Abstract
Formation of neural and sensory progenitors in the inner ear requires Sox2 in mammals, and in other species is thought to rely on both Sox2 and Sox3. How Sox2 and/or Sox3 promote different fates is poorly understood. Our mutant analysis in zebrafish showed that sox2 is uniquely required for sensory development while sox3 is uniquely required for neurogenesis. Moderate misexpression of sox2 during placodal stages led to development of otic vesicles with expanded sensory and reduced neurogenic domains. However, high-level misexpression of sox2 or sox3 expanded both sensory and neurogenic domains to fill the medial and lateral halves of the otic vesicle, respectively. Disruption of medial factor pax2a eliminated the ability of sox2/3 misexpression to expand sensory but not neurogenic domains. Additionally, mild misexpression of fgf8 during placodal development was sufficient to specifically expand the zone of prosensory competence. Later, cross-repression between atohla and neurogl helps maintain the sensory-neural boundary, but unlike mouse this does not require Notch activity. Together, these data show that sox2 and sox3 exhibit intrinsic differences in promoting sensory vs. neural competence, but at high levels these factors can mimic each other to enhance both states. Regional cofactors like pax2a and fgf8 also modify sox2/3 functions.
Keywords: SoxB1, sensory epithelia, statoacoustic ganglion, otic placode
INTRODUCTION
Development of the inner ear is a highly dynamic process in which neurons, sensory epithelia, and a variety of non-sensory cell types arise from a simple epithelial structure, the otic placode. The otic placode quickly forms a fluid-filled cyst, the otic vesicle, which then further elaborates the complex shape and array of cell types comprising the inner ear. Sensory epithelia and neural progenitors originate in a ventral region of the early otic vesicle. In mammals and birds, neuroblasts arise first and subsequently delaminate from the otic epithelium and are quickly replaced by developing sensory epithelia (Raft and Groves, 2015; Raft et al., 2007). In zebrafish, neuroblasts and sensory epithelia initially form simultaneously in abutting domains in the floor of the otic vesicle, after which neuroblasts delaminate and differentiate in a manner similar to tetrapod vertebrates (Haddon and Lewis, 1996; Kantarci et al., 2016; Millimaki et al., 2007). Despite differences in timing and degree of spatial overlap of neural vs. sensory development, many of the same regulatory genes operate in all vertebrate species. The transition from neural to sensory development in mammals is triggered in part by cross-repression between proneural and prosensory factors Ngn1 and Atoh1 (Raft et al., 2007). In principle a similar cross-repression could help stabilize spatial segregation of sensory and neural fates in zebrafish, although this has not been formally investigated.
In all vertebrates, members of the SoxB1 family of transcription factors are required for normal development of both sensory epithelia and neurons of the inner ear (reviewed by Raft and Groves, 2015). In non-mammalian vertebrate species, Sox3 is the first to be expressed during placodal development and appears to presage neural development, marked by expression of the proneural gene Neurog1 (Abello et al., 2010; Neves et al., 2007; Nikaido et al., 2007; Sun et al., 2007). Sox2 is expressed at later stages and is associated with development of sensory epithelia, marked by expression of the prosensory gene Atoh1 (Millimaki et al., 2010; Neves et al., 2007). However, Sox2 and Sox3 show overlapping domains of expression for extended periods of otic development. This raises questions about whether Sox2 and Sox3 act redundantly in otic development, and how their cell-type specific functions are regulated (i.e. how do they differentially activate Neurog1 vs. Atoh1). In mammals, Sox3 is not expressed in the otic vesicle, whereas Sox2 is expressed in the floor of the otic vesicle and is required for both neurons and sensory epithelia (Kiernan et al., 2005; Puligilla et al., 2010; Steevens et al., 2017). Moreover, replacement of the Sox3 coding region with Sox2 permits development of mice that are viable and morphologically normal, suggesting that Sox2 and Sox3 are largely redundant (Adikusuma et al., 2017). Nevertheless, it is still not understood how Sox2 can regulate both neurons and sensory epithelia in the same tissue, a problem that is presumably related to how Sox2 and Sox3 regulate different cell fates in non-mammalian vertebrates.
SoxB1 factors are well known for providing two seemingly contradictory functions. They can maintain pluripotency of stem cells and progenitors (Bylund et al., 2003; Goldsmith et al., 2016; Rizzino and Wuebben, 2016; Surzenko et al., 2013; Tucker et al., 2010), and they can promote early differentiation of various cell types and tissues (Amador-Arjona et al., 2015; Archer et al., 2011; Hoffmann et al., 2014; Okuda et al., 2010; Rogers et al., 2009). A number of studies have shown that the nature of SoxB1 function can vary depending on their level of expression (Boer et al., 2007; Hutton and Pevny, 2011; Kopp et al., 2008; Rizzino and Wuebben, 2016) or the availability of region- or stage-specific cofactors (Ambrosetti et al., 1997; Boer et al., 2007; Chew et al., 2005; Kamachi et al., 2001; Kondoh and Kamachi, 2010). It is unknown which of these variables regulates the ability of Sox2 and Sox3 (or Sox2 alone) to differentially regulate neural and sensory fates in the otic vesicle.
We have investigated the shared and unique functions of sox2 and sox3 in zebrafish through analysis of knockout lines and heat shock-inducible transgenes. Mutant analysis confirms that sox2 is uniquely required for normal sensory development whereas sox3 is uniquely required for neural development. Some misexpression studies also support these gene-specific functions. For example, misexpression of sox2 at a moderate level during placodal development expands the domain of sensory development in the otic vesicle while restricting the domain of neurogenesis. However, when misexpressed at high levels, sox2 and sox3 mimic each other and lead to dramatic expansion of sensory and neural fates throughout the medial and lateral walls of the otic vesicle, respectively. The ability to expand sensory fates, but not neural fate, requires the medial factor pax2a. Early misexpression of sox2 or sox3 did not accelerate the onset of sensory or neural development, rather expansion of these fates occurred gradually after formation of the otic vesicle. Moreover, misexpression of sox2 or sox3 at later stages temporarily halts expression of prosensory and proneural factors atohla and neurog1. Analysis of additional markers shows that misexpression of sox2 or sox3 expands anterior-ventral identity (including the zones of sensory-neural competence) throughout the otic vesicle. Together these data suggest that sox2 and sox3 promote sensory-neural competence while delaying onset of sensory and neural differentiation. Regionally expressed factors such as pax2a and fgf8 then help to diversify sox2/3 function to establish sensory and neural fates in spatially segregated domains. We also confirm that cross-repression between atoh1a and neurog1 help reinforce the sensory-neural boundary.
MATERIALS AND METHODS
Fish strains and developmental conditions
Wild-type zebrafish were derived from the AB line (Eugene, OR). Mutant alleles sox2x50, sox3x52 (Gou et al., 2017) and mibta52b (Itoh et al., 2003; Jiang et al., 1996) and genotyping methods were previously described. Transgenic line TG(brn3c:gap43-GFP) (Xiao et al., 2005) was used to visualize sensory hair cells. Transgenic lines TG(hsp70:fgf8a)x17, TG(hsp70:atoh1a)x20, TG(hsp70:sox2)x21 (Millimaki et al., 2010), TG(hsp70:sox3)x32 (Gou et al., 2017) and TG(hsp70:ngn1)x28 (Kantarci et al., 2016) used in the misexpression studies here were referred to as hs:fgf8, hs:atoh1a, hs:sox2, hs:sox3 and hs:neurog1 respectively. Embryos were developed under standard conditions at 28.5°C (Kimmel et al., 1995), except during and after heat shock, in fish water containing methylene blue. Embryos were staged based on standard morphological features (Kimmel et al., 1995). To prevent melanin formation in older embryos (>24 hpf), PTU (1-phenyl 2-thiourea, 0.3 mg/ml) was added to fish water during development.
