Abstract
Background
The Norwood operation is associated with high healthcare utilization, and prior studies demonstrate substantial variability in Norwood costs across centers. However, specific factors driving this cost variation are unclear. We assessed center variability in Norwood costs and underlying mechanisms in a multi-center cohort.
Methods
Clinical data from the Pediatric Heart Network Single Ventricle Reconstruction trial were linked with cost data from the Children’s Hospital Association Inpatient Essentials database. Center variation was assessed by modeling Norwood costs adjusted for baseline patient characteristics, and the relationship with complications, length of stay (LOS), and specific cost categories was examined. Those undergoing transplant or Stage II during the Norwood admission were excluded.
Results
Nine centers (332 patients) were included. Adjusted mean cost/case varied 4.6-fold across centers (range $50,559—$230,851; p<0.001). There was also variation across centers in the adjusted mean number of complications/case (2.6-fold variation) and adjusted mean LOS/case (1.9-fold variation). Differences in complications explained 63% of the cost variation across centers. After accounting for complications, differences in LOS explained 66% of the remaining cost variation. Seven specific complications were found to occur more frequently at high cost centers: pleural effusion, seizures, wound infection, thrombus, liver dysfunction, sepsis, necrotizing enterocolitis (all p<0.001). With regard to types of cost, room & board/supplies, and lab costs were the primary drivers of cost variation across centers.
Conclusions
This study identified several factors associated with center variation in Norwood costs, which may be targeted in subsequent initiatives aimed at both improving quality of care and reducing costs.
Classifications: CHD, hypoplastic left heart syndrome, Norwood operation, Health economics
In this era of rising health care costs, improved understanding of factors impacting resource utilization is increasingly important. Prior work has shown that patients with congenital heart disease account for the highest costs among all birth defects1, and that hospital costs for this population are disproportionally high when compared to other pediatric hospitalizations2. It is estimated that approximately $6 billion in inpatient costs annually are related to congenital heart disease3.
Among all types of congenital heart disease, patients undergoing the Norwood operation for hypoplastic left heart syndrome and related single right ventricle anomalies have generally been reported to account for the greatest resource utilization4. Moreover, significant variation across centers in costs related to the Norwood operation has been demonstrated5. However, the factors which influence this variation remain understudied. It is known that post-operative complications and prolonged length of stay can have a significant impact on hospital costs following congenital heart surgery on a patient level; however it is less clear how these factors, and specific complications, influence variation in costs across centers. A better understanding of these relationships may aid in informing the design of initiatives aimed at both improving patient outcomes and reducing costs of care across institutions. Therefore, the purpose of this study was to examine variation across centers in costs associated with the Norwood operation, and to evaluate the relationship of post-operative complications, length of stay (LOS), and other factors with this variation.
PATIENTS AND METHODS
Data Sources
The Single Ventricle Reconstruction (SVR) Trial (ClinicalTrials.gov number NCT00115934), conducted by the Pediatric Heart Network enrolled 555 infants across 15 centers undergoing the Norwood procedure for hypoplastic left heart syndrome or related single right ventricle anomalies from 2005–2008. Patients were randomized to receive either a modified Blalock-Taussig shunt or a right-ventricle-to-pulmonary artery shunt6.
The Inpatient Essentials database (formally known as the Case Mix database) is a large administrative database maintained by Children’s Hospital Association (CHA; Lenexa, KS). The database contains resource utilization and other patient data from 90 US children’s hospitals7. In addition to daily line item utilization, these are also categorized as lab, imaging, pharmacy, and other (supplies, clinical, room and nursing).
This study involving a retrospective analysis of de-identified data was reviewed by the Medical University of South Carolina Institutional Review Board and did not meet qualifications for human subjects research.
Linkage
Clinical data from the SVR trial were merged at the patient level with cost data from the Inpatient Essentials database for centers participating in both datasets during the trial period as previously described7. Briefly, probabilistic matching of indirect identifiers was used to link records, including center, gender, date of admission (+/− 1 day), date of discharge (+/− 1 day), and date of birth (+/− 1 day). These methods have been previously validated in the congenital heart disease population7,8.
