Abstract
Sphingolipids, including the two central bioactive lipids ceramide and sphingosine-1-phosphate (S1P), have opposing roles in regulating cancer cell death and survival, respectively, and there have been exciting developments in understanding how sphingolipid metabolism and signalling regulate these processes in response to anticancer therapy. Recent studies have provided mechanistic details of the roles of sphingolipids and their downstream targets in the regulation of tumour growth and response to chemotherapy, radiotherapy and/or immunotherapy using innovative molecular, genetic and pharmacological tools to target sphingolipid signalling nodes in cancer cells. For example, structure–function-based studies have provided innovative opportunities to develop mechanism-based anticancer therapeutic strategies to restore anti-proliferative ceramide signalling and/or inhibit pro-survival S1P–S1P receptor (S1PR) signalling. This Review summarizes how ceramide-induced cellular stress mediates cancer cell death through various mechanisms involving the induction of apoptosis, necroptosis and/or mitophagy. Moreover, the metabolism of ceramide for S1P biosynthesis, which is mediated by sphingosine kinase 1 and 2, and its role in influencing cancer cell growth, drug resistance and tumour metastasis through S1PR-dependent or receptor-independent signalling are highlighted. Finally, studies targeting enzymes involved in sphingolipid metabolism and/or signalling and their clinical implications for improving cancer therapeutics are also presented.
Sphingolipids are structural molecules of cell membranes with important roles in maintaining barrier function and fluidity1. Sphingolipids also regulate various biological processes such as growth, proliferation, migration, invasion and/or metastasis by controlling signalling functions within the cancer cell signal transduction network2,3. For example, the generation of ceramide and sphingosine is induced by chemotherapy, radiation and/or oxidative stress, and these sphingolipids mediate cell death, senescence and/or cell cycle arrest4. However, metabolic conversion of ceramide to sphingosine-1-phosphate (S1P), sphingomyelin or glucosylceramide has anti-apoptotic and pro-survival roles5,6. Over the past few decades, nearly all the major enzymes involved in sphingolipid metabolism have been identified and cloned, which has provided data that reveal that the abundance of sphingolipids is highly regulated by these metabolic enzymes, the altered expression or activity of which has key roles in the regulation of cancer signalling and/or treatment. These studies have led to clinical trials in patients with various types of tumours evaluating the potential of therapeutic strategies targeting the enzymes that catabolize ceramide or that generate S1P.
This Review discusses the recent mechanistic and clinical studies that have had a major influence in the understanding of the role of sphingolipids in various aspects of cancer biology and therapeutics. Specifically, mechanisms of ceramide-mediated cell death and S1P-dependent tumour growth, proliferation and metastasis are highlighted, with particular attention given to clinical cancer therapy, tumour immunology and immunotherapy. Finally, current and emerging therapeutic strategies targeting enzymes involved in sphingo-lipid metabolism and/or signalling for cancer therapy are presented.
Sphingolipid metabolism and cancer
Cellular stress induces the generation of sphingosine and/or ceramide (BOX 1; FIG. 1) through the activation of de novo synthesis pathways, sphingomyelin hydrolysis or the salvage pathway to mediate cancer cell death1,2. By contrast, many tumours exhibit increased ceramide metabolism mainly by increased activities of glucosyl-ceramide synthase (GCS), sphingomyelin synthase (SMS), ceramide kinase (CERK), acid ceramidase (AC) and/or sphingosine kinase (SPHK), which increases the generation of sphingolipids with pro-survival functions7,8. Multiple signalling nodes in sphingo-lipid metabolism, including sphingolipids, metabolic enzymes and/or receptors, represent novel therapeutic targets for the development of novel anticancer intervention strategies.
Box 1. Sphingolipidomics and cancer.
Ceramide is composed of a sphingosine long-chain base (LCB) containing 18 carbons (d18), which is amide-linked to a fatty acyl chain containing variable numbers of carbons, ranging from 14 to 26. The de novo synthesis of ceramide by (dihydro)ceramide synthases (CERS1–6) generates ceramide molecules with different fatty acyl chains that have distinct biological roles in cancer cells, possibly due to differential subcellular localization and/or trafficking in cells1. Moreover, recent data have suggested that in addition to the more abundant d18 LCB, ceramides that are generated endogenously via CERS1–6 can include an LCB with 16 or 20 carbons174, which might also have distinct biological roles in cancer. Thus, major bioactive sphingolipid molecules — including ceramides and other distinct sphingolipids, such as α-hydroxy-ceramides and 1-O-acyl-ceramides173 — can have distinct fatty acyl chain lengths (as well as distinct LCB sphingosine backbones174). The chemical structures of major sphingolipids are shown below.
The global measurement of these distinct sphingolipids and ceramides with different fatty acyl chain lengths or LCBs in cancer cells, tumour tissues or plasma is performed by quantitative sphingolipidomics using mass spectrometry-based assays171,172. The tissue localization of these distinct ceramides or sphingolipids can also be visualized using matrix-assisted laser desorption ionization (MALDI) imaging mass spectrometry153. Overall, quantitative measurements of sphingolipids by sphingolipidomics coupled with sub-tissue localization using MALDI imaging provide mechanistic and therapeutic insights into the roles of these bioactive molecules in cancer biology and therapy.
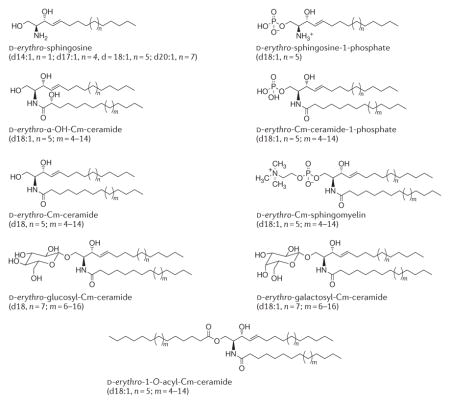
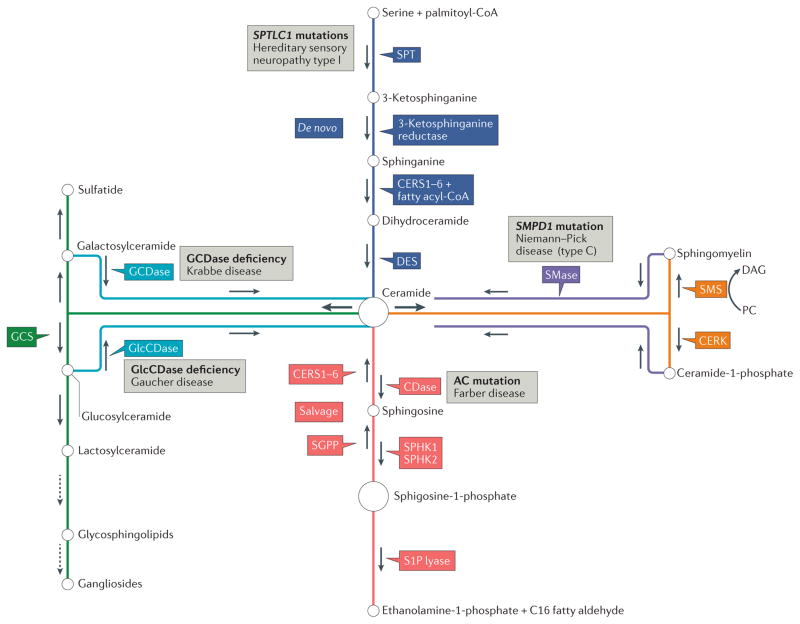
Figure 1. Pathways of sphingolipid metabolism and key enzymes.
Ceramide is the central molecule in sphingolipid metabolism and can be synthesized de novo (dark blue boxes) by the functions of serine palmitoyl-transferase (SPT), 3-ketosphinganine reductase, (dihydro)ceramide synthases (CERS1–6) and dihydroceramide desaturase (DES). Ceramide can also be generated by the hydrolysis of complex sphingolipids such as sphingomyelin (SM) by the action of sphingomyelinases (SMases) (purple box). Conversion of sphingosine to ceramide is catalysed by the salvage pathway (red boxes) via CERS1–6, the same enzymes involved in de novo ceramide synthesis. Ceramide is metabolized to generate sphingosine-1-phosphate (S1P) by the functions of ceramidases (CDases) and sphingosine kinase 1 (SPHK1) and SPHK2. S1P is further hydrolysed by S1P lyase to exit the sphingolipid metabolic cycle. The metabolism of ceramide to generate complex sphingolipids such as glycosphingolipids or gangliosides requires the synthesis of glucosylceramide (GlcCer) by glucosylceramide synthase (GCS) (green box), which is a precursor for the synthesis of complex sphingolipids (dotted arrows). Sphingomyelin synthase (SMS) enzymes convert ceramide to SM via insertion of a choline moiety into ceramide as a head group using phosphatidylcholine (PC) as a donor, yielding free diacylglycerol (DAG), and ceramide kinase (CERK) converts ceramide to ceramide-1-phosphate (C1P) (orange boxes). Ceramide is hydrolysed by CDases, including acid ceramidase (AC), to release free fatty acids and sphingosine. Ceramide can be synthesized by the hydrolysis of SM by SMases, including acid sphingomyelinase (ASMase; encoded by SMPD1). Mutations in enzymes involved in sphingolipid metabolism have been associated with various lysosomal storage disorders (grey boxes), including Farber, Gaucher, Niemann–Pick and Krabbe diseases. Patients with Gaucher disease have increased susceptibility to the development of cancer, such as myeloma25. Mutations in the genes encoding SPT subunits, such as SPTLC1, are associated with hereditary sensory neuropathy type I (REF. 27). GCDase, galactosylceramidase; GlcCDase, glucosylceramidase; SGPP, S1P phosphatase.
Sphingolipid structure and metabolism
Ceramide consists of a sphingosine long-chain base (LCB) and an amide-linked fatty acyl chain that varies from 14 to 26 carbons (C) in length1,2 (BOX 1). Endogenous ceramides are synthesized via the de novo pathway, which begins with the condensation of serine and palmitoyl-CoA by serine palmitoyltransferase (SPT) to form 3-ketosphinganine, which is reduced to sphin-ganine by 3-ketosphinganine reductase, leading to the synthesis of dihydroceramide by (dihydro)ceramide synthases9,10 — which have been referred to simply as ceramide synthases (CERS1–6)11, as they are rate-limiting enzymes for the synthesis of endogenous ceramides after dihydroceramide desaturation (FIG. 1). Dihydroceramide is desaturated to yield ceramide by dihydroceramide desaturase (DES; also known as DEGS1) via insertion of a double bond between C4 and C5 in the LCB (generating d18:1 LCB) 12.
CERS1–6 are specialized for ceramide synthesis with different fatty acyl chain lengths: CERS1 and CERS4 generate C18–20 ceramides, CERS5 and CERS6 generate C14–16 ceramides, and CERS2 selectively generates C22–24 ceramides12,13 (BOX 1). CERS3 mediates the synthesis of very-long-chain C28–32 ceramides with polyunsaturated (4–6 double bonds) fatty acid residues, which are specifically expressed in testes and skin tissues14. Ceramide is also generated by sphingomyelinases (SMases), which mediate sphingomyelin hydrolysis, or by the cerebrosidase enzymes glucosylceramidase (GlcCDase) and galactosylceramidase (GCDase) (FIG. 1), which catalyse glucosylceramide and/or galactosylceramide breakdown to ceramide1–4. The salvage pathway for ceramide generation utilizes recycling of sphingosine as a substrate for CERS1–6 (FIG. 1); the salvage pathway also involves the recycling of exogenous short-chain C2–6 ceramides for the generation of endogenous long-chain C14–26 ceramides, which exert various signalling functions in cancer cells, such as inhibition of Myc proto-oncogene protein (MYC) and telomerase15.
CERK16 and SMS17 convert ceramide to ceramide-1-phosphate (C1P) and sphingomyelin, respectively (FIG. 1). Ceramide is also utilized as a precursor for the generation of glucosylceramide by GCS18. Glucosylceramide is then transported from the early Golgi to the distal Golgi compartments by phosphatidylinositol-4-phosphate adaptor protein 2 (FAPP2; also known as PLEKHA8) for the synthesis of lactosylceramide and subsequently complex glycosphingolipids, which determine the lipid composition of the plasma membrane19. Non-vesicular C1P trafficking is regulated by glycolipid transfer protein domain-containing protein 1 (GLTPD1; also known as CPTP), which has been shown to induce prostaglandin signalling and inflammation in A549 human lung cancer cells20.
