Summary
Carfilzomib is a next-generation proteasome inhibitor that selectively and irreversibly binds to its target. In clinical studies, carfilzomib has shown efficacy in patients with relapsed and/or refractory multiple myeloma (MM) and has demonstrated a tolerable safety profile. In this phase 2, open-label, multicentre clinical trial, 35 patients with relapsed and/or refractory MM following 1–3 prior therapies, including at least one bortezomib-based regimen, received carfilzomib 20 mg/m2 in a twice-weekly, consecutive-day dosing schedule for ≤12 monthly cycles. The best overall response rate (ORR) was 17.1% and the clinical benefit response rate (ORR + minimal response) was 31.4%. The median duration of response was >10.6 months and the median time to progression was 4.6 months. The most common adverse events were fatigue (62.9%), nausea (60.0%), and vomiting (42.9%). No exacerbation of baseline peripheral neuropathy was observed. Single-agent carfilzomib was generally well tolerated for up to 12 treatment cycles and showed activity in patients with relapsed and/or refractory MM who had received prior treatment with bortezomib. These data, combined with an acceptable toxicity profile, support the potential use of carfilzomib in patients with relapsed and/or refractory MM and warrant continued investigation of carfilzomib as single agent or in combination with other agents.
Keywords: Multiple myeloma, Proteasome inhibitor, Relapsed, Refractory, Phase 2
Introduction
Multiple myeloma (MM) will affect an estimated 21,700 people and cause over 10,710 deaths in the United States (US) in 2012 (American Cancer Society [ACS] 2012). The US 5-year relative survival rate from 1999–2006 was just 39%, with a median survival of 4 years (Rajkumar 2009; Siegel et al, 2011a). Previously, therapy for relapsed and/or refractory MM (i.e., patients who are either relapsed and refractory OR relapsed or refractory) consisted primarily of combination therapies based around melphalan, prednisone, cyclophosphamide, dexamethasone or other broadly cytotoxic agents (Kyle and Rajkumar 2008); however, treatment options and corresponding patient outcomes have greatly improved in the past decade following the introduction of agents, such as thalidomide, lenalidomide and liposomal doxorubicin (Laubach et al, 2010; Plosker 2008; Rajkumar 2009). One notable therapy that has emerged in recent years has been the proteasome inhibitor bortezomib (Velcade®, Millennium Pharmaceuticals, Cambridge, MA), the first-in-class drug that validated the proteasome as a target for therapeutic approaches to MM (Hideshima et al, 2001; Kane et al, 2003). Proteasome inhibitors such as bortezomib are effective anti-cancer agents, as transformed cells generally have a higher level of proteasome activity than differentiated cells (Kanayama et al, 1991).
Although it is effective as an anti-myeloma drug, there are limitations to the use of bortezomib, including development of resistance and painful peripheral neuropathy (Boyette-Davis et al, 2011; Mohty et al, 2010). These adverse effects of bortezomib have limited its use in some patients, for whom there is a need for additional treatment options. A number of modifications to the clinical dosing scheme for bortezomib (eg, weekly dosing, subcutaneous (SC) administration) have been evaluated in an effort to address these issues, with encouraging results (Bringhen et al, 2010; Moreau et al, 2011). However, there is still a need for a next generation of proteasome inhibitors with greater efficacy and improved tolerability profiles. One such novel proteasome inhibitor currently under development is carfilzomib (formerly PR-171; Onyx Pharmaceuticals, South San Francisco, CA).
Carfilzomib is a potent and highly selective proteasome inhibitor that is structurally and mechanistically distinct from bortezomib (Demo et al, 2007). Carfilzomib selectively and irreversibly inhibits the chymotrypsin-like activity of the 20S proteasome, necessitating de novo protein synthesis to restore activity (Parlati et al, 2009). In preclinical studies, carfilzomib demonstrated more potent proteasome inhibition and minimal off-target activity against non-proteasomal proteases relative to bortezomib (Demo et al, 2007). In addition, consecutive-day dosing of carfilzomib was well-tolerated and led to prolonged irreversible proteasome inhibition (Demo et al, 2007). Carfilzomib has also shown a lack of histological or behavioural neurotoxicity with chronic dosing in animals (Arastu-Kapur et al, 2011; Kirk et al, 2008) and displayed cytotoxic activity against bortezomib-resistant cell lines (Kuhn et al, 2007).
Early clinical studies testing carfilzomib in patients with relapsed and/or refractory haematological malignancies including MM have been encouraging, with rapid responses beginning shortly after the onset of therapy, primarily mild or moderate haematological toxicities, and low rates of peripheral neuropathy (PN) (Alsina et al, 2007; O'Connor et al, 2009; Singhal et al, 2011). A minimal effective dose of 15 mg/m2 has been established. (O'Connor et al, 2009). Durable responses (from >90 to >280 days) have been achieved in patients using a twice-weekly, consecutive-day dosing schedule, and responses were observed in patients who had failed bortezomib therapy, treatment with immunomodulatory agents, and stem cell transplant (Alsina et al, 2007).
Following these promising results, 2 phase 2 studies were initiated to evaluate single-agent carfilzomib in patients with relapsed and refractory MM following at least 2 lines of therapy (PX-171-003) and in patients with relapsed and/or refractory MM following 1–3 prior therapies (PX-171-004). The 003 study, from which the results have been submitted to the US food and Drug Administration for regulatory review and accelerated approval of carfilzomib, involved a larger population of patients (N=266) (Siegel et al, 2010). The 004 study is a smaller study originally designed to investigate the impact of carfilzomib treatment in less heavily-treated patients. The results presented here are from the analysis of the lower dose, first cohort of a larger study in patients who had previously been exposed to bortezomib (Fig 1). Following an amendment to the study, a separate cohort that included only bortezomib-naïve patients was initiated to investigate a higher dose of carfilzomib; the data for bortezomib-naïve patients were analysed independently and published separately (Vij et al, 2012).
