Abstract
Cystathionine β-synthase (CBS) is the central enzyme in the trans-sulfuration pathway that converts homocysteine to cysteine. It is also one of the three major enzymes involved in the biogenesis of H2S. CBS is a complex protein with a modular three-domain architecture, the central domain of which contains a 272CXXC275 motif whose function has yet to be determined. In the present study, we demonstrated that the CXXC motif exists in oxidized and reduced states in the recombinant enzyme by mass spectroscopic analysis and a thiol labeling assay. The activity of reduced CBS is ∼2–3-fold greater than that of the oxidized enzyme, and substitution of either cysteine in CXXC motif leads to a loss of redox sensitivity. The Cys272–Cys275 disulfide bond in CBS has a midpoint potential of −314 mV at pH 7.4. Additionally, the CXXC motif also exists in oxidized and reduced states in HEK293 cells under oxidative and reductive conditions, and stressing these cells with DTT results in more reduced enzyme and a concomitant increase in H2S production in live HEK293 cells as determined using a H2S fluorescent probe. By contrast, incubation of cells with aminooxyacetic acid, an inhibitor of CBS and cystathionine γ-lyase, eliminated the increase of H2S production after the cells were exposed to DTT. These findings indicate that CBS is post-translationally regulated by a redox-active disulfide bond in the CXXC motif. The results also demonstrate that CBS-derived H2S production is increased in cells under reductive stress conditions.
Keywords: oxidation-reduction (redox), redox regulation, hydrogen sulfide, disulfide, allosteric regulation, homocysteine, cystathionine beta synthase, sulfur metabolism
Introduction
Cystathionine β-synthase (CBS)3 is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that plays a key role in the metabolism of sulfur-containing amino acids (1–3). CBS, the first enzyme of the trans-sulfuration pathway, catalyzes the condensation of serine and homocysteine to produce cystathionine, which is subsequently converted to cysteine, the limiting substrate in the synthesis of the antioxidant glutathione (4, 5). Alternatively, CBS, which is one of three key enzymes involved in the production of H2S, can catalyze the condensation of homocysteine and cysteine or condensation of two molecules of cysteine to produce H2S, a recently recognized endogenous gasotransmitter that mediates diverse biological functions (6–9). Mutations in the cbs gene represent the single most common cause of homocystinuria, which leads to a number of complications affecting the cardiovascular, ocular, skeletal, and central nervous systems (10).
Human CBS is a modular protein composed of three functional domains that is intricately regulated (11, 12). The N-terminal domain binds the heme cofactor, which seems to function as a redox sensor (13) that inhibits CBS activity by means of binding of CO or NO (14–17) or during reduction of nitrite (18, 19). Nitration of tryptophan residues in CBS results in alterations of the heme pocket, which lead to the loss of cysteinate coordination by the ligand Cys52 and concomitant inactivation of CBS (20). Additionally, a structural role for the heme in the proper folding of CBS has been described (21). The C-terminal domain contains a tandem of the so-called CBS domain, which forms a regulatory domain that binds S-adenosylmethionine, an allosteric activator (22–24). Cleavage of the regulatory domain from full-length CBS yields a truncated dimeric enzyme that is more active than full-length CBS but is unresponsive to S-adenosylmethionine (25). The central domain contains the PLP cofactor, which is essential for catalysis, and a 272CXXC275 motif, the function of which has yet to be determined (26, 27).
The quantitative significance of the trans-sulfuration pathway for provision of intracellular cysteine, which is required for glutathione synthesis and H2S, links the metabolism of homocysteine directly to cellular redox homeostasis (28). Therefore, it is not surprising that a number of enzymes in the sulfur metabolic pathway display sensitivity to redox changes. Because CBS is the limiting enzyme in the trans-sulfuration pathway, we focused on evaluating its activity with respect to regulation in response to the ambient redox state. Indeed, our previous report used S-methylcysteine, a substrate analog of cysteine, to show that S-glutathionylation at Cys346 enhances human CBS activity under oxidative stress conditions (29). Furthermore, in addition to heme, human CBS has a second putative redox-active center, a CXXC motif that is surface-exposed in full-length CBS that lacks the loop of residues 516–525 (26). However, because high concentrations of homocysteine and cysteine are used as substrates in the canonical CBS activity assay, which would reduce the disulfide of the CXXC motif, the activity of oxidized CBS cannot be assessed using this assay.
We hypothesized that redox changes in the CXXC motif in CBS might regulate enzyme activity under various redox conditions and that the CXXC motif would be a redox sensor. Here, we found that the CXXC motif exists in oxidized and reduced states in recombinant CBS and in HEK293 cells. The activity of reduced CBS increased by ∼2–3-fold compared with oxidized CBS in vitro and resulted in a concomitant increase in CBS-derived H2S in response to reductive stress in HEK293 cells. Additionally, the midpoint potential of the Cys272–Cys275 disulfide bond in CBS was determined. These observations indicate that CBS activity is allosterically regulated by a redox-active disulfide bond.
Results
Reduced CBS has a higher activity than oxidized CBS
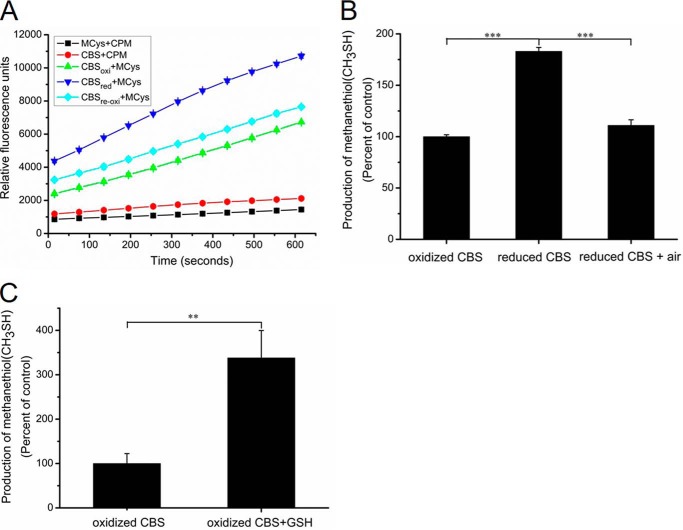
Previous reports showed that the CXXC motif in CBS exists in oxidized disulfide and reduced dithiol states in the structure of the truncated catalytic core enzyme that lacks the C-terminal regulatory domain (27). To investigate whether the activities of the reduced and oxidized forms of wildtype CBS differ, a modified assay in which S-methylcysteine served as the substrate was developed. The production of methanethiol (CH3SH) can be monitored by fluorescence spectroscopy in the presence of a fluorescent thiol probe (Fig. 1A). The relative activity of the reduced wildtype CBS was 1.8-fold higher than that of the oxidized CBS. When the reduced CBS was reoxidized by exposure to air, the activity of reoxidized CBS was restored to a level similar to that of the wildtype CBS (Fig. 1, A and B). Additionally, human CBS contains 11 cysteine residues, one of which, Cys52, coordinates the heme axially (10). The number of thiols in CBS protein was determined using a 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) assay. To fully expose the cysteine residues of the precipitated CBS proteins, 1% (w/v) SDS was used according to a previous report (37). A total of 9.8 ± 0.22 cysteines were modified per reduced CBS monomer, and the number decreased to 7.6 ± 0.12 when the reduced CBS were reoxidized by exposing them to air. This result indicated that the CXXC motif in reduced dithiol state can be oxidized to the disulfide state by exposure to air.
Figure 1.
The redox state of CBS affects its activity in vitro. A, recombinant wildtype CBS (1 mg/ml) was incubated with 10 mm DTT for 1 h at 4 °C, and the samples were then ultrafiltered to remove DTT. CBS activity was measured using S-methylcysteine (10 mm) as a substrate. The data points and error bars are the means ± S.D. of three independent experiments. MCys, S-methylcysteine; CBSoxi, no treatment; CBSred, DTT treatment; CBSreoxi, CBSred was reoxidized by exposure to air. B, methanethiol (CH3SH) production catalyzed by CBS was determined, and the initial reaction rates were calculated according to the data from Fig. 1A. The graph represents the relative activity of samples compared with the oxidized CBS and shows the means ± S.D. (n = 3). ***, p < 0.001. The specific activity of the oxidized CBS in the S-methylcysteine assay (using DTNB detection) was 0.45 ± 0.04 μmol methanethiol h−1 mg−1. C, recombinant CBS protein (20 μg) was treated with 20 mm GSH for 2 h at 4 °C. The activity was measured using S-methylcysteine (10 mm) as a substrate in the presence of 20 mm GSH. The CH3SH production was measured by gas chromatography. The graph represents the relative activity of sample compared with untreated CBS (oxidized CBS) and the means ± S.D. (n = 3). **, p < 0.01.