Ceramide is hydrolysed by ceramidases (CDases) to yield sphingosine, which is phosphorylated by SPHK1 (also known as SK1) or SPHK2 (also known as SK2) to generate S1P, which engages with five specific G protein-coupled receptors (GPCRs), S1PR1–5 (also known as S1P1–5), in an autocrine or paracrine manner to elicit pro-survival signalling in various cancer cells21. S1P is rapidly metabolized by S1P phosphatase (SGPP)22 to sphingosine or by S1P lyase 1 (SPL; also known as SGPL1)23 to yield ethanolamine-1-phosphate and hexadecanol (C16 fatty aldehyde) (FIG. 1).
The clinical relevance of sphingolipid metabolism has been established, as mutations in key metabolic enzymes are associated with lysosomal storage diseases such as Gaucher disease (glucocerebrosidase or GlcCDase deficiency), Farber disease (AC mutations), Krabbe disease (GCDase deficiency) and Niemann–Pick disease (mutations in SMPD1, which encodes acid sphingomyelinase (ASMase)), owing to aberrant accumulation of sphingolipids; thus, they are also referred to as sphin-golipidoses24 (FIG. 1). Moreover, some of these metabolic disorders are linked to cancer development; for example, patients with Gaucher disease have an increased risk of developing myeloma25.
Sphingolipid enzymes and cancer
The abundance of sphingolipid molecules is highly regulated by metabolic enzymes, the altered expression or activity of which has key roles in the induction of cancer cell death or survival (FIG. 1; TABLE 1).
Table 1.
Selected enzymes of sphingolipid metabolism and their roles in cancer
| Enzyme | Function or activity | Cancer relevance | Inhibitors | Refs |
|---|---|---|---|---|
| Ceramide synthesis and tumour suppression | ||||
| SPT | De novo ceramide synthesis | Increased activity in response to chemotherapy and radiotherapy in breast cancer cells | Myriocin | 26–28 |
| CERS1 | Synthesis of C18 (dihydro)ceramide | Induces mitophagy in head and neck and AML cell lines, mouse xenograft models and patient-derived AML cells | FB1; HDAC1 or HDAC2 | 31–33 |
| CERS6 | Synthesis of C16 (dihydro)ceramide | Induces caspase activation and cell death in lung cancer cells; preserves ER and Golgi integrity in head and neck cancer cells; elevated in breast tumour tissues; protects from GVHD in a mouse model of leukaemia | FB1 | 35–42, 142 |
| DES | Ceramide synthesis | Induces cell cycle arrest in neuroblastoma cells | Fenretinide; ABC294640; C8-CPC | 43 |
| ASMase | Ceramide generation | Induces apoptosis in lymphoblasts; promotes haematogenous tumour metastasis in mouse models | Tri-cyclic anti-depressants | 45–47 |
| NSMase | Ceramide generation | Mediates cell cycle arrest in breast cancer cells; exosome release | GW4869 | 44,48, 49 |
| SPL | S1P breakdown | Induces ceramide accumulation and colon cancer cell death | THI | 82,83 |
| Ceramide metabolism and pro-survival signalling | ||||
| CERT | Ceramide transport from ER to Golgi | Inhibits pro-apoptotic ceramide signalling in breast cancer cells and tumours in mouse models | CHC | 54–56 |
| CERK | C1P generation | Induces breast cancer cell survival in culture and mouse models | NVP-231 | 57–59 |
| GCS | GlcCer synthesis | Mediates drug resistance in patients with oral cancer and in breast cancer cells and xenografts | PPMP; PDMP | 60–64 |
| AC | Ceramide cleavage | Mediates resistance to cell death in prostate cancer cells and xenografts and elevated in tumours from patients | LCL-521 | 65–68 |
| SPHK1 | S1P generation | Mediates pro-survival signalling and metastasis in bladder cancer, lung cancer and melanoma cells in culture and in mouse models | PF543 | 75–78 |
| SPHK2 | Nuclear S1P generation | HDAC1 and HDAC2 inhibition in MCF-7 breast cancer cells; increases telomerase stability in lung cancer cells and xenografts | ABC294640 | 125,127 |
AC, acid ceramidase; AML, acute myeloid leukaemia; ASMase, acid sphingomyelinase; C1P, ceramide-1-phosphate; C8-CPC, C8-cyclopropenylceramide; CERK, ceramide kinase; CERS, ceramide synthase; CERT, ceramide transfer protein; CHC, 3-chloro-8β-hydroxycarapin-3,8-hemiacetal; DES, dihydroceramide desaturase; ER, endoplasmic reticulum; FB1, fumonisin B1; GCS, glucosylceramide synthase; GlcCer, glucosylceramide; GVHD, graft-versus-host disease; HDAC, histone deacetylase; NSMase, neutral sphingomyelinase; PDMP, 1-phenyl-2-decanoylamino-3-morpholino-1-propanol; PPMP, 1-phenyl-2-palmitoyl-3- morpholino-1-propanol; S1P, sphingosine-1-phosphate; SPHK, sphingosine kinase; SPL, S1P lyase 1; SPT, serine palmitoyltransferase; THI, tetrahydroxybutylimidazole.
SPT
Mammalian trimeric SPT consists of two large subunits (serine palmitoyltransferase 1 (SPTLC1), SPTLC2 or SPTLC3) and a small subunit (SPT small subunit A (SPTSSA) or SPTSSB) and is localized in the endoplasmic reticulum (ER)26. Specific missense mutations in the human SPTLC1 gene, which result in the accumulation of 1-deoxysphingoid bases owing to the utilization of L-alanine instead of L-serine as a substrate by the mutant SPT, are associated with hereditary sensory neuropathy type I (REF. 27). SPT-mediated elevation of cellular 1-deoxysphingoid base levels has been linked to paclitaxel-induced peripheral neuropathy in cell culture models as well as in serum samples from patients with breast cancer who received paclitaxel treatment28, which presents a major dose-limiting toxicity for paclitaxel treatment in patients with breast cancer. The metabolic switch from SPT-dependent generation of 1-deoxysphingoid bases to conventional sphingolipids, such as (dihydro)sphingosine and/or ceramide, by use of L-serine might be a potential therapeutic intervention to alleviate paclitaxel-induced neurotoxicity in various cancer settings27,28. However, this strategy requires testing in future clinical trials. In addition, the ORM proteins (encoded by ORMDL genes in humans, including ORMDL3) have been identified as key suppressors of SPT function that limit cellular sphingolipid levels in yeast and mammalian cells, including HEK293 and HeLa cells29,30. However, it remains unknown whether ORMDLs have roles in the regulation of cancer cell survival in the absence and/or presence of various cellular stress stimuli, such as chemotherapy.
CERS1–6
The discovery and cloning of CERS1–6 (REFS 9–11) were key to understanding the roles of ceramides with different fatty acyl chain lengths in cancer cell signalling. For example, CERS1 expression was found to be repressed in head and neck cancer cells by histone deacetylase 1 (HDAC1) and miR-574-5p, therefore inhibiting C18 ceramide generation31. In addition, CERS1-generated C18 ceramide induced cell death and tumour suppression in cell culture and xenograft mouse models of head and neck squamous cell carcinoma (HNSCC)32,33. Approximately 50% of mice with germline loss of Cers2, which is involved in reduction of long-chain (C22–24) ceramides, developed pheochromocytoma owing to possible defects in apoptosis34. Moreover, CERS6-generated C16 ceramide was identified as a transcriptional target of p53 that is activated by non-genotoxic folate stress to induce cell death in p53 wild-type human lung cancer cells35. CERS6-generated C16 ceramide was also shown to increase tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity by inducing nuclear translocation of caspase 3 in colon cancer cells36. Moreover, CERS6-generated C16 ceramide induced BAX-mediated apoptosis in response to irradiation in HeLa cells37. The mitochondria-associated anti-apoptotic protein Bcl-2-like protein 13 (BCL2L13) directly inhibited ceramide synthesis in glioblastoma cell lines and xenograft tumours in mice by binding to CERS2 and CERS6, therefore blocking their homodimerization and heterodimerization38. In addition to its apoptotic roles, increased levels of CERS6-generated C16 ceramide were detected in lung and oral cancer tissues compared with matched non-cancerous tissues39,40. Unexpectedly, CERS6-generated C16 ceramide was found to protect the integrity of the ER and Golgi membranes in HNSCC cells; downregulation of CERS6 altered trafficking of activating transcription factor 6 (ATF6) and induced its aberrant activation, resulting in ER-stress-mediated HNSCC cell death41,42.
Overall, these studies support the notion that CERS-generated ceramides with different fatty acyl chains have distinct roles in inducing cancer cell death and survival, which are dependent on the context and/or tissue or cell type.
DES
For many decades, exogenous dihydroceramide was considered an inactive sphingolipid molecule possibly owing to its poor solubility. However, knockdown of DES or pharmacological inhibition using fenretinide or C8-cyclopropenylceramide was reported to increase the accumulation of endogenous dihydroceramides, delaying the G1–S cell cycle transition in neuroblastoma cells43. These data suggest that endogenous dihydroceramide has signalling roles that inhibit cancer cell growth without inducing apoptosis. However, whether therapeutic targeting of DES inhibits apoptosis owing to a decrease in ceramide generation remains unknown.
SMases
Ceramide is also generated by the hydrolysis of sphingomyelin by SMases. Based on their pH-dependent optimal activity, there are three types of SMases: acid, neutral and alkaline44. Induction of ASMase44,45 expression increased ceramide generation, resulting in apoptotic cell death in human lymphoblasts, both in culture and in mice46. Interestingly, Smpd1−/− mice were protected from lung and spleen metastasis by melanoma cells, and transplantation of Smpd1 wild-type mouse platelets to Smpd1−/− mice reactivated tumour metastasis47. Mechanistically, melanoma cells exhibited increased platelet secretory ASMase activity, which induced C16 ceramide formation and led to activation and clustering of α5β1 integrin on melanoma cells, thereby promoting cancer cell adhesion and metastasis47. These data suggest that although induction of ASMase in cancer cells results in apoptosis, systemic ASMase activation in platelets leads to increased melanoma metastasis, highlighting the importance of cell-type-specific expression of enzymes involved in ceramide signalling in inducing cell death versus increasing survival and/or migration, similar to CERS6–C16 ceramide signalling in lung cancer (apoptotic)35 versus HNSCC (survival)41 cells.
In addition to the roles of neutral sphingomyelinase 2 (NSMase2; also known as SMPD3) in inducing ceramide-dependent cell cycle arrest48, it has been shown that exosome release from cells is dependent on NSMase2-generated ceramide, which was necessary for intra-endosomal membrane transport of multivesicular endosomes that are then secreted as exosomes49. However, whether NSMase2-dependent ceramide-enriched exosomes influence cancer cell cycle arrest remains to be determined.
In addition, alkaline sphingomyelinase (Alk-SMase; also known as NPP7) deficiency resulted in increased tumour size and number in mouse models of colon cancer induced by azoxymethane (AOM) and dextran sulfate sodium, possibly owing to reduced ceramide generation50.
Collectively, these data support the hypothesis that the hydrolysis of sphingomyelin by SMases generates ceramide, which mediates cancer cell death, growth arrest and/or tumour suppression.
SMS and CERT
The metabolic conversion of ceramide to sphingomyelin, which occurs mainly in the Golgi as well as in the plasma membrane, is catalysed by sphingomyelin synthase 1 (SMS1) localized in the Golgi and SMS2 localized in the Golgi and plasma membrane17,51. The synthesis of sphingomyelin is regulated by ceramide transport from the ER to the Golgi by ceramide transfer protein (CERT; also known as COL4A3BP) via a non-vesicular mechanism52,53. Loss of CERT expression was reported to augment epidermal growth factor receptor (EGFR) signalling in triple negative breast cancer (TNBC) cells through alterations in sphingomyelin levels at the plasma membrane54. Moreover, CERT knockdown sensitized ovarian cancer, colorectal cancer (CRC) and/or HER2-positive breast cancer cells to paclitaxel-induced cell death through induction of ceramide-mediated ER stress or lysosome-associated membrane glycoprotein 2 (LAMP2)-dependent auto-phagic flux55. Pharmacological inhibition of CERT using 3-chloro-8β-hydroxycarapin-3,8-hemiacetal (CHC) was also reported to effectively induce ceramide-dependent HeLa cell death56. Thus, these data suggest that inhibition of ceramide metabolism, at least via targeting ceramide trafficking by CERT, might provide novel strategies for inducing cancer cell death or attenuating drug resistance. However, it remains to be elucidated whether SMS inhibition has direct roles in inducing cell death or drug sensitivity in cancer cells or tumours.