Fig 1. PX-171-004 Study Design.
ECOG=Eastern Cooperative Oncology Group
Methods
Study design and eligibility
This was a phase 2 multicentre, open-label, single-arm study registered at ClinicalTrials.gov (NCT00530816). Written informed consent was obtained from all enrolled patients in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of each participating centre.
Patients with measurable relapsed and/or refractory MM who were 18 years of age or older were eligible to participate in this study. Measurable disease was defined as either or both of the following: serum M-protein ≥ 10 g/l and/or urine M-protein ≥ 200 mg/24 h. Prior to an amendment to the protocol, 4 patients were permitted to enter the study with disease measurable by serum free light chain (sFLC) only. Key inclusion criteria were: Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2, responsive (i.e., achieved minimal response [MR] or better) to standard first-line therapy, and relapsed or relapsed and refractory after 1–3 prior therapeutic regimens (including at least 1 bortezomib-containing regimen). Refractory disease was defined as ≤25% response or progressive disease (PD) either during therapy or within 60 days after completion of the last therapy. Induction therapy, stem cell transplant, and planned maintenance were considered as one regimen. Patients were also required to have: total white blood cell (WBC) count ≥ 2.0 × 109/l, absolute neutrophil count (ANC) ≥ 1.0 × 109/l, haemoglobin ≥ 80 g/l, platelet count ≥ 50 × 109/l, creatinine clearance (CrCl) ≥ 30 ml/min, and adequate hepatic function.
Excluded from the study were patients who: had received glucocorticoid therapy (prednisone >10 mg/day or equivalent) or chemotherapy with an approved or investigational anticancer treatment (including corticosteroid therapy) within the 3 weeks prior to first dose; received radiation therapy or immunotherapy in the 4 weeks prior to first dose or received localized radiation therapy within 1 week prior to first dose; participated in an investigational therapeutic study within 3 weeks or 5 drug half-lives prior to first dose (whichever time was greater); or had prior treatment with carfilzomib. Other exclusion criteria included cardiovascular disease (including congestive heart failure [New York Heart Association Grade III or IV], symptomatic ischaemia, uncontrolled conduction abnormalities, and myocardial infarction); acute active infection requiring systemic antibiotics, antivirals, or antifungals within 2 weeks prior to first dose; human immunodeficiency virus seropositivity or active hepatitis A, B, or C infection; or significant neuropathy (Grade 3, 4, or Grade 2 with pain) at the time of the study initiation.
Treatment
Intravenous (IV) carfilzomib (20 mg/m2) was administered over a period of 2–10 min on days 1, 2, 8, 9, 15, and 16 of every 28-day cycle for up to 12 cycles. During the first cycle, prophylactic dexamethasone at a dose of 4 mg/day was administered prior to each dose of carfilzomib to ameliorate fever, chills, shortness of breath, and/or rigors. In phase 1 studies, these symptoms had been observed in small subsets of study patients in Cycle 1 and occasionally in Cycle 2. In the current study, if any of these treatment-related effects were observed during treatment after dexamethasone had been discontinued, oral (PO) or IV dexamethasone 4 mg was permitted to be re-administered prior to subsequent doses of carfilzomib. Carfilzomib doses were held or reduced to 15 mg/m2 and 11 mg/m2 in response to certain adverse events (AEs), and following resolution, patients were permitted to resume treatment at the same dose level (eg, for reversible haematological AEs) or a reduced dose level, depending on the AE. No more than 2 dose reductions were permitted before removal from the study. Patients completing 12 cycles of treatment with carfilzomib were eligible to continue receiving carfilzomib on extension study PX-171-010 (NCT00884312).
Assessment of response and safety
The response-evaluable population comprised patients who had completed at least one cycle of treatment with carfilzomib, underwent at least one post-baseline response assessment, and had disease measurable by protein electrophoresis. Overall response rate (ORR) to carfilzomib included stringent complete response (sCR), complete response (CR), very good partial response (VGPR), and partial response (PR). The primary endpoint of ORR was based on response-evaluable subset population, which excluded 4 patients whose disease was followed by sFLC measurement only. Secondary endpoints were based on the whole response-evaluable population and included: the best ORR throughout the treatment period and clinical benefit response (CBR) rate (ORR + MR); duration of response (DOR), defined as time from first evidence of PR or better, i.e., first observation of PR that is subsequently confirmed to PD or death without PD; time to progression (TTP), defined as time from study entry (first dose of carfilzomib) to PD; and overall survival (OS, defined as time from first dose of carfilzomib to date of death). Progression-free survival (PFS, defined as time from start of treatment to PD or death due to any cause) was also included. Additional exploratory efficacy endpoints were duration of clinical benefit (defined as the time from first confirmed MR or higher to the first confirmed PD or death without PD) and duration of MR.
Patients were evaluated for response on Day 15 of Cycle 1, Day 1 of Cycles 2–12, and at the end of the study. Responses and progression were assessed according to the International Myeloma Working Group’s Uniform Response Criteria (Durie et al, 2006) for all except MR, which was assessed using the European Group for Blood and Marrow Transplantation (EBMT) criteria (Blade et al, 1998). In brief, MR was defined in the protocol as reduction of M-protein ≥25% and <50% in serum or ≥50% and <90% in urine, maintained for 6 weeks (MR was considered confirmed with an interval of at least 4 weeks between measurements for patients with an observation of PD or death at least 6 weeks from the first MR observation). Responses were reviewed, confirmed, and adjudicated by an independent review committee consisting of 4 individuals not associated with the trial (3 clinicians as voting members and 1 non-voting secretary). Independent Review Committee (IRC) assessments were documented in the case report form (CRF) and entered in a database maintained separately from the clinical database. Discordant cases between the IRC and investigator were re-reviewed by the IRC, which was also documented in the CRF.
