Abstract
Heterochromatin formation in budding yeast is regulated by the silent information regulator (SIR) complex. The SIR complex comprises the NAD-dependent deacetylase Sir2, the scaffolding protein Sir4, and the nucleosome-binding protein Sir3. Transcriptionally active regions present a challenge to SIR complex–mediated de novo heterochromatic silencing due to the presence of antagonistic histone post-translational modifications, including acetylation and methylation. Methylation of histone H3K4 and H3K79 is dependent on monoubiquitination of histone H2B (H2B-Ub). The SIR complex cannot erase H2B-Ub or histone methylation on its own. The deubiquitinase (DUB) Ubp10 is thought to promote heterochromatic silencing by maintaining low H2B-Ub at sub-telomeres. Here, we biochemically characterized the interactions between Ubp10 and the SIR complex machinery. We demonstrate that a direct interaction between Ubp10 and the Sir2/4 sub-complex facilitates Ubp10 recruitment to chromatin via a co-assembly mechanism. Using hydrolyzable H2B-Ub analogs, we show that Ubp10 activity is lower on nucleosomes compared with H2B-Ub in solution. We find that Sir2/4 stimulates Ubp10 DUB activity on nucleosomes, likely through a combination of targeting and allosteric regulation. This coupling mechanism between the silencing machinery and its DUB partner allows erasure of active PTMs and the de novo transition of a transcriptionally active DNA region to a silent chromatin state.
Keywords: epigenetics, deubiquitylation (deubiquitination), heterochromatin, yeast, sirtuin, chromatin, histone, SIR complex, ubiquitination
Introduction
Eukaryotes package their genome into specific chromatin structures to regulate gene expression in response to external stimuli. In contrast to open and transcriptionally active euchromatin, heterochromatin is a condensed form of chromatin that is refractory to gene transcription and contributes to genome stability. Despite being generally condensed, heterochromatin is subjected to multiple disruptive processes, including disassembly for DNA replication. The de novo establishment of heterochromatin requires factors that act with silencing complexes to ensure heterochromatin fidelity.
The assembly of heterochromatin domains is facilitated by silencing proteins that recognize specific histone post-translational modifications (PTMs)3 or the absence thereof (1, 2). The presence of particular combinations of histone tail PTMs is generally believed to “code” for regions of the genome that are active or inactive (3, 4). A general unmodified state or absence of histone PTMs is the code that promotes heterochromatic silencing in budding yeast. This is exemplified by the specific recognition of the unmodified histone H4 tail by the silencing machinery (5–7). Additionally, methylation of histone H3 lysine 4 (H3K4me) and H3 lysine 79 (H3K79me) antagonizes silencing (8–12). Heterochromatin assembly is coupled to enzymatic conversion of the histone code from the active to the inactive state. For example, lysine deacetylase enzymes are frequently core components of gene repression complexes, removing histone acetylation, which otherwise promotes transcription.
Three general steps in establishing heterochromatin involve the following: 1) recruitment of the silencing complex to the target locus; 2) nucleation via histone-modifying activities; and 3) spreading of the silencing complexes due to iterative cycles of recruitment and nucleation. In budding yeast, silent information regulator (SIR) complex–dependent heterochromatin is a well-studied and simplified silencing model (13–15). The SIR complex is composed of three proteins, Sir2, Sir3, and Sir4. Sir2 is the founding member of the sirtuin family and is a histone lysine deacetylase whose activity is necessary for nucleation of the SIR complex (16–18). Sir4 mediates protein–protein interactions within the complex and between other interacting partners. Sir3 is required for heterochromatic spreading and silencing due in part to strong association with unmodified H3 and H4 tails (6, 7, 19, 20).
Active regions of the genome that will undergo heterochromatin remodeling contain euchromatin-promoting PTMs. Several active marks are known to be anti-silencing, such as H3K4me3 and H3K79me3, as they interfere with SIR complex–mediated silencing and prevent association of the SIR complex with euchromatic regions (Fig. 1A) (8–12, 21–24). Tri-methylation of H3K4 and H3K79 are dependent on Rad6/Bre1-mediated monoubiquitination of H2BK123 (H2B-Ub) (25–27). Collectively, H2B-Ub, H3K4me, and H3K79me are strongly associated with genomic regions that undergo transcription (11, 22, 28). Reduction of these euchromatin-promoting marks is important to the assembly and spreading of repressed heterochromatin domains.
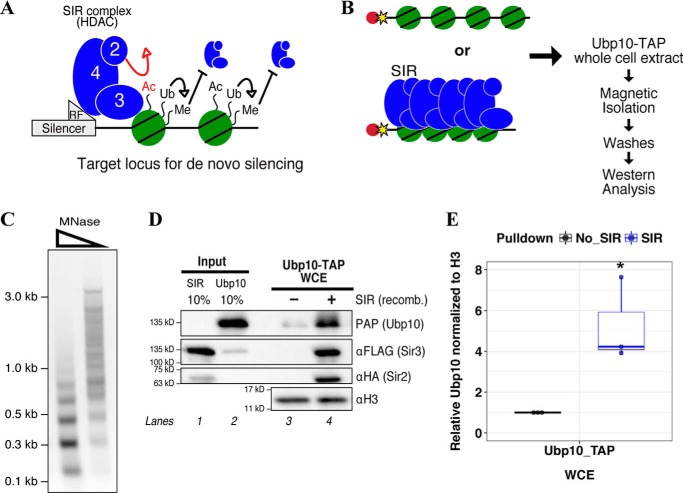
Figure 1.
Ubp10 is recruited to SIR complex–mediated heterochromatin. A, diagram of post-translational modifications that affect SIR complex binding to nucleosomes. SIR complex has inherent histone deacetylase activity by virtue of Sir2. SIR complex cannot erase histone ubiquitination or histone methylation on its own. Histone ubiquitination indirectly inhibits SIR complex association by promoting H3 lysine methylation. B, experimental design for chromatin/heterochromatin pulldowns using whole-cell extracts. WCEs were made from a strain expressing endogenous Ubp10 that is C-terminally TAP-tagged. Bead-conjugated chromatin or SIR complex–assembled heterochromatin were incubated in Ubp10-TAP extracts. Beads were magnetically isolated and washed. Bound proteins were detected by Western blot analysis. C, analysis of reconstituted chromatin by limited MNase digestion. From left, titration of MNase from high to low (tick marks represent 2-log ladder markers). D, Western blot analysis of heterochromatin pulldown compared with chromatin from whole-cell extracts. SIR complex and chromatin are recombinant proteins and were detected by Western blotting. SIR, SIR complex; recomb., recombinant; PAP, peroxidase-anti-peroxidase. E, Western blotting quantification of Ubp10-TAP enrichment on chromatin versus heterochromatin. Ubp10 signal in mock reaction quantified as 1 and Ubp10 signal in +SIR complex sample is relative to mock. Mock and relative signals were normalized to H3. An integration of both a box plot and a scatter plot is shown. Error bars were determined from three independent experiments. Asterisk indicates p value < 0.05.
Although the SIR complex is equipped to erase acetylation marks, which antagonize silencing, other proteins have been identified that work with the SIR complex to generate the fully “erased” heterochromatin histone code. One such protein is Ubp10, a deubiquitinase (DUB) with an identified role in silencing (29–32). Ubp10 regulates the levels of monoubiquitinated histone H2BK123 in yeast and localizes to sub-telomeric heterochromatin. A recent study identified that Ubp10 also acts within the nucleolus and interacts with factors that regulate ribosome biogenesis (33). Ubp10 genetically interacts with Sir4 and is mutually recruited with the SIR complex to the sub-telomeres (30, 31, 34). Changes to H2B-Ub levels when Ubp10 is deleted or overexpressed cause a disruption in telomeric silencing (32, 35). Despite a genetic role for Ubp10 in silencing, the mechanism of Ubp10 targeting and regulation during SIR complex–mediated silencing is not well-understood.
In this study, we detail the molecular mechanism of how SIR complex recruits Ubp10 to chromatin and stimulates Ubp10 DUB activity to help generate the silent histone code. Using multiple biochemical approaches, we show that Ubp10 directly interacts with the Sir protein sub-complex, Sir2/4, independent of chromatin and that this interaction likely supports the co-assembly of Ubp10 onto chromatin with the SIR complex. We characterize Ubp10 DUB activity using substrates with homogeneous H2B-Ub analogs. We discover that Sir4 recruits and allosterically enhances Ubp10 DUB activity on H2B-Ub mononucleosomes. Finally, we present a model for how this coupling mechanism allows for SIR complex–mediated heterochromatin to efficiently assemble and silence the target locus.