CERK and GCS
CERK phosphorylates ceramide to generate C1P, which induces eicosanoid synthesis and cell migration via the activation of cytosolic phospholipase A2α (cPLA2α)57. Increased CERK–C1P signalling promoted breast cancer cell survival and mammary tumour recurrence following HER2 inhibition in mouse models58. Moreover, a CERK inhibitor, NVP-231, inhibited breast and lung cancer cell proliferation by inducing ceramide-mediated cell cycle arrest and apoptosis59.
Ceramide can also be utilized by GCS as a precursor for glucosylceramide generation. In fact, GCS expression was associated with poor prognosis in patients with oral cavity cancers60. Molecular or pharmacological inhibition of GCS attenuated resistance to chemotherapeutic drugs in head and neck cancer or hepatocellular carcinoma cells61,62. In addition, inhibition of GCS restored p53-dependent apoptosis, which was reported to be ceramide-dependent, in ovarian cancer cells expressing mutant p53 (REF. 63). GCS-dependent accumulation of glucosylceramide, the precursor for complex glycosphingolipid synthesis, was required for the maintenance of breast cancer stem cell (CD44+ESA+CD24−) pluripotency, which might be associated with accumulation of glycosphingolipids such as globotriosylceramide (Gb3), which are known markers of stem cells64. These findings suggest that both CERK and GCS are key therapeutic targets for inducing ceramide-mediated cancer cell death or reversal of resistance to apoptosis.
CDases
There are three classes of CDases — acid, neutral and alkaline. AC65 was found to be upregulated in various cancer types, notably prostate cancer66,67. Radiation-induced AC expression was reported to mediate resistance to radiation therapy, resulting in prostate tumour relapse in mouse models, consistent with the observation of increased AC expression levels in tumour samples from patients with prostate cancer who had relapsed after radiation therapy66. Furthermore, AC promoted chromosome region maintenance 1 protein homologue (CRM1; also known as EXP1)-dependent nuclear export of PTEN through S1P-mediated AKT signalling in prostate cancer cells67. Moreover, a positive feedback mechanism was reported between CERS6 and AC activation, whereby induction of CERS6 expression increased AC expression through activation of JUN N-terminal kinase (JNK) in colon cancer cells68. Thus, these studies indicate that AC mediates the metabolic communication between ceramide catabolism and S1P synthesis to activate pro-survival S1P signalling in cancer cells. However, it remains unknown whether AC-mediated CERS6–C16 ceramide signalling induces colon cancer cell survival by inhibiting ATF6 and inducing ER stress.
Neutral ceramidase69 (NCDase) utilizes ceramide for sphingosine release, which is then utilized for S1P biosynthesis by SPHK1 and/or SPHK2, resulting in the inhibition of cell death through reduced levels of pro-apoptotic ceramide. NCDase is highly abundant in intestinal tissues, and inhibition of NCDase resulted in ceramide-mediated apoptosis and autophagy in colon cancer cells and tumour xenograft mouse models70. Moreover, NCDase-null mice were protected from the development of AOM-induced colon cancer70.
There are three alkaline CDases (alkaline ceramidase 1 (ACER1), ACER2 and ACER3), which also hydrolyse ceramide to sphingosine. Although the role of ACER1 (REF. 71) in cancer is currently unknown, activation of ACER2 increased cancer cell death in response to doxorubicin-induced DNA damage owing to sphingosine accumulation and reactive oxygen species signalling, despite a reduction in ceramide generation72. ACER3 selectively catabolizes C18:1 ceramides and C20:1 ceramides, which contain unsaturated forms of C18 and C20 fatty acids such as oleic acid or paullinic acid, respectively, whereas conventional C18 ceramide and C20 ceramide contain saturated fatty acids such as stearic acid or arachidic acid, respectively. In mice, deficiency in ACER3 exacerbated the development of colitis and colitis-associated CRCs, and these Acer3−/− mice exhibited increased systemic inflammation and a hyper-active innate immune system owing to the accumulation of lactosylceramides and monohexosylceramides73.
These studies collectively suggest that, in addition to conventional ceramides, accumulation of complex sphingolipids (such as lactosylceramides and exosylceramides (BOX 1)) due to alterations in CDase expression and/or activity influences cancer cell death versus survival. Accordingly, it will be important to control for and monitor the accumulation of various classes of sphingolipids while targeting these metabolic enzymes for cancer therapy.
SPHK1 and SPHK2
SPHK1 and SPHK2 belong to the diacylglycerol kinase family and mediate the generation of S1P from ceramide (FIG. 1). SPHK1 is localized predominantly in the cytosol and is detected in mice at embryonic day 7 (E7) of development, whereas SPHK2 is localized mainly in the nuclear membrane and also in the cytoplasm and is expressed at E11 (REF. 74), indicating their distinct biological roles and utility as different downstream signalling targets in cancer despite their metabolic redundancy for S1P generation. For example, SPHK1 (REF. 75) was found to be overexpressed in various tumours, including colon cancers, and Sphk1−/− mice that were exposed to AOM developed fewer colon tumours than Sphk1 wild-type mice76. In a meta-analysis of clinical studies, increased expression of SPHK1 mRNA was indicative of poor prognosis and decreased survival in patients with various cancers77. Moreover, the long non-coding RNA Khps1, which is regulated by the transcription factor E2F1, was found to be transcribed in an antisense orientation to SPHK1 and induced SPHK1 expression via recruitment of histone acetyltransferase p300–CREB-binding protein (CBP) and E2F1 to the SPHK1 promoter78.
Similar to SPHK1, tumours from patients with non-small-cell lung cancer exhibited increased levels of SPHK2 compared with matched normal tissues, and high SPHK2 expression was associated with resistance to gefitinib and lower overall survival (OS) compared with patients who had low SPHK2 expression79. In vitro, CRC cell proliferation was induced by SPHK2 overexpression owing to increased MYC signalling80. Moreover, epidermal growth factor (EGF)-mediated phosphorylation of SPHK2 (at Ser351 and Thr578) by ERK1 stimulated the migration of breast cancer cells towards EGF81.
Thus, the findings of these studies suggest that both SPHK1 and SPHK2 have important roles in inducing cancer cell survival, supporting the importance of sub-cellular localization of S1P generation in determining the activation of specific oncogenic signalling pathways.
SPL and SGPPs
SPL activity provides an exit route from sphingolipid metabolism via the rapid hydrolysis of S1P. SPL was downregulated at the protein level in colon cancer tissues, and SPL silencing promoted colon carcinogenesis through signal transducer and activator of transcription 3 (STAT3)-mediated miR-181b-1 expression, which occurred via S1P accumulation and/or S1PR signalling82. By contrast, reconstitution of SPL by over-expression increased p53-dependent and p38-dependent apoptosis owing to reduced S1P signalling in colon cancer cells and xenograft tumours83. Inhibition of SGPP1, which increases S1P accumulation, promoted gastric cancer cell migration84. Moreover, SGPP1 overexpression, indicative of reduced S1P signalling, in patients with gastric cancer was associated with improved OS compared with patients with low SGPP1 expression84. Thus, these studies suggest that activation of SPL and/or SGPP1 (and possibly SGPP2) would provide a novel anticancer therapeutic strategy (TABLE 1).
Sphingolipids in cancer cell death
The synthesis and/or accumulation of ceramide in response to cellular stress is known to mediate cancer cell death through various mechanisms, including induction of apoptosis, necroptosis, autophagy and ER stress. These ceramide-mediated cell death pathways are differentially regulated based on cell and/or tissue type, the subcellular localization of ceramide and/or the availability of downstream targets of ceramide.
Apoptosis
It was initially demonstrated that ceramide induces apoptosis (BOX 2) in leukaemic cells85. The roles of ceramide biogenesis in radiation-induced apoptosis were later revealed, which involved ceramide-rich macro-domains on the mitochondrial membrane that are required for BAX integration in HeLa cells37,86 (FIG. 2). The involvement of ceramide channels in the induction of mitochondrial apoptosis through mitochondrial outer membrane permeabilization (MOMP) was reported as a possible mechanism, at least for radiation-induced apoptosis87. NSMase-dependent sphingolipid metabolism promoted MOMP via coordinated activation of BAX and BAK, suggesting that BCL-2 homology 3 (BH3)-only proteins and the specific sphingolipid milieu at the mitochondrial membrane regulate apoptosis88. Moreover, silencing of the gene encoding lysosomal-associated transmembrane protein 4B (LAPTM4B) — which facilitates removal of ceramide from late endosomal (LE) organelles — induced sphingolipid accumulation in LE organelles, destabilized the lysosomal membrane and resulted in resistance to caspase 3 activation and apoptosis induced by chemotherapeutic drugs in A431 human epidermoid carcinoma cells89 (FIG. 2). This study suggests that subcellular compartmentalization of ceramide by LAPTM4B regulates sphingolipid-mediated caspase 3 activation and apoptosis.
Box 2. Ceramide — a bona fide inducer of apoptosis.
In 1993, the induction of apoptosis by ceramide was first demonstrated in leukaemic cells by treatment with exogenous ceramide85. Since then, there have been myriad studies showing that endogenously generated ceramide is a bona fide inducer of apoptosis that is regulated by various mechanisms in a cell-type-dependent and/or context-dependent manner (as discussed in the main text)36,37,86,89. However, there are also studies that have demonstrated that induction of ceramide might protect some cancer cells from cell death. For example, ceramide synthase 6 (CERS6)-generated C16 ceramide was shown to be important in protecting head and neck squamous cell carcinoma (HNSCC) cells from endoplasmic reticulum (ER) stress-mediated apoptosis41,42. This observation was consistent with other studies, which reported that ceramide synthase HYL-2, which generates C20 ceramide and C22 ceramide, protected Caenorhabditis elegans from anoxia-induced death175. Moreover, studies in HeLa cells revealed that overexpression of CERS2, which increased the generation of C24 ceramide, protected cells from ionizing radiation (IR)-induced apoptosis, whereas C16 ceramide generated by CERS5 and CERS6 was responsible for IR-mediated apoptosis in these cells176,131. Overall, these studies suggest that although ceramide mediates apoptosis in the majority of cancer cells via mitochondrial membrane perturbation and/or influencing cell death signalling, it might also have protective roles against apoptosis depending on the presence and/or absence of downstream ceramide targets, the subcellular localization of ceramide and the types of stress stimuli. As mechanisms of ceramide-mediated apoptosis and signalling pathways are elucidated in greater detail, the question of how these differential roles of ceramide in inducing apoptosis versus protection from apoptosis in cancer cells are regulated will be addressed in future studies.
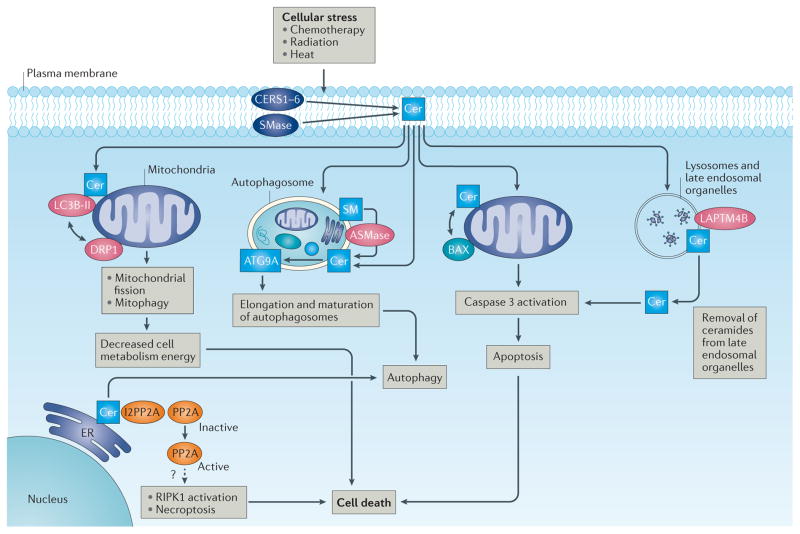
Figure 2. Intracellular ceramide signalling in cancer cell death and tumour suppression.