Study Population
As previously described, all patients enrolled in the SVR trial were eligible for inclusion7. Patients undergoing Stage II palliation or heart transplant during the same hospital admission (n=27) were excluded from analysis, since this study focused on the Norwood hospitalization, and it is known that these practices can vary widely across centers. One center enrolling <10 total patients was also excluded from this analysis, as their small sample size precluded meaningful evaluation of center-level data. Of note, we did not exclude those who died during the hospitalization as our prior work has shown that the inclusion/exclusion of survivors has little impact on overall center-level cost variation in this population9.
Data Collection
Detailed clinical information was obtained from the SVR trial dataset as previously described7. All serious adverse events and other complications collected in the trial were included, which were comprised of major complications in the cardiac, vascular, respiratory, neurologic, gastrointestinal, infectious, renal, and hematologic systems10.
Data on hospital costs and payor were obtained from the Inpatient Essentials database7. Costs were estimated from charges using hospital specific cost-to-charge ratios, adjusted for geographic region using the Centers for Medicare and Medicaid Services price wage index, and inflated to 2008 dollars using the medical component of the consumer price index.
Analysis
Due to non-normal distributions, continuous variables were summarized with medians and interquartile ranges, and categorical variables were depicted as frequencies and percentages.
Center variation in costs, post-operative complication rates, and post-operative LOS was examined using generalized linear mixed effects models with a random intercept for each hospital to account for within center clustering. Continuous outcomes were log transformed prior to modeling, and estimates were back-transformed onto the original scale for ease of interpretability. As described previously7, models were adjusted for baseline patient and operative characteristics found to be associated with higher costs (p<0.1) in univariate analyses including pre-operative intubation, pre-Norwood pulmonary artery banding, pre-Norwood atrial septectomy, presence of aberrant right subclavian artery, number of pre-Norwood surgeries, number of pre-Norwood complications, payor type, use of regional cerebral perfusion, cardiopulmonary bypass time, deep hypothermic circulatory arrest time, use of ultrafiltration, and presence of open sternum. Of note, shunt type was not found to be associated with cost in univariate analysis.
From the models, adjusted center-level costs, number of post-operative complications, and post-operative LOS were determined. To understand the proportion of variation in center-level costs explained by complications and LOS, adjusted center-level costs were modeled by the number of complications, and the percent of variation explained was determined using the coefficient of determination (R-square). We then additionally adjusted center-level costs by the number of complications, and modeled this measure with adjusted post-operative length of stay.
To examine the specific types of complications which occurred more frequently at high cost centers, centers were grouped into adjusted cost tertiles based on the distribution of the data, and the frequency of complications was examined across these cost tertiles. We only focused on those complications occurring in 5% or more of our overall study cohort, as it is both complications that are relatively common and that occur more frequently at high cost centers that may be the most “high leverage” complications to target in initiatives aiming to reduce cost variation. Specific categories of cost were also assessed across centers (n=8 for which data were available). All analyses were performed using SAS version 9.4 (SAS Institute Incorporated, Cary, North Carolina). A p-value <0.05 was considered statistically significant.
Results
Study population
Overall, 9 out of 15 centers participating in the SVR trial also participated in the Inpatient Essentials database and enrolled >10 patients during the trial period. From these 9 centers, records on 332 of 350 eligible patients were able to be matched between datasets (overall linkage rate of 95%). The 18 unmatched patients were distributed across 4 sites, with a range of 0–7 unmatched patients across these sites.
Patient Characteristics
Study population characteristics are displayed in Table 1. Overall, 62% were male, 78% had a prenatal diagnosis, and the median age at surgery was 4 days. The majority (86%) had hypoplastic left heart syndrome (vs. other single right ventricle anomalies) and 4% had obstructed pulmonary venous return. In this cohort, 52% were randomized to a right ventricle to pulmonary artery shunt.
Table 1.