Results
Ubp10 is recruited to SIR complex–mediated heterochromatin
We previously used stable isotope labeling of amino acids in cell culture and mass spectrometry (SILAC-MS) to identify proteins excluded from heterochromatin (36). We further analyzed these data and identified proteins that were preferentially recruited to heterochromatin and found the budding yeast deubiquitinase, Ubp10, as one of the top hits. Ubp10 was identified as a regulator of yeast heterochromatin by genetic analyses (29–31). Yeast two-hybrid analysis identified an interaction between Ubp10 and Sir4 suggesting a direct recruitment mechanism between the two proteins to chromatin (30, 34). To further understand how Ubp10 is recruited to heterochromatin and contributes to silencing, we began by performing chromatin pulldown assays similar to the SILAC-MS experiments using whole-cell extracts (WCE) in which Ubp10 is TAP-tagged endogenously. Briefly, chromatin was enzymatically assembled using purified budding yeast components, including unmodified histone octamers, Nap1 histone chaperone, Isw1a chromatin remodeler, and a biotinylated DNA template. Nucleosome spacing and periodicity were examined by performing a limited micrococcal nuclease (MNase) digestion (Fig. 1C). The chromatin substrates were then conjugated to magnetic streptavidin-coated beads for immobilization. Heterochromatin domains were further assembled by incubating recombinant SIR complex with chromatin (10, 36). Assembled chromatin, in the presence or absence of the SIR complex, was incubated in WCE with an ATP-regeneration system. Incubations were followed by magnetic isolation of chromatin beads and washes. Bound proteins were analyzed by Western blotting (Fig. 1D) using antibodies shown in Table S1. Ubp10 was differentially enriched on heterochromatin by roughly 5-fold in a SIR complex-dependent manner, re-confirming our previous SILAC-MS results (Fig. 1, D and E).
Ubp10 recruitment to chromatin is stimulated by co-assembly with SIR complex
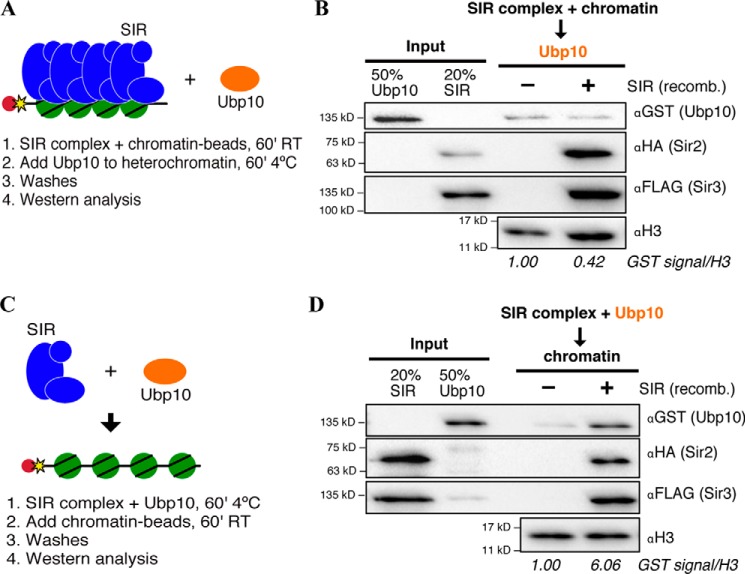
To determine whether Ubp10 is recruited to heterochromatin directly by the SIR complex, we performed chromatin and heterochromatin pulldowns in the presence of recombinant Ubp10 purified with a GST tag from Escherichia coli. We utilized order of addition experiments to help determine the context in which Ubp10 gets to SIR heterochromatin. As shown in Fig. 2A, heterochromatin was first assembled to mimic a maintained and stable state of heterochromatin before Ubp10 was added. Under these conditions, Ubp10 did not bind heterochromatin better than chromatin alone (Fig. 2B). One possible explanation for a lack of SIR complex-dependent enrichment of Ubp10 is that another protein is required to bridge Ubp10 to SIR complex. Another explanation is that Ubp10 can only be recruited to chromatin at the same time as the SIR complex, not after the SIR complex forms a stable assembly. We tested both possibilities simultaneously by changing the order of addition of the experiment (Fig. 2C). Ubp10 and SIR complex were pre-incubated and then added to chromatin. The pulldown under these conditions resulted in increased recruitment of Ubp10 by the SIR complex (6-fold increase) when compared with chromatin without SIR complex bound (Fig. 2D). These results demonstrate that Ubp10 co-assembles with the SIR complex onto chromatin. We note that heterochromatin pulldowns using WCEs were performed using pre-assembled heterochromatin and resulted in an enrichment of Ubp10 binding (Fig. 1D). It is possible that pre-assembled heterochromatin is subject to activities such as chromatin remodeling, which may increase the dynamics of the SIR complex, allowing Ubp10 to co-assemble. Overall, this co-assembly mechanism is strongly suggestive of Ubp10 recruitment by the SIR complex during de novo formation of heterochromatin when H2B-Ub nucleosomes are most likely to be encountered by the SIR complex in vivo.
Figure 2.
Ubp10 co-assembles with SIR complex onto chromatin. A, schematic for testing Ubp10 binding to pre-assembled heterochromatin using the recombinant (recomb) proteins, SIR complex and Ubp10. SIR complex was pre-incubated with chromatin beads before the addition of Ubp10 to the reaction. B, Western blot analysis of pre-assembled heterochromatin pulldown. Ubp10 signal in mock reaction quantified as 1, and Ubp10 signal in +SIR complex sample is relative to mock. Mock and relative signals were normalized to H3. Proteins were detected using antibodies specific for epitope tags, GST-Ubp10, 3×HA-Sir2, and Sir3–3×FLAG, whereas H3 was detected by its native epitope. C, schematic for testing co-assembly of Ubp10 and SIR complex to chromatin using recombinant proteins, SIR complex and Ubp10. SIR complex was pre-incubated with Ubp10 before the addition of chromatin beads to the reaction. D, Western blot analysis of co-assembly of Ubp10 and SIR complex on chromatin, analyzed as in B.
Direct interaction with the Sir2/4 sub-complex underlies co-assembly of Ubp10 with SIR complex to chromatin
Previous yeast two-hybrid data suggested that Sir4 is a binding partner of Ubp10 in vivo (Fig. 3A) (30, 34). To begin biochemical characterization of this interaction, we first tested whether the purified Sir2/4 sub-complex mediates differential recruitment of Ubp10 to chromatin in WCE. The recombinant Sir2/4 sub-complex, rather than Sir4 alone, is used to maintain Sir4 integrity during the purification process (37). Sir2/4 stimulated Ubp10 association with chromatin in WCE (Fig. 3B, lanes 2 and 3). In this experiment, recombinant Sir2/4 is able to recruit Sir3 from the extract. To control for any background recruitment activity by endogenous Sir3 supplied in the extract, SIR3 was deleted in the Ubp10-TAP strain. In the absence of Sir3, Ubp10 was recruited to chromatin in a Sir2/4-dependent manner indicating that Sir3 is not required to recruit Ubp10 (Fig. 3, B and C). We then tested whether Sir2/4-mediated recruitment of Ubp10 to chromatin also occurs in a purified system. Similar to the results with the full SIR complex components, Ubp10 is only differentially recruited to chromatin under conditions of co-assembly with Sir2/4 (Fig. 3, D and E). On its own, Sir3 did not preferentially recruit Ubp10 to chromatin under any condition (Fig. S1, B and C).
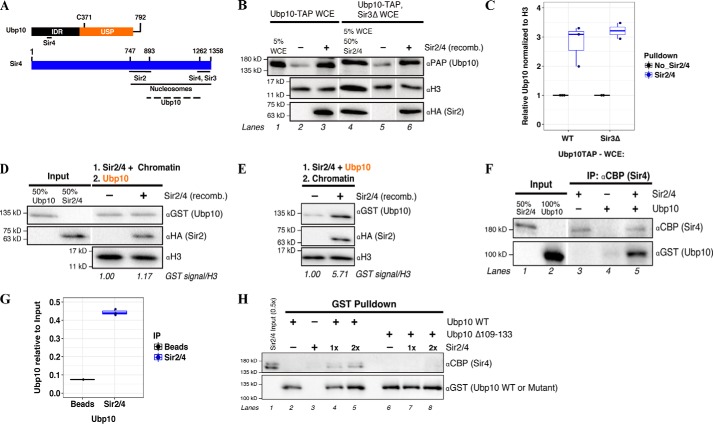
Figure 3.