Gene misexpression and morpholino injections
Misexpression of various genes was achieved by briefly incubating embryos heterozygous or homozygous for heat shock inducible transgenes in a water bath at 38 or 39°C for 30 or 60 minutes, except where noted in the text. For experiments involving activation of hs:sox3 at 12 hpf, heat shock was performed at 38°C because 39°C heat shock at this time led to severe axial truncation by 24 hpf, precluding meaningful interpretation of results. In contrast, activation of hs:sox3 at 39°C was readily tolerated at later stages. In all cases, after heat-shock embryos were maintained at 33°C until fixation. Wild-type embryos were also heat shocked to serve as controls for all misexpression studies. Knock-down of pax2a was achieved by injecting 5 ng of morpholino oligomers (mo), obtained from Gene Tools, Inc, into one-cell stage embryos. Sequence of pax2a-mo was previously described (Bricaud and Collazo, 2006). In all morpholino knock-downs, embryos were co-injected with p53-mo (Robu et al., 2007) to prevent non-specific cell death. Phenotypes described here were assessed in at least 15 embryos per probe and time point unless stated otherwise.
In situ hybridization and immunohistochemistry
Whole-mount in situ hybridization, two-color in situ hybridization and immunostaining were performed as previously described (Jowett and Yan, 1996; Phillips et al., 2001; Riley et al., 1999). Primary antibody used to label mature neurons of statoacoustic ganglion (SAG) is anti-Islet1/2 (Developmental Studies Hybridoma Bank 39.4D5, 1:100), secondary antibody is Alexa 546 goat anti-mouse IgG (ThermoFisher Scientific A-11003, 1:50). For cell proliferation analysis, anti-phospho-Histone H3 (EMD MILLIPORE 06–570, 1:350) and Alexa 546 goat anti-rabbit IgG (ThermoFisher Scientific A-11010, 1:50) were used.
Cell transplantation and cryo-sectioning
Donor hs:sox3/hs:sox3 embryos were injected with lineage tracer (10,000 MW, lysine-fixable tetramethylrhodamine labeled dextran in 0.2 M KCl) at one-cell stage. Donor cells were transplanted into unlabeled wild-type host embryos during late blastula stage. Embryos stained by whole-mount in situ hybridization were processed for cryo-sectioning as previously described (Vemaraju et al., 2012) and cut into serial 10-μm sections then mounted in 30% glycerol, except that sections from mosaic embryos were mounted in SlowFade Gold antifade reagent with DAPI (Life technologies).
Genotyping and data analysis
To identify sox2−/−, sox3−/− single mutants and sox2−/−; sox3−/− double mutants from sox2+/−; sox3+/−; brn3c:gap43-GFP triple carrier intercross, tails of individual embryos, post Islet1/2 immunostaining for Fig. 2B, were used for DNA extraction and single-embryo genotyping described previously (Gou et al., 2017). Quantification of gene expression area was performed using Photoshop measuring number of pixels. Areas shown in the figures were normalized relative to wild-type control embryos. Quantification of number of cells expressing certain gene or protein was done in either whole mounts (atoh1b, phospho-Histone H3 and Islet1/2 staining) or in serial sections (atoh1a, neurog1 staining in mosaic embryos). Hair cells were counted in fixed whole mount embryos by imaging TG(brn3c:gap43-GFP) fluorescence, a stable marker of mature hair cells (Xiao et al., 2005). Mature SAG neurons were counted in whole mounts stained with anti-Isl1/2 monoclonal antibody. Expression of Isl1/2 marks SAG cells that have completed migration, become post-mitotic, and sprouted projections to synaptic targets (Vemaraju et al., 2012). Statistical pair-wise comparisons were performed using students’ t-test. For experiments that involve more than two groups, significance was evaluated by ANOVA and Tukey’s post-hoc HSD tests.
Figure 2. Distinct roles for sox2 and sox3 in sensory and neural development.

(A, B) Box-and-whisker plots of the total number of hair cells at 38 hpf (A) and mature SAG neurons at 36 hpf (B) in control, sox2−/−, sox3−/−, and sox2−/−; sox3−/− double mutant embryos. Green lines represent means. Asterisks indicate statistically significant differences compared to controls (***P<0.001, Tukey’s HSD test following ANOVA). (C-H) Dorsolateral views (anterior to left) of expression of atoh1a (C-E) and neurog1 (F-H) at 24 hpf in control embryos, hs:sox2/+ heterozygotes and hs:sox3/+ heterozygotes. Embryos were heat shocked at 12.5 hpf, 38°C or 39°C for 30 minutes, as indicated. (I-L) Expression of atoh1a (I, J) and neurog1 (K, L) at 24 hpf in cross sections through the middle of the otic vesicle in control embryos (I, K) and hs:sox3/+ heterozygotes (J, L). Embryos were heat shocked at 12.5 hpf, 38°C for 30 minutes. Otic vesicle borders are outlined in C-L. (M, N) Quantification of the total number of hair cells (M) and mature SAG neurons (N) at 30 hpf in control and hs:sox2/+ embryos. (O, P) Quantification of the total number of hair cells (O) and mature SAG neurons (P) at 31 hpf in control and hs:sox3/+ embryos. Error bars represent standard deviation in M-P, and asterisks indicate statistically significant differences relative to controls (*P<0.05, ***P<0.001, student’s t-test, n>13).
RESULTS
Expression of sox3 in the otic vesicle
Although expression of sox3 is a well known marker of early development of the otic placode (Nikaido et al., 2007; Sun et al., 2007), expression in the otic vesicle has not been described. We therefore characterized expression of sox3 in the otic vesicle at 24 hpf with respect to neurog1 in the neurogenic domain, atoh1a in sensory maculae, and the medial marker pax2a (Fig. 1A). Cross sections through the utricular macula show that sox3 is expressed at a relatively low level and overlaps with both atoh1a and pax2a (Fig. 1B-D). Slightly more posterior sections passing through the widest part of the neurogenic domain show that sox3 overlaps extensively with neurog1, but neither gene overlaps with pax2a (Fig. 1E-G). Additionally, sox3 is expressed in a gradient, with high levels in the medial half of the neurogenic domain and falling rapidly towards the lateral half. The lateral domain corresponds to the region where neuroblasts delaminate, a function that requires overlap of neurog1 with a lateral domain of goosecoid expression (Kantarci et al., 2016). Thus, sox3 is widely expressed in the floor of the otic vesicle but shows highest expression in newly specified neuroblasts, with levels declining as neuroblasts mature and delaminate. Additionally, the sensory-neural boundary corresponds closely to the pax2a expression domain. We previously reported that sox2 expression is restricted to prosensory regions of the otic placode and vesicle (Millimaki et al., 2010) and that sensory epithelia coexpress and upregulate pax2a (Riley et al., 1999).
Figure 1. Expression of sox3 in the otic vesicle overlaps sensory and neurogenic domains.
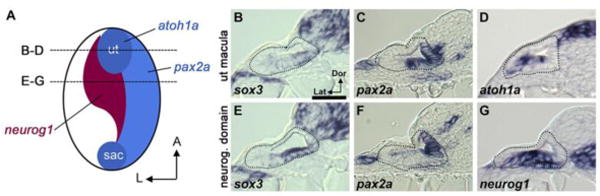
(A) Schematic depiction of the floor of the otic vesicle (anterior up, lateral to the left) showing expression domains of atoh1a in the utricular (ut) and saccular (sac) maculae, neurog1 in the neurogenic domain, and the medial marker pax2a. Dashed lines indicate the section planes shown in (B-D) and (E-G). (B-D) Expression of sox3 (B), pax2a (C) and atoh1a (D) in cross sections passing through the utricular macula at 24 hpf. (E-G) Expression of sox3 (E), pax2a (F) and neurog1 (G) in cross sections passing through the widest part of the neurogenic domain at 24 hpf. Otic vesicle borders are outlined in B-G.
