Abstract
Background
We report whether the etiology underlying associations of childhood ADHD with adolescent alcohol and marijuana involvement is consistent with causal relationships or shared predispositions, and whether it differs by gender.
Methods
In three population-based twin samples (N=3762; 64% monozygotic), including one oversampling females with ADHD, regressions were conducted with childhood inattentive or hyperactive-impulsive symptoms predicting alcohol and marijuana outcomes by age 17. To determine whether ADHD effects were consistent with causality, twin difference analyses divided effects into those shared between twins in the pair and those differing within pairs.
Results
Adolescents with more severe childhood ADHD were more likely to initiate alcohol and marijuana use earlier, escalate to frequent or heavy use, and develop symptoms. While risks were similar across genders, females with more hyperactivity-impulsivity had higher alcohol consumption and progressed further toward daily marijuana use than did males. Monozygotic twins with more severe ADHD than their co-twins did not differ significantly on alcohol or marijuana outcomes, however, suggesting a non-causal relationship. When co-occurring use of other substances and conduct/oppositional defiant disorders were considered, hyperactivity-impulsivity remained significantly associated with both substances, as did inattention with marijuana, but not alcohol.
Conclusions
Childhood ADHD predicts when alcohol and marijuana use are initiated and how quickly use escalates. Shared familial environment and genetics, rather than causal influences, primarily account for these associations. Stronger relationships between hyperactivity-impulsivity and heavy drinking/frequent marijuana use among adolescent females than males, as well as the greater salience of inattention for marijuana, merit further investigation.
Keywords: Attention Deficit Hyperactivity Disorder, Gender Differences, Alcohol, Cannabis, Longitudinal Studies, Discordant Twin Design
1. Introduction
Although adolescent alcohol use in the U.S. has declined over the past two decades, 46% of 12th graders report being intoxicated at least once (Johnston, et al., 2016), and binge drinking is common by young adulthood (Center for Behavioral Health Statistics and Quality, 2016). While the trend toward legalizing marijuana helps lower perceptions of its risks among adolescents (Pacek et al., 2015), they may be at higher risk for adverse long-term outcomes from marijuana than adults (Volkow et al., 2014). Accordingly, identifying early-emerging risks and how they influence adolescent alcohol and marijuana misuse is of vital importance.
Attention-deficit hyperactivity disorder (ADHD) has long been recognized as a risk factor for substance abuse (Barkley et al., 1990; Gittelman et al., 1985; Lambert and Hartsough, 1998). Youth with ADHD are more likely to initiate use earlier, escalate to frequent smoking and marijuana use (Sibley et al., 2014), and engage in binge drinking by adulthood than those without ADHD (Howard et al., 2015). Nevertheless, clinical samples of youth with ADHD, followed prospectively and assessed for alcohol/marijuana problems, are largely male (Lee et al., 2011), as boys are more likely to be referred for treatment by teachers (Derks et al., 2007). Consequently, many studies are insufficiently powered to detect gender differences (Williamson and Johnston, 2015). Recent population-based studies in Sweden and Finland found ADHD symptoms predict drug use in 15-year-olds (Selinus et al., 2016) and frequent alcohol/marijuana use in 17-year-olds (Sihvola et al., 2011) to a greater degree for girls than for boys, however. Sihvola et al. concluded that the potentially greater relevance of inattention to alcohol/marijuana problems among girls needs further investigation. Girls may experience greater social and academic consequences from inattention than boys (Becker et al., 2013; Elkins et al., 2011) and higher risk for heavy cigarette smoking (Elkins et al., 2017). Yet, while girls are more likely to have the primarily inattentive subtype of ADHD (Hinshaw et al., 2006), its consequences are less often studied.
Furthermore, although ADHD often becomes apparent during childhood, prior to initiation of substance use, it cannot be assumed that ADHD causes substance problems based on this temporal sequence alone (Weinberg et al., 2014). Rather than being causal, associations may result from overlapping genetic (Derks et al., 2014) and environmental (Galera et al., 2013) risks increasing the likelihood of both ADHD and alcohol/marijuana problems. For example, low socioeconomic status (SES) may both exacerbate childhood ADHD (Russell et al., 2015) and contribute to smoking (Hill et al., 2014), while smoking is not only associated with ADHD, but predicts alcohol/marijuana use among those with ADHD (Biederman et al., 2012). Additionally, externalizing disorders, such as conduct (CD) and oppositional defiant disorder (ODD), often co-occur with ADHD and may account for its associations with alcohol/marijuana problems (Pingault et al., 2013). However, in the IMAGE sample (Groenman et al., 2013), while substance use disorders were most common among ADHD youth with CD, their risk was not fully accounted for by CD. Moreover, although CD reduced their effects, hyperactive-impulsive symptoms of ADHD were still associated with substance initiation and increased likelihood of marijuana disorders in adolescents (Elkins et al., 2007). Because hyperactivity-impulsivity diminishes with age (Arnold et al., 2014), its relationship to substance involvement may change. While hyperactivity-impulsivity may be associated with initiation, inattention may be more related to marijuana problems by young adulthood (Bidwell et al., 2014), even when accounting for CD effects (Zohsel et al., 2016).
In the current study, we combined prospective and twin difference designs to clarify whether the etiology of adolescent alcohol/marijuana involvement in twins discordant for ADHD is consistent with a causal influence of ADHD or shared predispositions. We examined whether differences within pairs in number and subtype of ADHD symptoms produce differences in each twin’s substance involvement, along with gender moderation of effects. Because each pair of monozygotic (MZ) twins shares essentially the same genetic sequence, if differences in ADHD within MZ pairs are related to differential alcohol/marijuana involvement, this can only be due to specific, non-genetic factors, suggestive of causality (McGue et al., 2010). If differences exist within dizygotic (DZ), but not MZ, pairs, this would suggest that genetic factors influence both ADHD and substance involvement, as DZ pairs share fewer genes than do MZs. Absence of within-pair differences in both twin types would suggest shared familial background accounts for ADHD-substance relationships, as MZ and DZ twin pairs raised together share their rearing environment (including prenatal substance exposure).
