Abstract
Background
Tobacco use is prevalent among persons with alcohol abuse and dependence. Varenicline has been shown to be the most effective pharmacotherapy for smoking cessation and may decrease alcohol consumption. The purpose of this study was to evaluate the efficacy of 12 weeks of varenicline for increasing smoking abstinence rates in smokers with alcohol abuse or dependence.
Methods
Participants were eligible for enrollment if they were 18 years or older, smoked 10 or more cigarettes per day for at least 6 months, had current alcohol abuse or dependence, and were interested in quitting smoking. Participants were randomly assigned to receive 12 weeks of varenicline 1 mg twice daily or matching placebo. The primary end point was 7-day point prevalence smoking abstinence at week 12.
Results
The 7-day point prevalence smoking abstinence rate at 12 weeks was significantly higher with varenicline (n=16) than placebo (n=17) (43.8% vs 5.9%; P=.01). At 24 weeks, the 7-day point prevalence smoking abstinence rate was still significantly higher with varenicline than placebo (31.3% vs 0%; P=.02). At 12 weeks, mean (SD) drinks per drinking day was significantly lower with varenicline than placebo (5.7 [3.9] vs 9.0 [5.3] drinks; treatment effect estimate, −2.8 [90% CI, −6.6-−1.0]). Adverse events were minor and comparable to varenicline clinical trials.
Conclusions
Varenicline is safe and efficacious for increasing smoking abstinence rates in smokers with alcohol abuse or dependence. Varenicline may decrease alcohol consumption in this population of smokers.
Keywords: alcohol use disorder, nicotine, pharmacotherapy, smoking cessation
1.0 Introduction
Alcohol dependence is an important and prevalent public health problem affecting approximately 7% of the adult population of the United States (Substance Abuse and Mental Health Services Administration, 2015). Persons with alcohol abuse or dependence who actively seek treatment have long-term alcohol abstinence rates of 40% to 60%. Because of a high smoking prevalence and more frequent smoking, those with an alcohol use disorder are at increased risk for adverse health consequences from tobacco (Falk et al., 2006; Hurt et al., 1996). Smokers with alcohol abuse or dependence are less likely to quit smoking and have low abstinence rates when they do attempt to quit (Hughes and Kalman, 2006). Among smokers with alcohol abuse or dependence who are able to achieve alcohol abstinence, ongoing smoking increases the risk of relapse to alcohol use (Dawson, 2000).
Varenicline is 1 of 7 first-line medications currently available in the United States for treating tobacco dependence (Cahill et al., 2012; Clinical Practice Guideline Treating Tobacco Use, Dependence Update Panel Liaisons Staff, 2008; Jorenby et al., 2006). Varenicline is the most efficacious monotherapy for increasing smoking abstinence rates, with proven superiority over bupropion SR and nicotine-replacement therapy (Anthenelli et al., 2016; Cahill et al., 2012; Jorenby et al., 2006). In addition to helping smokers to stop smoking, varenicline has also been shown to decrease alcohol consumption in both animal models and human trials (Litten et al., 2013; Mitchell et al., 2012; Randall et al., 2015). Although some randomized trials have included participants who are in long-term stable recovery from alcohol abuse or dependence, active problem drinking has been an exclusion criterion for these early trials (Hays et al., 2011; Hays et al., 2009).
The goal of the current pilot study was to evaluate the efficacy of varenicline in smokers with current alcohol abuse or dependence. We hypothesized that 12 weeks of treatment with varenicline would be more effective than placebo for decreasing tobacco dependence rates, nicotine withdrawal symptoms, and alcohol consumption in this population.
2.0 Methods
The Mayo Clinic Institutional Review Board approved the study, which was preregistered at Clinical Trials.gov (NCT01347112) before recruitment and enrollment began. We recruited participants from the general population in the Rochester, Minnesota, area. Enrollment took place between July 2011 and April 2013. The study followed the CONSORT guidelines.
