Abstract
Commonalities in addictive behavior, such as craving, stimuli-driven drug seeking, and a high propensity for relapse following abstinence, have pushed for a unified theory of addiction that encompasses most abused substances. This unitary theory has recently been challenged -- citing distinctions in structural neural plasticity, biochemical signaling, and neural circuitry to argue that addiction to opioids and psychostimulants are behaviorally and neurobiologically distinct. Recent more selective examination of drug-induced plasticity have highlighted that these two drug classes promote an overall reward circuitry signaling overlap through modifying excitatory synapses in the nucleus accumbens – a key constituent of the reward system. We discuss adaptations in pre-/postsynaptic, and extrasynaptic glutamate signaling produced by opioids and psychostimulants, and their relevance to circuit remodeling and addiction related behavior -- arguing that these core neural adaptations are important targets for developing pharmacotherapies to treat addiction to multiple drugs.
Keywords: addiction, opioids, psychostimulants, glutamate, nucleus accumbens, plasticity
Several decades of research have led to an understanding that addiction is a progressive pathology resulting from drug-induced neural plasticity within brain regions involved in reward, emotion, decision-making, and habit [1–3]. Commonalities in the ability of abused drugs such as opioids and psychostimulants to promote similar neurochemical, psychological (e.g., incubation of craving) and behavioral effects (e.g., sensitization, high propensity to relapse) have pushed for a unified theory of substance abuse. This view has recently been called into question, suggesting that addiction to psychostimulants and opioids differ at the behavioral, neurobiological, and even structural and circuit level [4–8]. The continued abuse of psychostimulants and recent rise in illicit opioid use, including opioid-based pain medications for non-therapeutic use [2, 9–14], has led to a need for more thorough examination of how much plasticity produced by these two drug classes overlap and diverge to better develop targeted therapies for addiction as a whole. The purpose of this review is to highlight recent work on glutamatergic transmission within the nucleus accumbens (NAc) -- a key neurobiological substrate of reward and motivated behavior – using electrophysiological data from preclinical models. We contend that while psychostimulants and opioids do indeed exhibit important behavioral, biochemical, and physiological distinctions, as well as various dissimilarities in plasticity [15–17], they share a striking overlap in NAc glutamate plasticity at the presynaptic and postsynaptic level (Figure 1) that likely represents important focal points for development of future therapeutic strategies.
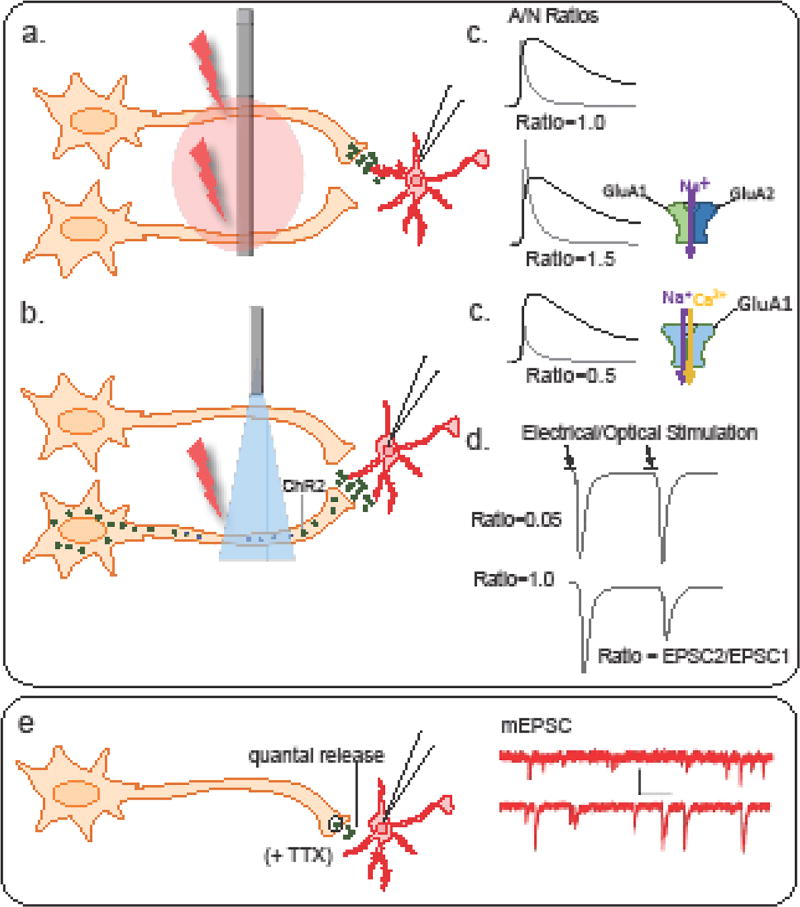
Figure 1. Measuring synaptic plasticity.
Excitatory strength as measured by changes in the ratio of currents mediated by AMPA and NMDA-type (A/N ratio) glutamate receptors involves evoked stimulation of axon terminals by (a) injecting electrical current or (b) applying short pulses of light to evoke glutamate release from all surrounding terminals or specific afferents, respectively. (c) Following drug treatment, increases in mean A/N ratio may suggest an increase in GluA2-containing AMPA-type receptors (or reductions in NMDAR-mediated currents), while reductions in A/N ratios may reflect an increased presence of GluA2-lacking AMPA receptors that exhibit reduced current at more depolarized potentials (rectification). (d) Alterations in transmitter release probability can be measured by comparing amplitudes of multiple EPSCs evoked electrically or optically (EPSC2/EPSC1). Reductions in paired pulse ratio (PPR) reflect an increase in release probability, while increases following treatment reflect a reduction. (e) Basal glutamate transmission is often measured by examining changes in the frequency or amplitude of EPSCs in the presence of TTX to eliminate activity-dependent transmission.
Psychostimulants and Opioids
Psychostimulants and opioids vary in chemical structure, pharmacodynamic profile, and physiological effects (Tanimoto coefficient < 0.85) [18, 19]. Despite these differences, these two drug classes do in fact share a number of core behavioral, neurochemical, and neurophysiological profiles. Behaviorally speaking, both drug classes promote motor sensitization, conditioned reward, drug-seeking and time-dependent increases in craving following extended periods of use [20, 21]. These parallels likely reflect overlapping neurotransmission and plasticity within shared circuits, although opioids do appear to involve more brain regions than psychostimulants with regards to relapse [7, 20, 22].
At the cellular and circuit level, psychostimulants produce similar effects on neuronal activity in the NAc [7, 20, 23]. This may reflect a shared ability to increase dopamine levels within the mesocorticolimbic dopamine system, albeit by different mechanisms. Psychostimulants do so by blocking reuptake (cocaine) or inverting dopamine transport (amphetamine), whereas opiates disinhibit DA neurons in the ventral tegmental area (VTA) through indirect inhibition of GABAergic input [24–26]. Notably, while it is well established that dopamine projections from the ventral tegmental area (VTA) to the NAc, data argue against a prominent role for dopamine in this circuit for opioid self-administration [7].
Preclinical Psychostimulant and Opioid Models
Numerous studies have examined effects of acute and repeated drug exposure using non-contingent models, whereby drug is administered via the experimenter with an intra-peritoneal or sub-cutaneous injection. These models include behavioral sensitization and conditioned place preference and can be used to measure rewarding/reinforcing properties of a drug, and drug-cue memories in their simplest form [7, 15, 20]. Unlike psychostimulants, the effects of non-contingent chronic opioid administration can also be studied through subcutaneous implantation of morphine pellets that promotes a steady state level of morphine in the body. This approach permits examination of tolerance as well as dependence and withdrawal – the latter of which can occur spontaneously or be precipitated by opioid receptor antagonist administration [27]. Importantly, repeated and intermittent models often incorporate multiple 24 h periods of withdrawal, whereas steady dosing does not – an important consideration when interpreting neural adaptations produced by opioids. Complicating matters further, while physical signs associated with opioid withdrawal generally subside within a week, affective/cognitive components of withdrawal syndromes can persist upwards of 80 days [27, 28].
In volitional (contingent) drug administration models, animals learn to lever press (or nose poke) for intravenous infusion or oral administration of the drug, where responding on a specific lever results in drug delivery. An important finding is that in rodents and humans, there is a progressive intensification (“incubation”) of drug craving following the conclusion of cocaine or heroin self-administration [29–31]. In rodents, this is reported as an increase in lever responding (i.e., drug-seeking) in the absence of drug delivery, however while the incubating effects of cocaine are known to persist as long as 60 days, incubation seems to peak at ~15 d and return to control levels by 60 d following heroin self-administration [21].
