Abstract
Objective
To determine the contribution of electrophysiologic testing in the diagnosis and anatomical classification of myoclonus.
Methods
Participants with a clinical diagnosis of myoclonus were prospectively recruited, each undergoing a videotaped clinical examination and battery of electrophysiologic tests. The diagnosis of myoclonus and its subtype was reviewed after 6 months in the context of the electrophysiologic findings and specialist review of the videotaped clinical examination.
Results
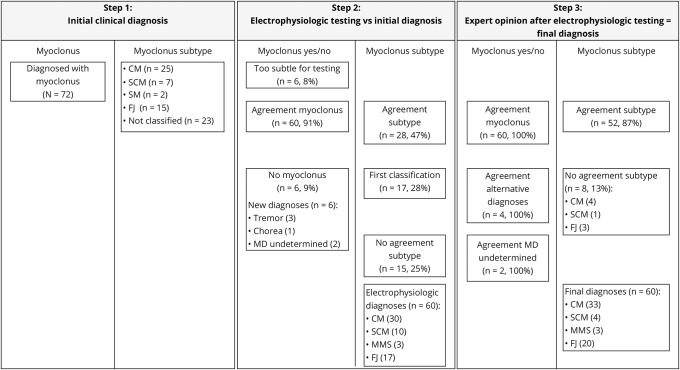
Seventy-two patients with myoclonus were recruited. Initial clinical anatomical classification included 25 patients with cortical myoclonus, 7 with subcortical myoclonus, 2 with spinal myoclonus, and 15 with functional myoclonic jerks. In 23 cases, clinical anatomical classification was not possible because of the complexity of the movement disorder. Electrophysiologic testing was completed in 66, with agreement of myoclonus in 60 (91%) and its subtype in 28 (47%) cases. Subsequent clinical review by a movement disorder specialist agreed with the electrophysiologic findings in 52 of 60; in the remaining 8, electrophysiologic testing was inconclusive.
Conclusions
Electrophysiologic testing is an important additional tool in the diagnosis and anatomical classification of myoclonus, also aiding in decision-making regarding therapeutic management. Further development of testing criteria is necessary to optimize its use in clinical practice.
Myoclonus is a frequently observed hyperkinetic movement disorder, which is often classified according to its anatomical origin: cortical myoclonus (CM), subcortical myoclonus (SCM), spinal myoclonus (SM), peripheral myoclonus (PM), or functional jerks (FJ) in case of a functional movement disorder.
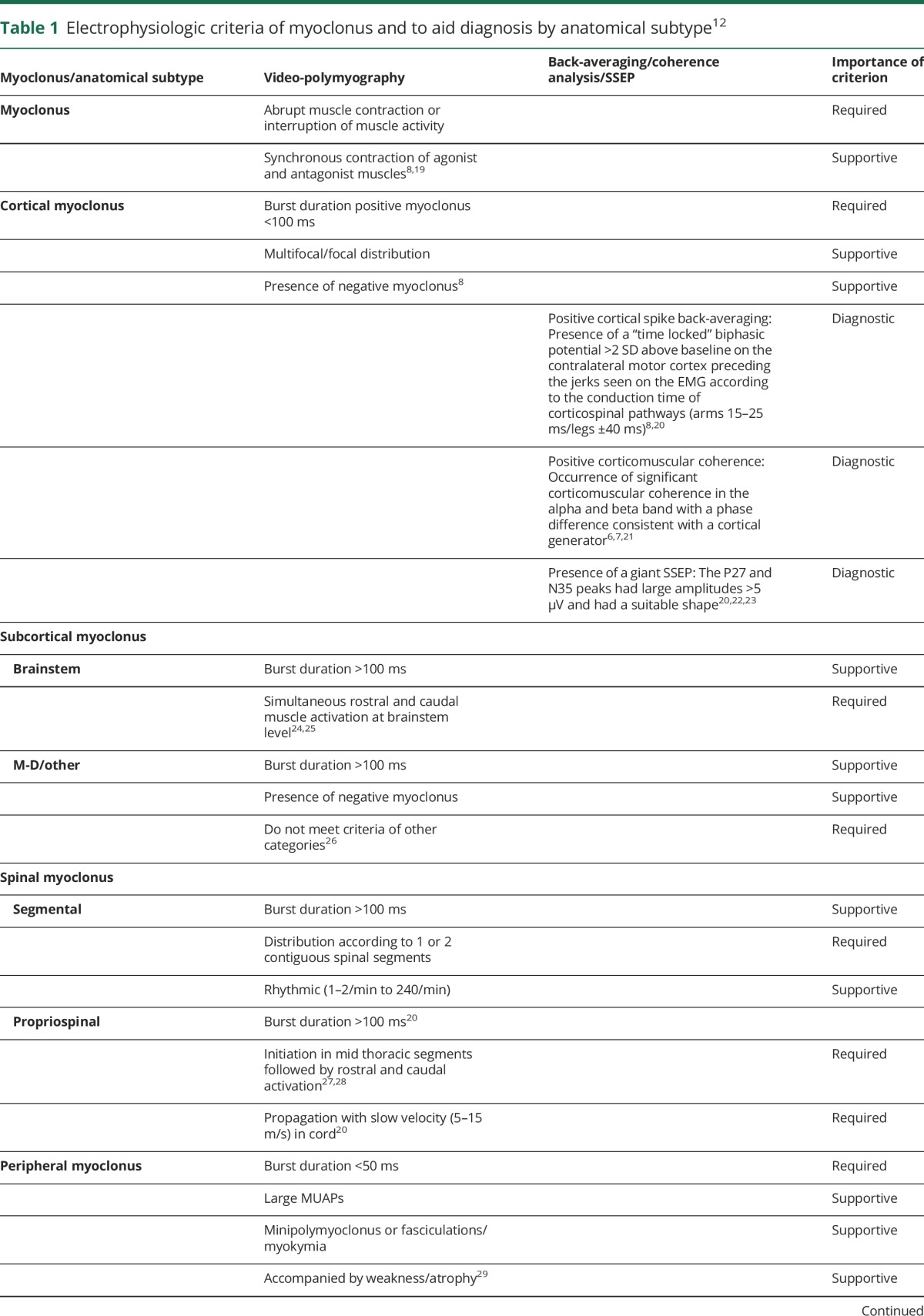
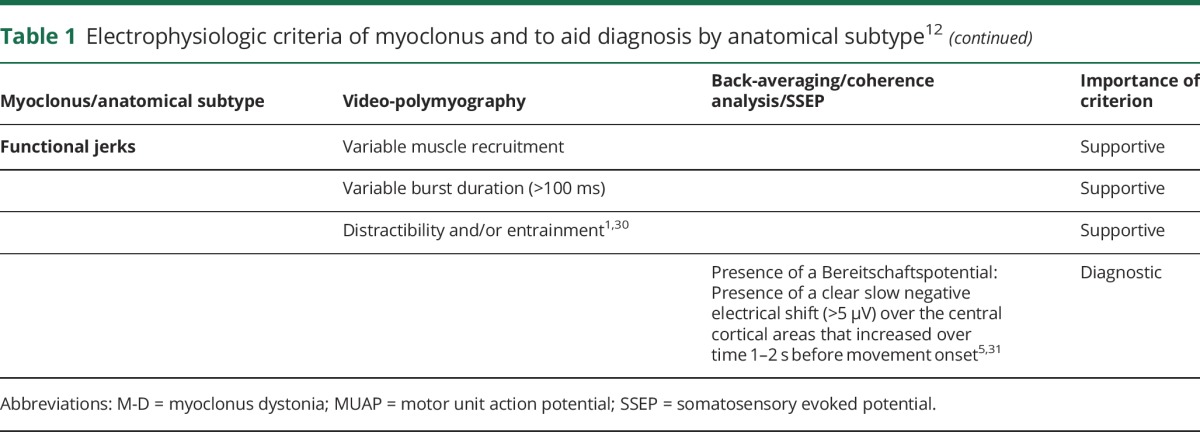