2.1 Participants
Participants were recruited through radio advertisements (74.9%), word of mouth (10.7%), Internet postings (5.9%), flyers (3.2%), and television advertisements (3.7%). Persons were eligible to participate if they: 1) were aged 18 years or older; 2) smoked on average 10 or more cigarettes per day for 6 months or more; 3) had alcohol dependence or abuse as assessed by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998) and the physician investigator; 4) were currently drinking; and 5) were interested in quitting smoking.
Persons were excluded from study participation if: 1) they had a cardiac condition (angina, myocardial infarction, or coronary angioplasty within the past 3 months), an untreated cardiac dysrhythmia, kidney disease, or cancer; 2) they had psychosis, bipolar disorder, or unstable or untreated moderate or severe depression as assessed by the Center for Epidemiologic Studies-Depression scale (Radloff, 1977); 3) they had current nonspecific suicidal thoughts as defined by the Columbia-Suicide Severity Rating Scale (Posner, 2007) or had ever made a suicide attempt; 4) they had a varenicline allergy; 5) another member of their household was already participating in the study; 6) they were undergoing current treatment with another investigational drug within the past 30 days; 7) they had untreated hypertension or a baseline blood pressure higher than 180 mm Hg systolic or 100 mm Hg diastolic; 8) they were currently using a tobacco-dependence treatment involving a drug, behavioral intervention, or both; or 9) they were concurrently using another nicotine product other than cigarettes. Women of childbearing potential or women who were pregnant, breastfeeding, or likely to become pregnant and who were not willing to use contraception during the medication phase of the trial also were excluded.
2.2 Medication
Participants were randomly assigned to varenicline or placebo for 12 weeks, with follow-up at 6 months. Pharmacy personnel dispensed study medication into containers labeled with study identification numbers. Study participants, investigators, and pharmacy staff were blinded to treatment assignment. Participants assigned to varenicline started at a dosage of 0.5 mg once daily for 3 days, which increased to 0.5 mg twice daily for days 4 to 7, and then to a target dosage of 1 mg twice daily for 11 weeks. Participants assigned to placebo received identical-appearing tablets with the same dosing instructions. Participants set a target quit day for the eighth day of the study.
2.3 Study Schedule
All interested persons called our clinical research center and completed a telephone prescreen. If they passed the telephone prescreen, they were invited to attend a one-on-one consent visit. If verified to be eligible for study participation, participants signed a written consent form and completed a baseline visit, at which time random assignment occurred, study drug was dispensed, brief counseling took place, and study assessments were completed. Participants were randomly assigned using a computer-generated sequence in a 1:1 ratio using Medidata Balance (Medidata Solutions, Inc). All participants received a personalized program consisting of brief behavioral counseling sessions (≈10 minutes) during in-person clinic follow-up visits, based on the “Smoke Free and Living It” manual (Croghan et al., 2012). Clinic study visits occurred every week for weeks 1 to 4 and every other week for weeks 6 to 12. This was followed by an in-person visit 1 week after the end of treatment (week 13), 2 telephone calls at weeks 16 and 20, and a final end-of-study in-person visit at week 24.
2.4 Measures
Sociodemographic characteristics, smoking and alcohol history information, and the Mini-International Neuropsychiatric Interview for alcohol and drug dependence (Sheehan et al., 1998) were collected at baseline. Tobacco dependence was measured with the Fagerström Test for Nicotine Dependence (Fagerstrom and Schneider, 1989; Heatherton et al., 1991). The Timeline Followback method from the consent/screen visit to the baseline visit was used to collect alcohol use (Sobell and Sobell, 1992). Vital signs, expired air CO, and alcohol levels using breath testing were collected at every study visit before any intervention. Participants also completed the Minnesota Nicotine Withdrawal Symptoms measure (Hughes, 2007). Adverse events (AEs) and concomitant medication data were collected at every study visit by querying the participants with a reference point of “since your last visit.” Data collection in this study used electronic data capture through Mayo Clinic Medidata Rave (Medidata Solutions, Inc).
