Abstract
Background/Objectives
A major risk factor of type 2 diabetes mellitus (T2DM) is a positive family history of diabetes. First degree relatives (FDR) of patients with T2DM are more insulin resistant and are reported to have larger abdominal subcutaneous adipocytes than adults without a family history. Our objectives were to assess whether a family history of T2DM is associated with larger abdominal adipocytes independent of age, sex, and abdominal subcutaneous fat and to assess whether FDR of T2DM is also independently related to femoral adipocyte size, as well as visceral fat and fasting plasma triglyceride (TG) concentrations.
Methods
We extracted adipocyte size, body composition, plasma TG and demographic data of non-diabetic research participants of previous studies conducted in our laboratory. We ascertained the family history of T2DM from the electronic medical records. Multivariate regression analysis was used to assess whether FDR of T2DM are more likely to have other risk factors after adjusting for known covariates.
Results
Of 604 participants, 148 were a FDR of T2DM. Although abdominal and femoral adipocyte size was greater in FDR of T2DM than those without a family history (0.74 ± 0.33 vs 0.63 ± 0.33 µg lipid/cell, P < 0.001; 0.81 ± 0.29 vs 0.72 ± 0.33 µg lipid/cell, P=0.01, respectively), this was confounded by FDR of T2DM being older, having greater BMI’s and percent body fat. A family history of T2DM was a significant predictor of abdominal adipocyte size after adjustment for age and body fat distribution parameters in females (total R2=0.5, p < 0.0001), but not in males. A family history of T2DM was not independently predictive of femoral adipocyte size, visceral fat area or TG.
Conclusions
FDR of T2DM females have larger abdominal, but not femoral, adipocytes, even after accounting for age and body fat distribution.
Introduction
A positive family history of type 2 diabetes mellitus (T2DM) is an important risk factor for this disease (1). Adults with one or more first degree relatives (FDR) with T2DM have a 30–70% increased risk of developing the disease in later life (2, 3). Recent work indicates that FDR of T2DM patients are more insulin resistant (4) than age, sex and BMI-matched controls without a family history of the disease. The biological underpinnings of the greater risk for T2DM are only beginning to emerge, but obesity is the strongest predisposing, concomitant factor.
Adipose tissue can expand by both hypertrophy and hyperplasia of adipocytes (5). It has been reported that hypertrophic, rather than hyperplastic, obesity is related to insulin resistance (6, 7) and that a family history of T2DM is associated with larger abdominal adipocytes relative to body fat (8). Furthermore, the larger adipocytes in FDR of T2DM are associated with impaired insulin sensitivity (9, 10). However, some of these findings could be due to incomplete adjustment for confounding variables. Another risk factor for insulin resistance is excess visceral fat (11–14), which can also be genetically determined (15). Several studies have indicated that FDR of patients with T2DM have greater visceral fat compared to those without the familial association (16–19).
Another feature of insulin resistance and T2DM is hypertriglyceridemia (20). Offspring of T2DM patients have higher postprandial plasma TG concentrations than counterparts without a family history of disease (21, 22), but the determinants of fasting plasma TG concentrations in FDR of T2DM has not been well studied.
In the present study, we primarily sought to test the hypothesis that a family history of T2DM predicts adipocyte size independent of other factors that influence this variable. Furthermore, we investigated the association between the family history of T2DM, visceral fat and fasting plasma triglyceride concentrations after accounting for covariates such as age, sex and body composition parameters.
Subjects and methods
We extracted age, sex, race/ethnicity, anthropometrics, body composition parameters, and adipocyte size data, as well as fasting plasma glucose, triglycerides, and insulin concentrations of volunteers who participated in 27 IRB-approved protocols conducted between 1995 and 2015 at Mayo Clinic in Rochester, MN. If volunteers participated in more than one protocol we used data from the first study. The criteria for enrollment for all of the included studies were such that the volunteers must have been non-smokers, weight stable, and free of acute illnesses and chronic diseases. These studies systematically excluded volunteers who regularly take medications that have sympathomimetic activity, or effects on glucose-lowering, insulin-sensitizing or lipid-lowering, as well as those who have thyroid disease unless they are rendered euthyroid as documented by normal TSH. The Mayo Clinic electronic medical record (EMR) was reviewed to ascertain family history of T2DM defined as having one or more parent or sibling FDR with T2DM. All participants provided informed written consent and consented that their EMR could be accessed for research.