Sir2/4 sub-complex directly interacts with Ubp10 during co-assembly onto chromatin. A, depiction of the domains and protein-binding sites on Ubp10 and Sir4. Ubp10 Cys-371 is the active cysteine involved in deubiquitinase activity. IDR, intrinsically disordered region; USP, ubiquitin-specific protease. The dotted line indicates the broad interaction region on Sir4 that Ubp10 is expected to bind as mapped by previous yeast two-hybrid data (28, 32). B, Western blot analysis of endogenous Ubp10-TAP recruitment to chromatin or Sir2/4-coated chromatin in whole-cell extracts made with sir3Δ. Lane 4, input for both recombinant Sir2/4 and Ubp10-TAP (sir3Δ cells) was combined. C, quantification of B. Signal for Ubp10-TAP is relative to input signal and then normalized to H3 signal. An integration of both a box plot and scatter plot is shown. Error bar indicates at least two independent experiments. D and E, similar experiments performed as in Fig. 2 except recombinant SIR complex is replaced with recombinant Sir2/4 sub-complex. Western blot analysis of recombinant GST-Ubp10 recruitment to pre-bound Sir2/4 chromatin (D) or co-assembly with Sir2/4 onto chromatin (E) is shown. Ubp10 signal in mock reaction quantified as 1 and Ubp10 signal in +SIR complex sample is relative to mock. Mock and relative signals were normalized to H3. Note: image (E) cropped from the same Western blot exposure as depicted in Fig. 2D. Input is as shown in Fig. 2D. F, Western blot analysis of Sir2/4 co-IP of Ubp10. Ubp10 and Sir2/4 were incubated together prior to immunoprecipitation with αCBP-conjugated to protein A beads. G, quantification of F. An integration of both a box plot and scatter plot is shown. Error bar indicates three independent experiments. H, Western blot analysis of GST pulldown of Sir2/4 sub-complex using GST-Ubp10 or GST-Ubp10 Δ109–133 bound to glutathione resin. Sir2/4 was added 1 or 2 times the picomoles of Ubp10 WT or mutant in the reaction.
The chromatin pulldown results show that Ubp10 likely interacts with the SIR complex on chromatin through Sir4. It is possible that chromatin acts as a bridging substrate for the interactions in our pulldown and in previously reported yeast two-hybrid experiments that initially suggested an interaction between Sir4 and Ubp10 (30). To rule this out, we next conducted a co-immunoprecipitation (co-IP) experiment using only purified Ubp10 and Sir2/4. For the capture step, we took advantage of the calmodulin-binding peptide (CBP) retained on Sir4 after tandem affinity purification. Ubp10 was detected only in the presence of Sir2/4, indicating a direct interaction between Ubp10 and Sir2/4 (Fig. 3, F and G). We next performed a reciprocal pulldown experiment and also tested the significance of the suggested Sir4-interacting region on Ubp10, which was previously mapped by yeast two-hybrids and shown to be necessary for telomeric silencing (34). Recombinant Ubp10 WT or Ubp10 Δ109–133 was immobilized on glutathione resin, and Sir2/4 was added to the reaction. The loss of the Sir4 interaction region on Ubp10 resulted in the loss of a direct interaction with Sir2/4 (Fig. 3H, lanes 7 and 8), whereas Ubp10 WT pulled down Sir2/4 (lanes 4 and 5). In contrast, Sir3 weakly interacts with Ubp10 by co-IP indicating Sir3 does not play a significant role in Ubp10 recruitment (Fig. S1D). Overall, these results strongly support that Ubp10 is brought to chromatin directly with the SIR complex primarily through the interaction with Sir4.
Ubp10 has independent histone-binding activity
Ubp10 appears to exhibit limited chromatin binding in the absence of Sir proteins (Fig. 2). Ubp10 bound to chromatin better at high concentrations; however, no binding to naked DNA was observed, suggesting that Ubp10 interacts with nucleosomes even in the absence of its ubiquitin substrate (Fig. 4A). Using recombinant proteins, including Ubp10, unmodified octamers, and octamers containing non-hydrolyzable H2B-Ub (H2B*Ub), we sought to determine which histones Ubp10 directly binds by performing a GST pulldown. Histones are highly conserved from yeast to humans, including the site and functions of H2B ubiquitination in its C-terminal tail (Lys-123 in Saccharomyces cerevisiae and Lys-120 in humans). We produced recombinant human histone octamers that contain stoichiometric H2B*Ub, where a non-hydrolyzable dithioether linkage attaches ubiquitin to lysine 120 (Fig. S1E)4 (38, 39). Importantly, these homogeneous populations of octamers are free of post-translational modifications except for H2B ubiquitination.
Figure 4.
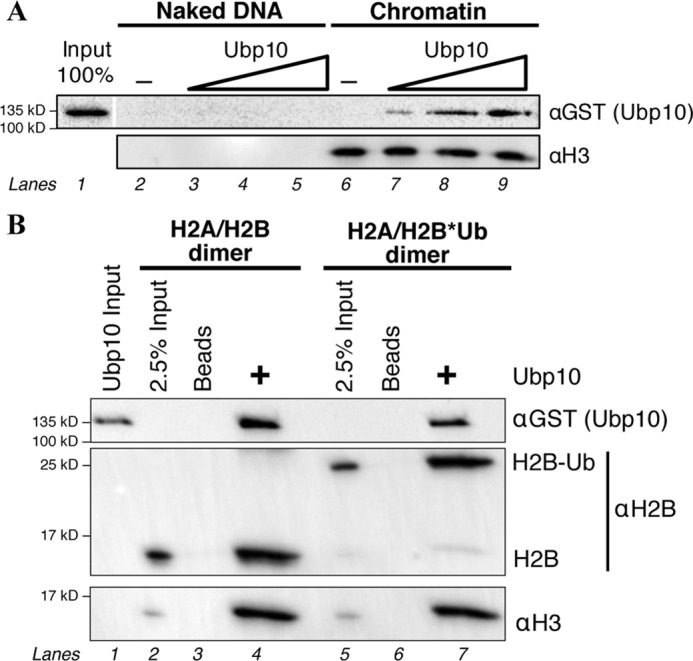
Up10 has independent histone-binding activity. A, Western blot of the titration of recombinant GST-Ubp10 with naked DNA or chromatin. Naked DNA and chromatinized DNA conjugated to beads were quantified and matched for pulldowns. B, Western blot of glutathione pulldown using recombinant Ubp10, unmodified octamers, and H2B*Ub-containing octamers.
Ubp10 pulled down not only H2A/H2B*Ub heterodimers as expected, but also unmodified H2A/H2B heterodimers and H3/H4 tetramers (Fig. 4B, lanes 4 and 7). In the pulldown conditions, H2A/H2B and H3/H4 form a stable dimer and tetramer, respectively. It is possible that Ubp10 can make simultaneous contacts with both the H2A/H2B dimer and H3/H4 tetramer or that Ubp10 may interact with either the H2A/H2B dimer or the H3/H4 tetramer. This result demonstrates that Ubp10 makes several interactions with the face of the nucleosome that permit limited binding on its own and may contribute to interactions during assembly with the SIR complex.
Assembly of H2B-Ub into nucleosomes reduces Ubp10-deubiquitinating activity
To determine the mechanism of Ubp10 activity regulation, we characterized Ubp10 DUB activity using ubiquitinated substrates. Ubp10 cleaves a variety of polyubiquitin chains of different linkages (Lys-63, Lys-11, and Lys-48) with the exception of a linear chain (linked through M1) (Fig. S2A). To assess Ubp10 DUB activity on relevant ubiquitinated substrates, we performed in vitro DUB assays using recombinant histones that are chemically ubiquitinated at defined sites (H2AKc119Ub or H2BKc120Ub). These substrates contain hydrolyzable Ub-histone linkages that differ from the native isopeptide bonds by only one atom (Fig. S1E).4
We reconstituted H2B-Ub mononucleosomes by salt-dialysis and observed, as expected, reduced migration of monoubiquitinated nucleosomes on a native polyacrylamide gel compared with unmodified mononucleosomes (Fig. 5A). We tested the specificity of Ubp10 DUB activity for different monoubiquitinated nucleosomes and found that Ubp10 cleaves both H2BKc120-Ub and H2AKc119-Ub (Fig. 5B, lanes 5–8, and Fig. S2C). Hydrolysis of H2B-Ub and H2A-Ub is a result of Ubp10 DUB catalytic activity as monoubiquitinated H2B remains uncleaved in the presence of ubiquitin-aldehyde, a highly specific DUB inhibitor (Fig. S2C, lane 5). Given that Ubp10 binds free H2A/H2B-Ub heterodimers, we tested whether Ubp10 also cleaves H2B-Ub outside of the nucleosomal context. There is a 6-fold increase in de-ubiquitinated H2B at 10 min when free H2A/H2B-Ub heterodimers are the substrate compared with H2B-Ub nucleosomes (Fig. 5B, lanes 4 and 8). The catalytic activity of Ubp10 is much lower when presented with an H2B-Ub nucleosome versus H2B-Ub dimers. As a control, the GST tag was cleaved from the N terminus of Ubp10, and we tested DUB activity with H2A/H2B-Ub heterodimers and H2B-Ub mononucleosomes. In the absence of the GST tag, we found that Ubp10 rapidly loses catalytic activity (Fig. S3). GST appears to either stabilize Ubp10 in a conformation that is required for catalytic activity or promotes the solubility/integrity of Ubp10. As such, all DUB activity assays were performed in the context of the GST tag.
Figure 5.