2.5 Study End Points
We used the same study end points for smoking that we used in a previous study (Ebbert et al., 2014). Seven-day point prevalence smoking abstinence rate was the primary study end point, which was defined in this study as self-reported, CO-confirmed, zero tobacco (chewing or smoking) use in the preceding week. A measured expired CO level (≤8 parts per million) was used to confirm the smoking abstinence status reported by the participant. A secondary end point for smoking was 7-day point prevalence smoking abstinence at weeks 12 and 26. Prolonged smoking abstinence was defined if participants self-reported an answer of “no” to both questions: 1) Since 14 days after your target quit date, have you used any tobacco on each of 7 consecutive days? and 2) Since 14 days after your target quit date, have you used any tobacco on at least 1 day in each of 2 consecutive weeks?
A secondary end point for alcohol use was the decrease in the number of heavy drinking days (≥5 standard alcohol drinks/day for men and ≥4 for women) during the final month of the treatment phase (weeks 9 through 12). Alcohol-use outcomes, collected at every visit, were determined using the Timeline Followback method for the 28-day period before the given visit. For weeks 12 and 24, alcohol-use outcomes for persons who discontinued the study before the given visit were imputed using the data for the 28-day period before the last study visit they attended.
2.6 Statistical Analysis
Data are presented as mean (SD) for continuous variables and frequency (percentage) for nominal variables. Tobacco-use outcomes were analyzed using an intention-to-treat approach. For these analyses, any participant who missed a visit was classified as smoking for that assessment. For all tobacco abstinence outcomes, groups were compared by using the Fisher exact test, and 1-tailed P values are reported. For the primary end point, a 1-tailed P value <20 was considered sufficient to suggest that a larger phase III study should be pursued (Rubinstein et al., 2005; Ratain and Sargent, 2009; Gan et al., 2010). Alcohol-use outcomes were analyzed using analysis of covariance, with treatment (varenicline vs placebo) as the independent variable and the baseline value of the given alcohol-use variable included as a covariate. To be consistent with the analyses reported for tobacco abstinence outcomes, the results of these analyses are summarized by presenting the point estimate and 90% CI for the estimated treatment effect.
The primary end point was prolonged smoking abstinence at week 12. In previous placebo-controlled trials of varenicline, the rates of prolonged smoking abstinence at week 12 for those receiving varenicline were 44%, vs 18% with placebo, for a smoking abstinence rate ratio of 2.5 (Gonzales et al., 2006; Jorenby et al., 2006). For alcoholic smokers, we anticipated lower smoking abstinence rates. Therefore, we hypothesized that the “true” prolonged smoking abstinence rate at week 12 for active alcoholic smokers receiving placebo would be no higher than 15% (Hughes et al., 2003). We believed that a regimen (i.e., varenicline) that increases this prolonged smoking abstinence rate to 32% in this patient population would be considered promising. Using these assumptions, we determined that a sample size of 70 participants (35 per group) was required for a clinical trial to have statistical power of 80% using a 1-tailed test at an α level of 0.20 to assess whether additional studies of varenicline are warranted.
The Minnesota Nicotine Withdrawal Symptoms (MNWS) measure was completed daily. The composite nicotine withdrawal score and the single item assessing craving were analyzed separately. MNWS baseline scores for each participant were obtained using values reported before the medication phase. Changes from baseline MNWS scores were determined from data acquired during the 16 days immediately after the target quit date. The change in daily MNWS score was the dependent variable using mixed linear models. A lag-1 autoregressive covariance structure was used, accounting for clustering of repeated measurements (Ebbert et al., 2014). Because these end points were of secondary interest and not part of the decision algorithm for assessing whether further studies are warranted, the results of the analyses of nicotine withdrawal and craving are presented using point estimates and 95% CIs, with 2-tailed P values. Analyses were performed using SAS Version 9.3 (SAS Institute Inc).
