Abstract
Epithelial pancreatic acinar cells perform crucial functions in food digestion, and acinar cell homeostasis required for secretion of digestive enzymes relies on SNARE-mediated exocytosis. The ubiquitously expressed Sec1/Munc18 protein mammalian uncoordinated-18c (Munc18c) regulates membrane fusion by activating syntaxin-4 (STX-4) to bind cognate SNARE proteins to form a SNARE complex that mediates exocytosis in many cell types. However, in the acinar cell, Munc18c's functions in exocytosis and homeostasis remain inconclusive. Here, we found that pancreatic acini from Munc18c-depleted mice (Munc18c+/−) and human pancreas (lenti-Munc18c-shRNA–treated) exhibit normal apical exocytosis of zymogen granules (ZGs) in response to physiologic stimulation with the intestinal hormone cholecystokinin (CCK-8). However, when stimulated with supraphysiologic CCK-8 levels to mimic pancreatitis, Munc18c-depleted (Munc18c+/−) mouse acini exhibited a reduction in pathological basolateral exocytosis of ZGs resulting from a decrease in fusogenic STX-4 SNARE complexes. This reduced basolateral exocytosis in part explained the less severe pancreatitis observed in Munc18c+/− mice after hyperstimulation with the CCK-8 analog caerulein. Likely as a result of this secretory blockade, Munc18c-depleted acini unexpectedly activated a component of the endoplasmic reticulum (ER) stress response that contributed to autophagy induction, resulting in downstream accumulation of autophagic vacuoles and autolysosomes. We conclude that Munc18c's role in mediating ectopic basolateral membrane fusion of ZGs contributes to the initiation of CCK-induced pancreatic injury, and that blockade of this secretory process could increase autophagy induction.
Keywords: exocytosis, autophagy, SNARE proteins, trypsin, pancreas, ER stress, pancreatitis, SNAREs
Introduction
The polarized epithelial pancreatic acinar cells perform crucial functions in food digestion by efficiently synthesizing digestive enzymes (1), storing them as dormant zymogen granules (ZGs)3 (1), eliminating defective or inappropriately activated enzymes by autophagy to ensure quality control (2), and faithfully exocytosing ZGs to the apical plasma membrane (PM) upon postprandial secretagogue stimulation (1, 3). Maintenance of acinar cellular homeostasis during these secretory processes requires well-orchestrated functions of an efficient endoplasmic reticulum (ER) network (4), mitochondria (5), autophagy (2, 6, 7), and SNARE-mediated exocytosis (8). Perturbations of any one of these processes were reported to contribute to pancreatic acinar cell injury and pancreatitis (9, 10). However, how defective exocytosis contributes to pancreatitis remains unclear.
Munc18c belongs to the Sec1/Munc18-like (SM) family of cytosolic proteins that regulate membrane fusion by interacting and activating cognate syntaxins to promote the assembly of distinct fusogenic SNARE complexes (11, 12). Mammals encode seven SM proteins, of which Munc18a, Munc18b, and Munc18c contribute to various exocytotic fusion events (11). Munc18a, expressed in neurons and endocrine cells, predominantly interacts with cognate syntaxin (STX)-1A to assert its function (12, 13), but also binds STX-2 and STX-3 (13). Munc18b is broadly expressed and also interacts with STX-1, -2, and -3 (14–16). Munc18c, also ubiquitously expressed in non-neuronal cells, interacts primarily with STX-4 and to lesser degree STX-2 (15, 16). Major insights into SM protein functions come from the Munc18a studies employing genetic deletion and in vitro reconstituted fusion assays. Munc18a was shown to be essential for secretion because Munc18a deletion in neurons caused complete absence of neurotransmitter secretion (17). In reconstituted systems, Munc18a was shown to play both positive (18) and negative regulatory actions (19). Specifically, Munc18a interaction with trans-SNARE complex promotes membrane fusion (12, 18), whereas Munc18 binary interaction with STX-1A stabilizes the “closed” form of STX-1A that is unable to bind cognate SNAREs to assemble into a fusogenic SNARE complex (12, 19). Munc18c has been shown to positively regulate physiologic exocytosis in many cell types, including GLUT4 vesicle exocytosis in adipocytes (20) and skeletal muscles (21), and insulin exocytosis in pancreatic β-cells (22, 23). Peculiarly, disruption of Munc18c association to STX-4 paradoxically promoted exocytosis in platelets (24) and also GLUT4 vesicle exocytosis in adipocytes (25). Although the physiologic roles of Munc18c are well studied in these cell types, its function in ZG exocytosis in pancreatic acinar cells has not been definitively established. In pancreatic acinar cells Munc18c is found on the basolateral plasma membrane (26). Whereas physiologic stimulation of acini had no effect on Munc18c, supraphysiologic (cholecystokinin (CCK), carbachol) and toxic (alcohol) stimulation induces PKCα-mediated phosphorylation of Munc18c, which activates the assembly of the basolateral PM SNARE complex (STX-4–SNAP23–VAMP8) (27–29) to effect basolateral exocytosis. With the activation of this SNARE complex, Munc18c dissociates from STX-4 into the cytosol to undergo proteolytic degradation (26, 27). These events that underlie basolateral exocytosis have been purported to contribute to pancreatitis (8, 9), supported by a report of VAMP8 deletion abrogating pancreatitis induced by alcohol and supraphysiologic stimulation (27). However, with the reports of Munc18c's complex positive (20, 22, 23, 30) and negative regulatory roles (24, 25) in exocytosis, it remains to be determined what role Munc18c plays in the cellular processes leading to pancreatic injury and pancreatitis.
We employed Munc18c-depleted mice (21, 22) and human pancreas (31) depleted of Munc18c to assess Munc18c functions in pancreatic acinar ZG exocytosis and cellular homeostasis under normal state and after pancreatitis induction (32). We found that Munc18c is a positive regulator of STX-4–mediated basolateral exocytosis, whereby Munc18c depletion reduced basolateral SNARE complex assembly and consequent basolateral exocytosis of ZGs. To translate these findings to a disease mechanism, we used the caerulein hyperstimulation model of pancreatitis (32) on this Munc18c-depleted mouse, which showed a much reduced severity of pancreatitis. Unexpectedly, we found that this Munc18c depletion–induced secretory blockade resulted in elevated ER stress–mediated autophagy induction which led to increased accumulation of autolysosomes. Munc18c thus could be useful as a potential therapeutic target for one of the most common life-threatening gastrointestinal diseases that currently has no specific treatment (33, 34).
Results
Munc18c-depleted mice are protected from caerulein-induced pancreatitis
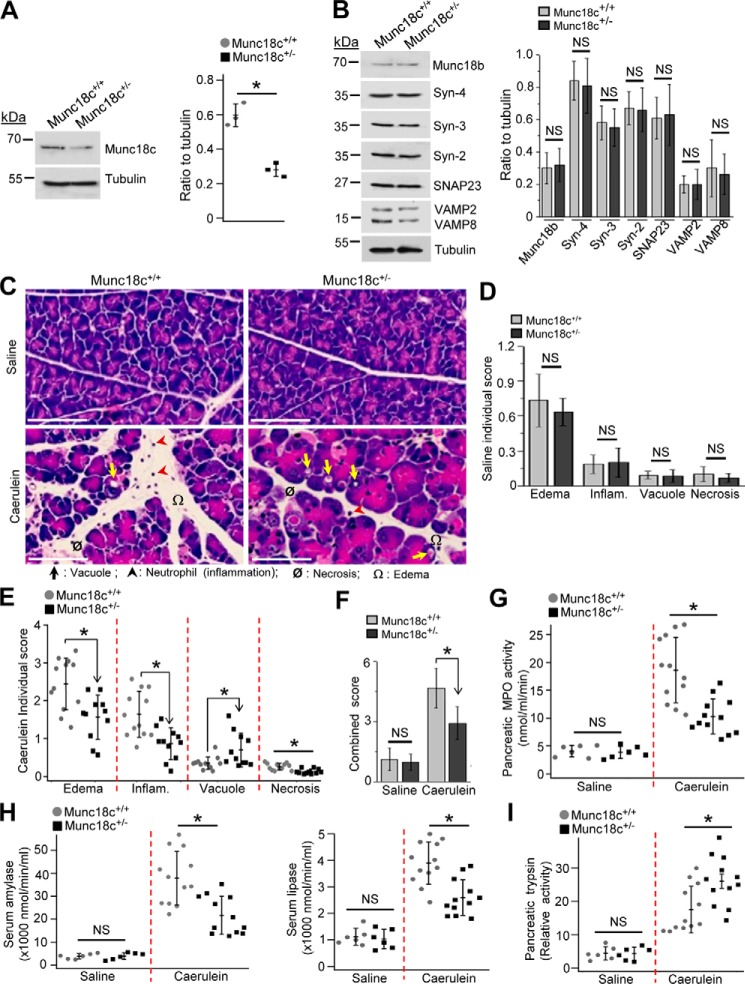
Munc18c is part of the putative SM-SNARE quaternary fusion complex (12, 18) that mediates pathological basolateral exocytosis (26–29) purported to contribute to pancreatitis (8, 9). This led us to question whether Munc18c depletion could alter the process of pancreatitis. We employed the heterozygous Munc18c+/− previously reported to reduce GLUT4 vesicle and insulin granule exocytosis (21, 22). We verified that Munc18c levels in Munc18c+/−mouse acini were reduced by 52%, without affecting the other SM and SNARE proteins (Fig. 1, A and B). Munc18c+/− versus WT mice were subjected to caerulein (CCK-8 analog) hyperstimulation (8 hourly injections) to induce pancreatitis (32), after which we assessed the histology (Fig. 1C) for pancreatic injury (edema, inflammation, vacuolization, and necrosis) scored individually (Fig. 1, D and E) and collectively (Fig. 1F). Whereas saline (control)-treated tissues showed no differences, caerulein treatment showed lower total and individual (all except an increase in autophagic vacuole formation) histology scores in the Munc18c+/− mice pancreatic tissues compared with WT littermates. These findings of reduced pancreatitis in the Munc18c+/− mice were consistent with the reduced tissue myeloperoxidase (MPO) activity (Fig. 1G) from the less polymorphonuclear leukocyte infiltration, and the reduced circulating levels of amylase and lipase (Fig. 1H and Fig. S1, C and D), which are clinical indicators of pancreatitis. However, pancreatic tissue trypsin activity was higher in Munc18c+/− mice (Fig. 1I), consistent with the increased number of autophagic vacuoles (Fig. 1E) where trypsinogen activation to trypsin (7) occurs. Munc18c depletion therefore reduced the severity of pancreatitis, but seemed to up-regulate autophagy activity. We therefore investigated the mechanisms to explain these two phenomena.
