Abstract
Objective
To compare neurodevelopmental outcomes of preterm infants with and without intervention for posthemorrhagic ventricular dilatation (PHVD) managed with an “early approach” (EA), based on ventricular measurements exceeding normal (ventricular index [VI] <+2 SD/anterior horn width <6 mm) with initial temporizing procedures, followed, if needed, by permanent shunt placement, and a “late approach” (LA), based on signs of increased intracranial pressure with mostly immediate permanent intervention.
Methods
Observational cohort study of 127 preterm infants (gestation <30 weeks) with PHVD managed with EA (n = 78) or LA (n = 49). Ventricular size was measured on cranial ultrasound. Outcome was assessed at 18–24 months.
Results
Forty-nine of 78 (63%) EA and 24 of 49 (49%) LA infants received intervention. LA infants were slightly younger at birth, but did not differ from EA infants for other clinical measures. Initial intervention in the EA group occurred at younger age (29.4/33.1 week postmenstrual age; p < 0.001) with smaller ventricles (VI 2.4/14 mm >+2 SD; p < 0.01), and consisted predominantly of lumbar punctures or reservoir taps. Maximum VI in infants with/without intervention was similar in EA (3/1.5 mm >+2 SD; p = 0.3) but differed in the LA group (14/2.1 mm >+2 SD; p < 0.001). Shunt rate (20/92%; p < 0.001) and complications were lower in EA than LA group. Most EA infants had normal outcomes (>−1 SD), despite intervention. LA infants with intervention had poorer outcomes than those without (p < 0.003), with scores <−2 SD in 81%.
Conclusion
In preterm infants with PHVD, those with early intervention, even when eventually requiring a shunt, had outcomes indistinguishable from those without intervention, all being within the normal range. In contrast, in infants managed with LA, need for intervention predicted worse outcomes. Benefits of EA appear to outweigh potential risks.
Classification of evidence
This study provides Class III evidence that for preterm infants with PHVD, an EA to management results in better neurodevelopmental outcomes than a LA.
Posthemorrhagic ventricular dilatation (PHVD) remains an important complication of intraventricular hemorrhage (IVH) in preterm infants, occurring in 30% to 50% of infants with an IVH grade III/IV.1–4 PHVD has been associated with cognitive and motor deficits, particularly when a ventriculoperitoneal (VP) shunt is needed.2,3,5–10 Intervention, required in 35% to 40% of preterm infants with PHVD, is aimed at preventing additional brain injury.2,5,8,11,12 Despite the severity of this condition, there is no consensus on the optimal timing of intervention for PHVD. Current practice for the management of PHVD can broadly be divided in 2 approaches, “early approach” (EA) and “late approach” (LA), with the application of approaches differing across centers worldwide. Sequential cranial ultrasound (cUS) is reliable for following the progression of PHVD. Ventricular dilatation can be quantified with measures such as the ventricular index (VI) and anterior horn width (AHW).13,14 Although previous studies have suggested beneficial effects of early intervention,2,6–9 studies comparing outcomes of the EA vs LA have not yet been reported.2,7,15,16
The primary objective of this multicenter, observational study in preterm infants with PHVD was to compare 18- to 24-month neurodevelopmental outcomes of infants with and without intervention in centers with an EA and LA. Secondary objectives were to assess the need for VP-shunt placement and incidence of intervention-related complications with both approaches. We hypothesized that with either the EA or LA, infants with progressive PHVD, assessed as needing intervention within the definitions used in this study, are at increased risk of adverse neurodevelopmental outcome compared to infants not needing intervention.
Methods
Patients
Preterm infants with gestation <30 weeks, with a large IVH (grade III/IV)1,4 and PHVD, born between January 2008 and December 2013 and admitted to the neonatal units of The Hospital for Sick Children (Canada), University Medical Center Utrecht, or Isala Women-Children's Hospital Zwolle (both the Netherlands), were eligible for this retrospective study. In Canada, infants were referred to The Hospital for Sick Children, and in the Netherlands, infants were either born in the University Medical Center Utrecht or referred from the Isala Women-Children's Hospital Zwolle to the University Medical Center Utrecht. Infants were selected from national and local neonatal databases with IVH grade III/IV and PHVD as search criteria. The 2 currently applied approaches to PHVD are as follows: (1) EA, the standard of care in Dutch centers, based on ventricular measurements with initially temporizing procedures, i.e., CSF drainage from lumbar punctures (LPs) or ventricular reservoir, if needed, followed by permanent neurosurgical intervention, i.e., VP-shunt placement; and (2) LA, the standard of care in most North American centers, based on clinical signs of increased intracranial pressure (ICP) with mostly permanent neurosurgical intervention as initial procedure. The participating centers were chosen for their difference in approach to PHVD: infants in the Dutch centers were considered as EA and infants in the Canadian center as LA (figure e-1, http://links.lww.com/WNL/A162). In the EA, infants were referred for neurosurgical assessment when ventricular measurements exceeded normal values; in the LA, infants were referred when there were signs of increased ICP and/or intervention was deemed necessary. Exclusion criteria were chromosomal disorders, congenital malformations, cystic periventricular leukomalacia as detected with cUS, CNS infection prior to intervention, inborn errors of metabolism, and death <10 days of birth. Relevant perinatal data and measures on neonatal clinical course were retrieved from the notes. Clinical signs of increased ICP included excessive increase in head circumference (>1 cm/wk), apneic spells, seizures, change in consciousness, and abnormal eye movements.
