Abstract
Introduction.
Ewing sarcoma (ES) is a rare and aggressive malignant neoplasm that mostly affects children and adolescents. Recent studies reported a gap of 20% in childhood cancer survival rates between the Northern/Western and the Eastern European countries. We aimed to analyse the survival of patients treated for ES at our institution, to evaluate its correspondence to current survival rates in the expert centres, and to assess changes in treatment outcomes over time.
Materials and methods.
A retrospective single-centre study was performed. Children under 18 years of age, diagnosed and treated for Ewing sarcoma/PNET at our institution from 2000 to 2014 were included. To assess the hypothesized improvement of treatment outcomes over time, a comparative analysis of two time periods – 2000–2007 and 2008–2014 – was carried out. Five-year overall survival (OS5y) and disease-free survival (DFS5y) were chosen as primary study end-points. Clinical and laboratory data were retrieved from patient records.
Results.
In total, 40 patients were included in the study: 24 (60%) males and 16 (40%) females. Twenty-eight children (70%) presented with local and 12 (30%) with primary metastatic disease. Over the analysed time frame, improvement in treatment outcomes was observed: DFS5y improved from 46% in 2000–2007 to 61% in 2008–2014 (p = 0.27), whereas OS5y changed minimally from 62% in 2000–2007 to 65% in 2008–2014. Increase in DFS5y was more prominent for localized disease –from 50% in 2000–2007 to 74% in 2008–2014 (p = 0.14). Prognosis of initial metastatic disease remained poor with DFS5y: 25% in 2000–2007 and 38% in 2008–2014. Patients’ median follow-up was 12.3 years (the range from 8.1 to 15.6) and 3.9 years (the range from 1.1 to 8.0) in the first and second study groups, respectively.
Conclusions.
OS5y of the entire patient cohort did not change considerably over time and remained slightly inferior compared to the best reported survival rates. There was an evident trend for improvement of DFS5y in localized disease. Survival of children with primary metastases remained poor despite slight increase in DFS5y. Implementation of international clinical trials, consolidation of multidisciplinary approach, patients’ concentration and widening of research activities could be beneficial for the treatment of children in the future.
Keywords: Ewing sarcoma, children, autologous haematopoietic stem cell transplantation, relapse
Abstract
JUINGO SARKOMOS GYDYMO REZULTATAI LIETUVOJE: VIENO GYDYMO CENTRO PATIRTIS
Santrauka
Įvadas. Juingo sarkoma (JS) yra retas piktybinis navikas, dažniausiai pasireiškiantis paauglystėje ir jauniems suaugusiems. Tarptautinių tyrimų duomenimis, onkologinėmis ligomis sergančių vaikų išgyvenamumas Šiaurės, Vakarų ir Rytų Europoje skiriasi beveik 20 %. Šio tyrimo tikslas buvo įvertinti vaikų, gydytų dėl Juingo sarkomos mūsų ligoninėje, išgyvenamumą ir palyginti jį su kitų centrų rodikliais, išanalizuoti gydymo rezultatų kitimą per tiriamąjį laikotarpį.
Tikslai ir metodai. Atlikta retrospektyvinė vieno iš dviejų Lietuvos vaikų onkologijos centrų duomenų analizė. Tyrime dalyvavo vaikai iki 18 metų, kuriems 2000–2014 m. diagnozuota ir gydyta Juingo sarkoma ar primityvus neuroektoderminis navikas. Siekiant įvertinti gydymo išeičių kitimą, pacientai buvo suskirstyti į dvi grupes priklausomai nuo gydymo laikotarpio: vaikai, kuriems Juingo sarkomos diagnozė nustatyta 2000–2007 ir 2008–2014 metais. Gydymo rezultatams įvertinti pasirinktas penkerių metų bendras išgyvenamumas (overall survival, OS5y) ir penkerių metų išgyvenamumas nesant ligos požymių (disease-free survival, DFS5y). Klinikiniai ir laboratoriniai duomenys surinkti iš pacientų ligos istorijų.