Figure 1.
Munc18c depletion in mice protects against caerulein-induced pancreatitis. A, representative Western blots (top panel, n = 3) and densitometry analysis (right, normalized to tubulin) showing 52% reduced expression of Munc18c in Munc18c+/− pancreatic acini. B, representative Western blots (left panel, n = 3) showing no change in expression (right panel, densitometry normalized to tubulin) of indicated proteins in WT and Munc18c+/− mouse acini. C, representative H&E-stained images of pancreas from control (top panels, 8 hourly intraperitoneal injections of 0.9% saline) and caerulein-administered (bottom panels, 8 hourly intraperitoneal injections of 50 μg/kg) WT (left) and Munc18c+/− (right) mice. Scale bars, 50 μm. Edema, inflammation, vacuolization, and necrosis are indicated by symbols. Larger area of histology images with magnified regions are in Fig. S1, A and B to better display the pancreatic injury. D and E, individual histology scores (on a scale of 0–4) of control (D, n = 6) and caerulein-treated (E, n = 12) pancreas. F, combined histology scores from D and E. G and H, quantitative measurements of activities of pancreatic myeloperoxidase (MPO) (G), serum amylase (H, left) and serum lipase (H, right) in control or caerulein-administered WT and Munc18c+/− mice. I, relative activity of pancreatic trypsin in control or caerulein-administered WT and Munc18c+/− mice. Enzyme activities were assayed on tissue samples from mice that were used for analysis in (D–F). Data with nonsignificant differences are expressed as ± S.D. Data with statistically significant differences are shown as scattered plots with S.D. *, p < 0.05, NS, not significant.
Munc18c depletion reduces secretion at supraphysiologic but not at physiologic CCK-8 stimulation
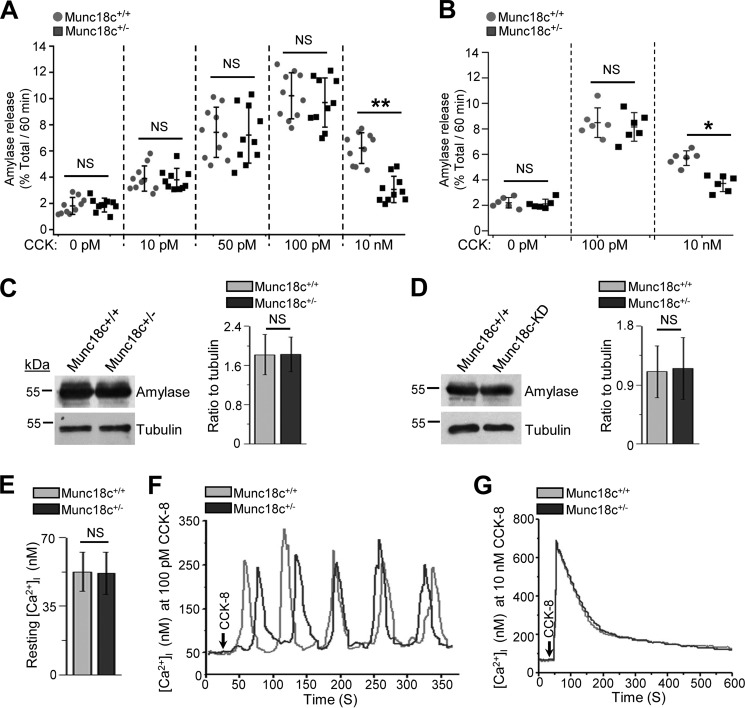
We examined whether Munc18c depletion affects stimulated amylase release. We used both Munc18c+/− mouse acini (Fig. 2A) and human pancreatic slices treated with lenti-Munc18c-shRNA/RFP (CACCTTGGTGTTCCCATTGTT) (23) to knockdown (KD) Munc18c expression (Fig. 2B). The latter showed 78% efficiency (expressed RFP) in transducing the human acini (data not shown) and reduced total pancreatic lysate Munc18c levels by 63% (Fig. S2, A and B). Both dispersed mouse acini (Fig. 2A) and human pancreatic slices (Fig. 2B) displayed classical biphasic pattern of amylase secretion (3, 31) with a gradual increase at submaximal CCK (10–20 pm) to a peak response (10–100 pm) followed by a progressive reduction from maximal levels at supraphysiologic doses (>1000 pm–20 nm). The reduction in secretion at supraphysiologic doses is because of progressive blockade in apical exocytosis and redirection of ZGs to fuse with the basolateral PM (26), whereby release of proteases into the interstitial space of the intact pancreas would lead to pancreatitis (8, 9). However, in dispersed acini, all released enzymes would end up in the media, but because the release at the basolateral PM is much less efficient than apical exocytosis, the amounts are much less than maximally released at the apical pole. Munc18c depletion in mouse acini (Fig. 2A) or human pancreatic slices (Fig. 2B) did not alter amylase secretion at submaximal to maximal CCK-8 stimulation. However, at supramaximal stimulation (10 nm CCK-8), Munc18c-depleted mouse acini (Fig. 2A) and human pancreatic slices (Fig. 2B) secreted 55 and 42% less amylase than WT acini and scrambled (sc)-shRNA transduced slices. Total amylase content was similar between Munc18c-depleted and WT acini of mouse acini and human pancreatic slices (Fig. 2, C and D).
Figure 2.
Munc18c depletion augments the inhibition in amylase secretion by supraphysiologic CCK-8-stimulation without affecting acinar Ca2+ homeostasis. A and B, WT and Munc18c+/− mice pancreatic acini (A), and control (lenti-scrambled shRNA) Munc18c KD (lenti-Munc18c-shRNA/RFP) human pancreatic slices (B) were stimulated with submaximal to supramaximal (10 pm–10 nm) concentrations of CCK-8 for 1 h at 37 °C. Amylase secreted was expressed as a percentage of total cellular amylase of the respective samples. Data shown as scattered plots with S.D. (n = 10 for A and n = 6 for B, each from three independent experiments). *, p < 0.05; **, p < 0.01. C and D, Western blot analysis of amylase expression (left panels) and densitometry quantification (normalized to loading control tubulin, right panels) showed no change between WT and Munc18c+/− mouse acini (C), and between control and Munc18c KD human pancreas (D). Data expressed as mean ± S.D. of three independent experiments. NS, not significant. E, resting [Ca2+]i was similar between WT (n = 20 cells) and Munc18c+/− (n = 22 cells) mouse acini. F and G, representative [Ca2+]i response curves from WT and Munc18c+/− mouse acini stimulated with 100 pm (F, 12 acinar cells each for WT and Munc18c+/− acini from three independent experiments) or 10 nm (G, 10 acinar cells each for WT and Munc18c+/− acini from three independent experiments) CCK-8 showed similar responses. Analysis of these [Ca2+]i responses in E–G are stated in the text, and showed no statistical differences. NS, not significant.