Body composition
Total body fat, lower body fat and fat free mass were measured from a dual-energy x-ray absorptiometry (DXA; Lunar/GE equipment, Madison, Wisconsin). Methods employed to assure body composition analysis is consistent over time and between instruments has been previously described (23).
Visceral, subcutaneous and total fat areas (cm2) were measured from a single-slice abdomen CT scan at the L2–L3 interspace using Slice-O-Matic (Tomovision, Montreal, Canada) (24).
Adipose tissue collection and analysis
Adipose tissue biopsy samples from abdominal and/or the femoral subcutaneous sites were obtained by small-needle aspiration under sterile conditions and local anesthesia before being processed for measurement of adipocyte size as previously described (25).
Assays
Fasting insulin concentrations were measured using one-step immunoenzymatic assay with the Beckman coulter Unicel DXI 800. Fasting plasma glucose (FPG) was measured by a glucose oxidase method. Plasma triglycerides were analyzed on a Roche/Hitachi Cobas c311.
Statistical analyses
All values are given as mean ± SD or median (25th, 75th quartiles). Baseline characteristics, body composition, adipocyte size, and laboratory data were compared between subjects with FDR with T2DM and those who did not have FDR with T2DM by using 2-sample t-tests for parametric testing or Wilcoxon tests for nonparametric testing. A p-value of < 0.05 was considered significant.
There was no difference in sex distribution between those with and without a family history of T2DM, but there were sex differences in adipocyte size and body fat distribution (25, 26). We performed separate multiple linear regression analyses using data from males and females to evaluate the independent contributions of family history of T2DM to the variance in adipocyte sizes, visceral fat area, and TG. When necessary these outcome variables were logarithmically transformed to meet the assumptions of linear regression analysis. Differences in baseline characteristics were controlled for in the model by forcing the following variable(s) into model as covariates: age, sex, percent body fat, lower body fat mass, subcutaneous fat area and/or visceral fat area. A variance inflation factor (VIF) was calculated to find mutually dependent predictors. We accepted variables with a VIF of < 10. Statistical analyses were performed with JMP software (version 10). There was consistent evidence from previous studies of an association between a family history of T2DM and increased adipocyte size (8–10). Therefore, our hypothesis was that a positive family history of T2DM was correlated with the enlargement of abdominal adipocyte, and one-tailed P value of less than 0.1 was considered statistically significance. Otherwise, a p-value of < 0.05 was considered significant. We estimated that we were able to able to measure the small effect size (Cohen's f2 = 0.02) in the multiple linear regression model with the use of a one-sided alpha level of 0.1 and a power of 80% with 483 subjects, suggesting that our sample size of 604 subjects was sufficient.
Results
Clinical characteristics, body composition, adipocyte size and metabolic parameters
Table 1 presents demographic data, body composition parameters and other group characteristics. Most participants were Caucasian (93%), and 4.1% were African American, 2.4% Asian and <1.0% were Native American. Twenty-five percent of our participants had a FDR with T2DM. On average, our volunteers with a family history of T2DM were older than those without a family history of T2DM. Body mass index, total fat mass, percent body fat, visceral and abdominal subcutaneous fat areas, as well as lower body fat mass was significantly greater in the group with a family history of T2DM. Abdominal and femoral adipocyte sizes were significantly greater in those with than those without family history of T2DM. Similarly, fasting glucose, insulin, and triglycerides concentrations were significantly greater in those with family history of T2DM.
Table 1.