3.0 Results
Of 188 people who called our clinical research center in response to advertising, 89 passed the telephone screen (Figure 1). After other exclusions and dropouts, 33 participants were randomly assigned to varenicline (n=16) or placebo (n=17), which was below our target enrollment goal of 35 participants per group. The varenicline and placebo group characteristics were comparable at baseline (Table 1). The 7-day point prevalence smoking abstinence rate at the end of treatment (12 weeks) was significantly higher in the varenicline than the placebo group (n=7 [44%] vs n=1 [6%]; P=.01). Prolonged smoking abstinence was also significantly higher with varenicline than placebo at end of treatment (n=6 [38%] vs n=1 [6%]; P=.03). At the end of study (24 weeks), the 7-day point prevalence smoking abstinence rate (n=5 [31%] vs 0%; P=.02) and prolonged smoking abstinence (n=4 [25%] vs 0%; P=.04) were both significantly higher with varenicline than with placebo.
Figure 1.
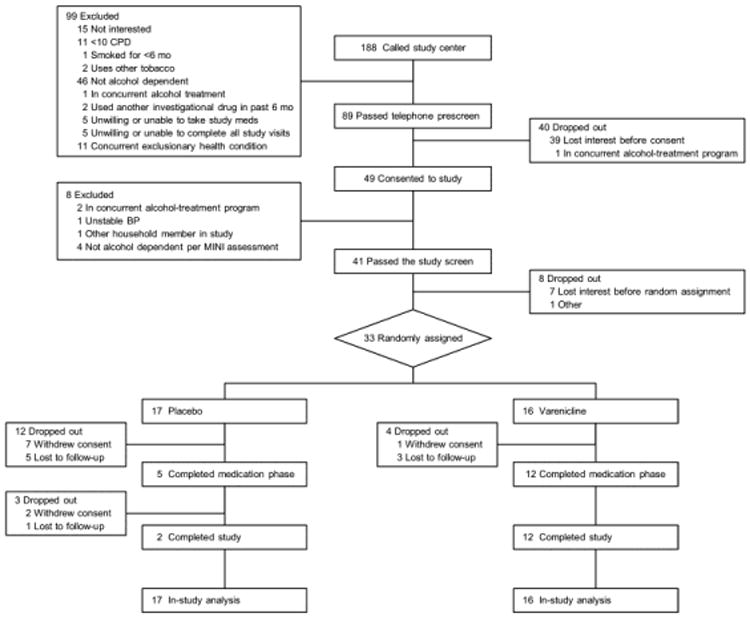
CONSORT Diagram Showing Flow of Study Participants. BP indicates blood pressure; CPD, cigarettes per day; MINI, Mini-International Neuropsychiatric Interview.
Table 1. Participant Characteristicsa.
| Characteristic | Varenicline (n=16) | Placebo (n=17) |
|---|---|---|
| Age, y | 40.2 (11.9) | 38.8 (10.4) |
| Men | 10 (63) | 11 (65) |
| Race | ||
| White, non-Hispanic | 14 (88) | 16 (94) |
| Otherb | 2 (12) | 1 (6) |
| Marital status | ||
| Never married | 6 (38) | 2 (12) |
| Separated/divorced | 6 (38) | 8 (47) |
| Married/living as married | 4 (25) | 7 (41) |
| Highest level of education | ||
| High school graduate or less | 7 (44) | 5 (29) |
| Some college | 7 (44) | 8 (47) |
| College graduate or higher | 2 (12) | 4 (24) |
| Current smoking rate, cigarettes/day | 19.1 (7.5) | 21.6 (7.3) |
| Fagerström Test for Nicotine Dependence | 6.0 (2.1) | 6.0 (2.1) |
| Ever made serious attempt to quit smoking | 13 (81) | 16 (94) |
| Other tobacco users in household | 12 (75) | 14 (82) |
Values are mean (SD) or No. of participants (%).
Other races include Asian (n=1) and black/African American (n=2).