Because Ca2+ is the primary fusogenic signal for ZG exocytosis (35), we assessed whether intracellular Ca2+ ([Ca2+]i) release was distorted by the Munc18c depletion. Munc18c+/−mouse acini compared with WT mouse acini showed similar [Ca2+]i at rest (Fig. 2E) and after CCK-8 stimulation at maximal 100 pm CCK-8 (Fig. 2F) which evoked Ca2+ oscillations (frequency, WT: 4 ± 0.36 spikes/4 min, Munc18c+/−: 4 ± 0.42 spikes/4 min, not significant (NS); amplitude, WT: 228.7 ± 34.3 nm, Munc18c+/−: 221.4 ± 37.1 nm, NS) and at supramaximal 10 nm CCK-8 stimulation (Fig. 2G) which evoked a high peak (amplitude, WT: 685.4 ± 61.2 nm, Munc18c+/−: 694 ± 52.3 nm, NS) followed by gradual decrease to low plateau response (35). These results suggest that the effect of Munc18c depletion on secretion must be at an exocytotic step downstream from [Ca2+]i release.
Munc18c depletion reduces supraphysiologic CCK-evoked basolateral PM exocytosis but does not affect physiologic apical exocytosis
To assess whether Munc18c depletion affects CCK-stimulated exocytosis, we used a syncollin-pHluorin imaging method employing adenovirus expression of syncollin-pHluorin (29, 31, 36) in Munc18c+/− and WT mouse acini. Ad-syncollin-pHluorin expression enables high spatiotemporal resolution of single ZG fusions (36). Here, the ZG content protein syncollin is fused to pH-sensitive GFP (pHluorin) which elicits high fluorescence at basic pH (ZGs fused to the PM exposes the ZG interior to pH 7.4 of the cell exterior) and reduced fluorescence at acidic pH (i.e. unfused ZGs). This enables tracking of sequential ZG fusions deep in the apical region (36). Physiologic 100 pm CCK-8 stimulation of WT (Fig. 3A, top panel) and Munc18c+/− mouse acini (Fig. 3A, bottom panel) resulted in equivalent number of exocytosis events (green hotspots) at the apical poles (within the inner circles), with very few fusion events at the basal or lateral PM (between inner and outer circles; analysis in Fig. 3A, right panel, normalized to cell area and recording time). At 10 nm CCK-8 stimulation of WT acini (Fig. 3B, top panel), apical fusion events were drastically reduced with increased fusion events at the basolateral PM, the latter exceeding the fusion events in the apical region (analysis in Fig. 3B, right panel). In Munc18c+/− mouse acini (Fig. 3B, bottom panel), 10 nm CCK-8 exhibited a severely reduced number of fusion events at the basolateral PM when compared with WT acini (analysis in Fig. 3B, right panel).
Figure 3.
Munc18c depletion reduces supraphysiologic CCK-evoked basolateral PM exocytosis. Ad–syncollin-pHluorin–infected WT and Munc18c+/− acini were stimulated with maximal (100 pm) or supramaximal (10 nm) CCK-8 and images were recorded by spinning disk microscopy. A and B, left panels show representative images of WT (top) and Munc18c+/− (bottom) acini at indicated time points after stimulation with 100 pm CCK-8 (A) or 10 nm CCK-8 (B). Scattered plots on right panels are the quantitative analysis (A, WT, 10 acini and Munc18c+/−, 12 acini; B, WT, 10 acini and Munc18c+/−, 12 acini; each from three independent experiments) of total fusion events and fusion events at apical and basolateral areas normalized to cell area and recording time. Apical and basolateral areas were demarcated by drawing two concentric circles on images considering the apical lumen as center (29, 36). Inner circle (A and B) that encompasses two-third area of the outer circle was designated as apical pole. The region between inner and outer circle (A and B) was considered as basolateral (29, 36, 59). Analyses were mean ± S.D. Scale bars, 10 μm. *, p < 0.05.
Munc18c depletion selectively impairs the assembly of basolateral SNARE complexes
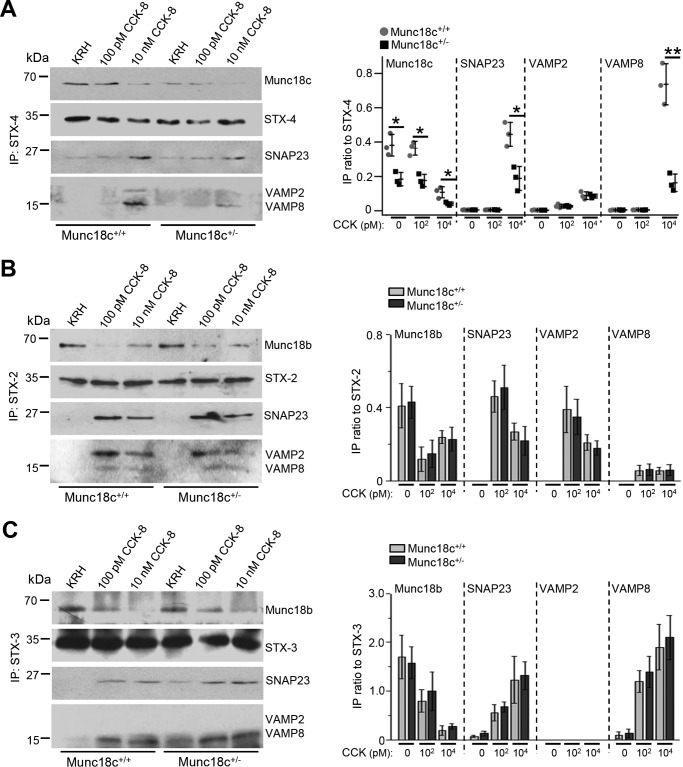
We have purported that Munc18c promotes the STX-4–SNAP23–VAMP8 SNARE complex formation (27). We therefore hypothesized that Munc18c depletion would reduce this SNARE complex assembly to explain the reduced basolateral exocytosis. We examined by co-immunoprecipitation assays the distinct SM-SNARE complexes formed in WT and Munc18c+/− mouse acini. STX-4 antibody (Fig. 4A) pulled down complete Munc18c–STX-4–SNAP23–VAMP8 complexes from WT acini only at 10 nm CCK-8 but not 100 pm CCK-8 stimulation, consistent with this SM-SNARE complex's role in basolateral exocytosis evoked at supraphysiologic stimulation (27). From the 10 nm CCK-8–stimulated Munc18c+/− acini, less of this SM-SNARE complex was pulled down, consistent with the observed reduced basolateral exocytosis. Our previous studies have suggested that Munc18b could activate STX-2 (27, 37) and STX-3 (27) in forming SNARE complexes with ZG VAMP2 and VAMP8, and also SNAP23 to mediate physiologic CCK-stimulated apical PM exocytosis and subsequent sequential ZG–ZG fusion (27, 37, 38). Indeed, after stimulation with physiologic 100 pm CCK-8, co-immunoprecipitation with STX-2 (Fig. 4B) or STX-3 antibodies (Fig. 4C) pulled down similar amounts of complete Munc18b–(STX-3–SNAP23–VAMP8) and Munc18b–(STX-2–SNAP23–VAMP2) complexes in WT and Munc18c+/− acini. This result would explain why Munc18c depletion did not alter apical exocytosis. These SM-SNARE complexes were notably reduced at 10 nm CCK-8 stimulation, consistent with the reduced apical exocytosis, but were similar between WT and Munc18c+/− acini. This also supports the thinking that Munc18c (like Munc18a) is required to activate STX-4 (like STX-1A) into an open conformation to assemble with cognate SNARE proteins into a complete SNARE complex (11).
Figure 4.
Munc18c depletion selectively impairs the assembly of syntaxin-4–VAMP8 SNARE complex induced by supraphysiologic CCK-8 stimulation. A–C, dispersed WT and Munc18c+/− pancreatic acini were stimulated in parallel with maximal (100 pm) or supramaximal (10 nm) CCK-8 for 30 min. 500 μg acini lysates were then immunoprecipitated with antibodies to (A) syntaxin-4, (B) syntaxin-2, and (C) syntaxin-3. Precipitated proteins were probed with indicated antibodies. Band intensity ratios of co-immunoprecipitated Munc18b/c, SNAP23, and VAMP8/2 to immunoprecipitated (IP) syntaxins are shown in the corresponding right panels. Input controls with their densitometry analysis are shown in Fig. S3. Data expressed as scattered plot with S.D. or mean ± S.D. of three independent experiments. *, p < 0.05; IP, immunoprecipitation.
Munc18c depletion–induced secretory blockade activates an ER stress response that increases autophagy induction
Increased vacuolization, predominantly autolysosomes (ALs) in pancreatic acinar cells during pancreatitis has been attributed to the perturbation of autophagy particularly at the distal step of AL degradation (2, 7). Peculiarly, whereas Munc18c depletion reduced the severity of pancreatitis, we noted a paradoxical increase in vacuole formation upon caerulein hyperstimulation (Fig. 1E).
