Scholar]
- Field C. Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia. 1983;56:341–347. doi: 10.1007/BF00379710. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Cifre J, Escalona JM, Galmés J, Gulías J, Lefi EK, Martinez SF, Moreno MT, Ribas-Carbó M, Riera D, et al. Understanding down regulation of photoynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Ann appl Biol. 2004;144:273–283. [Google Scholar]
- Ford ED, Cocke A, Horton L, Fellner M, Van Volkenburgh E. Estimation, variation and importance of leaf curvature in Zea mays hybrids. Agric For Meteorol. 2008;148:1598–1610. [Google Scholar]
- Frantz JM, Joly RJ, Mitchell CA. Intracanopy lighting influences radiation capture, productivity, and leaf senescence in cowpea canopies. J Amer Soc Hort Sci. 2000;125:694–701. [Google Scholar]
- Gilmore DW, Seymour RS, Halteman WA, Greenwood MS. Canopy dynamics and the morphological development of Abies balsamea: effects of foliage age on specific leaf area and secondary vascular development. Tree Physiol. 1995;15:47–55. doi: 10.1093/treephys/15.1.47. [DOI] [PubMed] [Google Scholar]
- Gordon D, Rosati A, Damiano C, DeJong TM. Seasonal effects of light exposure, temperature, trunk growth and plant carbohydrate status on the initiation and growth of epicormic shoots in Prunus persica. J Horticult Sci Biotechnol. 2006;81:421–428. [Google Scholar]
- Goudriaan J. Light distribution. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 3–22. [Google Scholar]
- Gutschick VP, Wiegel FW. Optimizing the canopy photosynthetic rate by patterns of investment in specific leaf mass. Am Nat. 1988;132:67–86. [Google Scholar]
- Han Q, Kawasaki T, Nakano T, Chiba Y. Spatial and seasonal variability of temperature responses of biochemical photosynthesis parameters and leaf nitrogen content within a Pinus densiflora crown. Tree Physiol. 2004;25:737–744. doi: 10.1093/treephys/24.7.737. [DOI] [PubMed] [Google Scholar]
- Hanba YT, Miyazawa SI, Kogami H, Terashima I. Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Aust J Plant Physiol. 2001;28:1075–1084. [Google Scholar]
- Hansen U, Schneiderheinze J, Rank B. Is the lower shade tolerance of Scots pine, relative to pedunculate oak, related to the composition of photosynhetic pigments. Photosynthetica. 2002;40:369–374. [Google Scholar]
- Hansen U, Schneiderheinze J, Stadelmann S, Rank B. The α-tocopherol content of leaves of pedunculate oak (Quercus robur L.) - variation over the growing season and along the vertical light gradient in the canopy. J Plant Physiol. 2003;160:91–96. doi: 10.1078/0176-1617-00952. [DOI] [PubMed] [Google Scholar]
- Hernández LF. Leaf angle and light interception in sunflower (Helianthus annuus L.). Role of the petiole’s mechanical and anatomical properties. Φyton. 2010;79:109–115. [Google Scholar]
- Hikosaka K. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta. 1996;198:144–150. doi: 10.1007/BF00325881. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. A model of dynamics of leaves and nitrogen in a plant canopy: an integration of canopy photosynthesis, leaf life span, and nitrogen use efficiency. Am Nat. 2003;162:149–164. doi: 10.1086/376576. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann Bot. 2005;95:521–533. doi: 10.1093/aob/mci050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K. Optimal nitrogen distribution within a leaf canopy under direct and diffuse light. Plant, Cell & Environment. 2014;37:2077–2085. doi: 10.1111/pce.12291. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Kumagai T, Ito A. Modeling canopy photosynthesis. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 239–268. [Google Scholar]
- Hikosaka K, Okada K, Terashima I, Katoh S. Acclimation and senescence of leaves: their roles in canopy photosynthesis. In: Yamamoto HY, Smith CM, editors. Photosynthetic responses to the environment. American Society of Plant Physiologists; Madison: 1993. pp. 1–13. [Google Scholar]