The incidence and severity of AEs were also assessed. The safety population included all patients who received at least 1 dose of carfilzomib. Safety assessments included AEs and serious AEs (SAEs), laboratory values, and vital signs. AEs were summarized based on the number and percentage of patients experiencing events according to the Medical Dictionary for Regulatory Activities (MedDRA®) AE dictionary system organ class and preferred term. The National Cancer Institute Common Terminology Criteria for Adverse Events, version 3 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf), was used to grade AEs.
Because of the significant rates of PN historically observed with the use of bortezomib, the incidence and severity of PN was carefully evaluated in this study. Detailed individual neuropathy histories were collected at screening, and comprehensive neurophysical examinations were conducted. In addition, patients were required to complete a PN-related quality of life (QoL) survey, the Functional Assessment of Cancer Therapy/ Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-NTx) version 4.0.(Huang et al, 2007) Following every 2 cycles of treatment, neurological exams were conducted and patients completed the FACT-GOG/NTx questionnaire.
Statistical analyses
Descriptive statistics, including 2-sided 95% confidence intervals (CIs), were used to summarize all study endpoints. All statistical analyses were performed using SAS® version 9.1 or later (SAS Institute, Inc., Cary, NC). Best ORR was determined based on a 2-sided 95% CI for evaluable patients whose best response was classified as sCR, CR, VGPR, or PR. Analysis of ORR was performed in subgroups defined according to a number of covariates as exploratory analyses by patient baseline characteristics of age, ECOG PS, International Staging System (ISS) for MM stage (Greipp et al, 2005), and serum β2-microglobulin concentration. Cytogenetic/fluorescent in situ hybridization (FISH) prognostic markers of high-risk disease were defined per Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART) criteria (Dispenzieri et al, 2007) analysed by either FISH [for del17p, t(4;14), or t(14;16)] or by metaphase cytogenetics (for del13 or hypodiploidy). ORR was estimated within each subgroup along with its 95% exact binomial CIs. Analysis for time-to-event secondary endpoints (PFS, TTP, DOR, and OS) was performed by preparing Kaplan-Meier estimates for quartiles including median and Kaplan-Meier curves. Also generated were 2-sided 95% CIs for the quartiles. The event-free rate was estimated for several time points (e.g., 9 months).
Simple descriptive statistics were used to summarize the AE profile of carfilzomib based on the number and percentage of patients experiencing system organ class events. Changes in clinical safety laboratory values and vital signs were summarized descriptively for each scheduled and unscheduled time point. Changes were calculated relative to the values collected at baseline and on the first day of each treatment cycle.
Results
Patient population
A total of 35 bortezomib-treated patients from 17 centres enrolled in this study between September 2007 and November 2008 with a data cutoff of November 2010. All patients received at least one dose of carfilzomib and therefore were evaluable for both safety and efficacy. The median age was 63 years (range 40–77) and the median time from diagnosis was 3.6 years (range 1.2–13.2) (Table I). A high tumour burden was demonstrated by an ISS stage of II or III in 42.9% of patients. A significant proportion of patients had poor/unfavourable markers by cytogenetic or FISH analysis (25.7%), and the median serum β2-microglobulin concentration at baseline was 3.3 mg/l (range 1.6–9.8). More than half of all patients had active PN at baseline: Grade 1 in 16 patients (45.7%) and Grade 2 in 3 patients (8.6%). At the time of study entry, a large proportion of patients also had active haematological abnormalities, including anaemia (85.7%), neutropenia (57.1%), and thrombocytopenia (28.6%), all of Grade 1/2 in severity with the exception of 1 patient with Grade 3 thrombocytopenia.
Table I.
Patient Baseline Characteristics.
| Characteristic | Carfilzomib 20 mg/m2 (N=35) |
|---|---|
| Median age, years (range) | 63 (40–77) |
| Male gender | 18 (51.4) |
| Median time from diagnosis, years (range) | 3.6 (1.2–13.2) |
| Immunoglobulin subtype, n (%) | |
| IgG | 24 (68.6) |
| IgA | 5 (14.3) |
| IgD | 1 (2.9) |
| Unknown/Missing | 5 (14.3) |
| Light chain subtype, n (%) | |
| Kappa | 23 (65.7) |
| Lambda | 12 (34.3) |
| ECOG PS, n (%) | |
| 0 | 15 (42.9) |
| 1 or 2 | 19 (54.3) |
| ISS Stage, n (%) | |
| I | 19 (54.3) |
| II | 8 (22.9) |
| III | 7 (20.0) |
| Baseline evaluation, n (%) | |
| Peripheral neuropathy ≥ Grade 1 | 19 (54.3) |
| Diabetes | 5 (14.3) |
| CrCl < 50 ml/min | 4 (11.4) |
| β2-microglobulin, mg/l (range) | 3.3 (1.6–9.8) |
| Cytogenetic/FISH prognostic markers, n (%) | |
| Normal/Favourable | 25 (71.4) |
| Unfavourable | 9 (25.7) |
Ig=immunoglobulin; ECOG PS=Eastern Cooperative Oncology Group performance status; ISS=International Staging System; CrCl=creatinine clearance; FISH=fluorescence in situ hybridization.