We propose that ADHD, particularly hyperactivity-impulsivity, indirectly increases adolescent involvement with alcohol and marijuana, primarily through shared externalizing propensities. This would be consistent with a study of cousins and siblings discordant for ADHD, which found shared familial factors mostly accounted for the relationship of ADHD to drug use disorders (Sundquist et al., 2015), though a small portion might be causal. By using the more conclusive twin difference design, we determined whether non-shared differences in inattention might also contribute incrementally to differential marijuana involvement, and whether possible causal influences might be more apparent in girls, as found recently for inattention and tobacco (Elkins et al., 2017). Furthermore, whether ADHD effects persist after accounting for non-shared exposures predating ADHD (e.g., birth weight differences), co-occurring externalizing disorders, and use of other substances was evaluated. By combining three datasets and including a cohort oversampling affected females, power to identify within-pair differences and moderation by gender was maximized.
2. Materials and methods
2.1. Participants
A total of 3,762 individual twins (52% female) from 1,881 like-sex twin pairs (64% MZ) visited with parents at baseline. Participants were from three community-ascertained cohorts recruited for the Minnesota Twin Family Study, a longitudinal investigation of the development of substance abuse (Iacono et al., 1999). Twin pairs identified from Minnesota birth records were eligible if they lived within a day’s drive of University of Minnesota and had no physical/psychological disability that precluded completing the assessment.
Prospective data from two cohorts (N=2,510) assessed at age 11 and followed to age 17 were utilized, along with cross-sectional data from the baseline assessment of a third cohort at age 17 (N=1,252). In one 11-year-old cohort, pairs were randomly allocated to screened or non-screened samples. The non-screened sample was recruited as described above. In the screened sample, a parent (usually the mother) was interviewed by phone to enrich the sample with twins exhibiting ADHD/CD symptoms and academic disengagement. Higher allocation of female pairs to the screened sample ensured participation of more affected females. Each parent-endorsed ADHD or disengagement item was assigned a weight of one; each CD symptom was assigned a weight of three. The family was recruited if at least one twin exceeded an empirically-validated score of five, which maximized sensitivity and specificity for identifying externalizing disorders (Keyes et al., 2009). The resulting cohort consisted of 998 twins (48% screened). Comparisons of participants and non-participants in all cohorts suggested minimal bias on demographic variables, producing a sample representative of Minnesota for the target birth years (e.g., 91–98% were White; for details, see Iacono et al., 1999; Keyes et al., 2009).
A flow chart describing the sample, data at each wave, and years during which cohorts were assessed, is provided in the Supplement1. Briefly, all cohorts overlapped at age 17. Age-17 data were available for 92.5% of the combined sample, with no selective loss at follow-up of those with more ADHD symptoms at baseline.
2.2. Measures and procedures
A complete description of the study was provided at the visit, followed by written informed consent from parents and written assent from twins. Participants were interviewed separately by different interviewers, each with a B.A. or M.A. in psychology (or a related field) and extensive training. Demographic measures included a SES composite consisting of the mean of four standardized scores: highest parental occupation status, mother’s and father’s highest degree, and household income. Measures of non-shared exposures included twin birth weight from birth certificates and parental report of previous neurological injuries.
Primary caregiver reports of twins, including lifetime ADHD with onset before age 12 (consistent with DSM-5), CD and ODD at baseline, and DSM-IV alcohol and marijuana abuse/dependence by age 17, were obtained with the Diagnostic Interview for Children and Adolescents-Revised (DICA–R; Reich, 2000), which was modified to include DSM-IV criteria. Twin reports of ADHD before age 12 (and CD/ODD), were obtained with a parallel version of the DICA-R. Self-reported alcohol and marijuana abuse/dependence were assessed at age 17 via a modified, expanded Substance Abuse Module (Robins et al., 1987), from the Composite International Diagnostic Interview (Robins et al., 1988).
Interviews were reviewed by two individuals with advanced clinical training, with symptoms assigned by consensus. A symptom was considered present if reported by parent or child, and if its frequency and severity met established guidelines. Symptom counts were harmonized with DSM-IV (e.g., the inattentive count for cohorts assessed on only six of nine DSM-IV inattentive symptoms was prorated by multiplying by 1.5), as different diagnostic systems were in place when each cohort was assessed. Substance abuse and dependence symptoms were combined into a single count.
Composite measures reflecting highest degree of alcohol/marijuana involvement by age 17 were derived from items added to the substance interview and a computerized measure, self-administered at each assessment. Earliest age of initiation reported across assessments was recorded for each substance. Frequency of use was coded to reflect typical escalation during adolescence (i.e., for alcohol frequency, 0 = never; 1= less than once a month; 2 = once a month/nearly weekly; 3 = weekly/daily; for marijuana frequency, 0 = never; 1 = once a month or less; 2 = weekly/nearly weekly; 3 = daily/nearly daily). Maximum quantity consisted of maximum drinks consumed in a 24-hour period for alcohol (0 = none; 1 = 1–3 drinks; 2 = 4–6; 3 = 7–10; 4 = 11–20; 5 = 21–29; 6 = ≥30 [>95th percentile]) or number of lifetime uses for marijuana (0 = none; 1 = 1–5 uses; 2 = 6–29; 3 = 30–199; 4 = ≥200 [95th percentile]).
2.3. Statistical analyses
Regression models were appropriate for each outcome’s distribution. Age of initiation was predicted via survival models implemented in the COXPH package in the R statistical program. Data were censored for those who had not initiated by age 17. A gamma between-within model recommended for co-twin survival analysis was used (Sjolander et al., 2013). For frequency, ordinal regression with proportional odds models was implemented with the R MIXOR package. For quantity and log-transformed DSM-IV abuse/dependence symptoms, linear mixed models were implemented in SAS PROC MIXED.
Individual-level models were fit using either the inattentive or hyperactive-impulsive symptom count to predict each substance outcome. Twin correlations were accounted for and appropriate standard errors generated (Carlin et al., 2005) through random intercepts at the cluster (pair) level, or shared frailty terms for survival models. Significant overall effects of ADHD were then divided into those (1) shared by twins in a pair (twin-pair average) and (2) non-shared (within-pair difference); the latter represents the ADHD effect after controlling for all shared measured and unmeasured confounders. Twin difference models were conducted separately by gender if gender moderation was significant at the individual-level. If the within-pair effect was significant, indicating that within-pair differences in alcohol or marijuana problems increased as within-pair differences in ADHD increased, whether this differed for MZ and DZ pairs was determined and separate estimates were obtained. These procedures parallel those used previously to identify etiological influences in the relationship of ADHD to smoking (Elkins et al., 2017).