Standard protocol approvals, registrations, and patient consents
Approval from the research ethics boards at each center was obtained, and the requirement for informed consent for this retrospective study with anonymized data was waived.
Cranial ultrasound
All available cUS scans of eligible infants, including scans from referring hospitals, were assessed by 3 examiners (L.M.L. and L.S.d.V. or J.T.) for presence, side (unilateral/bilateral), and grade (III/IV) of IVH and for presence of PHVD. To quantify PHVD, VI and AHW were measured.13,14 Ventricular size at different time points, including prior to intervention and maximum dilatation throughout the neonatal period, were recorded along with age (days) and postmenstrual age (weeks) on day of measurement. For the VI, the distance (mm) above the threshold for ventricular dilatation adjusted for increase in VI with increasing postmenstrual age (+2 SD line) was calculated. For the AHW, the distance (mm) above 6 mm was calculated. PHVD was defined as VI >+2 SD and/or AHW >6 mm.13,14 The presence of associated brain lesions considered important for outcome were also recorded: focal/ischemic white and deep gray matter injury, porencephalic cyst, subdural hemorrhage, and large cerebellar lesion (>4 mm).17
Intervention
Intervention for PHVD included LP, ventricular reservoir, and VP shunt. Intervention details included type of first intervention, type and number of subsequent intervention(s), age at intervention(s), and intervention-related complications. Complications included infection, reservoir or shunt obstruction, need for revision, additional acquired cerebral lesions (e.g., intraventricular, intraparenchymal, and subdural hemorrhage), and intervention-related death.
Neurodevelopmental outcome
Neurodevelopmental outcome at 18 to 24 months’ corrected age was assessed as part of national standard follow-up programs by experienced developmental specialists in the follow-up clinics of the participating centers. Assessments consisted of a structured neurologic and standardized developmental assessment, using Bayley Scales of Infant and Toddler Development (Third Edition) (BSID-III)18 and/or Griffiths Mental Development Scales (GMDS; all items)19 as primary outcome. For the BSID-III, the composite scores for cognitive and motor development have a mean of 100 and SD of 15, and were calculated based on the infant's corrected age. For the GMDS, the developmental quotient, based on cognitive and motor functioning, has a mean of 100 and SD of 12, and was also calculated based on corrected age. Scores below −2 SD indicate moderately abnormal function. Infants who were severely impaired and not testable were assigned a score of −3 SD (55 or 64, respectively). The presence of neurologic impairments, including cerebral palsy (CP),20 hearing and vision impairment, and childhood epilepsy, were recorded.
Data analysis
Data analyses were performed using IBM SPSS Statistics version 24 (IBM Corp., Armonk, NY).
Infants were divided into 4 groups based on their center's approach to PHVD and the need for any type of intervention: (1) EA without intervention, (2) EA with intervention, (3) LA without intervention, and (4) LA with intervention. To address our primary hypothesis, neurodevelopmental outcome scores were compared between infants with and without intervention for PHVD within the EA and the LA group. Differences in clinical measures and imaging were explored between the EA and the LA group, and between infants with and without intervention within the EA and the LA group. In addition, outcome was compared within the EA and the LA group between infants with only temporizing interventions (LP and/or reservoir) and with permanent intervention (VP shunt).
For measurements of ventricular size, inter- and intraobserver variability were assessed.
Details on intervention, rates of VP-shunt placement, and intervention-related complications were compared between the EA and the LA group for infants with intervention.
Categorical variables were compared using the χ2 or Fisher exact test, where appropriate. Continuous variables were compared using the Mann-Whitney U test. As we had one primary hypothesis, a p value <0.05 was considered significant.
Primary research question/classification of evidence
We compared 18- to 24-month neurodevelopmental outcomes of preterm infants with and without intervention for PHVD in centers applying EA and LA. This study provides Class III evidence that for preterm infants with PHVD, an EA to management results in better neurodevelopmental outcomes than a LA.
Results
Patients
A total of 193 eligible preterm infants were selected from the databases, of whom 127 infants were included: 30 were excluded for exclusion criteria and 36 for ventricular measurements not meeting PHVD criteria (figure 1). Infants in the EA group were older and larger at birth (p = 0.02), showed fewer signs of increased ICP (p < 0.001), and had a lower overall neonatal mortality rate (p < 0.01) (table e-1, http://links.lww.com/WNL/A163). No differences in other clinical measures were found across groups or between infants with and without intervention within the EA and LA groups.