Electrophysiologic testing is frequently useful in distinguishing myoclonus from other hyperkinetic movement disorders, and in identifying its anatomical origin.1–3 The tests used in the assessment of myoclonus include polymyography, EEG-EMG back-averaging, coherence analysis, and somatosensory evoked potential (SSEP).4–8 Table 1 summarizes the electrophysiologic criteria used in the diagnosis of myoclonus and its subtypes.
Table 1.
Electrophysiologic criteria of myoclonus and to aid diagnosis by anatomical subtype12
The sensitivity and specificity of electrophysiologic testing in patients with myoclonus are largely unknown, with the majority of work to date being limited by small cohorts, highly selected patient populations, or reliance on expert opinion to determine the diagnosis.9–11
Our recent retrospective analysis of 85 patients with myoclonus demonstrated the key clinical and electrophysiologic features in distinguishing myoclonus subtypes.12 In 74% of cases, the clinical diagnosis of myoclonus was confirmed with electrophysiologic testing, and electrophysiologic assessment of the myoclonus subtype aided diagnosis in 73% of cases. In this study, we sought to apply these principles to a prospectively recruited cohort of patients, evaluating the contribution of electrophysiologic testing in the diagnosis and management of myoclonus.
Methods
Participants
Participants with a clinical diagnosis of myoclonus were identified prospectively from inpatient and outpatient settings (July 2014 to June 2016). Exclusion criteria included ongoing inpatient care on the intensive care unit, language and/or literacy barriers, and age 6 years or younger. All participants were followed up for a minimum of 6 months, after which a final diagnosis was made.
Initial clinical classification
The initial clinical diagnosis of myoclonus and its anatomical subtype was provided by the participants' primary caring neurologist (adult or pediatric), with all participants undergoing a standardized and systematic assessment, including videotaped clinical examination.