Study population characteristics
| Total N = 312 | |
|---|---|
| Preoperative characteristics | |
| Male gender | 193 (62%) |
| Prenatal diagnosis | 243 (78%) |
| Gestational age, weeks | 38 [37–39] |
| Birthweight, grams | 3154 [2660–3400] |
| Age at surgery, days | 4 [3–6.5] |
| Preoperative intubation | 137 (44%) |
| Anatomic Diagnosis | |
| HLHS (vs. other single RV anomaly) | 269 (86%) |
| Aortic Atresia | 204 (65%) |
| Obstructed pulmonary venous return | 11 (4%) |
| Payor group | |
| Government | 125 (40%) |
| Private | 128 (41%) |
| Other | 59 (19%) |
| Operative factors | |
| Randomized RV-PA shunt | 161 (52%) |
| Open sternum | 207 (67%) |
Data are presented as number (%) and median (interquartile range) as appropriate. HLHS = hypoplastic left heart syndrome; RV = right ventricle; PA = pulmonary artery
Variation in Norwood Costs, Complications, and Length of Stay across Centers
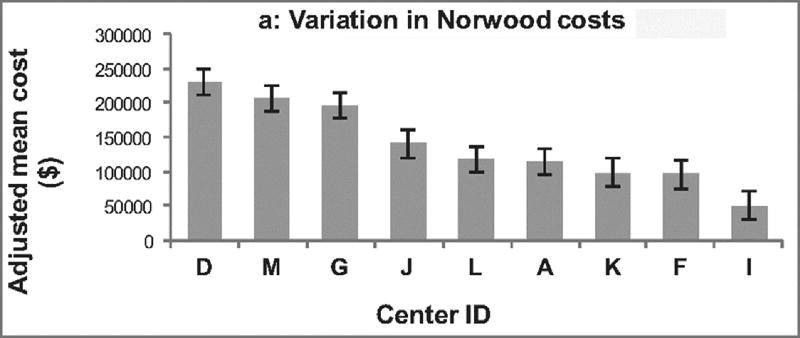
Variation in total adjusted cost/case across centers is displayed in Figure 1a. Adjusted cost/case varied by 4.6 fold across centers (range $50,559 – $230,851; p < 0.001).
Figure 1.
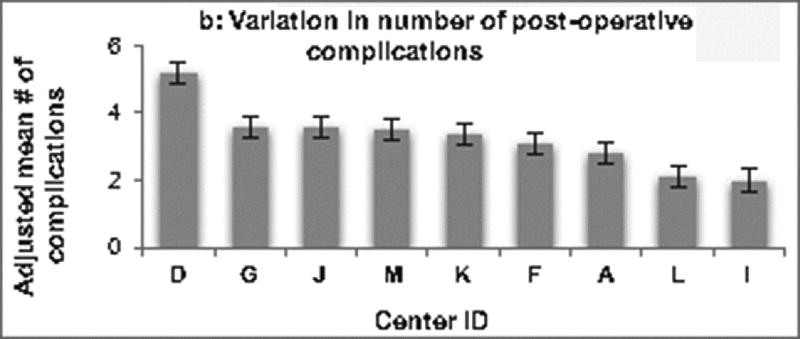
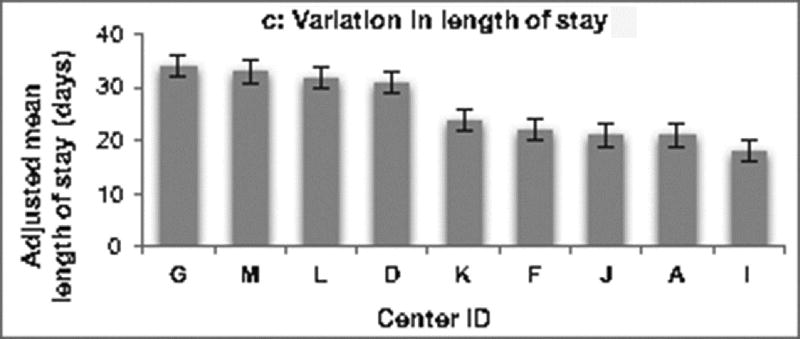
Variation across centers. Centers are displayed in descending order. (A) demonstrates variation in adjusted hospital costs across centers for the Norwood operation. Hospital costs adjusted for baseline patient characteristics varied 4.6 fold across centers (p <0.001). (B) Variation in the adjusted number of post-operative complications across centers is depicted. There was 2.6 fold variation across institutions (p = 0.004). (C) Shows the variation across centers in adjusted post-operative length of stay, which varied 1.9 fold across centers (p < 0.001).