Baseline characteristics of participants
| Variables | Total | Positive family history |
Negative family history |
P value |
|---|---|---|---|---|
| n | 604 | 148 | 456 | |
| Age (years) | 35.3 ± 10.8 | 38.6 ± 9.6 | 34.2 ± 11.0 | <0.0001 |
| Sex (male, %) | 40 | 31 | 41 | 0.28 |
| Caucasian (%) | 93 | 94 | 93 | 0.07 |
| Height (cm) | 171 ± 9 | 171 ± 10 | 171 ± 9 | 0.81 |
| Weight (kg) | 83.6 ± 21.7 | 89.9 ± 24.9 | 81.6 ± 20.2 | <0.0001 |
| BMI | 28.5 ± 7.1 | 30.6 ± 7.9 | 27.8 ± 6.7 | <0.0001 |
| FFM (kg) | 52.8 ± 11.6 | 54.5 ± 13.2 | 52.3 ± 11.0 | 0.04 |
| Fat mass (kg) | 29.3 ± 15.4 | 32.9 ± 14.9 | 28.2 ± 15.4 | 0.001 |
| Body fat (%) | 34.5 ± 11.7 | 37.1 ± 10.6 | 33.6 ± 11.9 | 0.002 |
| Lower body fat mass (kg) | 10.4 ± 5.3 | 11.4 ± 5.1 | 10.1 ± 5.3 | 0.01 |
| (n=580) | (n=144) | (n=436) | ||
| CT visceral fat area (cm2) | 66.7 | 98.2 | 61.5 | <0.0001 |
| (31.8, 134.6) | (45.1, 173) | (30.7, 117.0) | ||
| CT subcutaneous fat area (cm2) | 172.4 | 236.2 | 144.6 | 0.002 |
| (87.8, 308.5) | (137.2, 329.1) | (82.1, 293.4) | ||
| Abdominal adipocyte size (µg of lipid /cell) | 0.656 ± 0.336 | 0.743 ± 0.331 | 0.627 ± 0.333 | 0.0003 |
| (n=591) | (n=144) | (n=447) | ||
| Femoral adipocyte size (µg of lipid /cell) | 0.744 ± 0.320 | 0.809 ± 0.293 | 0.723 ± 0.326 | 0.01 |
| (n=463) | (n=111) | (n=352) | ||
| Fasting plasma glucose (mg/dL) | 93 ± 9 | 95 ± 11 | 92 ± 9 | 0.003 |
| Insulin (µu/mL) | 5.3 (3.4, 9.0) | 7.2 (3.9, 11.3) | 5.1 (3.3, 8.3) | 0.0003 |
| (n=556) | (n=136) | (n=420) | ||
| Triglycerides (mg/dL) | 90 (66, 128) | 96 (69,148) | 87 (65, 123) | 0.02 |
| (n=537) | (n=131) | (n=406) |
Data are presented as mean ± SD and median (IQR).
BMI, body mass index; CT, computed tomography; FFM, fat free mass
Variables associated with adipocyte sizes, visceral fat area, and TG
We investigated the association between adipocyte size, body fat distribution, fasting plasma triglyceride concentrations and family history of T2DM after adjusting for differences in baseline characteristics in males (Table 2) and females (Table 3).
Table 2.
Multiple linear regression analysis of adipocyte size, CT visceral fat area, and triglycerides as dependent variables for males
| Variables | Log abdominal adipocyte size |
Log femoral adipocyte size |
Log CT visceral fat area |
Log triglycerides | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Parameter Estimate |
P | Parameter Estimate |
P | Parameter Estimate |
P | Parameter Estimate |
P | |
| Family history of T2DM$ | −0.0229 | 0.46 | 0.0070 | 0.84 | 0.0395 | 0.22 | 0.0571 | 0.11 |
| Age (years) | 0.0005 | 0.70 | 0.0041 | 0.003 | 0.0069 | <0.0001 | −0.0039 | 0.01 |
| CT subcutaneous fat area (cm2) | 0.0005 | 0.01 | ||||||
| Lower body fat mass (kg) | 0.0096 | 0.13 | 0.0359 | <0.0001 | ||||
| Body fat (%) | 0.0272 | <0.0001 | ||||||
| CT visceral fat area (cm2) | 0.0008 | <0.0001 | 0.0010 | <0.0001 | ||||
| Adjusted R2 | 0.48 | <0.0001 | 0.33 | <0.0001 | 0.70 | <0.0001 | 0.18 | <0.0001 |
For Family history of T2DM: positive family history=1 and negative family history=0.
CT, computed tomography; T2DM, type 2 diabetes mellitus.
Table 3.
Multiple linear regression analysis of adipocyte size, CT visceral fat area, and triglycerides as dependent variables for females
| Variables | Log abdominal adipocyte size |
Log femoral adipocyte size |
Log CT visceral fat area |
Log triglycerides | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Parameter Estimate |
P | Parameter Estimate |
P | Parameter Estimate |
P | Parameter Estimate |
P | |
| Family history of T2DM$ | 0.0617 | 0.004 | 0.0129 | 0.53 | −0.0165 | 0.55 | 0.0147 | 0.57 |
| Age (years) | 0.0004 | 0.69 | 0.0030 | 0.001 | 0.0078 | <0.0001 | −0.0002 | 0.87 |
| CT subcutaneous fat area (cm2) | 0.0005 | <0.0001 | ||||||
| Lower body fat mass (kg) | 0.0011 | 0.69 | 0.0098 | <0.0001 | ||||
| Body fat (%) | 0.0302 | <0.0001 | ||||||
| CT visceral fat area (cm2) | 0.0002 | <0.0001 | 0.0015 | <0.0001 | ||||
| Adjusted R2 | 0.50 | <0.0001 | 0.14 | <0.0001 | 0.68 | <0.0001 | 0.18 | <0.0001 |
For Family history of T2DM: positive family history=1 and negative family history=0.