Patients had received a median of 3.0 prior therapies (range 1–13)(Table II). Five (14.3%) patients received 4 or more prior regimens, and all patients had been previously treated with bortezomib. More than half of all patients (20; 57.1%) received bortezomib in their treatment regimen immediately preceding study entry. Seven patients (20.0%) were refractory to bortezomib at any time prior to participating in this study, and 22 patients (62.9%) had disease that was refractory to their last therapy regardless of drug. The majority of patients (97.1%) had previously received corticosteroid therapy; other common treatments included alkylating agents (88.6%), thalidomide (68.6%), and lenalidomide (37.1%), and 80.0% of the patients enrolled had undergone autologous stem cell transplant.
Table II.
Prior Therapies.
| Carfilzomib 20 mg/m2 (N=35) |
|
|---|---|
| Median number of prior therapies (range) | 3.0 (1–13) |
| Prior therapy, n (%) | |
| Bortezomib | 35 (100.0) |
| Bortezomib in last regimen | 20 (57.1) |
| Thalidomide | 24 (68.6) |
| Lenalidomide | 13 (37.1) |
| Thalidomide or lenalidomide | 27 (77.1) |
| Corticosteroid | 34 (97.1) |
| Alkylating agent | 31 (88.6) |
| Anthracycline | 11 (31.4) |
| Stem cell transplant | 28 (80.0) |
| Refractory status, n (%) | |
| To bortezomib in any prior regimen | 7 (20.0) |
| To last therapy,* n (%) | 22 (62.9) |
Most patients received a combination regimen as their last therapy.
Efficacy
All 35 patients were included in the response-evaluable population, for which the best ORR was 17.1% as determined by an independent central assessment (1 CR, 1 VGPR, and 4 PR), and the CBR rate was 31.4% (Table III). Responses were observed even in those patients with more severe disease at baseline; >10% ORR was reported in subgroups analysed by patient baseline characteristics of ECOG PS and ISS stage prognostic markers, as well as poor/unfavourable markers characterized by cytogenetic analysis (6 patients) or FISH analysis (3 patients) (Table IV). Although there were some differences in absolute numbers (e.g., the ORRs between ISS Stages I, II, and III), the small numbers of patients in each subgroup, along with the wide confidence intervals, precluded any firm conclusions to be drawn from differences between or within subgroups. In the response-evaluable subset population (N=31), in which patients with disease measured by sFLC only were excluded, ORR was 16.1% (1 CR, 1 VGPR and 3 PR) as determined by an independent central assessment.
Table III.
Best Overall Response (Response-evaluable Population).
| Carfilzomib 20 mg/m2 (N=35) |
|
|---|---|
| * Best response, n (%) | |
| CR | 1 (2.9) |
| VGPR | 1 (2.9) |
| PR | 4 (11.4) |
| MR | 5 (14.3) |
| SD | 13 (37.1) |
| PD | 10 (28.6) |
| ORR (sCR + CR + VGPR + PR) | 6 (17.1) |
| CBR (ORR + MR) | 11 (31.4) |
Includes 4 patients whose disease was followed by sFLC, 1 of whom had a confirmed response.
CR=complete response; VGPR=very good partial response; PR=partial response; MR=minimal response; SD=stable disease; PD=progressive disease; ORR=overall response rate; CBR=clinical benefit response.
Table IV.
Overall Response Rate (ORR) of Baseline Characteristic Subgroup.*
| n | ORR, % | 95% CI | |
|---|---|---|---|
| Overall | 35 | 17.1 | 6.6–33.7 |
| Baseline characteristic | |||
| Age group | |||
| <65 years | 21 | 9.5 | 1.2–30.4 |
| ≥65 years | 14 | 28.6 | 8.4–58.1 |
| ECOG PS | |||
| 0 | 15 | 26.7 | 7.8–55.1 |
| 1 or 2 | 19 | 10.5 | 1.3–33.1 |
| ISS Stage | |||
| I | 19 | 15.8 | 3.4–39.6 |
| II | 8 | 25.0 | 3.2–65.1 |
| III | 7 | 14.3 | 0.4–57.9 |
| Cytogenetic/FISH prognostic markers | |||
| Normal/favourable | 25 | 16.0 | 4.5–36.1 |
| Poor/unfavourable | 9 | 11.1 | 0.3–48.2 |
Response-evaluable population.
ECOG PS=Eastern Cooperative Group performance status; ISS=International Staging System; FISH=fluorescence in situ hybridization.
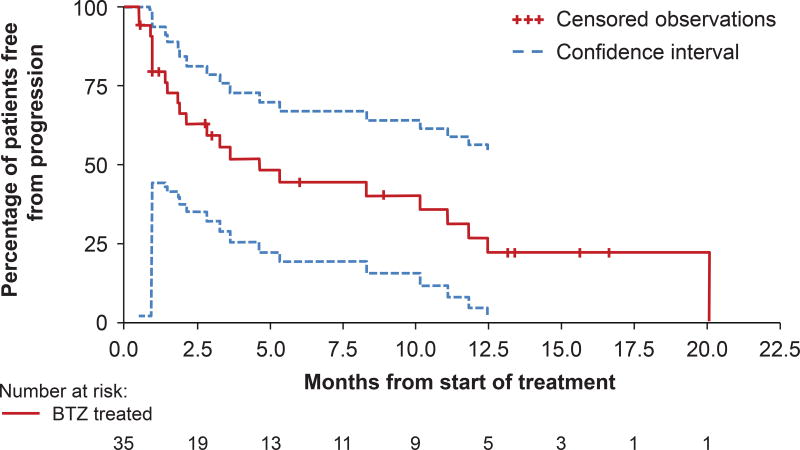
Responses were fairly rapid, with a median time to response (TTR) of 1.4 months to achieve ≥PR and 1.0 month to achieve ≥MR. The median DOR was >10.6 months with 4 out of the 6 patients with a DOR of 10.6 months or longer. A more precise estimate of the median DOR could not be determined as 4 of the 6 patients with responses had their DOR censored at the close of the study (Table V). Median duration of clinical benefit was 10.6 months and median duration of MR was 7.9 months. The median TTP was 4.6 months (95% CI: 1.9–11.1) (Fig 1) and the median PFS was 4.6 months (95% CI: 2.1–11.1). Median OS was calculated to be 29.9 months; however, survival data remain immature and neither the upper nor lower limit of 95% CI could be estimated as of the data cutoff.