To avoid partial oxidization of reduced CBS after the removal of DTT, the activity of CBS that had been pretreated with 20 mm GSH was measured using gas chromatography in reaction mixtures that contained 20 mm GSH. The activity of the reduced CBS was ∼3-fold higher than that of the oxidized CBS (Fig. 1C). These results showed that CBS activity was regulated under the different redox conditions, and reduced CBS can readily be reoxidized by exposing it to air in a buffer lacking reductants.
The CXXC motif in CBS exists in disulfide and thiol states under oxidative and reductive conditions
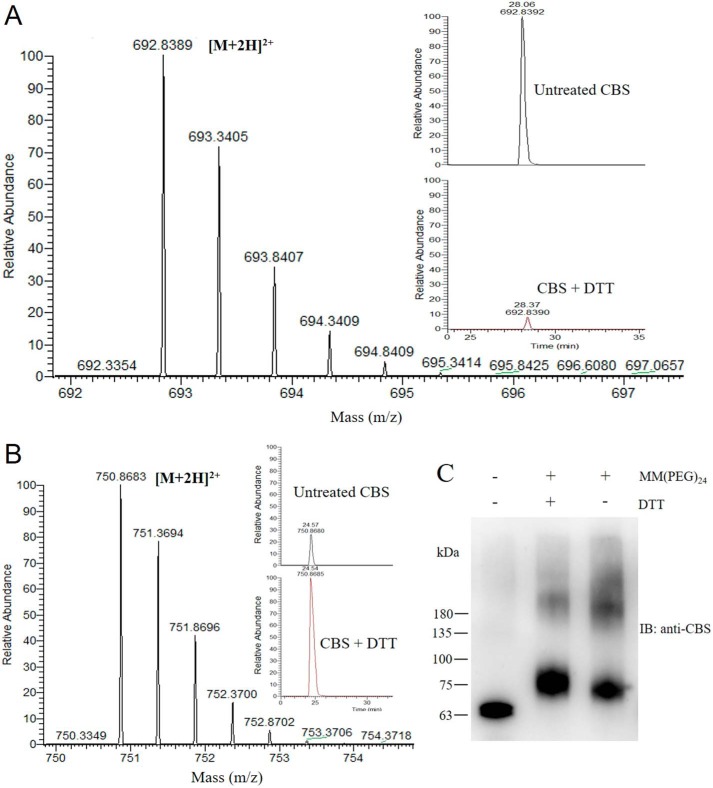
The vicinal cysteine residues of the CXXC motif are in the reduced state in the crystal structure of the modified CBS that lacks the loop from residues 516–525 (26). Although the vicinal cysteine residues of the CXXC motif can be oxidized to form a disulfide bond, which is observed in the crystal structure of the truncated catalytic core CBS (27), it is not known whether the oxidized disulfide bond of the CXXC motif exists in wildtype CBS. Purified wildtype CBS was treated with or without 10 mm DTT, and the resulting samples were analyzed using LC-MS/MS with a 99.8% of sequence coverage. The results showed that a unique peptide, KCPGCRIIGVDPE, contained a disulfide bond, which demonstrated that the oxidized form of the CXXC motif was detected. The redox state of the Cys272–Cys275 disulfide bond in purified CBS, and its susceptibility to reduction was determined. The unpaired Cys272 and Cys275 cysteines but not the Cys272–Cys275 disulfide-bond cysteines were alkylated with iodoacetamide. The monoisotopic mass [M+2H]2+ of the disulfide bond peptide was 692.8389 Da (observed nominal mass = 1384.6711 Da; expected nominal mass = 1384.6712 Da) (Fig. 2A and Fig. S1), and the monoisotopic mass [M+2H]2+ of the unpaired Cys272–Cys275 peptide that was alkylated with iodoacetamide was 750.8683 Da (observed nominal mass = 1500.7297 Da; expected nominal mass = 1500.7297 Da) (Fig. 2B and Fig. S2). Additionally, according to the peak area from the ion chromatogram, the relative ion abundance of the peptide containing Cys272–Cys275 disulfide bond in the reduced CBS was ∼6% of that in the oxidized CBS, which suggested that more than 90% of the disulfide bond in the CXXC motif was reduced after DTT treatment of the oxidized CBS (Fig. 2A).
Figure 2.
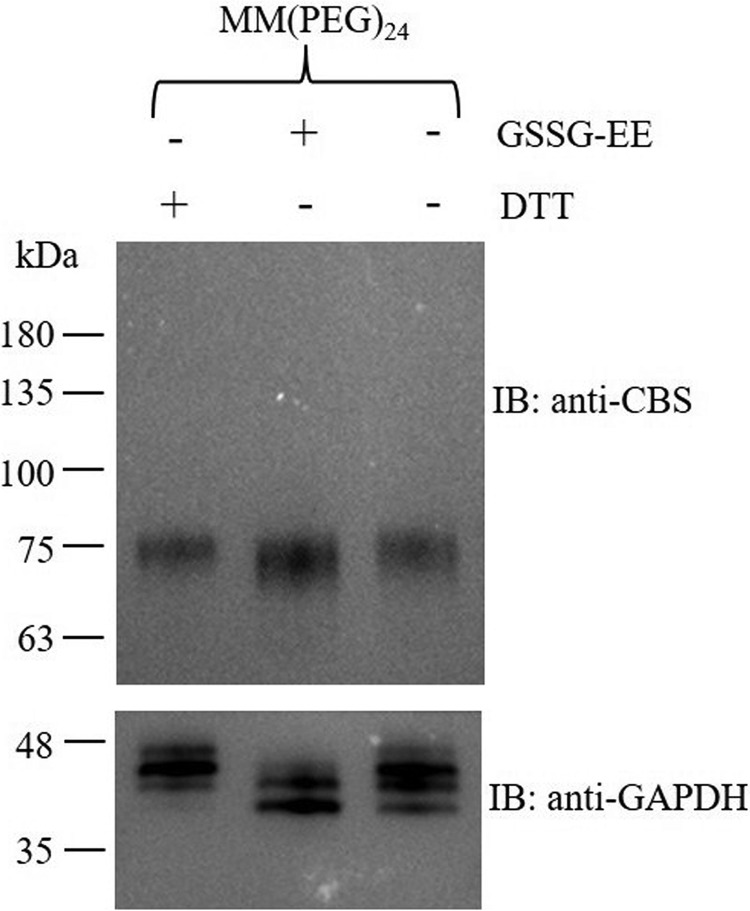
The 272CXXC275 motif in CBS protein exists in disulfide and thiol states under oxidative and reductive conditions. A, recombinant CBS protein was analyzed by LC-MS/MS. The mass spectrum of the KCPGCRIIGVDPE peptide that contained the oxidized form of the 272CXXC275 motif is shown. The monoisotopic mass [M+2H]2+ of this peptide was 692.8389 Da (observed nominal mass = 1384.6711 Da; expected nominal mass = 1384.6712 Da). The chromatographic peaks of the oxidized peptide from untreated CBS and reduced CBS are shown in the inset. B, recombinant CBS was treated with 10 mm DTT for 1 h and then was analyzed by LC-MS/MS. The mass spectrum of the KCPGCRIIGVDPE peptide showing the reduced form of 272CXXC275 motif is shown. The monoisotopic mass [M+2H]2+ of this iodoacetamide-derivatized peptide was 750.8683 Da (observed nominal mass = 1500.7297 Da; expected nominal mass = 1500.7297 Da). The chromatographic peaks of the reduced peptide from the untreated CBS and reduced CBS are shown in the inset. C, the recombinant CBS was incubated without or with 10 mm DTT for 1 h and then precipitated with trichloroacetic acid. The precipitated CBS was labeled with the methyl-PEG-maleimide reagent MM(PEG)24. The labeled samples were resolved on SDS-PAGE and immunoblotted for CBS. The molecular size markers in kDa are indicated at the left.
In addition to the mass spectrometry, methyl-PEG24-maleimide (MM[PEG]24) was used to label the thiols of the oxidized and reduced CBS. The difference between the molecular masses of the labeled oxidized and reduced forms of CBS was ∼3 kDa (Fig. 2C), which suggested that a single disulfide bond is formed in the purified CBS. Collectively, these results indicate that a reversible redox change in the vicinal cysteines involving a dithiol-disulfide reaction exists in wildtype CBS protein under different redox conditions.