In response to cellular stress, the increased generation of ceramide (Cer) via (dihydro)ceramide synthases (CERS1–6) and sphingomyelinases (SMases) results in cancer cell death and tumour suppression, which is regulated by various mechanisms involving both direct and indirect targets of ceramide. Ceramide accumulation in the mitochondrial membrane induces BAX recruitment to the mitochondria, resulting in mitochondrial outer membrane permeabilization (MOMP), caspase activation and apoptosis in response to radiation86–88. In addition, regulation of ceramide transport from late endosomal organelles by lysosomal-associated transmembrane protein 4B (LAPTM4B) stabilizes lysosomal membranes, leading to ceramide-mediated caspase 3 activation and apoptosis induced by chemotherapeutic drugs89. Activation of serine/ threonine-protein phosphatase 2A (PP2A) is mediated by direct binding between phosphatase 2A inhibitor I2PP2A and ceramide or FTY720, a sphingosine analogue drug, leading to receptor-interacting serine/threonine-protein kinase 1 (RIPK1)-dependent necroptosis in lung cancer cells91. Cellular stress in response to chemotherapy results in C18 ceramide trafficking to the outer mitochondrial membrane, which then directly binds the lipidated form of microtubule-associated protein 1 light chain 3β (LC3B-II) to recruit autophagosomes to mediate lethal mitophagy by the activation of dynamin-related protein 1 (DRP1) and mitochondrial fission96,97. Autophagy in cancer cells has been shown to be induced by accumulation of dihydroceramide and/or ceramide in the endoplasmic reticulum (ER) of glioma cells in response to tetrahydrocannabinol, a psychotropic cannabinoid. Accumulation of dihydroceramide and/or ceramide in the ER leads to permeabilization of the autophagosomal and lysosomal membranes, resulting in cathepsin release and apoptotic cell death94. Moreover, hydrolysis of sphingomyelin (SM) to generate ceramide via acid sphingomyelinase (ASMase) is important to promote the elongation and maturation of autophagosomal membranes via recruitment of autophagy-related protein 9A (ATG9A), suggesting a novel role for ceramide in the maturation of early autophagosomal membranes95.
Necroptosis
Induction of ceramide signalling was shown to be involved in necroptosis in response to ceramide exposure, to CERS1 overexpression and consequent C18 ceramide generation (possibly in the ER) and to treatment with the sphingosine analogue drug FTY720, also known as fingolomod (Gilenya; Novartis) in human lung cancer cells90–92 (FIG. 2). Interaction of ceramide or FTY720, but not phosphorylated FTY720 (P-FTY720), with phosphatase 2A inhibitor I2PP2A (also known as SET) activated the tumour suppressor serine/threonine-protein phosphatase 2A (PP2A)90,91, inducing necroptosis and growth suppression in lung tumour xenograft models, which was mediated by receptor-interacting serine/threonine-protein kinase 1 (RIPK1) kinase activity91,92 (FIG. 2). The involvement of direct activation of RIPK1 by PP2A and/or ceramide generation in FTY720-dependent necroptosis, however, is unclear and should be investigated in future studies.
Autophagy
The accumulation of dihydroceramide and ceramide induces autophagy, resulting in context-dependent cancer cell death or survival93. Tetrahydrocannabinol, a psychotropic cannabinoid (an active constituent of cannabis), triggered dihydroceramide accumulation in the ER of glioma cells, which promoted permeabilization of the autophagosomal and lysosomal membranes, resulting in cathepsin release and apoptotic cell death (FIG. 2), possibly via its conversion to ceramide by DES94. Accumulation of sphingomyelin (by silencing of SMPD1) in cancer cells led to the accumulation of elongated and unclosed autophagosomal membranes with reduced association of autophagy-related protein 9A (ATG9A) (FIG. 2), suggesting a negative role for sphingomyelin in the maturation of early autophagosomal membranes through alterations in ATG9A trafficking95.
A pivotal study demonstrated that the induction of ceramide stress using chemotherapeutic agents or exogenous ceramides promoted accumulation of CERS1-generated C18 ceramide at the outer mitochondrial membrane, leading to the recruitment of auto-phagosomes to mitochondria via interaction of ceramide with LC3B-II (formed by lipidation of microtubule-associated protein 1 light chain 3β (LC3B))96. This then selectively induced mitophagy and cell death in head and neck cancer96 and acute myeloid leukaemia (AML) cells97 (FIG. 2). Nutrient deprivation, which induces survival autophagy, had no effect on ceramide-induced lethal autophagy or mitophagy in head and neck cancer cells96, suggesting that induction of selective mitophagy, but not general autophagy, mediates cancer cell death in head and neck cancer and AML cells both in culture and in mouse models. It was also shown that ceramide-dependent lethal mitophagy is regulated by the activation of dynamin-related protein 1 (DRP1) (FIG. 2) and mitochondrial fission, which involved protein kinase A (PKA) inhibition and decreased phosphorylation of DRP1 at Ser637 in Fms-like tyrosine kinase 3 (FLT3)-positive AML cells in culture and in AML blasts obtained from patients and grown in the bone marrow of immuno-compromised mice97. These findings therefore indicate that CERS1-generated ceramide acts as a receptor on the mitochondrial membrane for LC3B-containing auto-phagosomes to induce selective and lethal mitophagy in head and neck cancer and AML cells.
ER stress and cell cycle arrest
Induction of ceramide-associated ER stress was reported as an upstream regulator of autophagy in glioma cells98. Additionally, doxorubicin treatment elevated ceramide levels, which activated calpain-mediated ATG5 cleavage, switching from protective ER stress and autophagy to ceramide-mediated apoptosis in SGPP1-depleted cells99. Moreover, methotrexate-induced folate stress increased the formation of ER stress aggregates enriched with CERS6, which was associated with the activation of p53 in lung cancer cells100. Thus, ceramide signalling might be involved in the induction of ER stress, although CERS6-generated C16 ceramide seems to protect against this process, at least in oral cancers41,42.
Deletion of Sphk1 in Tp53-null mice attenuated formation of thymic lymphomas, which was accompanied by elevation of ceramide levels, increased expression of cell cycle inhibitors and tumour cell senescence101. Endogenous ceramides, which were generated via the salvage pathway in response to C6 ceramide exposure, interacted with phosphatidylinositol 3-kinase C2β (PI3KC2β) and suppressed its activation, resulting in the inhibition of ovarian cancer cell motility in response to EGF stimulation in culture and attenuation of peritoneal tumour metastasis of human ovarian cancer xenografts in mice102.
Together, these studies suggest that the distinct sub-cellular compartmentalization and/or trafficking of sphingolipids and their various downstream targets, especially ceramides with different fatty acyl chains, dictate their roles and mechanisms of action for induction of cancer cell death and/or growth inhibition.
S1P in cancer growth and metastasis
The metabolic conversion of ceramide to S1P by the functions of SPHK1 and SPHK2 mediate cancer growth and metastasis via S1PR-dependent or S1PR-independent signalling. Interestingly, signal transduction pathways mediated by S1P through selective S1PR1–5 signalling have distinct roles in the regulation of cancer cell proliferation, migration and/or invasion in a context-dependent manner. Moreover, downstream signalling targets of S1P generated by SPHK1 versus SPHK2 independent of S1PR involvement seem to be distinct.
Pro-survival S1P–S1PR signalling
The recruitment and activation of SPHK1 at the plasma membrane leads to S1P generation and the subsequent engagement of S1P with its specific receptors, S1PR1–5, therefore activating oncogenic sphingolipid signalling in an autocrine or paracrine manner103 (FIG. 3).
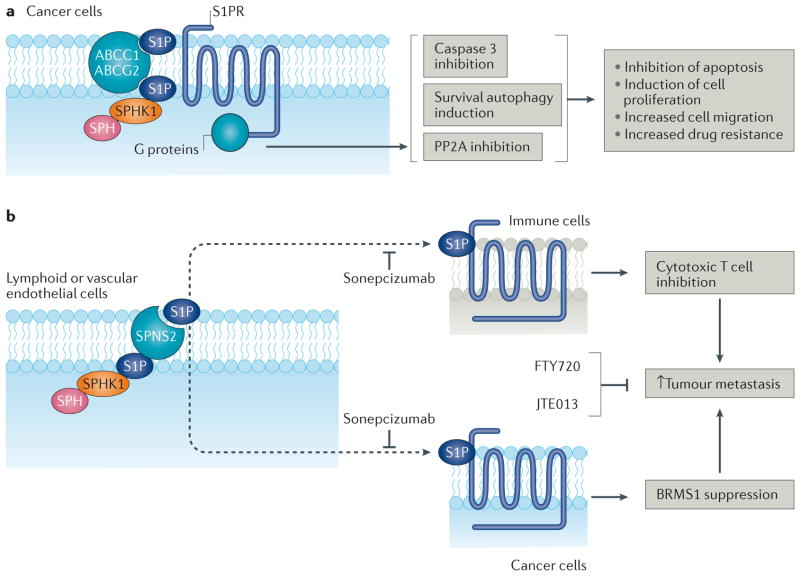
Figure 3. Oncogenic S1P–S1PR1-5 signalling.
a | Sphingosine kinase 1 (SPHK1)-generated sphingosine-1-phosphate (S1P) engages with G protein-coupled S1P receptors (S1PR1–5) to elicit oncogenic signalling103. S1P is secreted from cancer cells by ATP-binding cassette sub-family C member 1 (ABCC1), ATP-binding cassette sub-family G member 2 (ABCG2)104 or protein spinster homologue 2 (SPNS2)105, the latter of which is active selectively in endothelial cells, leading to autocrine or paracrine signalling. S1PR signalling inhibits apoptosis, induces cell proliferation and/or migration and increases drug resistance via inhibition of BAX–caspase 3 signalling, induction of survival autophagy and/or inhibition of serine/threonine-protein phosphatase 2A (PP2A)104,105,136. b | Circulating S1P increases tumour metastasis106–110. For example, SPNS2-dependent S1P secretion from endothelial cells attenuates cytotoxic T cell function, possibly by influencing S1PR functions on immune cells106 and/or cancer cells107, and therefore promotes tumour metastasis106,107. Moreover, secretion of SPHK1-generated S1P from host lymphoid or vascular endothelial cells activates S1PR2 signalling in tumour cells, which inhibits expression of breast cancer metastasis-suppressor 1 (BRMS1), a master suppressor of metastasis, leading to increased metastasis107. Mice deficient in SPNS2 or SPHK1 exhibited reduced lung colonization and metastasis regardless of tumour levels of S1P, suggesting a role for systemic S1P in the communication between host immune cells and cancer cells to increase tumour metastasis. Inhibition of systemic S1P (using sonepcizumab; also known as Sphingomab) or S1PR2 in cancer cells (using JTE013)107 and/or S1PR1 in cancer cells (using FTY720)109 should attenuate metastasis. SPH, sphingosine.
S1P
Oestradiol was reported to mediate export of S1P from breast cancer cells via ATP-binding cassette sub-family C member 1 (ABCC1) and ATP-binding cassette sub-family G member 2 (ABCG2), leading to S1PR signalling and cell growth and survival104 (FIG. 3). Protein spinster homologue 2 (SPNS2) selectively mediates S1P secretion in endothelial cells, but not in erythrocytes or platelets105 (FIG. 3). Interestingly, deletion of Spns2 resulted in a marked reduction in lung metastasis after tail vein injections of various types of mouse cancer cells and was associated with circulating lymphopaenia and increased infiltration of T cells and natural killer cells to the lungs, therefore enhancing tumour cell killing and decreasing metastatic burden in Spns2−/− mice106. These data were consistent with a study reporting the systemic roles of S1P as a transducer of host–cancer cell communication to induce metastasis of mouse bladder cancer or melanoma cells to the lungs of Sphk1+/+ control mice, which was reduced in Sphk1−/− mice107. The S1P-mediated host–cancer cell communication occurred by S1PR2-dependent repression of breast cancer metastasis-suppressor 1 (BRMS1), a master suppressor of tumour metastasis, regardless of the Sphk1 and/or Sphk2 status of tumours107 (FIG. 4). Moreover, attenuation of systemic S1P signalling in Sphk1−/− mice, or using the anti-S1P antibody sonepcizumab (Sphingomab; Lpath Therapeutics)108 in Sphk1 wild-type mice, reactivated BRMS1 and inhibited lung colonization and metastasis of bladder cancer and melanoma cancer allografts107 (FIG. 4). In addition, increased systemic S1P levels in Sphk2-knockout mice exacerbated chronic intestinal inflammation and led to the development of colitis-associated cancer (CAC) via S1PR1–nuclear factor-κB (NF-κB)–interleukin-6 (IL-6)–STAT3 signalling109. Inhibition of S1PR1 signalling using the pro-drug FTY720 attenuated CAC development in mice109 (FIG. 4). Moreover, osteoblast-derived systemic S1P also induced proliferation and docetaxel resistance in bone-metastasis-derived prostate cancer cells110. Thus, these data suggest a key role for systemic S1P signalling in regulation of tumour growth and/or metastasis, in part by inducing S1PR signalling in tumour cells.
