Summary
Adult stem cells reside in specialized microenvironments called niches that maintain stem cells in an undifferentiated and self-renewing state. Despite extensive studies on the signaling pathways that operate within stem cells and their niches, the mechanisms that restrict niche signal exclusively to stem cells remained elusive: such a mechanism is crucially important to ensure that stem cells undergo self-renewal while their progeny, often located just one cell diameter away from the niche, differentiate. Here, we review recent progress on the characterization of niche-stem cell interactions with special focus on emerging mechanisms that spatially restrict niche signaling.
Adult stem cells reside in specialized microenvironments called niches that maintain stem cells in an undifferentiated and self-renewing state. Despite extensive studies on the signaling pathways that operate within stem cells and their niches, the mechanisms that restrict niche signal exclusively to stem cells remained elusive: such a mechanism is crucially important to ensure that stem cells undergo self-renewal while their progeny, often located just one cell diameter away from the niche, differentiate. Here, we review recent progress on the characterization of niche-stem cell interactions with special focus on emerging mechanisms that spatially restrict niche signaling.
Keywords: Germline stem cells, Niche, Short range signaling, MT-nanotubes, Spermatogonial stem cells
Introduction
Cells need to communicate with their neighbors in the correct manner and at the right time to build and maintain functional tissues and organs. Only a handful of signaling pathways appear to mediate the majority of cell-to-cell communication within complex tissues. While much has been learned about the molecular mechanics of these pathways, how signal transduction is spatially and temporally regulated in such a precise manner in vivo remains less well understood. Adult tissue homeostasis, in particular, depends on the correct spatio-temporal regulation of signaling between stem cells and their cellular neighbors. Improper signaling can lead to maladaptive increases or decreases in stem cell numbers. Such changes can result in cancer or tissue degeneration. Mechanisms that adjust stem cell signaling in the face of ever changing conditions ensure the proper balance of stem cell self-renewal and differentiation needed for normal tissue function (reviewed in Morrison and Kimble, 2006,Rando 2006)). In this review, we will highlight recent insights into the mechanisms that fine-tune stem cell signaling in vivo, with a particular focus on the reproductive system. The general underlying mechanisms involved in regulating stem cell-niche signaling in the ovary and testis are likely used in other stem cell systems as well.
Stem cell niches and signaling
The “niche” hypothesis, first proposed by Schofield in 1978 (Schofield 1978), posits that local environments determine whether stem cells remain in an undifferentiated state in vivo. Since this original publication, numerous cellular and non-cellular niches have been described in the literature (reviewed in (Morrison and Spradling 2008) (Wagers 2012)(Scadden 2014)). Niches have also been found to regulate stem cell division rates and survival (reviewed in (Li and Xie 2005), (Morrison and Spradling 2008)). In “cellular niches”, dedicated niche cells form specialized microenvironments that promote stem cell self-renewal and/or prevent stem cell differentiation. Niche cells influence stem cell behavior by producing various signaling molecules, such as Delta, Hedgehog (Hh), bone morphologic proteins (BMPs), Wnt/Wingless (Wg), cytokines, chemokines and other growth factors (reviewed in (Li and Xie 2005), (Morrison and Spradling 2008)). In “non-cellular niches”, extracellular molecules, such as extracellular matrix (ECM) proteins, provide essential signals to create the niche. The ECM can also function to concentrate self-renewing signaling molecules that might come from distant sources, thus creating a specialized microenvironment for stem cells. Additional variables influence stem cell behavior. For example, pH, oxygen, ions, mechanical force and electrical stimuli can all modulate stem cell activity, adding to the complexity in niche-mediated stem cell regulation (reviewed in (Wagers 2012)).
While significant progress has been made in understanding which niche signals foster stem cell self-renewal, there is a considerable lack of understanding regarding the mechanisms that prevent inappropriate delivery of self-renewing signals to stem cell progeny that have left the niche. Further insights into these mechanisms will have important implications for our understanding of tissue homeostasis and disease.
Germline stem cell systems in invertebrate model organisms
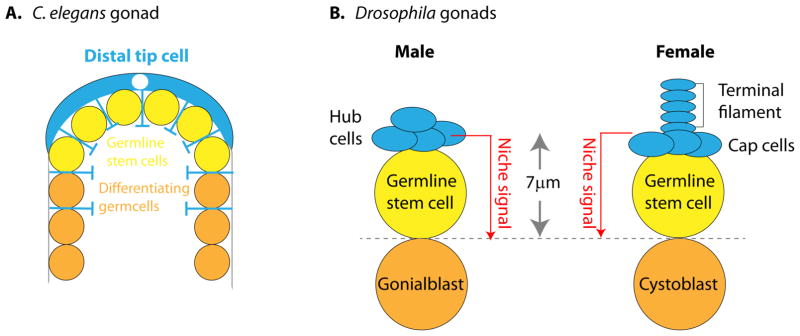
The germline stem cells (GSCs) of C. elegans and Drosophila have long served as useful models for studying stem cell niches. The simplicity and accessibility of worm and fly gonads, combined with the availability of robust and sophisticated genetic tools have greatly accelerated the characterization of the in vivo cellular niches that help to maintain these GSCs. The C. elegans gonad represents perhaps one of the simplest examples of a cell based stem cell niche. A distal tip cell (DTC), located at the tip of each gonad arm, extends a number of cellular projections that make contact with a small group of undifferentiated and mitotically active germ cells (Figure 1A). Ablation of the DTC causes germ cells at the tip of the gonad to exit mitosis and initiate the meiotic program. Further work has shown that the DTC acts to prevent undifferentiated germ cells from entering meiosis via Notch signaling pathway (see below, reviewed in (Byrd and Kimble 2009)(Kimble 2014)).
Figure 1. Short range niche signaling in C. elegans and Drosophila gonadal niches.