The redox state of CXXC motif regulates CBS activity
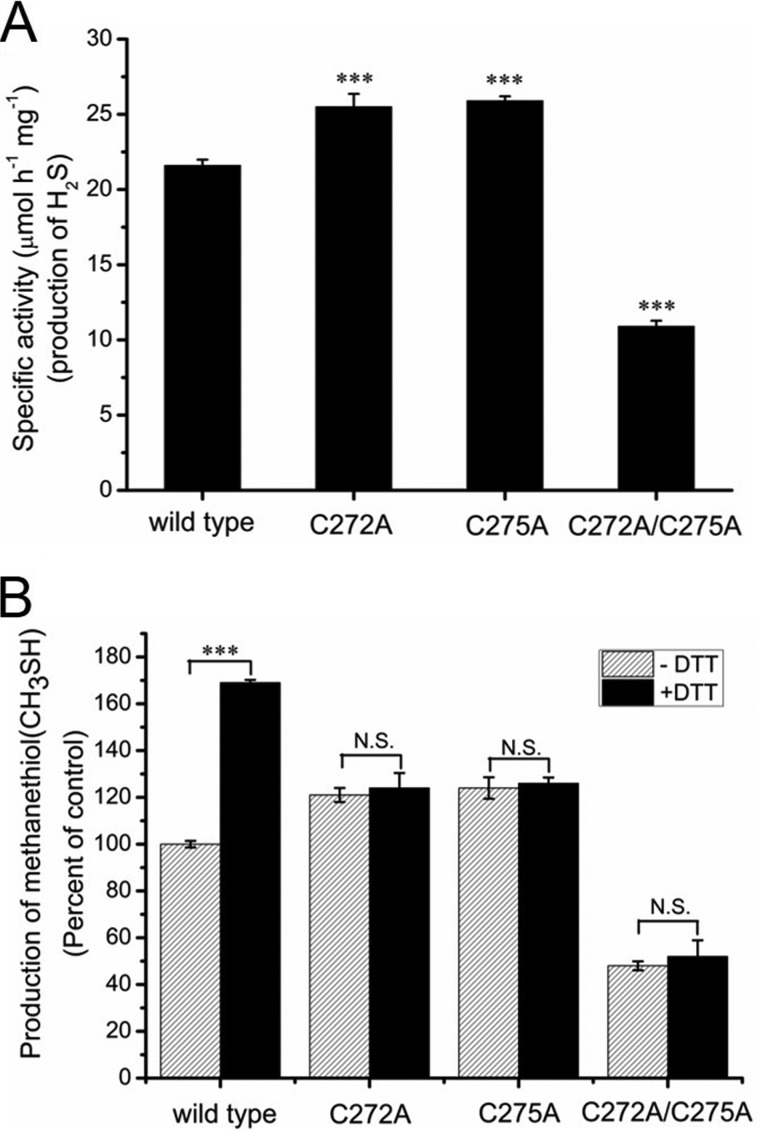
Because the Cys272–Cys275 was the unique disulfide bond detected in the oxidized CBS, we speculated that redox state of the CXXC motif would regulate CBS activity. The C272A, C275A, and C272A/C275A (double mutant) forms of the enzyme were purified, and the purity of each mutant protein was judged to be >90% by SDS-PAGE analysis. The yield of both purified C272A and C275A proteins (∼3 mg/liter culture) was ∼3-fold lower than the wildtype CBS (∼10 mg/liter culture), and the C272A/C275A protein was obtained in very low yield (∼0.5 mg/liter culture). The activities of the wildtype and mutant proteins were assessed in 100 mm Hepes buffer (pH 7.4) using the methylene blue assay. The basal activities of C272A and C275A mutant enzymes were 25.5 ± 0.86 and 25.9 ± 0.30 μmol mg−1 h−1, respectively, and were ∼1.2-fold higher than the wildtype CBS (21.6 ± 0.39 μmol mg−1 h−1). However, the activity of the C272A/C275A enzyme (10.9 ± 0.38 μmol mg−1 h−1) was ∼2-fold lower than the wildtype CBS (Fig. 3A).
Figure 3.
Redox state of 272CXXC275 motif regulates CBS activity. A, the specific activities of wildtype CBS and of the CBS variants C272A, C275A, and C272A/C275A (double mutant) were measured using the methylene blue assay. The data points and error bars indicate the means ± S.D. (n = 4). ***, p < 0.001 versus wildtype CBS. B, the wildtype CBS protein and CBS variants were incubated without or with 10 mm DTT. The activities of these samples were determined using S-methylcysteine as the substrate in a 200-μl reaction solution containing 30 μm CPM fluorescent probe and 100 mm Hepes, pH 7.4. The data points and error bars show the means ± S.D. of three independent experiments. ***, p < 0.001 versus control; N.S., not significant.
Previous studies indicated that the heme content in CBS protein affects the specific activity of enzyme (39). Therefore, the heme concentrations of wildtype and mutant CBS proteins were determined using the pyridine hemochrome assay (32, 33). The C272A, C275A, and C272A/C275A mutant enzymes have 0.92 ± 0.04, 1.14 ± 0.08, and 1.06 ± 0.01 hemes per CBS monomer, respectively, and are similar to that of wildtype CBS (0.98 ± 0.01 hemes per monomer). These results indicated that the lower activity of C272A/C275A double mutant may result from the conformational changes in CBS, not from the low heme content.
We next determined the effect of these mutations in the CXXC motif on the activity of CBS. Unlike wildtype CBS, no changes in the activity of these three mutants were observed before and after treatment with DTT (Fig. 3B). Collectively, these results were consistent with Cys272–Cys275 being the unique disulfide bond in purified CBS, with its redox state regulating CBS activity.
Redox potential of the Cys272–Cys275 disulfide bond
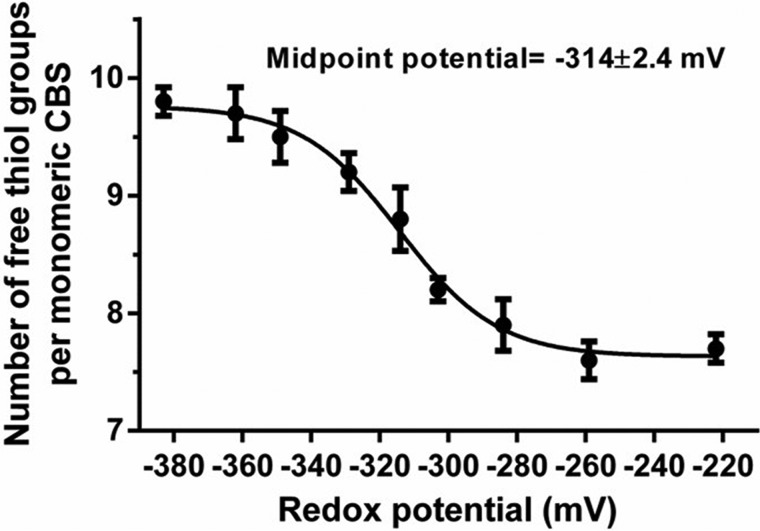
The midpoint potential of the Cys272–Cys275 disulfide bond was determined using DTT as the reductant. Because Cys272–Cys275 is the unique disulfide bond observed in purified wildtype CBS (Fig. 2), the DTNB assay was employed to determine the number of free thiol groups in the CBS protein, which was preincubated in solutions that were prepared by mixing different ratios of reduced dithiothreitol (DTTred) and oxidized dithiothreitol (DTToxi, trans-4,5-dihydroxy-1,2-dithiane; Sigma) followed by denaturation. The calculated midpoint potential for the CBS disulfide bond was −314 ± 2.4 mV (95% confidence interval: −320 to −308 mV) (Fig. 4), which is similar to the cytosolic glutathione redox potential (EGSH) of −300 to −320 mV in unstressed cells (40, 41).
Figure 4.
Redox potential of the Cys272–Cys275 disulfide bond in CBS. Recombinant CBS (100 μg) protein was preincubated in solutions containing various concentrations of reduced dithiothreitol (DTTred) and oxidized dithiothreitol (DTToxi, trans-4,5-dihydroxy-1,2-dithiane), pH 7.4, for 1 h at 37 °C and then precipitated with trichloroacetic acid. Precipitated CBS was dissolved with 100 mm Tris-HCl (pH 8.0) containing 1% (w/v) SDS. The number of free thiol groups was determined by titration with 0.5 mm DTNB. The data points and error bars show the means ± S.D. (n = 3).