NAC and medium spiny neurons
Drug-induced modifications in NAc glutamatergic neurotransmission has been identified as an underlying factor driving drug-taking, relapse, withdrawal and other drug-associated behavior for both drug classes [15–17]. The NAc is a heterogeneous structure that can be divided into core and shell region based on their anatomical connectivity and role in reward-related responses [32–34]. The NAc core is highly connected with motor circuitry and responsible for evaluation of reward and initializing reward-related motor activity [35–37]. Alternatively, the shell is highly connected with limbic and autonomic brain regions and is heavily involved in motivation and establishing learning associations [35, 38].
GABAergic medium-spiny projection neurons (MSNs) comprise a majority (>90%) of all neurons in the core and shell. MSNs receive coordinated input from glutamatergic afferents arising from several brain areas, including the basolateral amygdala (BLA), ventral hippocampus (vHPC), and medial prefrontal cortex (mPFC) [39]. Canonical understanding is that NAc medium spiny neurons (MSNs) can largely be divided into two sub-populations based on the expression of peptides, dopamine receptors (dopamine D1 vs. D2), and downstream projection targets [40–42], with a small fraction (~2–17%) expressing both D1- and D2-receptors [43, 44]. Unlike the distinct direct and indirect pathways of the dorsal striatum, information processing within the NAc and efferent regulation of basal ganglia output structures involves significantly more overlap between D1- and D2-MSN projections than previously believed [42, 44–46](12–16). Further, emerging evidence indicates that D1- and D2-MSN populations display different physiological characteristics, exhibit different forms of drug-induced plasticity, and generally exert antagonistic effects in drug-associated behaviors [42, 43, 47–49].
Psychostimulant and opioid effects on presynaptic glutamatergic release
Glutamatergic afferents in the NAc that collectively innervate single MSNs form a separate but unified system of information input [50]. This convergent neurotransmitter release promotes heterosynaptic plasticity and associative learning -- a common feature of addiction phenotypes [51–53] (see Figure 1 for presynaptic plasticity measurements). Examination of cocaine’s effects on glutamate release at isolated inputs to MSNs, specifically the infralimibic cortex (ILC) and the paraventricular nucleus of the thalamus (PVNT), show an increase in the probability of release at early and late withdrawal time points [54, 55]. Cocaine-dependent increases in glutamate release appear restricted to ILC and PVNT afferents as no changes are observed at BLA or ventral hippocampal synapses onto D1- or D2-MSNs following experimenter administered cocaine [56]. As for opioids, glutamate release probability is elevated at BLA- but not prelimbic cortex (PrC)-to-MSNs projections in the NAc core as early as 2 hours following an escalating regimen of morphine over 5 days [57]. In the NAc shell, increased miniature excitatory postsynaptic current (mEPSC) frequency at D1-MSNs and reduced frequency at D2-MSNs accords with respective increases and decreases in release probability from pooled afferents 10–14 d after non-contingent morphine [47]. One likely possibility is that these changes may reflect modifications in prefrontal signaling, as repeated non-contingent morphine does not alter release at BLA or PVNT afferents[58].
Compared to the shell, direct evidence for withdrawal-dependent alterations in transmitter release in the NAc core is substantially lacking, however increased release probability has been observed at PrL-to-Core pooled MSNs following cocaine and remifentanil self-administration, respectively [59, 60]. These are further substantiated by indirect evidence that cocaine-, heroin-, and morphine increase mEPSC frequency and/or suppresses presynaptic regulatory mechanisms such as metabotropic group II/III receptors (mGluR2/3R) and activator of G protein signaling (AGS3) at intermediate withdrawal periods [61, 62] [63–65] but see [66].
Although we lack an in-depth comparison of psychostimulant- or opioid-induced changes in presynaptic glutamate release based on withdrawal time points and mode of administration, the current data suggest that both drug classes collectively increase glutamate release on MSNs in the NAc, particularly at ILC afferents (Supplemental Table1) – begging the question whether tempering this release would be a beneficial therapeutic approach for treating drug addicted patients. To this end, clinical trials are identifying whether FDA approved medications believed to decrease glutamate release are potential therapeutic strategies for treating patients suffering from addiction. These medications are typically anti-seizure medications, such as zonisamide (tested to treat cocaine dependence), lamotrigine (tested to treat ketamine and alcohol dependence), and topiramate (tested to treat cocaine, alcohol, nicotine, or methamphetamine addictions) all believed to block varying types of voltage-gated sodium or calcium channel activity and in some cases, concomitant effects on glutamate transmission mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and kainate-type receptors [67]. Although the mechanism is unclear, lamotrigine is believed to inhibit glutamate release from cortical-to-ventral striatal inputs making this medication potentially worth testing as a pharmacological treatment for patients suffering from psychostimulant or opioid addiction [68]. Alternatively, agonists of putative autoreceptors and hetero-presynaptic mGluR2/3 receptors increase glutamate and dopamine release, a property that may position these drugs as potential treatment for dysphoria associated with early withdrawal [69]. The use of such anti-epileptics to dampen excitatory signaling still requires extensive preclinical testing of undesired side effects (i.e., fatigue, negative affect, depression) and examination of their ability to reverse drug-induced plasticity or produce plasticity on their own.
Extracellular (synaptic/perisynaptic) glutamate levels following psychostimulant or opioid exposure
Glial-dependent regulation of perisynaptic, extracellular glutamate strongly regulates synaptic plasticity through activation of extrasynaptic metabotropic glutamate and NMDA receptors [17, 70–72]. This regulation is primarily two-fold – occurring through the glutamate transporter, GLT-1, which is localized on glia in the vicinity of the synaptic cleft, and by release of glial-derived glutamate through the cystine-glutamate exchanger [59, 72, 73]. Both GLT-1 and the catalytic subunit of the exchanger (xCT) are downregulated after cocaine and heroin self-administration in the NAc core, as well as cocaine and morphine in the shell [17, 47, 74–79].
During withdrawal, downregulated xCT activity results in reduced glial-derived glutamate tone that normally activate presynaptic mGluR2/3 and postsynaptic mGluR5s that exert inhibitory tone on transmitter release. Effects of reduced presynaptic inhibitory tone are further exacerbated by drug-dependent reductions in GLT-1-dependent glutamate clearing [73, 80–82].
Electrophysiological and biochemical evidence suggests that re-exposure to drug and associated cues promotes rapid potentiation of glutamate transmission, and that this correlates with drug-seeking during reinstatement testing [59, 74, 83]. Although a direct causal link remains lacking, additional studies have shown that exposure to relapse-inducing stimuli following withdrawal from amphetamine, cocaine, and morphine, leads to a reduction in AMPAR signaling in the NAc shell and core [84–90]. These adaptations promote a scenario of uninhibited (potentiated) glutamate release triggered by re-exposure to drug or highly salient stimuli which may prolong postsynaptic signaling and synaptic spillover that perhaps represents and/or triggers a key form of short-term plasticity that closely shared by psychostimulants and opioids that is required to engage in drug-seeking behavior (i.e., reinstatement/relapse) [59, 60, 72, 74, 83, 91–95].
The above data suggest that proteins that regulate glial glutamate release and uptake may represent ideal targets for pharmacotherapies aimed at mitigating drug craving and relapse. A wide body of preclinical work supports this theory, as administration of either ceftriaxone (an antibiotic known to upregulate GLT-1 expression and function), or N-acetylcysteine (NAC; a prodrug that activates the exchanger) restores morphine, cocaine and heroin-induced pre- and postsynaptic plasticity, prevents stimuli-induced increases in glutamate release, blocks reinstatement of drug-seeking, and disrupts the rewarding properties of morphine [47, 59, 96]. However, bioavailability of NAC may limit its effectiveness in an outpatient setting, while no known clinical trials have tested ceftriaxone efficacy as a treatment for addiction or assessed unintended side-effects in humans.
Psychostimulant and opioid-dependent generation of silent synapses
Silent synapses are excitatory glutamatergic synapses that express functional NMDARs, but lack functionally stable AMPARs [97]. They are formed either by synaptogenesis-like mechanisms or by silencing pre-existing synapses [98], thus acting as intermediary structures for strengthening or weakening glutamatergic synaptic inputs, respectively [99]. Contingent cocaine administration as well as non-contingent cocaine and morphine generate silent synapses in the NAc during early withdrawal. Interestingly, cocaine-generated silent synapses are preferentially expressed on D1R-MSNs [100, 101], whereas those generated by morphine exposure occur primarily on D2R-MSNs [100]. Following cocaine self-administration silent synapses have been observed in the shell at BLA- and ILC-to-Shell MSNs synapses and at PrL-to-MSNs in the core [102–104].