Table V.
Time to Progression, Time to Response, and Duration of Response.*
| Carfilzomib 20 mg/m2 (N=35) |
|
|---|---|
| Median TTP, months (95% CI) | 4.6 (1.9–11.1) |
| Median TTR, months (95% CI) | |
| ≥PR (ORR) | 1.4 (0.5–1.9) |
| ≥MR (CBR) | 1.0 (0.5–6.7) |
| Median DOR, months (95% CI) | |
| ≥PR (ORR) | NE (10.6–NE) |
| ≥MR (CBR) | 10.6 (7.2–NE) |
| MR | 7.9 (5.8–14.6) |
Response-evaluable population.
NE=not estimated due to censoring
TTP=time to progression; TTR=time to response; DOR=duration of response; 95% CI=95% confidence interval; PR=partial response; MR=minimal response; ORR=overall response rate; CBR=clinical benefit response
Safety
The safety population included all 35 patients enrolled in the study. Patients started a median of 4 cycles of carfilzomib (range 1–12), and 9 patients (25.7%) completed 12 cycles of carfilzomib therapy. Although no patients from this study entered the PX-171-010 extension protocol, 1 patient continued to receive carfilzomib treatment on a single-patient extension protocol for an additional 11 months.
Carfilzomib was generally well tolerated. The most frequent AEs were fatigue (62.9%), nausea (60.0%), vomiting (42.9%), diarrhoea, and dyspnea (both 37.1%) (Table VI). Twenty patients (57.1%) experienced at least one severe (Grade ≥3) AE, the majority of them (15/20) Grade 3. The most common Grade 3/4 AEs were haematological, including thrombocytopenia (20.0%), anaemia (14.3%), and neutropenia (11.4%). Treatment-related AEs occurred in all patients and the most common were nausea (51.4%), fatigue (48.6%), increased serum creatinine (34.3%), diarrhoea (31.4%), vomiting (31.4%), dyspnea (28.6%), thrombocytopenia (28.6%), and anaemia (25.7%).
Table VI.
Safety Population Treatment-emergent Adverse Events of All Grades (≥20%) or ≥Grade 3 (≥5%)
| Treatment-emergent Adverse Event | Carfilzomib 20 mg/m2 (N=35) |
|
|---|---|---|
| All Grades n (%) |
≥Grade 3 n (%) |
|
| Haematological | ||
| Anaemia | 12 (34.3) | 5 (14.3) |
| Thrombocytopenia | 11 (31.4) | 7 (20.0) |
| Neutropenia | 9 (25.7) | 4 (11.4) |
| Lymphopenia | 6 (17.1) | 2 (5.7) |
| Non-haematological | ||
| Fatigue | 22 (62.9) | 1 (2.9) |
| Nausea | 21 (60.0) | 1 (2.9) |
| Vomiting | 15 (42.9) | 1 (2.9) |
| Dyspnea | 13 (37.1) | 2 (5.7) |
| Diarrhoea | 13 (37.1) | 0 (0) |
| Upper respiratory infection | 12 (34.3) | 2 (5.7) |
| Increased serum creatinine | 12 (34.3) | 1 (2.9) |
| Increased alanine aminotransferase | 10 (28.6) | 0 (0) |
| Headache | 9 (25.7) | 1 (2.9) |
| Hypoesthesia | 9 (25.7) | 0 (0) |
| Pyrexia | 9 (25.7) | 0 (0) |
| Asthenia | 8 (22.9) | 1 (2.9) |
| Constipation | 8 (22.9) | 0 (0) |
| Cough | 8 (22.9) | 0 (0) |
| Dizziness | 7 (20.0) | 0 (0) |
| Increased aspartate aminotransferase | 7 (20.0) | 0 (0) |
| Pain | 7 (20.0) | 0 (0) |
| Hypertension | 6 (17.1) | 2 (5.7) |
| Hypercalcaemia | 5 (14.3) | 2 (5.7) |
| Pneumonia | 3 (8.6) | 3 (8.6) |
| Epiglottitis | 2 (5.7) | 2 (5.7) |
Of the 26 patients that discontinued early, 16 patients discontinued due to disease progression and 6 patients due to treatment-emergent AEs; the only AE leading to discontinuation for more than 1 patient was hypercalcaemia (2 patients). Other reasons for discontinuation were physician discretion and near complete remission (1 patient each) as well as patient decision to come off study or withdrew consent (1 patient each). No deaths occurred on study (inclusive of 30 days of last carfilzomib dose). Nearly half of the patients (45.7%) received the protocol-specified dosing schedule on time and 62.9% received doses of 90–100% of the relative dose intensity specified in the protocol. Four patients had dose reductions due to AEs, although 3 of these patients were successfully re-escalated to the 20 mg/m2 dose.