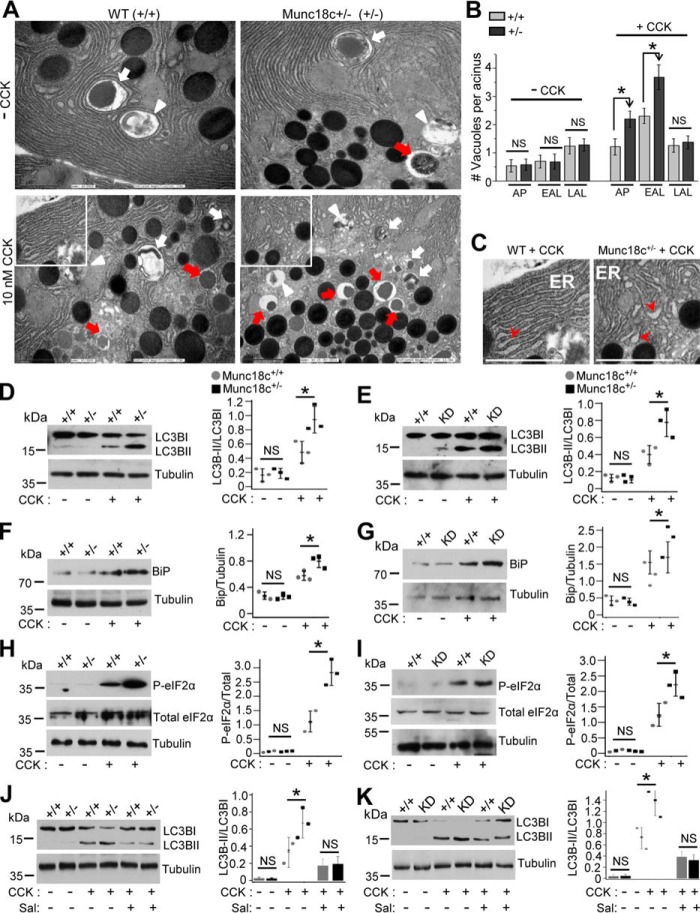
To assess the nature of the large vacuole formation, we performed transmission electron microscopy (TEM), which can distinguish the different autophagic vacuoles (AVs) Fig. 5, A and B and Fig. S4A) into autophagosome (AP) (double membrane with undigested content), early AL (EAL) (single membrane with undigested content), and late AL (LAL) (single membrane with amorphous electron-dense content). Analysis of these AVs at resting conditions showed no differences between WT and Munc18c+/− acini (Fig. 5A, top; analysis in Fig. 5B). After supraphysiologic CCK-8 stimulation, Munc18c+/−acini (Fig. 5A bottom) compared with WT acini accumulated 81% more APs, 60% more EALs, but no significant increase in LALs (analysis in Fig. 5B). Perturbations of the autophagic flux is confirmed biochemically by the increased conversion of cytosolic microtubule-associated protein 1 light chain (LC3) B-I to lipidated LC3B-II (39). Here, Munc18c+/− mouse (Fig. 5D) and Munc18c-shRNA KD human acini (Fig. 5E) displayed 69 and 73% more conversion of LC3B-I to LC3B-II, which taken along with the increase in APs and EALs is indicative of an increase in autophagy induction (39), which we examined next.
Figure 5.
Munc18c depletion amplifies autolysosome and autophagosome accumulation by enhancing ER stress–mediated autophagy induction. A, TEM images (out of 25 acini per group) showing appearance of the different autophagic vacuoles (larger figures shown in Fig. S4A): Autophagosomes (AP) (double membrane with undigested substances, indicated by white arrows), early autolysosomes (EAP) (single membrane with undigested substances, indicated by red arrows), and late autolysosome (LAL) (single membrane with amorphous electron dense substances, indicated by white arrowheads) in control (− CCK) WT and Munc18c+/− mouse acini (top panels). As shown, these distinct AVs are known to increase after 30 min of 10 nm CCK-8 stimulation (bottom panels), but with a larger abundance in Munc18c+/− acini (AP and EAL, not LAL). Scale bars, 2 μm. B, quantification of the distinct AVs in control and CCK-8–stimulated acini shown as mean ± S.D. C, magnification of regions (white box) from bottom panels of (A) showing appearance of more dilated ER (indicated by red arrowheads) in CCK-8–stimulated Munc18c+/− acini compared with WT acini. Scale bars, 2 μm. Additional TEM images showing the changes in ER morphology more clearly are in Fig. S4, B and C. D and E, left panels showing Western blot analyses of LC3B-I lipidation (LC3B-II) in control and 10 nm CCK-8–stimulated WT versus Munc18c+/− mouse acini (D), and control versus Munc18c-KD human acini (E). Quantification of lipidation as densitometric ratio of LC3B-II to LC3B-I are shown in corresponding right panels. F–K, left panels showing Western blot analyses of (F and G) BiP expression in WT and Munc18c+/− mouse acini (F) and control and Munc18c-KD human pancreas slices (G), (H and I) P-eIF2α (Ser-51), and total eIF2α in WT and Munc18c+/− mouse acini (H) and control and Munc18c-KD human pancreas slices (I), and (J and K) LC3B-I lipidation (LC3B-II) in presence of ER stress inhibitor salubrinal (Sal) (50 μm) in mouse (J) and human (K) pancreas. Densitometry analyses are shown in corresponding right panels as scattered plots with S.D. Tubulin shows equal loading in Western blotting. Data shown are representative of three independent experiments. *, p < 0.05.
The complex regulation of autophagy also includes ER stress responses (2, 6, 40, 41), which can be severely perturbed by the dysregulation in secretory pathways (42). In pancreatic acinar cells, supraphysiologic stimulation is known to increase ER stress that perturbs autophagy (4, 6). This led us to hypothesize that Munc18c depletion–induced basolateral exocytotic blockade along with the supramaximal CCK-induced apical blockade in acinar cells might have resulted in greatly increased proteostasis which would specifically input into the ER stress response in a manner that contributes to autophagy induction (43). This increase in autophagy induction could then accentuate supraphysiologic CCK-8–stimulated ER stress. Indeed, assessment of ER morphology by TEM showed that Munc18c+/− acini displayed more dilated and ribosome-free smooth ER (Fig. 5C and Fig. S4C), reminiscent of increased ER stress (6). We next assessed for biochemical evidence of ER stress. Levels of GRP78 (BiP), an established ER stress marker (44), were higher in CCK-8–hyperstimulated Munc18c+/− mouse (Fig. 5F) and Munc18c-depleted human acini (Fig. 5G) compared with their WT counterparts. To specifically assess whether this excess in ER stress was the major contributor to the augmented autophagy induction in the Munc18c-depleted acini, we examined eIF2α phosphorylation (serine-51), an indicator of PERK-eIF2α pathway activation. We focused on this PERK-eIF2α axis of the ER stress response as this is the major and established contributor to autophagy induction per se (45). In fact, we detected increased phosphorylation of eIF2α in CCK-8–hyperstimulated Munc18c+/− mouse (Fig. 5H) and human acini (Fig. 5I). To confirm that the increased autophagic induction was attributed to ER stress, we used the eIF2α signaling inhibitor salubrinal, which was able to reduce the LC3B-I to LC3B-II conversion in WT and Munc18c-depleted mouse (Fig. 5J) and human acini (Fig. 5K).