Electrophysiologic testing
The standardized electrophysiologic protocol included an initial polymyography, with participants excluded at this stage if the myoclonus was too subtle to adequately perform the assessment. For those meeting electrophysiologic criteria for myoclonus, further investigations included EEG-EMG back-averaging (if >25 jerks) or coherence analysis (if jerk frequency was >3 Hz). Where possible, those with CM and SCM underwent testing for SSEPs (figure e-1, http://links.lww.com/WNL/A164).
An experienced neurophysiologist (J.W.E. and J.H.v.d.H.) blinded to the original clinical diagnosis determined whether the findings were consistent with myoclonus, and the likely myoclonus subtype. Table 1 summarizes the electrophysiologic criteria used in determining diagnosis.12
Diagnostic review and 6-month follow-up
A neurologist with expertise in movement disorders (M.A.J.T.), blinded to the initial diagnoses, reviewed the clinical details, videotaped clinical examination, and results of the electrophysiologic testing. Each patient was reviewed again 6 months after their initial assessment to determine any changes to the clinical findings, with the final diagnosis being confirmed by the specialist (figure 1).
Figure 1. Overview of the stages of clinical assessment and diagnosis undertaken in this study.
CM = cortical myoclonus; FJ = functional jerks; MD = movement disorder; MMS = multiple myoclonus subtypes; SCM = subcortical myoclonus; SM = spinal myoclonus.
Severity of the myoclonus
The severity of the myoclonus was determined by 2 independent clinicians (R.Z. and J.C.v.Z. or J.M.G.) following review of the videotaped clinical examinations, scoring sections 2 and 4 of the Unified Myoclonus Rating Scale (UMRS), and the 7-point Global Clinical Impression–Severity (GCI-S) scale.
Power analysis