Requirements for sox2 and sox3 in sensory and neural development
To test whether sox2 and sox3 play redundant or distinct roles in otic development, we examined accumulation of hair cells and neurons of the statoacoustic ganglion (SAG) in embryos disrupted for sox2 or sox3 or both sox2 and sox3. sox2−/− mutants show a 25% reduction in the number of hair cells at 38.5 hpf (Fig. 2A) and often display 1-2 dying hair cells being extruded from the otic epithelium, similar to previous findings in sox2 morphants (Millimaki et al., 2010). In contrast, the number of mature SAG neurons at 36 hpf is normal in sox2−/− mutants (Fig. 2B). Conversely, sox3−/− mutants produce a normal number of hair cells (Fig. 2A) but the number of mature SAG neurons is reduced by 23% at 36 hpf (Fig. 2B). Interestingly, sox2−/−;sox3−/− double mutants do not show additional reduction in accumulation of hair cells or SAG neurons compared to sox2−/− and sox3−/− single mutants, respectively (Fig. 2A, B). These data show that sox2 and sox3 are uniquely required for sensory and neural development, respectively, and there is no synergistic interaction between sox2 and sox3.
Misexpression of sox2 and sox3.
To further explore the unique and overlapping functions of sox2 and sox3, we tested the effects of misexpressing sox2 or sox3 at different developmental stages and with varied expression levels using heat shock-inducible transgenes. We began by misexpressing sox2 or sox3 at 12.5 hpf (7 somites stage), when developing otic cells are still uncommitted. For moderate misexpression, transgenic hs:sox2/+ and hs:sox3/+ heterozygotes were heat shocked for 30 minutes at 38-39°C (see Materials & Methods), yielding a pulse of transgene activity lasting roughly 2-3 hours after the end of the heat shock period (Padanad et al., 2012). Moderate misexpression of sox2 at 12.5 hpf led to a modest expansion of sensory epithelia at 24 hpf as shown by a slightly expanded domain of atoh1a (Fig. 2D) and a 29% increase in mature hair cells by 30 hpf (Fig. 2M). Under these same conditions, there was a marked reduction in the neurogenic domain at 24 hpf marked by neurog1 (Fig. 2G) and the number of mature SAG neuron was reduced by 21% at 30 hpf (Fig. 2N). In contrast to sox2, moderate misexpression of sox3 expanded both sensory and neurogenic domains at 24 hpf (Fig. 2E, H). Sections through the middle of the otic vesicle revealed ectopic atoh1a expressing cells in the medial wall of the otic vesicle (Fig. 2J compare to 2I), while ectopic neurog1 expressing cells appeared in the lateral wall of the otic vesicle (Fig. 2L compare to 2K). At later stages there was a corresponding increase in the number of mature hair cells and SAG neurons (Fig. 2O, P). Thus, when misexpressed at moderate levels, sox2 promotes sensory development and impairs neurogenesis, whereas sox3 promotes both sensory and neural development.
To achieve higher levels of misexpression, we increased transgene copy-number by generating hs:sox2/hs:sox2 and hs:sox3/hs:sox3 homozygotes. High-level misexpression of sox2 at 12.5 hpf led to a dramatic expansion of both sensory (Fig. 3B compare to 3A) and neurogenic domains by 24 hpf (Fig. 3F compare to 3E), with ectopic atoh1a expressing cells filling the medial wall (Fig. 3I) and some ectopic neurog1 expressing cells spreading into the lateral wall (Fig. 3L). High-level misexpression of sox3 at 12.5 hpf usually led to similar but even more pronounced expansion of sensory and neurogenic domains at 24 hpf (Fig. 3C, G), such that almost all medial cells express atoh1a and almost all lateral cells express neurog1 (Fig. 3J, M). However, in a subset of hs:sox3/hs:sox3 embryos, the neurogenic domain expanded to encompass nearly the entire otic vesicle including the medial wall (Fig. 3H, N) with a corresponding contraction of the sensory domain (Fig. 3D, K). Thus, when expressed at high levels, sox2 and sox3 can greatly expand both sensory and neural fates, and in extreme cases sox3 can lead to acquisition of neural fate by nearly all otic cells.
Figure 3. Effects of high-level misexpression of sox2 or sox3 during early placode development.
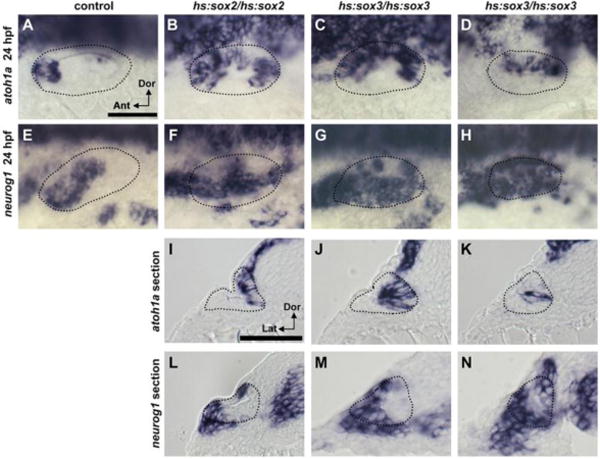
(A-H) Dorsolateral views (anterior to the left) of expression of atoh1a (A-D) and neurog1 (E-H) at 24 hpf in control (A, E), hs:sox2/hs:sox2 (B, F) and hs:sox3/hs:sox3 (C-H) embryos. (I-N) Expression of atoh1a (I-K) and neurog1 (L-N) at 24 hpf in cross sections (lateral to the left) through the middle of the otic vesicle in hs:sox2/hs:sox2 homozygotes (I, L) and hs:sox3/hs:sox3 homozygotes (J, K, M, N). hs:sox2/hs:sox2 homozygotes were heat shocked at 12.5 hpf, 39°C for 60 minutes, whereas hs:sox3/hs:sox3 homozygotes were heat shocked at 12.5 hpf, 38°C for 30 minutes. Otic vesicle borders are outlined.
We next tested the effects of misexpressing sox2 or sox3 at later stages of otic development. Moderate misexpression of sox2 at 18 hpf caused no obvious changes in expression of atoh1a or neurog1 at 24 hpf (data not shown), and high-level misexpression of sox2 at 18 hpf caused only a modest increase in sensory and neurogenic domains at 24 hpf (Fig. 4D, H). Moderate misexpression of sox3 at 18 hpf also led to a modest expansion of sensory and neurogenic domains at 24 hpf (Fig. 4B, F). In contrast, high-level misexpression of sox3 at 18 hpf strongly reduced expression of atoh1a and neurog1 at 24 hpf (Fig. 4C, G), indicating suppression of sensory and neural development.
Figure 4. Effects of misexpressing sox2 or sox3 at later stages.
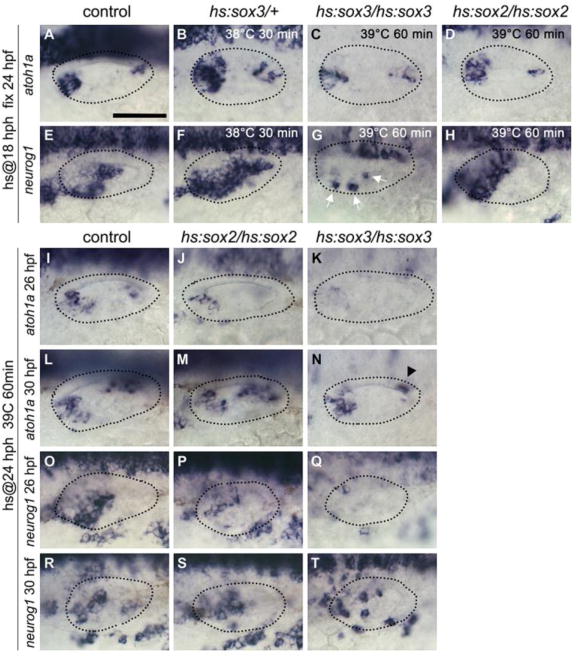
(A-H) Expression of atoh1a (A-D) and neurog1 (E-H) at 24 hpf in control (A, E), hs:sox3/+ (B, F), hs:sox3/hs:sox3 (C, G) and hs:sox2/hs:sox2 (D, H) embryos. Embryos were heat-shocked at 18 hpf with varying temperatures and durations as indicated. White arrows indicate otic expression of neurog1 in G. (I-N) Expression of atohla at 26 hpf (I-K) and 30 hpf (L-N) in control (I, L), hs:sox2/hs:sox2 (J, M) and hs:sox3/hs:sox3 (K, N) embryos following heat-shock (39°C for 60 minutes) at 24 hpf. Black arrowhead indicates atoh1a expression in the saccular macula in N, which is strongly reduced compare to L. (O-T) Expression of neurog1 at 26 hpf (O-Q) and 30 hpf (R-T) in control (O, R), hs:sox2/hs:sox2 (P, S) and hs:sox3/hs:sox3 (Q, T) embryos following heat-shock (39°C, 60 minutes) at 24 hpf. All images show dorsolateral views (anterior to the left) and otic vesicle borders are outlined.