Given the dimensional nature of ADHD and to maximize power for detecting within-pair effects, ADHD symptoms, rather than diagnoses, were utilized for regression analyses2. Even so, many adolescents had clinically-relevant ADHD: 337 males and 201 females had ≥ 5 symptoms of either the predominantly inattentive, hyperactive-impulsive, or Combined subtype, including impairment. Therefore, analyses of alcohol/marijuana initiation among those with or without clinical diagnoses of ADHD were also conducted. A nonparametric, weighted log-rank test for non-proportional hazard models was applied to compare survival curves (Harrington and Fleming, 1982) across subtype-defined groups.
3. Results
3.1. Demographic differences in alcohol and marijuana use
Table 1 details alcohol/marijuana involvement by age 17, which was consistent with aggregated trends among U.S. 12th graders from 1990-present (Johnston et al., 2016). Gender differences were smallest for initiation and largest at higher levels of consumption, with male adolescents’ use exceeding that of females. Thus, overall effects of ADHD on substance outcomes were adjusted for gender, as well as other shared demographic covariates (i.e., cohort, parental SES, age at assessment) in regression analyses. Adjusting for shared covariates had no effect on within-pair effects, which are due to experiences or characteristics the twins do not share. Adjusting for non-shared differences in birthweight or neurological problems was unnecessary, as correlations between these and ADHD symptoms were not significantly different from zero.
Table 1.
Descriptive Statistics for Lifetime Alcohol and Marijuana Involvement by Age 17 in Three Combined Cohorts by Gender
| Combined Cohorts | Males | Females | Male: Female Effect Sizec | |||||
|---|---|---|---|---|---|---|---|---|
| (N=3407–3647)a | (N=1627–1747) | (N=1780–1900) | ||||||
| Measure of Alcohol or Marijuana Involvement by Age 17 | % | N | % | N | % | N | OR | 95% CI |
| Ever used alcohol without parental permission | 68.7 | 2504 | 70.2 | 1226 | 67.3 | 1278 | 1.14 | .99, 1.32 |
| Ever intoxicated or drunk | 51.1 | 1786 | 52.2 | 869 | 50.2 | 917 | 1.10 | .96, 1.25 |
| Ever used marijuana | 31.4 | 1145 | 33.5 | 585 | 29.5 | 560 | 1.20 ** | 1.05, 1.38 |
| Weekly or daily drinkingb | 12.0 | 411 | 14.2 | 231 | 10.0 | 180 | 1.49 *** | 1.21, 1.83 |
| Weekly or daily marijuana useb | 15.8 | 540 | 19.1 | 310 | 12.8 | 230 | 1.60 *** | 1.33, 1.93 |
| Ever consumed more than 10 drinks in 24 hoursb | 26.2 | 905 | 37.1 | 611 | 16.3 | 294 | 3.03 *** | 2.58, 3.55 |
| ≥ 30 lifetime uses of marijuanab | 10.5 | 356 | 13.3 | 216 | 7.9 | 140 | 1.79 *** | 1.43, 2.24 |
| Mean | SD | Mean | SD | Mean | SD | d | 95% CI | |
| Age of alcohol initiation | 14.6 | 2.0 | 14.4 | 2.2 | 14.9 | 1.8 | .25*** | .17, .33 |
| Age of first alcohol intoxication | 15.1 | 1.7 | 14.9 | 1.8 | 15.3 | 1.6 | .25*** | .16, .34 |
| Age of marijuana initiation | 15.0 | 1.8 | 14.9 | 1.8 | 15.1 | 1.7 | .11 | −.01, .22 |
| DSM-IV Alcohol Abuse/Dependence symptom countd | .51 | 1.3 | .69 | 1.5 | .35 | 1.1 | .26*** | .20, .33 |
| DSM-IV Cannabis Abuse/Dependence symptom countd | .45 | 1.5 | .65 | 1.8 | .26 | 1.0 | .26*** | .19, .32 |
Ns varied by substance phenotype from 3407 (≥ 30 lifetime uses of marijuana) to 3647 (ever used) but always exceeded 90% of baseline sample.
Levels of use were combined into clinically significant categories above; however, continuous or ordinal measures were used in regression models.
Effect sizes are expressed as odds ratios (OR) for categorical outcomes or Cohen’s d for quantitative outcomes. ORs significantly greater than 1 indicate a higher level of use for males than females (e.g., 1.49 = a 49% increase in the likelihood for males). For Cohen’s d, .20 corresponds to a small effect. Because male gender was associated with earlier initiation; males and females were reverse-coded for comparing age of initiation.
These raw symptom counts were log-transformed in regression analyses to mitigate positive skew.
p<.05,
p<.01,
p<.001.
3.2. Overall effects of ADHD and gender on alcohol and marijuana involvement
Table 2 provides adjusted overall and within-pair effects. Effects on initiation are given as hazard ratios, reflecting increased likelihood of initiating use during any specific year; effects on frequency are given as odds ratios. Both reflect the increased likelihood (or odds) associated with each one symptom increase in inattention or hyperactivity-impulsivity and are in bold if significantly different from 1. For models predicting quantity or substance symptoms, ADHD symptoms, quantity measures, and alcohol and marijuana symptoms (log-transformed) were converted to standardized scores based on the entire sample (mean = 0; SD = 1). These estimates reflect the increase in quantity or symptoms (in SD units) associated with a 1 SD increase in inattention or hyperactivity-impulsivity and are in bold if significantly different from zero.
Table 2.