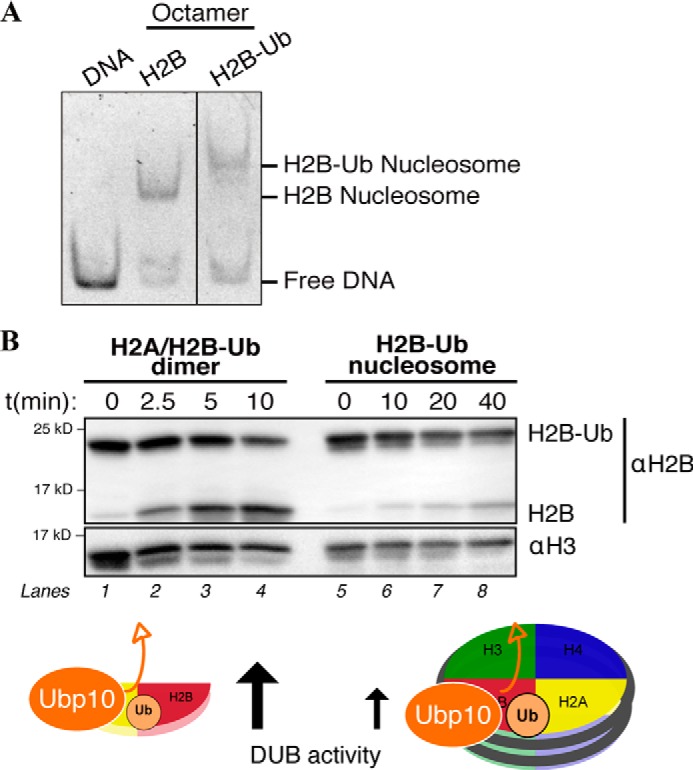
H2B-Ub nucleosomes are resistant to Ubp10 DUB activity. A, native gel of reconstituted mononucleosomes containing either unmodified human histone octamer (H2B) or H2B-Ub-assembled octamer. Mononucleosomes were reconstituted via salt dialysis using 147-bp DNA template containing a 601 Widom sequence. DNA–protein complexes were run on a 5% native polyacrylamide gel and stained with SYBR Gold. B, in vitro deubiquitinase assay (DUB assay) was performed using 5 nm recombinant Ubp10 and 100 nm nucleosomal H2A/H2B-Ub or 100 nm free H2A/H2B-Ub heterodimers. Deubiquitination activity was determined by SDS-PAGE followed by Western blot analysis using αH2B and looking at formation of cleaved H2B. Samples were quenched at indicated time points.
Sir2/4 enhances Ubp10 DUB activity on H2B-Ub mononucleosomes
A number of DUBs require binding partners for targeting and enhanced stimulation of deubiquitinase activity (40–42). Because Sir4 and Ubp10 directly interact, we wanted to test whether Sir4 binding stimulates Ubp10 DUB activity on H2B-Ub mononucleosomes. Indeed, when Sir2/4 was added equimolar to Ubp10, there was a substantial increase in Ubp10 DUB activity on H2B-Ub nucleosomes (Fig. 6A). At 10 min, there is an ∼33-fold increase in de-ubiquitinated H2B mononucleosomes when Ubp10 and Sir2/4 are present compared with Ubp10 alone (Fig. 6B). As expected, Sir2/4 does not cleave H2B-Ub on its own; thus, the increase in H2B-Ub cleavage observed is a Sir2/4-dependent effect on Ubp10 DUB activity (Fig. S4A). Similarly, the SIR complex enhanced Ubp10 DUB activity (Fig. 6C), whereas Sir3 alone prevented Ubp10-dependent cleavage of nucleosomal H2B-Ub (Fig. S1F). We also observed enhanced cleavage of free H2A/H2B-Ub heterodimers by Ubp10 in the presence of Sir2/4, indicating that stimulation can also occur outside the context of a nucleosome. However, Sir2/4 stimulation of Ubp10 DUB activity did not extend to a Lys-11-linked di-ubiquitin chain substrate (Fig. S2B), nor did Sir2/4 stimulate DUB activity of the SAGA DUB module, which contains Ubp8, another budding yeast DUB that targets H2B-Ub (Fig. S4B).
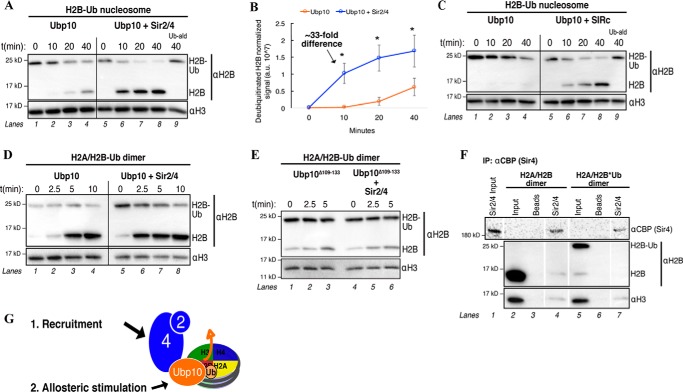
Figure 6.
Sir2/4 allosterically stimulates Ubp10 DUB activity on H2B-Ub nucleosomes. A, in vitro DUB assays performed as in Fig. 5. 5 nm Sir2/4 was added to 5 nm Ubp10 and 100 nm nucleosomal H2A/H2B-Ub. Samples were quenched at the indicated time points. Ubiquitin-aldehyde (Ub-ald) above lane indicates ubiquitin-aldehyde (DUB inhibitor) was added to reaction. B, quantification of A. Generation of H2B signal was plotted as a function of time. H2B signal (volume intensity) was normalized to H3 signal (loading control) to generate arbitrary values (107). Error bars indicate three independent experiments. Asterisks indicates a p value < 0.05. C, DUB assays as in A with 5 nm SIR complex, 5 nm Ubp10, and 100 nm nucleosomal H2A/H2B-Ub. D, in vitro DUB assays performed as in Fig. 5. 5 nm Sir2/4 was added to 5 nm Ubp10 and 100 nm H2A/H2B-Ub heterodimers. E, in vitro DUB assays performed as in Fig. 5. 5 nm Sir2/4 was added to 5 nm Ubp10 Δ109–133 and 100 nm H2A/H2B-Ub heterodimers. F, Sir2/4 co-IP in the presence of either unmodified core histone octamer (H2B) or H2B-Ub assembled octamer. Sir4 was immunoprecipitated by bead-conjugated αCBP, and associated proteins were detected by Western blotting. G, diagram depicting Sir2/4-dependent recruitment and allosteric stimulation of Ubp10 for enhanced DUB activity on a H2B-Ub nucleosome.
Sir4 binds Ubp10 within the N-terminal region, distal from the C-terminal catalytic DUB domain (Fig. 3A) (30, 34). We hypothesize that Sir4 allosterically stimulates Ubp10 DUB activity on H2B-Ub substrates. In support of an allosteric stimulation, we demonstrate that the loss of the Sir4 interaction site on Ubp10 results in the loss of stimulated DUB activity in the presence of Sir2/4 (Fig. 6E). Additionally, Sir2/4 does not directly interact with H2B-Ub (Fig. 6F, compare lanes 4 and 7), as this interaction could increase the local substrate concentration of both Ubp10 and H2B-Ub and thus enhance DUB activity. From these results, we conclude that the enhancement in Ubp10 DUB activity on H2B-Ub substrates is a combination of direct recruitment and specific allosteric stimulation by Sir2/4 (Fig. 6G).
Discussion
In this study, we employed multiple biochemical techniques to mechanistically determine how Ubp10 activity is coupled to the assembly of SIR complex heterochromatin. We demonstrate that Ubp10 co-assembles with SIR complex onto chromatin rather than to a pre-assembled heterochromatic structure. We identify that Sir4 directly binds Ubp10 off chromatin and that this interaction ultimately facilitates enhanced de-ubiquitination of H2B-Ub substrates. The enhancement of Ubp10 de-ubiquitination of H2B-Ub mononucleosomes likely occurs through a combination of recruitment and allosteric stimulation. As discussed below, we propose that SIR complex–regulated targeting of Ubp10 to chromatin and stimulation of Ubp10 DUB activity on H2B-Ub nucleosomes is important for the initiation of de novo heterochromatic silencing (Fig. 7).
Figure 7.