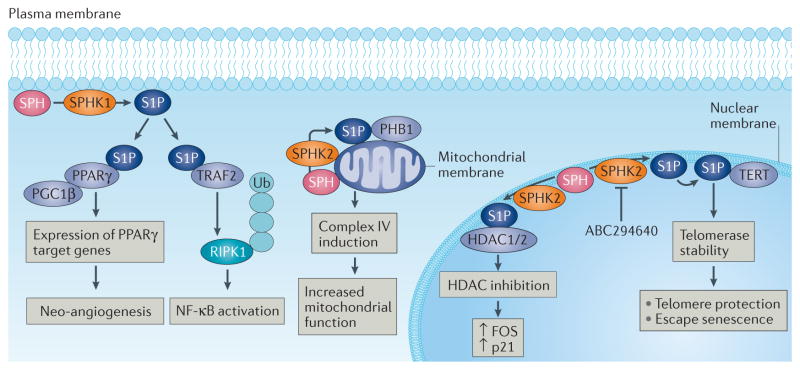
Figure 4. Receptor-independent intracellular S1P signalling.
Sphingosine kinase 1 (SPHK1)-generated sphingosine-1-phosphate (S1P) binds TNF receptor-associated factor 2 (TRAF2) to induce polyubiquitylation of receptor-interacting serine/threonine-protein kinase 1 (RIPK1), which then indirectly mediates nuclear factor-κB (NF-κB) activation121. S1P also directly associates with peroxisome proliferator-activated receptor-γ (PPARγ), which then mediates the recruitment of PPARγ co-activator 1β (PGC1β) to induce PPARγ-dependent gene expression and neo-angiogenesis124. Generation of S1P by SPHK2, which is localized in the nuclear membrane127, binds to histone deacetylase 1 (HDAC1) and HDAC2 and inhibits their activity at the nuclear membrane to induce epigenetic regulation of expression ofCDKN1A, which encodes p21, and FOS, which encodes proto-oncogene FOS125. SPHK2-generated S1P also binds prohibitin 2 (PHB2) on the mitochondrial membrane to induce cytochromec oxidase (complex IV) activity and mitochondrial respiration126. Generation of S1P in the nuclear envelope by SPHK2 results in S1P–telomerase reverse transcriptase (TERT) binding at the nuclear membrane, which then protects telomerase from E3 ubiquitin-protein ligase makorin 1 (MKRN1)-dependent ubiquitylation and degradation, therefore stabilizing telomerase and attenuating senescence induction127. This process is mediated by mimicking of TERT phosphorylation upon S1P–TERT binding, and SPHK2 knockdown, genetic loss or pharmacological inhibition using ABC294640 results in rapid telomerase degradation, leading to accelerated senescence and tumour suppression127. SPH, sphingosine; Ub, ubiquitin.
S1PR1–5
S1PR1 was identified as potential target to block STAT3 signalling in activated B cell-like diffuse large B cell lymphoma111. Furthermore, elevated S1PR1 signalling in T lymphoblastic lymphoma cells was associated with the blockade of intravasation and haematological dissemination owing to increased inter-cellular adhesion molecule 1 (ICAM1) expression and augmented cell–cell adhesion112.
In tumour cells, S1PR2 signalling induced AML growth and/or proliferation113 and was shown to activate ezrin–radixin–moesin (ERM) proteins to induce motility and invasion of HeLa cells in culture114, consistent with S1PR2-mediated tumour metastasis of bladder cancer and melanoma cells in mouse models107. By contrast, systemic S1PR2 signalling, mainly in endothelial cells and bone-marrow-derived cells (BMDCs), was reported to inhibit tumour angiogenesis in mouse models115. Thus, these data suggest that targeting S1PR2 selectively in cancer cells, at least in AML, melanoma and bladder or cervical cancers, but not in normal ECs and BMDCs, provides a potential anticancer therapeutic strategy.
S1PR3 signalling promoted the expansion of aldehyde dehydrogenase (ALDH)-positive cancer stem cells (CSCs) via ligand-independent Notch activation in response to stimulation with exogenous S1P116; CSCs overexpressing SPHK1 induced tumour growth in xenograft mouse models, which was mitigated by S1PR3 knockdown, and mammospheres derived from patients with breast cancer were found to contain both SPHK+ALDH1+ cells and S1PR3+ALDH1+ cells. However, it is unknown whether S1P–S1PR3 signalling promotes stem cell characteristics in cancer cells. Additionally, activation of S1PR3 by transforming growth factor-β (TGFβ)–SMAD3 signalling was shown to induce lung cancer progression and metastasis in a mouse model117.
In a clinical study, increased S1PR4 expression was associated with shorter disease-free survival in a cohort of 140 patients with oestrogen receptor (ER)-negative breast cancer118. A subsequent study demonstrated that SPHK2-generated S1P induces S1PR4 signalling, preventing the nuclear translocation of S1PR2 to promote the growth of ER-negative breast cancer cells119. However, the mechanism of how S1PR4 regulates nuclear translocation of S1PR2, and the nuclear signalling roles of S1PR2 in ER-negative breast cancer cell growth suppression, remain unknown. Nevertheless, this mechanism appears to be cell type and/or context-dependent, as there are multiple reports suggesting that S1PR2 signalling promotes tumour growth and/or metastasis in various other cancer types, such as AML, melanoma and bladder or cervical cancers107,113–115
A study from 2017 demonstrated that S1P–S1PR5 signalling promoted mitotic progression in HeLa cells, leading to chromosome segregation defects120. Mechanistically, S1P was secreted through SPNS2 and stimulated mitosis via S1PR5-dependent activation of the PI3K–AKT pathway, which required the activation of Polo-like kinase 1 (PLK1)120.
Overall, these data suggest that the selective targeting of S1PR1–5 signalling in various cancer types is efficacious to impede S1P-mediated cancer growth, proliferation and/or metastasis.
S1PR-independent S1P signalling
In addition to receptor-dependent signalling functions, endogenous S1P also regulates cancer cell signalling without S1PR involvement. For example, SPHK1-generated S1P directly binds to the ubiquitin ligase TNF receptor-associated factor 2 (TRAF2) in the cytoplasm, inducing Lys63-polyubiquitylation of RIPK1, phosphorylation of inhibitor of NF-κB kinase (IKK) and degradation of inhibitor of NF-κB (IκB), therefore activating NF-κB signalling in HEK293 and HeLa cells121. This, however, seems to be context-dependent, as SPHK1-generated S1P was not required for NF-κB activation and inflammation in macrophages122 or keratinocytes123. Moreover, SPHK1-generated S1P was reported as a ligand for peroxisome proliferator-activated receptor-γ (PPARγ) that regulates neo-angiogenesis in human endothelial cells, possibly by influencing transcription of PPARγ-responsive genes124, consistent with the roles of S1P in inducing tumour angiogenesis and/or metastasis (FIG. 4).
SPHK2-generated nuclear S1P bound directly to HDAC1 and HDAC2, inhibiting their enzymatic activity and therefore preventing deacetylation of histone H3 in human MCF7 breast cancer cells125. The S1P–HDAC1–HDAC2 repressor complex reportedly associated with the promoter regions of CDKN1A, which encodes p21, and FOS, which encodes proto-oncogene FOS, inducing their expression125 (FIG. 4). It remains unknown, however, whether SPHK2–S1P-mediated inhibition of HDAC1 and HDAC2 alters gene expression globally in cancer cells to promote or suppress tumour growth. In another study, SPHK2-generated S1P in mitochondria associated with prohibitin 2 (PHB2) to induce cytochrome c oxidase (complex IV) function and mitochondrial respiration126. In addition, SPHK2-derived S1P in the nuclear envelope directly interacted with telomerase reverse transcriptase (TERT), the catalytic subunit of telomerase, to prevent E3 ubiquitin-protein ligase makorin 1 (MKRN1)-dependent ubiquitylation and degradation of TERT, therefore inhibiting telomere damage and senescence127. Interestingly, S1P binding to TERT at the nuclear membrane mimicked TERT phosphorylation without protein kinase function, therefore regulating TERT stability at the nuclear envelope127. Inhibition of SPHK2 resulted in the rapid degradation of TERT, inducing severe telomere damage and rapid senescence, leading to cancer growth suppression127 (FIG. 4).
Thus, these data suggest that SPHK1-generated and SPHK2-generated S1P have distinct endogenous targets, regulating TRAF2 and PPARγ (SPHK1-generated S1P) versus HDAC1, HDAC2, PHB2 and telomerase (SPHK2-generated S1P) through sphingolipid–protein interactions independent of S1PR signalling.
Sphingolipids and cancer therapy
Cellular stress induced by chemotherapeutic agents and/or radiation is known to induce pro-cell death mechanisms and tumour suppression, at least in part through the induction of ceramide generation. Conversely, channelling ceramide for S1P generation results in resistance to chemotherapy and radiotherapy. Moreover, there have been new developments in understanding the roles of sphingolipids in the regulation of immune cell function in the context of cancer immunotherapy.
Chemotherapy and radiotherapy
Ceramide generated de novo was shown to mediate daunorubicin-induced apoptosis in human p388 leukaemia and U937 histiocytic lymphoma cells128. In a phase II clinical trial, elevated serum levels of C18 ceramide were markedly associated with improved response to gemcitabine plus doxorubicin combination therapy in patients with recurrent head and neck cancers129, a combination that induced CERS1-dependent C18 ceramide generation in HNSCC cells and xenograft tumour models in mice130. Ceramide biogenesis at the mitochondrial membrane by activation of CERS function was required for radiation-induced apoptosis in the germ line of Caenorhabditis elegans via activation of CEP-1 (the worm homologue of p53) and BH3-domain protein EGL-1–CED-3 caspase131. Interestingly, abrogation of ceramide signalling using an anti-ceramide neutralizing antibody prevented gastrointestinal damage in response to high doses of radiation, relieving gastrointestinal radiation syndrome associated mortality in mouse models132. However, it remains unknown whether an anti-ceramide antibody can also protect against endothelial cell apoptosis in the small intestinal lamina propria and crypt stem cell clonogens during anticancer radiotherapy. Thus, these data suggest that ceramide generation in cancer cells in response to chemotherapy and radiotherapy has an important role in tumour suppression. However, radiation-induced ceramide might also exert gastrointestinal toxicity through induction of intestinal stem cell death, which needs to be controlled to reduce general adverse effects of radiation therapy.
Drug resistance
The conversion of ceramide to glucosylceramide by GCS has been shown to mediate drug resistance in various cancers133. For example, doxorubicin treatment increased GCS expression in invasive ductal breast cancer cells through recruitment of transcription factor Sp1 (SP1) to the GCS (also known as UGCG) promoter134. Similarly, SPHK1 overexpression was reported at intrinsic or acquired resistance to cetuximab in CRC cell lines, xenograft mouse models and tumours obtained from patients135. Interestingly, S1PR1 inhibition using FTY720 sensitized resistant CRC cells and tumours to cetuximab135. In addition, overexpression of SPHK1 induced resistance to imatinib-mediated apoptosis in chronic myeloid leukaemia (CML) cells via inhibition of PP2A signalling through S1PR2 activation136. Moreover, miR-95-mediated silencing of SGPP1 promoted S1P-dependent resistance to radiation in mouse models of breast and prostate tumours137. Thus, increased sphingolipid metabolism towards glucosyl-ceramide and/or S1P generation results in resistance to anticancer therapy. Hence, while GCS and SPHK1/2 are potential therapeutic targets to overcome drug resistance, increased accumulation of their sphingolipid products — glucosylceramide and S1P, respectively — might be potential predictive biomarkers for chemotherapy resistance in various cancers.