Over the first 16 days after the target quit date, nicotine craving was significantly lower in those receiving varenicline than placebo (average difference, −1.79; 95% CI, −2.59-−0.99; P<001) (Figure 2). No significant differences were observed in nicotine withdrawal symptoms between varenicline and placebo participants (average difference, −0.13; 95% CI, −0.42-0.16; P=.41).
Figure 2.
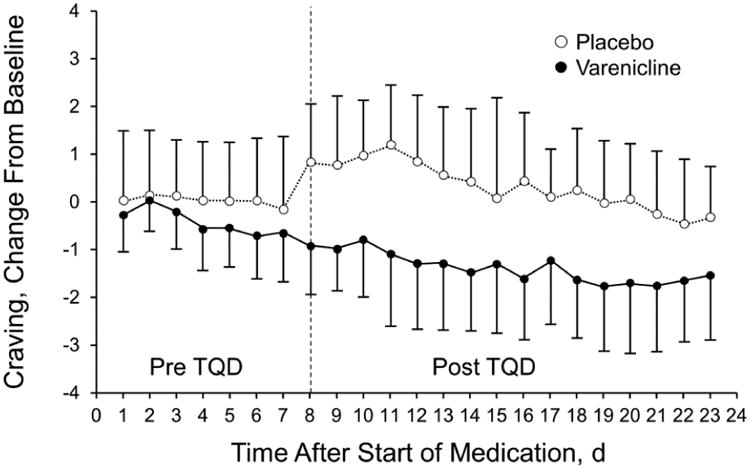
Nicotine Cravings. Mean (SD) change in nicotine cravings from baseline before and after the target quit date (TQD).
Baseline alcohol-use outcomes including average drinks per day, average drinks per drinking day, drinking days, and heavy drinking days were similar between the 2 groups (Table 2). At the end of treatment, average drinks per drinking day was significantly lower with varenicline than placebo (mean [SD], 5.7 [3.9] vs 9.0 [5.3]; treatment effect estimate, −2.8; 90% CI, −6.6-−1.0). At the end of study, the average drinks per drinking day was also significantly lower in the varenicline group than the placebo group (mean [SD], 5.0 [3.8] vs 7.6 [4.3]; treatment effect estimate, −2.3; 90% CI, −5.0-−0.4) (Table 2).
Table 2. Alcohol-Use Outcomes.
| Outcome | Groupa | Treatment Effect Estimate (90% CI) | |
|---|---|---|---|
|
| |||
| Varenicline (n=16) | Placebo (n=17) | ||
| Baseline | |||
| Average drinks/day | 5.6 (4.7) | 4.9 (2.5) | |
| Average drinks/drinking day | 8.7 (5.3) | 9.3 (4.7) | |
| Drinking days | 17.1 (7.2) | 16.7 (7.7) | |
| Heavy drinking days | 12.5 (8.7) | 12.4 (7.0) | |
| Week 12 (end of treatment) | |||
| Average drinks/day | 3.3 (3.1) | 4.0 (2.9) | −1.1 −2.5-−0.3) |
| Average drinks/drinking day | 5.7 (3.9) | 9.0 (5.3) | −2.8 (−6.6-−1.0) |
| Drinking days | 13.8 (9.7) | 14.4 (7.7) | −0.9 (−4.1-2.2) |
| Heavy drinking days | 7.9 (8.3) | 9.1 (7.2) | −1.3 (−4.7-2.1) |
| Week 24 | |||
| Average drinks/day | 2.9 (2.9) | 3.7 (3.1) | −1.1 (−2.6-0.4) |
| Average drinks/drinking day | 5.0 (3.8) | 7.6 (4.3) | −2.3 (−5.0-−0.4) |
| Drinking days | 12.9 (9.7) | 14.1 (8.3) | −1.5 (−4.8-1.8) |
| Heavy drinking days | 7.8 (8.5) | 8.8 (7.5) | −1.1 (−4.6-2.4) |
Values are mean (SD).