(A) In C. elegans, one distal tip cell (DTC) forms the niche for germline stem cells located at the distal end. DTC extends long projections that contact stem cells. (B) Asymmetric fate determination of Drosophila germline stem cells (GSCs) largely depends on the differential placement of two stem cell daughters to distinct locations: cells within the niche self-renew while cells outside the niche differentiate. The niche cell cluster (hub cells in males, terminal filament and cap cells in females) provides signals for stem cell self-renewal to the juxtaposed stem cells, but not other daughter cells displaced 1 cell diameter away from niche cells (Gonialblast in male, Cystoblast in female).
Drosophila gonads house slightly more complex cellular niches. In male Drosophila, a cluster of hub cells located at the apical tip of each testis provides a niche for GSCs. In the ovary, a small group of 5–7 cap cells help form the female GSC niche. Hub cells and cap cells both produce a number of ligands essential for germline stem cell (GSC) self-renewal. In males, hub cells produce Unpaired (Upd), a ligand in the Jak/Stat signaling pathway, and Decapentaplegic (Dpp) and Glass bottom boat (Gbb), ligands in the BMP pathway. BMP signaling also promotes GSC maintenance in the ovary (Michel et al. 2012) (Amoyel et al. 2013) (Luo et al. 2015). In both male and female Drosophila gonads, ectopic expression of niche ligands leads to expansion of GSC-like cells outside of the normal niche, and/or delays in the differentiation of GSC progeny, demonstrating that niche-produced factors play a major role in stem cell fate determination (Tulina and Matunis 2001) (Kiger et al. 2001)(Xie and Spradling 1998).
Mammalian SSC (spermatogonial stem cell) niche
Recent work has also begun to cast light on the complex nature of niche-stem cell interactions within the mammalian testis. Spermatogonia reside within the basal compartment of the seminiferous tubules and are classified as Asingle, Apaired, Aaligned, Intermediate and B-subtypes, based on morphological and molecular markers (J. M. Oatley and Brinster 2012)(S.-R. Chen and Liu 2015). Recent work using lineage tracing has shown that a Pax7+ subset of the Asingle population contains bona fide spermatogonial stem cells (SSCs) (Aloisio et al. 2014). Pax7+ Asingle SSCs are fast cycling stem cells and have long-term self-renewal capacity. Further quantitative analysis will provide insights into what percentage of bona fide stem cells are Pax7+ Asingle cells and what percentage of Pax7+ Asingle cells are stem cells. For example, in addition to Pax7, Id4 also marks a rare subset of Asingle spermatogonia potentially enriched for stem cells (Chan et al. 2014)(Sun et al. 2015). The relationship between Pax7+ Asingle cells and Id4+ Asingle cells remains unknown.
Glial cell line-derived neutrophic factor (GDNF), a member of the TGFβ super family of signaling molecules, and its receptor GDNF-family receptor-α1 (GFR-α1) comprise a core SSC self-renewal signaling pathway (J. M. Oatley and Brinster 2012)(S.-R. Chen and Liu 2015). GFR-α1 is expressed in subsets of Asingle, Apaired, Aaligned cells (Grasso et al. 2012), while Sertoli cells express GDNF (Meng et al. 2000). Interestingly, Apaired and Aaligned cells have the ability to fragment into single cells under certain conditions, perhaps suggesting these different cell types can dedifferentiate in response to niche signals (Nakagawa et al. 2010). Transplantation experiments show that Sertoli cells support the maintenance of SSCs (M. J. Oatley et al. 2011). GDNF heterozygous mutants display premature differentiation of SSCs (Meng et al. 2000) and decreases in GDNF expression correlate with decreases in functional SSCs during the course of aging (Ryu et al. 2006). Moreover, over-expression of GDNF appears to block germ cell differentiation, giving rise to an expansion of undifferentiated stem cell-like germ cells (Meng et al. 2000). These findings together point to GDNF produced by Sertoli cells as being a critical factor in the maintenance of SSCs.
While Pax7 and Id4 mark a rare subset of undifferentiated spermatogonia compared to the larger GFR-α1+ population, it remains unclear whether niche signaling directly influences the expression of these two transcription factors. This is in contrast to the clear niche-stem cell signaling relationship that exists between Sertoli-derived GDNF and GFR-α1- expressing spermatogonial cells. Whether a bona fide SSC niche specifically regulates Id4 and/or Pax7 expression in neighboring germ cells, conferring SSC identity, represents a point of significant interest.
While it is clear that GDNF represents a principle niche signal that regulates SSC self-renewal, several fundamental questions arise:
GDNF-GFR-α1 signaling likely occurs in a population of cells broader than bona fide SSCs (including Aaligned populations). Does this indicate that the bona fide niche (e.g. potentially a subset of Sertoli cells) provides additional unidentified SSC-specifying signals?
Sertoli cells are present throughout the seminiferous tubules, but the number and position of potential SSCs appears more limited. Whether only a subset of Sertoli cells serve as niches remains elusive.
Furthermore, Sertoli cells encapsulate not only SSCs but also all stages of differentiating germ cells. Sertoli cells are large cells that occupy space from the basement membrane to the lumen of the seminiferous tubules, thus contacting all stages of germ cells from SSCs to differentiating spermatids. This begs the question of how Sertoli cells can specify SSC identity, while encapsulating (and likely regulating) spermatid differentiation at the same time.
A few possibilities exist in regards to these questions. Oatley et al. (2009) showed that Leydig cells and select peritubular myoid cells express colony-stimulating factor 1 (CSF1). A SSC enriched Thy+ population of germ cells express the receptor for CSF1 and recombinant CSF1 appeared to enhance SSC self-renewal. A more recent study showed that interstitial macrophages also express CSF1, in addition to enzymes involved in retinoic acid enzyme biosynthesis (DeFalco et al. 2015). The phenotypes resulting from depletion of macrophages within the testis remain somewhat controversial: early studies suggest loss of macrophages disrupt meiotic progression within germ cells while more recent findings indicate that ablation of macrophages results in reduced numbers of Aaligned cells (Cohen et al. 1996)(Cohen, Hardy, and Pollard 1997)(Pollard et al. 1997)(DeFalco et al. 2015).