Rezultatai. Išanalizuoti 40 pacientų duomenys – 24 (60 %) berniukų ir 16 (40 %) mergaičių. Dvidešimt aštuoniems vaikams (70 %) diagnozuota lokali ligos forma, o 12 (30 %) – išplitusi liga (su atokiomis metastazėmis). Per tiriamąjį laikotarpį stebėtas gydymo rezultatų gerėjimas: DFS5y padidėjo nuo 46 % 2000–2007 iki 61 % 2008–2014 m. (p = 0,27), o OS5y pakito nedaug – nuo 62 % 2000–2007 iki 65 % 2008–2014 metais. DFS5y padidėjimas buvo didesnis sergant lokalia liga – pagerėjo nuo 50 % 2000–2007 iki 74 %. 2008–2014 metais (p = 0,14). Išplitusios ligos prognozė išliko nepalanki: DFS5y padidėjo nuo 25 % 2000–2007 iki 38 % 2008–2014 metais. Pacientų stebėsenos po gydymo mediana buvo 12,3 metai (svyravo nuo 8,1 iki 15,6 metų) ir 3,9 metų (svyravo nuo 1,1 iki 8 metų) atitinkamai pirmame ir antrame laikotarpiuose.
Išvados. Visos tiriamosios kohortos OS5y pasikeitė nereikšmingai ir išliko kiek žemesnis už geriausius skelbiamus išgyvenamumo rodiklius. Fiksuotas ryškesnis DFS5y padidėjimas lokalios ligos atveju. Kaip ir kitose šalyse vaikų su pirminėmis metastazėmis išgyvenamumas išliko mažas, nepaisant pagerėjusio DFS5y. Tarptautinių klinikinių tyrimų vykdymas, daugiadisciplininio bendradarbiavimo stiprinimas ir pacientų koncentracija galėtų dar labiau pagerinti ligonių pasveikimo rodiklius.
Raktažodžiai: Juingo sarkoma, vaikai, kraujodaros kamieninių ląstelių transplantacija, Lietuva
INTRODUCTION
Ewing sarcoma (ES) is the second most frequent bone tumour in children and young adults. Initially described by James Ewing in 1921 as bone malignancy (1), nowadays it comprises several entities of malignant soft tissue tumours that are genetically and biologically almost identical to ES. Since 2013, Ewing Sarcoma Family of Tumours (ESFT) encompasses skeletal ES, extraosseous ES, Askin’s and peripheral neuroectodermal tumour (PNET) (2).
ES can manifest as local or disseminated disease with distant metastases at the time of diagnosis. Treatment of ES is complex and includes chemotherapy, surgery, radiotherapy, and highdose chemotherapy with autologous haematopoietic stem cell transplantation (autoHSCT). Basically, the same cytotoxic agents (vincristine, ifosfamide, doxorubicin, etoposide with or without cyclophosphamide), varying in treatment intensity, are used for remission induction in Europe and North America (3). Until the 1980s, only radiation was used for local ES control, yet surgical management came into favour for local control after a number of studies reported improved outcomes (4).
Current overall survival for ES is approximately 70–74% (5). However, overall prognosis varies significantly in different patient subgroups. For instance, young adults aged 15 to 19 years have a survival rate of about 56% (6). In the case of initial metastatic disease, the survival rate is even worse and does not exceed 30%, except for isolated pulmonary metastasis that has a better clinical outcome of three-year event-free survival of up to 52% (3). Several randomized controlled trials reported some prognostic factors for ES. Presence of metastases at the time of diagnosis is the strongest predictive factor for an unfavourable outcome (7), however, in localized ES the initial tumour size or volume is recognized as the most valuable for prognosis (8). For localized tumours resected after induction chemotherapy, histologic response is an even stronger independent prognostic factor than the initial tumour size (8). Other important prognostic factors such as patient’s age, tumour location, tumour volume, metastases at presentation, serum lactate dehydrogenase level, and EWS-FLI1 fusion type have also been described (9, 10).