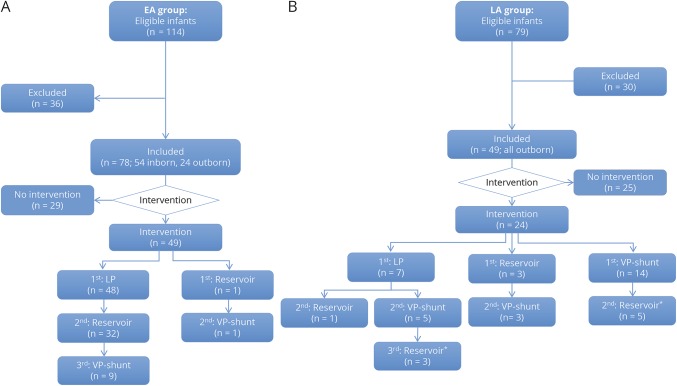
Figure 1. Flow diagram of eligible and included preterm infants in the EA and LA groups, and division of infants within the groups.
Flow diagram of eligible (total 193), excluded (total 66), and included (total 127) infants in the EA (A) and the LA (B) groups, and subsequent division of infants within the EA and LA groups based on intervention requirement. Type of first and subsequent interventions is also shown. *A ventricular reservoir was placed after VP shunt as temporary measure in case of shunt dysfunction. EA = early approach; LA = late approach; LP = lumbar puncture; VP = ventriculoperitoneal.
Cranial ultrasound
Good inter- and intraobserver agreement for ventricular measurements was found, with κ values >0.8. In the EA group, maximum ventricular sizes were smaller (p < 0.001, both VI and AHW) and associated lesions less common (p < 0.01) (table e-1, http://links.lww.com/WNL/A163). No other differences in cUS findings were found across groups or between infants with and without intervention within groups.
Intervention
Forty-nine infants (63%) in the EA and 24 infants (49%) in the LA group underwent intervention (table 1 and figure 1). Infants in the EA group were younger at first intervention (p < 0.001). While EA group infants mostly had an LP as initial intervention, most LA group infants received a VP shunt as initial intervention. Overall rate of VP-shunt placement was lower in the EA than LA group (20%/92%; p < 0.001). VP shunts were placed later, around term equivalent age, in the EA group.
Table 1.
Details on intervention for infants in the EA and LA groups
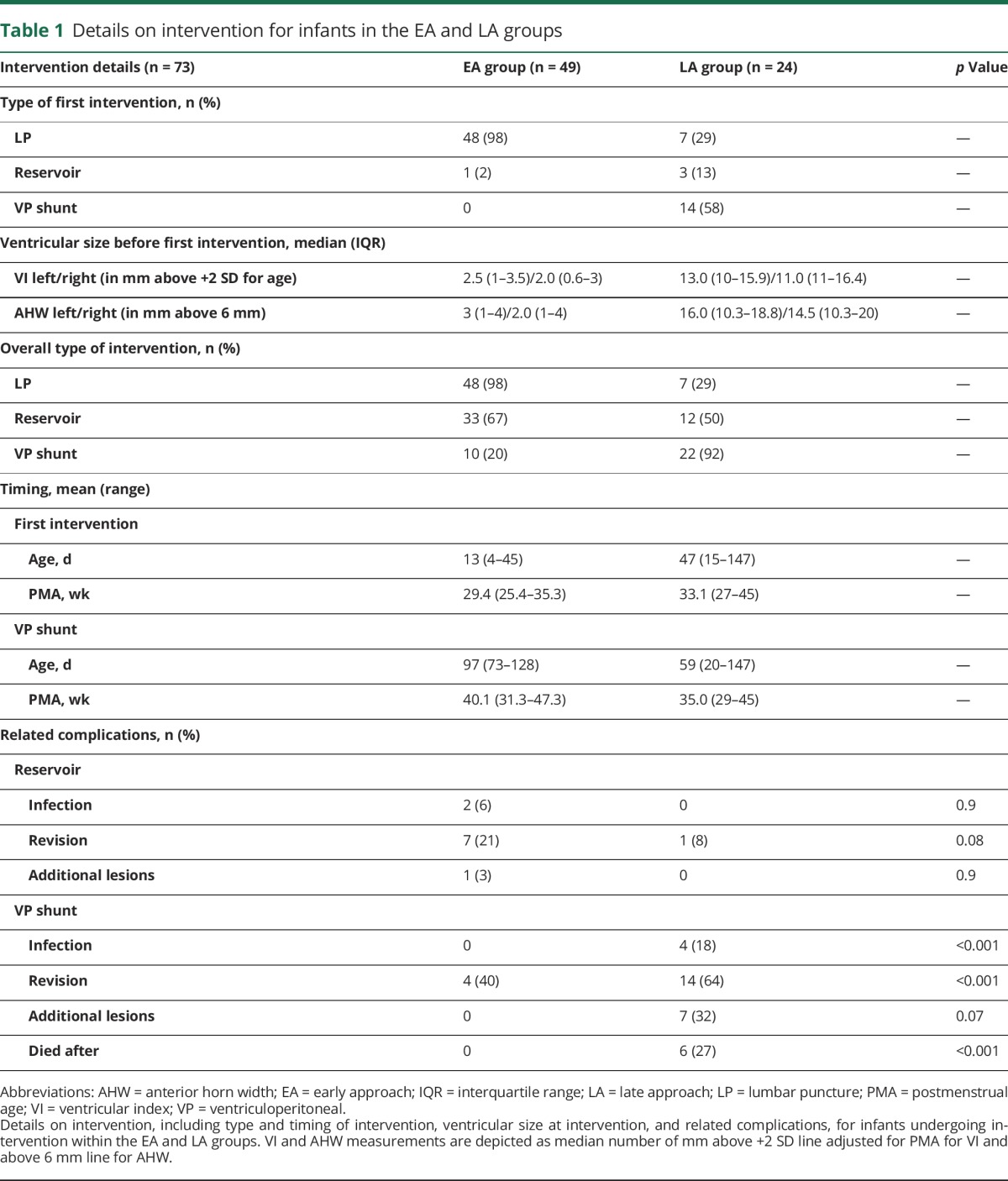
Although reservoir-related complications were slightly more common in the EA group, substantially more shunt-related complications occurred in the LA group, particularly infection and need for revision. Intervention-related death only occurred in the LA group.