CT, computed tomography; T2DM, type 2 diabetes mellitus.
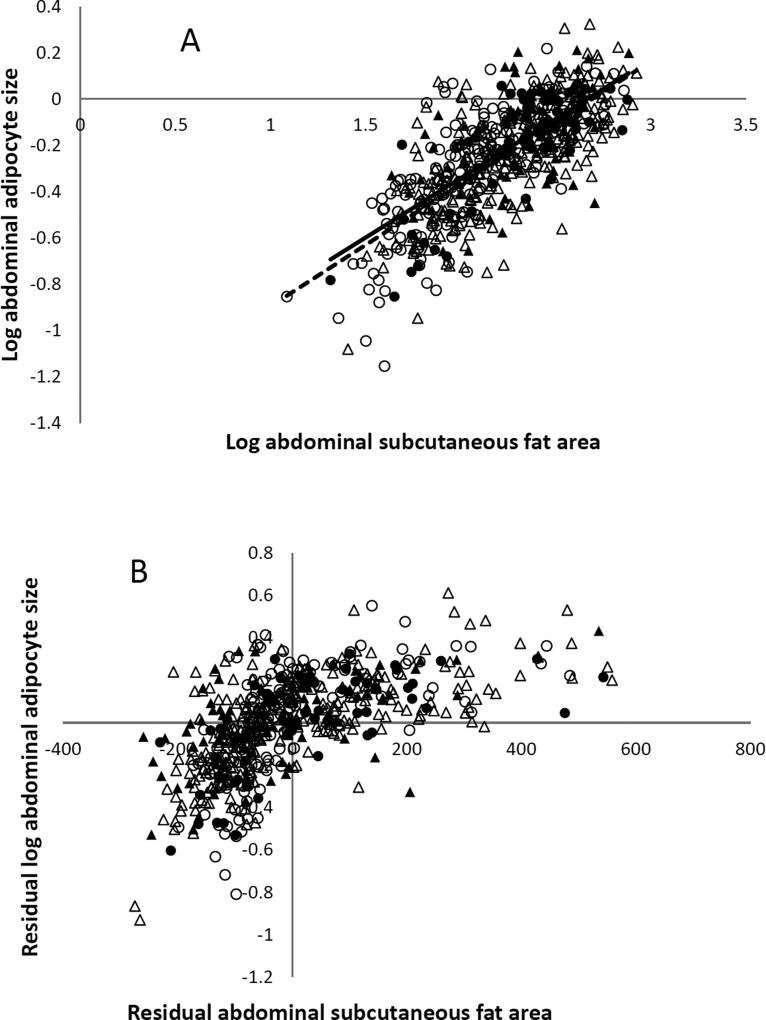
For males, the inter-individual variability in abdominal adipocyte size could be predicted by abdominal subcutaneous fat area (P=0.01) and visceral fat area, but there was no indication that a family history of T2DM was associated with fat cell size Table 2, Figures 1a and b; R2 = 0.41, P < 0.0001).
Figure 1. Abdominal adipocyte size and CT abdominal subcutaneous fat area.
A. The relation between log abdominal adipocyte size and abdominal subcutaneous area in males (circle) and females (triangle) with (fill) or without (no fill) family history of type 2 diabetes assessed by simple linear regression after grouping by positive (solid line) and negative (dash line) family history of type 2 diabetes. B. The relationship between log abdominal adipocyte size and abdominal subcutaneous area in males (circle) and females (triangle) assessed by partial regression leverage plot after adjusting for age, sex, and family history of type 2 diabetes (adjusted R2 = 0.43, P < 0.0001).