Moderate misexpression of sox2 or sox3 at 24 hpf had no discernable effect on subsequent sensory or neurogenic domains (data not shown), but high-level misexpression at 24 hpf strongly suppressed expression of atohla and neurogl by 26 hpf (Fig. 4I-k, O-Q). By 30 hpf, expression of atoh1a and neurog1 recovered to near normal in hs:sox2/hs:sox2 embryos (Fig. 4M, S), whereas hs:sox3/hs:sox3 embryos continued to show partial suppression of atoh1a and neurog1 (Fig. 4N, T). Thus, the ability of sox2 and sox3 to expand sensory and neurogenic domains is gradually lost during later stages of otic development and instead high-level misexpression strongly suppresses sensory and neural development. This suggests that sox2 and sox3 promote an early state of sensory and neural competence while delaying early fate-specification, similar to their roles in establishing the neural plate during gastrulation (Archer et al., 2011; Bylund et al., 2003; Okuda et al., 2010; Rogers et al., 2009) .
A delayed response to early misexpression of sox2 and sox3.
To better characterize the effects of early misexpression, we examined when changes in atoh1a and neurog1 expression first become evident following high-level misexpression of sox2 and sox3. In hs:sox2/hs:sox2 embryos heat shocked at 12.5 hpf, expression of atoh1a was normal through 16.5 hpf (Fig. 5B), with the first signs of moderate expansion appearing by 18.5 hpf (Fig. 5E). Expression of neurog1 was reduced in hs:sox2/hs:sox2 at 16.5 hpf but showed moderate expansion by 18.5 hpf (Fig. 5H, K). In hs:sox3/hs:sox3 embryos heat shocked at 12.5 hpf, the domain of atoh1a was partially expanded at 16.5 hpf and continued to expand through 18.5 hpf (Fig. 5C, F). High-level misexpression of sox3 did not accelerate the onset of neurog1 expression, but the neurog1 domain was already partially expanded by 16.5 hpf and continued to expand through 18.5 hpf (Fig. 5I, L). To test whether expansion of sensory and neurogenic domains involved elevated proliferation, we examined patterns of phospho-histone H3 staining after misexpressing sox2 or sox3 at 12.5 hpf. There was no significant change in the number of phospho-Histone H3 positive cells in the otic vesicle in hs:sox2/hs:sox2 or hs:sox3/hs:sox3 embryos at any point before or during sensory-neural expansion (Fig. 5M, N). Thus, early activation of sox2 or sox3 did not immediately or directly induce sensory and neural fates, nor promote cell proliferation to expand the sensory and neurogenic domains. Instead, the delayed response to misexpression suggests that transient elevation of sox2 or sox3 during placodal stages causes lasting changes in developmental programming that enhance competence to form sensory and neural fates in the otic vesicle.
Figure 5. sox2 and sox3 do not directly specify neural or sensory fates.
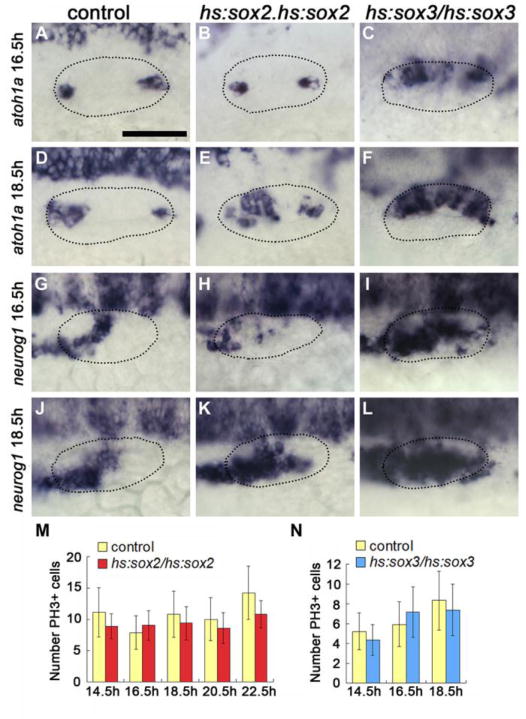
(A-F) Dorsolateral views (anterior to the left) showing otic expression of atoh1a at 16.5 hpf (A-C) and 18.5 hpf (D-F) in control (A, D), hs:sox2/hs:sox2 (B, E) and hs:sox3/hs:sox3 (C, F) embryos following heat-shock at 12.5 hpf. (G-L) Dorsolateral views showing otic expression of neurog1 at 16.5 hpf (G-I) and 18.5 hpf (J-L) in control (G J), hs:sox2/hs:sox2 (H, K) and hs:sox3/hs:sox3 (I, L) embryos following heat-shock at 12.5 hpf. Otic vesicle borders are outlined in all images. (M, N) Quantification of the number of phospho-Histone H3 positive (PH3+) cells in the otic vesicle of control and hs:sox2/hs:sox2 (M) or hs:sox3/hs:sox3 (N) embryos at multiple time points following heat-shock at 12.5 hpf. Error bars represent standard deviation. No statistically significant differences between control and hs:sox2/hs:sox2 or hs:sox3/hs:sox3 embryos at any time point examined (student’s t-test, n>15). In all panels, hs:sox2/hs:sox2 embryos were heat shocked at 39°C for 60 minutes, whereas hs:sox3/hs:sox3 embryos were heat shocked at 38°C for 30 minutes.
Effects of misexpression of sox2 and sox3 on patterning in the otic vesicle
The strong expansion of sensory and neurogenic domains following high-level misexpression of sox2 or sox3 suggested dramatic changes in patterning of the otic vesicle. To test this, we analyzed the expression of a variety of regional markers at 24 hpf after high-level misexpression of sox2 or sox3 at 12.5 hpf. Anterior markers fgf3 and pax5 were expanded throughout the medial wall of the otic vesicle at 24 hpf, as was fgf8 (Fig. 6A-C”), and the anterior-ventral marker hmx3a expanded to include nearly all cells in otic vesicle (Fig. 6E-E”). Medial expression of pax2a was not altered (Fig. 6D-D”) but the posterior-medial marker pou3f3b was lost in all embryos (Fig. 6H-H”). Posterior-lateral markers otx1b and gsc and the dorsal marker dlx3b were strongly reduced in hs:sox2/hs:sox2 embryos and were eliminated in hs:sox3/hs:sox3 embryos (Fig. 6F-F”, G-G”, J-J”). Similarly, tbx1, a marker of non-neural and non-sensory fates, was nearly eliminated in all embryos (Fig. 6I-I”). These data show that early misexpression of sox2 and sox3 leads to expansion of anterior-ventral identity in almost all cells in the otic vesicle, corresponding to the region shared by utricular sensory and neural fates. Despite this dramatic change in axial patterning, most embryos maintained a clear sensory-neural boundary, corresponding to the lateral boundary of the pax2a domain (see below).
Figure 6. Axial patterning in the otic vesicle following early high-level misexpression of sox2 or sox3.