A power calculation was performed based on our previously reported retrospective analysis.12 It was estimated that electrophysiologic testing would support the clinical diagnosis of the myoclonus anatomical subtype in approximately 70%. A change in clinical classification of >20%, due to electrophysiologic testing, was considered clinically relevant. Using the One Proportion Confidence Interval Formula: Exact (Clopper-Pearson), a 95% confidence level, 0.7 (proportion), 0.8 (upper limit), we estimated that a minimum of 56 participants would need to be recruited.
Statistical analysis
The clinical characteristics were analyzed using Kruskal-Wallis tests for continuous, nonnormally distributed data. Interrater reliability was assessed using the intraclass correlation coefficient (ICC) (2-way mixed, consistency, average measures),13 or Cohen κ14 where appropriate. A Chi-squared Automatic Interaction Detection (CHAID) (SPSS, IBM, Armonk, NY; parent nodes n < 3, child nodes n > 1) analysis was undertaken to generate a decision tree in order to quantify the importance of the clinical and electrophysiologic criteria in the diagnosis of the myoclonic subtypes.
Standard protocol approvals, registrations, and patient consents
Full written informed consent was obtained from all participants according to the Declaration of Helsinki. The study protocol was approved by the University Medical Centre Groningen ethics committee (M14.157933, approved July 2, 2014).
Results
Overall cohort
A total of 72 patients (32 male; 40 female) were recruited, with a median age of 29 years (range: 7–83 years), 59 from the outpatient setting and 13 from inpatient care.
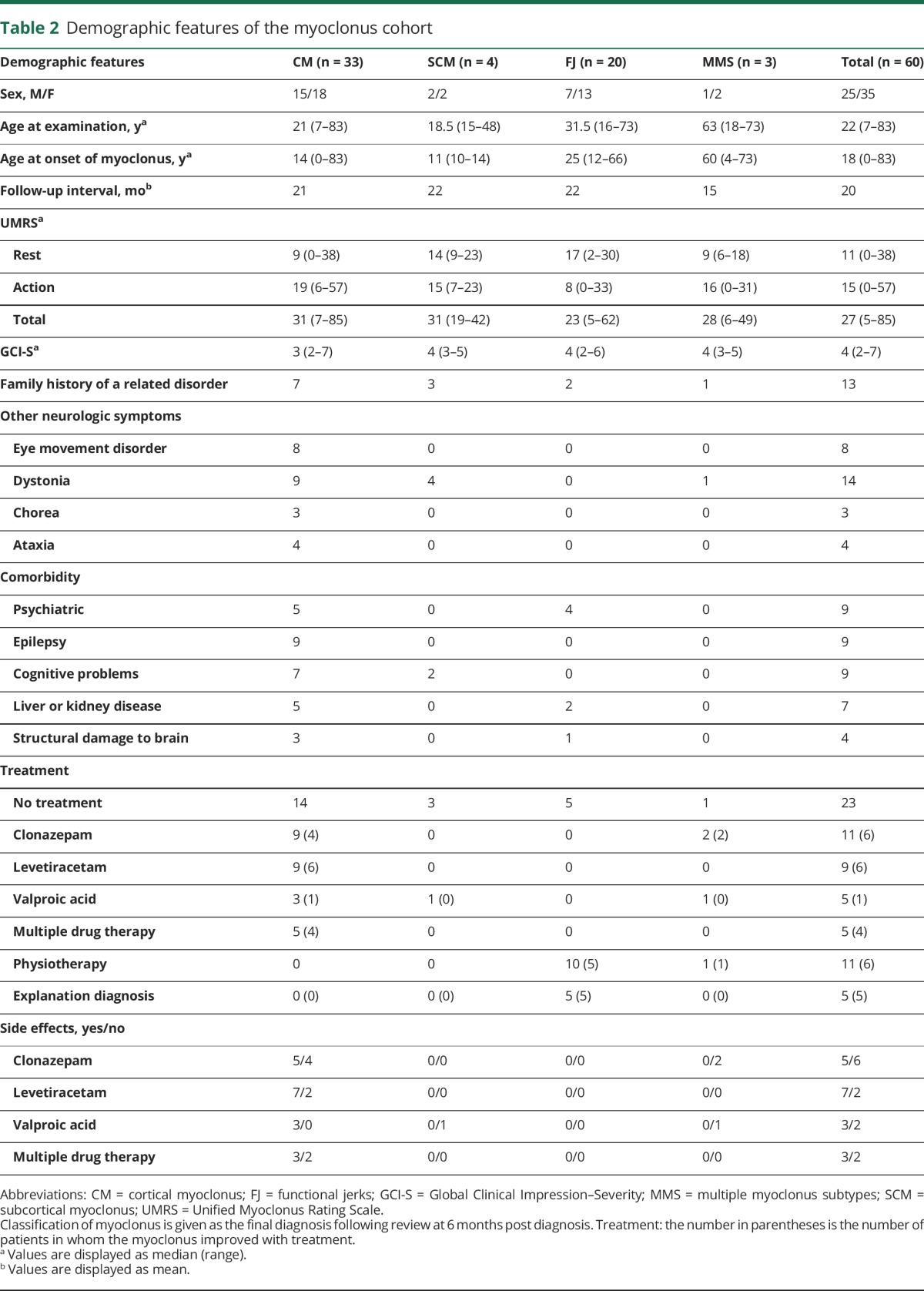
The demographic details and clinical characteristics of this cohort are summarized in table 2 and table e-1 (http://links.lww.com/WNL/A165), respectively.
Table 2.
Demographic features of the myoclonus cohort
Clinical diagnosis of myoclonus pre-electrophysiologic testing
Of the 72 individuals with myoclonus, these were subdivided into CM (n = 25), SCM (n = 7), SM (n = 2), and FJ (n = 15), with subtype diagnoses not possible in 23 patients (32%) because of the complexity of the movement disorder.
Electrophysiologic diagnoses