A total of 7 participants (5 varenicline, 2 placebo) reported 9 AEs that were considered to be possibly, probably, or definitely related to study medications. The AEs attributed to varenicline by study investigators were nausea (n=4), sleep disturbance (n=2), and vivid dreams (n=1). AEs attributed to the placebo effect were vivid dreams (n=1) and depressed mood (n=1). No serious AEs were reported.
4.0 Discussion
Among cigarette smokers with alcohol abuse or dependence, varenicline was more effective than placebo for increasing 7-day point prevalence and prolonged smoking abstinence rates at 12 and 24 weeks. Varenicline also decreased the average drinks per day at both 12 and 24 weeks compared with placebo. Varenicline was well tolerated, with mild AEs and no serious AEs, comparable to findings in larger varenicline clinical trials (Ebbert et al., 2014; Jorenby et al., 2006).
Varenicline has been demonstrated in preclinical studies to have a potential role in decreasing alcohol-use behaviors. Both nicotine and alcohol interact with nicotinic acetylcholine (nACh) receptors, which induce dopamine release in the nucleus accumbens (Nocente et al., 2013). Varenicline is a partial agonist-antagonist at the α4β2 nACh receptor subunit, which is involved in ethanol intake, ethanol withdrawal, and the acoustic startle response (Butt et al., 2004; Owens et al., 2003; Steensland et al., 2007; Tritto et al., 2001). In an animal study evaluating the effects of varenicline on dopamine response to alcohol, nicotine, or the combination, varenicline reduced dopamine levels in the nucleus accumbens in animals who were concurrently exposed to alcohol and nicotine, compared with sham treatment (Ericson et al., 2009). Varenicline has been observed to affect sensitivity to alcohol through α4β2 nACh partial agonism in rats previously exposed to alcohol. Both alcohol self-administration and seeking behavior were reduced in varenicline-treated animals, but only at higher doses (3 mg/kg) (Randall et al., 2015).
Several clinical studies have evaluated the efficacy of varenicline for decreasing alcohol-use behaviors (Fucito et al., 2011; Litten et al., 2013; McKee et al., 2009; Meszaros et al., 2013; Mitchell et al., 2012; Plebani et al., 2013). A recent systematic review evaluated the efficacy and safety of varenicline in the treatment of alcohol-use disorders (Erwin and Slaton, 2014). Seven randomized controlled trials were identified, 4 of which were conducted in alcohol-dependent patients (Fucito et al., 2011; Litten et al., 2013; Meszaros et al., 2013; Plebani et al., 2013). The largest of these studies was a multicenter, placebo-controlled, randomized clinical trial, which assigned participants with alcohol dependence to 12 weeks of varenicline (n=99) or placebo (n=101) (Litten et al., 2013). Participants smoking at least 1 cigarette in the week before enrollment (39%) averaged 11 cigarettes per day. The percentage of heavy drinking days was significantly lower with varenicline than with placebo (37.9% vs 48.4%; P=.03) (Litten et al., 2013). Varenicline was also associated with significantly fewer drinks per drinking day (5.8 vs 6.8; P=.03) and with a lower average number of cigarettes smoked (7.4 vs 11.7; P=.02) in participants who smoked. AEs were reported to be mild and similar to previous, large, smoking-cessation studies (Litten et al., 2013).
The simultaneous treatment of concomitant alcohol- and tobacco-use disorders poses a considerable clinical challenge. Cigarette smokers with past and current alcohol abuse or dependence are more tobacco dependent than smokers without this history (Hughes and Kalman, 2006; Hurt et al., 1995). Smokers with current or past alcohol abuse or dependence are less likely to quit in their lifetime (Hughes and Kalman, 2006). One of the main concerns about concurrent treatment of tobacco dependence and alcohol abuse or dependence is the potential for compromised alcohol abstinence when compared with sequential treatment of the alcohol abuse or dependence followed by treatment of the tobacco-use disorder (Joseph et al., 2004).