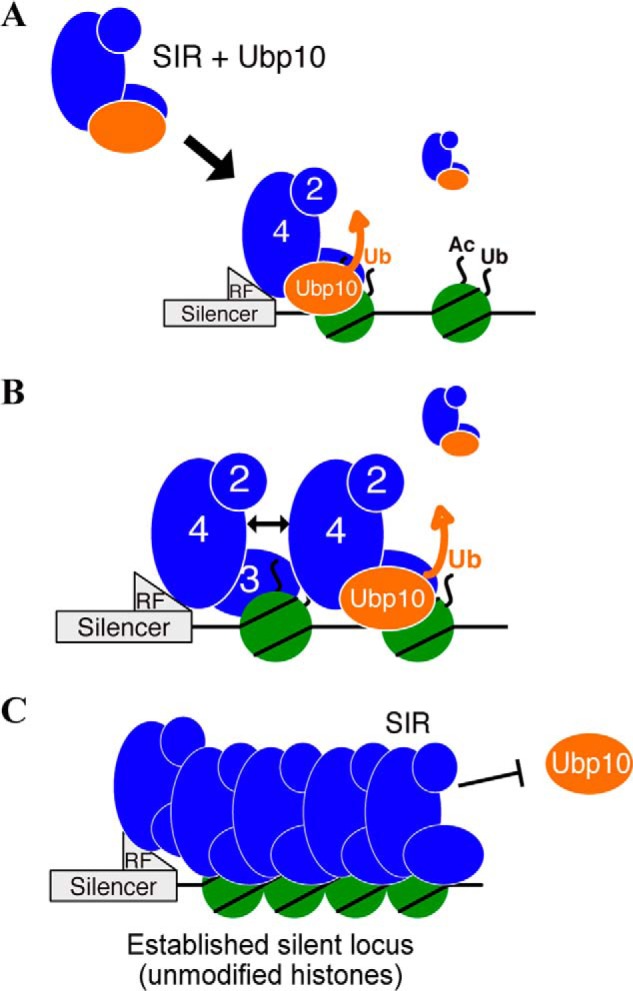
Model for Ubp10 function during de novo heterochromatin assembly. A, SIR complex recruits a H2BK123-Ub deubiquitinase, Ubp10, off chromatin and targets Ubp10 to monoubiquitinated chromatin (an active locus). Sir2/4 allosterically enhances (thick orange arrow) Ubp10 de-ubiquitination of H2BK123-Ub, which directly prevents subsequent H3 methylation. B, de-modification of the adjacent nucleosome allows SIR complex to engage the nucleosome, recruit another SIR-Ubp10 complex via self–self interactions (double-headed arrow), and promote de-modification of the next nucleosome. C, established heterochromatin domain is devoid of post-translational modifications and does not recruit Ubp10.
Ubp10 is not recruited to an established heterochromatin structure
We confirmed previous in vivo observations that Ubp10 is recruited to heterochromatin in a SIR complex-dependent manner using pre-assembled heterochromatin and whole-cell extracts (Fig. 1) (30–32). SIR complex–mediated recruitment of Ubp10 to chromatin appears to be dependent on the state of SIR complex assembly. Ubp10 binding was minimal on pre-assembled heterochromatin, most likely due to the masking of the binding site for Ubp10 on Sir4. This suggests that once a stable and compact heterochromatin structure, devoid of histone PTMs, is formed, Ubp10 recruitment is specifically limited. Our results may explain seemingly paradoxical observations in which ubp10Δ strains do not exhibit large increases in H2B-Ub or gene expression at the HM loci and sites of natural telomeric silencing (32, 35, 43). Similarly, pre-established, endogenous sites of heterochromatin (HML and HMR) in vivo appear to be maintained upon deletions of DOT1 or SET1 (24). At established heterochromatin, presumably Ubp10 has already de-ubiquitinated H2B during de novo assembly and is not required for further maintenance. In addition, Ubp10 DUB activity is also important for regulating non-histone-ubiquitinated proteins, notably Rpa190 (the largest subunit of RNA polymerase I) (33). Thus, it is potentially important for Ubp10 to not be trapped at established heterochromatic regions to promote a soluble pool of Ubp10 to act elsewhere in the genome.
Ubp10 co-assembly with SIR complex may facilitate de novo HC establishment
Collectively, our order of addition experiments and complementary co-IP and pulldown results (Figs. 2 and 3) demonstrate that the direct interaction between Ubp10 and Sir4 likely underlies the co-assembly mechanism. Our studies confirm previous yeast two-hybrid interaction data suggesting Sir4 and Ubp10 directly interact (30, 34) and chromatin immunoprecipitation results that argue Ubp10 and SIR complex mutually recruit each other to regulate silencing at sub-telomeres (31). Our data extend this idea in that an upstream event, Sir2/4 binding Ubp10 in the nucleoplasm prior to chromatin association, may reflect the recruitment of Ubp10 during the initiation of heterochromatin assembly in vivo.
A de novo silencing event of an active locus, where the SIR complex would assemble in a genomic region for the first time, may be the context where Ubp10 is most important. H2B-Ub appears to be important for nucleosome re-assembly after chromatin-disruptive processes, such as DNA replication and RNA transcription (44, 45). A recent study suggests that Asf1-mediated incorporation of H2B-Ub during nucleosome assembly is needed to regulate heterochromatin establishment (46). Based on our model, Ubp10 recruitment would act downstream to clear H2B-Ub from chromatin to facilitate SIR complex–mediated compaction of nucleosomes. This mechanism likely occurs during each round of re-assembly of SIR-mediated heterochromatin, even if ubiquitinated H2B is only encountered infrequently.
Sir2/4 specifically targets Ubp10 to H2B-Ub nucleosomes
On its own, Ubp10 binds to chromatin only at relatively high concentrations and associates with unmodified H2A/H2B heterodimers and, surprisingly, H3/H4 tetramers. These results indicate Ubp10 can interact with a nucleosome through low-affinity histone interactions in the absence of its ubiquitin substrate (Fig. 4). However, we are unable to distinguish whether one molecule of Ubp10 makes simultaneous or independent contacts with H2A/H2B and H3/H4. In contrast, we show that Ubp10 is recruited to chromatin and targeted to H2B-Ub nucleosomes when in complex with the main silencing machinery in budding yeast. A parallel targeting mechanism to nucleosomes is seen with the targeting of Ubp8 to regions of active transcription by the SAGA DUB module (41, 47). Ubp8 displays little DUB activity independent of the SAGA DUB module. However, Ubp8 is incorporated into the DUB module off chromatin, targeted to H2B-Ub nucleosomes in a Sgf11-dependent manner, and exhibits DUB activity on H2B-Ub chromatin. Overall, our results support the theme of incorporating DUBs into larger protein complexes for recruitment and precise targeting (48). This mechanism appears to be an important layer of regulation in DUB biology in light of the diverse number of substrates that are ubiquitinated (49).
Sir2/4 allosterically stimulates Ubp10 activity to generate the silent histone code
The “histone code” that drives heterochromatin establishment in budding yeast requires simply the absence of histone PTMs. Sir2-dependent deacetylation is the sole erasure activity of the SIR complex, yet SIR complex binding is not severely affected by acetylated chromatin in the absence of deacetylase activity (10). Instead, Sir3, the Sir protein important for spreading of the SIR complex, is particularly sensitive to histone acetylation and H3K79me (6, 8, 9, 11, 12, 23, 50). It remains unknown whether H2B-Ub alone can directly inhibit or reduce SIR complex binding to the nucleosome. However, H2B-Ub indirectly inhibits SIR complex silencing through the promotion of H3K4 and H3K79 methylation. The kinetics of establishing heterochromatin is delayed in the absence of Jhd2, the H3K4 demethylase, indicating that erasing methylation marks is a key feature in heterochromatin assembly (24). Whereas Jhd2 is known as an H3K4 demethylase (51), there remains no known demethylase for H3K79me. Instead, the removal of this stable methylation mark is likely to be dependent on multiple cycles of DNA replication to be diluted.
We propose a model in which the SIR complex, in conjunction with Ubp10, can overcome the challenge of anti-silencing H3 methylation marks on chromatin. Alone, Ubp10 is an inefficient DUB in the presence of its target substrate as it is less active on an H2B-Ub nucleosome compared with H2A/H2B-Ub dimers (Fig. 5). However, when in complex with Sir2/4, Ubp10 DUB activity is greatly stimulated on H2B-Ub substrates, both mononucleosomes and H2A/H2B-Ub dimers (Fig. 6). Furthermore, the data strongly argue that this increase in DUB activity is regulated by allosteric stimulation and is specific to Ubp10 (Fig. 6). The observed resistance of H2B-Ub nucleosomes to Ubp10 DUB activity may be multifaceted. First, the mononucleosomal context of H2B-Ub may structurally present a challenge to Ubp10 regarding the accessibility of the isopeptide bond. Sir2/4 recruitment of Ubp10 to the nucleosome may better position the DUB domain of Ubp10 to cleave monoubiquitin. Second, a Sir2/4-bound Ubp10 may have a decreased off rate on the nucleosome versus Ubp10 alone. This Sir2/4-dependent tethering of Ubp10 increases the probability of cleaving monoubiquitinated H2B. Finally, Ubp10 DUB activity is allosterically stimulated by Sir2/4. Allosteric stimulation appears to be a common means to regulate and restrict DUBs to specifically act on their substrate (48, 52, 53). None of the above-mentioned mechanisms for Ubp10 and the SIR complex are mutually exclusive and likely work in combination to achieve specific targeting of Ubp10 to H2B-Ub chromatin in regions that will undergo silencing.
Our study demonstrates how the SIR complex can coordinate with other epigenetic erasure proteins to transition an active domain into a silent domain. This specific coupling of deacetylation and H2B de-ubiquitination by a chromatin-modifying complex to achieve gene repression has also been recently shown in a mammalian system (54). We propose that Ubp10 is important for de novo heterochromatin establishment and that Sir2/4 enhancement of Ubp10 DUB activity on H2B-Ub nucleosomes represents a mechanism that minimizes the opportunity for subsequent H3 methylation (Fig. 7). The eventual dilution of the anti-silencing mark H3K79me and the prevention of new H3K79 methylation by removing H2B-Ub would facilitate heterochromatin establishment, as has been observed previously for de novo silencing kinetics (9, 24, 55). In conjunction with previous genetic analyses of Ubp10 in silencing, we present a more refined and mechanistically detailed model for how Ubp10 promotes assembly of SIR complex–mediated heterochromatin.