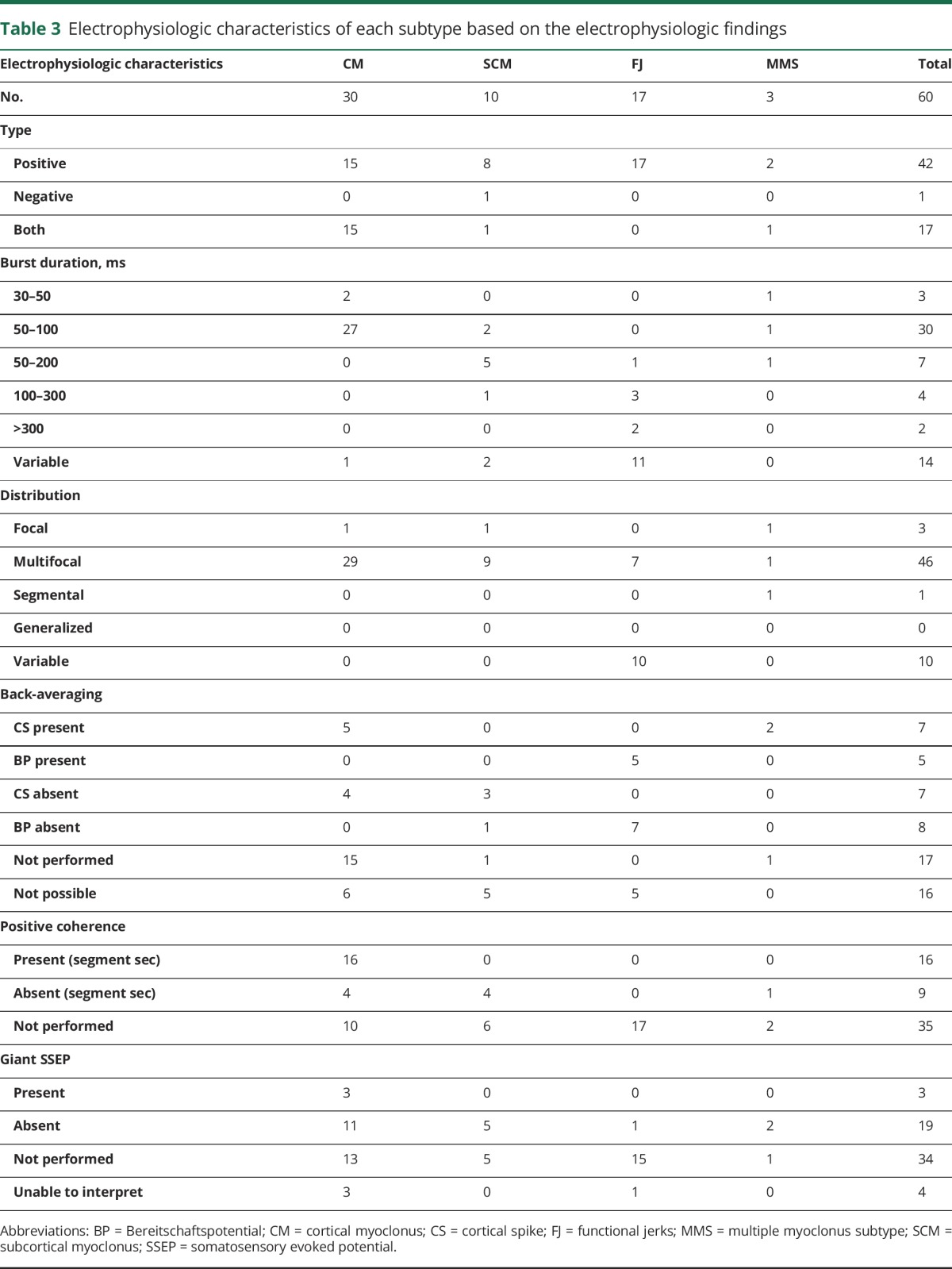
In 6 patients (8%), clinically diagnosed with distal multifocal CM, the myoclonic jerks were of such small amplitude that the polymyographic recordings were indeterminate and unable to be interpreted. Of the remaining 66 patients, electrophysiologic testing supported a diagnosis of myoclonus in 60 (91%), with these subdivided into CM (n = 30), SCM (n = 10), multiple myoclonus subtypes (MMS) (n = 3), and FJ (n = 17). A cortical origin was detected in 5 of 9 patients (60%) with CM using back-averaging, and 16 of 20 (80%) using coherence analysis. SSEP analysis demonstrated giant potentials in 3 of 14 patients (21%) with CM, and a Bereitschaftspotential was identified in 5 of 12 patients (42%) with FJ.
A full summary of the electrophysiologic characteristics of this cohort can be seen in table 3.
Table 3.
Electrophysiologic characteristics of each subtype based on the electrophysiologic findings
Comparison of clinical and electrophysiologic diagnoses
There was agreement between the clinical diagnosis and electrophysiologic testing in a diagnosis of myoclonus for 91% (60/66) of the study cohort. Of these 60 cases, there was agreement of its subtype in 28 cases (47%) (14 CM, 2 SCM, and 12 FJ) and disagreement in 15 cases (25%). Of the remaining 17 cases (28%) without a clinical subclassification, electrophysiologic testing proved helpful, subdividing these into 12 CM, 2 SCM, and 3 FJ (table e-2, http://links.lww.com/WNL/A165).
Clinical opinion of the movement disorder specialist
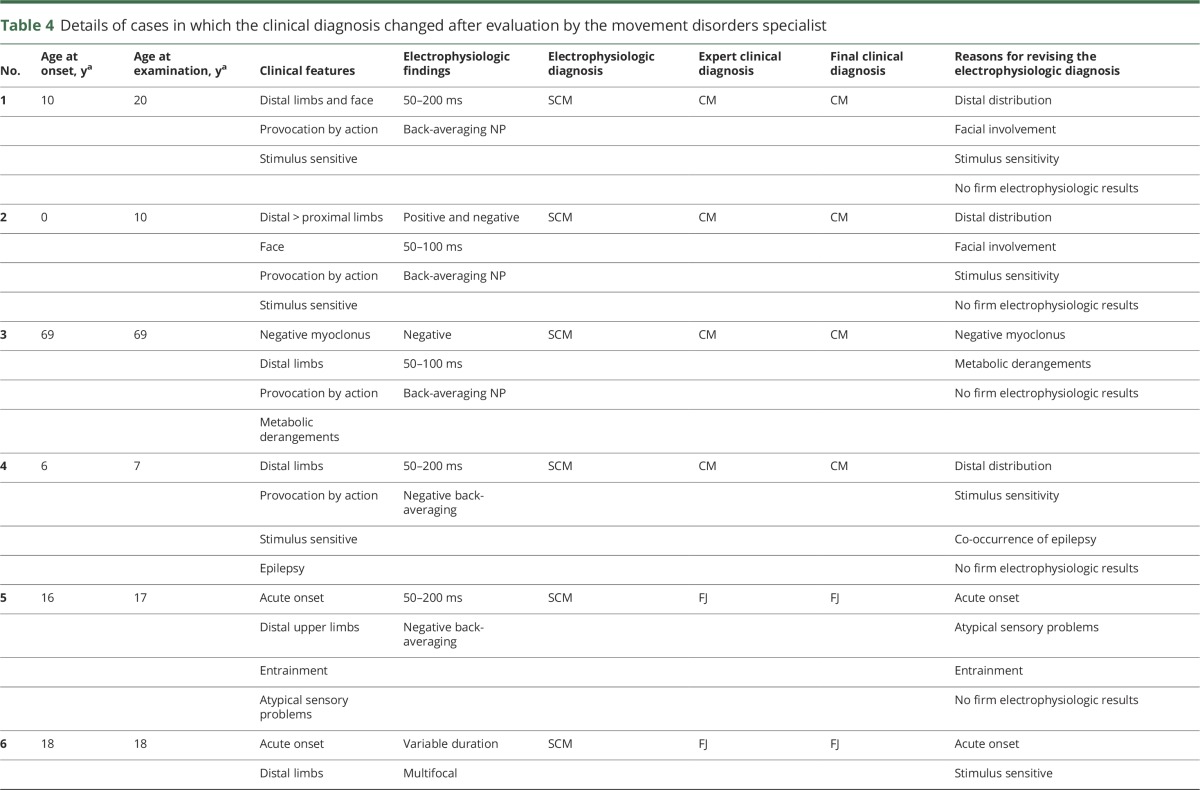
There was agreement between the electrophysiologic testing and specialist movement disorder opinion in 66 cases, and agreement on its subtype in 52 of 60 cases (87%), considered a “substantial” agreement (κ = 0.78). A summary of the 8 cases in which there was disagreement between expert clinical diagnosis and electrophysiologic testing is provided in table 4; in each, there was a lack of conclusive electrophysiologic findings to facilitate a diagnosis of myoclonus subtype.