Mechanistic and temporal dynamics of silent synapses generation
Silent synapses are believed to represent key substrates for long-term neurocircuit alterations [105]. The conserved nature of silent synapse generation across drug classes suggests this may be a shared mechanism in facilitating circuit remodeling that initiates early stages of plasticity responsible for developing addictive behavior, however the mechanisms by which these synapses are generated appear to differ. Cocaine-induced synapse generation is associated with selective insertion of GluN2-containing NMDARs -- which are often expressed at newly developed synapses [102] -- increased dendritic spines [100, 106], and activation of the cAMP response element-binding protein (CREB), which promotes spinogenesis [106], suggesting that these are nascent synapses. On the other hand, exposure to morphine does not increase the synaptic expression of GluN2B-containing NMDARs, and increases the number of filopodia-like dendritic spines while decreasing thin-long spines [100]. As generation of these synapses is blocked by endocytosis-blocking peptides and AMPAR levels are correlated with spine head volume, morphine-induced generation of silent synapses is likely mediated by AMPAR removal at D2R-MSNs that ultimately weakens excitatory input to these neurons.
Despite these differences, a number of temporal and age-related similarities exist between cocaine and morphine silent synapses. First, both processes require repeated drug exposure, and is a gradual yet transient process that peaks at WD1-2 and returns to basal levels by WD7 [100, 102]. Second, although silent synapse development is normally more prominent during development (10%) vs. adulthood (~3%), both drugs produce large and comparable increases in silent synapses in middle-adolescent (~P35) and adults (~P65) in pooled MSNs (~25–30%) [102]. Lastly, when administered alongside conditioned cues silent synapse generation is increased in the NAc -- highlighting the possibility that this process may reflect a common mechanism for drug-associative learning [47, 107]. As exposure to relapse-inducing stimuli during withdrawal is sufficient to “restore” drug-induced increases in synaptic strength [85, 87, 90, 108, 109], it will be interesting to see if this plasticity is also associated with generation of silent synapses.
Overlapping neurocircuit reorganization in the NAc following psychostimulants or opioid treatment
Previous examination of structural plasticity in NAc MSNs showed that cocaine and amphetamine increases dendritic branching and spine density, while morphine had the opposite effect – causing long-lasting decreases in dendritic branching and number of spines [110]. Based on differential effects of opiates and psychostimulants on striatal immediate early gene (Fos) expression, it has been suggested that these opposing effects may be explained by engagement of direct versus indirect striatal pathways (i.e., D1R- vs. D2R-MSNs) [7]. This notion is supported by recent electrophysiological data in MSN subpopulations [100]. As withdrawal from cocaine progresses, newly generated synapses on D1R-MSNs are stabilized by the insertion of AMPARs [103, 104] precipitating stable, mushroom-like spines that enhance glutamatergic input at D1R-MSNs relative to D2R-MSNs [100]. Following repeated morphine treatment, the removal of AMPARs on preexisting D2R-MSN synapses generates “weakened”, transient spine structures that precipitates subsequent loss of dendritic spines and decreases in glutamatergic input on D2R-MSNs relative to D1R-MSNs [100]. Although easily interpreted as a major difference in drug effect, both these processes would likely produce a similar result in overall NAc circuitry (Figure 3) – that is a relative increase in excitatory drive on D1-MSN pathways responsible for promoting addiction-related behavior [47, 100, 106] [111].
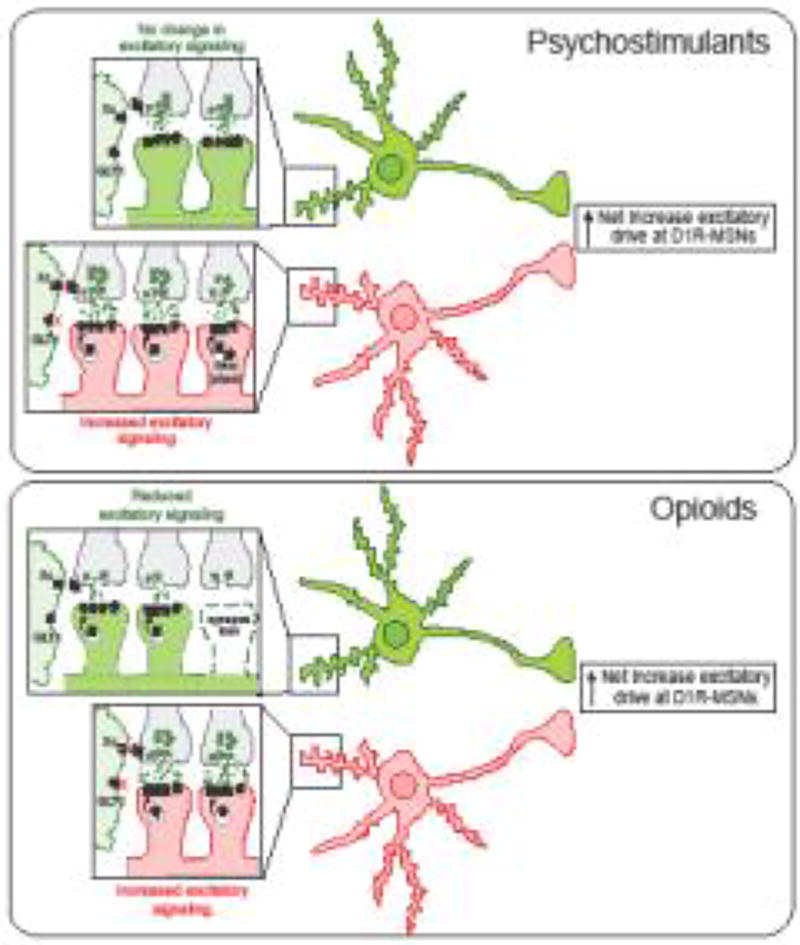
Figure 3. Psychostimulants and opioids promote an overall increase in excitatory drive at NAc D1R-MSN pathways.
Anatomical, pathway, model, and drug-specific differences in NAc plasticity certainly exist, however we propose a general (hypothetical model) based on current knowledge that these drugs have a similar overall effect on NAc circuitry. Both drug classes promote increases in silent synapse formation during early withdrawal. Psychostimulants (top) promote generation of new synapses on D1-MSNs that display increased GluN2B expression and subsequent insertion of GluA2-lacking or GluA2-containing (depending on the NAc subregion), while opioids (bottom) generate these on D2R-MSNs through endocytosis of AMPARs – resulting in increased excitatory strength at D1R-MSNs and reduced strength at D2R-MSNs, respectively. It is possible that increases in presynaptic release observed as early as 24 h post drug exposure are initiated by reductions in inhibitory autoreceptor tone typically generated by the cysteine-glutamate exhchanger, which in conjunction with reduced glutamate clearance from decreased GLT-1 expression, permit enhanced postsynaptic signaling that lead to maturation of D1R-MSN silent synapses. Although psychostimulants do not appear to alter pre- or postsynaptic plasticity at D2R-MSNs, opioids reduce both presynaptic and postsynaptic drive, the latter of which involves removal of AMPARs and ultimately synapse pruning. Based on this data, we posit that although the mechanisms by which overall changes occur may differ, both psychostimulants and opioids promote a progressive pathology with a similar net effect on NAc microcircuitry that promotes a relative (net) increase excitatory drive at D1R-MSNs that is likely important for drug-seeking and conditioned reward.
Psychostimulant and opioid-dependent glutamate receptor plasticity
AMPAR plasticity
Acute withdrawal
A wealth of data supports the notion that activation of and enduring adaptations in NAc AMPAR signaling plays a key role in psychostimulant- and opioid-dependent behavioral sensitization, conditioned reward, craving, and the continued propensity for relapse -- making these receptors a key target for studying how drugs of abuse promote persistent addicted behavior and guiding development of future pharmacotherapies [27, 47, 87, 95, 100, 112–118].