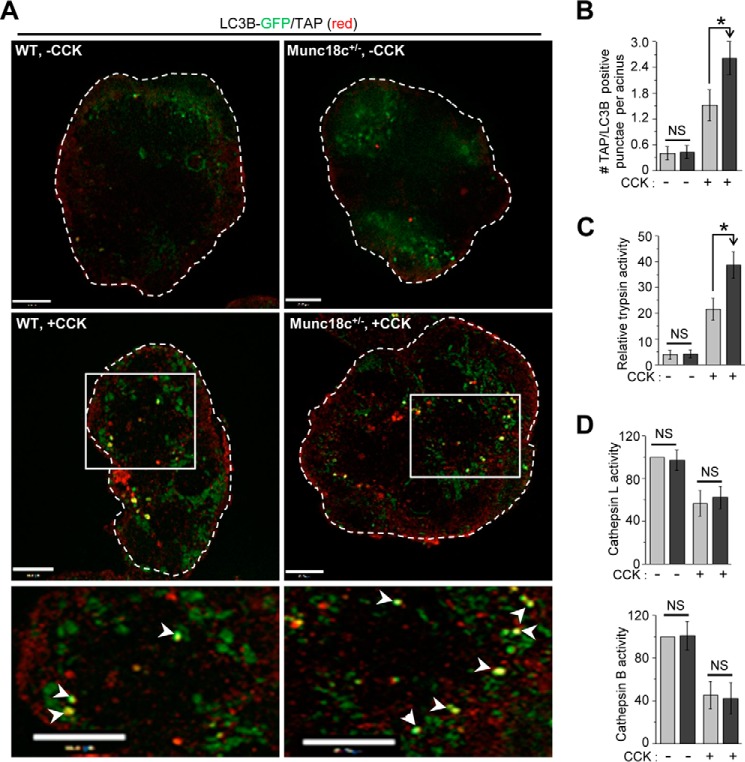
We then assessed the protease activities within the ALs that are known to be perturbed by supraphysiologic CCK-8 stimulation as an underlying mechanism of pancreatic injury and pancreatitis (2, 6, 7). Supraphysiologic CCK-8 induced defects in the processing and altered activity of cathepsins, causing an imbalance between cathepsin B (i.e. more) and cathepsin L (i.e. less) within the ALs, which results in increased trypsinogen activation (7). Release of proteases particularly cathepsin B into the cytosol was recently implicated as the major cause of acinar injury (46). Trypsinogen activation was detected using the trypsinogen activation peptide (TAP) marker for activated trypsin (47) within the acinar vacuoles, and whole tissue lysate assay for trypsin (48). After CCK-8 hyperstimulation, we observed a 47% increase in LC3B/TAP-positive vacuoles (Fig. 6A; analysis in Fig. 6B) in Munc18c+/− acini compared with WT acini. The TAP's presence in LC3B-positive vacuoles would indicate that these vacuoles are autolysosomes (7). Consistently, Munc18c+/− acini 42% more trypsin activity after 10 nm CCK-8 stimulation (Fig. 6C), whereas trypsin activities were similar at basal conditions between control and Munc18c+/− acini.
Figure 6.
Munc18c depletion promotes autolysosomal trypsinogen activation. A, representative merged confocal immunofluorescence images of LC3B GFP (green) and TAP (red) in WT and Munc18c+/− mouse acini that were either kept as control (no CCK-8, top panels) or stimulated with 10 nm CCK-8 for 30 min (bottom panels). Selected areas from the stimulated images are magnified to better view their co-localization shown as yellow hotspots (pointed to with white arrowheads) indicative of activated trypsin within the autolysosomes. n = 3 independent experiments. Scale bars, 10 μm. Full sequence of images are shown in Fig. S5. B, quantification of TAP-positive LC3B GFP puncta from 40 acinar cells from three independent experiments performed as described previously (7). Data expressed as mean ± S.D. C, relative activity of trypsin in lysates from WT and Munc18c+/− mouse acini stimulated as in (A). Data expressed as mean ± S.D. from three independent experiments. D, relative activities of cathepsin L (top) and cathepsin B (bottom) in the lysosomal fractions from WT and Munc18c+/− mouse acini that were either kept as control or CCK-stimulated as in (A). The activities of cathepsins in controls were taken as 100. Data expressed as mean ± S.D. from three independent experiments. *, p < 0.05, NS = not significant.
Finally, we assessed for abnormal cathepsin activities caused by supraphysiologic CCK-8 stimulation (Fig. 6D), purported to account for the defective AL degradation, which leads to AL accumulation (2, 7). Cathepsin activities (cathepsin B and L levels) were similar between Munc18c-depleted and WT mouse acini. These data indicate that the increased in AP and AL formation in Munc18c-depleted acini is attributed to the increase in autophagy induction caused by the secretory (basolateral and apical exocytosis) blockade, which in turn increased proteostasis input into ER stress, but against a defective AL clearance caused by the supraphysiologic CCK stimulation.
Discussion
In summary, we showed the Munc18c depletion in mouse and human pancreatic acini could reduce basolateral exocytosis by reducing the formation of fusogenic STX-4–SNAP23–VAMP8 complexes. This would only occur during supramaximal stimulation, whereas Munc18c depletion would not affect physiologic stimulated apical exocytosis. This effect of Munc18c on basolateral exocytosis was sufficient to reduce caerulein-induced pancreatitis, indicating that Munc18c could be a potential therapeutic target to treat pancreatitis in humans.
Unexpectedly, we found that the blockade of basolateral exocytosis by Munc18c depletion likely increased input into ER stress response in a manner that increased autophagy induction. This was accentuated by the fact that supramaximal CCK stimulation has long been known to cause apical blockade by a yet unknown mechanism. Such a severe secretory blockade in pancreatic acinar cells may result in greatly increased proteostasis causing increased accumulation of misfolded proteins that culminates in amplifying the ER unfolded protein response and thus ER stress (4, 49), which is a potent trigger for autophagy induction as the adaptive mechanism for proteostatic degradation (50). This would therefore promote autophagosome and consequent autolysosome formation. However, in the normal autophagic process, the autolysosomes would undergo normal degradation. Unfortunately, supramaximal stimulation of pancreatic acini impairs this terminal step in the autophagic flux by causing an imbalance in cathepsin processing which results in deficient lysosome degradation and consequent accumulation of active trypsin within the increased number of autolysosomes (7). These events, independently provoked by supramaximal CCK stimulation, are not affected by the Munc18c deficiency per se. Nonetheless, the increased ER stress input into autophagy induction caused by Munc18c deficiency in the background of reduced autolysosome degradation caused by supramaximal CCK stimulation, culminates in increased number of autolysosomes and activated trypsin within the autolysosomes.
Peculiarly, our work suggests that it may not be the increased number of autolysosomes with active trypsin per se that causes pancreatitis. Rather, it seems that the larger contributor to pancreatitis, at least in our Munc18c deficiency models (both mice and human), is basolateral exocytosis which would deliver zymogen cargo into the interstitial space between acinar cells, where protease activation leads to interstitial pancreatitis (51, 52). Furthermore, it is not simply intra-acinar activation of trypsinogen per se that causes pancreatic injury, but the release of the lysosomal proteases, particularly cathepsin B, in the cytosol that causes acinar cell death (46). We think that Munc18c and its cognate SNARE proteins do not directly regulate autophagy or ER stress per se in the pancreatic acinar cells but rather they contribute to these processes by their role, when deficient, in inducing secretory blockade. Nevertheless, it would be of interest to further explore whether other SNARE proteins might directly influence acinar autophagic processes (53).
Experimental procedures
Animals and reagents
Munc18c hemizygous (Munc18c+/−) mice (Munc18c−/− are embryonic lethal) (21) were obtained from crossing of Munc18c+/− and wild type C57bL6. Mice were kept at 22 °C, 40% humidity, 12-h:12-h light-dark cycle and given chow and water ad libitum. All studies were performed in accordance with the University of Toronto regulations for animal experiments. Substrate for trypsin (Boc-Gln-Ala-Arg-MCA) and chymotrypsin (Suc-Ala-Ala-Pro-Phe-AMC) were from Peptides International (Louisville, KY) and Calbiochem, respectively. Serum amylase, lipase, and pancreatic myeloperoxidase (MPO), assay kits were from BioVision (Milpitas, CA). CCK-8 was from Research Plus Inc. (Barnegat, NJ). Sulfated caerulein and all other reagents unless specified were from Sigma.
Human pancreas slice preparation, culture, and lentivirus transduction
Pancreas slices were prepared from healthy portions of human pancreas that were retrieved from resected pancreas of pancreatic cancer patients (5 patients; 3 males and 2 females, ages ranging from 57 to 65 years old), with written consent. Slicing was conducted in ice-cold extracellular solution (in mm: 125 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 2 sodium pyruvate, 0.25 ascorbic acid, 3 myo-inositol, 6 lactic acid, 7 glucose) with a vibrating blade microtome (VT-1000S Vibratome, Leica Microsystems, Mannheim, Germany). 10 slices (in extracellular solution) per Petri dish were transduced with 1010 pfu/ml of control (scrambled) shRNA or Munc18c shRNA lentiviruses (23) for 2 h. For long-term culture (24 h), lentivirus-transduced slices were placed on cell culture inserts (0.4 μm, diameter 30 mm, EMD Millipore) that were coated with 3 mg/ml type 1 rat tail collagen. Inserts were then floated on optimized Waymouth's MB 752/1 medium (11 mm glucose, 1% FBS, 0.1 mg/ml soybean trypsin inhibitor, 1 μg/ml dexamethasone, and penicillin (100 units/ml)/streptavidin (100 μg/ml) in 6-well plates as described by Marciniak et al. (54).
Mice acini isolation, culture, and adenovirus transduction
Dispersed pancreatic acini were prepared from 2- to 4-month-old mice using our established mechanical and enzymatic dissociation technique as described earlier (36). Dispersed acini were cultured in acinar culture media: DMEM/F12 with 10% normal goat serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a cell culture incubator. LC3B GFP expressing adenovirus (1010 pfu/ml) were added to the culture 12 h prior to imaging.