Table 4.
Details of cases in which the clinical diagnosis changed after evaluation by the movement disorders specialist
Final clinical diagnoses
Follow-up review after 6 months resulted in no changes to clinical diagnosis in all 60 patients, with the final subclassification including 33 CM (55%), 4 SCM (7%), 3 MMS (5%), and 20 FJ (33%). The CHAID analysis demonstrated (1) polymyographic measurement of the myoclonic burst duration, (2) exacerbation of the myoclonus with action, and (3) facial involvement to be the most important criteria in determining myoclonic subtype (figure e-2, http://links.lww.com/WNL/A164).
Severity of myoclonus
The median UMRS severity score was 27 (Rest 11/128, Action 15/144) and GCI-S score 4/7. No significant statistical difference was observed between the subtypes of myoclonus (p = 0.2). The interrater concordance was “excellent” (ICC = 0.94 [95% confidence interval: 0.9–0.96]) and “good” (ICC = 0.72 [95% confidence interval: 0.58–0.82]) for the UMRS and GCI-S, respectively.
Underlying etiology of the myoclonus
Of the 40 patients diagnosed with an organic movement disorder, an underlying etiology was identified in 21 patients (53%). In 12 patients, a causative genetic mutation was identified, and 9 were found to have an acquired cause including metabolic disturbances (n = 3), drug-induced myoclonus (n = 1), and structural brain lesions (n = 2). Of those with an underlying genetic etiology, the highest rate was among those with CM (n = 10), with mutations in the NKX2.1 (n = 2) and NPC1 (n = 2) genes being most common. A single case of a contiguous gene deletion (578 kb, 16p11.2) involving the PRRT2 gene was identified with an extended phenotype including psychomotor retardation, hemiplegic migraine, epilepsy, myoclonus, and dystonia. All patients with a myoclonic epilepsy syndrome had evidence of epileptiform discharges on EEG, with the CM in those with juvenile myoclonic epilepsy and Lafora disease demonstrating an epileptic origin. All 4 patients with SCM had a clinical diagnosis of myoclonus dystonia, with a RELN variant identified in one patient. Table 5 summarizes the etiologic diagnoses and additional clinical characteristics.
Table 5.
Underlying etiologic diagnoses and additional clinical characteristics

Discussion
This prospective study has sought to demonstrate the benefit of electrophysiologic testing alongside clinical examination, in determining the diagnosis of myoclonus and its subtype in an unselected cohort. We have shown that this combined approach leads to changes in the initial diagnosis of myoclonus and its subtype in 53% of cases.
Overall, agreement of a diagnosis of myoclonus between the examining clinicians and the electrophysiologic findings was 91% (n = 60), decreasing to 47% (n = 28) with anatomical subtype. These findings contrast with results from similar studies in tremor cohorts (n = 210) where agreement between the 2 assessment forms was 87%, potentially reflecting greater clinical familiarity and larger patient cohorts.15–17 We identified several clinical groups in which there was some consistency in the change in diagnosis following electrophysiologic testing. These included those with multifocal myoclonus (principally distinguishing between CM and SCM), combined movement disorders (e.g., myoclonus in the presence of dystonia), and functional jerks. The findings from this study also reflect the difficulty in determining a conclusive clinical diagnosis with myoclonus, and lend weight to the importance of electrophysiologic testing, particularly in nonspecialist centers.
Higher-level electrophysiologic techniques were used to determine whether the myoclonus was of cortical origin or an FJ. The yield of back-averaging and coherence analysis to confirm a cortical origin was 60% and 80%, respectively. The additive value of these techniques was lower than the 100% seen in previous studies, potentially attributable to the heterogeneity of our cohort in contrast to smaller, more selected study groups (n = 20/n = 3).9,18 A CHAID analysis demonstrated that a combination of polymyography (burst duration) and clinical phenomenology provided the greatest accuracy (95%) in determining myoclonus subtype.