Additional factors may influence niche size. For example, Follicle Stimulating Hormone and Testosterone for different sources influence the activity of Sertoli cells (J. M. Oatley and Brinster 2012)(Smith and Walker 2014). Other studies have shown that the basement membrane that lines seminiferous tubules and Peritubular Myoid (PM) cells may also help to promote the maintenance of SSCs. Recently obtained data show that PM cells express GDNF and can support SSC self-renewal in culture (L.-Y. Chen et al. 2014). The vasculature of the testis also appears to play an important role in stem cell renewal (Yoshida et al. 2007). Careful analysis using live cell imaging of mouse gonads showed that Asingle cells tend to reside close to the vascular network, while their differentiating daughters move away from these regions and disperse through the basal compartment of the testis. However, a more recent study on Id4+ SSCs showed that this population of SSCs does not associate with the vasculature (Chan et al. 2014). Therefore, caution should be taken in regarding the vasculature as a possible niche component. In addition, a valve-like terminal segment of the seminiferous tubules also supported SSC maintenance in hamster testis, suggesting niches may come in different varieties (Aiyama et al. 2015). Thus contributions of various different cell types might explain why not all Sertoli cells function as the SSC niche: the combination of signals from Sertoli cells, Leydig cells, macrophages and possibly additional cells may be required to fully define the functional niche.
Alternatively, it is possible that cell intrinsic fate determinant(s) are segregated during SSC divisions, conferring SSC identity to those that inherit the determinants. In this scenario, GDNF-expressing Sertoli cells could be the only population that provides SSC niche function, which determines SSC identity in combination with cell-intrinsic fate determinants within SSCs themselves.
The most challenging question is how a single population of Sertoli cells can simultaneously regulate SSC and differentiating germ cells. Because tight junctions have been observed between Sertoli cells and germ cells, it is tempting to speculate that at each stage, germ cells are subjected to spatially segregated and distinct signaling events. In combination with this type of compartmentalization, secretion from Sertoli cells may also be polarized, i.e. GDNF is only secreted toward the SSC area, whereas other factor(s) are secreted toward a different domain of the Sertoli cell surface. Alternatively, germ cells and/or Sertoli cells may extend distinct set of nanotubes/cytonemes to mediate specific signaling (see the section “Protrusion mediated access to ligand source”). Considering that Drosophila trachea air sac primordium extend distinct sets of cytonemes (FGF-specific cytonemes and Dpp-specific cytonenes) toward different target cells (Roy et al. 2014), this remains a distinct possibility.
What restricts niche signaling?
As described above, many signaling pathways have been shown to function in GSC systems. However, the mechanisms that restrict niche signaling to foster the appropriate number of stem cells needed for tissue homeostasis under different environmental conditions remain poorly understood. Recent work using simple model systems may provide important clues as to the types of mechanisms that may be utilized to limit signaling in different contexts. Below, we describe several biological processes that can modulate the range of the niche signaling within model systems.
i) Tissue geometry
Tissue architecture, and more specifically the exact spatial positioning of cells relative to one another, can dictate cell fate. As illustrated in Figure1, GSCs in model systems directly adhere to their niche cells. Drosophila GSCs typically align their spindles perpendicularly toward the hub or cap cells, placing one daughter cell in direct contact with the niche while displacing the other daughter away from the niche (Yamashita, Jones, and Fuller 2003). This positioning ensures an asymmetric outcome of the stem cell division, i.e. self-renewal and differentiation (Figure 1B). Given the proximity of GSCs and their differentiating daughter cells, the effective range of niche signals has to be tightly restricted. Considering that many niche ligands, including Dpp, act over a long range (~100 μm) in other contexts, such as developing imaginal discs, mechanisms that limit the effective range of these ligands within the niche to 1 cell diameter (~7μm) must be in place.
ii) Juxtacrine or contact dependent signaling
By its very nature, contact-dependent or “juxtacrine” signaling allows for highly selective cell-to-cell communication. The Notch pathway (reviewed in (Kopan and Ilagan 2009)) represents one of the best studied examples of juxtacrine signaling. Notch and its ligands (Delta and Serrate in Drosophila) are both transmembrane proteins. Thus, activation of the pathway occurs only when communicating cells are in direct contact with one another. These molecules are not released into the extracellular space, minimizing the possibility of ectopic signal activation. The Notch pathway functions in a number of stem cell niches (Liu et al. 2010). For example, within the C. elegans gonad, Notch signaling keeps GSCs in an undifferentiated state. Within this system, maintenance of undifferentiated germ cells depends on the repression of three pathways: gld-1, gld-2 and a third meiotic entry pathway that remains poorly understood (Kadyk and Kimble 1998)(Eckmann et al. 2004)(Hansen et al. 2004)(Fox et al. 2011). The DTC expresses the Notch ligand LAG-2, while the germline expresses the receptor GLP-1. Notch pathway activation within germ cells induces the transcription of a number of target genes that act in concert with additional factors to repress the ability of germ cells from entering meiosis through the aforementioned pathways (Brenner and Schedl 2016). Thus, by using the Notch pathway, the DTC directly and precisely regulates the size of the GSC population (Byrd and Kimble 2009)(Kimble 2014). Notch signaling also acts in Drosophila gonads to control GSC numbers. In this case, the Notch pathway does not mediate direct communication between niche cells and GSCs, but rather acts during the formation of the niche itself. In the developing Drosophila ovary, expression of the Notch ligand Delta by developing terminal filament cells induces pathway activation in immediately adjacent somatic cells, specifying them to become cap cells (Song et al. 2007). Limited cell-cell communication is essential for the formation of properly sized niches. For example, ectopic activation of the Notch pathway in a greater number of cells within the developing gonad leads to the formation of ectopic niches and the inappropriate expansion of the GSC population in adults (Ward et al. 2006)(Song et al. 2007). Similarly, during male gonad development, Notch pathway regulates differentiation of somatic gonadal precursors (SGPs), precursors of hub cells. Notch pathway within SGPs acts together with EGF pathway to signal from primordial germ cells (PGCs) to determine appropriate niche size (Kitadate and Kobayashi 2010).