Results of Overall (Individual-Level) and Twin Difference Analyses of Baseline ADHD Symptoms on Alcohol and Marijuana Initiation, Frequency of Use, Maximum Quantity, and DSM-IV Substance Abuse/Dependence Symptoms by Age 17
| ADHD Inattentive Symptoms (raw- or z-score) | |||||||
|---|---|---|---|---|---|---|---|
| Alcohol or Marijuana Measure | Gender Moderationa | Overall Effectc (N=3407-3630)b |
Within-Pair Twin Difference Effect | ||||
| Dizygotic Pairs (N=591-650 pairs)b |
Monozygotic Pairs (N=1051-1154 pairs)b |
||||||
| Age at Initiation of Use | Hazard Ratiod | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Alcohol initiation | No | 1.08*** | 1.06, 1.11 | 1.04 | 0.99, 1.10 | 1.02 | 0.96, 1.08 |
| Marijuana initiation | No | 1.19*** | 1.15, 1.24 | 1.04 | 0.97, 1.11 | 1.06 | 0.99, 1.15 |
| Frequency of Use by Age 17 | Odds Ratiod | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Progression toward Weekly/Daily Drinking | No | 1.08*** | 1.03, 1.13 | 1.05 | 0.97, 1.13 | 0.98 | 0.89, 1.08 |
| Progression toward Daily Marijuana Use | No | 1.20*** | 1.13, 1.28 | 1.06 | 0.96, 1.18 | 1.06 | 0.96, 1.19 |
| Maximum Quantity by Age 17 (z)e | β | 95% CI | β | 95% CI | β | 95% CI | |
| Maximum Drinks in 24 Hours | No | 0.07*** | 0.04, 0.10 | 0.03 | −0.03, 0.09 | 0.01 | −0.04, 0.06 |
| Number of Lifetime Uses of Marijuana | No | 0.08*** | 0.05, 0.11 | 0.01 | −0.05, 0.07 | 0.04 | −0.01, 0.09 |
| Substance Use Disorder Symptoms(z)e | β | 95% CI | β | 95% CI | β | 95% CI | |
| DSM-IV Alcohol Abuse/Dependence | No | 0.07*** | 0.03, 0.10 | 0.04 | −0.03, −0.11 | −0.04 | −0.10, 0.02 |
| DSM-IV Marijuana Abuse/Dependence | No | 0.07*** | 0.04, 0.10 | −0.02 | −0.08, 0.06 | 0.01 | −0.05, 0.06 |
| ADHD Hyperactive-Impulsive Symptoms (raw- or z-score) | |||||||
| Age at Initiation of Use | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Alcohol Initiation | No | 1.14*** | 1.11, 1.17 | 1.06 * | 1.00, 1.13 | 1.04 | 0.97, 1.13 |
| Marijuana Initiation | No | 1.22*** | 1.18, 1.27 | 1.08 * | 1.00, 1.17 | 1.00 | 0.91, 1.11 |
| Frequency of Use by Age 17 | Odds Ratio d | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Progression toward Weekly/Daily Drinking | No | 1.20 *** | 1.14, 1.27 | 1.19 ** | 1.08, 1.30 | 1.02 | 0.90, 1.16 |
| Progression toward Daily Marijuana Use | Yes | ||||||
| Female | Female>Malea | 1.40 *** | 1.27, 1.55 | 1.34 ** DZ> MZf | 1.13, 1.59 | 1.03 | 0.87, 1.23 |
| Male | 1.19 *** | 1.08, 1.31 | 1.03 | 0.89, 1.21 | 0.83 | 0.66, 1.04 | |
| Maximum Quantity by Age 17 (z)e | β | 95% CI | β | 95% CI | β | 95% CI | |
| Maximum Number of Drinks in 24 Hours | Yes | ||||||
| Female | Female>Malea | 0.15 *** | 0.11, 0.19 | 0.15 *** DZ> MZf | 0.11, 0.19 | 0.03 | −0.04, 0.10 |
| Male | 0.08 *** | 0.04, 0.13 | 0.01 | −0.08, 0.11 | −0.05 | −0.14, 0.03 | |
| Number of Lifetime Uses of Marijuana | No | 0.10 *** | 0.07, 0.13 | 0.08 * | 0.02, 0.15 | −0.05 | −0.11, 0.01 |
| Substance Use Disorder Symptoms (z)e | β | 95% CI | β | 95% CI | β | 95% CI | |
| DSM-IV Alcohol Abuse/Dependence | No | 0.12 *** | 0.08, 0.15 | 0.08 * DZ> MZf | 0.01, 0.16 | −0.04 | −0.11, 0.03 |
| DSM-IV Marijuana Abuse/Dependence | No | 0.08 *** | 0.05, 0.12 | 0.04 DZ> MZf | −0.04, 0.11 | −0.03 | −0.09, 0.04 |
Gender moderation test statistics and significance levels are given in section 3.2.
Overall effects include all adolescents with data on the outcome; twin difference analyses include pairs in which both twins have data.
Overall effects were covariate-adjusted for age (except initiation), cohort, parental SES, and gender (except when presented separately by gender).
Effects for initiation are given as hazard ratios; effects for frequency are given as odds ratios. Those significantly greater than 1.0 correspond to the increased likelihood of initiating at each age (ages 8–18) or progressing a level of frequency associated with a 1 symptom increase in ADHD. Within-pair estimates reflect differential likelihood of initiating or progressing associated with a twin having 1 more ADHD symptom than his or her cotwin.
Effects for maximum quantity of use and symptoms are given as standardized beta coefficients (β), because ADHD symptoms, maximum quantity, and log-transformed abuse/dependence symptoms were all converted to standardized (z) scores, with a mean of 0 and SD of 1. Overall estimates thereby reflect the increase in quantity or symptoms (in SD units) associated with a 1 SD increase in ADHD; within-pair estimates reflect the difference (in SD units) associated with a twin being 1 SD higher in ADHD than his or her cotwin.
DZ>MZ indicates that the within-pair effect was significantly greater for dizygotic than for monozygotic twin pairs.
p<0.05
p<0.01
p<0.001.
Adolescents with more inattentive or hyperactive-impulsive symptoms (top or bottom of Table 2, respectively) were more likely to have initiated alcohol and marijuana use earlier, have higher quantity and frequency of use, and symptoms of alcohol or marijuana abuse/dependence. Hazard ratios (for initiation) and odds ratios (for frequency) were significantly larger for marijuana than alcohol, as their associated confidence intervals did not overlap (except frequency in males). Rate of marijuana initiation increased by 19% for each inattention symptom (compared to 8% for alcohol) and by 22% for each hyperactive-impulsive symptom (14% for alcohol). Effects on quantity and symptoms were similar for both substances.
There was no significant gender moderation of inattention effects on any outcome. While hyperactivity-impulsivity effects on initiation and symptoms did not differ by gender, hyperactivity-impulsivity was associated more strongly with maximum drinks consumed [F (1, 1680) = 3.91, p <.05] and marijuana frequency (z = 2.29, p =.02) for females than males. Estimates are provided separately by gender for these outcomes. Each hyperactive-impulsive symptom was associated with a 40% increase in odds for females (19% for males) of progressing in marijuana frequency (e.g., weekly to daily). For maximum drinks, a 1 SD increase in hyperactivity-impulsivity was associated with a .15 SD increase in drinks for females (.08 for males).