- Hikosaka K, Sudoh S, Hirose T. Light acquisition and use by individuals competing in a dense stand of an annual herb, Xanthium canadense. Oecologia. 1999;118:388–396. doi: 10.1007/s004420050740. [DOI] [PubMed] [Google Scholar]
- Hirose T, Oikawa S. Mean residence time of leaf number, area, mass, and nitrogen in canopy photosynthesis. Oecologia. 2012;169:927–937. doi: 10.1007/s00442-012-2266-3. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Pons TL, van Rheenen JWA. Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia. 1988;77:145–150. doi: 10.1007/BF00379180. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, van Rheenen JWA. Canopy development and leaf nitrogen distribution in a stand of Carex acutiformis. Ecology. 1989;70:1610–1618. [Google Scholar]
- Hoshika Y, Watanabe M, Inada N, Mao Q, Koike T. Photosynthetic response of early and late leaves of white birch (Betula platyphylla var. japonica) grown under free-air ozone exposure. Environ Pollut. 2013;182:242–247. doi: 10.1016/j.envpol.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Hunt LA. Relationships between photosynthesis, transpiration and nitrogen in the flag and penultimate leaves of wheat. In: Day W, Atkin RK, editors. Proceedings of a NATO advanced research workshop on wheat growth and modelling, held April 9-12, 1984, in Bristol, United Kingdom. Plenum Press; New York: 1985. pp. 149–156. [Google Scholar]
- Huzulák J, Eliásh P. Within-crown pattern of ecophysiological features in leaves of Acer campestre and Carpinus betulus. Folia Geobot Phytotax. 1975;10:337–350. [Google Scholar]
- Kervella J, Pagès L, Génard M. Genotypic differences in the leaf emergence rate of young peach trees. J Amer Soc Hort Sci. 1995;120:278–282. [Google Scholar]
- Kikuzawa K. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct Ecol. 2003;17:29–38. [Google Scholar]
- Kitajima K, Mulkey SS, Wright J. Variation in crown light utilization characteristics among tropical canopy trees. Ann Bot. 2005;95:525–547. doi: 10.1093/aob/mci051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T. Autumn coloring, photosynthetic performance and leaf development of deciduous broad-leaved trees in relation to forest succession. Tree Physiol. 1990;7:21–32. doi: 10.1093/treephys/7.1-2-3-4.21. [DOI] [PubMed] [Google Scholar]
- Koike T, Sanada M, Lei TT, Kitao M, Lechowicz MJ. Senescence and the photosynthetic performance of individual leaves of deciduous broadleaved trees as related to forest dynamics. In: Murata N, editor. Research in photosynthesis Proceedings of the IXth International Congress on Photosynthesis, Nagoya, Japan, August 30-September 4, 1992. IV. Kluwer Academic Publishers; Dordrecht - Boston - London: 1992. pp. 703–706. [Google Scholar]
- Koppel AT, Frey JM. Izmentshivost hvoi yeli yevropeiskoi v zavisimosti ot radiatsionnogo rezhima vnutri krony. (Variability of Norway spruce needles related to radiation regime within the crown) Lesovedeniye. 1984;0(3):53–59. [Google Scholar]
- Kull O, Koppel A, Noormets A. Seasonal changes in leaf nitrogen pools in two Salix species. Tree Physiol. 1998;18:45–51. doi: 10.1093/treephys/18.1.45. [DOI] [PubMed] [Google Scholar]
- Le Roux X, Sinoquet H, Vandame M. Spatial distribution of leaf dry weight per area and leaf nitrogen concentration in relation to local radiation regime within an isolated tree crown. Tree Physiol. 1999;19:181–188. doi: 10.1093/treephys/19.3.181. [DOI] [PubMed] [Google Scholar]
- Lewandowska M, Jarvis PG. Changes in chlorophyll and carotenoid content, specific leaf area and dry weight fraction in Sitka spruce, in response to shading and season. New Phytol. 1977;79:247–256. [Google Scholar]
- Logan BA, Demmig-Adams B, Adams WW., III Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. upon a sudden increase in growth PPFD in the field. J Exp Bot. 1998;49:1881–1888. [Google Scholar]