Notably, Notch is not the only example of a juxtacrine signal: several ligands once thought to function as secreted factors were later found to act in a juxtacrine manner. For example, pro-TGF-α, tethered to the plasma membrane of a mouse bone marrow stromal cell binds to EGFR on an adjacent hematopoietic progenitor cell (Anklesaria et al. 1990). In addition, bone marrow niche cells lacking membrane-bound Steel factor (ligand for c-Kit), despite retaining the expression of a secreted form, failed to maintain HSCs (Barker 1997)(Ding et al. 2012). Several other molecules including cytokines and growth factors (e.g. c-Kit, TGF-α and Amphiregulin) also act in a juxtacrine manner in specific contexts (reviewed in (Singh and Harris 2005)). In all these examples, juxtacrine signaling guarantees that cell-cell communication will be spatially restricted to those neighbors that immediately contact one another, thus making them an ideal candidate to participate in the type of spatially-limited signaling observed in most stem cells niches.
iii) Limit the amount of ligand production and/or secretion
Several potential mechanisms may control the range of niche signaling despite relying on the secreted ligands. For example, the simple modulation of ligand production at the level of transcription or translation could influence the local signaling gradient. In addition, the availability of niche ligands can also be regulated at the level of secretion. Exocytosis represents a highly regulated process, both in terms of the amount and the subcellular location of the molecules targeted for secretion. Polarized exocytosis play an important role in most eukaryotic cells (He and Guo 2009). A multi-protein complex, the exocyst, is required for this polarized exocytosis. Exocyst localizes to sites of active exocytosis and mediates the targeting and tethering of post-Golgi vesicles to the plasma membrane prior to membrane fusion. In the Drosophila male GSC niche, Michel et. al. showed that BMP secretion and E-cadherin membrane targeting require exocytosis and recycling endosomes in the GSC niche (Michel et al. 2011). They observed the colocalization of E-Cadherin and BMP ligand at adherens junctions within the niche cell membrane. It is still unclear if adherens junctions are functionally required for BMP ligand secretion or not. Regardless, this study suggests that specific mechanisms regulate the secretion of ligands, potentially allowing for the precise control of signal availability to stem cells within the niche.
iv) Modulation of the ligand diffusion outside the ligand-producing cells
Regulating how a ligand diffuses through a tissue represents another potential mechanism by which to modulate the range of signaling. The extracellular matrix (ECM) can influence how far a ligand can travel from its source. In addition, several ECM components function as reservoirs of ligands: ECM components bind to soluble ligands to regulate their diffusion characteristics, thus regulating local availability of the ligands (reviewed in (Hynes 2009)). For example, fibronectin, vitronectin, collagens and proteoglycans are known to bind BMPs and other growth factors including FGFs and HGFs. ECM molecules can influence the solubility and activity of these ligands. Enzymes, such as metalloproteinases (Matrix metalloproteinases: MMP), induce the remodeling of ECM components and permit the release of factors as necessary (Hynes 2009). The Drosophila HSPG (heparan sulfate proteoglycan) protein Dally is essential for concentrating Dpp molecules on the surface of cells in wing discs (Akiyama et al. 2008). In the female GSC niche, Dally is specifically expressed in niche cells to ensure a high-level of BMP signaling, and thus promotes GSC identity (Guo and Wang 2009). HSPG is thought to function as a co-receptor to activate the receptor on the surface of target cells. In this manner, HSPG enhances the specificity between ligand-producing and –receiving cells. Strikingly, ectopic expression of Dally in the Drosophila ovary expands the number of undifferentiated germline stem cells, suggesting that Dally influences the range of niche signaling (Hayashi, Kobayashi, and Nakato 2009). Similarly, in the male GSC niche, the secreted ECM protein Magu/Pentagone (Pent) is specifically expressed in hub cells and modulates Dpp signal activation exclusively in the GSC population (Zheng et al. 2011). Notably, ECM proteins do not always restrict ligand availability, but can also increase the distance over which signals act. Type IV collagens bind to Dpp and regulate BMP signaling in both the Drosophila embryo and ovary (Akiyama et al. 2008)(Guo and Wang 2009). Interaction between Dpp and type IV collagen appears to promote long-range gradient formation in the embryo, while it restricts the range of BMP pathway activation in the ovary through sequestration of the Dpp ligand.