Despite dramatic improvement in childhood cancer survival, treatment outcomes in Eastern European countries are still inferior to those in Northern and Western Europe. Recent publications reported a considerable gap in survival rates – up to 20% – between the Northern/Western and Eastern European countries (11–13). However, EUROCARE–5 population-based study revealed that since 2000, the most significant improvement in treatment outcomes has been achieved in Eastern Europe (11). As an example of a small Eastern European country, Lithuania has put considerable efforts to improve childhood cancer survival rates. Initiation of a clinical trial NOPHO ALL-2008 has led to a significant improvement of event-free survival in patients treated for acute lymphoblastic leukaemia (14). In addition, patients’ concentration, gain of experience and adequate diagnostic and treatment facilities have dramatically improved survival of patients with acute myeloid leukaemia (15).
Evaluation of treatment outcomes of children treated for ES in Lithuania has not been performed yet. Therefore we aimed to assess the survival of children diagnosed with and treated for ES at the Centre for Paediatric Oncology and Haematology (Children’s Hospital, Affiliate of Vilnius University Hospital Santaros Klinikos), one of two paediatric oncology centres in the country.
MATERIALS AND METHODS
Study population and design
We performed a retrospective single-centre analysis of treatment outcomes in children below 18 years of age diagnosed and treated for ES at our institution from 2000 to 2014. The analysed patient cohort was retrieved from the institutional database and cross-checked with the Lithuanian Cancer Registry. The survival status and clinical data were evaluated in March 2016. The study was approved by the institutional ethics board.
The diagnosis of ESFT was based on standard histopathological criteria until 2008 and included molecular diagnostics after 2008. To assess the hypothesized improvement of outcomes, the entire cohort was split into two time periods – 2000–2007 and 2008–2014. All children up to 18 years of age at the time of diagnosis were included.
Primary study endpoints were five-year overall and disease-free survival (OS5y and DFS5y). Patient data, tumour characteristics, treatment used, and events were retrieved from patients’ records. An event was defined as a relapse, disease progression, death, or secondary cancer whatever first occurred.
Statistical analysis
For the two periods, OS5y and DFS5y were plotted using the Kaplan-Meier methodology. Calculating the probability of OS5y, the event was defined as death of any cause at any time. For the probability of DFS5y, patients were censored at the time of the first relapse or tumour progression. Statistical analysis was performed using IBM SPSS Statistics 23.0 software. p values of ≤0.05 (two-sided) were considered as indicating statistical significance.
RESULTS
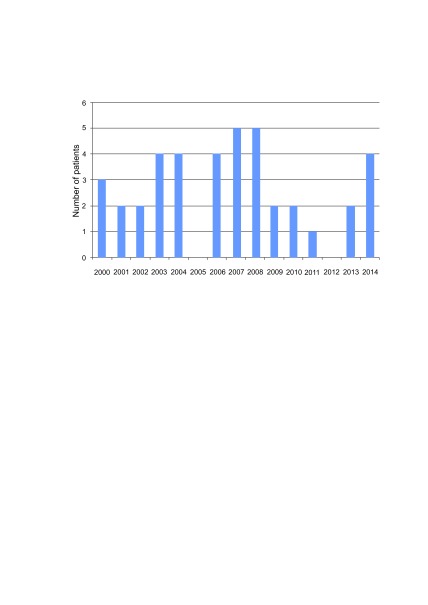
A total of 40 children were diagnosed and treated for ES from 2000 to 2014. Twenty-four (60%) children were treated during the first study period (2000‒2007) and 16 (40%) during the second period (2008‒2014). Median annual number of new cases was 3 (ranged from 0 to 5 cases per year) (Fig. 1).
Fig. 1.
Annual number of new cases (n = 40)
Patients’ characteristics
Patients’ baseline characteristics are summarized in Table 1. The median age was similar in both study groups (10.9 ± 4.5 in 2000‒2007 vs. 11.6 ± 4.9 in 2008–2014) at the time of diagnosis, three infants were affected only in the first study period. There was a male predominance during the second period (68.7% vs. 50%). Extremities were the most common primary tumour site, with much less frequent occurrence in pelvic bones or internal organs and soft tissues. Diagnostics for chromosomal mutations were performed in 56.3% of patients only in the second study period. Primary tumours were more frequently localized. Over the study period, most of the patients were treated according to EuroEwing99, although in some cases in the first study period other treatment regimens were chosen on individual basis. Remission was achieved in 41.6% of patients in 2000–2007 and in 62.5% of patients in 2008–2014. Mortality (there were no cases of treatment-related mortality) was lower in the second study period, with 75.0% currently alive patients compared to 58.0% from the first study period. More than half of the patients received high-dose chemotherapy followed by autoHSCT in both study periods, however the rate of remission after the HSCT was higher in 2008–2014 (73% vs. 31%).