- Medhurst JL, Beadle CL. Photosynthetic capacity and foliar nitrogen distribution in Eucalyptus nitens is altered by high-intensity thinning. Tree Physiol. 2005;25:981–991. doi: 10.1093/treephys/25.8.981. [DOI] [PubMed] [Google Scholar]
- Milroy SP, Bange MP, Sadras VO. Profiles of leaf nitrogen and light in reproductive canopies of cotton (Gossypium hirsutum) Ann Bot. 2001;87:325–333. [Google Scholar]
- Miyaji KI. Longevity and productivity of leaves of a cultivated annual, Glycine max Merrill. II. Productivity of leaves in relation to their longevity, plant density and sowing time. New Phytol. 1984;97:479–488. [Google Scholar]
- Miyazawa SI, Satomi S, Terashima I. Slow leaf development of evergreen broad-leaved tree species in Japanese warm temperate forests. Ann Bot. 1998;82:859–869. [Google Scholar]
- Miyazawa SI, Suzuki AA, Sone K, Terashima I. Relationships between light, leaf nitrogen and nitrogen remobilization in the crowns of mature evergreen Quercus glauca trees. Tree Physiol. 2004;24:1157–1164. doi: 10.1093/treephys/24.10.1157. [DOI] [PubMed] [Google Scholar]
- Miyazawa SI, Terashima I. Slow development of leaf photosynthesis in an evergreen broad-leaved tree, Castanopsis sieboldii: relationships between leaf anatomical characteristics and photosynthetic rate. Plant Cell Environ. 2001;24:279–291. [Google Scholar]
- Munger PH, Chandler JM, Cothren JT, Hons FM. Soybean (Glycine max) - velvetleaf (Abutilon theophrasti) interspecific competition. Weed Sci. 1987;35:647–653. [Google Scholar]
- Niinemets Ü. Acclimation to low irradiance in Picea abies: influences of past and present light climate on foliage structure and function. Tree Physiol. 1997a;17:723–732. doi: 10.1093/treephys/17.11.723. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Distribution patterns of foliar carbon and nitrogen as affected by tree dimensions and relative light conditions in the canopy of Picea abies. Trees. 1997b;11:144–154. [Google Scholar]
- Niinemets Ü. Energy requirement for foliage construction depends on tree size in young Picea abies trees. Trees. 1997c;11:420–431. [Google Scholar]
- Niinemets Ü. Cohort-specific tuning of foliage physiology to interacting stresses in evergreens. Tree Physiol. 2014;34:1301–1304. doi: 10.1093/treephys/tpu099. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Within-canopy variations in functional leaf traits: structural, chemical and ecological controls and diversity of responses. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 101–141. [Google Scholar]
- Niinemets Ü, Anten NPR. Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A, Nedbal L, Govindjee, editors. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Springer Verlag; Berlin: 2009. pp. 363–399. [Google Scholar]
- Niinemets Ü, Bilger W, Kull O, Tenhunen JD. Acclimation to high irradiance in temperate deciduous trees in the field: changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell Environ. 1998;21:1205–1218. [Google Scholar]
- Niinemets Ü, Cescatti A, Lukjanova A, Tobias M, Truus L. Modification of light-acclimation of Pinus sylvestris shoot architecture by site fertility. Agric For Meteorol. 2002a;111:121–140. [Google Scholar]
- Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T. Complex adjustments of photosynthetic capacity and internal mesophyll conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 2006a;29:1159–1178. doi: 10.1111/j.1365-3040.2006.01499.x. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Ellsworth DS, Lukjanova A, Tobias M. Site fertility and the morphological and photosynthetic acclimation of Pinus sylvestris needles to light. Tree Physiol. 2001;21:1231–1244. doi: 10.1093/treephys/21.17.1231. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Ellsworth DS, Lukjanova A, Tobias M. Dependence of needle architecture and chemical composition on canopy light availability in three North American Pinus species with contrasting needle length. Tree Physiol. 2002b;22:747–761. doi: 10.1093/treephys/22.11.747. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü, Keenan TF. Measures of light in studies on light-driven plant plasticity in artificial environments. Frontiers in Plant Science. 2012;3:156. doi: 10.3389/fpls.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Keenan TF, Hallik L. Tansley review. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015;205:973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kull O. Stoichiometry of foliar carbon constituents varies along light gradients in temperate woody canopies: implications for foliage morphological plasticity. Tree Physiol. 1998;18:467–479. doi: 10.1093/treephys/18.7.467. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Kull O. Sensitivity to photoinhibition of photosynthetic electron transport in a temperate deciduous forest canopy: Photosystem II centre openness, non-radiative energy dissipation and excess irradiance under field conditions. Tree Physiol. 2001;21:899–914. doi: 10.1093/treephys/21.12-13.899. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Kull O, Tenhunen JD. Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ. 2004a;27:293–313. [Google Scholar]
- Niinemets Ü, Lukjanova A. Needle longevity, shoot growth and branching frequency in relation to site fertility and within-canopy light conditions in Pinus sylvestris. Ann For Sci. 2003;60:195–208. [Google Scholar]
- Niinemets Ü, Sonninen E, Tobias M. Canopy gradients in leaf intercellular CO2 mole fractions revisited: interactions between leaf irradiance and water stress need consideration. Plant Cell Environ. 2004b;27:569–583. [Google Scholar]
- Niinemets Ü, Tobias M, Cescatti A, Sparrow AD. Size-dependent variation in shoot light-harvesting efficiency in shade-intolerant conifers. Int J Plant Sci. 2006b;167:19–32. [Google Scholar]
- Noormets A, Kull O, Koppel A. Nitrogen dynamics in Salix leaves during the first production year. In: Perttu K, Koppel A, editors. Short rotation willow coppice for renewable energy and improved environment Proceedings of a joint Swedish-Estonian seminar on energy forestry and vegetation filters; Tartu. 24-26 September 1995; Uppsala: Swedish University of Agricultural Sciences; 1996. pp. 51–59. [Google Scholar]
- Norman JM, Jarvis PG. Photosynthesis in Sitka spruce (Picea sitchensis (Bong.) Carr.). III. Measurements of canopy structure and interception of radiation. J Appl Ecol. 1974;11:375–398. [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T. Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 2005;28:916–927. [Google Scholar]
- Oguchi R, Hikosaka K, Hiura T, Hirose T. Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate forest. Oecologia. 2006;149:571–582. doi: 10.1007/s00442-006-0485-1. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Hikosaka K, Hirose T. Leaf lifespan and lifetime carbon balance of individual leaves in a stand of an annual herb, Xanthium canadense. New Phytol. 2006;172:104–116. doi: 10.1111/j.1469-8137.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Nishi Y, Watanabe A, Terashima I. Possible mechanisms of adaptive leaf senescence. Plant Biol. 2001;3:234–243. [Google Scholar]
- Padilla JM, Otegui ME. Co-ordination between leaf initiation and leaf appearance in field-grown maize (Zea mays): genotypic differences in response of rates to temperature. Ann Bot. 2005;96:997–1007. doi: 10.1093/aob/mci251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DT. Growth and water relations of cotton (Gossypium hirsutum), spurred anoda (Anoda cristata), and velvetleaf (Abutilon theophrasti) during simulated drought and recovery. Weed Sci. 1988;36:318–324. [Google Scholar]