This study is limited by the lack of a definitive diagnostic test or marker. We have sought to reduce this by ensuring a minimum 6-month follow-up period to allow for any changes in clinical symptomatology. However, this lack of objective testing also serves to reinforce the potential gain of routine electrophysiologic testing to both aid, and provide additional evidence of the diagnosis of myoclonus and its subtype. Our cohort also likely reflects a more complex group of patients than might be expected in routine clinical practice, because of recruitment from a single specialist movement disorder center. We also acknowledge that while the electrophysiologic tests discussed are readily available within our center, such access varies considerably between centers and internationally.
Electrophysiologic testing is an important contributing diagnostic tool for the classification of myoclonus and its subtypes. While this clearly constitutes an important element of clinical work for neurologists with an interest in movement disorders, this algorithm of testing is also likely to be of use for those working in the fields of metabolic disorders, pediatrics, and epilepsy. Further development of the electrophysiologic criteria for myoclonus subtypes, and application of this work to larger, unselected patient cohorts is essential to improve its objectivity and diagnostic value.
Glossary
- CHAID
Chi-squared Automatic Interaction Detection
- CM
cortical myoclonus
- CS
cortical spike
- FJ
functional jerks
- GCI-S
Global Clinical Impression–Severity
- ICC
intraclass correlation coefficient
- MMS
multiple myoclonus subtype
- PM
peripheral myoclonus
- SCM
subcortical myoclonus
- SM
spinal myoclonus
- SSEP
somatosensory evoked potential
- UMRS
Unified Myoclonus Rating Scale
Author contributions
R. Zutt: design of the study, collecting data, analysis and interpretation of the data, drafting and revising the manuscript. J.W. Elting: design of the study, collecting data, revising the manuscript. J.C. van Zijl: collecting data, revising the manuscript. J.H. van der Hoeven, C.M. Roosendaal, and J.M. Gelauff: collecting data, revising the manuscript. K.J. Peall: analysis and interpretation of the data, drafting and revising the manuscript. M.A.J. Tijssen: design of the study, collecting data, analysis and interpretation of the data, drafting and revising the manuscript.
Study funding
No targeted funding reported.
Disclosure
R. Zutt and J.W. Elting report no disclosures relevant to the manuscript. J.C. van Zijl is funded by the MD/PhD scholarship of the University of Groningen. J.H. van der Hoeven and C.M. Roosendaal report no disclosures relevant to the manuscript. J.M. Gelauff is funded by a scholarship from the Research School of Behavioural and Cognitive Neurosciences (BCN), part of the University of Groningen. K.J. Peall is funded by an MRC Clinician-Scientist Fellowship (MR/P008593/1). M.A.J. Tijssen is funded by STW Technology Society–NeuroSIPE, Netherlands Organization for Scientific Research–NWO Medium, Fonds NutsOhra, Prinses Beatrix Fonds, Gossweiler Foundation, Phelps Stichting, Stichting wetenschapsfonds dystonie vereniging, and educational grants from Ipsen, Allergan, Merz, Actelion, and Medtronic. Go to Neurology.org/N for full disclosures.
References
- 1.van der Salm SM, de Haan RJ, Cath DC, van Rootselaar AF, Tijssen MA. The eye of the beholder: inter-rater agreement among experts on psychogenic jerky movement disorders. J Neurol Neurosurg Psychiatry 2013;7:742–747. [DOI] [PubMed] [Google Scholar]
- 2.Erro R, Bhatia KP, Edwards MJ, Farmer SF, Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord 2013;28:1868–1873. [DOI] [PubMed] [Google Scholar]
- 3.Esposito M, Edwards MJ, Bhatia KP, Brown P, Cordivari C. Idiopathic spinal myoclonus: a clinical and neurophysiological assessment of a movement disorder of uncertain origin. Mov Disord 2009;24:2344–2349. [DOI] [PubMed] [Google Scholar]
- 4.Shibasaki H, Nakamura M, Nishida S, Kakigi R, Ikeda A. Wave form decomposition of “giant SEP” and its computer model for scalp topography. Electroencephalogr Clin Neurophysiol 1990;77:286–294. [DOI] [PubMed] [Google Scholar]
- 5.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol 2006;117:2341–2356. [DOI] [PubMed] [Google Scholar]
- 6.Grosse P, Cassidy MJ, Brown P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: physiological principles and clinical applications. Clin Neurophysiol 2002;113:1523–1531. [DOI] [PubMed] [Google Scholar]