CBS exists in oxidized and reduced states in HEK293 cells under oxidative and reductive conditions
A thiol labeling assay was employed to determine whether the CXXC motif in CBS exists as the oxidized or reduced form respectively in HEK293 cells. Briefly, the cells were incubated with DTT or oxidized glutathione ethyl ester (GSSG-EE) to induce reductive stress or oxidative stress, respectively. Subsequently, the cells were treated with TCA to avoid artificial thiol-disulfide modifications, and then the free thiol groups in proteins were labeled with MM[PEG]24, which has a molecular mass of 1240 Da. As shown in Fig. 5, the difference between the average molecular masses of the labeled CBS under reductive and oxidative stress was ∼2–3 kDa, which suggested that a single disulfide bond is formed. These results indicated that the CXXC motif in CBS protein exists in reduced and oxidized states in mammalian cells and that it can be influenced by relevant cellular redox stresses. Unexpectedly, glyceraldehyde-3-phosphate dehydrogenase, which was used as a loading control, also exists in reduced and oxidized states in cells (Fig. 5). Additionally, the cytotoxicity of 0.5 mm DTT was assessed by using the cell counting kit-8 assay (supporting Experimental procedures). The results showed that DTT treatment for 24 h did not significantly affect the viability of HEK293 cells (Fig. S3).
Figure 5.
CBS exists in oxidized and reduced states in HEK293 cells under oxidative and reductive conditions. HEK293 cells were exposed to 0.5 mm DTT or 0.5 mm oxidized GSSG-EE for 10 min followed by washing three times with ice-cold PBS buffer. Then the cells were treated with 15% (v/v) trichloroacetic acid at 4 °C for 30 min before being alkylated with 10 mm MM(PEG)24. The samples were resolved on SDS-PAGE and immunoblotted (IB) for CBS. The molecular size markers in kDa are indicated at the left.
Reductive stress enhances CBS-derived H2S production in cultured cells
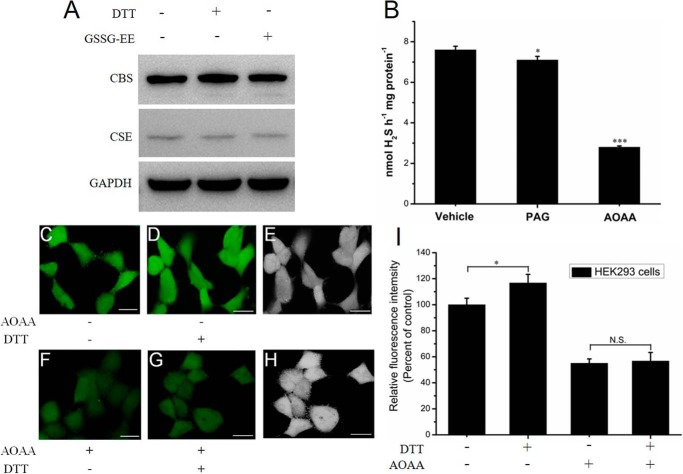
The activity of purified CBS was regulated as a function of the different redox states (Fig. 1). It was therefore anticipated that cleavage of the Cys272–Cys275 disulfide bond would increase the catalytic activity of the enzyme to concomitantly enhance CBS-derived H2S production. This was tested using fluorescent images of the endogenous H2S production in live HEK293 cells exposed to 0.5 mm DTT. Compared with the very low level of expression of cystathionine γ-lyase (CSE), CBS is highly expressed in HEK293 cells as indicated by Western blot analysis (Fig. 6A). These results are consistent with a previous report (42). Moreover, the expression levels of CBS and CSE were not significantly changed when cells were incubated with DTT or GSSG-EE for 10 min. We also determined the activities of CBS and CSE in cell lysates using the methylene blue assay. As shown in Fig. 6B, compared with the H2S-producing activity of the control group (7.6 ± 0.18 nmol h−1 mg−1), the activity of the cell lysate in the presence of 1 mm AOAA or 2 mm dl-propargylglycine was 2.8 ± 0.06 or 7.1 ± 0.18 nmol h−1 mg−1, respectively. Therefore, the amount of CSE-derived H2S was 0.5 nmol h−1 mg−1, and CBS-derived H2S was 4.3 nmol h−1 mg−1. The results indicated that the H2S-producing activity of CBS was 8.6 times higher than that of CSE, which suggested that CBS is the major enzyme that catalyzes the biosynthesis of H2S in the cytoplasm of HEK293 cells.
Figure 6.
Increasing activity of CBS in its reduced state increases the H2S signaling in live HEK293 cells. A, HEK293 cells were exposed to 0.5 mm DTT or 0.5 mm oxidized GSSG-EE for 10 min. The cell lysates (50 μg) were resolved on SDS-PAGE and immunoblotted for CBS. B, H2S production was measured in HEK293 cells using the methylene blue method. The inhibitors 1 mm AOAA and 2 mm dl-propargylglycine (PAG) were used to inhibit CBS and CSE, respectively. The data are presented as the means ± S.D. (n = 3). *, p < 0.05; ***, p < 0.001 versus vehicle. C, HEK293 cells were incubated with 2 μm SF7-AM for 30 min at 37 °C, washed, and then imaged using confocal microscopy. D, the same dishes of cells shown in C were incubated with 0.5 mm DTT for 30 min at 37 °C and then imaged. E, brightfield images of the same dishes of cells shown in D. Scale bar, 25 μm. F, HEK293 cells were incubated with 0.5 mm AOAA for 24 h to inhibit CBS activity and were then washed. Subsequently, the cells were incubated with 2 μm SF7-AM for 30 min at 37 °C, washed, and then imaged. G, The same dishes of cells shown in F were incubated with 0.5 mm DTT for 30 min at 37 °C and then imaged. H, brightfield images of the same dishes of cells shown in G. Scale bar, 25 μm. I, quantification of the confocal fluorescence images of H2S signaling in HEK293 cells, with data from C, D, F, and G for comparison. The graph represents the relative fluorescence intensity compared with that of non-treated cells (C) and shows the means ± S.D. (n = 3). *, p < 0.05; N.S., not significant.
Initial studies reported relatively high hydrogen sulfide concentrations of 30 to >100 μm in mammalian tissues (43–46), and these values are much higher than those determined in this study. Actually, the reevaluated endogenous H2S concentrations are orders of magnitude lower than those previously measured, with recent studies suggesting a concentration range of 15 to 120 nm in mouse liver and brain tissues (47, 48).
We next investigated the effect of reductive stress conditions on the CBS-derived H2S production using the H2S fluorescent probe SF7-AM in live HEK293 cells. Live cells were incubated with SF7-AM and imaged before and after treatment with DTT. The cells displayed a clear increase in the intracellular fluorescence compared with vehicle control (Fig. 6, C–E and I). To validate the contribution of CBS to the enhancement of H2S signaling, the cells were incubated with AOAA, an inhibitor of CBS and CSE, and then were imaged before and after treatment with DTT. The results showed that intracellular fluorescence was not significantly changed (Fig. 6, F–H and I), which indicated that reductive stress caused the increase in CBS activity in mammalian cells and the concomitant enhancement of the intracellular H2S production.
Discussion
A previous study indicated that under the conditions of the in vitro assay, the heme group, rather than the CXXC motif in CBS, is the redox sensor (27). However, because the high concentration of homocysteine and cysteine in the reaction mixture would reduce the CXXC disulfide, the role of the CXXC motif in redox regulation of CBS cannot be assessed in this assay. In addition to the CXXC motif, the heme in CBS has been proposed to be a redox sensor (49). Binding of exogenous ligands like NO and CO to the heme in a ferrous form of CBS is correlated with loss of enzyme activity (14–17). However, because of the low heme redox potential (−350 mV) of wildtype CBS (49), the existence of a ferrous form of CBS in vivo under physiological conditions remains an open question.
To address these gaps in our understanding of the redox regulation of CBS, we have employed S-methylcysteine to determine the activities of both oxidized and reduced CBS. Indeed, the dithiol form of the CXXC motif in CBS is associated with a more active form of the enzyme (Fig. 1). Additionally, reduced CBS is readily oxidized by exposing it to air in the absence of reductants, as evidenced by determination of thiols using DTNB assay. The activity of reduced CBS is 1.8-fold higher than oxidized CBS as indicated by the fluorescent thiol assay, whereas reduced CBS has a ∼3-fold increase in activity as shown by the gas chromatographic assay (Fig. 1C). The basis for the discrepancy between these two data sets could be the result of reoxidization of the partially reduced CBS in the fluorescent thiol assay, which lacks reductants.