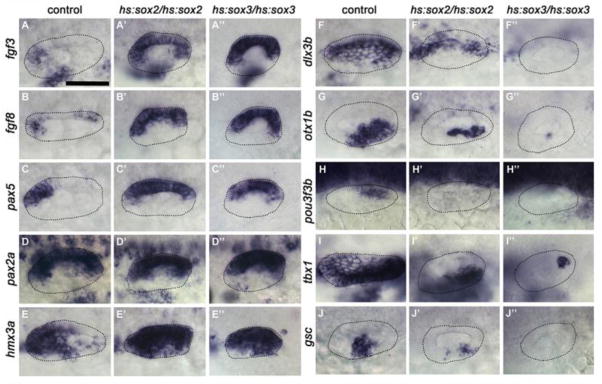
Dorsolateral views (anterior to the left) showing expression of various regional markers at 24 hpf in the otic vesicle of control (A-J), hs:sox2/hs:sox2 (A’-J’) and hs:sox3/hs:sox3 (A″-J″) embryos following heat-shock at 12.5 hpf. hs:sox2/hs:sox2 embryos were heat shocked at 39°C for 60 minutes, hs:sox3/hs:sox3 embryos were heat shocked at 38°C for 30 minutes. Otic vesicle borders are outlined in all images.
Mosaic misexpression of sox3
Because global misexpression could potentially alter surrounding tissues that normally provide signals needed for proper patterning of the otic vesicle, we transplanted hs:sox3/hs:sox3 cells into wild-type host embryos to test the effects of mosaic misexpression. We reasoned that if early misexpression of sox3 acts cell-autonomously to enhance sensory-neural competence, then transplanted hs:sox3/hs:sox3 cells should be able to adopt sensory and neural fates in ectopic locations within the otic vesicle. In support, when mosaic embryos were heat shocked at 12.5 hpf, 26.7% of transplanted hs:sox3/hs:sox3 cells located in regions outside endogenous sensory epithelia expressed atoh1a ectopically at 24 hpf (n=32 embryos, 105 transplanted cells) (Fig. 7A, B, E), whereas no wild-type cells transplanted into wild-type host embryos expressed atoh1a ectopically (n=8 embryos, 63 ectopic transplanted cells) (Fig. 7E). Similarly, 31.9% of transplanted hs:sox3/hs:sox3 cells located outside the endogenous neurogenic domain expressed neurog1 ectopically at 24 hpf (n=9 embryos, 72 transplanted cells) (Fig. 7C, D, F), whereas no control transplants expressed neurog1 ectopically (n=11 embryos, 83 ectopic transplanted cells) (Fig. 7F). These data support the idea that elevating sox3 at 12.5 hpf cell-autonomously enhances pro-sensory and pro-neural competence of otic cells.
Figure 7. Effects of early misexpression of sox3 in genetic mosaics.
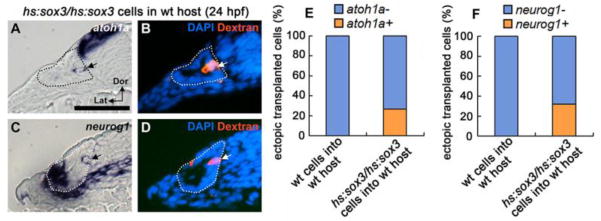
(A-D) Expression of atoh1a (A) and neurog1 (C) at 24 hpf in cross sections of wild-type hosts into which fluorescent dextran-labeled hs:sox3/hs:sox3 transgenic cells were transplanted. Mosaic embryos were heat shocked at 12.5 hpf, 38°C for 30 minutes. (B, D) Fluorescent image of dextran and DAPI staining on the same sample shown in A and C respectively. Sections pass through the middle of the otic vesicle, just posterior to the utricular macula. Arrows indicate transgenic cells that ectopically express atoh1a or neurog1. Otic vesicle borders are outlined. (E, F) Quantification of the percentage of transgenic cells located outside endogenous sensory or neural domains that ectopically express atoh1a (E) or neurog1 (F).
Interaction with pax2a influences sox2 and sox3 function
Previous studies suggested that SoxB1 factors can physically or genetically interact with other transcription factors to modify their functions (Ambrosetti et al., 1997; Boer et al., 2007; Chew et al., 2005; Kamachi et al., 2001; Kondoh and Kamachi, 2010). Because pax2a expression is restricted to the medial wall of the otic vesicle and helps regulate sensory development (Riley et al., 1999), we hypothesized that Pax2a locally biases the activity of Sox2 and Sox3 to promote sensory fate. To test this, we examined the effects of misexpressing sox2 or sox3 in pax2a−/− mutants or pax2a morphants. Disruption of pax2a function did not block formation of endogenous sensory epithelia (Fig. 8C; and Riley et al., 1999) but suppressed the ability of early high-level misexpression of sox2 to expand the sensory domain of atoh1a at 24 hpf (compare Fig. 8B, D). Importantly, knocking down pax2a did not suppress the ability of sox2 to expand the neurogenic domain of neurog1 (Fig. 8F, H). Similarly, disruption of pax2a suppressed the ability of sox3 misexpression to expand the sensory domain of atoh1a whereas expansion of the neurogenic domain of neurog1 still occurred (data not shown). These data support the hypothesis that the pro-sensory effect of Sox2 and Sox3 requires Pax2a, whereas the pro-neural function does not.
Figure 8. Pax2a is required for prosensory but not proneural expansion.
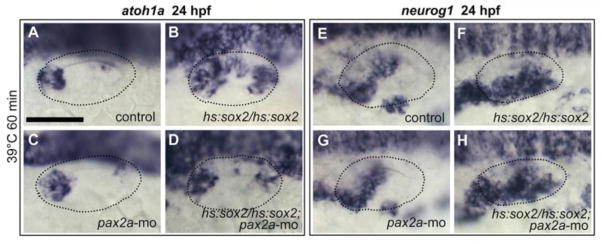
(A-H) Dorsolateral views (anterior to the left) showing otic expression of atoh1a (A-D) and neurog1 (E-H) at 24 hpf in control embryos (A, E), pax2a-morphants (C, G), hs:sox2/hs:sox2 homozygotes (B, F) and hs:sox2/hs:sox2 homozygotes injected with pax2a-mo (D, -H). Embryos were heat shocked at 12.5 hpf, 39°C for 60 minutes. Otic vesicle borders are outlined in all images.
Mutual repression between atoh1a and neurog1.
In mouse embryos, neurogenesis and sensory development occur sequentially from the same spatial domain. The transition from neurogenesis to sensory development is regulated in part by mutual repression between Neurog1 and Atoh1 (Raft et al., 2007). To test whether a similar cross-repression helps reinforce or maintain the sensory-neural boundary in zebrafish, we activated hs:atohla/+ or hs:neurogl/+ at 24 hpf when sensory and neurogenic domains are already well established and then examined subsequent effects on neurog1 or atoh1a expression at 30 hpf. We previously reported that serial activation of hs:atoh1a by heat shocking embryos 24 hpf and 27 hpf provides optimal expansion of sensory epithelia (Sweet et al., 2011). Under these same conditions, serial activation of hs:atoh1a eliminated expression of neurog1 at 30 hpf (Fig. 9C, D). Conversely, serial activation of hs:neurog1 at 24 hpf and 27 hpf strongly repressed expression of atoh1a at 30 hpf (Fig. 9A, B). These data support the idea that the mutual antagonism between atoh1a and neurog1 helps maintain the sensory-neural boundary during otic development in zebrafish.
Figure 9. Roles for atoh1a-neurog1 cross-repression but not Notch in sensory-neural segregation.