Experimental procedures
DNA templates
The 3-kb DNA template used for chromatin pulldowns was PCR-generated from plasmid pUC18-G5cyc1G−, bearing five Gal4-binding sites upstream of a CYC1 promoter-driven G-less cassette, using a biotinylated primer as described previously (10). DNA used for reconstituting mononucleosomes was PCR-generated from plasmid 601 using primers that primed 147 bp with the Widom 601 sequence in the center of the template. PCR products were purified using Macherey-Nagel Nucleospin DNA purification kit.
Cloning and purification of GST-Ubp10 (full length and mutant)
PCR was used to amplify the Ubp10 ORF from S. cerevisiae genomic DNA. pGEX-6-P1 and the Ubp10 PCR insert were digested with XhoI and EcoRI and followed by T4 DNA ligation to make pAJ285. For the Sir-interaction Ubp10 mutant, pAJ285 was digested with HindIII and SacI. A Gibson assembly reaction was then performed using digested pAJ285 and a Ubp10 Δ109–133 gBlock (IDT) fragment to make GST-Ubp10 Δ109–133, pAJ342. GST-Ubp10 WT or mutant was expressed in E. coli BL21 Codon-Plus cells as described previously (34). Cells were harvested and lysed in Lysis Buffer containing PBS, pH 7.4, additionally containing 350 mm NaCl, 1 mm EDTA, 1 mm EGTA, 15 mm DTT, 0.5% Triton X-100, 1 mm PMSF, 2 mg/ml lysozyme. The soluble extract was added to glutathione resin (Thermo Fisher Scientific) and eluted off with 10 mm glutathione. A Mono Q column was used to further purify GST-Ubp10. Fractions were dialyzed in PB150, and aliquots were stored at −80 °C. Refer to Table S2 for plasmid or strain information.
Sir protein purifications
Recombinant Sir2, Sir3, and Sir4 were purified as described previously (10), except that Sir2/4 was tandem affinity-purified from strain AJY136. The integrated Sir2/4 yeast strain (AJY136) was generated by first removing the CEN6 from 3×HA-Sir2/LEU2 and TAP-Sir4/URA3 using Gibson assembly. pAJ327 (TAP-Sir4/URA3-CEN6) and pAJ328 (3XHA-Sir2/LEU2-CEN6) were linearized and integrated into AJY65 using the lithium acetate transformation to generate AJY136. Refer to Table S2 for plasmid or strain information.
Chromatin reconstitution and nucleosome assembly
Enzymatic assembly of chromatin templates, MNase analysis, and quantification of DNA were performed as described previously (10). Mononucleosomes were reconstituted using salt dialysis, as described previously (56), and stored at 4 °C for no longer than a month.
Whole-cell extract preparation
The Ubp10-TAP yeast strain was obtained from the yeast TAP collection (Open Biosystems). Cells were grown to ∼1 × 107 cells/ml in YPD at 30 °C with shaking. Cells were pelleted at 4000 rpm and washed in Wash Buffer: 20 mm Tris-HCl, pH 7.5, 150 mm NaCl. Cells were spun, resuspended in 1 ml of Wash Buffer, pelleted, and flash-frozen. Pellets were thawed and resuspended in Lysis Buffer (50 mm HEPES-KOH, pH 7.5, 325 mm NaCl, 10% glycerol, 0.5% Nonidet P-40, 1 mm EDTA, 1 mm PMSF, 1 μg/ml bestatin/leupeptin/pepstatin, 1 mm benzamidine), and 0.1 mg/ml BSA. Glass beads were added to the biomass in a final volume of 1 ml and lysed using a bead mill (Mini-Beadbeater, BioSpec): 2× 45-s bead beating at 4 °C with a 2-min rest in between on ice. The lysate was collected and spun 14,000 rpm for 5 min at 4 °C. Soluble lysate was transferred to a new tube, spun for 15 min, and collected in a new tube. Bradford readings were taken and lysates flash-frozen and stored at −80 °C. Refer to Table S2 for plasmid or strain information.
Chromatin and heterochromatin pulldowns
Bead-conjugated 3.0-kb pUC18-G5cyc1G− chromatin template (113 ng of DNA) was incubated with SIR complex, Sir3 (23 pmol for WCE experiments or 2.3 pmol for experiments using all recombinant proteins) and Sir2/4 (0.6 pmol), for 1 h at room temperature with rotation in 30 μl of Pulldown Buffer150 (PB150): 50 mm HEPES, pH 7.5, 10 mm magnesium acetate, 5 mm EGTA, 0.1 mm EDTA, 0.02% Nonidet P-40, 5% glycerol, 150 mm potassium acetate, 1 mm DTT, 1 mm PMSF, 1 μg/ml bestatin/leupeptin/pepstatin, 1 mm benzamidine, and 0.1 mg/ml BSA. For experiments in WCEs, SIR complex-bound chromatin was incubated in 100 μg of WCE, an ATP-regeneration system (30 mm creatine phosphate, 3 mm ATP, 4.1 mm magnesium acetate, and 6.4 μg/ml creatine kinase, final concentration), and PB150 in 20 μl for 1 h at room temperature with rotation, followed by 1 h rotation at 4 °C. Samples were washed in cold PB100, twice in 500 μl and once in 150 μl. In the purified in vitro system, pulldown experiments were performed as in the above procedure with several modifications: 2.25 pmol of GST-Ubp10 was used in place of WCE, and the ATP-regeneration system was not used. Samples were boiled, separated from magnetic beads, run on a 12% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. Proteins were detected by Western blot analysis.
Generation of hydrolyzable and non-hydrolyzable monoubiquitinated histone octamers
The non-hydrolyzable ubiquitinated histone mimics (H2B*Ub) were prepared by cross-linking of recombinant human H2BK120C and His-tagged UbG76C, as described previously (38). The hydrolyzable mimic (H2BKc120ub) was prepared from recombinant human H2BK120C and His-tagged Ub. An isopeptide bond was formed between the C terminus of Ub and a derivatized aminoethylcysteine side chain at position 120. A series of blocking and de-blocking steps were taken to ensure site specificity of the reaction, which were confirmed by mass spectrometry analysis. Detailed method will be reported elsewhere.4
Co-immunoprecipitations
After tandem affinity purification of Sir2/4, Sir4 retains the CBP sequence. αCBP (ICL RCBP-45A-Z) was conjugated to protein A Dynabeads (Life Technologies, Inc., 10002D) for 30 min at room temperature. Beads were washed with PBS and resuspended in Pulldown Buffer100 (PB100): 50 mm HEPES, pH 7.5, 10 mm magnesium acetate, 5 mm EGTA, 0.1 mm EDTA, 0.02% Nonidet P-40, 5% glycerol, 100 mm potassium acetate, 1 mm DTT, 1 mm PMSF, 1 μg/ml bestatin/leupeptin/pepstatin, 1 mm benzamidine, and 0.1 mg/ml BSA. 0.6 pmol of Sir4 and 2 pmol of Ubp10 were added to beads in a 20-μl reaction. When histone octamers (4× molar amounts of Sir2/4 or Ubp10) were used; tubes were blocked with buffer containing 2 mg/ml BSA and 0.1% Nonidet P-40, and reactions were performed in 250 μl. Proteins were incubated for 60 min on ice. Beads were added to the proteins and rotated end-over-end for 60 min at 4 °C. Beads were magnet separated, resuspended in SDS-Laemmli Buffer, and boiled at 95 °C. Ubp10 and Sir2/4 samples were run on an 8% polyacrylamide-SDS gel, and Sir2/4 or Ubp10 with histone octamers were run on a 4–20% gradient gel (Bio-Rad). Sir2/4 and Ubp10 were transferred to a nitrocellulose membrane, and histones were transferred onto an Immobilon PSQ PVDF membrane (Millipore). Proteins were detected by Western blot analysis.
GST pulldown
For Ubp10 pulldowns with Sir2/4, 2 pmol of GST-Ubp10 or GST-Ubp10 Δ109–133 were incubated with glutathione SuperFlow agarose resin (Pierce catalog no. 25237) for 120 min at 4 °C with rotation in Pulldown Buffer150 (PB150): 50 mm HEPES, pH 7.5, 10 mm magnesium acetate, 5 mm EGTA, 0.1 mm EDTA, 0.02% Nonidet P-40, 5% glycerol, 150 mm potassium acetate, 1 mm DTT, 1 mm PMSF, 1 μg/ml bestatin/leupeptin/pepstatin, 1 mm benzamidine, and 0.1 mg/ml BSA. Ubp10-bound resin was washed once in 200 μl of PB150. Sir2/4 was added at 2 pmol (1×) or 4 pmol (2×) to Ubp10-resin and incubated for 30 min at 4 °C with rotation in PB150. Beads were spun at 500 × g for 1 min at 4 °C and washed in 50 μl of PB150. Samples were boiled in SDS-Laemmli Buffer and supernatants were run on an 8% polyacrylamide-SDS gel. Proteins were transferred onto a nitrocellulose membrane and detected by Western blotting.