v) Protrusion mediated access to ligand source
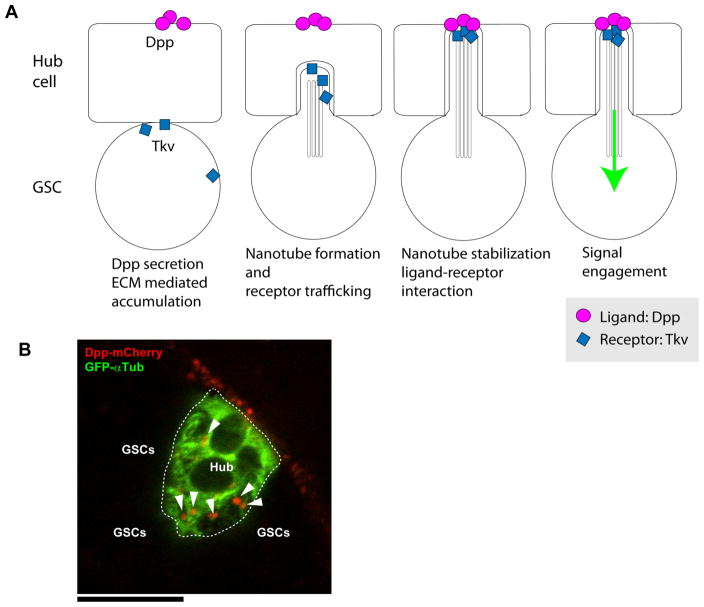
Our recent discovery of MT (microtubule based)-nanotubes suggests another potential mechanism that influences which cells can respond to niche signals (Figure 2A) (Inaba et al. 2015). MT-nanotubes are MT-based protrusions that extend from GSCs into the hub cell area. MT-nanotubes are sensitive to fixation similar to other thin protrusions reported to date, such as cytonemes and tunneling nanotubes, explaining why they have escaped detection in previous studies. 3D reconstitution of confocal stacks revealed that the MT-nanotubes invaginate into the hub cell cluster but do not breach the membranes of hub cells. Double plasma membranes from both cells appeared to wrap around the core microtubule bundle extending from the GSC. Tkv receptor protein expressed within GSCs translocates to the tips of MT-nanotubes, where it interacts with Dpp ligand expressed by hub cells (Figure 2A, B). Dpp ligand fused to mCherry expressed within hub cells exhibits a punctate pattern within hub cell-hub cell junctions, likely representing the point where MT-nanotubes foster efficient signal reception (Inaba et al. 2015, Figure 2B). IFT (intraflagellar transport) proteins appeared to participate in MT-nanotube formation and/or maintenance. Perturbation of MT-nanotubes compromises activation of Dpp signaling within GSCs, leading to GSC loss, indicating that MT-nanotubes promote signal reception (Inaba et al. 2015). Similar to the cytonemes whose formation and/or stabilization requires ligand-receptor interactions (Roy et al. 2014), MT-nanotube formation depends on interactions between Dpp and Tkv. Taken together, these data suggest that GSCs sense niche produced ligands and extend/stabilize MT-nanotubes towards the source of these signals. MT-nanotube formation in turn allows GSCs around the hub to experience the necessary threshold of signal activation needed for their self-renewal. MT-nanotube-mediated signaling represents one of the first examples in which cells utilize cellular protrusions to foster short-range signaling. These, and other similar structures, may promote efficient signal transduction in response to a limited amount of ligand produced by a local source, such as niche cells. By contrast, MT-nanotubes do not promote stem cell self-renewal via Jak/Stat signaling between hub cells and GSCs. The specificity of MT-nanotubes for BMP signaling suggests that stem cells likely use multiple mechanisms to receive signals from the niche.
Figure 2. MT-nanotube mediated niche-stem cell signaling.
(A) Model for MT-nanotube mediated signaling. Dpp induces MT-nanotube formation, and receptor–ligand interaction occurs at the surface of MT-nanotubes, leading to signaling activation in GSCs. (B) Dpp-mCherry (red) expressed in hub cells together with GFP-α-Tubulin (green, hub cell cortex) using hub specific unpaired (Upd) promoter. DppmCherry forms punctae along hub cell cortex (arrowheads). Scale bar: 10μm. Entire hub area is encircled by white broken line. GSCs are attached to hub from surrounding area (not visible here).
Other examples of protrusion mediated niche-stem cell regulation have been reported. For example, cap cells express Hedgehog protein and use short filopodia (cytonemes) to transport this ligand to escort cells (Rojas-Ríos, Guerrero, and González- Reyes 2012). In addition co-cultured osteoblast and human hematopoietic progenitor cells form long distance cytoplasmic connections (tunneling/membrane nanotubes; TNTs), which mediate trafficking of SARA endosomes between cells to regulate Smad signaling (Gillette et al. 2009). These observations give rise to an intriguing possibility that cytoplasmic contents may be directly transported between niche cells and stem cells. However, it remains unclear whether TNTs actually function to foster in vivo niche-stem cell interactions.
vi) Ligand diffusion vs. contact-dependent/protrusion-mediated signaling?
Integration of protrusion-mediated signaling and ECM-mediated diffusion may serve to fine-tune the delivery of niche ligands to stem cells. For example, the ECM may help to increase the local concentration of ligands specifically around protrusions, making the protrusion-mediated restriction of ligand delivery even tighter. Whether both mechanisms function together in the same niche or whether these mechanisms play distinct roles in the regulation of signaling remains an open question.
Many niches rely on signaling molecules that are presumably secreted into the extracellular space. A potential benefit of using diffusible ligands within niches includes the ability to adaptively adjust stem cell numbers in response to physiological change. For example, if niche signaling solely depended on juxtacrine signaling, re-establishment of the stem cell population after stem cell loss would be difficult. However, by using diffusible ligands, niches can influence cell fate at a distance, potentially allowing for the dedifferentiation of otherwise differentiating cells. Dedifferentiation has been observed in a number of systems including the Drosophila ovary and testis (Kai and Spradling 2004)(Brawley and Matunis 2004). Therefore, diffusible ligands may allow adaptable stem cell regeneration, if controlled properly. In such a context, ECM-mediated control of ligand diffusion/concentration may complement protrusion-dependent restriction of the niche signaling to maintain long-term tissue homeostasis.
vii) Intrinsic factors that mediate the ability of a cell to respond to a signal
Intrinsic factors within individual cells also help to sharpen the boundary between which cells experience signal transduction in response to ligands and which do not. For example, in the differentiating daughters of female GSCs (Xia et al. 2010) and male GSCs (Chang et al. 2013), BMP signaling is actively repressed by the HECT-domain ubiquitin E3 ligase SMAD ubiquitination regulatory factor (Smurf), which targets Tkv for degradation. smurf mutant ovaries exhibit an expansion of GSC-like cells outside of the niche (Xia et al. 2010), indicating that the degradation of Tkv promotes female germ cell differentiation. Likewise, smurf mutant testes also show increased/expanded Mad phosphorylation and an increased number of GSCs and transit-amplifying cell divisions (Chang et al. 2013). These results indicate that prompt inactivation of Dpp signaling is essential for timely differentiation of germ cells. However, in both cases, how the degradation activity is regulated differently between stem cells and their differentiating daughters remains unclear, and thus there must be additional mechanism(s) for decoding a cells’ location within the tissue.