Relation between ventricular dilatation and need for intervention
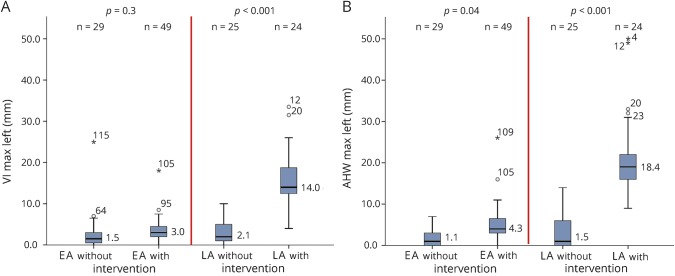
VI was comparable for infants in the EA group (p = 0.3), regardless of need for intervention, while AHW was larger in the infants undergoing intervention (p = 0.04). In the LA group, infants with intervention had larger ventricles than those without intervention (p < 0.001, both VI and AHW) (figure 2).
Figure 2. Median maximum ventricular measurements for infants without and with intervention in EA and LA groups.
Maximum ventricular measurements, including VI in millimeters above the +2 SD line adjusted for postmenstrual age on the y-axis in panel A and AHW in millimeters above the 6 mm line on the y-axis in panel B, for the infants without and with intervention in the EA and the LA groups depicted on the x-axis. Similar graphs were obtained for median maximum VI and AHW measurements for the right lateral ventricles. AHW = anterior horn width; VI = ventricular index.
Neurodevelopmental outcome
Eleven infants in the EA group and 20 infants in the LA group died between 10 days and 6 weeks of birth. Six LA group infants died as a result of PHVD intervention-related complications; in the remaining infants, death was related to systemic illness. Follow-up rate for infants who survived up to 24 months’ corrected age was high for both the EA (62/67) and LA (27/29) groups (table 2 and table e-1). The majority of survivors in the EA group (55/62) had cognitive and motor outcome scores within the normal range (within 1 SD of mean). In contrast, most survivors in the LA group had moderately to severely abnormal outcomes (14/27) (table 2).
Table 2.
Neurodevelopmental outcome of survivors without and with intervention within the EA and LA groups
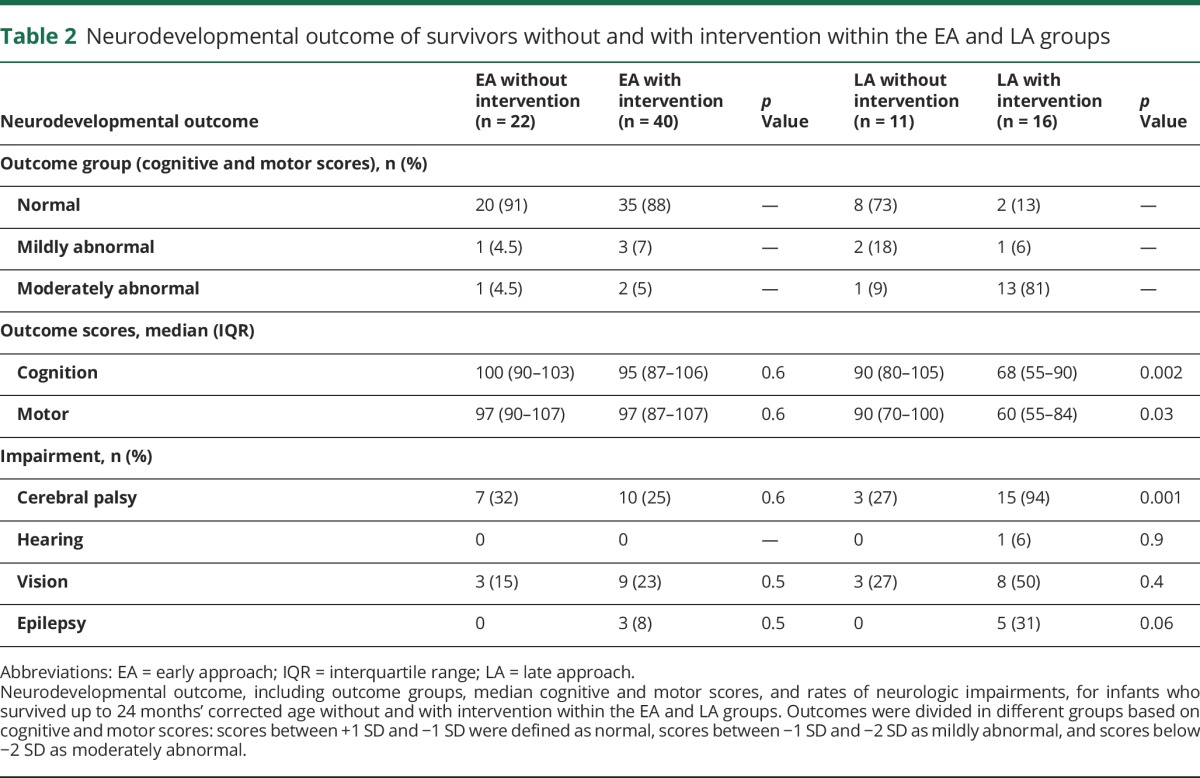
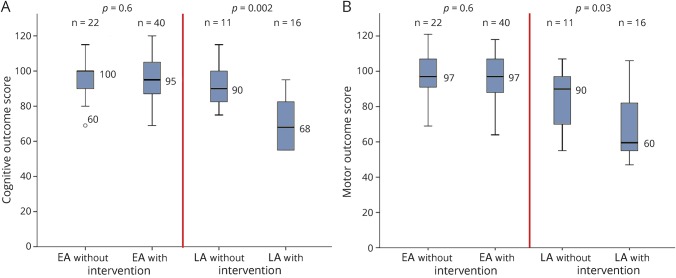
Survivors in the EA group had similar cognitive (p = 0.6) and motor (p = 0.6) scores and low rates of neurologic impairments (all p ≥ 0.5), regardless of need for intervention. When considering only survivors in the EA group, those with only temporizing procedures and those with permanent VP-shunt placement had similar cognitive (95 vs 95; p = 0.6) and motor (97 vs 99; p = 0.3) scores.
In the LA group, survivors with intervention had lower cognitive (p = 0.002) and motor (p = 0.03) scores than those without intervention. The difference remained significant when accounting for grade IV IVH (both p < 0.04). Similarly, survivors with intervention showed higher rates of impairments than those without, especially CP (p = 0.001) (table 2 and figure 3). Only 2 survivors with nonpermanent interventions had outcome data precluding a meaningful comparison of this with shunts.
Figure 3. Median outcome scores for survivors without and with intervention in EA and LA groups.