The cysteine residue is characterized by five angles, and the different angles can permit 20 different possible combinations of disulfide bond. These disulfide bonds can be classified into three main types: the spiral, hook, and staple bonds (50). Based on this classification standard, the Cys272–Cys275 disulfide bond in CBS is classified as a −/+RHHook catalytic bond of the type observed in oxidoreductases (e.g. thioredoxin) and isomerases (e.g. protein disulfide isomerase) rather than an allosteric disulfide bond, which has the −RHStaple configuration. However, the distance between the CXXC motif and the active site is ∼20 Å, and it appears unlikely that these cysteines play a catalytic role. Additionally, mutation of the cysteines in the CXXC motif results in retention of enzyme activity (Fig. 3A), which excludes an essential role for these cysteines in catalysis. Moreover, in the present study, we have provided clear evidence that the CXXC motif in CBS exists in both oxidized disulfide bond and reduced dithiol states (Fig. 2), and changes in redox state of CXXC motif regulate the activity of CBS (Fig. 1).
The surface exposure of the CXXC motif observed in the structure of the modified human full-length CBS is probably important for access to the bond by the cellular GSH/GSSG molecules (26). The midpoint potential of the CBS disulfide bond is −314 mV (Fig. 4) at pH 7.4. In mammalian cells, the cytosolic glutathione redox potential (EGSH) of unstressed cells is typically found in the range between −300 to −320 mV (40, 41), which is similar to the midpoint potential of the CBS disulfide bond. Therefore, the CXXC motif in CBS is likely to exist as an equivalent mixture of the oxidized and reduced states, which was confirmed by thiol-labeling assays in HEK293 cells (Fig. 5). This midpoint potential (−314 mV) of the CBS disulfide bond allows CBS activity to be fine-tuned in response to stress in vivo.
Under reductive stress conditions, an increase in the proportion of the reduced CXXC motif in CBS is primarily responsible for an increase in CBS activity, thus leading to the enhancement of H2S production (Fig. 6). These results seem at first glance to be contradictory to our previous observations that under oxidative stress S-glutathionylation of CBS at Cys346 increases its activity (29). However, because glutathionylation of CBS at Cys346 is efficient with GSH but not with GSSG, it implies that the thiol of Cys346 should first be oxidized to a sulfenic acid by increasing concentrations of reactive oxygen species before being attacked by GSH under conditions of cellular oxidative stress (29). Actually, a high level of reactive oxygen species does not necessarily indicate a more oxidized intracellular redox state. It has been shown that in tumors, high levels of reactive oxygen species are often counterbalanced with high levels of reductants such as GSH, NADPH, and vitamin C (51–53). Therefore, CBS activity would be coordinately regulated by both thiol-disulfide exchange reactions and reactions with partially reactive oxygen species at the level of the CXXC motif and Cys346.
Conformational changes are the hallmarks of protein dynamics and often intimately related to protein functions (54). Changes in the redox state of the CXXC motif, which can be mediated by thiol-disulfide exchange reactions, will probably lead to the conformational changes of CBS and concomitant influence of CBS activity. Indeed, mutagenesis of cysteine residues in CXXC motif significantly affected the specific activity of CBS (Fig. 3A). Because the crystal structure of full-length oxidized CBS is not available, the structural basis for regulating the CBS activity by the redox state of the CXXC motif is not known, and this issue needs to be investigated.
The present study provided evidence that CBS activity is regulated by the redox state (thiol or disulfide) of the CXXC motif. As a result, reduced CBS has higher H2S-producing activity in cells exposed to reductive challenge (Fig. 6). A recent study demonstrated that the intracellular redox state, which was measured using a redox-sensitive organic reporter molecules assembled on gold nanoshells, undergoes reductive changes associated with the induction of hypoxia based on the use of surface-enhanced Raman spectroscopy nanosensors (55). Hypoxia is a feature of solid tumors and generally occurs at distances greater than 100 μm from functional blood vessels (56). The increase in reductive stress under hypoxic conditions is predicted to increase production of CBS-derived H2S.
In summary, our findings imply that CBS activity is controlled by the redox state (thiol or disulfide) of the CXXC motif in the presence of different oxidants or reductants in solution. This may cause the CXXC motif to act as a redox sensor. This study also demonstrates that there is an increase in H2S production under reductive conditions via reduction of the CXXC motif in CBS. However, the physiological effects of enhanced H2S under reductive stress conditions remain to be fully elucidated.
Experimental procedures
Materials
MM[PEG]24 was purchased from Thermo Fisher Scientific (Waltham, MA). The anti-cystathionine β-synthase (anti-CBS) antibody was obtained from ABclonal (Wuhan, China). The anti-cystathionine γ-lyase (anti-CSE) antibody was purchased from Abcam (Cambridge, MA). The fluorescent thiol probe 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin (CPM) and hydrogen sulfide probe (SF7-AM) were obtained from Sigma–Aldrich. Methanethiol (CH3SH) and dimethyl sulfide were purchased from ANPEL Laboratory Technologies Inc. (Shanghai, China). GSSG-EE was synthesized by Ontores Biotechnologies (Hangzhou, China). The cell counting kit-8 was purchased from Dojindo (Kumamoto, Japan). Unless otherwise specified, all chemicals were used as received.
Protein expression and purification
Mutagenesis of CBS was performed using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). The mutations were verified by DNA sequencing (Sangon, Shanghai, China). The expression and purification of the wildtype and variant versions of human CBS were performed as described previously for the wildtype proteins (30). Generally, cell pellets obtained from a 2-liter culture were resuspended in 200 ml of PBS supplemented with 20 mg of lysozyme, 5 mg of PLP, and a protease inhibitor tablet (Roche Applied Science). The cells were lysed by sonication, and the supernatant was obtained by centrifugation at 12,000 rpm for 30 min. Subsequently, the supernatant was loaded on a GSTrap FF column (GE Healthcare) that had been equilibrated with PBS. The column was subsequently washed with at least 20 column volumes of PBS, and glutathione S-transferase (GST)-fused CBS was eluted with 20 mm GSH in 50 mm Tris (pH 8.0). The GST-fused CBS was cleaved with thrombin at a final concentration of 5 units/mg protein at 4 °C, and the GST tag was removed using a Q Sepharose FF column (GE Healthcare) that had been equilibrated with 50 mm Tris buffer (pH 8.0). The CBS protein was eluted with a 200-ml linear gradient of 0 to 500 mm NaCl in 50 mm Tris-HCl (pH 8.0). Fractions containing the pure CBS protein were pooled and desalted, and the aliquoted enzyme was stored at −80 °C. The purities of all protein preparations were judged to be >90% by SDS-PAGE analysis.
H2S production assays
The H2S measurements were performed using the methylene blue assay according to Asimakopoulou et al. (31) with some modifications. First, H2S production in the reaction is trapped by Pb(NO3)2 to produce PbS. Subsequently, in acid solution PbS reacts with N,N-dimethyl-p-phenylenediamine-sulfate to produce methylene blue in the presence of FeCl3. The concentration of methylene blue is determined from the absorbance at 670 nm. In the case of the CBS enzyme, each test consisted of a 200-μl reaction mixture containing 2.5 μg of the purified CBS, 5 mm cysteine, 5 mm homocysteine, and 100 mm Hepes buffer (pH 7.4). For the cell lysate, HEK293 cells were washed three times with ice-cold PBS buffer and then scraped from 100-mm plates. The cells were harvested by centrifugation for 5 min, and the supernatant was carefully discarded. Next, 500 μl of Tris-HCl buffer (50 mm, pH 8.0) was added to the tube (500 μl buffer/1 plate cells), and the cell suspension was frozen and thawed three times using a liquid nitrogen/water bath. The reaction mixtures (200 μl) containing 180 μl of the cell lysate, 5 mm cysteine, and 5 mm homocysteine were prepared. The inhibitors aminooxyacetic acid (1 mm) or dl-propargylglycine (2 mm) were added to the reaction 10 min before the cysteine was added to the solution. After 30 min of incubation at 37 °C, the reaction was terminated by adding 1% (w/v) Pb(NO3)2 to trap the H2S followed by 15% (v/v) TCA to precipitate the proteins. Subsequently, N,N-dimethyl-p-phenylenediamine-sulfate (dissolved in 7.2 m HCl) was added to the reaction mixture immediately followed by addition of FeCl3 in 1.2 m HCl. The reaction mixtures were centrifuged at 12,000 rpm for 10 min to remove precipitation. The absorbance of the supernatant was measured at 670 nm. The reaction mixture without the substrates was used as the negative control. The H2S content was calculated against a calibration curve of standard H2S solutions.