For Ubp10 pulldowns with histones, 2.25 pmol of recombinant GST-Ubp10 was incubated with 4.5 (2×) pmol of recombinant Homo sapiens histone octamer in PB150 for 60 min rotating at 4 °C. Glutathione resin was added to the IP sample and incubated for 60 min rotating at 4 °C. Beads were spun 700 × g for 1 min at 4 °C and washed once in PB500 (0.5 m potassium acetate). Beads were spun, resuspended in SDS-Laemmli Buffer, and boiled at 95 °C. Samples were run on a 4–20% SDS-polyacrylamide gel, and Ubp10 was transferred to a nitrocellulose membrane, and histones were transferred to a PVDF PSQ (0.22 μm). Proteins were detected by Western blot analysis.
In vitro deubiquitinase assays
In vitro DUB assays were performed in 15-μl reactions with DUB Buffer (40 mm Tris, pH 7.6, 100 mm NaCl, 0.1 mg/ml BSA, 5% glycerol, and 2 mm DTT). Low-retention tubes were blocked with 2 mg/ml BSA and 0.1% Nonidet P-40. Recombinant proteins were dialyzed in DUB buffer with 100 μm ZnSO4 prior to experiments. 100 nm free H2A/H2B-Ub heterodimers or 100 nm nucleosomal H2A/H2B-Ub heterodimer (50 nm nucleosome sample with two copies of H2A/H2B-Ub) were incubated with 5 nm recombinant GST-Ubp10 or GST-Ubp10 Δ109–133. When indicated, SIR complex or Sir proteins were incubated with GST-Ubp10 at equimolar concentrations for 1 h on ice prior to the experiment. Ub-aldehyde was used for inhibited control samples at 2.5 μm final. Reactions were incubated at 23 °C in a thermoshaker, and time point samples were quenched in 5× SDS-Laemmli Buffer. Boiled samples were run on a 15% SDS-polyacrylamide gel and either stained with Coomassie Blue or transferred to Immobilon PSQ PVDF membranes for Western blot analysis. Proteins were probed by Western blot analysis.
We observed differences in detection and transfer efficiencies between monoubiquitinated H2B and H2B that influenced the detection signal by the H2B antibody. Monoubiquitin potentially influences access of the H2B antibody to its epitope, which resides within the last 25 amino acids of the C-terminal tail of H2B. We thus used arbitrary units as a mode of measure to compare volumetric intensities of de-ubiquitinated H2B between different conditions within the same experiment. Quantifications of Western blottings were performed using ImageLab (Bio-Rad). For data analysis, volumetric signal intensity for H2B (cleaved H2B-Ub product) was determined by subtracting background signal from the H2B signal. This provided the de-ubiquitinated H2B signal that was then normalized to the H3 signal per lane (loading control) and divided by 107. Arbitrary units (107) were then plotted as a function of time. Error bars were determined from three individual experiments, and p values were generated using Welch's t test.
Author contributions
A. Z. and A. M. J. conceptualization; A. Z. and E. D. D. data curation; A. Z. formal analysis; A. Z., N. O. A.-A., T. Y., and A. M. J. funding acquisition; A. Z. validation; A. Z., N. O. A.-A., E. D. D., T. Y., and A. M. J. investigation; A. Z., N. O. A.-A., E. D. D., T. Y., and A. M. J. methodology; A. Z. and A. M. J. writing-original draft; A. Z., N. O. A.-A., E. D. D., T. Y., and A. M. J. writing-review and editing; A. M. J. supervision.
Supplementary Material
Acknowledgments
We thank M. Churchill for helpful discussions. We also thank D. Kuljis for initial cloning of Ubp10, and A. Jacobson and C. Liu for the ubiquitin chains. We are very appreciative for the generous gift of recombinant SAGA DUB module from C. Wolberger. We also thank R. Sheridan for help with R.
This work was supported in part by National Institutes of Health Grants T32GM008730 (to A. Z.), R01GM098401 (to T. Y.), and R35GM119575 (to A. M. J.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4 and Tables S1 and S2.
N. O. Al-Afaleq and T. Yao, manuscript in preparation.
- PTM
- post-translational modification
- SIR
- silent information regulator
- H2B-Ub
- monoubiquitination of histone H2B
- DUB
- deubiquitinase
- Ub
- ubiquitin
- WCE
- whole-cell extract
- co-IP
- co-immunoprecipitation
- CBP
- calmodulin binding peptide
- GST
- glutathione S-transferase
- PMSF
- phenylmethylsulfonyl fluoride
- SILAC-MS
- stable isotope labeling of amino acids in cell culture-mass spectrometry
- TAP
- tandem affinity purification
- MNase
- micrococcal nuclease.
References
- 1. Vaquero A., Scher M., Erdjument-Bromage H., Tempst P., Serrano L., and Reinberg D. (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444 10.1038/nature06268 [DOI] [PubMed] [Google Scholar]
- 2. Margueron R., Justin N., Ohno K., Sharpe M. L., Son J., Drury W. J. 3rd., Voigt P., Martin S. R., Taylor W. R., De Marco V., Pirrotta V., Reinberg D., and Gamblin S. J. (2009) Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767 10.1038/nature08398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strahl B. D., and Allis C. D. (2000) The language of covalent histone modifications. Nature 403, 41–45 10.1038/47412 [DOI] [PubMed] [Google Scholar]
- 4. Suganuma T., and Workman J. L. (2011) Signals and combinatorial functions of histone modifications. Annu. Rev. Biochem. 80, 473–499 10.1146/annurev-biochem-061809-175347 [DOI] [PubMed] [Google Scholar]
- 5. Hecht A., Laroche T., Strahl-Bolsinger S., Gasser S. M., and Grunstein M. (1995) Histone H3 and H4 N termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80, 583–592 10.1016/0092-8674(95)90512-X [DOI] [PubMed] [Google Scholar]
- 6. Armache K. J., Garlick J. D., Canzio D., Narlikar G. J., and Kingston R. E. (2011) Structural basis of silencing: Sir3 BAH domain in complex with a nucleosome at 3.0 A resolution. Science 334, 977–982 10.1126/science.1210915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F., Li G., Altaf M., Lu C., Currie M. A., Johnson A., and Moazed D. (2013) Heterochromatin protein Sir3 induces contacts between the amino terminus of histone H4 and nucleosomal DNA. Proc. Natl. Acad. Sci. U.S.A. 110, 8495–8500 10.1073/pnas.1300126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Leeuwen F., Gafken P. R., and Gottschling D. E. (2002) Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109, 745–756 10.1016/S0092-8674(02)00759-6 [DOI] [PubMed] [Google Scholar]
- 9. Katan-Khaykovich Y., and Struhl K. (2005) Heterochromatin formation involves changes in histone modifications over multiple cell generations. EMBO J. 24, 2138–2149 10.1038/sj.emboj.7600692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson A., Li G., Sikorski T. W., Buratowski S., Woodcock C. L., and Moazed D. (2009) Reconstitution of heterochromatin-dependent transcriptional gene silencing. Mol. Cell 35, 769–781 10.1016/j.molcel.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitada T., Kuryan B. G., Tran N. N., Song C., Xue Y., Carey M., and Grunstein M. (2012) Mechanism for epigenetic variegation of gene expression at yeast telomeric heterochromatin. Genes Dev. 26, 2443–2455 10.1101/gad.201095.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behrouzi R., Lu C., Currie M. A., Jih G., Iglesias N., and Moazed D. (2016) Heterochromatin assembly by interrupted Sir3 bridges across neighboring nucleosomes. Elife 5, e17556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kueng S., Oppikofer M., and Gasser S. M. (2013) SIR proteins and the assembly of silent chromatin in budding yeast. Annu. Rev. Genet. 47, 275–306 10.1146/annurev-genet-021313-173730 [DOI] [PubMed] [Google Scholar]
- 14. Oppikofer M., Kueng S., and Gasser S. M. (2013) SIR-nucleosome interactions: structure-function relationships in yeast silent chromatin. Gene 527, 10–25 10.1016/j.gene.2013.05.088 [DOI] [PubMed] [Google Scholar]
- 15. Rusche L. N., Kirchmaier A. L., and Rine J. (2003) The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72, 481–516 10.1146/annurev.biochem.72.121801.161547 [DOI] [PubMed] [Google Scholar]
- 16. Tanny J. C., Dowd G. J., Huang J., Hilz H., and Moazed D. (1999) An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell 99, 735–745 10.1016/S0092-8674(00)81671-2 [DOI] [PubMed] [Google Scholar]
- 17. Imai S., Armstrong C. M., Kaeberlein M., and Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 10.1038/35001622 [DOI] [PubMed] [Google Scholar]
- 18. Oppikofer M., Kueng S., Martino F., Soeroes S., Hancock S. M., Chin J. W., Fischle W., and Gasser S. M. (2011) A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J. 30, 2610–2621 10.1038/emboj.2011.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hecht A., Strahl-Bolsinger S., and Grunstein M. (1996) Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383, 92–96 10.1038/383092a0 [DOI] [PubMed] [Google Scholar]
- 20. Onishi M., Liou G. G., Buchberger J. R., Walz T., and Moazed D. (2007) Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol. Cell 28, 1015–1028 10.1016/j.molcel.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 21. Santos-Rosa H., Bannister A. J., Dehe P. M., Géli V., and Kouzarides T. (2004) Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J. Biol. Chem. 279, 47506–47512 10.1074/jbc.M407949200 [DOI] [PubMed] [Google Scholar]