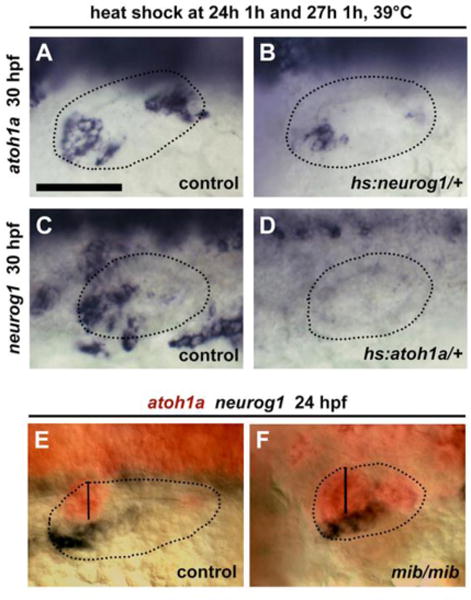
(A, B) Expression of atoh1a at 30 hpf in a control (A) and hs:neurog1/+ (B) embryo following serial heat shock at 24 and 27 hpf, for 60 min at 39°C. (C, D) Expression of neurog1 at 30 hpf in a control (C) and hs:atoh1a/+ (D) embryo following serial heat shock at 24 and 27 hpf, for 60 min at 39°C. (E, F) Co-staining of atohla (red) and neurog1 (black) expression by two-color in situ hybridization in a control embryo (E) and a mib homozygote mutant (F) at 24 hpf. Otic vesicle borders are outlined in all images. The width of the utricular macula is marked by vertical lines. All images show dorsolateral views with anterior to the left.
Because Neurog1-Atoh1 cross-repression in mouse is mediated in part by Notch signaling (Raft et al., 2007), we examined whether disruption of Notch signaling in mind bomb (mib) mutants alters the sensory-neural boundary in zebrafish. Two-color in situ hybridization showed that mib mutants exhibit strong upregulation of both atoh1a and neurog1, but the atoh1a-neurog1 boundary is nevertheless maintained (Fig. 9E, F). This indicates that Notch activity represses expression of both atoh1a and neurog1 but is not required for spatial segregation of sensory and neural fates in zebrafish.
Early expansion of sensory potential by Fgf
The earliest sign of sensory development occurs at roughly 10.5 hpf (tail bud stage) when atoh1b is induced at the medial edge of the otic placode (Millimaki et al., 2007), less than one hour after Fgf-dependent induction of pax8 in the nascent otic anlagen (Phillips et al., 2001). Expression of atoh1b is later required for timely activation of sox2 in the sensory domain (Millimaki et al., 2010). We showed previously that maintaining expression of atoh1b requires Fgf (Millimaki et al., 2007), but this is difficult to interpret because blocking Fgf at 10 hpf destabilizes otic development (Léger and Brand, 2002). Additionally, high-level misexpression of Fgf at 10 hpf enlarges the entire otic placode (Padanad et al., 2012), including the early domain of atoh1b expression. We therefore tested whether misexpressing Fgf at a low level could expand the domain of atoh1b without increasing the number of pax8-expressing otic cells. For this experiment, hs:fgf8/+ heterozygotes were subjected to a very mild heat shock at 35°C beginning at 10 hpf and embryos were fixed at 10.8hpf to examine expression of atoh1b. Under these conditions, the size of the pax8 domain was not altered (Fig. 10A, B, E) but the domain of atoh1b expression expanded by 34% based on measuring spatial area or by counting atoh1b+ cells (Fig. 10C, D, F, G). The enlarged domain of atoh1b was not maintained after the heat shock, as the number of atoh1b+ cells declined to normal by 14 hpf (not shown) in a process previously shown to involve Notch-dependent domain-restriction (Millimaki et al., 2007). Nevertheless, these data indicate that the level of Fgf signaling can influence the proportion of pre-otic cells able to express prosensory markers.
Figure 10. Low-level misexpression of Fgf8 expands sensory potential in the early placode.
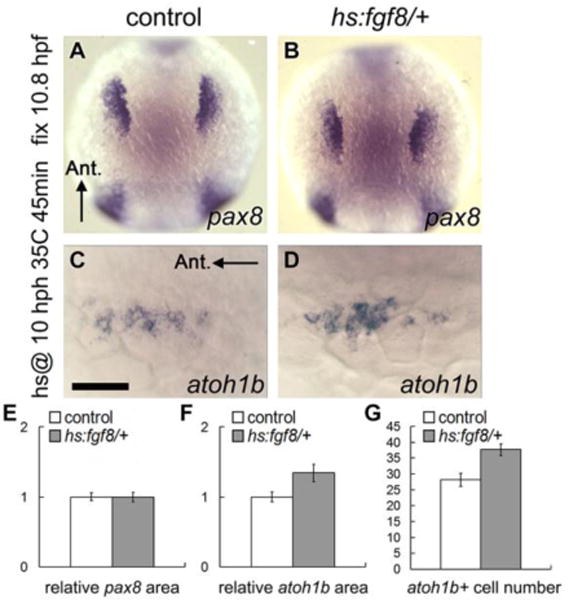
(A-D) Dorsal views showing expression of pax8 (A, B, anterior up) and atoh1b (C, D, anterior to the left) at 10.8 hpf in control (A, C) and hs:fgf8/+ (B, D) embryos that were heat shocked at 10 hpf, 35°C for 45 minutes. (E-G) Quantification of relative surface area of otic/epibranchial pax8 domain (E), atoh1b domain (F) and the number of atoh1b-expressing cells (G) in control and hs:fgf8/+ embryos following heat shock at 10 hpf, 35°C for 45 minutes. Error bars represent standard error of the mean. Asterisks indicate statistically significant differences compare to control (*P<0.05, student’s t-test, n>22).
DISCUSSION
Our findings support a model in which sox2 and sox3 provide unique functions during placodal development to establish sensory and neurogenic competence, respectively (Fig. 11A). Previous studies suggest that SoxB1 functions can vary based on intrinsic differences in protein structure, expression level, or availability of cofactors. It appears that all three variables influence sox2 and sox3 functions during otic development in zebrafish. First, several observations suggest that the functions of sox2 and sox3 are intrinsically different. Although expression of sox2 and sox3 overlap in the prosensory region, mutant analysis shows that only sox2 is required for normal sensory development, and double mutants show no further impairment. Additionally, moderate misexpression of sox2 expands sensory domain while reducing the neurogenic domain. Second, the functions of both genes depend on their level of expression. Specifically, sox2 and sox3 can mimic each other when misexpressed at high levels, leading to dramatic expansion of the sensory and neurogenic domains in the otic vesicle (Fig. 11B). Third, regionally expressed cofactors appear to modify the functions of Sox2 and Sox3: The medial factor pax2a is required to expand the sensory domain following misexpression of either sox2 or sox3, whereas the neurogenic domain is unaffected (Fig. 11A, B). Thus the overlap of multiple mechanisms allows otherwise similar SoxB1 factors to perform distinct functions in abutting domains of the otic vesicle.
Figure 11. Summary and model of sensory-neural patterning.
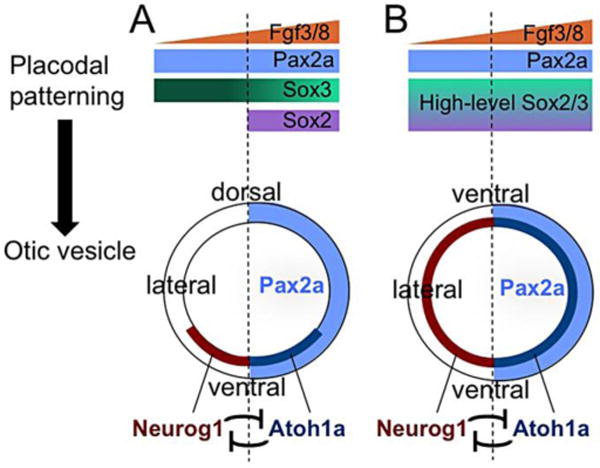
(A) Under normal conditions, a gradient of Fgf from the mesendoderm and hindbrain induces the formation of otic placode, in which pax2a and sox3 are uniformly expressed. By 12 hpf, however, sox3 expression is reduced in medial cells and sox2 is restricted to medial cells, changes that reflect ongoing Fgf signaling. Later, expression of pax2a becomes limited to the medial wall of the otic vesicle. Sensory competence is favored by the overlap between sox2, pax2a, elevated Fgf, and reduced sox3. Neurogenic competence is favored by elevated sox3, the absence of sox2, and moderate Fgf. These early patterns help establish spatial segregation of sensory and neurogenic domains in the floor of the otic vesicle (with the anterior half represented here), in which cross-repression between Neurog1 and Atoh1a helps reinforce or maintain segregation. (B) When Sox2/3 is transiently overexpressed at 12.5 hpf, prosensory and proneural competence increases in all otic cells. Later, sensory and neurogenic domains expand throughout the otic vesicle. This is accompanied by loss of non-sensory and non-neural fates and expansion of anterior-ventral identity throughout the otic vesicle, the region normally shared by utricular sensory and neural fates. Despite these changes in axial patterning, medial-lateral segregation of sensory and neural domains, and the medial domain of Pax2a, are maintained.