3.3. Twin difference effects
Twin difference effects are presented separately within DZ and MZ pairs to identify the source of differences. Within-pair differences in inattention were not significantly associated with differences in any alcohol/marijuana outcomes for MZ or DZ pairs. Hyperactivity-impulsivity differences within MZ pairs were also unrelated to outcomes. However, hyperactivity-impulsivity differences within DZ pairs were significant (except for marijuana symptoms), although only those within female DZ pairs were significant for marijuana frequency and maximum drinks. Furthermore, within-pair differences in hyperactivity-impulsivity were significantly greater for DZ than MZ pairs on two outcomes for each substance. These results are consistent with hyperactivity-impulsivity relationships to alcohol/marijuana being primarily attributable to genetic differences and inattention effects to shared familial influences affecting both inattention and alcohol/marijuana use.
3.4. Mediation by other externalizing disorders or use of other substances
Because twin difference results were not consistent with causal influence, effects were likely attributable to factors other than ADHD alone. As noted earlier, ADHD effects may be mediated by CD/ODD or other substance use. In addition, while ADHD treatment with stimulant medications does not have a consistent effect on risk for alcohol or drug disorders (Humphreys et al., 2013), medication use is often confounded with ADHD severity (Looby, 2008).3 Consequently, individual-level analyses were repeated with either (1) ever used the other substance (either alcohol or marijuana), (2) ever used tobacco, (3) log-transformed CD/ODD symptoms at baseline, or (3) ever used prescription stimulants, as a covariate.
Stimulant medication had no impact on size or significance of effects. Inattention remained a significant predictor of almost all marijuana outcomes when accounting for CD/ODD, alcohol, or tobacco use; only the effect for marijuana symptoms did not, when adjusting for CD/ODD (p = .22). However, inattention was no longer a significant predictor of alcohol outcomes for nine of twelve models adjusting for either CD/ODD, marijuana, or tobacco use (except for alcohol initiation, which remained significant when adjusting for marijuana initiation or CD/ODD, p<.0001 or p = .04, respectively; or alcohol symptoms, when adjusting for tobacco, p = .01). Conversely, all hyperactivity-impulsivity effects on both substances remained significant when adjusting for use of another substance or CD/ODD, except outcomes moderated by gender. Figure 1 illustrates associations between hyperactivity-impulsivity and marijuana frequency by gender, adjusting for SES, alcohol or tobacco use, CD/ODD, and the portion due to within-pair effects. After adjusting for tobacco use, odds ratios for overall hyperactivity-impulsivity effects were reduced in size (consistent with partial mediation of ADHD-drug effects by tobacco; Lee et al., 2017), yet remained significant for both genders (except males, after adjusting for CD/ODD)4. Within-pair differences were significant for female DZ pairs only.
Figure 1.
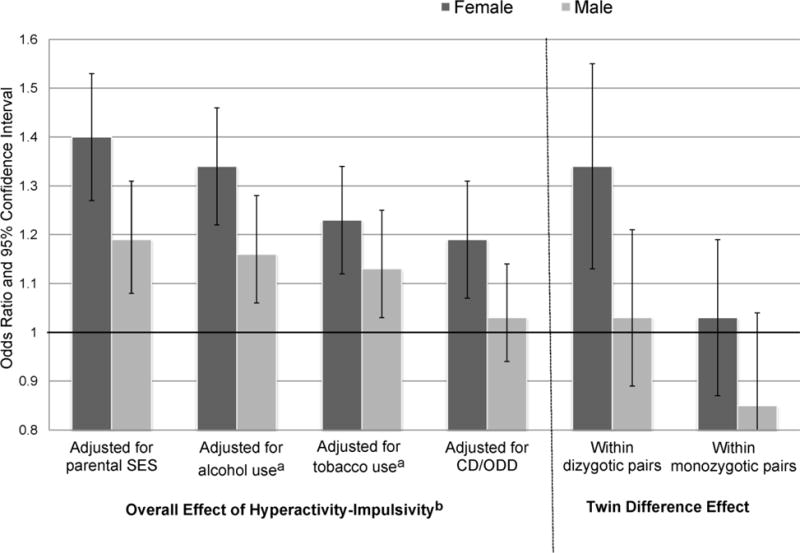
Overall Effects of ADHD Hyperactive-Impulsive Symptoms on Frequency of Marijuana Use, By Gender, Before and After Adjusting for Alcohol, Tobacco Use and Conduct/Oppositional Defiant Disorders, and Twin Difference Effects Within Dizygotic and Monozygotic Pairs
SES=socioeconomic status; CD/ODD=conduct/oppositional defiant disorder symptoms. Error bars indicate 95% confidence intervals. Marijuana frequency is coded: 0 = never; 1 = once a month or less; 2 = weekly/nearly weekly; 3 = daily/nearly daily. Odds ratios greater than 1 correspond to the increased odds of progressing one level of frequency to the next (i.e., from weekly to daily use) associated with each hyperactive-impulsive symptom. Twin difference effects reflect the differential likelihood of progressing in frequency associated with a twin having one more hyperactive-impulsive symptom than his or her co-twin.
aAdjusted for ever used alcohol or tobacco. When frequency of alcohol use was also considered, overall effects of hyperactivity-impulsivity remained significant for both genders (OR = 1.30 for females; 1.17 for males; p <.0001 and p = .001, respectively). When frequency of tobacco use was considered, only the overall effect for females remained significant (p<.05).
bOverall effect included N=3407 individuals; twin difference effects included 591 dizygotic or 1051 monozygotic pairs.
3.5. Clinical relevance of ADHD for initiation
Figure 2 displays cumulative hazard functions from survival analyses, or probability of initiating use in the next year for (a) alcohol or (b) marijuana, based on ADHD subtype diagnoses (2.3). For alcohol, presence of hyperactivity-impulsivity was most important. Adolescents with hyperactive-impulsive or Combined subtypes were at higher risk for initiating alcohol at earlier ages, compared to those who were inattentive-only [Combined vs. Inattentive: χ2(1) = 5.8, p < .05; Hyperactive vs. Inattentive: χ2 = 9.6, p < .01] or those with no ADHD [Combined vs. No Diagnosis: χ2(1) = 12.9, p =.001; Hyperactive vs. No Diagnosis: χ2 = 20.9, p <.0001], whereas the inattentive-only and no diagnosis groups were at similarly low risk [χ2 (1) = .03, p = .85]. However, all subtypes were at higher risk for marijuana initiation [Combined vs. No Diagnosis: χ2 (1) = 33.4; Hyperactive vs. No Diagnosis: χ2 = 30.1; Inattentive vs. No Diagnosis: χ2 = 18.1, all ps < .0001; Benjamini Hochberg adjustment for multiple testing applied], suggesting inattention is primarily relevant for marijuana initiation.
Figures 2.