Tumour immunology and immunotherapy
The roles of S1P–S1PR signalling in the induction of immune cell egress from lymphoid organs to the blood has been established138. Many studies suggest that sphingolipids also regulate the antitumour functions of various immune cell types. For example, ceramide inhibited the function of myeloid-derived suppressor cells (which normally inhibit cytotoxic T lymphocytes) by activation of lysosomal cathepsin B and cathepsin D, leading to attenuation of autophagy and induction of ER stress, therefore enhancing cytotoxic T lymphocyte function and suppression of CMS4-met-derived soft tissue sarcoma tumour growth in mouse models139. In patients with Gaucher disease, who are susceptible to developing myeloma25, clonal immunoglobulin was reactive against lyso-glucosylceramide (an acyl group derivative of glucosylceramide) owing to a deficiency in glucocerebrosidase/glucosylceramidase, which is highly elevated in these patients140. Interestingly, in Gaucher disease, complement signalling via complement C5a and C5a receptor 1 (C5aR1) induced the accumulation of glucosylceramide and tissue inflammation141. Moreover, CERS6-generated C16 ceramide increased T cell response to alloantigens during allogeneic haematopoietic stem cell transplantation (allo-HSCT) in a mouse model of leukaemia, an effective immunotherapy for various haematological malignancies142. Interestingly, data revealed that genetic loss of CERS6 prevented development of graft-versus-host disease in mouse models, a major toxic effect limiting the anti-leukaemic efficacy of allo-HSCT142. In addition, α-galactosylceramide — a marine-sponge-derived antitumour agent composed of ceramide conjugated to galactose via an α-linkage — was shown to activate natural killer cells143.
S1P–S1PR signalling has also been associated with the regulation of immune cell function in cancer models. STAT3-induced S1PR1 signalling provided a positive feedback loop for STAT3 activation in cancer cells and immune cells within the tumour micro-environment to promote malignant progression144. Furthermore, enterobacteria-secreted particles mediated the production of S1P-containing exosome-like structures by intestinal epithelial cells, which drove T helper 17 (TH17) cell-mediated development of colon cancer through activation of CC-chemokine ligand 20 (CCL20), prostaglandin E2 (PGE2) and MYD88 signalling145. Phosphoproteomic analysis of multiple sclerosis (MS) brain lesions revealed that phosphorylation of S1PR1 at Ser351 exacerbated TH17 cell-mediated auto-immune neuroinflammation146. However, it remains unknown whether S1PR1 phosphorylation controls immune cell function in cancer and/or tumour suppression. Interestingly, S1P secretion from dying cells constituted a ‘find-me signal’ for macrophage recruitment, promoting erythropoietin signalling in macrophages and enhancing phagocytosis of apoptotic cells and immune tolerance147. Collectively, these findings suggest that ceramide and S1P metabolism have key roles in the regulation of tumour immunology and that targeting S1P signalling might be a promising strategy to improve antitumour immunotherapy.
Therapeutic targeting of sphingolipids
FTY720, a sphingosine analogue drug derived from myriocin, has been successfully used to therapeutically target sphingolipid signalling for the treatment of patients with relapsing MS148,149 (TABLE 2). The pro-drug FTY720, which is phosphorylated by SPHK2 to generate P-FTY720, a structural analogue of S1P but functional antagonist for S1PR1, promoted tumour suppression via S1PR-dependent109 or receptor-independent91 mechanisms in colon and lung cancer cell lines and mouse models, respectively. FTY720, but not P-FTY720, inhibited CML stem cell proliferation and expansion in vitro, therefore reversing imatinib (Gleevec; Novartis) resistance150. Although FTY720 concentrations of 10–20 μM are required to effectively induce cancer cell death in cell culture studies, tumour suppression in human lung cancer xenograft mouse models was achieved using physiologically relevant concentrations of FTY720 (3 or 10 mg per kg daily) that suppressed the immune system via inhibition of S1PR1-dependent lymphocyte egress in mouse models, leading to induction of necroptosis in vivo91,150. Interestingly, lipidomics-based measurements showed that lung tumour tissues mainly accumulated the pro-drug FTY720 with anticancer function, whereas serum primarily contained the immune suppressive P-FTY720 after treatment with FTY720 in mice91. Thus, these data support that FTY720 might be considered for anticancer therapy in various cancer settings. As P-FTY720 does not seem to be involved in CML or lung cancer cell death, one future approach might be to use FTY720 in combination with an SPHK2 inhibitor (such as ABC294640 (REF. 151)), which should prevent P-FTY720 synthesis and immune suppression to improve FTY720-mediated anticancer therapy.
Table 2.
List of anticancer drugs targeting sphingolipid metabolism
| Name | Target or activity | Stage of development | Refs |
|---|---|---|---|
| Ceramide inducers and analogues | |||
| C8-CPC | DES | Preclinical | 43 |
| CHC | CERT; ceramide trafficking; sphingomyelin synthesis | Preclinical | 56 |
| NVP-231 | CERK | Preclinical | 59 |
| LCL521 and LCL204 | AC | Preclinical | 66–68 |
| Pyridinium ceramide (LCL-124 and LCL-461) | Cancer mitochondria and/or mitophagy | Preclinical | 152,153 |
| Nanoliposomal ceramide | Survivin | Preclinical | 154 |
| Inhibitors of S1P metabolism and signalling | |||
| FTY720 | S1PR1; I2PP2A | FDA-approved (for multiple sclerosis) | 91, 148–150 |
| JTE013 | S1PR2 | Preclinical | 107 |
| AB1 | S1PR2 | Preclinical | 161 |
| SK1-I | SPHK1 | Preclinical | 156 |
| PF543 | SPHK1 | Preclinical | 157–159 |
| VPC03090 | S1PR1; S1PR3 | Preclinical | 160 |
| Sphingomab (sonepcizumab) | S1P | Phase II | 162 |
| ABC294640 | SPHK2; DES | Phase Ib and II | 163–170 |
AC, acid ceramidase; C8-CPC, C8-cyclopropenylceramide; CERK, ceramide kinase; CERT, ceramide transfer protein; CHC, 3-chloro-8β-hydroxycarapin-3,8-hemiacetal; DES, dihydroceramide desaturase; FDA, US Food and Drug Administration; I2PP2A, phosphatase 2A inhibitor I2PP2A; S1P, sphingosine-1-phosphate; S1PR, S1P receptor; SPHK, sphingosine kinase.
In addition, highly soluble pyridinium ceramides (TABLE 2), which selectively accumulate in the mitochondria of cancer cells, induced lethal mitophagy and tumour suppression in head and neck and prostate cancer cell lines and xenograft tumours in mice152,153. Moreover, inhibition of survivin by nanoliposomal ceramide (NLC) resulted in the complete remission of the natural killer type of aggressive large granular lymphocytic leukaemia in cell lines and mouse models154 (TABLE 2). NLC is now being tested in a phase I clinical trial for the treatment of patients with advanced solid tumours155.
Targeting SPHK–S1P–S1PR signalling
The processes of S1P generation and signalling have also been promising targets for anticancer therapy. For example, SK1-I, a sphingosine analogue and competitive inhibitor of SPHK1, attenuated glioblastoma growth and/or proliferation in cell lines and xenograft models156. In addition, a potent SPHK1 selective inhibitor, PF-543 (REFS 157,158), was initially reported to lack cytotoxicity in cancer cells despite reducing S1P generation and accumulation157. However, recent studies have demonstrated the efficacy of PF-543 in inhibition of TNBC or CRC growth and/or proliferation in cell culture or in xenograft mouse models159. There are also S1PR1 and S1PR3 (VPC03090)160 or S1PR2 (AB1)161 antagonists under preclinical evaluation (TABLE 2), which might be tested as anticancer drugs in future clinical trials.
The anti-S1P antibody sonepcizumab108 has been evaluated in a phase II clinical trial for the treatment of patients with renal clear cell carcinoma (RCC) who were refractory to anti-VEGF therapy162. Forty patients with RCC who had received a median of three prior therapies were recruited to this multi-centre trial, and a median OS of 21.7 months was reported; however, the primary end point based on 2 month progression-free survival was not achieved162. Although a limited number of patients (10%) exhibited a partial response with a median response duration of 5.9 months with no major toxicity, the efficacy of sonepcizumab alone in metastatic RCC was not impressive. Interestingly, biomarker-focused studies showed an increase in systemic S1P concentrations with sonepcizumab treatment162, suggesting that metastatic S1P signalling was still active in patients given the therapy, which might be the reason for this limited efficacy of the drug in the clinic. Thus, future studies are needed to understand how systemic S1P induces tumour growth and metastasis to improve the neutralization of S1P signalling using lipid-specific antibodies in patients. In addition, testing the efficacy of sonepcizumab in combination with various SPHK1 and SPHK2 inhibitors or S1PR2 antagonist to treat patients with metastatic RCC or lung cancer is warranted.
The SPHK2 inhibitor ABC294640 suppressed the growth of lung or pancreatic tumours via different mechanisms, including inhibition of telomerase stability or suppression of MYC and ribonucleoside-diphosphate reductase subunit M2 (RRM2) expression127,163 (TABLE 2). It was also shown that ABC294640 has an off-target effect, mediating dihydroceramide accumulation owing to DES inhibition in prostate cancer cells and tumours164. Nevertheless, a phase I clinical trial evaluating ABC294640 (Yeliva; RedHill Biopharma) for the treatment of patients with solid tumours has been successfully completed, which reported a rapid and biphasic reduction in plasma levels of S1P165. Furthermore, ABC294640 is currently being tested in a phase II clinical trial for the treatment of patients with hepatocellular carcinoma who exhibit resistance to sorafenib166. Moreover, targeting of SPHK2 using ABC294640 for the treatment of patients with refractory multiple myeloma167,168, diffuse large B cell lymphoma169 or Kaposi sarcoma170 is under investigation in phase Ib and phase II clinical trials.
Overall, these studies demonstrate that induction of ceramide or inhibition of S1P metabolism and/or signalling are novel and innovative strategies for anticancer therapy in the clinic.
Conclusions
There have been myriad discoveries elucidating the roles and mechanisms of sphingolipids in cancer signalling during the past decade, which has required the development of analytical, molecular, structural, genetic and pharmacological tools. For example, the development of a highly quantitative analytical methodology using mass spectrometry171,172 (BOX 1) to measure sphingolipid molecules — including 1-O-acyl-ceramide173, d16 or d18 LCB-containing ceramides and conventional d18 LCB-containing ceramides and/or sphingolipids (sphingosine)174 — in cancer cells, tissues and/or blood was key to understanding the alterations of these signalling molecules and their metabolism in cancer versus non-cancerous cells and/or tissues. Moreover, the cloning of almost all of the key enzymes involved in sphingolipid metabolism helped to generate genetic models to study their roles in cancer biology and/or treatment, which are now coupled with structure–function-based anticancer drug design and development.
Mechanistically, it is well established that the sub-cellular localization and downstream targets of sphingolipids, especially ceramide and S1P, determine their distinct anticancer versus pro-oncogenic functions, which are dependent on context and cell type. For example, mitochondrial accumulation of ceramide, regardless of its fatty acyl composition, using pyridinium ceramide152,153 promoted cancer cell death by inducing mitophagy96,97. Moreover, nuclear membrane localization and roles of SPHK2-generated S1P–protein binding increased telomerase stability and function by allosterically mimicking protein phosphorylation127. These paradigms might also be important for the regulation of other S1P targets such as TRAF2 (REF. 121), PPARγ124, HDAC1 and HDAC2 (REF. 125) and/or PHB2 (REF. 126) to control senescence and induce cancer-associated inflammation and/or survival.
In addition, understanding the roles of sphingolipid signalling in the regulation of communication between tumours and host cells associated with the tumour microenvironment105–110, including stromal cells, endothelial cells, osteoclasts or platelets, will help to develop novel therapeutic strategies to inhibit cancer growth, proliferation and/or metastasis. Moreover, additional studies are needed to determine how sphingolipid signalling can be altered to increase the antitumour functions of T cells and decrease the suppressor functions of myeloid-derived suppressor cells and/or tumour-associated macrophages to improve the anticancer efficacy of immunotherapy.
However, the rapid metabolic interconversions, trafficking and signalling roles within biological (organelle) membranes of sphingolipids present biochemical and biophysical challenges for defining the roles of ceramides in the regulation of apoptosis versus survival175,176 (BOX 2). Future studies are needed to determine how enzymes of sphingolipid metabolism are inter-regulated, possibly by protein–protein and/or lipid–protein interactions. For this, additional molecular and analytical tools, including antibodies with higher selectivity and/or specificity against the enzymes of sphingolipid metabolism and/or bioactive sphingolipids, should be developed. Overall, an increased understanding of the mechanisms by which sphingolipids control cancer cell signalling and metabolism will help improve future anticancer therapy.