- Pieters GA, van den Noort ME, van Nijkerken JA. Growth adaptation of leaves and internodes of poplar to irradiance, day length and temperature. Tree Physiol. 1999;19:933–942. doi: 10.1093/treephys/19.14.933. [DOI] [PubMed] [Google Scholar]
- Pons TL. Regulation of leaf traits in canopy gradients. In: Hikosaka K, Niinemets Ü, Anten NPR, editors. Canopy photosynthesis: from basics to applications. Springer Verlag; Berlin: 2016. pp. 143–168. [Google Scholar]
- Pons TL, Jordi W. Induction of leaf senescence and shade acclimation in leaf canopies - variation with leaf longevity. In: Lambers H, Poorter H, van Vuuren MMI, editors. Inherent variation in plant growth Physiological mechanisms and ecological consequences. Backhuys Publishers; Leiden: 1998. pp. 121–137. [Google Scholar]
- Pons TL, Pearcy RW. Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiol Plant. 1994;92:636–644. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Tansley review. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Portsmuth A, Niinemets Ü. Structural and physiological plasticity to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct Ecol. 2007;21:61–77. [Google Scholar]
- Powell GR, Vescio SA. Syllepsis in Larix laricina: occurrence and distribution of sylleptic long shoots and their relationships with age and vigour in young plantation-grown trees. Can J For Res. 1986;16:597–607. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. The R Foundation for Statistical Computing; Vienna: 2012. ISBN: 3-900051-07-0. [Google Scholar]
- Rapp M, Santa Regina I, Rico M, Gallego HA. Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forests. For Ecol Manage. 1999;119:39–49. [Google Scholar]
- Regnier EE, Harrison SK. Compensatory responses of common cocklebur (Xanthium strumarium) and velvetleaf (Abutilon theophrasti) to partial shading. Weed Sci. 1993;41:541–547. [Google Scholar]
- Reich PB, Kloeppel BD, Ellsworth DS, Walters MB. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia. 1995;104:24–30. doi: 10.1007/BF00365558. [DOI] [PubMed] [Google Scholar]
- Sadras VO, Echarte L, Andrade FH. Profiles of leaf senescence during reproductive growth of sunflower and maize. Ann Bot. 2000;85:187–195. [Google Scholar]
- Sadras VO, Hall AJ, Connor DJ. Light-associated nitrogen distribution profile in flowering canopies of sunflower (Helianthus annuus L.) altered during grain growth. Oecologia. 1993;95:488–494. doi: 10.1007/BF00317432. [DOI] [PubMed] [Google Scholar]
- Sato K, Kim JM. Relationships between environmental conditions and production and consumption activities of individual leaves in the population of rice plant in a paddy field. I. Changes in photosynthesis and dark respiration of individual leaves under field conditions. Jap J Crop Sci. 1980a;49:243–250. [Google Scholar]
- Sato K, Kim JM. Relationships between environmental conditions and production and consumption activities of individual leaves in the population of rice plant in a paddy field. IV. Leaf positional and seasonal changes in the rates of net photosynthesis and dark respiration in paddy fields of different plant spacing and fertilization. Jap J Crop Sci. 1980b;49:263–269. [Google Scholar]
- Sato K, Kim JM. Relationships between environmental conditions and production and consumption activities of individual leaves in the population of rice plant in a paddy field. V. Leaf positional and seasonal changes of some organic and mineral components of the individual leaf in the population of different plant spacing and fertilization. Jap J Crop Sci. 1980c;49:270–275. [Google Scholar]
- Scarascia-Mugnozza GE, Hinckley TM, Stettler RF, Heilman PE, Isebrands JG. Production physiology and morphology of Populus species and their hybrids grown under short rotation. III. Seasonal carbon allocation patterns from branches. Can J For Res. 1999;29:1419–1432. [Google Scholar]