Determination of heme content
Heme concentration was determined by the pyridine hemochrome assay as described previously (32, 33). The reaction mixture (200 μl) contained purified CBS (20 μm), 100 μl of 40% (v/v) pyridine, and 500 μm potassium ferricyanide (dissolved in 0.2 m NaOH). The reaction was initiated by the addition of 2 μl of 0.5 m sodium dithionite (dissolved in 0.5 m NaOH) and incubated for 5 min at room temperature. Heme concentration was determined from the absorbance at 557 nm using the extinction coefficient of 23.98 mm−1 cm−1 for the pyridine hemochromogen.
Measurement of CBS activity using a fluorescent probe
Purified wildtype CBS (1 mg/ml) was treated with 10 mm DTT for 1 h at 4 °C, and then DTT was removed by ultrafiltration using a 1-ml spin column. As a result, the DTT concentration was diluted ∼1000-fold by washing with the nitrogen-bubbled 100 mm Hepes (pH 7.4) buffer. The reduced CBS solutions were reoxidized by exposing them to air, pipetting up and down 6–8 times, and incubating for 2 h at 4 °C. The activities of reduced CBS (DTT treatment), oxidized CBS (no treatment), and reoxidized CBS were determined using S-methylcysteine as a substrate according to our previous report with some modifications (29). CBS can catalyze the decomposition of the S-methylcysteine, a thiol-free substrate analog of cysteine, to produce methanethiol (CH3SH), which can be continuously monitored with the fluorescent thiol probe CPM. The reaction mixture (200 μl) containing 10 mm S-methylcysteine, 30 μm CPM thiol fluorescent detection reagent, and 100 mm Hepes buffer (pH 7.4) was added to a 96-well plate, and the reaction was started by the addition of 5 μg of CBS. The 96-well plate was placed in a multifunctional microplate reader. The fluorescence of the mixture was monitored at 460 nm (λex = 400 nm) for 10 min at 37 °C. The activity of oxidized CBS was set as 100%. Alternatively, DTNB was used in the reaction mixture to detect production of methanethiol using an extinction coefficient of 13,600 m−1 cm−1 at 412 nm for 2-nitro-5-thiobenzoic acid anion (29, 34).
Measurement of CBS activity using a gas chromatographic assay
CBS activity that leads to production of methanethiol (CH3SH) was also determined using a gas chromatographic assay according to our previous report with some modifications (29). Recombinant CBS protein (20 μg) was preincubated in a 100 mm Hepes buffer (1 ml) containing 20 mm GSH for 2 h at 4 °C in a sealed bottle (20 ml), and the reaction was initiated by the addition of 10 mm S-methylcysteine. After incubation at 37 °C with gentle shaking for 10 min, 0.5-ml aliquots from the gas phase of the sealed bottles were collected using gas-tight syringes and injected in a GC 9860 gas chromatograph (Shanghai Qiyang Information Technology Co., Ltd., Shanghai, China) that was equipped with a SE-30 column (30 m × 0.53 mm × 1.0 μm). CH3SH production was detected using a pulsed flame photometric detector. A methanethiol standard was obtained from ANPEL Laboratory Technologies Inc. (Shanghai, China). An internal standard of dimethyl sulfide was added to the reaction mixtures. The activity of the untreated CBS protein was defined as 100%. The peak area of CH3SH was normalized to the peak area of an internal standard.
Mass spectrometry
A solution of CBS (50 μl at 5 mg/ml) was treated with 10 mm DTT for 1 h at room temperature. Untreated CBS was used as a control. The samples were ultrafiltered and buffer-exchanged using a 1-ml spin column with 20 mm phosphate buffer (pH 7.8) and then denatured using 6 m guanidine hydrochloride. Subsequently, the free thiol groups of the samples were derivatized with iodoacetamide at a final concentration of 25 mm. The samples were concentrated, and the buffer was exchanged using a 1-ml spin column with 20 mm phosphate buffer (pH 7.8) and then digested using a protein:endoproteinase Glu-C ratio of 20:1 at 37 °C for 16–18 h. The digested samples were acidified with 10% (v/v) formic acid and directly used for LC-MS/MS analyses. All analyses were performed using a Thermo EASY-nLC1000 Nano HPLC system with a Thermo EASY column SC200 (150 μm × 100 mm, RP-C18) at a flow rate of 0.3 μl/min. The mobile phases A and B consisted of 0.1% (v/v) formic acid in H2O and 0.1% (v/v) formic acid in 84% (v/v) acetonitrile, respectively. The peptides were eluted using the following gradient: t = 0–50 min: 0–60% B; t = 51–53 min: 60–100% B; and t = 54–60 min: 100% B. A HPLC system was directly coupled to a Q Exactive hybrid quadrupole Orbitrap mass spectrometer (Thermo Finnigan). Positive ions were generated by electrospray, and the Q Exactive spectrometer was operated in data-dependent acquisition mode.
A survey scan from m/z 300–1800 was acquired in the Q Exactive spectrometer (resolution = 70,000 at m/z 200, with an accumulation target value of 3,000,000 ions) with lockmass enabled. Up to 10 of the most abundant ions were sequentially isolated and fragmented within the linear ion trap using collisionally induced dissociation with an activation q of 0.25. The m/z ratios selected for MS/MS were dynamically excluded for 10 s. The MS data were searched against the database from the National Center for Biotechnology and Information. The search parameters were: precursor tolerance, 10 ppm; and product ion tolerances, ±0.1 Da. Methionine oxidation, deamidation of glutamine and asparagines, and carbamidomethylation of cysteine were selected as variable modifications. The relative ion abundance of the peptides containing the oxidized or reduced CXXC motif was calculated according to the peak areas from the ion chromatogram. The sequence coverage of wildtype CBS protein obtained by MS analysis was 99.8%.
Labeling of unpaired cysteine thiols in CBS
Recombinant wildtype CBS 100 μl (1 mg/ml) was treated with or without 10 mm DTT for 1 h at 4 °C and then was precipitated with 15% (v/v) trichloroacetic acid at 4 °C for 30 min. The precipitated protein was washed three times with cold acetone and then redissolved in 100 μl of phosphate buffer (100 mm) containing 1% (w/v) SDS, pH 7.0. Subsequently, the methyl-PEG-maleimide reagent, MM[PEG]24, was added to a final concentration of 10 mm. After 16 h of incubation at 25 °C, the labeled samples were resolved on SDS-PAGE, protein-transferred to a PVDF membrane, and blotted with anti-CBS and goat anti-rabbit peroxidase antibodies. The blots were visualized using chemiluminescence (Tanon, Shanghai, China). The molecular mass of MM(PEG)24 is 1240 Da.
HEK293 cells were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum and a 1% (v/v) penicillin-streptomycin solution at 37 °C and 5% (v/v) CO2. After the cells had reached ∼80% confluence in a 10-cm plate, DTT or oxidized glutathione ethyl ester (GSSG-EE) was added to a final concentration of 0.5 mm. After 10 min of incubation, the cells were washed three times with ice-cold PBS supplemented with 0.5 mm DTT or GSSG-EE and then incubated with a final concentration of 15% (v/v) TCA for 30 min at 4 °C to avoid artificial thiol-disulfide modifications (35). The pellets obtained by centrifugation at 12,000 rpm for 10 min were redissolved in 100 μl of phosphate buffer (100 mm) containing 1% (w/v) SDS, pH 7.0. Subsequently, MM(PEG)24 was added to a final concentration of 10 mm. After 16 h of incubation at 25 °C, the labeled samples were resolved on SDS-PAGE and immunoblotted for CBS.
Redox potential of the CBS disulfide bond
DTNB assay was employed to determine the redox potential of the CBS disulfide bond. Briefly, redox buffers were prepared in a 100 mm Hepes buffer containing various concentrations of reduced dithiothreitol (DTTred) and oxidized dithiothreitol (DTToxi, trans-4,5-dihydroxy-1,2-dithiane; Sigma), pH 7.4. The total dithiothreitol concentration in the redox buffers was 10 mm, and all solutions were deoxygenated by bubbling with nitrogen for 30 min. The DTToxi/DTTred redox potential was calculated according to the Nernst equation,
| (1) |
where E0 = −352 mV at pH 7.4, R is the gas constant, T is the absolute temperature, and F is Faraday's constant (n = 2), and [DTToxi] and [DTTred] are molar concentrations of oxidized and reduced DTT, respectively (36). Subsequently, recombinant wildtype CBS (100 μg) was preincubated in solutions (500 μl) with various concentrations of DTToxi and DTTred for 1 h at 37 °C and then precipitated with 15% (v/v) trichloroacetic acid at 4 °C for 30 min. The precipitated protein was washed three times with cold acetone and then was dissolved in 200 μl of 100 mm Tris-HCl (pH 8.0) containing 1% (w/v) SDS. To fully expose the cysteine residues of the precipitated CBS proteins, 1% (w/v) SDS was used in the DTNB assay according to a previous report (37). Control samples contained the same amount of DTNB in the same buffer, but the CBS protein was omitted. The final mixture was incubated with 0.5 mm DTNB for 10 min at room temperature, and the samples were analyzed spectrophotometrically at 412 nm using an extinction coefficient of 13,600 m−1 cm−1 for 2-nitro-5-thiobenzoic acid anion (29). The number of free thiol groups of CBS protein was determined, and the midpoint potential of the CBS disulfide bond was calculated.