Variation across centers in adjusted rate of complications and LOS was also examined. As displayed in Figure 1b, the adjusted number of complications per patient varied 2.6 fold across centers (range 2.0–5.2 complications per patient, p = 0.004). Figure 1c demonstrates there was also substantial variability in adjusted post-operative LOS, with 1.9 fold variation observed across centers (range 18–34 days, p=0.004).
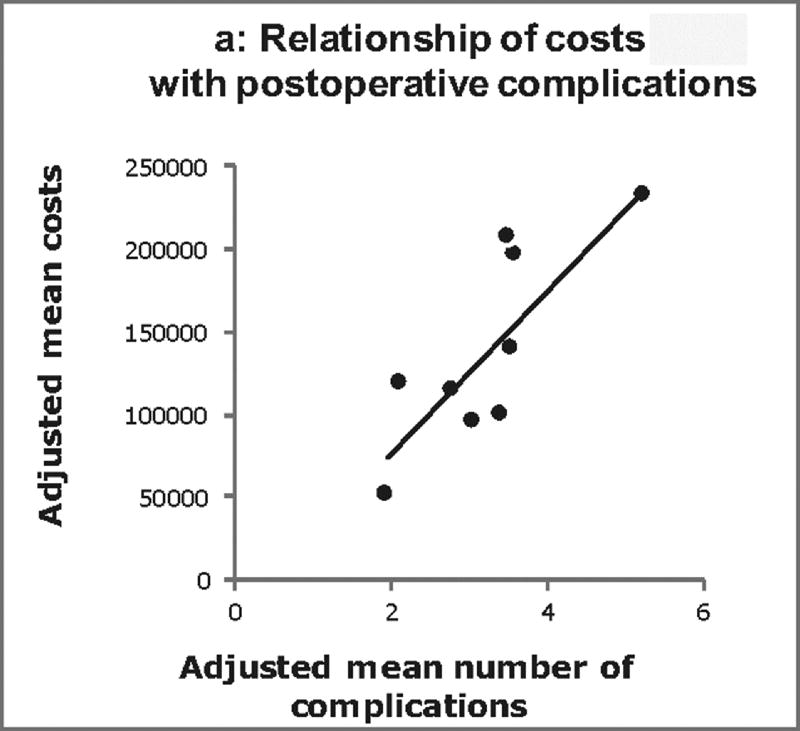
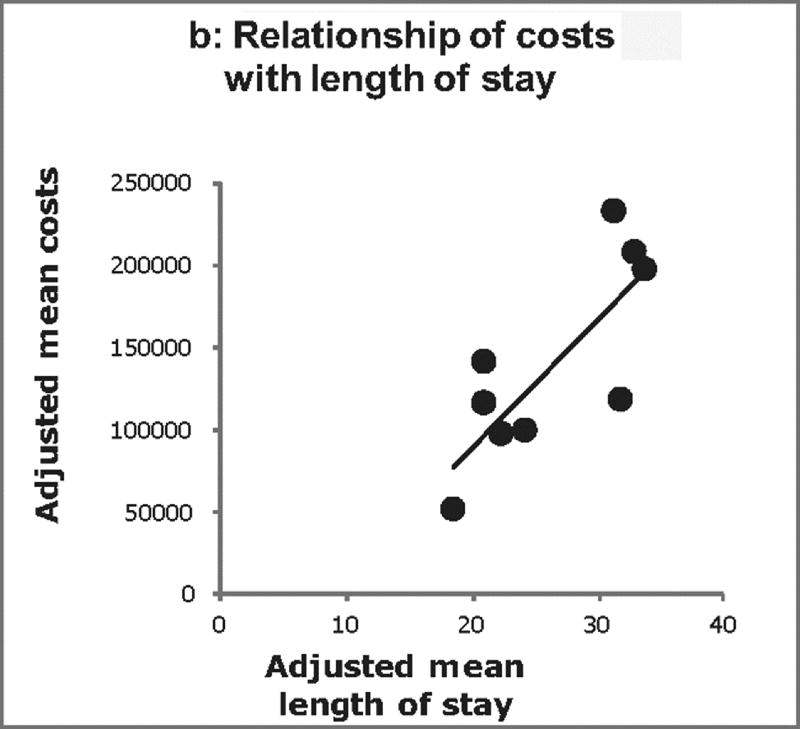
The relationship between adjusted costs, complications, and LOS across centers was further examined (Figure 2). Positive correlations were found between both center costs and complications (p<0.001; Figure 2a), and center costs and LOS (p<0.001; Figure 2b). Overall, differences in the number of complications explained 63% of the total variation in costs across centers. After accounting for complications, differences in post-operative LOS explained 66% of the residual variation in costs across centers.