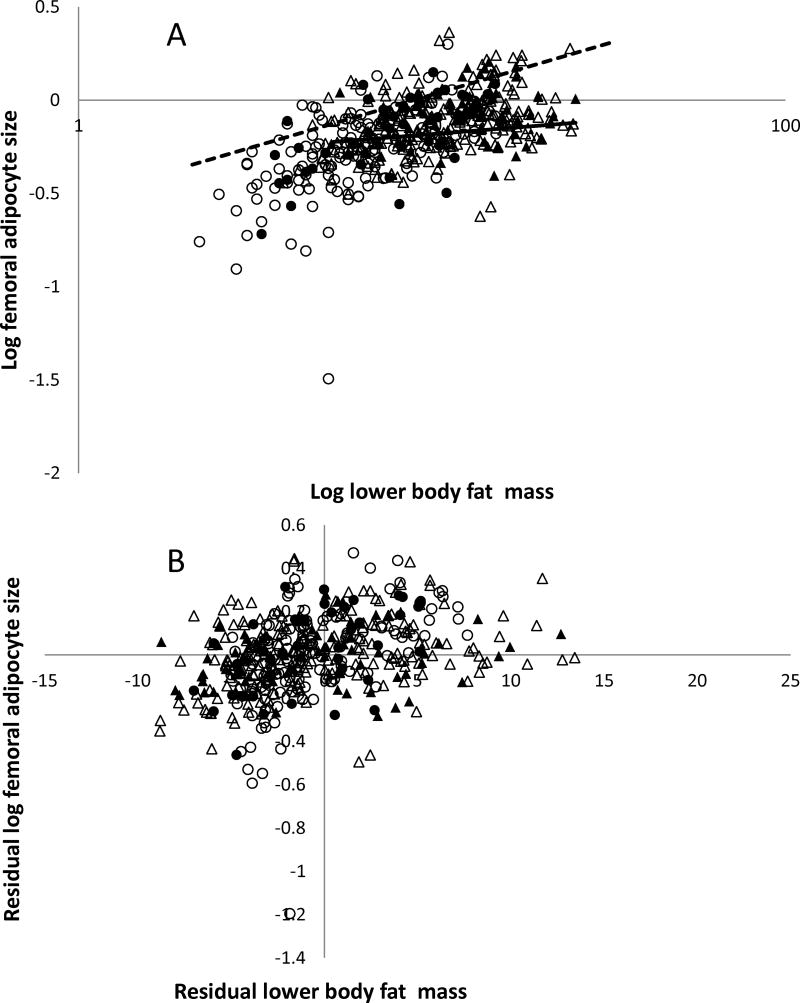
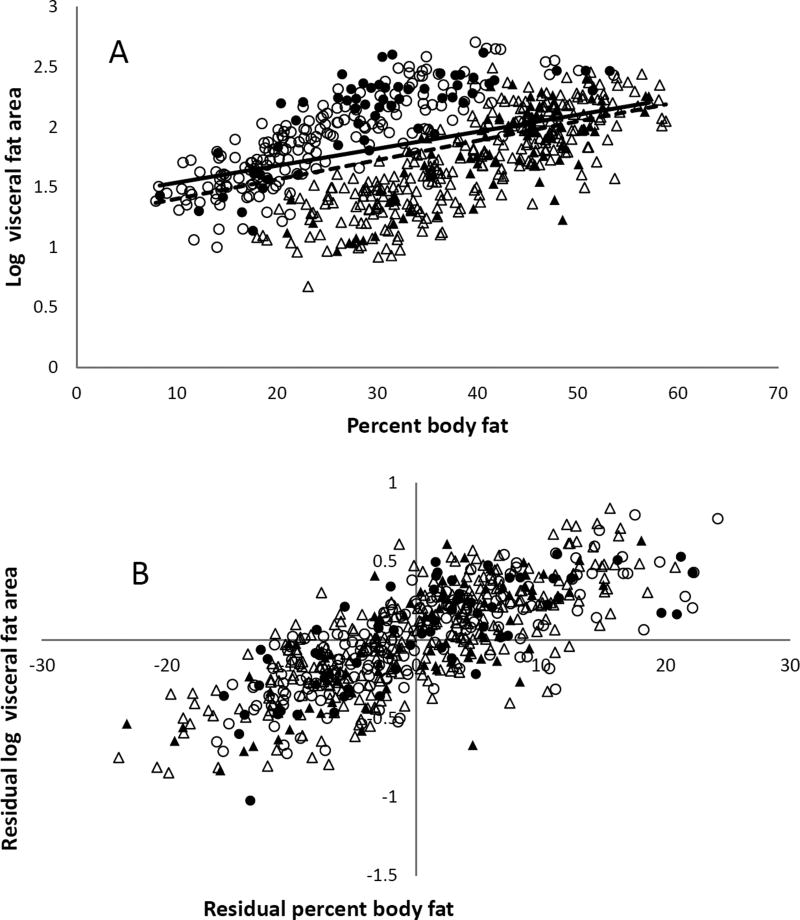
For both females and males, femoral adipocyte size was predicted by lower body fat mass and age (Tables 2 and 3, Figures 2A and 2B), but not a family history of T2DM. Visceral fat area was predicted by percent body fat and age, but not a family history of T2DM for both females and males (Tables 2 and 3, Figure 3A and 3B).