Although enduring changes in AMPAR signaling likely contribute to relapse, understanding the pharmacodynamic effects of early psychostimulant and opioid exposure may provide important insight into how drugs of abuse begin to co-opt the reward circuit and modify behavior. Electrophysiological characterization of AMPAR signaling using whole-cell recordings from pooled or separated populations of MSNs in the NAc shell suggested that neither acute cocaine or amphetamine, nor repeated cocaine or morphine, is sufficient to trigger changes in synaptic strength (A/N ratios; Figure 1, Box2) or the amplitude of AMPAR currents in the first 24 h when administered non-contingently [85, 119–123]. On the other hand, 24 h following the end of cocaine self-administration, mEPSC amplitude is reduced in recordings from pooled MSN [124]. This early adaptation may be important for future development of enhanced AMPAR signaling, as stimulation of D1R during early abstinence increased cocaine-induced reductions in AMPAR signaling, and prevented subsequent enhancement of synaptic strength [124]. In the NAc core, mEPSC amplitude and frequency, as well as GluA1 surface expression is elevated in pooled MSNs and tissue punches during early withdrawal from acute amphetamine and repeated cocaine, however cocaine-dependent increases were only observed following 20–28 d (but not 7 d) of injections, the latter suggesting that extended exposure may promote significant un-silencing of synapses normally generated during early cocaine exposure [84, 102, 125].
Protracted withdrawal
Repeated non-contingent cocaine, amphetamine and morphine, as well as cocaine self-administration increased A/N ratios, basal AMPAR transmission (mEPSC amplitude and frequency), and expression of GluA2-lacking receptors in the NAc shell – an effect that occurs almost exclusively in D1R-MSNs [47, 53, 100, 121, 123, 125–127]. A single cocaine exposure has also been shown to upregulate NAc shell GluA2-lacking AMPAR signaling at D1R-MSN synapses, but like repeated exposure appears to require a period of withdrawal [127]. While non-contingent effects of cocaine on mEPSCs extend to the core in pooled MSNs, effects of repeated amphetamine (pooled MSNs) and morphine (identified MSNs) remain localized to the shell [47, 84].
Similar to non-contingent exposure, early biochemical and electrophysiological studies in pooled afferent and MSN populations showed elevated AMPAR signaling in the NAc core and shell following intermediate and long-term withdrawal (with or without extinction) from short- and long-access cocaine and heroin self-administration that was electrophysiologically shown to reflect a combination of GluA2-lacking and GluA2-containing receptors [81, 82, 85, 119, 124, 128–132]. These adaptations are almost exclusively isolated to D1R-MSN synapses in both the core and shell [53, 133] but see [127]. Although sufficient opioid self-administration data is lacking, these findings suggest that a core feature of opioid and stimulant exposure is a withdrawal-dependent progressive increase in NAc AMPAR signaling, regardless of exposure number and mode of drug administration (Figure 2).
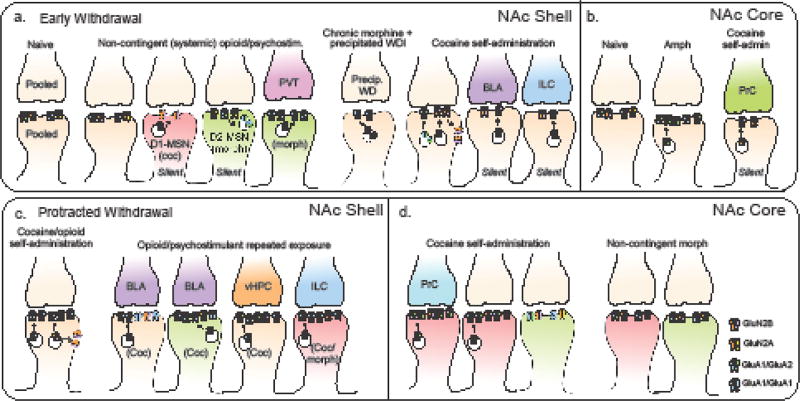
Figure 2. Summary of known postsynaptic adaptations in glutamate receptor signaling and silent synapse expression following early and protracted withdrawal from psychostimulants or opioids.
Repeated exposure to psychostimulants and opioids promotes a number of overalapping postsynaptic modifications in excitatory postsynaptic currents mediated by the ionotropic AMPA- and NMDA-type receptors, as well as promote expression of silent synapses that express functional NMDA but not AMPA receptors (silent) on MSNs in the NAc shell (a, c) and core (b, d). These adaptations have been reported at synapses on D1R-MSNs (red), D2R-MSNs (green), and pooled populations of MSNs (tan), as well specific afferents arising from regions that include the paraventricular ventricular nucleus of the thalamus (PVT, pink), medial prefrontal cortex infralimbic (ILC, blue) and prelimbic (PrC, green) regions, basolateral amygdala (BLA, purple) and ventral hippocampus (orange). While anatomical, cellular, temporal, and mechanistic nuances certainly exist, known adaptations to data suggest that both drug classes increase silent synapse number during early withdrawal in both the core and shell, which are either stabilized or weakened as withdrawal becomes more protracted by subsequent insertion or removal of AMPARs that are permeable (GluA1/GluA1) or impermeable (GluA1/GluA2) to calcium.
Prefrontal-accumbens plasticity
NAc MSN excitatory synapses receive glutamatergic input from limbic and paralimbic regions -- each presumably contributing emotional and motivational information through variable forms of plasticity. The mPFC-to-NAc pathway has long been proposed to undergo drug-induced synaptic plasticity that is important for regulating opioid and psychostimulant relapse [8]. This pathway can be divided into two primary projections consisting of the ILC-to-Shell and PrC-to-Core projections. Optogenetic examination of these pathways has shown that ILC-to-Shell synapses are strengthened by increased insertion of GluA2-lacking receptors selectively at D1R-MSN synapses following withdrawal from non-contingent cocaine and morphine exposure (WD7-14), as well as short-and long-access cocaine self-administration (WD10–45) [47, 53, 104, 126, 127], but see [134]. PrC-to-Core synapses have been examined far less, however elevated AMPAR transmission in pooled MSNs following cocaine self-administration reflects insertion of canonical GluA2-containing AMPARs [104].
Allocortical-accumbens plasticity
Neighboring synapses in the NAc shell receiving input from the vHPC or BLA also display increased synaptic strength and AMPAR-EPSCs [53, 103, 127, 134]. Similar to PrL inputs, enhanced glutamate signaling at vHPC synapses appears to involve insertion of GluA2-containing AMPARs [53, 134]. While either non-contingent cocaine or morphine alter AMPAR transmission in pooled or identified MSNs [58, 134], data reporting effects on postsynaptic signaling within BLA projections appear to be more susceptible to variations in model. Increases at BLA-to-Shell synapses likely reflect increased insertion of GluA2-lacking receptors on D2R-MSNs and perhaps GluA2-containing AMPARs in D1R-MSNs, as Pascoli et al. [53] examined rectification indices of AMPA transmission but not BLA-specific quantal transmission [53, 103, 127].
Behavioral and clinical relevance
Studies employing pharmacological and optogenetic manipulations suggest that restoration of opioid or psychostimulant-induced upregulation of NAc AMPARs exerts an inhibitory effect on opioid dependence/withdrawal, reward-seeking behavior, and a progressive intensification (“incubation”) of drug craving across [47, 58, 100, 103, 104, 122, 124, 128, 131, 135–137]. Several classes of AMPAR antagonists have undergone clinical trials for substance abuse treatment, including tezampanel (NGX424) and topiramate, which reduced cocaine self-administration (in rats), craving and increases the likelihood to remain abstinent from cocaine use which (humans) [114, 138–141].
Emerging evidence suggests that drugs that indirectly target AMPARs may provide an alternative approach, with fewer side-effects as direct effects. For example, positive allosteric modulators of AMPA receptors known as Ampakines, have been shown to alter psychostimulant behavior and modulate peptides that modulate drug seeking and glutamate plasticity (e.g., brain-derived neurotrophic factor, bdnf) [73]. Additionally, using synaptic stimulation patterns known to activate mGluR5s restores cocaine- and opioid-induced AMPAR signaling and disrupts relapse and conditioned reward across drug classes [47, 53, 76, 84, 126, 131, 142]. Although not addiction studies, ligands or compounds that modulate these receptors have been advanced to testing in clinical trials for various medical conditions and have some efficacy in alleviating somatic signs, anxiety and irritability associated with early opioid withdrawal in humans[143, 144]. Further examination of systemically available positive allosteric modulators of mGluR5 have shown favorable selectivity, side effect profiles, and anti-addiction properties [144–147].