Figure 2.
Center-level relationship between Norwood costs, postoperative complications, and length of stay (A) displays the relationship between adjusted Norwood costs and adjusted number of post-operative complications at the center level. Each dot represents one center. Similarly, (B) displays the relationship between adjusted Norwood costs and adjusted post-operative length of stay. There is a positive correlation between both costs and complications and costs and length of stay (p < 0.001 for both). Overall in our models, differences in the number of complications explained 63% of the total variation in costs across centers. After accounting for complications, differences in post-operative length of stay explained 66% of the residual variation in costs across centers.
Specific Complications and Cost Categories
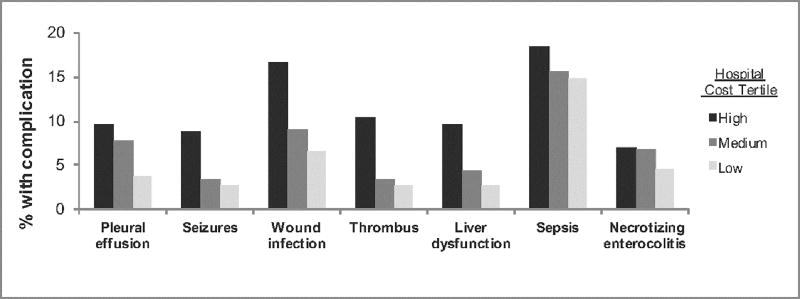
We evaluated the specific types of complications associated with higher costs. Complications occurring in 5% or more of the overall study population were examined, and centers were classified into low, medium, and high tertiles based on adjusted costs as described in the methods. Seven specific complications were found to occur significantly more frequently at high cost centers (Figure 3): pleural effusion requiring drainage, seizures, wound infection, thrombus, liver dysfunction, sepsis, and necrotizing enterocolitis (all p<0.001). Of note, extracorporeal membrane oxygenation and chronic respiratory failure were included in the group of complications which were examined across the cost tertiles; these complications did not occur more frequently in the high cost group.
Figure 3.
Specific complications associated with cost variation. Centers were categorized into low, medium, and high cost tertiles based on adjusted Norwood costs. The figure depicts the 7 complications occurring more frequently in the high cost hospitals (p<0.001 for all complications comparing hospital tertiles).
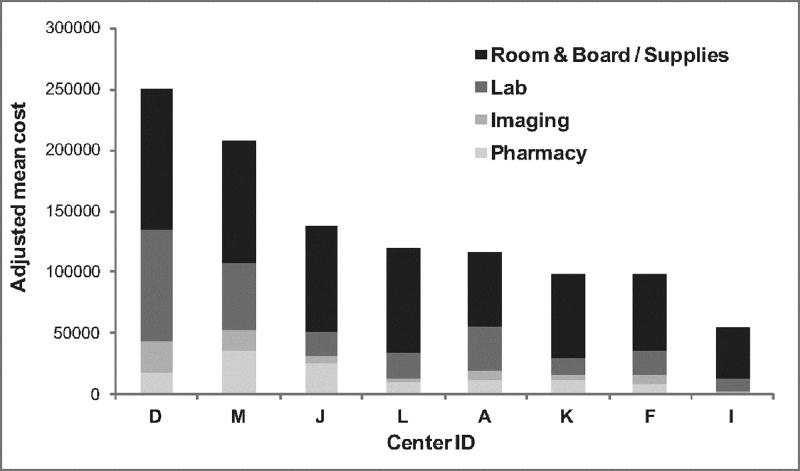
Finally, differences in cost categories were described across centers in the 8 centers with this data available. Centers with the highest costs generally had higher costs across all categories; however this was dominated primarily by costs associated with room & board/supplies, and lab costs (Figure 4).