Intracellular calcium ([Ca2+]i) measurement
Time course of acinar [Ca2+]i was measured with ratiometric Ca2+ indicator Fura-2 AM (1.0 μm with 0.05% pluronic F-127), using a Zeiss inverted epifluorescence microscope as described before (55). Briefly, Fura-2 AM–loaded acini were sequentially illuminated through 340/25 nm and 380/25 nm excitation filters and imaged through 40× UV, 1·3 NA, oil immersion objective through a 500/20 nm longpass filter. 512 pixel × 512 pixel images were captured at 3 frames/s with 35 ms exposure on a Hamamatsu Flash 4.0 cooled CCD camera. A 340/380 ratio (R) that is proportional of [Ca2+]i was calculated for each image after background substraction for each wavelength. Fura-2 signal was calibrated with no Ca2+ (+1 mm EGTA) to obtain minimum ratio (Rmin) and with high Ca2+ (+10 mm CaCl2) to obtain maximum ratio (Rmax) of 340/380 in presence of ionomycin (1 μm). The intracellular R of Fura-2 was then expressed [Ca2+]i using the formula [Ca2+]i = Kd × [(R − Rmin)/(Rmax − R)] × Sf/Sb, where Sf and Sb are the emission intensity at 380 nm for Ca2+-free and Ca2+-bound Fura-2, respectively.
Induction of caerulein pancreatitis, preparation of serum and tissue samples, and histological scoring of pancreatitis
These assays were performed as we described previously (27). Briefly, caerulein was administered through intraperitoneal (i.p.) injections at a dose of 50 μg/kg body weight at hourly intervals (8 injections) to sex-matched littermate mice (age, 2–4 months; body weight, 20–25 g) that were fasted for 18 h with water given ad libitum. Mice from control groups received equal volume of 0.9% saline. Mice were sacrificed at 1 h after the eighth injection. Whole blood samples were collected by puncturing the heart with a heparin-coated syringe and centrifuged at 4 °C. The pancreata were removed on ice and a portion of it was fixed in 10% formalin in PBS for further histological processing and hematoxylin and eosin (H&E) staining. Serum and remaining portions of pancreas were snap frozen in liquid nitrogen and stored in −80 °C for biochemical assays. H&E stained slides were scanned with a slide scanner (Axioscan, Zeiss) at 40× magnification and images were analyzed for pancreatic injury on a scale of 0–3.
Enzyme assays
Dispersed pancreatic acini were stimulated with CCK-8 in Krebs-Ringer-HEPES (KRH) buffer (NaCl 104, KCl 5, KH2PO4 1, MgCl2 1.2, and HEPES 25; in mm, pH 7.4) supplemented with 1.2 mm CaCl2, 2.5 mm d-glucose, 2 mm l-glutamine, MEM amino acids (Invitrogen), MEM nonessential amino acids (Invitrogen), 0.015% (w/v) soybean trypsin inhibitor, and 0.2% (w/v) BSA. Secreted and acinar amylase content were measured using an established colorimetric method as we reported previously (27, 29, 36, 56). Activities of serum amylase, lipase, and MPO were determined at room temperature using enzyme assay kits (BioVision) as per manufacturer's guidelines. Trypsin and chymotrypsin activities were measured as we reported earlier (31, 48). Briefly, pancreatic tissue or acinar cells were lysed 1:10 (w/v) in ice-cold trypsin assay buffer (50 mmol/liter Tris, pH 8.1, 150 mmol/liter NaCl, 1 mmol/liter CaCl2, 0.01% BSA). 50 μl of clarified (1000 g, 2 min) lysates were mixed with 50 μl of 400 μmol/liter enzyme substrate within wells of a 96-well black μClear plates (Greiner Bio One, Frickenhausen, Germany). Release of protease cleaved 7-amino-4-methylcoumarin (AMC) was measured immediately using a fluorescent plate reader (FLUOstar Optima, Isogen Life Science, De Meern, The Netherlands) for 10 min at room temperature. Protease activity was determined as relative fluorescent units per second, normalized to total amylase (relative fluorescent units per second per microgram of amylase). Lysosomal cathepsin L (CL) and cathepsin B (CB) activities were determined by commercial kits (Abcam, ab65306, ab65300). Briefly, lysosomal pellets were lysed in 1:5 (w/v) ice-cold CL/CB buffers. 10 μl of lysates were further diluted (1:10) in CL/CB buffers and mixed with 200 μm CL substrate Ac-FR-AFC or CB Substrate Ac-RR-AFC for 2 h in presence or absence of CL/CB inhibitor (in 1:50 dilution). The release of amino-4-trifluoromethylcoumarin (AFC) as enzyme activity was measured with a fluorimeter (as described above) at 400 nm excitation and emission at 460 nm for CL and 505 nm for CB. The relative fluorescent units (RFUs) were normalized to protein levels. RFU of lysosomal CL/CB was determined by subtracting the RFU measured in the presence of CL/CB inhibitor and data expressed as percentage of change in activity taking WT control lysosomal activity as 100.
Confocal microscopy
Immunofluorescence and live cell imaging were performed with a spinning disc confocal imaging system equipped with Olympus IX81 inverted fluorescence microscope (Center Valley, PA), a Yokogawa CSU X1 spinning disc confocal scan head (Yokogawa Electric Corporation, Tokyo, Japan), a diode-pumped solid state laser set (405 nm, 491 nm, 561 nm, 605 nm) (Spectral Applied Research, Concord, ON, Canada), and a Hamamatsu C9100–13 EM-CCD (Hamamatsu Photonics, Shizuoka, Japan); driven and image data were analyzed by Volocity 3DM software (Perkin Elmer Corporation, Waltham, MA) (29, 31). Images were captured at a magnification of 94.3× (63× oil immersion objective, 1.35 NA). For immunofluorescence, freshly prepared or LC3B GFP transduced and agonist stimulated acini were washed with ice-cold PBS, fixed in 4% paraformaldehyde in PBS for 30 min, and permeabilized in 0.1% Triton X-100 buffer for 20 min. Samples were then blocked 1 h in 10% goat serum and immunostained with primary antibodies to TAP (ABIN1173333, Antibodies online; 1:100). 0.4-μm thick optical sections were captured with 0.3 μm Z-spacing. Live cell exocytosis imaging was performed (29) on syncollin-pHluorin adenovirus transduced cultured acini (36). All stimulations were done using sulfated CCK-8 in KRH buffer in a heated chamber equilibrated at 37 °C. Images were acquired at 6 frames/min for 10 min.
Electron microscopy
This was performed essentially as described earlier (27, 29). Briefly, agonist stimulation was terminated by adding 2 volumes of ice-cold KRH buffer. The acini were then pelleted and washed two times by centrifugation (300 × g, 4 °C) with ice-cold PBS, fixed immediately with a Karnovsky-style fixative (3.2% paraformaldehyde and 2.5% glutaraldehyde in a 0.1 mol/liter sodium cacodylate buffer with 5 mmol/liter CaCl2, pH 6.5) for 1 h and then postfixed with 1% osmium tetroxide for 30 min. Samples were pre-embedded in 1% uranyl acetate for 1 h, were then dehydrated and infiltrated with Epon 812 resin. Ultrathin sections (80 nm) were counterstained with 4% uranyl acetate followed by Reynold's lead citrate and then examined and photographed in a Philips transmission electron microscope.
Immunoprecipitation (IP) and Western blot analysis
This was performed as described elsewhere (29). Treatments on stimulated and unstimulated acini were stopped immediately by adding 2 volumes of ice-cold KRH buffer. Acini were then washed two times with ice-cold PBS, harvested by centrifugation (300 × g, 4 °C), and lysed by sonication in lysis buffer (25 mm HEPES, 100 mm KCl, 1.5% Triton X-100 with protease inhibitors). 1 mg of protein extract from each condition was initially precleared with 50 μl of protein A-Sepharose beads (Molecular Probes, Eugene, OR) for 2 h at 4 °C and then subjected to IP with specific 2 μg anti–"STX-2 (110022), –"STX-3 (110033), or –"STX-4 (110042) antibodies (from Synaptic Systems) linked to protein A-Sepharose beads. Beads obtained from the IP were washed three times with ice-cold lysis buffer and eluted from the beads by boiling in sample buffer (2 m Tris/HCl, pH 6.8, 20% SDS, 30% glycerol, 0.03% phenol red) and separated on 12–15% gradient SDS-PAGE followed by electrophoretic transfer onto nitrocellulose membrane (EMD Millipore). Membranes were immunodecorated with appropriate primary antibodies and identified by standard Western blot analysis technique with an ECL (enhanced chemiluminescence) Detection Kit (GE Healthcare). Densitometry analysis of the blots were performed by Image J software taking maximum band intensity as 100. The primary antibody dilutions used were anti-STXs (1:1000), anti-SNAP23 (1:1000), anti-Munc18b (1:1000), anti-VAMP2 (1:200), anti VAMP8 (1:500) (29), anti-Munc18c (AF5659, R&D Systems; 1:200), anti-BiP (PA1–014A, Thermo Fisher, 2 μg/ml), anti-eIF2α (9227S, 1:1000), anti-Phospho eIF2α (3597S, 1:1000), anti-LC3B (2775S, 1:800) from Cell Signaling Technology, and anti-tubulin (1:5000) from Sigma.