Median cognitive outcome scores on the y-axis in panel A and median motor outcome scores on the y-axis in panel B for the infants who survived up to 24 months’ corrected age without and with intervention in the EA and LA groups depicted on the x-axis. Comparison between infants without and with intervention within both groups is also shown. EA = early approach; LA = late approach.
Discussion
In this multicenter, observational study in preterm infants with PHVD, we compared 18- to 24-month neurodevelopmental outcomes of infants with and without intervention in centers with an EA to PHVD, based on ventricular measurements, and a LA, based on clinical signs of increased ICP. We demonstrate that infants receiving EA have essentially normal early cognitive and motor outcomes, regardless of intervention, even when a VP shunt is eventually required. In contrast, infants receiving LA have adverse cognitive and motor outcomes if intervention is ultimately needed. With the LA, VP-shunt rate and shunt-related complications were also more prevalent.
The worse neurodevelopmental outcomes in infants with late intervention suggest that progressive ventricular dilatation and prolonged pressure is deleterious to the immature white matter (WM). Even when CSF pressure is brought back to normal at a later stage, the WM may no longer have the capacity to recover.21 Consistent with the hypothesis of permanent WM injury, in many infants with late intervention, the ventricles remained severely dilated after ventricular reservoir or VP-shunt placement. WM damage with severe and prolonged ventricular dilatation may be directly related to pressure and interference of perfusion, and indirectly to distortion, ischemia, free radical formation, and inflammation.12 Studies using near-infrared spectroscopy and Doppler techniques have shown that cerebral perfusion, oxygenation, and flow are progressively impaired with increasing dilatation, and gradually improve following CSF drainage.22–24 Progressive ventricular dilatation has also been associated with increasingly abnormal amplitude-integrated EEG background and delayed latencies of evoked potentials. These neurophysiologic changes occur even before signs of increased ICP develop, and normalize following CSF drainage.24–27 In addition, preterm infants with PHVD have higher apparent diffusion coefficient values in WM and smaller total brain, deep gray matter, and cerebellar volumes than those without.21,28–30 Together with these observations, our findings suggest that intervening early may prevent WM damage.
Although our findings support an EA to PHVD, specific thresholds for intervention need to be defined. Previous studies have described that preterm infants with early intervention, based on ventricular size or within 3 to 4 weeks of birth, had better outcomes than those with somewhat later intervention.6–9,31 A large retrospective study showed that 2-year outcome was better in preterm infants with intervention initiated when VI crossed the +2 SD compared to the +2 SD + 4-mm line.2 The vast majority of our LA infants had intervention when ventricular size was well above normal values and after the first month of life.