Figure 4.
Categories of costs across centers. Centers are depicted in order of decreasing adjusted cost. Different shades illustrate the proportion of total costs comprised of standard cost categories. Centers with the highest costs generally had increased costs across all categories; however, this was dominated primarily by costs associated with room & board/supplies (black), and lab costs (dark gray).
COMMENT
This study examined variation in Norwood costs across centers. We found significant inter-institutional variation with nearly 5-fold variation in adjusted costs across centers. A high proportion of this variation was associated with differences in postoperative complication rates and LOS. Additionally, we found seven specific postoperative complications that occurred more commonly in high-cost centers, and identified that room and board and supply costs along with lab costs appeared to account for a high degree of the observed cost variation.
Methodology allowing integration of clinical trial data and administrative cost information at the patient level through matching on “indirect identifiers” was employed in this study. This technique was highly successful linking 95% of eligible records between the two databases, resulting in a unique dataset of merged clinical trial and cost data to support an analysis that would otherwise not be possible. The benefits and unique opportunities supported by better integration and sharing of data have been highlighted across several fields in medicine11,12. For example, improved mechanisms for integrating disparate sources of data and facilitating data sharing across different entities has been identified as in integral part of the Cancer Moonshot 2020 initiative12. In pediatric cardiovascular disease, this has also been recognized as a priority, and The National Heart, Lung, and Blood Institute convened a working group in 2015 to develop a common vision for an integrated network for congenital heart disease research. The Working Group concluded that a comprehensive integration of data sources in the field may both improve clinical research efficiency and support novel analyses13.
The present study utilized data linkage methodologies to better understand variation in costs of care across congenital heart centers. Despite the significant improvements in congenital heart disease outcomes over the past three decades, there is a growing body of literature demonstrating that significant variation in outcomes across centers remains9,14–16. Further, variation in both quality and costs of care has been demonstrated4,17,18. This study documents nearly five-fold variation in Norwood hospitalization costs across centers participating in the SVR trial. Given that these centers were all generally mid-to-high volume centers, this is likely an underestimate of the variation that would be seen across a broader cohort6.
While multiple studies have now documented variation in perioperative outcomes and costs across congenital heart disease centers5,15,19, relatively few have examined underlying mechanisms. This study was uniquely positioned to addressed this gap due to the detailed clinical data collected in the SVR trial. We found that variation across centers in postoperative complications and LOS accounted for a significant portion of the variability in costs. Compared to prior studies9, we found that the complications examined in our study explained a greater degree of the cost variation. This is likely due to the extensive list of complications and adverse events captured within the SVR trial as compared to other data sources.
Importantly, our study was able to expand upon these general findings and those from other studies to understand specific complications that occurred more commonly at higher cost centers. These included pleural effusion requiring drainage, seizures, wound infection, thrombus, liver dysfunction, sepsis, and necrotizing enterocolitis. Many of these have also been shown previously to be associated with excess costs on a patient level20,21. This more granular information is critical as it may aid in better informing targeted interventions that have the potential to both reduce complications and achieve cost savings7,17,20. Our data regarding the types of cost that appear to influence overall cost variation suggest that room and board/supplies and lab costs are the primary drivers. While the former is likely closely tied to our findings regarding overall LOS, curtailing unnecessary lab tests may be another potential target for improvement efforts.