During the study 6 (17.1%) patients experienced neuropathy-related treatment-emergent AEs; the majority of PN reports were Grade 1 and considered not related to carfilzomib treatment (4 patients). Additionally there was 1 patient with Grade 2 and 1 patient with Grade 3 (both possibly related to treatment) and no reports of Grade 4 PN. There were no dose reductions or discontinuations due to PN, which is especially notable as 54.3% of all study patients had Grade 1–2 PN at baseline. Moreover, prolonged treatment with carfilzomib as seen in this study was not associated with any significant increase in neuropathy, as supported by the absence of a meaningful change from baseline to the last assessment (n=30) on the on the FACT/GOG-NTx results.
Discussion
In this study, the best ORR for the response-evaluable population was 17.1% and the CBR was 31.4%. Additional statistical testing between subgroups was not performed due to the small numbers within subgroups; however, responses of at least 10% were observed even in high-risk patients and those with more severe disease, including poor/unfavourable cytogenetics and ISS stage III based on the subgroup analyses. These results indicate single-agent activity in this population and are interesting because of the very limited data available on retreatment with monotherapies in patients with MM refractory to bortezomib (Mohty et al, 2012; van de Donk et al, 2011). Because the number of patients in this study was relatively small, caution must be used in interpreting the results. However, the results of this study are comparable to those from previous carfilzomib studies with patients who had similar medical and treatment histories (Alsina et al, 2007; Siegel et al, 2010).
The majority of carfilzomib doses in this study were administered at the 20 mg/m2 level. Subsequent studies have demonstrated the tolerability of higher doses including 27 mg/m2 on the same schedule and 56 mg/m2 when given as a 30-min infusion (Papadopoulos et al, 2011; Siegel et al, 2010). Based on the results of other carfilzomib studies with a dosing regimen of 27 mg/m2, including the 003-A1 study and the independently analysed bortezomib-naïve cohorts from the 004 study, it is likely that increased anti-MM activity would be observed even at higher dose levels as a single agent (Siegel et al, 2010; Vij et al, 2010; Vij et al, 2012).
At a median follow-up of 13.4 months (95% CI: 8.9–16.6), the median TTP was 4.6 months (95% CI: 1.9–11.1). In part, this short median TTP is the result of early disease progression in approximately 50% of patients, most probably due to advanced disease at the time of study entry. For patients remaining on study for more than 4 cycles, progression events were often substantially delayed, a finding consistent with the promising DOR seen in both patients with ≥PR and those with ≥MR. The median DOR of 7.9 months (95% CI: 5.8–14.6) reached for patients experiencing MR suggests that MR may be an equally valid response endpoint and supports the concept that sustained MR is associated with a clinically meaningful benefit (Niesvizky et al, 2008; Richardson et al, 2005). These results reinforce the suggestion of durable disease control with carfilzomib monotherapy for a significant number of heavily pretreated patients in this study.
Carfilzomib treatment was generally tolerable, with the majority of patients receiving carfilzomib at the planned dose and according to schedule. The fact that patients started a median of 4 cycles of treatment correlates well with median time to response and indicates that patients are able to tolerate carfilzomib for a long enough interval to see evidence of response. Reinforcing the tolerability profile of carfilzomib from other studies (Siegel et al, 2011b; Singhal et al, 2011), 25.7% of patients completed a full 12 cycles of treatment and 1 patient continued on a single-patient extension protocol.
The most common treatment-related AEs were gastrointestinal (nausea, diarrhoea, vomiting, and constipation), constitutional (fatigue), respiratory (dyspnea), and myelosuppressive (thrombocytopenia, anaemia, and neutropenia). When compared with a larger study of carfilzomib with a similar but more heavily treated bortezomib-relapsed and refractory population (PX-171-003) (Siegel et al, 2010), the overall AE rates were comparable, although certain toxicities were reported less frequently in this study, namely haematological AEs ≥Grade 3. Additionally, although an actual comparison cannot be made due the differences in trial sizes and patient populations, toxicity compared relatively favourably to bortezomib—in the Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial comparing treatment of bortezomib and dexamethasone in patients with relapsed MM, 9% of patients completed the planned bortezomib therapy compared with the 25.7% here, and 37% had AEs resulting in discontinuation of bortezomib compared with the 17.1% that discontinued due to an AE here (Richardson et al, 2005).
PN is a common occurrence in patients with MM and is both caused and exacerbated by treatment with bortezomib and thalidomide. PN has been reported in up to 70% of patients treated with thalidomide and approximately 37% of patients treated with bortezomib (Mohty et al, 2010). Therefore, the occurrence of PN was carefully monitored in this study. The incidence of neuropathy at baseline for this study was >50%, but exacerbations were not observed upon carfilzomib treatment. Similarly, previous studies have found that baseline PN does not impact the efficacy and tolerability of carfilzomib (Martin et al, 2010). Additionally, patients’ self-reported experiences and quality of life in the current study were minimally impacted from the initiation of the study through to their last assessment, further supporting the PN-specific tolerability of this agent.
In summary, single-agent carfilzomib at 20 mg/m2 achieved significant responses in this heavily pre-treated population, including treatment with bortezomib and the immunomodulatory agents thalidomide and lenalidomide. Treatment with carfilzomib was well tolerated for up to 12 cycles, and a lack of significant toxicities supports the use of single-agent carfilzomib or potential combination regimens. The results of the larger phase 2 study of carfilzomib therapy in heavily pretreated patients with relapsed and refractory MM, including a significant proportion of patients previously treated with bortezomib (PX-171-003-A1, NCT00511238), further demonstrate the efficacy of carfilzomib in this population (Siegel et al, 2010; Siegel et al 2011c). Ongoing studies are investigating the use of carfilzomib in combination with other agents and its use in patients with newly diagnosed MM.
Fig 2. Time to Progression.
Median time to progression = 4.6 months (95% CI 1.9–11.1).