Even without intervention, PHVD may resolve spontaneously when VI is >+2 SD.3 Thus, it is possible that with the EA, too many infants undergo LPs or ventricular reservoir placement. LPs may also have avoided neurosurgical and/or permanent intervention in our cohort, even though several randomized controlled trials (RCTs) have not demonstrated a beneficial effect of LPs for outcome.5,11 In the EA group, two-thirds of infants needed a reservoir after initial LPs, with a subsequent one-third of those going on to VP-shunt placement. Results of the multicenter RCT “Early Versus Late Ventricular Intervention Study” (ELVIS; ISRCTN43171322), comparing early (VI >+2 SD) vs later (VI >+2 SD + 4 mm) intervention for outcome and VP-shunt requirement, may inform the optimal timing and ventricular size for early intervention. Meanwhile, our data support the EA using VI crossing the +2 SD + 4 mm line and/or AHW crossing 6 mm as cutoff for intervention.
Increase in AHW and rounding of the frontal horns, so-called “ballooning,” may precede changes in VI as a sign of increased ICP. Thus, AHW may be a more sensitive marker for early and mild ventricular dilatation than VI, and better predictor of need for intervention. Identifying predictors of spontaneous stabilization of PHVD may prevent unnecessary interventions and help in determining the optimal time of early intervention. Given the importance of these measures in assessing the timing of intervention, performing frequent cUS in preterm infants following a large IVH is strongly recommended.
The rationale for the LA is the risks of complication from neurosurgical intervention and the inserted device, and a lack of definitive evidence that an EA improves outcome.7,16 Most studies by Northern American groups had intervention-related complications as primary outcome, rather than neurodevelopmental outcome.32,33 In our study, the EA was associated with lower VP-shunt requirement, older age at shunt placement, and reduced risk of shunt-related complications. The low rate of reservoir complications following early intervention, and higher rate of VP-shunt placement and complications following late intervention, are consistent with prior reports.2,8,10,16,32,34–36 In the Utrecht center, it is standard practice to have a dedicated team of nurses perform reservoir taps according to strict guidelines, which has markedly reduced infection rates.34,36 As neurosurgical techniques are similar across centers, the higher rate of shunt-related complications in the LA group may be related to the younger age of the infants at time of VP-shunt placement. Using a VP shunt as first procedure, when protein and blood cell levels in CSF are high and the ventricles severely dilated, may result in shunt dysfunction and subdural hemorrhages from ventricular collapse. These risks may be attenuated by initially performing temporizing procedures, thereby removing CSF debris, keeping CSF pressure lower, and allowing for the infants to gain weight and become more stable. Our current findings suggest that the potential benefit of the EA for neurodevelopmental outcome outweighs the risk of intervention-related complications.
Several limitations of this retrospective study need to be addressed. Infants were not randomized between EA and LA based on ventricular size but we took advantage of substantial center-based differences in PHVD treatment approach. LA group infants were slightly younger and smaller at birth, and had a higher incidence of grade IV IVH and significant associated brain lesions. All LA group infants were born in other perinatal centers and referred once there were signs of increased ICP and/or intervention was deemed necessary. Part of the EA group infants were also born in another perinatal center. However, these infants were referred because of increase in ventricular size rather than clinical symptoms. To avoid confounding from center-specific differences, we focused our comparisons within the EA and the LA. It is important that the rate and location of grade IV IVH and associated brain lesions in the LA group did not differ between infants with and without intervention, while outcome was poorer and CP rate higher in the infants with intervention. Of interest, when looking across centers, LA group infants not requiring intervention had comparable outcomes, rates of neurologic impairments, and ventricular measures to infants in the EA group, with and without intervention. The similarities between the LA group infants without intervention and all EA group infants support the LA group infants being similar to the EA group infants and not more prone to less favorable outcome. This rather suggests the poorer outcomes in LA group infants with intervention to be inherent to the approach.
cUS protocols differed between the EA and LA groups, with cUS being less frequently repeated in the LA group. As intervention decisions in the LA group were based primarily on presence of clinical signs, the frequency of cUS in this group should not have influenced timing of intervention.
Infants who died within the first 10 days of birth were excluded from further analyses as PHVD was still in an early and progressive stage. These infants were similar in the EA and LA group with death following critical illness or redirection of care. Including their measurements may have underestimated ventricular size and incorrectly associated mild ventricular dilatation with unfavorable outcome.
The main strength of the study is that the 2 currently applied approaches to PHVD could be addressed by comparing the outcomes of sizeable groups of preterm infants with and without intervention for PHVD in centers well known for their expertise in neonatal neurology, neonatal neurosurgery, and long-term follow-up. The expertise of the study teams enabled standardized approaches to brain imaging measures and neurodevelopmental outcome assessments.