- Schoettle AW, Smith WK. Interrelationships among light, photosynthesis and nitrogen in the crown of mature Pinus contorta ssp. latifolia. Tree Physiol. 1999;19:13–22. doi: 10.1093/treephys/19.1.13. [DOI] [PubMed] [Google Scholar]
- Sharma S, Rafiqul Hoque ATM, Analuddin K, Hagihara A. Phenology and litterfall production of mangrove Rhizophora stylosa Griff. in the subtropical region, Okinawa Island, Japan; Proceedings of International Conference on Environmental Aspects of Bangladesh (ICEAB10); Japan. Sept 2010; BenJapan, Thurgoona, New South Wales: ICEAB2010; 2010. pp. 87–90. [Google Scholar]
- Shiraiwa T, Sinclair TR. Distribution of nitrogen among leaves in soybean canopies. Crop Sci. 1993;33:804–808. [Google Scholar]
- Slot M, Wirth C, Schumacher J, Mohren GMJ, Shibistova O, Lloyd J, Ensminger I. Regeneration patterns in boreal Scots pine glades linked to cold-induced photoinhibition. Tree Physiol. 2005;25:1139–1150. doi: 10.1093/treephys/25.9.1139. [DOI] [PubMed] [Google Scholar]
- Suwa R, Hagihara A. Seasonal changes in canopy photosynthesis and foliage respiration in a Rhizophora stylosa stand at the northern limit of its natural distribution. Wetlands Ecology and Management. 2008;16:313–321. [Google Scholar]
- Tadaki Y. Studies on the production of structure of forest. XVII. Vertical change in specific leaf area in forest canopy. J Jap For Soc. 1970;52:263–268. [Google Scholar]
- Tadaki Y, Itatiya K, Tochiaki K, Miyauchi H, Matsuda U. Studies on the production of structure of forest. XVI. Primary productivity of Abies veitchii forests in subalpine zone of Mt. Fuji. Bull Gov For Exp Stat, Tokyo. 1970;229:1–22. [Google Scholar]
- Takeda T, Yajima M. An improvement of semiempirical method for estimating the total photosynthesis of the crop population. I. On light-photosynthesis curve of rice leaves. Proceedings of the Crop Science Society of Japan. 1975a;44:343–349. [Google Scholar]
- Takeda T, Yajima M. An improvement of semiempirical method for estimating the total photosynthesis of the crop population. II. On light attenuation and vertical distribution of Pmax in rice plant population. Proceedings of the Crop Science Society of Japan. 1975b;44:350–356. [Google Scholar]
- Terazawa K, Kikuzawa K. Effects of flooding on leaf dynamics and other seedling responses in flood-tolerant Alnus japonica and flood-intolerant Betula platyphylla var. japonica. Tree Physiol. 1994;14:251–261. doi: 10.1093/treephys/14.3.251. [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Volin JC, Oleksyn J, Reich PB. Interaction of ozone pollution and light effects on photosynthesis in a forest canopy experiment. Plant Cell Environ. 1995;18:895–905. [Google Scholar]
- Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro-Díez P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 2012;35:839–856. doi: 10.1111/j.1365-3040.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- Urban L, Le Roux X, Sinoquet H, Jaffuel S, Jannoyer M. A biochemical model of photosynthesis for mango leaves: evidence for the effect of fruit on photosynthetic capacity of nearby leaves. Tree Physiol. 2003;23:289–300. doi: 10.1093/treephys/23.5.289. [DOI] [PubMed] [Google Scholar]
- Urban L, Léchaudel M, Lu P. Effect of fruit load and girdling on leaf photosynthesis in Mangifera indica L. J Exp Bot. 2004;55:2075–2085. doi: 10.1093/jxb/erh220. [DOI] [PubMed] [Google Scholar]
- Valladares F. Light and the evolution of leaf morphology and physiology. Current Topics in Plant Biology. 2003;4:47–61. [Google Scholar]
- Valladares F, Gianoli E, Gómez JM. Ecological limits to plant phenotypic plasticity. New Phytol. 2007;176:749–763. doi: 10.1111/j.1469-8137.2007.02275.x. [DOI] [PubMed] [Google Scholar]