- 7.Brown P, Farmer SF, Halliday DM, Marsden J, Rosenberg JR. Coherent cortical and muscle discharge in cortical myoclonus. Brain 1999;122:461–472. [DOI] [PubMed] [Google Scholar]
- 8.Shibasaki H, Yamashita Y, Kuroiwa Y. Electroencephalographic studies myoclonus. Brain 1978;101:447–460. [DOI] [PubMed] [Google Scholar]
- 9.Caviness JN, Adler CH, Beach TG, Wetjen KL, Caselli RJ. Small-amplitude cortical myoclonus in Parkinson's disease: physiology and clinical observations. Mov Disord 2002;17:657–662. [DOI] [PubMed] [Google Scholar]
- 10.Sinha S, Satishchandra P, Gayathri N, Yasha TC, Shankar SK. Progressive myoclonic epilepsy: a clinical, electrophysiological and pathological study from South India. J Neurol Sci 2007;252:16–23. [DOI] [PubMed] [Google Scholar]
- 11.Binelli S, Agazzi P, Canafoglia L, et al. Myoclonus in Creutzfeldt-Jakob disease: polygraphic and video-electroencephalography assessment of 109 patients. Mov Disord 2010;25:2818–2827. [DOI] [PubMed] [Google Scholar]
- 12.Zutt R, Elting JW, van der Hoeven JH, Lange F, Tijssen MA. Myoclonus subtypes in tertiary referral center: cortical myoclonus and functional jerks are common. Clin Neurophysiol 2017;128:253–259. [DOI] [PubMed] [Google Scholar]
- 13.Fermanian J. Measuring agreement between 2 observers: a quantitative case [in French]. Rev Epidemiol Sante Publique 1984;32:408–413. [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 15.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998;13:5–10. [DOI] [PubMed] [Google Scholar]
- 16.Caviness JN, Alving LI, Maraganore DM, Black RA, McDonnell SK, Rocca WA. The incidence and prevalence of myoclonus in Olmsted County, Minnesota. Mayo Clin Proc 1999;74:565–569. [DOI] [PubMed] [Google Scholar]
- 17.van der Stouwe AM, Elting JW, van der Hoeven JH, et al. How typical are “typical” tremor characteristics? Sensitivity and specificity of five tremor phenomena. Parkinsonism Relat Disord 2016;30:23–28. [DOI] [PubMed] [Google Scholar]
- 18.Rossi Sebastiano D, Soliveri P, Panzica F, et al. Cortical myoclonus in childhood and juvenile onset Huntington's disease. Parkinsonism Relat Disord 2012;18:794–797. [DOI] [PubMed] [Google Scholar]
- 19.Tassinari CA, Rubboli G, Shibasaki H. Neurophysiology of positive and negative myoclonus. Electroencephalogr Clin Neurophysiol 1998;107:181–195. [DOI] [PubMed] [Google Scholar]
- 20.Shibasaki H, Hallett M. Electrophysiological studies of myoclonus. Muscle Nerve 2005;31:157–174. [DOI] [PubMed] [Google Scholar]
- 21.Grosse P, Guerrini R, Parmeggiani L, Bonanni P, Pogosyan A, Brown P. Abnormal corticomuscular and intermuscular coupling in high-frequency rhythmic myoclonus. Brain 2003;126:326–342. [DOI] [PubMed] [Google Scholar]
- 22.Shibasaki H, Yamashita Y, Neshige R, Tobimatsu S, Fukui R. Pathogenesis of giant somatosensory evoked potentials in progressive myoclonic epilepsy. Brain 1985;108:225–240. [DOI] [PubMed] [Google Scholar]
- 23.Obeso JA, Rothwell JC, Marsden CD. Somatosensory evoked potentials in myoclonus. Adv Neurol 1986;43:373–384. [PubMed] [Google Scholar]
- 24.Bakker MJ, van Dijk JG, van den Maagdenberg AM, Tijssen MA. Startle syndromes. Lancet Neurol 2006;5:513–524. [DOI] [PubMed] [Google Scholar]
- 25.Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain 1991;114:1903–1928. [DOI] [PubMed] [Google Scholar]
- 26.Marelli C, Canafoglia L, Zibordi F, et al. A neurophysiological study of myoclonus in patients with DYT11 myoclonus-dystonia syndrome. Mov Disord 2008;23:2041–2048. [DOI] [PubMed] [Google Scholar]
- 27.Brown P, Thompson PD, Rothwell JC, Day BL, Marsden CD. Axial myoclonus of propriospinal origin. Brain 1991;114:197–214. [PubMed] [Google Scholar]
- 28.Brown P, Rothwell JC, Thompson PD, Marsden CD. Propriospinal myoclonus: evidence for spinal “pattern” generators in humans. Mov Disord 1994;9:571–576. [DOI] [PubMed] [Google Scholar]
- 29.Zutt R, Elting JW, Tijssen MAJ. Myoclonus. In: Wolters Baumann EC, editors. Parkinson Disease and Other Movement Disorders. 1st ed. Amsterdam: VU University Press; 2014:513–533. [Google Scholar]
- 30.Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol 2006;5:695–700. [DOI] [PubMed] [Google Scholar]
- 31.van der Salm SM, Tijssen MA, Koelman JH, van Rootselaar AF. The bereitschaftspotential in jerky movement disorders. J Neurol Neurosurg Psychiatry 2012;83:1162–1167. [DOI] [PubMed] [Google Scholar]