NMDAR Plasticity
Numerous preclinical studies have illustrated the importance of NMDAR signaling in psychostimulant and opioid addiction pathology [59, 73, 148–150]. A majority of biochemical assessments during early withdrawal showed no change (many do not distinguish core and shell), with additional studies showing reductions in GluN1 or GluN2B expression -- the latter of which generally agrees with observed reductions in GluN-mediated EPSCs (core and shell were not distinguished here either) [81, 151–154].
More anatomically and physiologically discrete observations suggest a more likely scenario that involves a shift in subunit composition and/or localization of ‘detectable’ receptors (synaptic vs. extrasynaptic). For example, electrophysiological recordings in the NAc shell indicate that decay kinetics of GluN-mediated currents and sensitivity to GluN2B antagonists is increased 1–2 d following repeated non-contingent cocaine, which is accompanied by an increase in the cell surface levels of GluN1 and GluN2B subunits [100, 102, 106]. Alternatively, biochemical data show that total GluN2A (but not GluN2B) expression is upregulated in the NAc following chronic morphine, which matches with observed NMDAR-mediated current decay kinetics [152, 155, 156] and a lack of altered sensitivity to ifenprodil following repeated morphine [100].
Corresponding increases in GluN1/GluN2B (and GluN2A) have been observed during early withdrawal from cocaine self-administration [157–159] but see [157–160]. Although upregulation of GluN2B expression in the shell did not equate to differences in the sensitivity of evoked synaptic currents to GluN2B antagonism, decay kinetics of spontaneous NMDAR-EPSCs were increased following blockade of glutamate reuptake, suggesting a diffusion of receptors to extrasynaptic locations that are inaccessible during synaptic recordings [159]. Whether opioid self-administration promotes similar changes in GluN2 subunits remains unclear, however this apparent divergence in subunit plasticity may reflect a unique role for GluN2A in opioid dependence/withdrawal (Box 3).
Protracted Withdrawal
Unlike acute withdrawal, protracted withdrawal appears to upregulate GluN2B (and/or GluN1) expression (core/shell total and surface) during intermediate withdrawal (7–21 d) regardless of drug class or mode of administration [104, 123, 130, 154, 161–163] [158, 160] but see [41, 129, 159]. Aligning with these findings, electrophysiological studies show an increase in GluN2B-mediated EPSCs in NAc shell and core following non-contingent morphine and self-administered heroin [81, 123, 164]. Thus, while increased GluN2B expression following cocaine promotes a continuum of GluN2B plasticity during early withdrawal [100, 102, 106], there appears to be a progressive shift in primary subunit composition from GluN2A to GluN2B during opioid withdrawal. However, caution is warranted, as data regarding subunit changes after prolonged withdrawal from chronic morphine, or acute withdrawal following intermittent morphine are lacking.
Functional and Clinical relevance of NMDAR plasticity
Pharmacological blockade of NMDARs, particularly those expressing the GluN2B subunit, inhibits opioid and psyschostimulant conditioned reward [165–167] and drug-seeking [81, 168] -- effects that appear attributable in part, to activation of receptors in the NAc [164, 165, 169]. Given the conserved nature of increased GluN2B expression at silent synapses (cocaine) and intermediate withdrawal points across drug classes, it is tempting to speculate that upregulation of GluN2B in the NAc plays a key role in initiating or solidifying remodeling of NAc based reward circuits during withdrawal. Supporting this contention, increases in GluN1 and/or GluN2B are often paralleled by increases in GluA1 receptor expression [154, 160, 162, 163, 170], and cocaine-generated synapses that subsequently exhibit increased expression of AMPAR-mediated currents exhibit increased sensitivity to ifenprodil [100, 103, 104]. Moreover, systemic treatments that prevent drug-induced increases in GluN2B expression prevent increases in GluA1 expression, development or expression of sensitization [100, 103, 104, 154, 163, 170], and reinstatement of opioid conditioned reward and drug-seeking [81, 171]. However, although the subject of much debate, the notion that GluN2B-expressing receptors upregulates AMPARs seems to run counter to many studies indicating these sub-types exert a negative effect on AMPAR insertion/expression. How might this upregulation occur? One speculation is that it involves alterations extrasynaptic NMDAR-mediated regulation of slow-inward currents (SICs) or voltage-gated potassium channels due to a shift towards increased GluN2B:GluN2A-expressing receptors [81, 123, 172–174]. These changes may in turn promote increases in MSN intrinsic excitability that are known to precede and perhaps promote increased AMPAR signaling during withdrawal [123, 172, 173].
To date, clinical studies using NMDAR modulators have had relatively limited efficacy in the treatment of addiction, while partial agonists such as D-cycloserine may benefit extinction learning during cue exposure therapy [73, 175, 176], however the lack of consistency across trials indicate that additional studies are needed for these compounds. Alternatively, recent data indicate a need to develop and study novel ligands that weakly activate NMDARs, such as acamprosate, indirectly alter NMDAR function, or as the above data suggest, permit targeting of specific subpopulations of NMDARs based on subunit expression.
Concluding Remarks
Although the mechanism may differ across drug classes, the above data highlight a core feature of drug-induced plasticity at the circuit level -- a progressive increase in the ratio between excitatory synaptic drive at D1R- over D2R-MSN synapses during withdrawal (Figure 3). This shift in plasticity reflects a combination of presynaptic and postsynaptic changes that are not necessarily mutually exclusive. For example, withdrawal from cocaine and morphine promote increased glutamate release at D1R-MSNs, which is paralleled by a reduction in release at D2R-MSNs in morphine-treated mice [47, 54, 124, 125]. At the postsynaptic level, the development of cocaine-induced adaptations reflects generation of new silent synapses and concomitant spinogenesis preferentially on D1R-MSNs during early withdrawal that become stabilized with the progression of withdrawal through insertion of AMPARs in both the core and shell [100, 103, 104, 137]. Alternatively, during the early stages of withdrawal from repeated morphine administration, AMPARs are removed from synapses on D2-MSNs [100]. Over time, evidence suggests that these “weakened” glutamatergic synapses on D2R-MSNs are removed -- precipitating loss of dendritic spines and thus AMPAR-mediated glutamate transmission [47, 100]. While pathway-specific plasticity produced by cocaine and opioids varies, enhanced glutamatergic transmission within the PFC-to-NAc pathway is shared across drug classes and drug models [47, 53, 54, 126, 127], further reinforcing the importance of plasticity within this pathway in addiction.
Previous work identified opposing effects of psychostimulants and opioids on spine dynamics in NAc MSNs, suggesting that mechanisms underlying plasticity in these neurons is fundamentally different across drug classes. However, data from identified sub-populations of MSNs suggest that the overall effect of these microcircuit changes may be the same at macro level – increased excitatory drive at D1-MSNs. Comprising two parallel and distinct striatal pathways, activation of D1R- vs. D2R-MSNs appear to be diametrically opposed in their effects on behavior, with activation of D1R-MSN generally promoting behavior and reward and the D2R-MSN pathway more aligned with inhibition of behavior and aversion [66, 133, 177, 178]. Therefore, a shift in excitatory drive in favor of the D1R-MSN pathway may a critical modification that permits drugs of abuse to more readily control behavior during the development and persistence of addiction.
While the addiction field has made important leaps in understanding the neurobiological consequences of drug exposure and how these adaptations relate to addictive behavior, many issues need to be addressed going forward (Outstanding Questions). It is our hope that the above discussion has highlighted a number of important considerations going forward in the field of opioid and psychostimulant addiction research. Opioids and psychostimulants are known to promote plasticity across a number of brain regions which contribute to plasticity observed in the NAc and retain important implications for addiction in their own regard. Furthermore, these two drug classes undoubtedly produce distinct neurobiological, behavioral and psychological effects -- thus generalizations across drugs of abuse should be made with extreme caution [7]. However, these drugs also produce a number of similar adaptations, particularly changes in glutamate synaptic strength within the NAc, that when reversed by pharmacological or genetic means, disrupt reward and drug-seeking -- arguing that these adaptations are critical adaptations for treatments aimed at mitigating addiction to target (Figure 3, Key Figure).
Outstanding Questions.
Do intrinsic differences in postsynaptic signaling mechanisms (e.g., receptor subunits, ion channels) permit drugs of abuse exert selective effects on specific sub-circuits of the NAc?
How do drugs of abuse selectively alter afferent specific plasticity within the NAc? Are their divergent forms of presynaptic regulation at sub-types of MSNs (e.g., mGluR2/3 versus eCBR)?