Subcellular fractionation
Acinar lysosomes were fractionated by differential centrifugation (57, 58) using a SW40Ti rotor (Beckman) at 4 °C. Briefly, control and agonist-stimulated acini were pelleted at 500 rpm at 4 °C. Pellets were resuspended in 0.3 m sucrose solution (1:10 acini to sucrose, w/v), and homogenized three times using a glass homogenizer. Unbroken cells and nuclei were pelleted at 200 × g, 15 min. Resultant supernatant was spun at 1300 × g, 15 min to obtain ZG fraction. Post-ZG supernatants were pelleted at 12,000 × g, 12 min to obtain lysosome-enriched fraction.
Statistical analysis
Statistical analyses were performed by one-way analysis of variance (ANOVA) using ORIGIN (Microcal, Amherst, MA) or 2-tailed Student's t test using EXCEL (Microsoft). Data presented as mean ± S.D. p < 0.05 is considered statistically significant (NS, not significant).
Author contributions
S. D. and H. Y. G. conceptualization; S. D. data curation; S. D., T. L., and A. I. O. investigation; S. D., T. L., A. I. O., L. X., D. H., N. A. F., H. X., M. S. C., and P. T. methodology; S. D. writing-original draft; T. L., A. I. O., T. A. J., and P. T. formal analysis; D. C. T. and H. Y. G. resources; D. C. T., P. T., and H. Y. G. writing-review and editing; H. Y. G. supervision; H. Y. G. funding acquisition; H. Y. G. project administration.
Supplementary Material
Acknowledgments
We are grateful to Dr. Junichi Sadoshima (Rutgers New Jersey Medical School, Newark, New Jersey) for sending us the LC3B GFP adenovirus. We thank the patients, surgeons, pathologists, and staff of the University Health Network Program in Biospecimen Sciences who provided the normal pancreatic tissue in this project. Some of the equipment used in this study was supported by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund Project Number 19442.
This work was supported by Canadian Institute of Health Research (CIHR) Grant MOP 119352 (to H. Y. G.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5.
- ZG
- zymogen granule
- CCK
- cholecystokinin
- STX
- syntaxin
- SNAP23
- synaptosomal-associated protein of 23kDa
- SNARE
- soluble NSF (N-ethylmaleimide sensitive factor) attachment protein receptor
- VAMP
- vesicle-associated membrane protein
- ER
- endoplasmic reticulum
- eIF2α
- eukaryotic initiation factor 2α
- AP
- autophagosome
- AV
- autophagic vacuoles
- AL
- autolysosome
- EAL
- early autolysosome
- LAL
- late autolysosome
- TAP
- trypsinogen activation peptide
- MPO
- myeloperoxidase
- KD
- knockdown
- TEM
- transmission electron microscopy
- NA
- numerical aperture
- CL
- cathepsin L
- CB
- cathepsin B
- RFU
- relative fluorescent units
- IP
- immunoprecipitation.
References
- 1. Palade G. (1975) Intracellular aspects of the process of protein synthesis. Science 189, 867 10.1126/science.189.4206.867-b [DOI] [PubMed] [Google Scholar]
- 2. Gukovskaya A. S., Gukovsky I., Algül H., and Habtezion A. (2017) Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology 153, 1212–1226 10.1053/j.gastro.2017.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams J. A., and Yule D. I. (2012). Stimulus-secretion coupling in pancreatic acinar cells. In Physiology of the Gastrointestinal Tract (Johnson L. R., ed.), 5th Ed, pp. 1361–1398, Elsevier, Amsterdam [Google Scholar]
- 4. Kubisch C. H., and Logsdon C. D. (2008) Endoplasmic reticulum stress and the pancreatic acinar cell. Expert Rev. Gastroenterol. Hepatol. 2, 249–260 10.1586/17474124.2.2.249 [DOI] [PubMed] [Google Scholar]
- 5. Mukherjee R., Mareninova O. A., Odinokova I. V., Huang W., Murphy J., Chvanov M., Javed M. A., Wen L., Booth D. M., Cane M. C., Awais M., Gavillet B., Pruss R. M., Schaller S., Molkentin J. D., et al. (2016) Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: Inhibition prevents acute pancreatitis by protecting production of ATP. Gut 65, 1333–1346 10.1136/gutjnl-2014-308553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonucci L., Fagman J. B., Kim J. Y., Todoric J., Gukovsky I., Mackey M., Ellisman M. H., and Karin M. (2015) Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc. Natl. Acad. Sci. U.S.A. 112, E6166–E6174 10.1073/pnas.1519384112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mareninova O. A., Hermann K., French S. W., O'Konski M. S., Pandol S. J., Webster P., Erickson A. H., Katunuma N., Gorelick F. S., Gukovsky I., and Gukovskaya A. S. (2009) Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J. Clin. Invest. 119, 3340–3355 10.1172/JCI38674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dolai S., Liang T., Cosen-Binker L. I., Lam P. P. L., and Gaisano H. Y. (2012) Regulation of physiologic and pathologic exocytoses in pancreatic acinar cells. The Pancreapedia 10.3998/panc.2012.12 [DOI] [Google Scholar]
- 9. Gaisano H. Y., and Gorelick F. S. (2009) New insights into the mechanisms of pancreatitis. Gastroenterology 136, 2040–2044 10.1053/j.gastro.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 10. Sah R. P., Garg P., and Saluja A. K. (2012) Pathogenic mechanisms of acute pancreatitis. Curr. Opin. Gastroenterol. 28, 507–515 10.1097/MOG.0b013e3283567f52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Archbold J. K., Whitten A. E., Hu S. H., Collins B. M., and Martin J. L. (2014) SNARE-ing the structures of Sec1/Munc18 proteins. Curr. Opin. Struct. Biol. 29, 44–51 10.1016/j.sbi.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 12. Südhof T. C., and Rothman J. E. (2009) Membrane fusion: Grappling with SNARE and SM proteins. Science 323, 474–477 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pevsner J., Hsu S. C., and Scheller R. H. (1994) n-Sec1: A neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. U.S.A. 91, 1445–1449 10.1073/pnas.91.4.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kauppi M., Wohlfahrt G., and Olkkonen V. M. (2002) Analysis of the Munc18b-syntaxin binding interface. Use of a mutant Munc18b to dissect the functions of syntaxins 2 and 3. J. Biol. Chem. 277, 43973–43979 10.1074/jbc.M208315200 [DOI] [PubMed] [Google Scholar]
- 15. Tellam J. T., Macaulay S. L., McIntosh S., Hewish D. R., Ward C. W., and James D. E. (1997) Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 272, 6179–6186 10.1074/jbc.272.10.6179 [DOI] [PubMed] [Google Scholar]
- 16. Tellam J. T., McIntosh S., and James D. E. (1995) Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J. Biol. Chem. 270, 5857–5863 10.1074/jbc.270.11.5857 [DOI] [PubMed] [Google Scholar]
- 17. Verhage M., Maia A. S., Plomp J. J., Brussaard A. B., Heeroma J. H., Vermeer H., Toonen R. F., Hammer R. E., van den Berg T. K., Missler M., Geuze H. J., and Südhof T. C. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 10.1126/science.287.5454.864 [DOI] [PubMed] [Google Scholar]
- 18. Shen J., Tareste D. C., Paumet F., Rothman J. E., and Melia T. J. (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 10.1016/j.cell.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 19. Misura K. M., Scheller R. H., and Weis W. I. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404, 355–362 10.1038/35006120 [DOI] [PubMed] [Google Scholar]
- 20. Thurmond D. C., Kanzaki M., Khan A. H., and Pessin J. E. (2000) Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol. Cell. Biol. 20, 379–388 10.1128/MCB.20.1.379-388.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh E., Spurlin B. A., Pessin J. E., and Thurmond D. C. (2005) Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54, 638–647 10.2337/diabetes.54.3.638 [DOI] [PubMed] [Google Scholar]
- 22. Oh E., and Thurmond D. C. (2009) Munc18c depletion selectively impairs the sustained phase of insulin release. Diabetes 58, 1165–1174 10.2337/db08-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu D., Xie L., Karimian N., Liang T., Kang Y., Huang Y. C., and Gaisano H. Y. (2015) Munc18c mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose stimulated insulin secretion in human pancreatic beta-cells. Mol. Metabol. 4, 418–426 10.1016/j.molmet.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houng A., Polgar J., and Reed G. L. (2003) Munc18-syntaxin complexes and exocytosis in human platelets. J. Biol. Chem. 278, 19627–19633 10.1074/jbc.M212465200 [DOI] [PubMed] [Google Scholar]