Figure 2. Femoral adipocyte size and lower body fat mass.
A. The relation between log femoral adipocyte size and lower body fat mass in males (circle) and females (triangle) with (fill) or without (no fill) family history of type 2 diabetes assessed by simple linear regression after grouping by positive (solid line) and negative (dash line) family history of type 2 diabetes. B. The relationship between log femoral adipocyte size and lower body fat mass in males (circle) and females (triangle) assessed by partial regression leverage plot after adjusting for age, sex, and family history of type 2 diabetes (adjusted R2 = 0.28, P < 0.0001).
Figure 3. Log CT visceral fat area and percent body fat.
A. The relation between log visceral fat area and percent body fat in males (circle) and females (triangle) with (fill) or without (no fill) family history of type 2 diabetes assessed by simple linear regression after grouping by positive (solid line) and negative (dash line) family history of type 2 diabetes. B. The relationship between log visceral fat area and percent body fat in males (circle) and females (triangle) assessed by partial regression leverage plot after adjusting for age, sex, and family history of type 2 diabetes (adjusted R2 = 0.70, P < 0.0001).
For females, the inter-individual variability in abdominal adipocyte size was predicted by abdominal subcutaneous fat area, visceral fat area (P < 0.0001) and the family history of T2DM (P = 0.004; Figures 1a and b; R2 = 0.45, P < 0.0001).
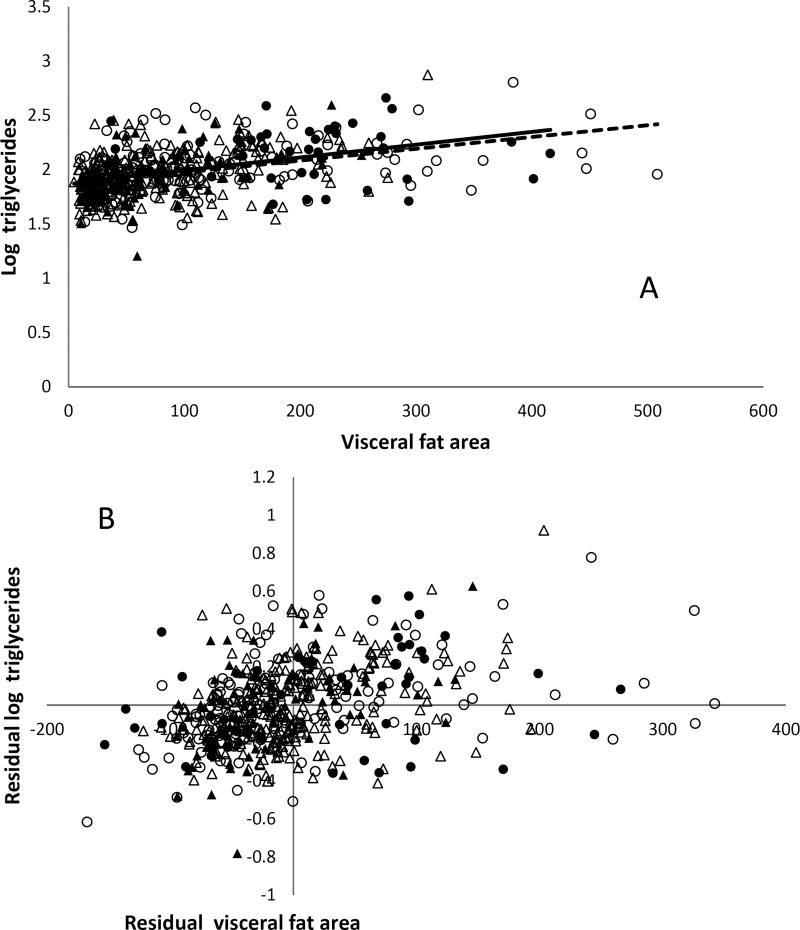
Fasting plasma triglyceride concentrations were predicted by visceral fat area (P < 0.0001) and age (P=0.01) in males (Table 2), but just visceral fat area in females (Table 3, Figure 4A and 4B).
Figure 4. Serum triglyceride concentrations and CT visceral fat area.