Confocal images of endogenous H2S production in live HEK293 cells
Confocal fluorescence imaging studies were performed with a Leica SP8 laser scanning confocal microscopy with a 63× oil objective. The hydrogen sulfide fluorescent probe SF7-AM was excited using a 488-nm Argon laser, and the emissions between 500 and 650 nm were collected using a detector (38). The cells were imaged at 37 °C with 5% (v/v) CO2 during the course of the experiment. All imaging experiments were carried out in 35-mm glass-bottomed dishes (NEST, Wuxi, China). HEK293 cells were incubated with 2 μm SF7-AM for 30 min at 37 °C with 5% (v/v) CO2. The medium was exchanged, and images were acquired for cells in three different fields. For DTT stimulation, 10 μl of 50 mm DTT was added directly to dishes for a final concentration of 0.5 mm. After 30 min of incubation at 37 °C with 5% (v/v) CO2, the same dishes of cells were imaged. For the inhibitor experiments, 10 μl of 50 mm AOAA was added to dishes to a final concentration of 0.5 mm. After 24 h of incubation, the cells were incubated with 2 μm SF7-AM for 30 min. Then the medium was exchanged, and images were acquired for the cells in three different fields. Subsequently, 0.5 mm DTT was added to the same dishes for an additional 30 min of incubation at 37 °C, and the cells were imaged. Image analysis was performed using ImageJ (National Institute of Health). The images were quantified using the mean pixel intensity after setting a common threshold for all images.
Author contributions
W. N. conceptualization; W. N. resources; W. N., J. W., and M. W. data curation; W. N. funding acquisition; W. N., J. W., J. Q., M. W., P. W., F. C., and S. Y. investigation; W. N. methodology; W. N. writing-original draft.
Supplementary Material
This work was supported by Grant 31471086 from the National Natural Science Foundation of China, Grant 2017GY-143 from the Key Science and Technology Program of Shaanxi Province, China, Grant G2017KY0001 from the Fundamental Research Funds for the Central Universities of China, and Grant Z2017233 from the Seed Foundation of Innovation and Creation for Graduate Students in Northwestern Polytechnical University. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supporting text and Figs. S1–S3.
- used are: CBS
- cystathionine β-synthase
- PLP
- pyridoxal 5′-phosphate
- AOAA
- aminooxyacetic acid
- MM(PEG)24
- methyl-PEG24-maleimide
- GSSG-EE
- oxidized glutathione ethyl ester
- CSE
- cystathionine γ-lyase
- DTNB
- 5,5′-dithiobis-2-nitrobenzoic acid
- CPM
- 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin
- GST
- glutathione S-transferase.
References
- 1. Banerjee R., and Zou C. G. (2005) Redox regulation and reaction mechanism of human cystathionine-β-synthase: a PLP dependent heme sensor protein. Arch. Biochem. Biophys. 433, 144–156 10.1016/j.abb.2004.08.037 [DOI] [PubMed] [Google Scholar]
- 2. Miles E. W., and Kraus J. P. (2004) Cystathionine β-synthase: structure, function, regulation, and location of homocystinuria causing mutations. J. Biol. Chem. 279, 29871–29874 10.1074/jbc.R400005200 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R., Evande R., Kabil O., Ojha S., and Taoka S. (2003) Reaction mechanism and regulation of cystathionine β-synthase. Biochim. Biophys. Acta 1647, 30–35 10.1016/S1570-9639(03)00044-X [DOI] [PubMed] [Google Scholar]
- 4. Chen X., Jhee K. H., and Kruger W. D. (2004) Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 279, 52082–52086 10.1074/jbc.C400481200 [DOI] [PubMed] [Google Scholar]
- 5. Singh S., Padovani D., Leslie R. A., Chiku T., and Banerjee R. (2009) Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 10.1074/jbc.M109.010868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kabil O., and Banerjee R. (2010) The redox biochemistry of hydrogen sulfide. J. Biol. Chem. 285, 21903–21907 10.1074/jbc.R110.128363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kabil O., and Banerjee R. (2014) Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox. Signal. 20, 770–782 10.1089/ars.2013.5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kabil O., Motl N., and Banerjee R. (2014) H2S and its role in redox signaling. Biochim. Biophys. Acta 1844, 1355–1366 10.1016/j.bbapap.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura H. (2010) Hydrogen sulfide: from brain to gut. Antioxid. Redox. Signal. 12, 1111–1123 10.1089/ars.2009.2919 [DOI] [PubMed] [Google Scholar]
- 10. Kraus J. P., Janosík M., Kozich V., Mandell R., Shih V., Sperandeo M. P., Sebastio G., de Franchis R., Andria G., Kluijtmans L. A., Blom H., Boers G. H., Gordon R. B., Kamoun P., Tsai M. Y., et al. (1999) Cystathionine β-synthase mutations in homocystinuria. Hum. Mutat. 13, 362–375 10.1002/(SICI)1098-1004(1999)13:5%3C362::AID-HUMU4%3E3.0.CO%3B2-K [DOI] [PubMed] [Google Scholar]
- 11. Taoka S., Widjaja L., and Banerjee R. (1999) Assignment of enzymatic functions to specific regions of the PLP-dependent heme protein cystathionine β-synthase. Biochemistry 38, 13155–13161 10.1021/bi990865t [DOI] [PubMed] [Google Scholar]
- 12. Majtan T., Pey A. L., and Kraus J. P. (2016) Kinetic stability of cystathionine β-synthase can be modulated by structural analogs of S-adenosylmethionine: potential approach to pharmacological chaperone therapy for homocystinuria. Biochimie 126, 6–13 10.1016/j.biochi.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 13. Singh S., Madzelan P., and Banerjee R. (2007) Properties of an unusual heme cofactor in PLP-dependent cystathionine β-synthase. Nat. Prod. Rep. 24, 631–639 10.1039/B604182P [DOI] [PubMed] [Google Scholar]
- 14. Vicente J. B., Colaço H. G., Mendes M. I., Sarti P., Leandro P., and Giuffrè A. (2014) NO* binds human cystathionine β-synthase quickly and tightly. J. Biol. Chem. 289, 8579–8587 10.1074/jbc.M113.507533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carballal S., Cuevasanta E., Marmisolle I., Kabil O., Gherasim C., Ballou D. P., Banerjee R., and Alvarez B. (2013) Kinetics of reversible reductive carbonylation of heme in human cystathionine β-synthase. Biochemistry 52, 4553–4562 10.1021/bi4004556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taoka S., and Banerjee R. (2001) Characterization of NO binding to human cystathionine β-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J. Inorg. Biochem. 87, 245–251 10.1016/S0162-0134(01)00335-X [DOI] [PubMed] [Google Scholar]
- 17. Vicente J. B., Colaço H. G., Sarti P., Leandro P., and Giuffrè A. (2016) S-Adenosyl-l-methionine modulates CO and NO• binding to the human H2S-generating enzyme cystathionine β-synthase. J. Biol. Chem. 291, 572–581 10.1074/jbc.M115.681221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carballal S., Cuevasanta E., Yadav P. K., Gherasim C., Ballou D. P., Alvarez B., and Banerjee R. (2016) Kinetics of nitrite reduction and peroxynitrite formation by ferrous heme in human cystathionine β-synthase. J. Biol. Chem. 291, 8004–8013 10.1074/jbc.M116.718734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gherasim C., Yadav P. K., Kabil O., Niu W. N., and Banerjee R. (2014) Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine β-synthase. PLoS One 9, e85544 10.1371/journal.pone.0085544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celano L., Gil M., Carballal S., Durán R., Denicola A., Banerjee R., and Alvarez B. (2009) Inactivation of cystathionine β-synthase with peroxynitrite. Arch. Biochem. Biophys. 491, 96–105 10.1016/j.abb.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majtan T., Singh L. R., Wang L., Kruger W. D., and Kraus J. P. (2008) Active cystathionine β-synthase can be expressed in heme-free systems in the presence of metal-substituted porphyrins or a chemical chaperone. J. Biol. Chem. 283, 34588–34595 10.1074/jbc.M805928200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., and Hardie D. G. (2004) CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 113, 274–284 10.1172/JCI19874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCorvie T. J., Kopec J., Hyung S. J., Fitzpatrick F., Feng X., Termine D., Strain-Damerell C., Vollmar M., Fleming J., Janz J. M., Bulawa C., and Yue W. W. (2014) Inter-domain communication of human cystathionine β synthase: structural basis of S-adenosyl-l-methionine activation. J. Biol. Chem. 289, 36018–36030 10.1074/jbc.M114.610782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ereño-Orbea J., Majtan T., Oyenarte I., Kraus J. P., and Martínez-Cruz L. A. (2014) Structural insight into the molecular mechanism of allosteric activation of human cystathionine β-synthase by S-adenosylmethionine. Proc. Natl. Acad. Sci. U.S.A. 111, E3845–E3852 10.1073/pnas.1414545111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kery V., Poneleit L., and Kraus J. P. (1998) Trypsin cleavage of human cystathionine β-synthase into an evolutionarily conserved active core: structural and functional consequences. Arch. Biochem. Biophys. 355, 222–232 10.1006/abbi.1998.0723 [DOI] [PubMed] [Google Scholar]