Table 1.
Patients’ characteristics by to study periods (n = 40)
| 2000-2007 (n = 24) |
2008-2014 (n = 16) |
|
|---|---|---|
| Frequency (%) | 60 | 40 |
| Age at diagnosis, years (mean ± SD) | 10.9 ± 4.5 | 11.6 ± 4.9 |
| Infants (n (%)) | 3 (12.5) | 0 (0) |
| Sex (n (%)) | ||
| Girls | 12 (50) | 5 (31.3) |
| Boys | 12 (50) | 11 (68.7) |
| Duration of symptoms, months (mean ± SD) | 3.0 ± 2.7 | 3.7 ± 4.3 |
| Serum LDH concentration (mean ± SD) | 713.7 ± 710.6 | 383.1 ± 317.7 |
| Primary tumour site (n (%)) | ||
| Pelvis | 0 (0) | 1 (6.3) |
| Axial skeleton | 7 (29.2) | 4 (25) |
| Extremities | 15 (62.5) | 9 (56.2) |
| Internal organs and soft tissues | 2 (8.3) | 2 (12.5) |
| Cytogenetics (n (%)) | ||
| EWSR1/FLI1 | n.a. | 9 (56.3) |
| Dissemination at the time of diagnosis (n (%)) | ||
| Metastatic disease | 8 (33.3) | 4 (25) |
| Local disease | 16 (66.7) | 12 (75) |
| Treatment protocol (n (%)) | ||
| EuroEwing99 | 18 (75) | 16 (100) |
| VAC | 1 (4) | 0 |
| ET-2 | 1 (4) | 0 |
| IESS I | 1 (4) | 0 |
| IESS II | 1 (4) | 0 |
| NB90 | 1 (4) | 0 |
| Refused treatment | 1 (4) | 0 |
| HSCT (n (%)) | ||
| Performed | 14 (58.3) | 9 (56.3) |
| Not performed | 10 (41.7) | 7 (43.7) |
| Continuous complete remission (n (%)) | ||
| Alive in remission | 14 (58.3) | 12 (75) |
| Dead | 10 (41.7) | 4 (25) |
| Follow-up (years from diagnosis) | ||
| Median [min-max] | 12.3 (8.1–15.6) | 3.9 (1.1–8.0) |
n.a. – not assessed
Treatment outcomes
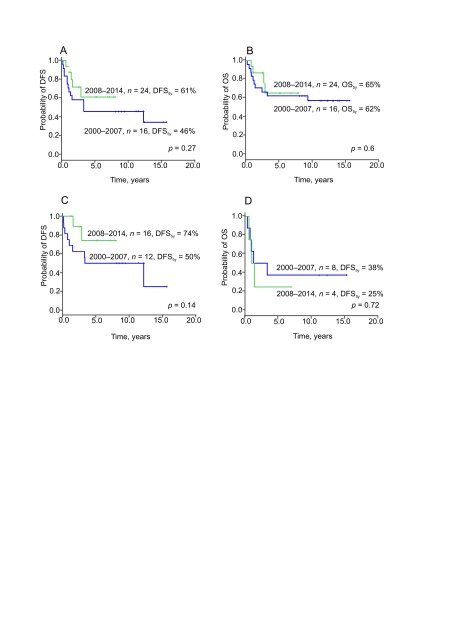
Improvement in treatment outcomes of ES was achieved over the study period (Fig. 2): DFS5y improved from 46% in 2000–2007 to 61% in 2008–2014 (p = 0.27, Fig. 2A) whereas OS5y did not change markedly: 62% in 2000–2007 and 65% in 2008–2014 (p = 0.60, Fig. 2B). Improvement in DFS5y was more prominent for localized disease: it improved from 50% in 2000–2007 to 74% in 2008–2014 (p = 0.14, Fig. 2C). Prognosis of initial metastatic disease remained poor with DFS5y of 25% in 2000–2007 and 38% in 2008–2014 (p = 0.72, Fig. 2D). Over the analysed period, no patient died due to treatment-related toxicity. One child developed a secondary carcinoma of oesophagus 12 years after the initial diagnosis that was attributed to the radiotherapy in the sternal region. Median follow-up was 12.3 years (range from 8.1 to 15.6 years) and 3.9 years (range from 1.1 to 8.0 years) in the first and second study groups, respectively.