Concluding remarks
Our understanding of potential regulatory mechanisms that control communication between niche cells and stem cells has greatly improved with the study of model organisms including Drosophila GSCs. These studies revealed remarkable complexity and precision in signaling mechanism regulating stem cell identity, differentiation and asymmetric divisions. At the same time, these studies have raised many interesting questions that will be addressed in future work. How are multiple mechanisms that control cell-cell signaling integrated into a single asymmetric event? Which event happens first? How flexible is the system? Does the effective range of signaling change to adapt to developmental and physiological changes? Are these mechanisms mutually dependent or do they provide redundancy to protect against the failure of one another? What ultimately happens when the spatial specificity of signaling is disturbed? These and other interesting questions about niche regulation await future study.
Acknowledgments
Grant sponsor: National Institutes of Health, grant number: R01AG047318 (to MB), 1R01GM118308-01 (to YY)
Grant sponsor: Howard Hughes Medical Institute
We thank Yamashita and Buszczak lab members for discussion. The research in the Yamashita laboratory is supported by Howard Hughes Medical Institute, National Institutes of Health (1R01GM118308-01). YMY is supported by MacArthur Foundation. MB is supported by the National Institutes of Health (R01AG047318)
Abbreviations
- GSC
Germline stem cells
- MT-nanotubes
Microtubule-based nanotubes
- SSCs
Spermatogonial stem cells
- ECM
Extracellular matrix
- DTC
Distal tip cell
- SGPs
Somatic gonadal precursors
- PGCs
Primordial germ cells
- MMP
Matrix metalloproteinase
- HSPG
Heparan sulfate proteoglycan
- IFT
Intraflagellar transport
- PM cells
Peritubular Myoid cells
- TNTs
Tunneling/membrane nanotubes
References
- Aiyama Yoshimi, Tsunekawa Naoki, Kishi Kasane, Kawasumi Miyuri, Suzuki Hitomi, Kanai-Azuma Masami, Kurohmaru Masamichi, Kanai Yoshiakira. A Niche for GFRα1-Positive Spermatogonia in the Terminal Segments of the Seminiferous Tubules in Hamster Testes. Stem Cells. 2015;33(9):2811–24. doi: 10.1002/stem.2065. [DOI] [PubMed] [Google Scholar]
- Akiyama Takuya, Kamimura Keisuke, Firkus Cyndy, Takeo Satomi, Shimmi Osamu, Nakato Hiroshi. Dally Regulates Dpp Morphogen Gradient Formation by Stabilizing Dpp on the Cell Surface. Developmental Biology. 2008;313(1):408–19. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisio Gina M, Nakada Yuji, Saatcioglu Hatice D, Peña Christopher G, Baker Michael D, Tarnawa Edward D, Mukherjee Jishnu, et al. The Journal of Clinical Investigation. 9. Vol. 124. American Society for Clinical Investigation; 2014. PAX7 Expression Defines Germline Stem Cells in the Adult Testis; pp. 3929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel Marc, Sanny Justina, Burel Michael, Bach Erika A. Hedgehog Is Required for CySC Self-Renewal but Does Not Contribute to the GSC Niche in the Drosophila Testis. Development (Cambridge, England) 2013;140(1):56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anklesaria P, Teixidó J, Laiho M, Pierce JH, Greenberger JS, Massagué J. Cell- Cell Adhesion Mediated by Binding of Membrane-Anchored Transforming Growth Factor Alpha to Epidermal Growth Factor Receptors Promotes Cell Proliferation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(9):3289–93. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JE. Early Transplantation to a Normal Microenvironment Prevents the Development of Steel Hematopoietic Stem Cell Defects. Experimental Hematology. 1997;25(6):542–47. [PubMed] [Google Scholar]
- Brawley Crista, Matunis Erika. Regeneration of Male Germline Stem Cells by Spermatogonial Dedifferentiation in Vivo. Science (New York, NY) 2004;304(5675):1331– 34. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brenner John L, Schedl Tim. Germline Stem Cell Differentiation Entails Regional Control of Cell Fate Regulator GLD-1 in Caenorhabditis Elegans. Genetics. 2016;202(3):1085–1103. doi: 10.1534/genetics.115.185678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd Dana T, Kimble Judith. Scratching the Niche That Controls Caenorhabditis Elegans Germline Stem Cells. Seminars in Cell & Developmental Biology. 2009;20(9):1107– 13. doi: 10.1016/j.semcdb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Frieda, Oatley Melissa J, Kaucher Amy V, Yang Qi-En, Bieberich Charles J, Shashikant Cooduvalli S, Oatley Jon M. Functional and Molecular Features of the Id4+ Germline Stem Cell Population in Mouse Testes. Genes & Development. 2014;28(12):1351– 62. doi: 10.1101/gad.240465.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Yi-Jie, Pi Haiwei, Hsieh Chang-Che, Fuller Margaret T. Smurf-Mediated Differential Proteolysis Generates Dynamic BMP Signaling in Germline Stem Cells during Drosophila Testis Development. Developmental Biology. 2013;383(1):106–20. doi: 10.1016/j.ydbio.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Liang-Yu, Brown Paula R, Willis William B, Eddy Edward M. Peritubular Myoid Cells Participate in Male Mouse Spermatogonial Stem Cell Maintenance. Endocrinology. 2014;155(12):4964–74. doi: 10.1210/en.2014-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Su-Ren, Liu Yi-Xun. Regulation of Spermatogonial Stem Cell Self-Renewal and Spermatocyte Meiosis by Sertoli Cell Signaling. Reproduction (Cambridge, England) 2015;149(4):R159–67. doi: 10.1530/REP-14-0481. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Chisholm O, Arceci RJ, Stanley ER, Pollard JW. Absence of Colony- Stimulating Factor-1 in Osteopetrotic (csfmop/csfmop) Mice Results in Male Fertility Defects. Biology of Reproduction. 1996;55(2):310–17. doi: 10.1095/biolreprod55.2.310. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Hardy MP, Pollard JW. Colony-Stimulating Factor-1 Plays a Major Role in the Development of Reproductive Function in Male Mice. Molecular Endocrinology (Baltimore, Md) 1997;11(11):1636–50. doi: 10.1210/mend.11.11.0009. [DOI] [PubMed] [Google Scholar]