Do alterations in glial-dependent modulation of glutamate signaling differentially impact D1R-versus D2R-MSN signaling?
What are the temporal, subunit, cell-type, and pathway-specific adaptations in NAc NMDAR signaling following self-administration of psychostimulants versus opioids?
Do the apparent divergent effects of psychostimulants and opioids on MSN intrinsic excitability reflect cell-type specific differences and how does contribute to overall similar changes in NAc microcircuitry glutamate transmission discussed here?
While much is known about drug-induced plasticity in the NAc, what other brain regions (e.g., prefrontal cortex, ventral pallidum, basolateral amygdala, ventral tegmental area) exhibitoverlapping and/or divergent forms of plasticity?
Text Box 1: Nucleus accumbens glutamate receptors
AMPA Receptors
Of the three main families of glutamate-gated ion channels, AMPARs are the most dynamic – with receptor trafficking in and out of synapses contributing to changes in synaptic strength in many forms of plasticity [179]. Assembling as homo- or heterotetramers made up of GluA1-4, approximately 90% of GluA1 subunits in NAc MSNs are associated with GluA2 (GluA2-containing) creating receptors that are permeable to Na+ but impermeable to Ca2+ and have a linear current-voltage (I–V) relationship [180, 181]. However, GluA1 expression is known to increase during experience-induced plasticity, which in turn favors formation of GluA1-homomeric AMPARs (GluA2-lacking) that are permeable to Ca2+ [182]. These GluA2-lacking receptors are permeable to Ca2+ [183] and correlate with enhanced channel conductance and long-term potentiation (LTP) leading to increased synaptic strength [181, 184].
NMDA Receptors
NMDA-type glutamate receptors (GluNs) often act in concert with co-localized AMPARs as synaptic coincidence detectors to facilitate plasticity [184]. Unlike AMPARs, NMDARs require glutamate binding and membrane depolarization to be activated. This depolarization expels extra extracellular Mg2+ from the channel pore, allowing Na+, K+, and Ca2+ to pass. Postsynaptic (or presynaptic) Ca2+ influx through NMDARs in turn activates intracellular signaling cascades that ultimately are responsible for changes in synaptic efficacy. NMDARs exist as heterotetramers composed of two obligatory GluN1 subunits, and two additional GluN2 or GluN3 subunits [184]. Differences in GluN2 subunit compositions (GluN2A-D) largely determines channel open time, intracellular coupling, and sometimes cellular localization. Most GluN1/GluN2A receptors are incorporated into synapses, while GluN1/GluN2B receptors are present at both synaptic and extrasynaptic sites [184]. Notably, evidence also suggests that expression of GluN subunits may be selective for MSN sub-populations, however data regarding this distribution is complex [185, 186].
Text Box 2: Measuring alterations in excitatory signaling
Modifications in transmission at excitatory synapses in the NAc often involve a combination of postsynaptic receptor and ion channel number/conductance[187], presynaptic neurotransmitter release, and glial-cell dependent regulation of non-synaptic extracellular glutamate [59, 73]. Ex vivo whole-cell patch clamp electrophysiology a very useful tool commonly used to assess these changes following exposure to drugs of abuse. Initial assessments of synaptic strength often begin by measuring synaptic AMPAR and NMDAR-mediated currents. This involves electrically or optically-evoking presynaptic glutamate release through axonal/terminal stimulation that binds to postsynaptic MSNs causing an influx of ions receptors that can be measured with a recording electrode as excitatory postsynaptic currents (EPSCs). Basal strength of excitatory synapses is difficult to compare between cells and acute slices using this approach due to variability in density of afferent fibers stimulated and number of synapses activated, thus responses are normalized isolating specific currents to construct a ratio of AMPAR:NMDAR currents that is independent of these variables. Importantly, by assessing AMPAR-mediated EPSCs at −80 mV and NMDAR-mediated currents at +40 mV, this method can circumvent the potential confound of changes in the proportion of Ca2+-permeable AMPARs (GluA2-lacking) that would influence the AMPAR component when recorded at more depolarized (+40 mV) potentials.
Alterations in the A/N ratio, can reflect a change in AMPAR or NMDAR-mediated currents, making it important to ascertain the underlying mechanism. This can be done by examining post-synaptic responses to non-evoked release of neurotransmitter vesicles -- events that can be isolated as AMPAR- or NMDAR-dependent based on solution composition and voltage-clamp potential. Although terminology can vary, spontaneous EPSCs (sEPSCs) include action-potential-dependent and -independent currents, whereas miniature (mEPSCs) reflect events in the absence of action potentials -- thus corresponding to the response elicited by a single vesicle of transmitter [188].
Alterations in s/mEPSC amplitude is generally considered to reflect postsynaptic plasticity, whereas changes in frequency can be attributed to presynaptic release probability or transient postsynaptic modifications (increased receptor or synapse number) [105, 189]. To determine whether changes align with presynaptic modifications a paired pulse ratio (PPR) can be used to measure pre-synaptic efficacy [187]. Here, two EPSCs are evoked at varying inter-stimulus intervals (e.g., 20–200 ms), with the ratio defined as the amplitude of the second EPSC divided the first. Notably, discrepancies between mEPSCs and PPR should be interpreted with caution, as PPRs are generated from a limited number of stimulated synapses, whereas the mEPSCs reflect input from “all” afferents.
Text Box3: Potential targets for treating opioid dependence
Preclinical models of psychostimulant addiction often include non-contingent models (experimenter administration) to measure pharmacodynamic drug effects, rewarding/reinforcing properties of a drug, and drug-cue memories in their simplest form. Self-administration approaches on the other hand employ volitional drug intake where animals perform operant tasks (e.g., pressing a lever) to receive an intravenous infusion of drug. Following daily self-administration, animals typically undergo a period of forced-abstinence (withdrawal) or extinction training, at which point re-exposure to the drug, drug-paired cue/contexts or stressors can be used to evoke renewed drug seeking.
Although not prevalent with psychostimulants, preclinical and clinical data indicate that following abrupt cessation of chronic or acute opioids can lead to precipitation of physical withdrawal and negative emotional states [27, 28, 135]. Preclinical approaches to examining tolerance and dependence often employ subcutaneous implantation of morphine pellets that promotes a steady state level of morphine in the body. Withdrawal can subsequently occur spontaneously or be precipitated by opioid receptor antagonist (Naloxone) administration [27]. This is drastically different from repeated intermittent non-contingent models that typically incorporate multiple 24 h periods of withdrawal throughout the experiment – an important consideration when interpreting neural adaptations produced by opioids. Complicating matters further, while physical signs of opioid withdrawal generally subside within a week, affective/cognitive components of withdrawal syndromes can persist upwards of 80 days [27, 28].
Manifestation of opioid-induced withdrawal signs align temporally with reductions in NAc core and shell GluA1 surface expression 1 or 72 h following repeated or acute morphine, respectively, while naloxone-precipitated withdrawal from chronic morphine is associated with reductions in NAc GluA2-lacking AMPARs [27, 100, 135, 190]. Alternatively, AMPAR-mediated signaling and surface expression is elevated in the NAc shell and core immediately (3–4 h) following an acute morphine injection (Anderson and Hearing, in press)[135], indicating that increases and decreases in AMPAR signaling may be important for the rewarding effects and withdrawal-associated aversive states, respectively. Biochemical and electrophysiological evidence also show time-dependent effects on NMDAR signaling whereby predominating GluN2A-expressing receptors during early withdrawal give-way to GluN2B-expressing receptors following more protracted withdrawal [100, 152, 156], suggesting a role for GluN2A receptors in development or expression of morphine tolerance or withdrawal, respectively. Supporting this contention, GluN2A knockout mice show marked loss of typical withdrawal abstinence behaviors precipitated by naloxone, which can be restored by rescuing GluN2A expression selectively in the NAc [191, 192]. These data highlight potentially unique adaptations produced by opioids during early withdrawal that may be early intervention targets for mitigating progressive increases drug use to avoid aversive effects associated with dependence and withdrawal.
Supplementary Material
Trends Box.
This review will provide an inimitable perspective on the striking overlap of neural adaptations in nucleus accumbens (NAc) excitatory produced by psychostimulants and opioids across preclinical and clinical data.
We postulate that previously conceived notions of divergent plasticity may in fact have similar consequences at the behavioral and neural circuit level, and that neural adaptations that are conserved across multiple drug classes provide likely candidate mechanisms underlying core features of addiction and may provide more targetable mechanisms for future pharmacotherapies.