Fig. 2.
Disease-free survival (all patients, n = 40) (A), overall survival (all patients, n = 40) (B), DFS (localised disease, n = 28) (C), DFS (metastatic disease, n = 12) (D)
High-dose chemotherapy and autologous HSCT
Twenty-three (57.5%) out of 40 enrolled patients received high-dose chemotherapy with autologous stem cell rescue. Characteristics of the stem cell recipients are summarized in Table 2. The median age at the time of transplant differed notably in the earlier and later study periods (4.4 vs. 10.6 years, respectively). In the second study period, higher proportions of patients received high-dose chemotherapy due to a poor response to chemotherapy in primary local disease as compared to the first period (55.6% vs. 35.7%). In the first study period, four patients were in complete remission and three had stable disease prior to high-dose chemotherapy, compared to five out of nine in complete remission and one out of nine with stable disease in the second study period. The preparative regimen was based on busulfan/melphalan in all cases in 2000–2007 and in five cases in 2008–2014. The remaining four children in the second study period received the combination of treosulfan and melphalan.
Table 2.
Characteristics of transplanted patients (n = 23)
| 2000-2007 (n = 14) |
2008-2014 (n = 9) |
|
|---|---|---|
| Frequency (%) | 61 | 39 |
| Years at HSCT (median ± SD) | 4.4 ± 1.9 | 10.6 ± 2.6 |
| Indications for high-dose chemotherapy (n (%)) | ||
| Poor response to chemotherapy in primary local disease | 5 (35.7) | 5 (55.6) |
| Primary metastatic disease | 6 (42.9) | 3 (33.3) |
| Relapse | 3 (21.4) | 1 (11.1) |
| Remission before HSCT (n (%)) | ||
| Complete remission | 4 (28.6) | 5 (55.5) |
| Stable disease/partial remission | 10 (77.4) | 4 (44.5) |
| Preparative regimen (n (%)) | ||
| Busulfan/Melphalan | 14 (100) | 5 (55) |
| Treosulfan/Melphalan | 0 (0) | 4 (45) |
| Outcome after HSCT (n (%)) | ||
| Remission | 3 (21.4) | 4 (44.5) |
| Progression | 5 (35.7) | 3 (33.3) |
| Relapse | 6 (42.9) | 2 (22.2) |
| Continuous complete remission (n (%)) | ||
| Alive in remission | 7 (50) | 7 (77.8) |
| Dead | 7 (50) | 2 (22.2) |
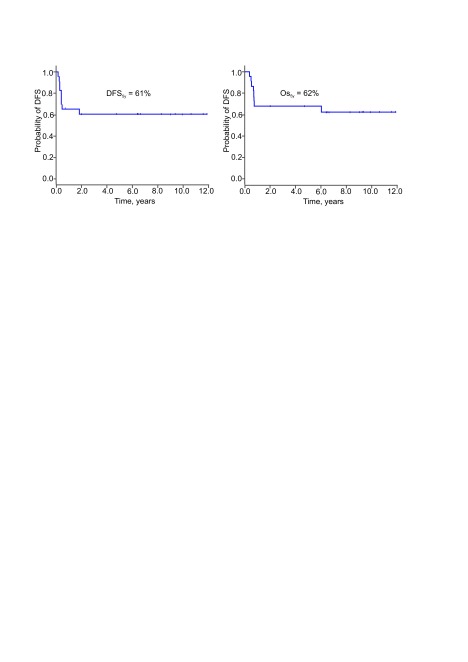
The main adverse event that compromised the outcomes following high-dose chemotherapy and autologous HSCT was a relapse – median time to relapse was 5 months (range 2–22 months, the 25th and the 75th percentile were 3.4 and 5.4 months, respectively). There was no death due to transplant regimen-associated toxicity. This translated into identical post-transplant DFS5y and OS5y (61% and 62%, respectively, Fig. 3).