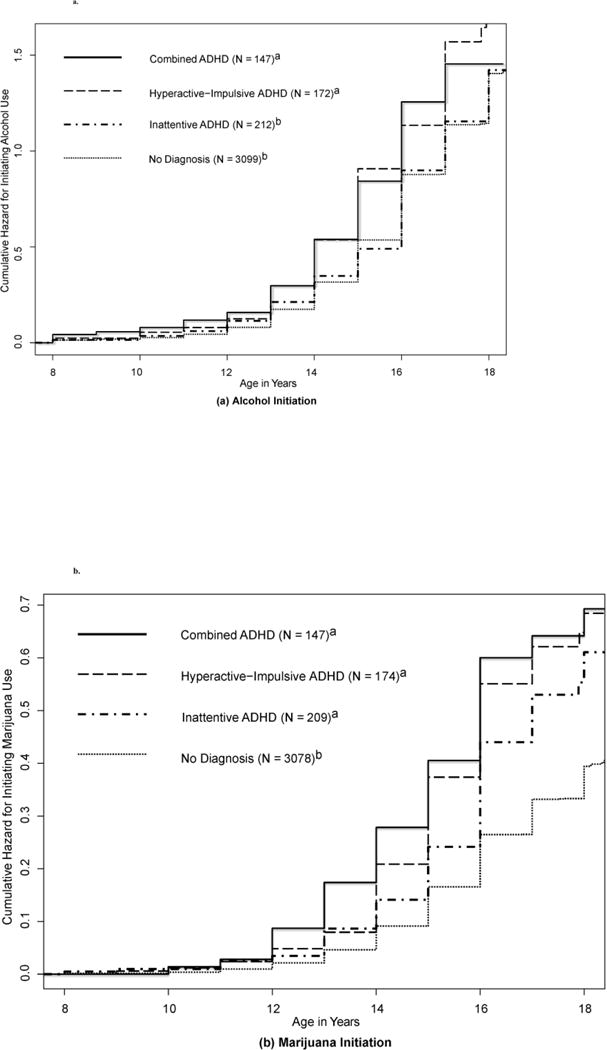
a and b
Cumulative Hazard Functions for (a) Alcohol and (b) Marijuana Initiation, by ADHD Subtype Diagnosis
The y-axis is the probability of initiation use in the next year for each group. Hazard functions of groups sharing the same superscript (i.e., either a or b) did not differ significantly at p < .05. When effects on marijuana initiation were adjusted based on alcohol initiation prior to or at the same age as marijuana (as it was for 80% of marijuana users), effect sizes for both types of ADHD symptoms on marijuana initiation were substantially reduced, though still highly significant (p < .0001).
4. Discussion
We evaluated whether associations of childhood ADHD to adolescent alcohol and marijuana involvement differ by gender and whether causal influence or shared propensities explain these associations. To our knowledge, this is the first twin difference study to examine effects of ADHD on adolescent alcohol/marijuana involvement, in a sample containing a number of females significantly affected by ADHD. Both male and female adolescents with more ADHD symptoms at baseline were more likely to initiate use earlier, with progression to heavier, more frequent use and symptoms of substance use disorders by age 17. Unlike our previous findings for tobacco, in which females with more severe ADHD were consistently at higher risk for smoking than males, due in part to possible gender-specific causal influences (Elkins et al., 2017), gender was not a significant moderator of ADHD effects for most outcomes, with two exceptions: larger effects of hyperactivity-impulsivity on alcohol consumption and marijuana frequency were observed in female than male adolescents, consistent with Sihvola et al (2011). While these results confirm the importance of ADHD-associated risks for alcohol/marijuana use, the absence of differences within MZ pairs suggests that shared genetic and environmental propensities primarily account for these associations, rather than a specific causal influence of ADHD.
4.1. Roles of hyperactivity-impulsivity and inattention
As hypothesized, hyperactivity-impulsivity effects were indirect, confounded by shared influences on both hyperactivity-impulsivity and alcohol/marijuana involvement. Within-pair effects were significant only for less genetically-related (DZ) pairs, consistent with genetic risk accounting for the relationship of hyperactivity-impulsivity to alcohol problems (Derks et al., 2014; Quinn et al., 2016), perhaps through transmission of a liability toward behavioral disinhibition and impulsivity (Iacono et al., 2008). That some hyperactivity-impulsivity associations were more pronounced for females is supported by evidence that females may require greater exposure to genetic and environmental risks for ADHD (Taylor et al., 2016) and alcoholism (Foster et al., 2015) to develop. For instance, higher ADHD prevalence has been observed for female than male adolescents presenting with alcohol or marijuana use disorders (Korsgaard et al., 2016). Preliminary evidence suggests impulsivity may predict greater induced alcohol craving among young women than men (Yarmush et al., 2016).
Because no twin difference effects of inattention on alcohol/marijuana outcomes were significant, this implicates shared familial background, rather than causal influence. This does not mean these associations are unimportant (Burt et al., 2009), only that they are attributable to shared genetic and environmental risks increasing the likelihood of both ADHD and alcohol/marijuana problems. The relatively larger magnitude of inattention effects for marijuana initiation and frequency, even when accounting alcohol/tobacco use and CD/ODD, suggests inattention may have a more specific relationship to marijuana than to alcohol. As shown in Figure 2b for marijuana initiation, at ages 14–16, inattention-only and no-diagnosis groups begin to diverge. By ages 16–18, risk for initiation in the inattention-only group approaches that of the hyperactive-impulsive and Combined groups. Thus, inattention may become increasingly salient to marijuana use by young adulthood (Bidwell et al., 2014).
4.2. Conclusions and implications
Etiological similarities outweighed differences in the relationship of ADHD to alcohol/marijuana problems for both genders. The relatively stronger association between hyperactivity-impulsivity and maximum drinks for females than males is concerning, as higher alcohol consumption is associated with increased risk for sexual victimization during young adulthood (Testa and Livingston, 2009). Furthermore, the strength of ADHD relationships to marijuana initiation and frequency, though likely non-causal, was comparable to those found for tobacco (Elkins et al., 2017).
There are limitations to these findings. Because the sensitivity of twin difference analysis decreases when twin correlations on a putative causal factor are high (Frisell et al., 2012), high MZ twin correlations for ADHD (.6–.7, depending on cohort) may have reduced power for identifying partially causal effects. Additionally, ADHD persistence, which may affect whether substance dependence develops (Breyer et al., 2014), was not assessed. There are a number of strengths, however, including multiple informants and comprehensive measurement of ADHD and alcohol/marijuana involvement with structured clinical interviews.