In a recent study of the effects of concurrent treatment for alcohol and tobacco dependence on alcohol abstinence (Cooney et al., 2015), 151 smokers with alcohol abuse or dependence receiving treatment in an intensive 3-week outpatient program were randomly assigned 2:1 to a concurrent smoking-cessation program (CSC) or a planned delayed smoking-cessation program (DSC). Participants in the CSC group (n=105) received tobacco treatment behavioral counseling, consisting of 12 sessions, and combination nicotine-replacement therapy (patch plus gum or lozenge) concurrently with the 3-week alcohol treatment program. The DSC was scheduled to start tobacco-dependence treatment approximately 3 months after the start of the alcohol-treatment program. The 7-day point prevalence smoking abstinence rate at 13 weeks was 19.0% in the CSC group, vs 0% in the DSC group. Drinking outcomes were not significantly different between DSC and CSC groups (Cooney et al., 2015). Six of the 7 daily assessed alcohol-relapse risk variables (number of drinks yesterday, urge to drink, alcohol abstinence self-efficacy, positive alcohol expectancies, alcohol abstinence motivation, negative affect, and self-control demands) were not significantly different between the 2 groups. Daily smoking abstinence was associated with improvement in alcohol-relapse risk factors. The data suggest that concurrent treatment of alcohol abuse and dependence and tobacco dependence may reduce the risk of alcohol relapse. Medications such as varenicline, which have a dual effect for decreasing tobacco and alcohol use, may have a role in this setting.
We previously conducted an open-label pilot study to obtain preliminary evidence for the efficacy of varenicline in the treatment of cigarette smokers who were in stable recovery more than 6 months (mean, 29 months) after alcohol-dependence treatment (Hays et al., 2011). A total of 32 participants who smoked a mean (SD) of 20.3 (5.0) cigarettes per day received 12 weeks of varenicline (1 mg twice daily). At end of treatment, the 7-day biochemically confirmed point prevalence smoking abstinence rate was 31%. The 12-week prolonged smoking abstinence rate was 28% (Hays et al., 2011). No serious AEs were reported and, like the present study, AEs were similar to large clinical trials involving varenicline. No alcohol relapse occurred among the participants. Varenicline may be effective for the treatment of tobacco dependence during active drinking or during stable recovery in patients with alcohol abuse and dependence.
The main limitation of the current study is the small sample size, which was underpowered because of low recruitment rates. Among the participants passing the telephone screen, less than half were eventually enrolled. In general, it was difficult to recruit smokers who were actively drinking compared with our previous study that recruited those who were in stable alcohol recovery. Before the study, power analysis was based on the target enrollment of 70 participants. With approval from the funding agency, we entered a 1-year extension to boost the number of enrolled participants. After the 1-year extension (bringing total trial length to 3 years), the decision was made to close enrollment and analyze the data for the 33 enrolled participants. Before this decision, the investigators remained blinded to treatment assignment, and no unblinding of participant data took place. Future studies evaluating dual active tobacco and alcohol dependence must take similar recruitment and retention difficulties into consideration. Other limitations include the generalizability of the results to other populations, because participants were predominantly male (64%) and white (91%).
5.0 Conclusions
Varenicline may be effective for increasing smoking abstinence rates and decreasing alcohol-use behaviors among smokers with current alcohol-use disorders.
Highlights.
Varenicline improved smoking abstinence in those with alcohol abuse or dependence.
Mean alcoholic drinks per drinking day was lower after treatment with varenicline.
Adverse events were minor and comparable to those in large trials of varenicline.
Acknowledgments
We thank the staff of the Mayo Clinic Nicotine Research Program for their assistance in the current clinical trial.
Role of the Funding Source: This current study was supported by grant R21 DA 30645 to Dr Richard D. Hurt and Pfizer IIR to Dr J. Taylor Hays.