Fig. 3.
Disease-free survival (A) and overall survival (B) following autologous haematopoietic stem cell transplantation (all patients, n = 23)
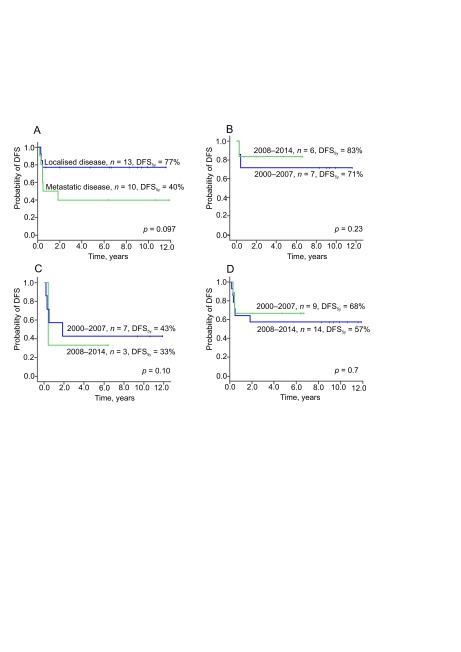
As for the entire cohort, in transplant recipients, major improvement in DFS5y and OS5y were notable for localized disease (improved from 40% to 77%, p = 0.097 and from 71% to 83%, p = 0.23, respectively, Fig. 4A and B) and less prominent if initial metastases were present (improved from 33% to 43%, p = 0.10 and from 57% to 68%, p = 0.7, respectively, Fig. 4C and D).
Fig. 4.
Disease-free survival following autologous haematopietic stem cell transplantation: by disease stage (n = 23) (A), for localised disease (n = 10) (B) for metastatic disease (n = 10) (C), by time period for all recipients (n = 23) (D)
DISCUSSION
We performed a retrospective analysis of a diagnostic and treatment approach with the aim to assess survival of Lithuanian children with ES by evaluating treatment outcomes during two periods. Our data demonstrated an improved DFS5y from 46% in 2000–2007 to 61% in 2008–2014. None of the 40 included children died from treatment-associated toxicity, which reflects sufficient experience of medical staff despite the small number of patients. Our previous research on acute myeloid leukaemia, one of the most challenging paediatric malignancy with regard to treatment-related toxicity, also demonstrated sufficient knowledge and experience accumulated at our institution for successful management of highly aggressive paediatric tumours (15).
The most prominent improvement in DFS5y (50% to 74%) was observed in localized disease. One of the reasons that could have contributed to better survival in local disease was the quality of local control, namely, the extent of surgical removal. Unfortunately, the retrospective nature of our study did not allow us to retrieve reliable data regarding surgery of the patients treated during the first study period. Therefore we can only speculate on the improved quality of the surgical approach. Available publications also support this consideration: local therapy remains one of the main milestones in the management of paediatric bone tumours in general (16), while radical surgery was demonstrated to have a major impact on improved survival in patients with chest wall ES in particular (17).
Another important issue that could have had a beneficial impact on the treatment outcomes in local tumours was a stricter pathological review that resulted in a more precise selection of patients with poor response to induction who benefited from treatment intensification with high-dose chemotherapy and autologous HSCT. This could explain a higher proportion of this indication to autoHSCT among children treated during the later study period as compared to the earlier one. On the other hand, improved pathological review could have resulted in proper administration of radiotherapy that several patients could have erroneously omitted in the earlier period leading to the subsequent relapse and death. The aforementioned more aggressive local approach translated into 74% DFS5y in local disease, which is in range with the survival rates of 65–75% reported by other groups (3, 17, 18).