Acknowledgments
The authors would like to thank all of the patients and their families who contributed to this study. Thanks also go to the staff from the participating study sites and all of the participating research nurses and data coordinators. The authors also acknowledge the statistical support of Sunhee Kwon Ro (Onyx Pharmaceuticals, Inc.), the safety analysis support of Leanne M. McCulloch, PharmD (Onyx Pharmaceuticals, Inc.), and critical review of the manuscript by Thomas Renau, PhD (Onyx Pharmaceuticals, Inc.). Medical writing and editorial assistance was provided by Melissa Kirk, PhD and Brian Szente, PhD (Fishawack Communications) and funded by Onyx Pharmaceuticals. The study was supported by Onyx Pharmaceuticals, Inc. and the Multiple Myeloma Research Consortium.
Grant : NIH/NCI P30 CA16672.
Footnotes
Authors’ contributions
RV designed the research, performed research, contributed vital new reagents or analytical tools, and analysed data. DSS designed research, performed research and analysed data. SJ designed research, performed research and analysed data. AJJ performed research and analysed data. AKS designed research, performed research and analysed data. KM performed research. NB performed research. AB performed research. LAK designed research, contributed vital new reagents or analytical tools, and analysed data. SW performed research and analysed data. AFW designed research, contributed vital new reagents or analytical tools, and analysed data. MW performed research and analysed data. The investigators and representatives from Onyx Pharmaceuticals Inc. designed the study. The data were collected and analysed by medical and statistical representatives from Onyx Pharmaceuticals Inc. in conjunction with the investigators. All authors had access to the primary data and participated in writing and editing this paper. All participating institutions received support from Onyx Pharmaceuticals Inc. for the conduct of the study.
Conflicts of Interest
The authors declare the following: RV: consultancy and research funding for Onyx Pharmaceuticals Inc. DSS: consultancy, honoraria, and board of directors or advisory committee membership for Millennium and Celgene. SJ: honoraria for Millennium, Celgene, Onyx Pharmaceuticals Inc., and Merck; board of directors or advisory committee membership for Ortho Biotech, Imedex, Medicom World Wide, Optum Health Education, and PER Group. AJJ: consultancy for Ortho Biotech, Celgene, Millennium, Onyx Pharmaceuticals Inc., Bristol-Myers Squibb, and Exelixis; honoraria for Ortho Biotech, Celgene, Millennium, Bristol-Myers Squibb, and Exelixis; speakers bureau for Ortho Biotech, Celgene, and Millennium; board of directors membership for Millennium, Onyx Pharmaceuticals Inc., and Bristol-Myers Squibb; advisory committee membership for Onyx Pharmaceuticals Inc. and Bristol-Myers Squibb. AKS: consultancy and research funding for Celgene, Millennium, Novartis, Bristol-Myers Squibb, and Onyx Pharmaceuticals Inc. KM: No relevant financial relationship(s) to disclose. NB: honoraria and speakers bureau for Celgene. AB: No relevant financial relationship(s) to disclose. LAK: consultancy for VLST Biotech, Threshold, and Onyx Pharmaceuticals Inc. SW: No relevant financial relationship(s) to disclose. AFW: employed by and equity ownership in Onyx Pharmaceuticals Inc. MW: research funding for Onyx Pharmaceuticals Inc.
References
- ACS. Cancer Facts & Figures 2012. American Cancer Society Inc; Atlanta, GA, USA: 2012. [Google Scholar]
- Alsina M, Trudel S, Vallone M, Molineaux C, Kunkel L, Goy A. Phase 1 Single Agent Antitumor Activity of Twice Weekly Consecutive Day Dosing of the Proteasome Inhibitor Carfilzomib (PR-171) in Hematologic Malignancies. Blood (ASH Annual Meeting Abstracts) 2007;110:A411. [Google Scholar]
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, Muchamuel T, Bennett MK, Driessen C, Ball AJ, Kirk CJ. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17:2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. British Journal of Haematology. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017–1024. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, Guglielmelli T, Benevolo G, Callea V, Rizzo V, Cangialosi C, Musto P, De Rosa L, Liberati AM, Grasso M, Falcone AP, Evangelista A, Cavo M, Gaidano G, Boccadoro M, Palumbo A. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F, Shenk KD, Smyth MS, Sun CM, Vallone MK, Woo TM, Molineaux CJ, Bennett MK. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Rajkumar SV, Gertz MA, Fonseca R, Lacy MQ, Bergsagel PL, Kyle RA, Greipp PR, Witzig TE, Reeder CB, Lust JA, Russell SJ, Hayman SR, Roy V, Kumar S, Zeldenrust SR, Dalton RJ, Stewart AK. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17:387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- Kanayama H, Tanaka K, Aki M, Kagawa S, Miyaji H, Satoh M, Okada F, Sato S, Shimbara N, Ichihara A. Changes in expressions of proteasome and ubiquitin genes in human renal cancer cells. Cancer Res. 1991;51:6677–6685. [PubMed] [Google Scholar]
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Jiang J, Muchamuel T, Dajee M, Swinarski D, Aujay M, Bennett MK, Yang J, Lewis E, Laidig G, Molineaux CJ. The Selective Proteasome Inhibitor Carfilzomib Is Well Tolerated in Experimental Animals with Dose Intensive Administration. Blood (ASH Annual Meeting Abstracts) 2008;112:A2765. [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach JP, Richardson PG, Anderson KC. The evolution and impact of therapy in multiple myeloma. Med Oncol. 2010;27(Suppl 1):S1–6. doi: 10.1007/s12032-010-9442-2. [DOI] [PubMed] [Google Scholar]