- 26. Ereño-Orbea J., Majtan T., Oyenarte I., Kraus J. P., and Martínez-Cruz L. A. (2013) Structural basis of regulation and oligomerization of human cystathionine βsynthase, the central enzyme of transsulfuration. Proc. Natl. Acad. Sci. U.S.A. 110, E3790–E3799 10.1073/pnas.1313683110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taoka S., Lepore B. W., Kabil O., Ojha S., Ringe D., and Banerjee R. (2002) Human cystathionine β-synthase is a heme sensor protein: evidence that the redox sensor is heme and not the vicinal cysteines in the CXXC motif seen in the crystal structure of the truncated enzyme. Biochemistry 41, 10454–10461 10.1021/bi026052d [DOI] [PubMed] [Google Scholar]
- 28. Mosharov E., Cranford M. R., and Banerjee R. (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39, 13005–13011 10.1021/bi001088w [DOI] [PubMed] [Google Scholar]
- 29. Niu W. N., Yadav P. K., Adamec J., and Banerjee R. (2015) S-Glutathionylation enhances human cystathionine-β-synthase activity under oxidative stress conditions. Antioxid. Redox. Signal. 22, 350–361 10.1089/ars.2014.5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niu W. N., Wu P., Chen F., Wang J., Shang X. Y., and Xu C. L. (2017) Discovery of selective cystathionine β-synthase inhibitors by high-throughput screening with a fluorescent thiol probe. Med. Chem. Commun. 8, 198–201 10.1039/C6MD00493H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asimakopoulou A., Panopoulos P., Chasapis C. T., Coletta C., Zhou Z., Cirino G., Giannis A., Szabo C., Spyroulias G. A., and Papapetropoulos A. (2013) Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 169, 922–932 10.1111/bph.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barr I., and Guo F. (2015) Pyridine hemochromagen assay for determining the concentration of heme in purified protein solutions. Bio. Protoc. 5, e1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berry E. A., and Trumpower B. L. (1987) Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161, 1–15 10.1016/0003-2697(87)90643-9 [DOI] [PubMed] [Google Scholar]
- 34. Sedlak J., and Lindsay R. H. (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25, 192–205 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 35. Botello-Morte L., Bes M. T., Heras B., Fernández-Otal Á., Peleato M. L., Fillat M. F. (2014) Unraveling the redox properties of the global regulator FurA from Anabaena sp. PCC 7120: disulfide reductase activity based on its CXXC motifs. Antioxid. Redox. Signal. 20, 1396–1406 10.1089/ars.2013.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hisatomi O., Nakatani Y., Takeuchi K., and Takahashi F., Kataoka H. (2014) Blue light-induced dimerization of monomeric aureochrome-1 enhances its affinity for the target sequence. J. Biol. Chem. 289, 17379–17391 10.1074/jbc.M114.554618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan K. Y., and Wasserman B. P. (1993) Direct colorimetric assay of free thiol groups and disulfide bonds in suspensions of solubilized and particulate cereal proteins. Cereal Chem. 70, 22–26 [Google Scholar]
- 38. Lin V. S., Lippert A. R., and Chang C. J. (2013) Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. U.S.A. 110, 7131–7135 10.1073/pnas.1302193110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kery V., Elleder D., and Kraus J. P. (1995) δ-Aminolevulinate increases heme saturation and yield of human cystathionine β-synthase expressed in Escherichia coli. Arch. Biochem. Biophys. 316, 24–29 10.1006/abbi.1995.1005 [DOI] [PubMed] [Google Scholar]
- 40. Meyer A. J., and Dick T. P. (2010) Fluorescent protein-based redox probes. Antioxid. Redox. Signal. 13, 621–650 10.1089/ars.2009.2948 [DOI] [PubMed] [Google Scholar]
- 41. Dooley C. T., Dore T. M., Hanson G. T., Jackson W. C., Remington S. J., and Tsien R. Y. (2004) Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22284–22293 10.1074/jbc.M312847200 [DOI] [PubMed] [Google Scholar]
- 42. Mustafa A. K., Gadalla M. M., Sen N., Kim S., Mu W., Gazi S. K., Barrow R. K., Yang G., Wang R., and Snyder S. H. (2009) H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y. H., Yao W. Z., Geng B., Ding Y. L., Lu M., Zhao M. W., and Tang C. S. (2005) Endogenous hydrogen sulfide in patients with COPD. Chest 128, 3205–3211 10.1378/chest.128.5.3205 [DOI] [PubMed] [Google Scholar]
- 44. Ogasawara Y., Isoda S., and Tanabe S. (1994) Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 17, 1535–1542 10.1248/bpb.17.1535 [DOI] [PubMed] [Google Scholar]
- 45. Zhao W., Zhang J., Lu Y., and Wang R. (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 20, 6008–6016 10.1093/emboj/20.21.6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han Y., Qin J., Chang X., Yang Z., and Du J. (2006) Hydrogen sulfide and carbon monoxide are in synergy with each other in the pathogenesis of recurrent febrile seizures. Cell. Mol. Neurobiol. 26, 101–107 10.1007/s10571-006-8848-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Furne J., Saeed A., and Levitt M. D. (2008) Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485 10.1152/ajpregu.90566.2008 [DOI] [PubMed] [Google Scholar]
- 48. Levitt M. D., Abdel-Rehim M. S., and Furne J. (2011) Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox. Signal. 15, 373–378 10.1089/ars.2010.3525 [DOI] [PubMed] [Google Scholar]
- 49. Singh S., Madzelan P., Stasser J., Weeks C. L., Becker D., Spiro T. G., Penner-Hahn J., and Banerjee R. (2009) Modulation of the heme electronic structure and cystathionine β-synthase activity by second coordination sphere ligands: the role of heme ligand switching in redox regulation. J. Inorg. Biochem. 103, 689–697 10.1016/j.jinorgbio.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cook K. M., and Hogg P. J. (2013) Post-translational control of protein function by disulfide bond cleavage. Antioxid. Redox. Signal. 18, 1987–2015 10.1089/ars.2012.4807 [DOI] [PubMed] [Google Scholar]
- 51. Li L. Z. (2012) Imaging mitochondrial redox potential and its possible link to tumor metastatic potential. J. Bioenerg. Biomembr. 44, 645–653 10.1007/s10863-012-9469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keshari K. R., Kurhanewicz J., Bok R., Larson P. E., Vigneron D. B., and Wilson D. M. (2011) Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proc. Natl. Acad. Sci. U.S.A. 108, 18606–18611 10.1073/pnas.1106920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hyodo F., Matsumoto K., Matsumoto A., Mitchell J. B., and Krishna M. C. (2006) Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 66, 9921–9928 10.1158/0008-5472.CAN-06-0879 [DOI] [PubMed] [Google Scholar]
- 54. Liu H., Dastidar S. G., Lei H., Zhang W., Lee M. C., and Duan Y. (2008) Conformational changes in protein function. Methods Mol. Biol. 443, 258–275 10.1007/978-1-59745-177-2_14 [DOI] [PubMed] [Google Scholar]
- 55. Jiang J., Auchinvole C., Fisher K., and Campbell C. J. (2014) Quantitative measurement of redox potential in hypoxic cells using SERS nanosensors. Nanoscale 6, 12104–12110 10.1039/C4NR01263A [DOI] [PubMed] [Google Scholar]
- 56. Helmlinger G., Yuan F., Dellian M., and Jain R. K. (1997) Interstitial pH and pO2 gradients in solid tumours in vivo: high resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 10.1038/nm0297-177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.