A. The relation between log triglycerides and visceral fat area in males (circle) and females (triangle) with (fill) or without (no fill) family history of type 2 diabetes assessed by simple linear regression after grouping by positive (solid line) and negative (dash line) family history of type 2 diabetes. B. The relationship between log triglycerides and visceral fat area in males (circle) and females (triangle) assessed by partial regression leverage plot after adjusting for age, sex, and family history of type 2 diabetes (adjusted R2 = 0.18, P < 0.0001).
Discussion
A positive family history of T2DM is a risk factor for metabolic abnormalities, including insulin resistance, greater visceral adiposity, postprandial hypertriglyceridemia and larger subcutaneous abdominal adipocytes (8–10, 21, 22). However, upon review of the literature we questioned whether these findings might be the result of incomplete statistical adjustment for confounding variables. We used data from over six hundred research participants with and without a FDR with T2DM to test for statistically independent effects of family history of T2DM after adjusting for other factors that predict adipocyte size. We found that a family history of T2DM is associated with larger abdominal adipocytes in females, but not in males. We did not find that a family history of T2DM was independently associated with femoral fat cell size, visceral fat or fasting TG concentrations.
The previous studies (8–10) indicate adults with at least one FDR with T2DM have larger abdominal subcutaneous adipocytes adjusted for body weight status (8), including fat mass or BMI (10). We judged that specific measures of abdominal subcutaneous fat (measured by CT), would be a more direct way of adjusting for abdominal fat cell size. Our findings confirmed that, after adjusting for abdominal subcutaneous area and visceral fat area, lower body fat, and age, a family history of T2DM was a good predictor of larger abdominal adipocytes in females. Of interest, in our population not only was a family history not predictive of abdominal fat cell size in males, the parameter estimate was actually negative. This suggests that the lack of association was not the slightly smaller sample size of males with a family history of T2DM. It has been suggested that enlarged abdominal adipocytes contribute to insulin resistance and increased risk of disease onset in adults with FDR with T2DM (8–10). Indeed, the increase in abdominal adipocyte size in subjects with at least two FDR with T2DM is inversely correlated with insulin sensitivity measured by a euglycemic hyperinsulinemic clamp (9).
To the best of our knowledge, this study is the first to also examine whether femoral adipocyte size is increased in those with a family history of T2DM. Although we expected that femoral adipocyte size would be increased in those with a FDR with T2DM, we found no such association. The statistical predictors of femoral adipocyte size were age and lower body fat mass as measured by DXA. In retrospect, given the epidemiological association between a predominant lower body fat distribution and greater metabolic health (28), our findings are perhaps not surprising as lower body fat appears to be a benign depot to store excess energy.
Visceral obesity is associated with insulin resistance with respect to glucose metabolism and an increased risk for development of T2DM (14, 29–31). A familial predisposition to T2DM could contribute to a preferential accumulation of visceral fat, and overall body fat mass and/or distribution (16). Healthy, but insulin-resistant FDR of T2DM were reported to have increased visceral adiposity compared with people without a family history of T2DM, despite similar BMI and overall fat mass (19). However, if the data from the groups in this study were separated by sex, the statistical significance of the difference in visceral fat area disappeared (19). Other investigators have reported that a family history of T2DM is not a predictor for visceral fat (17, 18). Our results are consistent with the findings that visceral fat is no greater in adults with FDR with T2DM after accounting for age and percent body fat.
We didn’t find an association between family history of T2DM and fasting plasma TG concentrations, consistent with previous findings (32–34). One study reported greater plasma TG concentrations in white offspring of parents with T2DM (35), however, these subjects were more obese than those in this study.
There are limitations to this study. Because the approaches to measuring adipocyte size vary from laboratory to laboratory it is potentially difficult to directly compare the results with other studies. We have carefully evaluated our fat cell size measurement approaches (25), however, and take care not to over-digest the sample (causing losses of large cells) and to count large immature adipocytes with more than one lipid droplet as single cells rather than multiple cells (25). All of our research volunteers underwent robust body composition measures that were obtained consistently. This allowed us to pool a large sample size to achieve the power to detect the significant predictors in the multiple linear regression analyses.
In summary, familial association with T2DM is not statistically associated with femoral adipocyte size, visceral fat accumulation or fasting plasma TG concentrations. However, having a FDR with T2DM is an independent predictor of abdominal adipocyte size in females, but not males, after controlling for age, abdominal subcutaneous fat area, visceral fat area, and lower body fat. This association is consistent with the recent findings that limited peripheral adipose storage capacity may be important in the pathogenesis of insulin resistance (27) and the increased risk of T2DM development in those with a family history of the disease. Our findings suggest that future studies will need to be powered to perform separate analyses for males and females.