Footnotes
Contributors: J.O. Ebbert, I.T. Croghan, R.D. Hurt, and J.T. Hays contributed to the conception and design of the research. All authors contributed to the acquisition and analysis of the data and the interpretation of the data. All authors contributed to drafting and critically revising the manuscript, agrees to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Conflict of Interest: R.T.H. reports research grants from Pfizer and the NIH. J.T.H. reports research grants from Pfizer. J.O.E. reports grants from Pfizer during the conduct of the study and grants from Takeda, the U.S. Department of Defense and the NIH outside the submitted work. R.D.H. reports a research grant from the NIH. All other others have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–20. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- Butt CM, King NM, Stitzel JA, Collins AC. Interaction of the nicotinic cholinergic system with ethanol withdrawal. J Pharmacol Exp Ther. 2004;308:591–9. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Clinical Practice Guideline Treating Tobacco Use, Dependence Update Panel Liaisons Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med. 2008;35:158–76. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Sevarino KA, Levy L, Kranitz LS, Sackler H, Cooney JL. Concurrent alcohol and tobacco treatment: Effect on daily process measures of alcohol relapse risk. J Consult Clin Psychol. 2015;83:346–58. doi: 10.1037/a0038633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croghan IT, Trautman JA, Winhusen T, Ebbert JO, Kropp FB, Schroeder DR, Hurt RD. Tobacco dependence counseling in a randomized multisite clinical trial. Contemp Clin Trials. 2012;33:576–82. doi: 10.1016/j.cct.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–49. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, Hurt RD. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: A randomized trial. JAMA. 2014;311:155–63. doi: 10.1001/jama.2013.283185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Ann Pharmacother. 2014;48:1445–55. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–82. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–71. [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan HK, Grothey A, Pond GR, Moore MJ, Siu LL, Sargent D. Randomized phase II trials: Inevitable or inadvisable? J Clin Oncol. 2010;28:2641–7. doi: 10.1200/JCO.2009.26.3343. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hays JT, Croghan IT, Schroeder DR, Ebbert JO, Hurt RD. Varenicline for tobacco dependence treatment in recovering alcohol-dependent smokers: An open-label pilot study. J Subst Abuse Treat. 2011;40:102–7. doi: 10.1016/j.jsat.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Decker PA, Croghan IT, Offord KP, Patten CA. A randomized, controlled trial of bupropion sustained-release for preventing tobacco relapse in recovering alcoholics. Nicotine Tob Res. 2009;11:859–67. doi: 10.1093/ntr/ntp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–6. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., III Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. J Stud Alcohol. 2004;65:681–91. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R NCIG Study Group. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–86. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Ajagbe TO, Batki SL. Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: A randomized, placebo-controlled pilot trial. J Clin Psychopharmacol. 2013;33:243–7. doi: 10.1097/JCP.0b013e3182870551. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocente R, Vitali M, Balducci G, Enea D, Kranzler HR, Ceccanti M. Varenicline and neuronal nicotinic acetylcholine receptors: A new approach to the treatment of co-occurring alcohol and nicotine addiction? Am J Addict. 2013;22:453–9. doi: 10.1111/j.1521-0391.2013.12037.x. [DOI] [PubMed] [Google Scholar]
- Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, Picciotto MR, Wehner JM, Collins AC. Alpha 4 beta 2 nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27:1867–75. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 2013;133:754–8. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K. Suicidality issues in clinical trials: Columbia suicide adverse event identification in FDA safety analyses 2007. [cited 2017 May 11];2007 Internet. Available from: http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4306s1-01-CU-Posner.ppt.
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015;232:2443–54. doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratain MJ, Sargent DJ. Optimising the design of phase II oncology trials: The importance of randomisation. Eur J Cancer. 2009;45:275–80. doi: 10.1016/j.ejca.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Human Press; New Jersey: 1992. pp. 41–2. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) [cited 2017 May 11];Results from the 2015 national survey on drug use and health: detailed tables. Internet. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-46b.
- Tritto T, Marley RJ, Bastidas D, Stitzel JA, Collins AC. Potential regulation of nicotine and ethanol actions by alpha4-containing nicotinic receptors. Alcohol. 2001;24:69–78. doi: 10.1016/s0741-8329(01)00135-5. [DOI] [PubMed] [Google Scholar]