- Martin T, Singhal SB, Vij RMW, Stewart AK, Jagganath S, Lonial S, Jakubowiak AJ, Kukreti V, Bahlis NJ, Alsina M, Chanan-Khan AA, Somlo G, Buadi F, Reu FJ, Zonder JA, Song K, Stadtmauer EA, Wong AF, Vallone M, Chang Y-L, Kauffman M, Orlowski RZ, Siegel DSD. Baseline peripheral neuropathy does not impact the efficacy and tolerability of the novel proteasome inhibitor carfilzomib (CFZ): results of a subset analysis of a phase 2 trial In patients with relapsed and refractory multiple myeloma (R/R MM) Blood (ASH Annual Meeting Abstracts) 2010;116:A3031. [Google Scholar]
- Mohty B, El-Cheikh J, Yakoub-Agha I, Moreau P, Harousseau JL, Mohty M. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica. 2010;95:311–319. doi: 10.3324/haematol.2009.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and 'retreatment' approaches in the era of novel agents. Leukemia. 2012;26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- Niesvizky R, Richardson PG, Rajkumar SV, Coleman M, Rosinol L, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Boral AL, Esseltine DL, Anderson KC, Blade J. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143:46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos K, Lee P, Singhal S, Holahan J, Tolcher A, Patnaik A, Vesole D, Rosen S, Rosen P, Bilotti E, Woo T, Lee S, Hannah A, Siegel D. PX-171-007: A phase 1b study evaluating the safety and efficacy of a 30-minute IV infusion of carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma (MM) (Abstract 0898) Haematologica (Abstracts from the 16th Annual Congress of the EHA) 2011;96:374. [Google Scholar]
- Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, Micklem DR, Ruurs P, Sylvain C, Lu Y, Shenk KD, Bennett MK. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- Plosker GL. Pegylated liposomal Doxorubicin: a review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs. 2008;68:2535–2551. doi: 10.2165/0003495-200868170-00008. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV. Multiple myeloma. Curr Probl Cancer. 2009;33:7–64. doi: 10.1016/j.currproblcancer.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Siegel D, Martin T, Wang M, Vij R, Jakubowiak AJ, Jagannath S, Lonial S, Kukreti V, Bahlis NJ, Alsina M, Chanan-Khan AA, Somlo G, Buadi F, Reu FJ, Zonder JA, Song K, Stadtmauer E, Wong AF, Vallone M, Chang Y-L, Kauffman M, Orlowski RZ, Stewart AK, Singhal SB The Multiple Myeloma Research Consortium. Results of PX-171-003-A1, an open-label, single-arm, phase 2 (ph 2) study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2010;116:A985. [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011a;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Siegel S, Kaufman J, Wang M, Martin T, Jagannath S, Niesvizky R, Reu F, Alsina M, Badros A, Gabrail N, Kukreti V, Singhal S, Le M, Kotlovker D, Bomba D, Hannah A, Vij R. A summary of safety and efficacy data achieved with long-term carfilzomib (CFZ) treatment in patients with relapsed and/or refractory multiple myeloma (R/R MM) Haemotologica. 2011b;96 Abstract 0302. [Google Scholar]
- Siegel D, Martin T, Wang M, Vij R, Lonial S, Kukreti V, Bahlis N, Alsina M, Somlo G, Buadi F, Reu F, Song K, Kunkel LA, Wong AF, Vallone M, Orlowski R, Stewart AK, Singhal S, Jagannath S, Jakubowiak A. PX-171-003-A1, an Open-label, Single-arm Phase (Ph) 2 Study of Carfilzomib (CFZ) in Patients (pts) with Relapsed and Refractory Multiple Myeloma (R/R MM): Long-term Follow-up and Subgroup Analysis. Journal of Clinical Oncology. 2011c;29 Abstract 8027. [Google Scholar]
- Singhal S, Siegel DS, Martin T, Vij R, Wang L, Jakubowiak AJ, Lonial S, Kukreti V, Zonder JA, Wong AF, McCulloch L, Badros AZ, Niesvizky R, Orlowski RZ, Stewart AK, Kotlovker D, Jagannath S. Integrated Safety From Phase 2 Studies of Monotherapy Carfilzomib in Patients with Relapsed and Refractory Multiple Myeloma (MM): An Updated Analysis. Blood (ASH Annual Meeting Abstracts) 2011;118:1876. [Google Scholar]
- van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H, Kropff M, Schey S, Avet-Loiseau H, Ludwig H, Goldschmidt H, Sonneveld P, Johnsen HE, Blade J, San-Miguel JF, Palumbo A. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37:266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Vij R, Kaufman JL, Jakubowiak AJ, Stewart AK, Jagannath S, Kukreti V, McDonagh KT, Alsina M, Bahlis NJ, Belch A, Reu FJ, Gabrail NY, Matous J, Vesole DH, Orlowski RZ, Le MH, Lee P, Wang M, MMRC T. Carfilzomib: high single agent response rate with minimal neuropathy even in high-risk patients (Abstract) Blood (ASH Annual Meeting Abstracts) 2010;116 Abstract 1938. [Google Scholar]
- Vij R, Wang M, Kaufman J, Lonial S, Jakubowiak A, Stewart A, Kukreti V, Jagannath S, McDonagh K, Alsina M, Bahlis N, Reu F, Gabrail N, Belch A, Matous J, Lee P, Rosen P, Sebag M, Vesole D, Kunkel L, Wear S, Wong A, Orlowski R, DS S. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naïve patients with relapsed and/or refractory multiple myeloma. Blood. 2012 doi: 10.1182/blood-2012-03-414359. Epub May 3, 2012; DOI 0.1182/blood-2012-03414359. [DOI] [PMC free article] [PubMed] [Google Scholar]