Acknowledgments
PA, KH, PR and MDJ designed the research. PA, PR and KH performed the research. PA and KH analyzed the data and wrote the paper. Drs. Michael Jensen and Kazanna Hames are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by grant NCRR UL1 TR000135, National Institutes of Health grants, DK-45343, DK-40484, DK-50456 and BIRCWH K12HD065897. Dr. Anthanont was a postdoctoral research fellow supported by Thammasat University, Thailand.
Footnotes
Declaration of Interests
All authors have approved the final manuscript.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Kahn CR, Vicent D, Doria A. Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu Rev Med. 1996;47:509–531. doi: 10.1146/annurev.med.47.1.509. [DOI] [PubMed] [Google Scholar]
- 2.Scott RA, Langenberg C, Sharp SJ, Franks PW, Rolandsson O, Drogan D, et al. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56:60–69. doi: 10.1007/s00125-012-2715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner R, Thorand B, Osterhoff MA, Muller G, Bohm A, Meisinger C, et al. Family history of diabetes is associated with higher risk for prediabetes: a multicentre analysis from the German Center for Diabetes Research. Diabetologia. 2013;56:2176–2180. doi: 10.1007/s00125-013-3002-1. [DOI] [PubMed] [Google Scholar]
- 4.Vaag A, Henriksen JE, Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:782–788. doi: 10.1172/JCI115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch J, Knittle JL. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 1970;29:1516–1521. [PubMed] [Google Scholar]
- 6.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women: importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE [Electronic Resource] 2011;6:e18284. doi: 10.1371/journal.pone.0018284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring) 2012;20:932–938. doi: 10.1038/oby.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PloS One. 2014;9:e105262. doi: 10.1371/journal.pone.0105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Despres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 12.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard C, Rice T, Lemieux S, Despres JP, Perusse L, Rao DC. Major gene for abdominal visceral fat area in the Quebec Family Study. Int J Obes Relat Metab Disord. 1996;20:420–427. [PubMed] [Google Scholar]
- 16.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 17.Johanson EH, Jansson PA, Lonn L, Matsuzawa Y, Funahashi T, Taskinen MR, et al. Fat distribution, lipid accumulation in the liver, and exercise capacity do not explain the insulin resistance in healthy males with a family history for type 2 diabetes. J Clin Endocrinol Metab. 2003;88:4232–4238. doi: 10.1210/jc.2002-021961. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell BD, Zaccaro D, Wagenknecht LE, Scherzinger AL, Bergman RN, Haffner SM, et al. Insulin sensitivity, body fat distribution, and family diabetes history: the IRAS Family Study. Obes Res. 2004;12:831–839. doi: 10.1038/oby.2004.100. [DOI] [PubMed] [Google Scholar]
- 19.Nyholm B, Nielsen MF, Kristensen K, Nielsen S, Ostergard T, Pedersen SB, et al. Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol. 2004;150:207–214. doi: 10.1530/eje.0.1500207. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo C, Hartnett S, Hanley AJ, Rewers MJ, Wagenknecht LE, Karter AJ, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98:1622–1630. doi: 10.1210/jc.2012-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA. Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med. 1999;131:27–31. doi: 10.7326/0003-4819-131-1-199907060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Normand-Lauziere F, Frisch F, Labbe SM, Bherer P, Gagnon R, Cunnane SC, et al. Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PloS One. 2010;5:e10956. doi: 10.1371/journal.pone.0010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthanont P, Jensen MD. Does basal metabolic rate predict weight gain? Am J Clin Nutr. 2016;104:959–963. doi: 10.3945/ajcn.116.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 25.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56–63. doi: 10.1093/ajcn/87.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 29.Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 30.Pouliot M, Despres JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 31.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, et al. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989;321:337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- 33.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 34.Stewart MW, Humphriss DB, Mitcheson J, Webster J, Walker M, Laker MF. Lipoprotein composition and serum apolipoproteins in normoglycaemic first-degree relatives of non-insulin dependent diabetic patients. Atherosclerosis. 1998;139:115–121. doi: 10.1016/s0021-9150(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 35.Laws A, Stefanick ML, Reaven GM. Insulin resistance and hypertriglyceridemia in nondiabetic relatives of patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1989;69:343–347. doi: 10.1210/jcem-69-2-343. [DOI] [PubMed] [Google Scholar]