Prior studies have suggested that these types of detailed analyses are a key first step in focusing efforts to reduce variation. For example, the Northern New England Cardiovascular Disease Study Group pioneered work examining variation in adult cardiothoracic surgery outcomes22. They conducted detailed analyses which demonstrated that differences in low output failure across centers accounted for the majority (80%) of the variation in mortality following coronary artery bypass. This finding informed efforts to develop tools to better identify, prevent, and treat low output, and to provide feedback to sites regarding best practices and outcomes, along with other collaborative activities This approach was successful in reducing death related to low cardiac output by 85% and overall mortality in participating centers declined from 5% to <2%22–25.
The success of similar approaches to focus and implement improvement initiatives has also been demonstrated recently in the pediatric cardiovascular population. For example, the Pediatric Heart Network Collaborative Learning Study aimed to increase rates of early extubation after congenital heart surgery. The group first collected data to demonstrate wide variation in timing of postoperative extubation across centers as well as a variety of underlying peri-operative practices related to sedation and other aspects of care. Informed by the data from a center with high rates of early extubation, the study team designed and implemented a clinical practice guideline based on these specific practices, which was then implemented across centers and successful in improving early extubation rates across these sites compared to control sites who continued usual care26.
Limitations
Although the SVR trial captured an extensive list of complications, it is unlikely that every possible postoperative complication was captured for this study’s analysis. Further, the information may not be generalizable to all patients undergoing a Norwood operation as this analysis was limited to data from medium and high volume centers participating in both the SVR trial and the Inpatient Essentials database during the study period. Similarly, center variation was only examined for those participating institutions. Postoperative complications, LOS, and other costs are often interconnected, and it is challenging to evaluate their independent impact. Although the estimated costs in this study are derived using standard methods across hospitals by the Children’s Hospital Association, and adjusted for geographic region, there may still be some differences related solely to hospital accounting practices rather than actual resources utilized. Our group is currently working to develop the methods to allow analysis of standardized costs in the congenital heart population, which will address these limitations in future investigations. Finally, this study only evaluates the inpatient costs for the first stage of Norwood palliation, and further studies evaluating costs across multiple hospitalizations, second and third-stages of palliation, and outpatient care will be necessary to determine longitudinal costs for hypoplastic left heart syndrome.
Conclusions
In conclusion, this study demonstrated wide variation in Norwood costs across centers. A high proportion of the variation in costs was associated with differences in complication rates and LOS. We identified specific complications that were more common at centers with higher costs, as well as other costs, particularly lab-related, that accounted for a significant proportion of the cost variation seen. These data may help to inform initiatives that hold the potential to both improve clinical outcomes and lower costs of care in this population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robbins JMBT, Tilford JM, et al. Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects-United States, 2003. MMWR Morbidity and mortality weekly report. 2007;(56):25–29. [PubMed] [Google Scholar]
- 2.Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA. Pediatric inpatient hospital resource use for congenital heart defects. Birth defects research Part A, Clinical and molecular teratology. 2014;100(12):934–943. doi: 10.1002/bdra.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogon BE, Kanter K, Alsoufi B, Maher K, Oster ME. Outcomes and hospital costs associated with the Norwood operation: beyond morbidity and mortality. Cardiology in the young. 2014:1–7. doi: 10.1017/S1047951114002224. [DOI] [PubMed] [Google Scholar]