With increased numbers of marijuana users accounting for the rising incidence of marijuana use disorders (Hasin et al., 2015), developing strategies to reduce frequent use has become increasingly important (Pedersen et al., 2016). Perceptions of risk associated with marijuana continue to decline among adolescents (Pacek et al., 2015), and those with ADHD appear even less likely to believe that marijuana causes difficulty with thinking and slowed responses (Harty et al., 2015). Our findings suggest that adolescents with ADHD need better preparation to make informed choices regarding alcohol and marijuana.
Supplementary Material
Highlights.
ADHD predicts when alcohol and marijuana use are initiated and escalation of use
Shared familial factors account for this relationship, rather than ADHD causing use
Hyperactivity-impulsivity predicts heavy drinking/daily marijuana use more in females
ADHD associations with marijuana tend to be larger than for alcohol
Inattention predicts marijuana use only, when other substances/externalizing included
Acknowledgments
Role of funding source
This work was supported by National Institute on Drug Abuse grants R01DA038065 to Irene Elkins, R37DA005147 and R01DA013240 to William Iacono; and National Institute on Alcohol Abuse and Alcoholism grant R01AA009367 to Matt McGue. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Power was estimated at 80% for detecting MZ-within-pair effects accounting for 0.7% of the variance in alcohol/marijuana outcomes. Power calculations were based on an average MZ correlation in substance outcomes of .60, a comparable correlation for ADHD, and approximately 1100 MZ pairs with outcome data.
Stimulant medication use, including methylphenidate- and amphetamine-based formulations, was reported for 225 twins at baseline or follow-up. Among the clinically-relevant ADHD cases, those treated with stimulants (N=155) had more ADHD symptoms (M = 11.6; SD = 3.0) than those never medicated [M = 9.1; SD = 2.6; t (536) = 9.65, p <.0001].
When co-occurring quantity, frequency, or symptoms related to use of another substance by age (17) were considered in addition to ever used, hyperactivity-impulsivity associations with corresponding alcohol/marijuana outcomes remained significant (p<.05) only for alcohol symptoms and maximum drinks/marijuana frequency in females only (i.e., marijuana quantity/symptoms were no longer significant when adjusted for tobacco quantity/symptoms). Inattention associations, though reduced in size by adjustment, remained significant for all marijuana outcomes but only one alcohol outcome: maximum drinks, adjusted for marijuana quantity.
Contributors
Irene Elkins was involved in the conceptualization of the project, data preparation, analysis and interpretation, and in drafting and editing the manuscript. Gretchen Saunders and Stephen Malone were each involved in conceptualization of the data analysis, data analysis and interpretation, and drafting and editing the manuscript. Margaret Keyes was involved in data preparation, interpretation, and drafting and editing the manuscript. William Iacono and Matt McGue were involved in the conceptualization of the overall project, oversight of data collection, conceptualization of these analyses, and drafting and editing the manuscript. All authors read and approved of final submission.
Conflict of Interest
No conflict declared.
References
- Arnold LE, Ganocy SJ, Mount K, Youngstrom EA, Frazier T, Fristad M, Horwitz SM, Birmaher B, Findling R, Kowatch RA, Demeter C, Axelson D, Gill MK, Marsh L. Three-year latent class trajectories of attention-deficit/hyperactivity disorder (ADHD) symptoms in a clinical sample not selected for ADHD. J Am Acad Child Adolesc Psychiatry. 2014;53:745–760. doi: 10.1016/j.jaac.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Becker SP, McBurnett K, Hinshaw SP, Pfiffner LJ. Negative social preference in relation to internalizing symptoms among children with ADHD predominantly inattentive type: Girls fare worse than boys. J Clin Child Adolesc Psychol. 2013;42:784–795. doi: 10.1080/15374416.2013.828298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Henry EA, Willcutt EG, Kinnear MK, Ito TA. Childhood and current ADHD symptom dimensions are associated with more severe cannabis outcomes in college students. Drug Alcohol Depend. 2014;135:88–94. doi: 10.1016/j.drugalcdep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Hammerness P, Batchelder H, Faraone SV. Cigarette smoking as a risk factor for other substance misuse: 10-year study of individuals with and without attention-deficit hyperactivity disorder. Br J Psychiatry. 2012;201:207–214. doi: 10.1192/bjp.bp.111.100339. [DOI] [PubMed] [Google Scholar]
- Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychol Addict Behav. 2014;28:238–246. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, Iacono WG. Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: Results from a cross-lagged monozygotic twin differences design. Dev Psychol. 2009;45:1752–1760. doi: 10.1037/a0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: A critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016. (HHS Publication No. SMA 16-4984, NSDUH Series H-51). [Google Scholar]
- Derks EM, Hudziak JJ, Boomsma DI. Why more boys than girls with ADHD receive treatment: A study of Dutch twins. Twin Res Hum Genet. 2007;10:765–770. doi: 10.1375/twin.10.5.765. [DOI] [PubMed] [Google Scholar]
- Derks EM, Vink JM, Willemsen G, van den Brink W, Boomsma DI. Genetic and environmental influences on the relationship between adult ADHD symptoms and self-reported problem drinking in 6024 Dutch twins. Psychol Med. 2014;44:2673–2683. doi: 10.1017/S0033291714000361. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, Malone S, Keyes M, Iacono WG, McGue M. The impact of attention-deficit/hyperactivity disorder on preadolescent adjustment may be greater for girls than for boys. J Clin Child Adolesc Psychol. 2011;40:532–545. doi: 10.1080/15374416.2011.581621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, Saunders GRB, Malone SM, Keyes MA, Samek DR, McGue M, Iacono WG. Increased risk of smoking in female adolescents who had childhood ADHD. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17010009. doi://doi.org/10.1176/appi.ajp.2017.17010009. [DOI] [PMC free article] [PubMed]
- Foster KT, Hicks BM, Iacono WG, Mcgue M. Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med. 2015;45:3047–3058. doi: 10.1017/S0033291715001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- Galera C, Pingault JB, Fombonne E, Michel G, Lagarde E, Bouvard MP, Melchior M. Attention problems in childhood and adult substance use. J Pediatr. 2013;163:1677–1683. doi: 10.1016/j.jpeds.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I Psychiatric status. Arch Gen Psychiatry. 1985;42:937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, Sergeant JA, Buitelaar JK, Faraone SV. Substance use disorders in adolescents with attention deficit hyperactivity disorder: A 4-year follow-up study. Addiction. 2013;108:1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- Harrington DP, Fleming TR. A class of rank test procedures for censored survival-data. Biometrika. 1982;69:553–566. [Google Scholar]