Acknowledgments
The author thanks N. Oleinik and C. Frichtel for their editorial assistance. The author is also thankful to Z. Szulc for his assistance with the chemical structures of sphingolipid molecules and the members of his laboratory for their helpful discussions. The author apologizes to those investigators whose publications were not mentioned in this Review owing to space limitations. This work is supported by research grants from the NIH (R01-DE16572, R01-CA88932, R01-CA173687 and P01-CA203628), and the South Carolina SmartState Endowment for Lipidomics and Drug Discovery.
Glossary
- Lactosylceramide
A type of ceramide (globoside) that is incorporated with lactose.
- Glycosphingolipids
A subtype of glycolipids that contain amino alcohol sphingosine, which include cerebrosides, gangliosides and globosides.
- Lysosomal storage diseases
A group of inherited metabolic disorders that result from defective lysosomal function and are mainly associated with accumulation of sphingolipids and/or glycosphingolipids.
- Pheochromocytoma
A rare tumour of the adrenal gland.
- Eicosanoid
A class of bioactive lipid derived from polyunsaturated fatty acids, which include prostaglandins, leukotrienes and thromboxanes.
- Hexosylceramides
Ceramide molecules that contain a hexosyl group, such as monohexosylceramide (glucosylceramide).
- Necroptosis
A programmed necrosis involving receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3 signalling that ruptures the plasma membrane, leading to cellular rupture and death.
- Mitochondrial outer membrane permeabilization (MOMP)
A key step in the execution of apoptosis, regulated by BCL-2 family member proteins, that leads to the release of pro-cell death factors, such as cytochrome c, from the internal mitochondrial membrane to engage with caspase signalling.
- Mitophagy
A form of autophagy that selectively degrades damaged mitochondria through the actions of double-membraned autophagosomes.
- Survival autophagy
A type of macroautophagy that mediates a vacuolar and self-digesting mechanism responsible for the removal of damaged proteins and/or organelles by double-membraned autophagosomes associated with lysosomes, providing nutrients for cell survival during stress conditions such as starvation.
- Mitochondrial fission
The partition of the mitochondrial membrane between two forming daughter mitochondria, which is regulated by a set of proteins including dynamin-related protein 1 (DRP1), parkin and PTEN-induced putative kinase 1 (PINK1).
- Lymphopaenia
A condition defined by the presence of abnormally low levels of lymphocytes (white blood cells or immune cells) in the blood.
- Gastrointestinal radiation syndrome
A syndrome caused by exposure to high doses of radiation that induces substantial cell death in the gastrointestinal tract.
- Allogeneic haematopoietic stem cell transplantation (Allo-HSCT)
A transplantation of multipotent haematopoietic stem cells derived from bone marrow, peripheral blood or umbilical cord blood from a genetically dissimilar donor for the treatment of patients with multiple myeloma or leukaemia.
- Graft-versus-host disease
A medical complication that might occur after allogeneic haematopoietic stem cell transplantation, in which transplanted immune cells from a donor (graft) recognize the recipient (host) tissues as foreign (non-self), attacking the host cells and resulting in tissue or organ damage.
Footnotes
Competing interests statement
The author declares no competing interests.
FURTHER INFORMATION
Lipidomics web: http://www.hollingscancercenter.org/research/shared-resources/lipidomics/index.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- 3.Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-α activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- 4.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 5.Cuvillier O, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJ, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 7.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 8.Morad SA, et al. Ceramide-antiestrogen nanoliposomal combinations — novel impact of hormonal therapy in hormone-insensitive breast cancer. Mol Cancer Ther. 2012;11:2352–2361. doi: 10.1158/1535-7163.MCT-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman K, et al. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro) ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. This work provides biochemical details of how CERS proteins function in de novo ceramide synthesis. [DOI] [PubMed] [Google Scholar]
- 10.Laviad EL, Kelly S, Merrill AH, Jr, Futerman AH. Modulation of ceramide synthase activity via dimerization. J Biol Chem. 2012;287:21025–21033. doi: 10.1074/jbc.M112.363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? : Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 12.Kraveka JM, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichur S, et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Jennemann R, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 15.Ogretmen B, et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. This study demonstrates the mechanisms by which exogenous ceramide is utilized for endogenous ceramide generation via the salvage or recycling pathway. [DOI] [PubMed] [Google Scholar]
- 16.Wijesinghe DS, Lamour NF, Gomez-Munoz A, Chalfant CE. Ceramide kinase and ceramide-1- phosphate. Methods Enzymol. 2007;434:265–292. doi: 10.1016/S0076-6879(07)34015-9. [DOI] [PubMed] [Google Scholar]
- 17.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watters RJ, et al. Targeting glucosylceramide synthase synergizes with C6-ceramide nanoliposomes to induce apoptosis in natural killer cell leukemia. Leuk Lymphoma. 2013;54:1288–1296. doi: 10.3109/10428194.2012.752485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Angelo G, et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature. 2013;501:116–120. doi: 10.1038/nature12423. This manuscript describes mechanisms of ceramide trafficking by FAPP2 for glucosylceramide generation. [DOI] [PubMed] [Google Scholar]
- 20.Simanshu DK, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500:463–467. doi: 10.1038/nature12332. This manuscript describes the mechanism of C1P trafficking to regulate inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang WC, et al. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB J. 2016;30:2945–2958. doi: 10.1096/fj.201600394R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamora-Pineda J, Kumar A, Suh JH, Zhang M, Saba JD. Dendritic cell sphingosine-1-phosphate lyase regulates thymic egress. J Exp Med. 2016;213:2773–2791. doi: 10.1084/jem.20160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandhoff K. Metabolic and cellular bases of sphingolipidoses. Biochem Soc Trans. 2013;41:562–568. doi: 10.1042/BST20130083. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbloom BE, et al. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105:4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 26.Han G, et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc Natl Acad Sci USA. 2009;106:8186–8191. doi: 10.1073/pnas.0811269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bode H, et al. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum Mol Genet. 2016;25:853–865. doi: 10.1093/hmg/ddv611. [DOI] [PubMed] [Google Scholar]
- 28.Kramer R, et al. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J. 2015;29:4461–4472. doi: 10.1096/fj.15-272567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslow DK, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siow DL, Wattenberg BW. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J Biol Chem. 2012;287:40198–40204. doi: 10.1074/jbc.C112.404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers-Needham M, et al. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol Med. 2012;4:78–92. doi: 10.1002/emmm.201100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koybasi S, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. This study describes a selective role for CERS1-generated C18 ceramide in head and neck cancer cell death. [DOI] [PubMed] [Google Scholar]
- 33.Thomas RJ, et al. HPV/E7 induces chemotherapy-mediated tumor suppression by ceramide-dependent mitophagy. EMBO Mol Med. 2017;9:1030–1051. doi: 10.15252/emmm.201607088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park WJ, et al. Development of pheochromocytoma in ceramide synthase 2 null mice. Endocr Relat Cancer. 2015;22:623–632. doi: 10.1530/ERC-15-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fekry B, et al. CerS6 is a novel transcriptional target of p53 protein activated by non-genotoxic stress. J Biol Chem. 2016;291:16586–16596. doi: 10.1074/jbc.M116.716902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White-Gilbertson S, et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen SA, et al. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc Natl Acad Sci USA. 2014;111:5682–5687. doi: 10.1073/pnas.1316700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J Clin Invest. 2016;126:254–265. doi: 10.1172/JCI79775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Senkal CE, et al. Alteration of ceramide synthase 6/ C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/ Golgi membrane network. J Biol Chem. 2011;286:42446–42458. doi: 10.1074/jbc.M111.287383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiffmann S, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- 43.Rahmaniyan M, et al. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286:24754–24764. doi: 10.1074/jbc.M111.250779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Airola MV, et al. Structure of human nSMase2 reveals an interdomain allosteric activation mechanism for ceramide generation. Proc Natl Acad Sci USA. 2017;114:E5549–E5558. doi: 10.1073/pnas.1705134114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorelik A, Illes K, Heinz LX, Superti-Furga G, Nagar B. Crystal structure of mammalian acid sphingomyelinase. Nat Commun. 2016;7:12196. doi: 10.1038/ncomms12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santana P, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 47.Carpinteiro A, et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol Med. 2015;7:714–734. doi: 10.15252/emmm.201404571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamseddine AA, et al. P53-dependent upregulation of neutral sphingomyelinase-2: role in doxorubicin-induced growth arrest. Cell Death Dis. 2015;6:e1947. doi: 10.1038/cddis.2015.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. This manuscript describes the mechanisms underlying ceramide-mediated exosome release. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, et al. Enhanced colonic tumorigenesis in alkaline sphingomyelinase (NPP7) knockout mice. Mol Cancer Ther. 2015;14:259–267. doi: 10.1158/1535-7163.MCT-14-0468-T. [DOI] [PubMed] [Google Scholar]
- 51.Barceló-Coblijn G, et al. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci USA. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanada K, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. This work provides the details of CERT-dependent ceramide transport from the ER to the Golgi. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heering J, et al. Loss of the ceramide transfer protein augments EGF receptor signaling in breast cancer. Cancer Res. 2012;72:2855–2866. doi: 10.1158/0008-5472.CAN-11-3069. [DOI] [PubMed] [Google Scholar]
- 55.Lee AJ, et al. CERT depletion predicts chemotherapy benefit and mediates cytotoxic and polyploid-specific cancer cell death through autophagy induction. J Pathol. 2012;226:482–494. doi: 10.1002/path.2998. [DOI] [PubMed] [Google Scholar]
- 56.Hullin-Matsuda F, et al. Limonoid compounds inhibit sphingomyelin biosynthesis by preventing CERT protein-dependent extraction of ceramides from the endoplasmic reticulum. J Biol Chem. 2012;287:24397–24411. doi: 10.1074/jbc.M112.344432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wijesinghe DS, et al. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J Lipid Res. 2014;55:1298–1309. doi: 10.1194/jlr.M048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne AW, Pant DK, Pan TC, Chodosh LA. Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res. 2014;74:6352–6363. doi: 10.1158/0008-5472.CAN-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastukhov O, et al. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br J Pharmacol. 2014;171:5829–5844. doi: 10.1111/bph.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JW, et al. Prognostic value of glucosylceramide synthase and P-glycoprotein expression in oral cavity cancer. Int J Clin Oncol. 2016;21:883–889. doi: 10.1007/s10147-016-0973-1. [DOI] [PubMed] [Google Scholar]
- 61.Roh JL, Kim EH, Park JY, Kim JW. Inhibition of glucosylceramide synthase sensitizes head and neck cancer to cisplatin. Mol Cancer Ther. 2015;14:1907–1915. doi: 10.1158/1535-7163.MCT-15-0171. [DOI] [PubMed] [Google Scholar]
- 62.Stefanovic M, et al. Targeting glucosylceramide synthase upregulation reverts sorafenib resistance in experimental hepatocellular carcinoma. Oncotarget. 2016;7:8253–8267. doi: 10.18632/oncotarget.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu YY, et al. Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Res. 2011;71:2276–2285. doi: 10.1158/0008-5472.CAN-10-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta V, et al. Ceramide glycosylation by glucosylceramide synthase selectively maintains the properties of breast cancer stem cells. J Biol Chem. 2012;287:37195–37205. doi: 10.1074/jbc.M112.396390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eliyahu E, Park JH, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21:1403–1409. doi: 10.1096/fj.06-7016com. [DOI] [PubMed] [Google Scholar]
- 66.Cheng JC, et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J Clin Invest. 2013;123:4344–4358. doi: 10.1172/JCI64791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beckham TH, et al. Acid ceramidase induces sphingosine kinase 1/S1P receptor 2-mediated activation of oncogenic Akt signaling. Oncogenesis. 2013;2:e49. doi: 10.1038/oncsis.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tirodkar TS, et al. Expression of ceramide synthase 6 transcriptionally activates acid ceramidase in a c-Jun N-terminal kinase (JNK)-dependent manner. J Biol Chem. 2015;290:13157–13167. doi: 10.1074/jbc.M114.631325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Airola MV, et al. Structural basis for ceramide recognition and hydrolysis by human neutral ceramidase. Structure. 2015;23:1482–1491. doi: 10.1016/j.str.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Barros M, et al. Role of neutral ceramidase in colon cancer. FASEB J. 2016;30:4159–4171. doi: 10.1096/fj.201600611R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liakath-Ali K, et al. Alkaline ceramidase 1 is essential for mammalian skin homeostasis and regulating whole-body energy expenditure. J Pathol. 2016;239:374–383. doi: 10.1002/path.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mao Z, et al. Alkaline ceramidase 2 (ACER2) and its product dihydrosphingosine mediate the cytotoxicity of N-(4-hydroxyphenyl)retinamide in tumor cells. J Biol Chem. 2010;285:29078–29090. doi: 10.1074/jbc.M110.105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K, et al. Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system. Cell Death Dis. 2016;7:e2124. doi: 10.1038/cddis.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest. 2014;124:4823–4828. doi: 10.1172/JCI77685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure. 2013;21:798–809. doi: 10.1016/j.str.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 76.Kawamori T, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y, et al. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e90362. doi: 10.1371/journal.pone.0090362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Postepska-Igielska A, et al. LncRNA Khps1 regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure. Mol Cell. 2015;60:626–636. doi: 10.1016/j.molcel.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q, et al. Prognostic significance of sphingosine kinase 2 expression in non-small cell lung cancer. Tumour Biol. 2014;35:363–368. doi: 10.1007/s13277-013-1051-1. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Liu X, Zuo Z, Hao C, Ma Y. Sphingosine kinase 2 promotes colorectal cancer cell proliferation and invasion by enhancing MYC expression. Tumour Biol. 2016;37:8455–8460. doi: 10.1007/s13277-015-4700-8. [DOI] [PubMed] [Google Scholar]