In contrast to our expectations, OS5y in both study periods remained almost identical (62% and 65%). This could reflect a beneficial effect of highdose chemotherapy and autoHSCT in the case of relapse or disease progression in patients undertreated during primary therapy in 2000–2007. As for primary metastatic disease, the DFS5y increased over time insignificantly, from 25% to 38%. Poor survival in patients with initially disseminated disease is in consistence with the data of other research groups that reported overall survival in the range of 30%–50% (3). Management of primary disseminated disease remains the main challenge in ES demonstrating that current treatment approaches are not sufficient to control disseminated malignancy.
Since the 1970s, improvement in ES treatment closely depended on national and international clinical trials conducted by cooperative groups. A collaborative approach and data accumulation allowed the development of chemotherapy guidelines and the definition of a risk group, thus application of most appropriate patient care that is crucial to ensure successful treatment and quality of survival (10, 19). In 2017, the international EWING2008 clinical trial was launched at out institution and we expect to improve the survival of children with ES in the long run.
The improvement demonstrated in our study became possible due to closer collaboration between paediatric oncologists, surgeons, radiologists, pathologists, radiotherapists that, along with advances in diagnostic imaging, surgery and radiotherapy facilities, contributed to the more appropriate control of local disease. Since regular multidisciplinary tumour board meetings of different specialists have been introduced in accordance with the recommendations of good clinical practice, common discussion proved to play an important role in increasing survival rates of childhood cancer by enabling more precise diagnoses, disease staging, and common treatment decisions (20–23).
Our study had several limitations. First, being retrospective it was limited to the data quality available in the retrieved patients’ records. Second, this was not a population-based study as we analysed treatment rates of children treated only in one of the two paediatric oncology centres in the country. However, we estimated that the analysed cohort represented more than a half of children affected by ES in Lithuania over the study period as in the 2000–2007 period children with most solid tumours other that central nervous system malignancies were concentrated at our institution. During the 2008–2014 period, paediatric solid tumours became increasingly divided between two paediatric oncology centres. The beneficial effect of patient concentration in a small country was demonstrated in our studies with childhood acute lymphoblastic and myeloid leukaemia enabling to achieve survival rates comparable to the numbers reported by research-intensive institutions (14, 15). Third, the small number of children corresponding to the decreasing population (the number of inhabitants in Lithuania in 2000–2014 decreased from 3.51 to 2.94 million, (24)) did not allow us to reach any statistically significant conclusions. Despite these limitations, this is the first attempt in the analysis of treatment outcomes in children treated for ES in our country.
In summary, our study demonstrated that the outcomes of patients with ES treated at our institution are comparable, although slightly inferior to the currently reported survival rates. Participation in international clinical trials, regular multidisciplinary tumour board meetings, and widening of research on paediatric solid tumours are necessary to improve treatment quality for patients with ES. Concentration of patients with such rare diseases as ES and implementation of shared care principles defined by the European Standards of Care for Children with Cancer (25) could maximize the survival rate.
Gabrielius Jakutis, Lina Ragelienė, Jelena Rascon
References
- Ewing J. Classics in oncology. Diffuse endothelioma of bone. James Ewing. Proceedings of the New York Pathological Society, 1921. CA Cancer J Clin. 1972; 22(22): 95–8. [DOI] [PubMed] [Google Scholar]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014; 46(46): 95–104. [DOI] [PubMed] [Google Scholar]