- 22. Ng H. H., Robert F., Young R. A., and Struhl K. (2003) Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11, 709–719 10.1016/S1097-2765(03)00092-3 [DOI] [PubMed] [Google Scholar]
- 23. Ng H. H., Ciccone D. N., Morshead K. B., Oettinger M. A., and Struhl K. (2003) Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: a potential mechanism for position-effect variegation. Proc. Natl. Acad. Sci. U.S.A. 100, 1820–1825 10.1073/pnas.0437846100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osborne E. A., Dudoit S., and Rine J. (2009) The establishment of gene silencing at single-cell resolution. Nat. Genet. 41, 800–806 10.1038/ng.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robzyk K., Recht J., and Osley M. A. (2000) Rad6-dependent ubiquitination of histone H2B in yeast. Science 287, 501–504 10.1126/science.287.5452.501 [DOI] [PubMed] [Google Scholar]
- 26. Sun Z. W., and Allis C. D. (2002) Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418, 104–108 10.1038/nature00883 [DOI] [PubMed] [Google Scholar]
- 27. Chandrasekharan M. B., Huang F., and Sun Z. W. (2009) Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. U.S.A. 106, 16686–16691 10.1073/pnas.0907862106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pavri R., Zhu B., Li G., Trojer P., Mandal S., Shilatifard A., and Reinberg D. (2006) Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125, 703–717 10.1016/j.cell.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 29. Singer M. S., Kahana A., Wolf A. J., Meisinger L. L., Peterson S. E., Goggin C., Mahowald M., and Gottschling D. E. (1998) Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics 150, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahana A., and Gottschling D. E. (1999) DOT4 links silencing and cell growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 6608–6620 10.1128/MCB.19.10.6608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emre N. C., Ingvarsdottir K., Wyce A., Wood A., Krogan N. J., Henry K. W., Li K., Marmorstein R., Greenblatt J. F., Shilatifard A., and Berger S. L. (2005) Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere-proximal Sir2 association and gene silencing. Mol. Cell 17, 585–594 10.1016/j.molcel.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 32. Gardner R. G., Nelson Z. W., and Gottschling D. E. (2005) Ubp10/Dot4p regulates the persistence of ubiquitinated histone H2B: distinct roles in telomeric silencing and general chromatin. Mol. Cell. Biol. 25, 6123–6139 10.1128/MCB.25.14.6123-6139.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson L. A., Reed B. J., Charette J. M., Freed E. F., Fredrickson E. K., Locke M. N., Baserga S. J., and Gardner R. G. (2012) A conserved deubiquitinating enzyme controls cell growth by regulating RNA polymerase I stability. Cell Rep. 2, 372–385 10.1016/j.celrep.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reed B. J., Locke M. N., and Gardner R. G. (2015) A conserved deubiquitinating enzyme uses intrinsically disordered regions to scaffold multiple protein interaction sites. J. Biol. Chem. 290, 20601–20612 10.1074/jbc.M115.650952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orlandi I., Bettiga M., Alberghina L., and Vai M. (2004) Transcriptional profiling of ubp10 null mutant reveals altered subtelomeric gene expression and insurgence of oxidative stress response. J. Biol. Chem. 279, 6414–6425 10.1074/jbc.M306464200 [DOI] [PubMed] [Google Scholar]
- 36. Johnson A., Wu R., Peetz M., Gygi S. P., and Moazed D. (2013) Heterochromatic gene silencing by activator interference and a transcription elongation barrier. J. Biol. Chem. 288, 28771–28782 10.1074/jbc.M113.460071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanny J. C., Kirkpatrick D. S., Gerber S. A., Gygi S. P., and Moazed D. (2004) Budding yeast silencing complexes and regulation of Sir2 activity by protein–protein interactions. Mol. Cell. Biol. 24, 6931–6946 10.1128/MCB.24.16.6931-6946.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Long L., Furgason M., and Yao T. (2014) Generation of nonhydrolyzable ubiquitin-histone mimics. Methods 70, 134–138 10.1016/j.ymeth.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Long L., Thelen J. P., Furgason M., Haj-Yahya M., Brik A., Cheng D., Peng J., and Yao T. (2014) The U4/U6 recycling factor SART3 has histone chaperone activity and associates with USP15 to regulate H2B deubiquitination. J. Biol. Chem. 289, 8916–8930 10.1074/jbc.M114.551754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohn M. A., Kowal P., Yang K., Haas W., Huang T. T., Gygi S. P., and D'Andrea A. D. (2007) A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell 28, 786–797 10.1016/j.molcel.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 41. Morgan M. T., Haj-Yahya M., Ringel A. E., Bandi P., Brik A., and Wolberger C. (2016) Structural basis for histone H2B deubiquitination by the SAGA DUB module. Science 351, 725–728 10.1126/science.aac5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheuermann J. C., de Ayala Alonso A. G., Oktaba K., Ly-Hartig N., McGinty R. K., Fraterman S., Wilm M., Muir T. W., and Müller J. (2010) Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 465, 243–247 10.1038/nature08966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schulze J. M., Hentrich T., Nakanishi S., Gupta A., Emberly E., Shilatifard A., and Kobor M. S. (2011) Splitting the task: Ubp8 and Ubp10 deubiquitinate different cellular pools of H2BK123. Genes Dev. 25, 2242–2247 10.1101/gad.177220.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batta K., Zhang Z., Yen K., Goffman D. B., and Pugh B. F. (2011) Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 25, 2254–2265 10.1101/gad.177238.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trujillo K. M., and Osley M. A. (2012) A role for H2B ubiquitylation in DNA replication. Mol. Cell 48, 734–746 10.1016/j.molcel.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu M. Y., Lin C. Y., Tseng H. Y., Hsu F. M., Chen P. Y., and Kao C. F. (2017) H2B ubiquitylation and the histone chaperone Asf1 cooperatively mediate the formation and maintenance of heterochromatin silencing. Nucleic Acids Res. 45, 8225–8238 10.1093/nar/gkx422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samara N. L., Datta A. B., Berndsen C. E., Zhang X., Yao T., Cohen R. E., and Wolberger C. (2010) Structural insights into the assembly and function of the SAGA deubiquitinating module. Science 328, 1025–1029 10.1126/science.1190049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sahtoe D. D., and Sixma T. K. (2015) Layers of DUB regulation. Trends Biochem. Sci. 40, 456–467 10.1016/j.tibs.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 49. Peng J., Schwartz D., Elias J. E., Thoreen C. C., Cheng D., Marsischky G., Roelofs J., Finley D., and Gygi S. P. (2003) A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 21, 921–926 10.1038/nbt849 [DOI] [PubMed] [Google Scholar]
- 50. Ehrentraut S., Hassler M., Oppikofer M., Kueng S., Weber J. M., Mueller J. W., Gasser S. M., Ladurner A. G., and Ehrenhofer-Murray A. E. (2011) Structural basis for the role of the Sir3 AAA+ domain in silencing: interaction with Sir4 and unmethylated histone H3K79. Genes Dev. 25, 1835–1846 10.1101/gad.17175111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liang G., Klose R. J., Gardner K. E., and Zhang Y. (2007) Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nat. Struct. Mol. Biol. 14, 243–245 10.1038/nsmb1204 [DOI] [PubMed] [Google Scholar]
- 52. Huang O. W., and Cochran A. G. (2013) Regulation of deubiquitinase proteolytic activity. Curr. Opin. Struct. Biol. 23, 806–811 10.1016/j.sbi.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 53. Wolberger C. (2014) Mechanisms for regulating deubiquitinating enzymes. Protein Sci. 23, 344–353 10.1002/pro.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lan X., Atanassov B. S., Li W., Zhang Y., Florens L., Mohan R. D., Galardy P. J., Washburn M. P., Workman J. L., and Dent S. Y. (2016) USP44 is an integral component of N-CoR that contributes to gene repression by deubiquitinating histone H2B. Cell Rep. 17, 2382–2393 10.1016/j.celrep.2016.10.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Larin M. L., Harding K., Williams E. C., Lianga N., Doré C., Pilon S., Langis É., Yanofsky C., and Rudner A. D. (2015) Competition between heterochromatic loci allows the abundance of the silencing protein, Sir4, to regulate de novo assembly of heterochromatin. PLoS Genet. 11, e1005425 10.1371/journal.pgen.1005425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dyer P. N., Edayathumangalam R. S., White C. L., Bao Y., Chakravarthy S., Muthurajan U. M., and Luger K. (2004) Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 375, 23–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.