- DeFalco Tony, Potter Sarah J, Williams Alyna V, Waller Brittain, Kan Matthew J, Capel Blanche. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Reports. 2015;12(7):1107–19. doi: 10.1016/j.celrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Lei, Saunders Thomas L, Enikolopov Grigori, Morrison Sean J. Nature. 7382. Vol. 481. Nature Publishing Group, a division of Macmillan Publishers Limited; 2012. Endothelial and Perivascular Cells Maintain Haematopoietic Stem Cells; pp. 457–62. All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann Christian R, Crittenden Sarah L, Suh Nayoung, Kimble Judith. GLD-3 and Control of the Mitosis/meiosis Decision in the Germline of Caenorhabditis Elegans. Genetics. 2004;168(1):147–60. doi: 10.1534/genetics.104.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox Paul M, Vought Valarie E, Hanazawa Momoyo, Lee Min-Ho, Maine Eleanor M, Schedl Tim. Cyclin E and CDK-2 Regulate Proliferative Cell Fate and Cell Cycle Progression in the C. Elegans Germline. Development (Cambridge, England) 2011;138(11):2223–34. doi: 10.1242/dev.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette Jennifer M, Larochelle Andre, Dunbar Cynthia E, Lippincott-Schwartz Jennifer. Intercellular Transfer to Signalling Endosomes Regulates an Ex Vivo Bone Marrow Niche. Nat Cell Biol. 2009;11(3):303–11. doi: 10.1038/ncb1838. ncb1838 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso Margherita, Fuso Andrea, Dovere Lisa, de Rooij Dirk G, Stefanini Mario, Boitani Carla, Vicini Elena. Distribution of GFRA1-Expressing Spermatogonia in Adult Mouse Testis. Reproduction (Cambridge, England) 2012;143(3):325–32. doi: 10.1530/REP-11-0385. [DOI] [PubMed] [Google Scholar]
- Guo Zheng, Wang Zhaohui. The Glypican Dally Is Required in the Niche for the Maintenance of Germline Stem Cells and Short-Range BMP Signaling in the Drosophila Ovary. Development (Cambridge, England) 2009;136(21):3627–35. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- Hansen Dave, Wilson-Berry Laura, Dang Thanh, Schedl Tim. Control of the Proliferation versus Meiotic Development Decision in the C. Elegans Germline through Regulation of GLD-1 Protein Accumulation. Development (Cambridge, England) 2004;131(1):93–104. doi: 10.1242/dev.00916. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, Nakato H. Drosophila Glypicans Regulate the Germline Stem Cell Niche. The Journal of Cell Biology. 2009;187(4):473–80. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Bing, Guo Wei. The Exocyst Complex in Polarized Exocytosis. Current Opinion in Cell Biology. 2009;21(4):537–42. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes Richard O. The Extracellular Matrix: Not Just Pretty Fibrils. Science (New York, NY) 2009;326(5957):1216–19. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba Mayu, Buszczak Michael, Yamashita Yukiko M. Nature. 7560. Vol. 523. Nature Publishing Group, a division of Macmillan Publishers Limited; 2015. Nanotubes Mediate Niche- Stem-Cell Signalling in the Drosophila Testis; pp. 329–32. All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk LC, Kimble J. Genetic Regulation of Entry into Meiosis in Caenorhabditis Elegans. Development (Cambridge, England) 1998;125(10):1803–13. doi: 10.1242/dev.125.10.1803. [DOI] [PubMed] [Google Scholar]
- Kai Toshie, Spradling Allan. Differentiating Germ Cells Can Revert into Functional Stem Cells in Drosophila Melanogaster Ovaries. Nature. 2004;428(6982):564– 69. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem Cell Self-Renewal Specified by JAK-STAT Activation in Response to a Support Cell Cue. Science (New York, NY) 2001;294(5551):2542–45. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kimble Judith. StemBook. Harvard Stem Cell Institute; 2014. Nov, C. Elegans Germline Stem Cells and Their Niche. [DOI] [PubMed] [Google Scholar]
- Kitadate Yu, Kobayashi Satoru. Notch and Egfr Signaling Act Antagonistically to Regulate Germ-Line Stem Cell Niche Formation in Drosophila Male Embryonic Gonads. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14241–46. doi: 10.1073/pnas.1003462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan Raphael, Ilagan Maria Xenia G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell. 2009;137(2):216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Linheng, Xie Ting. Stem Cell Niche: Structure and Function. Annual Review of Cell and Developmental Biology. 2005 Jan;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. Annual Reviews. [DOI] [PubMed] [Google Scholar]