- Gaspar N Hawkins DS Dirksen U Lewis IJ Ferrari S Le Deley MC et al. . Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol. 2015; 33(33): 3036–46. [DOI] [PubMed] [Google Scholar]
- Donati D Yin J Di Bella C Colangeli M Bacci G Ferrari S et al. . Local and distant control in non-metastatic pelvic Ewing’s sarcoma patients. J Surg Oncol. 2007; 96(96): 19–25. [DOI] [PubMed] [Google Scholar]
- www.curesearch.org [Google Scholar]
- Grevener K Haveman LM Ranft A van den Berg H Jung S Ladenstein R et al. . Management and outcome of ewing sarcoma of the head and neck. Pediatr Blood Cancer. 2016; 63(63): 604–10. [DOI] [PubMed] [Google Scholar]
- Ladenstein R Potschger U Le Deley MC Whelan J Paulussen M Oberlin O et al. . Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010; 28(28): 3284–91. [DOI] [PubMed] [Google Scholar]
- Oberlin O Deley MC Bui BN Gentet JC Philip T Terrier P et al. . Prognostic factors in localized Ewing’s tumours and peripheral neuroectodermal tumours: the third study of the French Society of Paediatric Oncology (EW88 study). Br J Cancer. 2001; 85(85): 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterill SJ Ahrens S Paulussen M Jurgens HF Voute PA Gadner H et al. . Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000; 18(18): 3108–14. [DOI] [PubMed] [Google Scholar]
- Takenaka S Naka N Obata H Joyama S Hamada K Imura Y et al. . Treatment outcomes of Japanese patients with Ewing sarcoma: differences between skeletal and extraskeletal Ewing sarcoma. Jpn J Clin Oncol. 2016; 46(46): 522–8. [DOI] [PubMed] [Google Scholar]
- Gatta G Botta L Rossi S Aareleid T Bielska-Lasota M Clavel J et al. . Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5–a population-based study. The Lancet Oncology. 2014; 15(15): 35–47. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K Pieters R Reaman GH Hjorth L Downie P Calaminus G et al. . Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol. 2013; 14(14): e95–e103. [DOI] [PubMed] [Google Scholar]
- Gatta G Peris-Bonet R Visser O Stiller C Marcos-Gragera R Sanchez MJ et al. . Geographical variability in survival of European children with central nervous system tumours. European journal of cancer. 2017; 82: 137–48. [DOI] [PubMed] [Google Scholar]
- Vaitkeviciene G, Matuzeviciene R, Stoskus M, Zvirblis T, Rageliene L, Schmiegelow K. Cure rates of childhood acute lymphoblastic leukemia in Lithuania and the benefit of joining international treatment protocol. Medicina. 2014; 50(50): 28–36. [DOI] [PubMed] [Google Scholar]
- Kairiene I, Pasauliene R, Lipunova N, Vaitkeviciene G, Rageliene L, Rascon J. Improved outcome of childhood acute myeloid leukemia in an Eastern European country: Lithuanian experience. European Journal of Pediatrics. 2017. [DOI] [PubMed] [Google Scholar]
- Bolling T, Hardes J, Dirksen U. Management of bone tumours in paediatric oncology. Clin Oncol (R Coll Radiol). 2013; 25(25): 19–26. [DOI] [PubMed] [Google Scholar]
- Bedetti B Wiebe K Ranft A Aebert H Schmidt J Jurgens H et al. . Local control in Ewing sarcoma of the chest wall: results of the EURO-EWING 99 trial. Ann Surg Oncol. 2015; 22(22): 2853–9. [DOI] [PubMed] [Google Scholar]
- Froeb D Ranft A Boelling T Paulussen M Klco-Brosius S Jurgens H et al. . Ewing sarcoma of the hand or foot. Klin Padiatr. 2012; 224(224): 348–52. [DOI] [PubMed] [Google Scholar]
- Bolling T Braun-Munzinger G Burdach S Calaminus G Craft A Delattre O et al. . Development of curative therapies for Ewing sarcomas by interdisciplinary cooperative groups in Europe. Klin Padiatr. 2015; 227(227): 108–15. [DOI] [PubMed] [Google Scholar]
- Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2010; 102(102): 125–34. [DOI] [PubMed] [Google Scholar]
- Brar SS, Hong NL, Wright FC. Multidisciplinary cancer care: does it improve outcomes? J Surg Oncol. 2014; 110(110): 494–9. [DOI] [PubMed] [Google Scholar]
- Berlanga P Segura V Juan Ribelles A Sanchez de Toledo P Acha T Castel V et al. . Paediatric tumour boards in Spain: a national survey. Clin Transl Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- Wright FC, Lookhong N, Urbach D, Davis D, Mc Leod RS, Gagliardi AR. Multidisciplinary cancer conferences: identifying opportunities to promote implementation. Ann Surg Oncol. 2009; 16(16): 2731–7. [DOI] [PubMed] [Google Scholar]
- www.stat.gov [Google Scholar]
- www.siope.eu [Google Scholar]