This multicenter study in a large cohort of preterm infants with PHVD demonstrates that intervention at an early stage, based on ventricular measurements and initially using temporizing procedures, is associated with favorable neurodevelopmental outcomes, even when a VP shunt is eventually needed. In contrast, later intervention, when the lateral ventricles are severely dilated and signs of increased ICP have developed, increases the risk of unfavorable outcome and intervention-related complications. These findings warrant reconsideration of PHVD management in LA centers. Further studies in larger cohorts with long-term follow-up are needed to refine cutoff values of ventricular size that are optimal for improving outcomes of preterm infants with PHVD. In addition, until strategies to prevent severe IVH are identified, a continued search for tools to identify newborn infants at risk of irreversible WM injury is warranted. Meanwhile, our findings support the EA with LPs or taps from a ventricular reservoir, draining adequate amounts of CSF, guided by measurements of ventricular size from cUS, before moving on to VP-shunt placement.
Acknowledgment
The authors thank Dr. Liset Hoftiezer (PhD student, Department of Neonatology, Women-Children's Hospital, Zwolle, the Netherlands) and Gwendolyn Thomas (medical student, Department of Neonatology, Wilhelmina Children's Hospital, University Medical Center Utrecht, the Netherlands) for their help with data collection; developmental specialists in the follow-up clinics of the 3 participating centers, Sunnybrook Health Sciences Centre (Toronto, Canada) and Mount Sinai Hospital (Toronto, Canada), Dr. Djien Liem (Pediatrician–Neonatologist, Department of Neonatology, Amalia Children's Hospital, Radboudumc, Nijmegen, the Netherlands), and Dr. Koen Dijkman (Pediatrician–Neonatologist, Department of Neonatology, Maxima Medical Center, Veldhoven, the Netherlands) for their contributions to collecting neurodevelopmental outcome data.
Glossary
- AHW
anterior horn width
- BSID-III
Bayley Scales of Infant and Toddler Development (Third Edition)
- CP
cerebral palsy
- cUS
cranial ultrasound
- EA
early approach
- GMDS
Griffiths Mental Development Scales
- ICP
intracranial pressure
- IVH
intraventricular hemorrhage
- LA
late approach
- LP
lumbar puncture
- PHVD
posthemorrhagic ventricular dilatation
- RCT
randomized controlled trial
- VI
ventricular index
- VP
ventriculoperitoneal
- WM
white matter
Footnotes
Editorial, page 351
Class of Evidence: NPub.org/coe
Author contributions
L.M.L. conducted the multicenter study. L.M.L., S.P.M., G.v.W.-M., L.G.L., and L.S.d.V. contributed to the conception and design of the study. All authors contributed equally to the data acquisition, including data on clinical course, brain imaging and measurements of ventricular size, surgical intervention and related complications, and neurodevelopmental outcome. L.M.L., S.P.M., G.v.W.-M., F.G., L.G.L., and L.S.d.V. contributed to the data analysis and interpretation. L.M.L., S.P.M., G.v.W.-M., L.G.L., and L.S.d.V. contributed to drafting the manuscript and accompanying tables and figures. All authors contributed equally to revising the manuscript and included tables and figures. All authors have approved the final version of the manuscript for publication.
Study funding
Dr. Lara M. Leijser was awarded the Early Investigators' Exchange Program Award from the International Pediatric Research Foundation to conduct this multicenter study.
Disclosure
L. Leijser has funding from the International Pediatric Research Foundation and the Research Training Centre, The Hospital for Sick Children. S. Miller has funding from the CIHR (Canadian Institutes of Health Research), SickKids Foundation, Kids Brain Health, and Ontario Brain Institute. G. van Wezel-Meijler, A. Brouwer, J. Traubici, I. van Haastert, H. Whyte, F. Groenendaal, A. Kulkarni, K. Han, P. Woerdeman, P. Church, E. Kelly, H. van Straaten, and L. Ly report no disclosures relevant to the manuscript. L. de Vries has funding from ZonMw (The Netherlands Organisation for Health Research and Development) and Wellcome. Go to Neurology.org/N for full disclosures.