- 81.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 82.Degagne E, et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest. 2014;124:5368–5384. doi: 10.1172/JCI74188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oskouian B, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci USA. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao XY, et al. Inhibition of sphingosine-1-phosphate phosphatase 1 promotes cancer cells migration in gastric cancer: clinical implications. Oncol Rep. 2015;34:1977–1987. doi: 10.3892/or.2015.4162. [DOI] [PubMed] [Google Scholar]
- 85.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. This is a key manuscript demonstrating that ceramide induces apoptosis. [DOI] [PubMed] [Google Scholar]
- 86.Siskind LJ, et al. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang KT, et al. Ceramide channels: destabilization by Bcl-xL and role in apoptosis. Biochim Biophys Acta. 2015;1848:2374–2384. doi: 10.1016/j.bbamem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chipuk JE, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. This manuscript provides mechanisms for BAX and BAK regulation by sphingolipid metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blom T, et al. LAPTM4B facilitates late endosomal ceramide export to control cell death pathways. Nat Chem Biol. 2015;11:799–806. doi: 10.1038/nchembio.1889. [DOI] [PubMed] [Google Scholar]
- 90.Mukhopadhyay A, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saddoughi SA, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. This study shows that ceramide binds I2PP2A to activate PP2A-dependent necroptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saddoughi SA, Ogretmen B. Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res. 2013;117:37–58. doi: 10.1016/B978-0-12-394274-6.00002-9. [DOI] [PubMed] [Google Scholar]
- 93.Jiang W, Ogretmen B. Autophagy paradox and ceramide. Biochim Biophys Acta. 2014;1841:783–792. doi: 10.1016/j.bbalip.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernandez-Tiedra S, et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy. 2016;12:2213–2229. doi: 10.1080/15548627.2016.1213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corcelle-Termeau E, et al. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy. 2016;12:833–849. doi: 10.1080/15548627.2016.1159378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sentelle RD, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. This manuscript shows that ceramide directly binds LC3B-II to recruit autophagosomes to mitochondria for mitophagy induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dany M, et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood. 2016;128:1944–1958. doi: 10.1182/blood-2016-04-708750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salazar M, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lepine S, Allegood JC, Edmonds Y, Milstien S, Spiegel S. Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. J Biol Chem. 2011;286:44380–44390. doi: 10.1074/jbc.M111.257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fekry B, Esmaeilniakooshkghazi A, Krupenko SA, Krupenko NI. Ceramide synthase 6 is a novel target of methotrexate mediating its antiproliferative effect in a p53-dependent manner. PLoS ONE. 2016;11:e0146618. doi: 10.1371/journal.pone.0146618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heffernan-Stroud LA, et al. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene. 2012;31:1166–1175. doi: 10.1038/onc.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitatani K, et al. Ceramide limits phosphatidylinositol-3-kinase C2β-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene. 2016;35:2801–2812. doi: 10.1038/onc.2015.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pitson SM, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takabe K, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Weyden L, et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature. 2017;541:233–236. doi: 10.1038/nature20792. This manuscript describes a mechanism for S1P secretion via SPNS2 from lymphoid endothelial cells to regulate metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ponnusamy S, et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–775. doi: 10.1002/emmm.201200244. This manuscript describes a role for systemic S1P in inducing tumour metastasis by providing communication between host and cancer cells via S1PR2 signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Visentin B, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. This manuscript provides validation of an anti-S1P antibody that neutralizes S1P signalling to promote tumour suppression. [DOI] [PubMed] [Google Scholar]
- 109.Liang J, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. This manuscript describes a role for systemic S1P in inducing STAT3-dependent inflammation and colitis-associated colon cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brizuela L, et al. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol Oncol. 2014;8:1181–1195. doi: 10.1016/j.molonc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood. 2012;120:1458–1465. doi: 10.1182/blood-2011-12-399030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feng H, et al. T-Lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell. 2010;18:353–366. doi: 10.1016/j.ccr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Powell JA, et al. Targeting sphingosine kinase 1 induces MCL1-dependent cell death in acute myeloid leukemia. Blood. 2017;129:771–782. doi: 10.1182/blood-2016-06-720433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adada MM, et al. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015;29:4654–4669. doi: 10.1096/fj.15-274340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Du W, et al. S1P2, the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772–781. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 116.Hirata N, et al. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J, et al. TGF-β/SMAD3 pathway stimulates sphingosine-1 phosphate receptor 3 expression: implication of sphingosine-1 phosphate receptor 3 in lung adenocarcinoma progression. J Biol Chem. 2016;291:27343–27353. doi: 10.1074/jbc.M116.740084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohotski J, et al. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2012;106:1453–1459. doi: 10.1038/bjc.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohotski J, Rosen H, Bittman R, Pyne S, Pyne NJ. Sphingosine kinase 2 prevents the nuclear translocation of sphingosine 1-phosphate receptor-2 and tyrosine 416 phosphorylated c-Src and increases estrogen receptor negative MDA-MB-231 breast cancer cell growth: the role of sphingosine 1-phosphate receptor-4. Cell Signal. 2014;26:1040–1047. doi: 10.1016/j.cellsig.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 120.Andrieu G, et al. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci Signal. 2017;10:eaah4007. doi: 10.1126/scisignal.aah4007. [DOI] [PubMed] [Google Scholar]
- 121.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. This manuscript describes SPHK1-generated S1P signalling in TRAF2 binding and NF-κB activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiong Y, et al. Sphingosine kinases are not required for inflammatory responses in macrophages. J Biol Chem. 2013;288:32563–32573. doi: 10.1074/jbc.M113.483750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Etemadi N, et al. TRAF2 regulates TNF and NF-κB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. Elife. 2015;4:e10592. doi: 10.7554/eLife.10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parham KA, et al. Sphingosine 1-phosphate is a ligand for peroxisome proliferator-activated receptor-γ that regulates neoangiogenesis. FASEB J. 2015;29:3638–3653. doi: 10.1096/fj.14-261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. This work describes a role for SPHK2-generated S1P in binding HDAC1 and HDAC2 and inhibiting histone deacetylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strub GM, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Panneer Selvam S, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal. 2015;8:ra58. doi: 10.1126/scisignal.aaa4998. This manuscript describes a mechanism for SPHK2-generated S1P to directly bind TERT to stabilize telomerase and prevent telomere damage via the phosphomimic function of S1P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bose R, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 129.Saddoughi SA, et al. Results of a phase II trial of gemcitabine plus doxorubicin in patients with recurrent head and neck cancers: serum C18-ceramide as a novel biomarker for monitoring response. Clin Cancer Res. 2011;17:6097–6105. doi: 10.1158/1078-0432.CCR-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Senkal CE, et al. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 131.Deng X, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. This work describes mechanistic details of how ceramide mediates cell death in response to radiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rotolo J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122:1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu YY, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X, et al. Doxorubicin influences the expression of glucosylceramide synthase in invasive ductal breast cancer. PLoS ONE. 2012;7:e48492. doi: 10.1371/journal.pone.0048492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosa R, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- 136.Salas A, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang X, et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013;73:6972–6986. doi: 10.1158/0008-5472.CAN-13-1657. [DOI] [PubMed] [Google Scholar]
- 138.Fang V, et al. Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-γ response. Nat Immunol. 2017;18:15–25. doi: 10.1038/ni.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu F, et al. Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget. 2016;7:83907–83925. doi: 10.18632/oncotarget.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nair S, et al. Clonal immunoglobulin against lysolipids in the origin of myeloma. N Engl J Med. 2016;374:555–561. doi: 10.1056/NEJMoa1508808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pandey MK, et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543:108–112. doi: 10.1038/nature21368. [DOI] [PubMed] [Google Scholar]
- 142.Sofi MH, et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017;2:e91701. doi: 10.1172/jci.insight.91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Le Nours J, et al. Atypical natural killer T-cell receptor recognition of CD1d-lipid antigens. Nat Commun. 2016;7:10570. doi: 10.1038/ncomms10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee H, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deng Z, et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat Commun. 2015;6:6956. doi: 10.1038/ncomms7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garris CS, et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol. 2013;14:1166–1172. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo B, et al. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity. 2016;44:287–302. doi: 10.1016/j.immuni.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 149.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 150.Neviani P, et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J Clin Invest. 2013;123:4144–4157. doi: 10.1172/JCI68951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11:524–534. doi: 10.4161/cbt.11.5.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Senkal CE, et al. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- 153.Beckham TH, et al. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J Pharmacol Exp Ther. 2013;344:167–178. doi: 10.1124/jpet.112.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu X, et al. Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116:4192–4201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02834611.
- 156.Kapitonov D, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schnute ME, et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012;444:79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- 158.Wang J, Knapp S, Pyne NJ, Pyne S, Elkins JM. Crystal structure of sphingosine kinase 1 with PF-543. ACS Med Chem Lett. 2014;5:1329–1333. doi: 10.1021/ml5004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ju T, Gao D, Fang ZY. Targeting colorectal cancer cells by a novel sphingosine kinase 1 inhibitor PF-543. Biochem Biophys Res Commun. 2016;470:728–734. doi: 10.1016/j.bbrc.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 160.Kennedy PC, et al. Characterization of a sphingosine 1-phosphate receptor antagonist prodrug. J Pharmacol Exp Ther. 2011;338:879–889. doi: 10.1124/jpet.111.181552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li MH, et al. Antitumor activity of a novel sphingosine-1-phosphate 2 antagonist, AB1, in neuroblastoma. J Pharmacol Exp Ther. 2015;354:261–268. doi: 10.1124/jpet.115.224519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pal SK, et al. A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer. 2017;123:576–582. doi: 10.1002/cncr.30393. [DOI] [PubMed] [Google Scholar]
- 163.Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget. 2016;7:60181–60192. doi: 10.18632/oncotarget.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Venant H, et al. The sphingosine kinase 2 inhibitor ABC294640 reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Mol Cancer Ther. 2015;14:2744–2752. doi: 10.1158/1535-7163.MCT-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Britten CD, et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:4642–4650. doi: 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02939807.
- 167.Venkata JK, et al. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood. 2014;124:1915–1925. doi: 10.1182/blood-2014-03-559385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02757326.
- 169.Qin Z, et al. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther. 2014;13:154–164. doi: 10.1158/1535-7163.MCT-13-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.US National Library of Medicine. ClinicalTrials.gov. 2014 https://clinicaltrials.gov/ct2/show/NCT02229981.
- 171.Bielawski J, et al. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2009;579:443–467. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- 172.Shaner RL, et al. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50:1692–1707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Senkal CE, et al. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017;25:686–697. doi: 10.1016/j.cmet.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Russo SB, Tidhar R, Futerman AH, Cowart LA. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J Biol Chem. 2013;288:13397–13409. doi: 10.1074/jbc.M112.428185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Menuz V, et al. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science. 2009;324:381–384. doi: 10.1126/science.1168532. [DOI] [PubMed] [Google Scholar]
- 176.Mesicek J, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]