- Liu Jianing, Sato Chihiro, Cerletti Massimiliano, Wagers Amy. Notch Signaling in the Regulation of Stem Cell Self-Renewal and Differentiation. Current Topics in Developmental Biology. 2010 Jan;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- Luo Lichao, Wang Huashan, Fan Chao, Liu Sen, Cai Yu. Wnt Ligands Regulate Tkv Expression to Constrain Dpp Activity in the Drosophila Ovarian Stem Cell Niche. The Journal of Cell Biology. 2015;209(4):595–608. doi: 10.1083/jcb.201409142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, et al. Regulation of Cell Fate Decision of Undifferentiated Spermatogonia by GDNF. Science (New York, NY) 2000;287(5457):1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Michel M, Kupinski AP, Raabe I, Bokel C. Hh Signalling Is Essential for Somatic Stem Cell Maintenance in the Drosophila Testis Niche. Development. 2012;139(15):2663– 69. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- Michel M, Raabe I, Kupinski AP, Perez-Palencia R, Bokel C. Local BMP Receptor Activation at Adherens Junctions in the Drosophila Germline Stem Cell Niche. Nat Commun. 2011;2:415. doi: 10.1038/ncomms1426. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and Symmetric Stem-Cell Divisions in Development and Cancer. Nature. 2006;441(7097):1068–74. doi: 10.1038/Nature04956. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Toshinori, Sharma Manju, Nabeshima Yo-ichi, Braun Robert E, Yoshida Shosei. Functional Hierarchy and Reversibility within the Murine Spermatogenic Stem Cell Compartment. Science (New York, NY) 2010;328(5974):62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley Jon M, Brinster Ralph L. The Germline Stem Cell Niche Unit in Mammalian Testes. Physiological Reviews. 2012;92(2):577–95. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley Melissa J, Racicot Karen E, Oatley Jon M. Sertoli Cells Dictate Spermatogonial Stem Cell Niches in the Mouse Testis. Biology of Reproduction. 2011;84(4):639–45. doi: 10.1095/biolreprod.110.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW, Dominguez MG, Mocci S, Cohen PE, Stanley ER. Effect of the Colony-Stimulating Factor-1 Null Mutation, Osteopetrotic (csfm(op)), on the Distribution of Macrophages in the Male Mouse Reproductive Tract. Biology of Reproduction. 1997;56(5):1290–1300. doi: 10.1095/biolreprod56.5.1290. [DOI] [PubMed] [Google Scholar]
- Rando Thomas A. Stem Cells, Ageing and the Quest for Immortality. Nature. 2006;441(7097):1080–86. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Rojas-Ríos Patricia, Guerrero Isabel, González-Reyes Acaimo. Cytoneme- Mediated Delivery of Hedgehog Regulates the Expression of Bone Morphogenetic Proteins to Maintain Germline Stem Cells in Drosophila. PLoS Biology. 2012;10(4):e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Sougata, Huang Hai, Liu Songmei, Kornberg Thomas B. Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein. Science (New York, NY) 2014;343(6173):1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Buom-Yong, Orwig Kyle E, Oatley Jon M, Avarbock Mary R, Brinster Ralph L. Effects of Aging and Niche Microenvironment on Spermatogonial Stem Cell Self- Renewal. Stem Cells (Dayton, Ohio) 2006;24(6):1505–11. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden David T. Nice Neighborhood: Emerging Concepts of the Stem Cell Niche. Cell. 2014;157(1):41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The Relationship between the Spleen Colony-Forming Cell and the Haemopoietic Stem Cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- Singh Amar B, Harris Raymond C. Autocrine, Paracrine and Juxtacrine Signaling by EGFR Ligands. Cellular Signalling. 2005;17(10):1183–93. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Smith Lee B, Walker William H. The Regulation of Spermatogenesis by Androgens. Seminars in Cell & Developmental Biology. 2014 Jul;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Xiaoqing, Call Gerald B, Kirilly Daniel, Xie Ting. Notch Signaling Controls Germline Stem Cell Niche Formation in the Drosophila Ovary. Development (Cambridge, England) 2007;134(6):1071–80. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Sun Feng, Xu Qing, Zhao Danfeng, Chen Charlie Degui. Id4 Marks Spermatogonial Stem Cells in the Mouse Testis. Scientific Reports. 2015 Jan;5:17594. doi: 10.1038/srep17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of Stem Cell Self-Renewal in Drosophila Spermatogenesis by JAK-STAT Signaling. Science (New York, NY) 2001;294(5551):2546– 49. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Wagers Amy J. The Stem Cell Niche in Regenerative Medicine. Cell Stem Cell. 2012;10(4):362–69. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Ward Ellen J, Shcherbata Halyna R, Reynolds Steven H, Fischer Karin A, Hatfield Steven D, Ruohola-Baker Hannele. Stem Cells Signal to the Niche through the Notch Pathway in the Drosophila Ovary. Current Biology : CB. 2006;16(23):2352–58. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Xia Laixin, Jia Shunji, Huang Shoujun, Wang Hailong, Zhu Yuanxiang, Mu Yanjun, Kan Lijuan, et al. The Fused/Smurf Complex Controls the Fate of Drosophila Germline Stem Cells by Generating a Gradient BMP Response. Cell. 2010;143(6):978–90. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Xie Ting, Spradling Allan C. Decapentaplegic Is Essential for the Maintenance and Division of Germline Stem Cells in the Drosophila Ovary. Cell. 1998;94(2):251–60. doi: 10.1016/S0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of Asymmetric Stem Cell Division by the APC Tumor Suppressor and Centrosome. Science. 2003;301(5639):1547– 50. doi: 10.1126/science.1087795301/5639/1547. [pii] [DOI] [PubMed] [Google Scholar]
- Yoshida Shosei, Sukeno Mamiko, Nabeshima Yo-Ichi. Science (New York, NY) 5845. Vol. 317. American Association for the Advancement of Science; 2007. A Vasculature-Associated Niche for Undifferentiated Spermatogonia in the Mouse Testis; pp. 1722–26. [DOI] [PubMed] [Google Scholar]
- Zheng Qi, Wang Yiwen, Vargas Eric, DiNardo Stephen. Magu Is Required for Germline Stem Cell Self-Renewal through BMP Signaling in the Drosophila Testis. Developmental Biology. 2011;357(1):202–10. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]