The ability of sox2 and sox3 misexpression to dramatically expand sensory and neurogenic domains correlates with global expansion of the anterior-ventral markers of the otic vesicle (Fig. 6). This phenotype is remarkable in several ways. First, regional fates in the otic vesicle normally rely on inductive interactions from surrounding tissues. However, early misexpression of sox2 or sox3 stably specifies anterior-ventral identity. That is, cells are not respecified by signals that normally establish distinct regional identities. Although global misexpression of sox2/3 potentially alters such signals, genetic mosaics containing isolated transgenic cells also show a high incidence of sensory or neural development in ectopic locations. With such sparse distribution of transgenic cells it is unlikely that signaling interactions from surrounding tissues are significantly altered, suggesting that sensory and neural competence persists regardless of changing regional signals. We speculate that sox2 and sox3 function in the otic vesicle much as they do in the early neural plate, wherein SoxB1 factors stably specify a zone of neurogenic potential while simultaneously preventing premature neural differentiation. Subsequently, rising levels of neurogenic bHLH transcription factors repress expression of SoxB1 factors as cells begin to differentiate. Such a transition is seen in the neurogenic domain of the otic vesicle, wherein sox3 is expressed in a gradient with levels declining towards the lateral edge where neuroblasts delaminate (Fig. 1E; Kantarci et al., 2016). The transition is also evident in sensory epithelia when hair cells upregulate atoh1a while losing expression of sox2 (Millimaki et al., 2010).
The second notable feature of the phenotype caused by early misexpression of sox2 or sox3 is that the medial-lateral segregation of sensory and neurogenic fates is maintained and continues to respect the pax2a expression boundary. Although pax2a expression is initially expressed throughout the otic placode, it overlaps with the medial/prosensory domain of sox2 as early as 12 hpf (6 somites, Gou et al., 2017). As the otic vesicle forms, expression of pax2a becomes restricted to the medial wall but continues to overlap with sox2 in the ventromedial quadrant. Expression of pax2a does not depend on sox2 but is nevertheless required for expansion of sensory epithelia by sox2/3 misexpression. Thus the sox2-pax2a partnership defines the prosensory compartment throughout early otic development. In contrast, the ability of sox3 to promote neural competence appears to require the absence of sox2 (Fig. 2G), whereas pax2a is superfluous. Neurogenic competence neither requires pax2a nor is it impaired by misexpression of pax2a (Kantarci et al., 2016).
Basis for unique functions of Sox2 and Sox3
Although most studies conclude that Sox2 and Sox3 functions are largely redundant, there are several examples in which these proteins exhibit distinct functions. For instance, human embryonic stem cells (hESCs) express both Sox2 and Sox3, but their functions are not identical. Either factor is sufficient to maintain pluripotency, but Sox2 alone can promote hESC self-renewal whereas Sox3 cannot (Wang et al., 2012). In neural progenitors, too, some functions of Sox2 and Sox3 are non-redundant as each factor activates a different set of neural makers (Archer et al., 2011; Rogers et al., 2009; Rogers et al., 2014). The mechanistic basis for such differences is unknown but possibly reflects structural differences in the transactivation domain that alter the ability to interact with different cofactors (Cox et al., 2010; Kondoh and Kamachi, 2010). Additionally, Sox2 and Sox3 can show markedly different affinities for specific DNA sequences (Collignon et al., 1996), indicating that small changes in their HMG DNA-binding domains also facilitate distinct functions.
Comparison with chick and mouse
In chick, Sox2 and Sox3 show overlapping expression during otic development. Expression of Sox3 begins during early placodal development, marking the nascent neurogenic domain and promoting subsequent expression of Ngn1 (Abello et al., 2010). Expression of Sox2 begins later and is retained in sensory epithelia, whereas Sox3 expression is lost after the neural-sensory transition (Neves et al., 2007). These data support a functional bias for Sox3 in neurogenic competence and Sox2 for sensory competence, similar to what we found in zebrafish. Overexpression of Sox2 in chick can induce Ngn1 (Evsen et al., 2013), though under these conditions it may mimic the normal function of Sox3 as we have found in zebrafish.
In mouse, Sox2 is expressed in the otic vesicle and is required for both neural and sensory development (Kiernan et al., 2005; Puligilla et al., 2010; Steevens et al., 2017), whereas Sox3 expression is not detected. How Sox2 alone mediates both functions is not understood, but comparison with zebrafish suggests several possibilities. First, the level of Sox2 expression could be sufficiently high in mouse that it can fulfill both functions, similar to our misexpression studies in zebrafish. Second, functional output could be influenced by interactions with Pax2. The early neurogenic domain in mouse straddles the Pax2 expression boundary (Burton et al., 2004), and it is likely that most neuroblasts arise from the lateral (Pax2-negative) domain. The lateral neurogenic domain overlaps a ventrolateral domain of Goosecoid (Vitelli et al., 2003), which in zebrafish is required for neuroblasts to delaminate and is repressed by pax2a (Kantarci et al., 2016). In mouse, vestibular and auditory neurons do not detectably express Pax2 (Lawoko-Kerali et al., 2002), and loss of Pax2 does not block formation of vestibular or spiral ganglia (Burton et al., 2004). In contrast, Pax2 is abundantly expressed in sensory epithelia, especially in differentiating hair cells (Lawoko-Kerali et al., 2002), and loss of Pax2 perturbs development of sensory epithelia (Burton et al., 2004; Zou et al., 2006). It is possible that Pax2 physically interacts with Sox2 to activate sensory-specific enhancers, analogous to Pax6-Sox2 activation of lens-specific enhancers (Kamachi et al., 2001). Alternatively, Pax2 and Sox2 can also bind independently to widely separated binding sites within specific enhancers to drive expression in sensory epithelia (Robert-Moreno et al., 2011).
Relative roles Neurog1-Atoh1a cross-repression and Notch
The transition from neural to sensory development in birds and mammals is triggered in part by Neurog1-dependent activation of Notch (Brooker et al., 2006; Daudet et al., 2007; Daudet and Lewis, 2005; Neves et al., 2011), as well as cross-repression between Neurog1 and Atoh1 (Raft et al., 2007). In zebrafish, too, atoh1a and neurog1 show cross-repression which presumably helps maintain and sharpen the sensory-neural border. However, Notch activity plays no role in spatial segregation between these domains in zebrafish, since a sharp boundary persists in mib mutants. Rather Notch acts independently in both domains to limit atoh1a and neurog1 activity. This function is critical for establishing the alternating pattern of hair cells and support cells in sensory epithelia (Haddon et al., 1998; Millimaki et al., 2007; Riley et al., 1999) and to limit the pace of neuroblast specification and differentiation (Kantarci et al., 2015).