- 4.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatrics. 2011;128(5):e1181–1186. doi: 10.1542/peds.2010-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquali SK, Gaies MG, Jacobs JP, William Gaynor J, Jacobs ML. Centre variation in cost and outcomes for congenital heart surgery. Cardiology in the young. 2012;22(6):796–799. doi: 10.1017/S104795111200159X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. The New England journal of medicine. 2010;362(21):1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHugh KE, Pasquali SK, Hall MA, Scheurer MA. Impact of postoperative complications on hospital costs following the Norwood operation. Cardiology in the young. 2015:1–7. doi: 10.1017/S1047951115002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquali SK, Jacobs JP, Shook GJ, et al. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. American heart journal. 2010;160(6):1099–1104. doi: 10.1016/j.ahj.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquali SK, Jacobs ML, He X, et al. Variation in congenital heart surgery costs across hospitals. Pediatrics. 2014;133(3):e553–560. doi: 10.1542/peds.2013-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohye RG, Gaynor JW, Ghanayem NS, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. The Journal of thoracic and cardiovascular surgery. 2008;136(4):968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross JS, Krumholz HM. Ushering in a new era of open science through data sharing: the wall must come down. Jama. 2013;309(13):1355–1356. doi: 10.1001/jama.2013.1299. [DOI] [PubMed] [Google Scholar]
- 12.Wang X. Cancer Moonshot 2020: a new march of clinical and translational medicine. Clinical and translational medicine. 2016;5(1):11. doi: 10.1186/s40169-016-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquali SK, Jacobs JP, Farber GK, et al. Report of the National Heart, Lung, and Blood Institute Working Group: An Integrated Network for Congenital Heart Disease Research. Circulation. 2016;133(14):1410–1418. doi: 10.1161/CIRCULATIONAHA.115.019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean PN, McHugh KE, Conaway MR, Hillman DG, Gutgesell HP. Effects of race, ethnicity, and gender on surgical mortality in hypoplastic left heart syndrome. Pediatric cardiology. 2013;34(8):1829–1836. doi: 10.1007/s00246-013-0723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHugh KE, Hillman DG, Gurka MJ, Gutgesell HP. Three-stage palliation of hypoplastic left heart syndrome in the University HealthSystem Consortium. Congenital heart disease. 2010;5(1):8–15. doi: 10.1111/j.1747-0803.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 16.Pasquali SK, He X, Jacobs JP, Jacobs ML, O'Brien SM, Gaynor JW. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. The Annals of thoracic surgery. 2012;94(2):573–579. doi: 10.1016/j.athoracsur.2012.03.065. discussion 579–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquali SK, Jacobs JP, Bove EL, et al. Quality-Cost Relationship in Congenital Heart Surgery. The Annals of thoracic surgery. 2015;100(4):1416–1421. doi: 10.1016/j.athoracsur.2015.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquali SK, Jacobs JP. The role of databases in improving the quality of care for congenital heart disease. World journal for pediatric & congenital heart surgery. 2013;4(2):139–141. doi: 10.1177/2150135113480221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquali SK, Ohye RG, Lu M, et al. Variation in perioperative care across centers for infants undergoing the Norwood procedure. The Journal of thoracic and cardiovascular surgery. 2012;144(4):915–921. doi: 10.1016/j.jtcvs.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquali SK, He X, Jacobs ML, et al. Excess costs associated with complications and prolonged length of stay after congenital heart surgery. The Annals of thoracic surgery. 2014;98(5):1660–1666. doi: 10.1016/j.athoracsur.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquali SK, He X, Jacobs ML, et al. Hospital variation in postoperative infection and outcome after congenital heart surgery. The Annals of thoracic surgery. 2013;96(2):657–663. doi: 10.1016/j.athoracsur.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Likosky DS, Nugent WC, Clough RA, et al. Comparison of three measurements of cardiac surgery mortality for the Northern New England Cardiovascular Disease Study Group. The Annals of thoracic surgery. 2006;81(4):1393–1395. doi: 10.1016/j.athoracsur.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor GT, Birkmeyer JD, Dacey LJ, et al. Results of a regional study of modes of death associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. The Annals of thoracic surgery. 1998;66(4):1323–1328. doi: 10.1016/s0003-4975(98)00762-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. Jama. 1996;275(11):841–846. [PubMed] [Google Scholar]
- 25.Likosky DS, Goldberg JB, DiScipio AW, et al. Variability in surgeons' perioperative practices may influence the incidence of low-output failure after coronary artery bypass grafting surgery. Circulation Cardiovascular quality and outcomes. 2012;5(5):638–644. doi: 10.1161/CIRCOUTCOMES.112.967091. [DOI] [PubMed] [Google Scholar]
- 26.Mahle WT, Nicolson SC, Hollenbeck-Pringle D, et al. Utilizing a Collaborative Learning Model to Promote Early Extubation Following Infant Heart Surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17(10):939–947. doi: 10.1097/PCC.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]