- 25. Kanda H., Tamori Y., Shinoda H., Yoshikawa M., Sakaue M., Udagawa J., Otani H., Tashiro F., Miyazaki J., and Kasuga M. (2005) Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Invest. 115, 291–301 10.1172/JCI22681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaisano H. Y., Lutz M. P., Leser J., Sheu L., Lynch G., Tang L., Tamori Y., Trimble W. S., and Salapatek A. M. (2001) Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J. Clin. Invest. 108, 1597–1611 10.1172/JCI9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cosen-Binker L. I., Binker M. G., Wang C. C., Hong W., and Gaisano H. Y. (2008) VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J. Clin. Invest. 118, 2535–2551 10.1172/JCI34672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cosen-Binker L. I., Lam P. P., Binker M. G., and Gaisano H. Y. (2007) Alcohol-induced protein kinase Cα phosphorylation of Munc18c in carbachol-stimulated acini causes basolateral exocytosis. Gastroenterology 132, 1527–1545 10.1053/j.gastro.2007.01.042 [DOI] [PubMed] [Google Scholar]
- 29. Dolai S., Liang T., Lam P. P. L., Fernandez N. A., Chidambaram S., and Gaisano H. Y. (2012) Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology 143, 832–843e7 10.1053/j.gastro.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 30. Khan A. H., Thurmond D. C., Yang C., Ceresa B. P., Sigmund C. D., and Pessin J. E. (2001) Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J. Biol. Chem. 276, 4063–4069 10.1074/jbc.M007419200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang T., Dolai S., Xie L., Winter E., Orabi A. I., Karimian N., Cosen-Binker L. I., Huang Y. C., Thorn P., Cattral M. S., and Gaisano H. Y. (2017) Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J. Biol. Chem. 292, 5957–5969 10.1074/jbc.M117.777433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorelick F. S., and Lerch M. M. (2017) Do Animal Models of Acute Pancreatitis Reproduce Human Disease? Cell. Mol. Gastroenterol. Hepatol. 4, 251–262 10.1016/j.jcmgh.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pandol S. J., Saluja A. K., Imrie C. W., and Banks P. A. (2007) Acute pancreatitis: bench to the bedside. Gastroenterology 132, 1127–1151 10.1053/j.gastro.2007.01.055 [DOI] [PubMed] [Google Scholar]
- 34. Peery A. F., Dellon E. S., Lund J., Crockett S. D., McGowan C. E., Bulsiewicz W. J., Gangarosa L. M., Thiny M. T., Stizenberg K., Morgan D. R., Ringel Y., Kim H. P., Dibonaventura M. D., Carroll C. F., Allen J. K., et al. (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187.e1–3 10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersen O. H., and Tepikin A. V. (2008) Polarized calcium signaling in exocrine gland cells. Ann. Rev. Physiol. 70, 273–299 10.1146/annurev.physiol.70.113006.100618 [DOI] [PubMed] [Google Scholar]
- 36. Fernandez N. A., Liang T., and Gaisano H. Y. (2011) Live pancreatic acinar imaging of exocytosis using syncollin-pHluorin. Am. J. Physiol. Cell Physiol. 300, C1513–C1523 10.1152/ajpcell.00433.2010 [DOI] [PubMed] [Google Scholar]
- 37. Pickett J. A., Thorn P., and Edwardson J. M. (2005) The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J. Biol. Chem. 280, 1506–1511 10.1074/jbc.M411967200 [DOI] [PubMed] [Google Scholar]
- 38. Behrendorff N., Dolai S., Hong W., Gaisano H. Y., and Thorn P. (2011) Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J. Biol. Chem. 286, 29627–29634 10.1074/jbc.M111.265199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., et al. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 10.4161/auto.5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroemer G., Mariño G., and Levine B. (2010) Autophagy and the integrated stress response. Mol. Cell 40, 280–293 10.1016/j.molcel.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rashid H. O., Yadav R. K., Kim H. R., and Chae H. J. (2015) ER stress: Autophagy induction, inhibition and selection. Autophagy 11, 1956–1977 10.1080/15548627.2015.1091141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hess D. A., Strelau K. M., Karki A., Jiang M., Azevedo-Pouly A. C., Lee A. H., Deering T. G., Hoang C. Q., MacDonald R. J., and Konieczny S. F. (2016) MIST1 links secretion and stress as both target and regulator of the UPR. Mol. Cell. Biol. 36, 2931–2944 10.1128/MCB.00366-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hetz C., and Papa F. R. (2018) The unfolded protein response and cell fate control. Mol. Cell 10.1016/j.molcel.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 44. Dolai S., Pal S., Yadav R. K., and Adak S. (2011) Endoplasmic reticulum stress-induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J. Biol. Chem. 286, 13638–13646 10.1074/jbc.M110.201889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walter P., and Ron D. (2011) The unfolded protein response: From stress pathway to homeostatic regulation. Science 334, 1081–1086 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- 46. Talukdar R., Sareen A., Zhu H., Yuan Z., Dixit A., Cheema H., George J., Barlass U., Sah R., Garg S. K., Banerjee S., Garg P., Dudeja V., Dawra R., and Saluja A. K. (2016) Release of cathepsin B in cytosol causes cell death in acute pancreatitis. Gastroenterology 151, 747–758.e5 10.1053/j.gastro.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frossard J. L. (2000) Trypsinogen activation peptide in acute pancreatitis. Lancet 356, 766–767 10.1016/S0140-6736(05)73665-5 [DOI] [PubMed] [Google Scholar]
- 48. Orabi A. I., Shah A. U., Muili K., Luo Y., Mahmood S. M., Ahmad A., Reed A., and Husain S. Z. (2011) Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini. J. Biol. Chem. 286, 14090–14097 10.1074/jbc.M110.196832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kubisch C. H., and Logsdon C. D. (2007) Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1804–G1812 10.1152/ajpgi.00078.2007 [DOI] [PubMed] [Google Scholar]
- 50. Song S., Tan J., Miao Y., and Zhang Q. (2017) Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement of UPR and the core autophagy machinery. J. Cell. Physiol. [DOI] [PubMed] [Google Scholar]
- 51. Fernández-del Castillo C., Schmidt J., Warshaw A. L., and Rattner D. W. (1994) Interstitial protease activation is the central event in progression to necrotizing pancreatitis. Surgery 116, 497–504 [PubMed] [Google Scholar]
- 52. Hartwig W., Jimenez R. E., Werner J., Lewandrowski K. B., Warshaw A. L., and Fernández-del Castillo C. (1999) Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology 117, 717–725 10.1016/S0016-5085(99)70466-X [DOI] [PubMed] [Google Scholar]
- 53. Nair U., Jotwani A., Geng J., Gammoh N., Richerson D., Yen W. L., Griffith J., Nag S., Wang K., Moss T., Baba M., McNew J. A., Jiang X., Reggiori F., Melia T. J., and Klionsky D. J. (2011) SNARE proteins are required for macroautophagy. Cell 146, 290–302 10.1016/j.cell.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marciniak A., Selck C., Friedrich B., and Speier S. (2013) Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ. PloS One 8, e78706 10.1371/journal.pone.0078706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kidd J. F., Pilkington M. F., Schell M. J., Fogarty K. E., Skepper J. N., Taylor C. W., and Thorn P. (2002) Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J. Biol. Chem. 277, 6504–6510 10.1074/jbc.M106802200 [DOI] [PubMed] [Google Scholar]
- 56. Lam P. P., Cosen Binker L. I., Lugea A., Pandol S. J., and Gaisano H. Y. (2007) Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic 8, 605–617 10.1111/j.1600-0854.2007.00557.x [DOI] [PubMed] [Google Scholar]
- 57. Saluja A., Saito I., Saluja M., Houlihan M. J., Powers R. E., Meldolesi J., and Steer M. (1985) In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am. J. Physiol. 249, G702–G710 10.1152/ajpgi.1985.249.6.G702 [DOI] [PubMed] [Google Scholar]
- 58. Tartakoff A. M., and Jamieson J. D. (1974) Subcellular fractionation of the pancreas. Meth. Enzymol. 31, 41–59 10.1016/0076-6879(74)31006-3 [DOI] [PubMed] [Google Scholar]
- 59. Wang Y., Chiu C. T., Nakamura T., Walker A. M., Petridou B., Trousdale M. D., Hamm-Alvarez S. F., Schechter J. E., and Mircheff A. K. (2007) Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am. J. Physiol. Endocrinol. Metab. 292, E1122–E1134 10.1152/ajpendo.00381.2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.