References
- 1.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight less than 1,500 gm. J Pediatr 1978;92:529–534. [DOI] [PubMed] [Google Scholar]
- 2.de Vries LS, Liem KD, van Dijk K, et al. ; Dutch Working Group of Neonatal Neurology. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in the Netherlands. Acta Paediatr 2002;91:212–217. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BP, Inder TE, Rooks V, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed 2002;87:F37–F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe JJ. Volpe's Neurology of the Newborn. 6th ed. Philadelphia: Elsevier; 2017;637–698. [Google Scholar]
- 5.Ventriculomegaly Trial Group. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Arch Dis Child 1990;65:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams-Chapman I, Hansen NI, Stoll BJ, et al. ; NICHD Research Network. Neurodevelopmental outcome of ELBW infants with posthemorrhagic hydrocephalus, requiring shunt insertion. Pediatrics 2008;121:e1167–e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer AJ, Brouwer MJ, Groenendaal F, et al. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed 2012;97:F50–F55. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasakumar P, Limbrick D, Munro R, et al. Posthemorrhagic ventricular dilatation: impact on early neurodevelopmental outcome. Am J Perinatol 2013;30:207–214. [DOI] [PubMed] [Google Scholar]
- 9.Radic JA, Vincer M, McNeely PD. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr 2015;15:580–588. [DOI] [PubMed] [Google Scholar]
- 10.Alan N, Manjila S, Minich N, et al. Reduced ventricular shunt rate in very preterm infants with severe intraventricular hemorrhage: an institutional experience. J Neurosurg Pediatr 2012;10:357–364. [DOI] [PubMed] [Google Scholar]
- 11.International PHVD Drug Trial Group. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. Lancet 1998;352:433–440. [PubMed] [Google Scholar]
- 12.Whitelaw A, Jary S, Kmita G, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 2010;125:e852–e858. [DOI] [PubMed] [Google Scholar]
- 13.Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 1981;56:900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies MW, Swaminathan M, Chuang SL, et al. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2000;82:F218–F223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev 2001;1:CD000216. [DOI] [PubMed] [Google Scholar]
- 16.Mazzola CA, Choudhri AF, Auguste KI, et al. ; Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 2014;14:8–23. [DOI] [PubMed] [Google Scholar]
- 17.Benders MJ, Kersbergen KJ, de Vries LS. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin Perinatol 2014;41:69–82. [DOI] [PubMed] [Google Scholar]
- 18.Bayley N. Bayley's Scales of Infant and Toddler Development. 3rd ed. San Antonio: The Psychological Corporation; 2006. [Google Scholar]
- 19.Griffiths R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years of Life. London: Test Agency Ltd; 1984. [Google Scholar]
- 20.Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr 2001;90:271–277. [PubMed] [Google Scholar]
- 21.Brouwer MJ, de Vries LS, Kersbergen KJ, et al. Effects of posthemorrhagic ventricular dilatation in the preterm infant on brain volumes and white matter diffusion variables at term-equivalent age. J Pediatr 2016;168:41–49. [DOI] [PubMed] [Google Scholar]
- 22.Kempley ST, Gamsu HR. Changes in cerebral artery blood flow velocity after intermittent cerebrospinal fluid drainage. Arch Dis Child 1993;69:74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Alfen-van der Velden AA, Hopman JC, Klaessens JH, et al. Cerebral hemodynamics and oxygenation after serial CSF drainage in infants with PHVD. Brain Dev 2007;29:623–629. [DOI] [PubMed] [Google Scholar]
- 24.Norooz F, Urlesberger B, Giordano V, et al. Decompressing posthaemorrhagic ventricular dilatation significantly improves regional cerebral oxygen saturation in preterm infants. Acta Paediatr 2015;104:663–669. [DOI] [PubMed] [Google Scholar]
- 25.de Vries LS, Pierrat V, Minami T, et al. The role of somatosensory evoked responses in infants with rapidly progressive ventricular dilatation. Neuropediatrics 1990;21:136–139. [DOI] [PubMed] [Google Scholar]
- 26.Olischar M, Klebermass K, Hengl B, et al. Cerebrospinal fluid drainage in posthaemorrhagic ventricular dilatation leads to improvement in amplitude-integrated electroencephalographic activity. Acta Paediatr 2009;98:1002–1009. [DOI] [PubMed] [Google Scholar]
- 27.Klebermass-Schrehof K, Rona Z, Waldhör T, et al. Can neurophysiological assessment improve timing of intervention in posthaemorrhagic ventricular dilatation? Arch Dis Child Fetal Neonatal Ed 2013;98:F291–F297. [DOI] [PubMed] [Google Scholar]
- 28.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging 2002;16:621–632. [DOI] [PubMed] [Google Scholar]
- 29.Messerschmidt A, Prayer D, Brugger PC, et al. Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol 2008;12:455–460. [DOI] [PubMed] [Google Scholar]
- 30.Jary S, De Carli A, Ramenghi LA, Whitelaw A. Impaired brain growth and neurodevelopment in preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr 2012;101:743–748. [DOI] [PubMed] [Google Scholar]
- 31.Bassan H, Eshel R, Golan I, et al. ; External Ventricular Drainage Study Investigators. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur J Paediatr Neurol 2012;16:662–670. [DOI] [PubMed] [Google Scholar]
- 32.Limbrick DD Jr, Mathur A, Johnston JM, et al. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr 2010;6:224–230. [DOI] [PubMed] [Google Scholar]
- 33.Lam HP, Heilman CB. Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. J Matern Fetal Neonatal Med 2009;22:1097–1101. [DOI] [PubMed] [Google Scholar]
- 34.Brouwer AJ, Groenendaal F, van den Hoogen A, et al. Incidence of infections of ventricular reservoirs in the treatment of post-haemorrhagic ventricular dilatation: a retrospective study (1992–2003). Arch Dis Child Fetal Neonatal Ed 2007;92:F41–F43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kormanik K, Praca J, Garton HJ, et al. Repeated tapping of ventricular reservoir in preterm infants with post-hemorrhagic ventricular dilatation does not increase the risk of reservoir infection. J Perinatol 2010;30:218–221. [DOI] [PubMed] [Google Scholar]
- 36.Brouwer AJ, Groenendaal F, Han KS, de Vries LS. Treatment of neonatal progressive ventricular dilatation: a single-centre experience. J Matern Fetal Neonatal Med 2015;28(suppl 1):2273–2279. [DOI] [PubMed] [Google Scholar]