Data discussed in this review will focus on electrophysiological (and to some extent biochemical) findings that touch on multiple forms of plasticity at NAc MSN glutamate synapses -- addressing what is known regarding the pharmacodynamic effects of psychostimulants and opioids from studies employing non-contingent models of drug administration, followed by examination of plasticity associated with intravenous drug self-administration and the relevance of these adaptations in preclinical models
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Reyes M, et al. Clinical effects of daily methamphetamine administration. Clin Neuropharmacol. 1991;14(4):352–8. doi: 10.1097/00002826-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Isenschmid DS. Cocaine - Effects on Human Performance and Behavior. Forensic Sci Rev. 2002;14(1–2):61–100. [PubMed] [Google Scholar]
- 6.Zacny JP, et al. A dose-response analysis of the subjective, psychomotor and physiological effects of intravenous morphine in healthy volunteers. J Pharmacol Exp Ther. 1994;268(1):1–9. [PubMed] [Google Scholar]
- 7.Badiani A, et al. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12(11):685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34(12):689–95. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 10.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 11.Ehrman R, et al. Conditioned tolerance in human opiate addicts. Psychopharmacology (Berl) 1992;108(1–2):218–24. doi: 10.1007/BF02245311. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF. Neural Mechanisms of Drug Reinforcementa. Annals of the New York Academy of Sciences. 1992;654(1):171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 13.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 14.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 15.Peters J, De Vries TJ. Glutamate mechanisms underlying opiate memories. Cold Spring Harb Perspect Med. 2012;2(9):a012088. doi: 10.1101/cshperspect.a012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35(2):185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalivas PW, et al. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett P, Barnard JM, Downs GM. Chemical Similarity Searching. Journal of Chemical Information and Computer Sciences. 1998;38(6):983–996. [Google Scholar]
- 19.Martin YC, Kofron JL, Traphagen LM. Do Structurally Similar Molecules Have Similar Biological Activity? Journal of Medicinal Chemistry. 2002;45(19):4350–4358. doi: 10.1021/jm020155c. [DOI] [PubMed] [Google Scholar]
- 20.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63(2):348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 22.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221(2–3):227–34. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 24.Harris JE, Baldessarini RJ. Uptake of (3H)-catecholamines by homogenates of rat corpus striatum and cerebral cortex: effects of amphetamine analogues. Neuropharmacology. 1973;12(7):669–79. doi: 10.1016/0028-3908(73)90120-2. [DOI] [PubMed] [Google Scholar]
- 25.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277(1):119–27. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chartoff EH, Connery HS. It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:116. doi: 10.3389/fphar.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothwell PE, Thomas MJ, Gewirtz JC. Protracted manifestations of acute dependence after a single morphine exposure. Psychopharmacology (Berl) 2012;219(4):991–8. doi: 10.1007/s00213-011-2425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrario CR, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61(7):1141–51. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24(5–6):356–62. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimer L, et al. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 33.Zahm DS, Brog JS. On the significance of subterritories in the "accumbens" part of the rat ventral striatum. Neuroscience. 1992;50(4):751–67. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 34.Everitt BJ, et al. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 35.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95(1):1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorn P, et al. Putting a spin on the dorsal–ventral divide of the striatum. Trends in Neurosciences. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Heimer L, et al. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9(3):354–81. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15(5 Pt 1):3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355(3):418–26. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 41.Lobo MK, et al. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9(3):443–52. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 42.Smith RJ, et al. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23(4):546–52. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–85. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupchik YM, et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18(9):1230–2. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cazorla M, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81(1):153–64. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders A, et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015;521(7550):85–9. doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hearing MC, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci U S A. 2016;113(3):757–62. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matamales M, et al. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4(3):e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valjent E, et al. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32(10):538–47. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24(1):85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 51.Hogarth L, et al. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- 52.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–12. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 53.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–64. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 54.Suska A, et al. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci U S A. 2013;110(2):713–8. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann PA, et al. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology. 2016;41(9):2399–410. doi: 10.1038/npp.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17(9):1198–207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan K, et al. Morphine treatment enhances glutamatergic input onto neurons of the nucleus accumbens via both disinhibitory and stimulating effect. Addict Biol. 2016 doi: 10.1111/adb.12438. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Y, et al. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530(7589):219–22. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scofield M, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacological reviews. 2016;68(3):816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen HW, et al. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014;39(5):1169–77. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowers MS, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42(2):269–81. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao L, et al. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(24):8746–51. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16(11):2231–5. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- 64.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moussawi K, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108(1):385–90. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328(5986):1709–12. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 67.Leppik IE. Zonisamide: chemistry, mechanism of action, and pharmacokinetics. Seizure. 2004;13(Suppl 1):S5–9. doi: 10.1016/j.seizure.2004.04.016. discussion S10. [DOI] [PubMed] [Google Scholar]
- 68.Thomas SP, Nandhra HS, Jayaraman A. Systematic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD) J Ment Health. 2010;19(2):168–75. doi: 10.3109/09638230903469269. [DOI] [PubMed] [Google Scholar]
- 69.Markou A. The role of metabotropic glutamate receptors in drug reward, motivation and dependence. Drug News Perspect. 2007;20(2):103–8. doi: 10.1358/dnp.2007.20.2.1083435. [DOI] [PubMed] [Google Scholar]
- 70.Fellin T. Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J Neurochem. 2009;108(3):533–44. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- 71.Haydon PG, et al. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56(Suppl 1):83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20(6):610–22. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 75.Kau KS, et al. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155(2):530–7. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9(1):59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madayag A, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27(51):13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue-and heroin-induced drug-seeking. Biol Psychiatry. 2008;63(3):338–40. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–9. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moran MM, et al. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25(27):6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen HW, et al. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014;34(16):5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trantham-Davidson H, et al. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–10. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77(5):867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jedynak J, et al. Cocaine and Amphetamine Induce Overlapping but Distinct Patterns of AMPAR Plasticity in Nucleus Accumbens Medium Spiny Neurons. Neuropsychopharmacology. 2016;41(2):464–76. doi: 10.1038/npp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kourrich S, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas MJ, et al. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4(12):1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 87.Brebner K, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310(5752):1340–3. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 88.Famous KR, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28(43):11061–70. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt HD, et al. ADAR2-dependent GluA2 editing regulates cocaine seeking. Mol Psychiatry. 2015;20(11):1460–6. doi: 10.1038/mp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt HD, et al. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC gamma. J Neurosci. 2013;33(35):14160–9. doi: 10.1523/JNEUROSCI.2284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venton BJ, Robinson TE, Kennedy RT. Transient changes in nucleus accumbens amino acid concentrations correlate with individual responsivity to the predator fox odor 2,5-dihydro-2,4,5-trimethylthiazoline. J Neurochem. 2006;96(1):236–46. doi: 10.1111/j.1471-4159.2005.03549.x. [DOI] [PubMed] [Google Scholar]
- 92.Gass JT, et al. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16(2):215–28. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakabayashi KT, Kiyatkin EA. Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core. J Neurophysiol. 2012;108(1):285–99. doi: 10.1152/jn.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gipson CD, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110(22):9124–9. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29(11):610–6. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Sari Y, et al. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10(9):3178–82. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanse E, Seth H, Riebe I. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci. 2013;14(12):839–50. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- 99.Marie H, et al. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45(5):741–52. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 100.Graziane NM, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19(7):915–25. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koya E, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15(11):1556–62. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63(1):40–7. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–51. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–67. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. Journal of Neuroscience. 2011;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitaker LR, et al. Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biological psychiatry. 2016;80(3):246–256. doi: 10.1016/j.biopsych.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rothwell PE, Kourrich S, Thomas MJ. Synaptic adaptations in the nucleus accumbens caused by experiences linked to relapse. Biol Psychiatry. 2011;69(11):1124–6. doi: 10.1016/j.biopsych.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt HD, et al. Group I metabotropic glutamate receptor-mediated activation of PKC gamma in the nucleus accumbens core promotes the reinstatement of cocaine seeking. Addict Biol. 2015;20(2):285–96. doi: 10.1111/adb.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33(6):267–76. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bock R, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature Neuroscience. 2013;16(5):632-+. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67(1):11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlezon WA, Jr, Rasmussen K, Nestler EJ. AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse. 1999;31(4):256–62. doi: 10.1002/(SICI)1098-2396(19990315)31:4<256::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 114.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25(3):341–60. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 115.Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697(1–2):76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- 116.Karler R, Calder LD, Turkanis SA. DNQX blockade of amphetamine behavioral sensitization. Brain Res. 1991;552(2):295–300. doi: 10.1016/0006-8993(91)90095-d. [DOI] [PubMed] [Google Scholar]