- Vapaavuori EM, Nurmi A, Vuorinen AH, Kangas T. Seasonal variation in the photosynthetic capacity of a willow (Salix cv. Aquatica gigantea) canopy. 2. Comparison of the structure and function of chloroplasts at different levels in the canopy. Tree Physiol. 1989;5:445–457. doi: 10.1093/treephys/5.4.445. [DOI] [PubMed] [Google Scholar]
- Vapaavuori EM, Vuorinen AH. Seasonal variation in the photosynthetic capacity of a willow (Salix cv. Aquatica gigantea) canopy. I. Changes in the activity and amount of ribulose 1,5-bisphosphate carboxylase-oxygenase and the content of nitrogen and chlorophyll at different levels in the canopy. Tree Physiol. 1989;5:423–444. doi: 10.1093/treephys/5.4.423. [DOI] [PubMed] [Google Scholar]
- Virdis A, Motzo R, Giunta F. Key phenological events in globe artichoke (Cynara cardunculus var. scolymus) development. Ann appl Biol. 2009;155:419–429. [Google Scholar]
- Vos J, van der Putten PEL. Effects of partial shading of the potato plant on photosynthesis of treated leaves, leaf area expansion and allocation of nitrogen and dry matter in component plant parts. Eur J Agron. 2001;14:209–220. [Google Scholar]
- Walcroft A, Le Roux X, Diaz-Espejo A, Dones N, Sinoquet H. Effects of crown development on leaf irradiance, leaf morphology and photosynthetic capacity in a peach tree. Tree Physiol. 2002;22:929–938. doi: 10.1093/treephys/22.13.929. [DOI] [PubMed] [Google Scholar]
- Warren CR, Adams MA. Distribution of N Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ. 2001;24:597–609. [Google Scholar]
- Weih M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiol. 2009;29:1479–1490. doi: 10.1093/treephys/tpp081. [DOI] [PubMed] [Google Scholar]
- Weikert RM, Wedler M, Lippert M, Schramel P, Lange OL. Photosynthetic performance, chloroplast pigments, and mineral content of various needle age classes of spruce (Picea abies) with and without the new flush: an experimental approach for analysing forest decline phenomena. Trees. 1989;3:161–172. [Google Scholar]
- Weinbaum SA, Muraoka TT, Plant RE. Intracanopy variation in nitrogen cycling through leaves is influenced by irradiance and proximity to developing fruit in mature walnut trees. Trees. 1994;9:6–11. [Google Scholar]
- Werner C, Ryel RJ, Correia O, Beyschlag W. Effects of photoinhibition on whole-plant carbon gain assessed with a photosynthesis model. Plant Cell Environ. 2001;24:27–40. [Google Scholar]
- Wright IJ, Leishman MR, Read C, Westoby M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Functional Plant Biology. 2006;33:406–419. doi: 10.1071/FP05319. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, et al. The world-wide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wu R, Hinckley TM. Phenotypic plasticity of sylleptic branching: Genetic design of tree architecture. CRC Crit Rev Plant Sci. 2001;20:467–485. [Google Scholar]
- Yamasaki M. In: Kamata N, Liebhold AM, Quiring DT, Clancy KM, editors. What causes spatio-temporal variations in leaf herbivory levels within a canopy of Fagus crenata; Proceedings: IUFRO Kanazawa 2003 International Symposium “Forest Insect Population Dynamics and Host Influences” Kanazawa University 21st-Century COE Program; Kanazawa: Kanazawa University; 2003. pp. 31–36. [Google Scholar]
- Yamasaki M, Kikuzawa K. Temporal and spatial variations in leaf herbivory within a canopy of Fagus crenata. Oecologia. 2003;137:226–232. doi: 10.1007/s00442-003-1337-x. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Koike N, Ishida A. Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ. 2002;25:1341–1356. [Google Scholar]
- Yasumura Y, Hikosaka K, Hirose T. Nitrogen resorption and protein degradation during leaf senescence in Chenopodium album grown in different light and nitrogen conditions. Functional Plant Biology. 2007;34:409–417. doi: 10.1071/FP06307. [DOI] [PubMed] [Google Scholar]
- Yin X-Y, Kropff MJ. The effect of temperature on leaf appearance in rice. Ann Bot. 1996;77:215–221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.