- Harty SC, Pedersen SL, Gnagy EM, Pelham WE, Jr, Molina BS. ADHD and marijuana-use expectancies in young adulthood. Subst Use Misuse. 2015;50:1470–1478. doi: 10.3109/10826084.2015.1018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72:1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Amos A, Clifford D, Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: Review of the evidence. Tob Control. 2014;23:89–97. doi: 10.1136/tobaccocontrol-2013-051110. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. J Consult Clin Psychol. 2006;74:489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Howard AL, Molina BSG, Swanson JM, Hinshaw SP, Belendiuk KA, Harty SC, Arnold LE, Abikoff HB, Hechtman L, Stehli A, Greenhill LL, Newcorn JH, Wigal T. Developmental progression to early adult binge drinking and marijuana use from worsening versus stable trajectories of adolescent attention deficit/hyperactivity disorder and delinquency. Addiction. 2015;110:784–795. doi: 10.1111/add.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: A meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnston ID, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. Institute for Social Research, University of Michigan; Ann Arbor: 2016. [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG. The enrichment study of the Minnesota Twin Family Study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Res Hum Genet. 2009;12:489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgaard HO, Torgersen S, Wentzel-Larsen T, Ulberg R. Substance abuse and personality disorder comorbidity in adolescent outpatients: Are girls more severely ill than boys? Child Adolesc Psychiatry Ment Health. 2016;10:8. doi: 10.1186/s13034-016-0096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lee CT, McClernon FJ, Kollins SH, Fuemmeler BF. Childhood ADHD symptoms and future illicit drug use: The role of adolescent cigarette use. J Pediatr Psychol. 2017 doi: 10.1093/jpepsy/jsx098. https://doi/10.1093/jpepsy/jsx098. [DOI] [PMC free article] [PubMed]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looby A. Childhood attention deficit hyperactivity disorder and the development of substance use disorders: Valid concern or exaggeration? Addict Behav. 2008;33:451–463. doi: 10.1016/j.addbeh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspect Psychol Sci. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Mauro PM, Martins SS. Perceived risk of regular cannabis use in the United States from 2002 to 2012: Differences by sex, age, and race/ethnicity. Drug Alcohol Depend. 2015;149:232–244. doi: 10.1016/j.drugalcdep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ER, Hummer JF, Rinker DV, Traylor ZK, Neighbors C. Measuring protective behavioral strategies for marijuana use among young adults. J Stud Alcohol Drugs. 2016;77:441–450. doi: 10.15288/jsad.2016.77.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault JB, Cote SM, Galera C, Genolini C, Falissard B, Vitaro F, Tremblay RE. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: A 15-year longitudinal population-based study. Mol Psychiatry. 2013;18:806–812. doi: 10.1038/mp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Pettersson E, Lundstrom S, Anckarsater H, Langstrom N, Gumpert CH, Larsson H, Lichtenstein P, D’Onofrio BM. Childhood attention-deficit/hyperactivity disorder symptoms and the development of adolescent alcohol problems: A prospective, population-based study of Swedish twins. Am J Med Genet B Neuropsychiatr Genet. 2016;171:958–970. doi: 10.1002/ajmg.b.32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. Authors; St. Louis: 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle LH. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Russell AE, Ford T, Russell G. Socioeconomic associations with ADHD: Findings from a mediation analysis. PLoS One. 2015;10:e0128248. doi: 10.1371/journal.pone.0128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinus EN, Molero Y, Lichtenstein P, Anckarsater H, Lundstrom S, Bottai M, Gumpert CH. Subthreshold and threshold attention deficit hyperactivity disorder symptoms in childhood: Psychosocial outcomes in adolescence in boys and girls. Acta Psychiat Scand. 2016;134:533–545. doi: 10.1111/acps.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Coxe S, Kipp H, Gnagy EM, Meinzer M, Ross JM, Lahey BB. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol. 2014;123:362–374. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Korhonen T, Pulkkinen L, Raevuori A, Marttunen M, Kaprio J. Prospective relationships of ADHD symptoms with developing substance use in a population-derived sample. Psychol Med. 2011;41:2615–2623. doi: 10.1017/S0033291711000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolander A, Lichtenstein P, Larsson H, Pawitan Y. Between-within models for survival analysis. Stat Med. 2013;32:3067–3076. doi: 10.1002/sim.5767. [DOI] [PubMed] [Google Scholar]
- Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Attention-deficit/hyperactivity disorder and risk for drug use disorder: A population-based follow-up and co-relative study. Psychol Med. 2015;45:977–983. doi: 10.1017/S0033291714001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Lichtenstein P, Larsson H, Anckarsater H, Greven CU, Ronald A. Is there a female protective effect against attention-deficit/hyperactivity disorder? Evidence from two representative twin samples. J Am Acad Child Adolesc Psychiatry. 2016;55:504–512 e502. doi: 10.1016/j.jaac.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Livingston JA. Alcohol consumption and women’s vulnerability to sexual victimization: Can reducing women’s drinking prevent rape? Subst Use Misuse. 2009;44:1349–1376. doi: 10.1080/10826080902961468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;371:879. doi: 10.1056/NEJMc1407928. [DOI] [PubMed] [Google Scholar]
- Weinberg N, Lopez M, Compton WM. Epidemiology of drug abuse: Building blocks for etiologic research. In: Madras B, Kuhar MJ, editors. The Effects of Drug Abuse on the Human Nervous System. Elsevier, Inc; 2014. pp. 51–76. [Google Scholar]
- Williamson D, Johnston C. Gender differences in adults with attention-deficit/hyperactivity disorder: A narrative review. Clin Psychol Rev. 2015;40:15–27. doi: 10.1016/j.cpr.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Yarmush DE, Manchery L, Luehring-Jones P, Erblich J. Gender and impulsivity: Effects on cue-induced alcohol craving. Alcohol Clin Exp Res. 2016;40:1052–1057. doi: 10.1111/acer.13030. [DOI] [PubMed] [Google Scholar]
- Zohsel K, Baldus C, Schmidt MH, Esser G, Banaschewski T, Thomasius R, Laucht M. Predicting later problematic cannabis use from psychopathological symptoms during childhood and adolescence: Results of a 25-year longitudinal study. Drug Alcohol Depend. 2016;163:251–255. doi: 10.1016/j.drugalcdep.2016.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