- 117.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 118.Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 2013;3(2):a012021. doi: 10.1101/cshperspect.a012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25(40):9144–51. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159(1):414–26. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 121.Kim J, et al. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69(11):1026–34. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 122.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12(8):1036–41. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 123.Wu X, et al. Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J Neurosci Res. 2012;90(6):1270–83. doi: 10.1002/jnr.23025. [DOI] [PubMed] [Google Scholar]
- 124.Ortinski PI, et al. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37(7):1671–82. doi: 10.1038/npp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dobi A, et al. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31(5):1895–904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481(7379):71–5. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- 127.Terrier J, Luscher C, Pascoli V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology. 2016;41(7):1779–89. doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferrario CR, et al. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35(3):818–33. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ghasemzadeh MB, et al. Region-specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 131.McCutcheon JE, et al. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–43. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Purgianto A, et al. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38(9):1789–97. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bock R, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16(5):632–8. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Britt JP, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Russell SE, et al. Nucleus Accumbens AMPA Receptors Are Necessary for Morphine-Withdrawal-Induced Negative-Affective States in Rats. J Neurosci. 2016;36(21):5748–62. doi: 10.1523/JNEUROSCI.2875-12.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu L, et al. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 137.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gottwald MD, Aminoff MJ. New frontiers in the pharmacological management of Parkinson's disease. Drugs Today (Barc) 2008;44(7):531–45. doi: 10.1358/dot.2008.44.7.1217105. [DOI] [PubMed] [Google Scholar]
- 139.Pascuzzi RM, et al. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11(3):266–71. doi: 10.3109/17482960903307805. [DOI] [PubMed] [Google Scholar]
- 140.Kampman KM, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 141.Kampman KM, et al. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13(1):74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- 142.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13(12):1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur Neuropsychopharmacol. 2016;26(2):186–194. doi: 10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2(1):83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65(8):717–20. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639(1–3):47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reichel CM, et al. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36(4):782–92. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Danysz W, Zajaczkowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol. 1995;6(5 And 6):455–474. [PubMed] [Google Scholar]
- 149.McGeehan AJ, Olive MF. The anti-relapse compound acamprosate inhibits the development of a conditioned place preference to ethanol and cocaine but not morphine. Br J Pharmacol. 2003;138(1):9–12. doi: 10.1038/sj.bjp.0705059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spanagel R, et al. Acamprosate suppresses the expression of morphine-induced sensitization in rats but does not affect heroin self-administration or relapse induced by heroin or stress. Psychopharmacology (Berl) 1998;139(4):391–401. doi: 10.1007/s002130050730. [DOI] [PubMed] [Google Scholar]
- 151.Martin G, et al. Chronic morphine treatment alters NMDA receptor-mediated synaptic transmission in the nucleus accumbens. J Neurosci. 1999;19(20):9081–9. doi: 10.1523/JNEUROSCI.19-20-09081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murray F, et al. Nucleus accumbens NMDA receptor subunit expression and function is enhanced in morphine-dependent rats. Eur J Pharmacol. 2007;562(3):191–7. doi: 10.1016/j.ejphar.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 153.Ortinski PI. Cocaine-induced changes in NMDA receptor signaling. Mol Neurobiol. 2014;50(2):494–506. doi: 10.1007/s12035-014-8636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29(21):6955–63. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bajo M, et al. Chronic morphine treatment alters expression of N-methyl-D-aspartate receptor subunits in the extended amygdala. J Neurosci Res. 2006;83(4):532–7. doi: 10.1002/jnr.20756. [DOI] [PubMed] [Google Scholar]
- 156.Martin G, et al. Chronic morphine treatment alters N-methyl-D-aspartate receptors in freshly isolated neurons from nucleus accumbens. J Pharmacol Exp Ther. 2004;311(1):265–73. doi: 10.1124/jpet.104.067504. [DOI] [PubMed] [Google Scholar]
- 157.Crespo JA, et al. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann N Y Acad Sci. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- 158.Lu L, et al. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85(6):1604–13. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 159.Ortinski PI, Turner JR, Pierce RC. Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation and cocaine self-administration. J Neurosci. 2013;33(22):9451–61. doi: 10.1523/JNEUROSCI.5730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Self DW, et al. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jacobs EH, et al. Morphine causes a delayed increase in glutamate receptor functioning in the nucleus accumbens core. Eur J Pharmacol. 2005;511(1):27–30. doi: 10.1016/j.ejphar.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 162.Liu XS, et al. Dopamine D3 receptor-regulated NR2B subunits of N-methyl-d-aspartate receptors in the nucleus accumbens involves in morphine-induced locomotor activity. CNS Neurosci Ther. 2014;20(9):823–9. doi: 10.1111/cns.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang X, et al. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32(2):377–87. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 164.Shen H, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108(48):19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma YY, et al. Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem Res. 2011;36(3):383–91. doi: 10.1007/s11064-010-0342-9. [DOI] [PubMed] [Google Scholar]
- 166.Miyatake M, et al. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur J Neurosci. 2005;22(6):1476–88. doi: 10.1111/j.1460-9568.2005.04325.x. [DOI] [PubMed] [Google Scholar]
- 167.Suzuki T, et al. Effects of the non-competitive NMDA receptor antagonist ifenprodil on the morphine-induced place preference in mice. Life Sci. 1999;64(12):PL151–6. doi: 10.1016/s0024-3205(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 168.Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192(4):571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 169.Kao JH, Huang EY, Tao PL. NR2B subunit of NMDA receptor at nucleus accumbens is involved in morphine rewarding effect by siRNA study. Drug Alcohol Depend. 2011;118(2–3):366–74. doi: 10.1016/j.drugalcdep.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 170.Scheggi S, et al. Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience. 2002;109(2):267–74. doi: 10.1016/s0306-4522(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 171.Liu SB, et al. Gentiopicroside attenuates morphine rewarding effect through downregulation of GluN2B receptors in nucleus accumbens. CNS Neurosci Ther. 2012;18(8):652–8. doi: 10.1111/j.1755-5949.2012.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16(3):173–84. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- 173.Wu X, et al. Effects of morphine withdrawal on the membrane properties of medium spiny neurons in the nucleus accumbens shell. Brain Res Bull. 2013;90:92–9. doi: 10.1016/j.brainresbull.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 174.Sepulveda J, Oliva P, Contreras E. Neurochemical changes of the extracellular concentrations of glutamate and aspartate in the nucleus accumbens of rats after chronic administration of morphine. Eur J Pharmacol. 2004;483(2–3):249–58. doi: 10.1016/j.ejphar.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 175.Bisaga A, et al. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111(1–2):97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tomek SE, et al. NMDA Receptor Modulators in the Treatment of Drug Addiction. Pharmaceuticals (Basel) 2013;6(2):251–68. doi: 10.3390/ph6020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hikida T, et al. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 178.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 180.Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–33. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pellegrini-Giampietro DE. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26(1):9–11. doi: 10.1016/s0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 182.Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49(5):663–70. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 183.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–34. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 184.Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fantin M, et al. NR2A and NR2B subunit containing NMDA receptors differentially regulate striatal output pathways. J Neurochem. 2007;103(6):2200–11. doi: 10.1111/j.1471-4159.2007.04966.x. [DOI] [PubMed] [Google Scholar]
- 186.Jocoy EL, et al. Dissecting the contribution of individual receptor subunits to the enhancement of N-methyl-d-aspartate currents by dopamine D1 receptor activation in striatum. Front Syst Neurosci. 2011;5:28. doi: 10.3389/fnsys.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Manabe T, et al. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70(4):1451–9. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 188.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9(6):423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 189.Graziane N, Dong Y. Electrophysiological Analysis of Synaptic Transmission. Springer; New York: 2015. [Google Scholar]
- 190.Glass MJ, et al. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210(2):750–61. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci. 2003;23(16):6529–36. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miyamoto Y, et al. Behavioural adaptations to addictive drugs in mice lacking the NMDA receptor epsilon1 subunit. Eur J Neurosci. 2004;19(1):151–8. doi: 10.1111/j.1460-9568.2004.03086.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.