The role of Fgf
The earliest sign of prosensory development in zebrafish is the induction of atoh1b in the medial portion of the otic placode (Millimaki et al., 2007). Activation of atoh1b requires Fgf signaling and is limited to medial cells in close proximity to the hindbrain-source of Fgf. We showed that low-level activation of hs:fgf8 is sufficient to increase the number of atoh1b-expressing cells without increasing the total number of pax8-positive otic cells (Fig. 10). The early otic domain of atoh1b does not reflect overt sensory specification since the domain is later restricted to only a few prospective hair cells by Notch-dependent lateral inhibition (Millimaki et al., 2007). However, the early atoh1b domain can nevertheless be viewed as an early marker of prosensory competence or potential, since knockdown of atoh1b blocks differentiation of the first hair cells and delays expression of atoh1a by many hours (Millimaki et al., 2007; Millimaki et al., 2010). While the above data suggest that elevating Fgf expands prosensory competence, it is unclear whether there is a corresponding contraction of neurogenic competence. However, we showed previously that weak activation of hs:fgf8 does cause downregulation of sox3 to a discrete lower level in the otic placode (Bhat and Riley, 2011; Padanad and Riley, 2011). Whether this molecular change reflects reduced neurogenic competence remains to be determined.
HIGHLIGHTS.
Sox2 uniquely promotes sensory competence while Sox3 promotes neural competence.
Transient overexpression of Sox2/3 expands both sensory and neurogenic domains.
Sensory-neural segregation involves Atoh1a-Neuorg1 cross-repression but not Notch.
Sensory competence and Sox2 function are favored by Pax2a and elevated Fgf.
Acknowledgments
This work was supported by National Institutes of Health/NIDCD grant R01-DC03806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339:166–78. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Adikusuma F, Pederick D, McAninch D, Hughes J, Thomas P. Functional Equivalence of the SOX2 and SOX3 Transcription Factors in the Developing Mouse Brain and Testes. Genetics. 2017;206:1495–1503. doi: 10.1534/genetics.117.202549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, Terskikh AV. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proceedings of the National Academy of Sciences. 2015;112:E1936–E1945. doi: 10.1073/pnas.1421480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosetti DC, Basilico C, Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 1997;17:6321–9. doi: 10.1128/mcb.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer TC, Jin J, Casey ES. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Developmental Biology. 2011;350:429–440. doi: 10.1016/j.ydbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N, Riley BB. Integrin-α5 Coordinates Assembly of Posterior Cranial Placodes in Zebrafish and Enhances Fgf-Dependent Regulation of Otic/Epibranchial Cells. PLoS One. 2011;6:e27778. doi: 10.1371/journal.pone.0027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer B, Kopp J, Mallanna S, Desler M, Chakravarthy H, Wilder PJ, Bernadt C, Rizzino A. Elevating the levels of Sox2 in embryonal carcinoma cells and embryonic stem cells inhibits the expression of Sox2:Oct-3/4 target genes†. Nucleic Acids Research. 2007;35:1773–1786. doi: 10.1093/nar/gkm059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–51. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Developmental Biology. 2004;272:161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Reciprocal Transcriptional Regulation of Pou5f1 and Sox2 via the Oct4/Sox2 Complex in Embryonic Stem Cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- Cox JL, Mallanna SK, Luo X, Rizzino A. Sox2 Uses Multiple Domains to Associate with Proteins Present in Sox2-Protein Complexes. PLoS One. 2010;5:e15486. doi: 10.1371/journal.pone.0015486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Evsen L, Sugahara S, Uchikawa M, Kondoh H, Wu DK. Progression of Neurogenesis in the Inner Ear Requires Inhibition of Sox2 Transcription by Neurogenin1 and Neurod1. The Journal of Neuroscience. 2013;33:3879–3890. doi: 10.1523/JNEUROSCI.4030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith S, Lovell-Badge R, Rizzoti K. SOX2 is sequentially required for progenitor proliferation and lineage specification in the developing pituitary. Development. 2016;143:2376–2388. doi: 10.1242/dev.137984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Y, Guo J, Maulding K, Riley BB. sox2 and sox3 cooperate to regulate otic/epibranchial placode induction in zebrafish. Developmental Biology. 2017 doi: 10.1016/j.ydbio.2018.01.011. Manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–44. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–28. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann SA, Hos D, Küspert M, Lang RA, Lovell-Badge R, Wegner M, Reiprich S. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development. 2014;141:39–50. doi: 10.1242/dev.098418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SR, Pevny LH. SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Developmental Biology. 2011;352:40–47. doi: 10.1016/j.ydbio.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind Bomb Is a Ubiquitin Ligase that Is Essential for Efficient Activation of Notch Signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Jowett T, Yan YL. Double fluorescent in situ hybridization to zebrafish embryos. Trends in Genetics. 1996;12:387–389. doi: 10.1016/s0168-9525(96)90091-8. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–86. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci H, Edlund RK, Groves AK, Riley BB. Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling. PLoS Genetics. 2015;11:e1005037. doi: 10.1371/journal.pgen.1005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci H, Gerberding A, Riley BB. Spemann organizer gene Goosecoid promotes delamination of neuroblasts from the otic vesicle. Proceedings of the National Academy of Sciences. 2016;113:E6840–E6848. doi: 10.1073/pnas.1609146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KKH, Tang ASP, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KSE. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Kamachi Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol. 2010;42:391–9. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small Increases in the Level of Sox2 Trigger the Differentiation of Mouse Embryonic Stem Cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Léger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. The Journal of Comparative Neurology. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–9. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. The Journal of Comparative Neurology. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giráldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Developmental Dynamics. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Ogura E, Kondoh H, Kamachi Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 2010;6:e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanad MS, Bhat N, Guo B, Riley BB. Conditions that influence the response to Fgf during otic placode induction. Developmental Biology. 2012;364:1–10. doi: 10.1016/j.ydbio.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanad MS, Riley BB. Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol. 2011;351:90–8. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–65. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 Induces Neuronal Formation in the Developing Mammalian Cochlea. The Journal of Neuroscience. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Groves AK. Segregating neural and mechanosensory fates in the developing ear: patterning, signaling, and transcriptional control. Cell Tissue Res. 2015;359:315–332. doi: 10.1007/s00441-014-1917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riley BB, Chiang MY, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Rizzino A, Wuebben EL. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochimica et Biophysica Acta (BBA) - Gene Regulatory. Mechanisms. 2016;1859:780–791. doi: 10.1016/j.bbagrm.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Naranjo S, de la Calle-Mustienes E, Gómez-Skarmeta JL, Alsina B. Characterization of New Otic Enhancers of the Pou3f4 Gene Reveal Distinct Signaling Pathway Regulation and Spatio-Temporal Patterns. PLoS One. 2011;5:e15907. doi: 10.1371/journal.pone.0015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 Activation by Knockdown Technologies. PLoS Genetics. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Harafuji N, Archer T, Cunningham DD, Casey ES. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech Dev. 2009;126:42–55. doi: 10.1016/j.mod.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers N, McAninch D, Thomas P. Dbx1 Is a Direct Target of SOX3 in the Spinal Cord. PLoS One. 2014;9:e95356. doi: 10.1371/journal.pone.0095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steevens AR, Sookiasian DL, Glatzer JC, Kiernan AE. SOX2 is required for inner ear neurogenesis. Sci Rep. 2017;7:4086. doi: 10.1038/s41598-017-04315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–86. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Surzenko N, Crowl T, Bachleda A, Langer L, Pevny L. SOX2 maintains the quiescent progenitor cell state of postnatal retinal Müller glia. Development. 2013;140:1445–1456. doi: 10.1242/dev.071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358:113–21. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker ES, Lehtinen MK, Maynard T, Zirlinger M, Dulac C, Rawson N, Pevny L, LaMantia AS. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137:2471–2481. doi: 10.1242/dev.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemaraju S, Kantarci H, Padanad MS, Riley BB. A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear. PLoS Genet. 2012;8:e1003068. doi: 10.1371/journal.pgen.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Viola A, Morishima M, Pramparo T, Baldini A, Lindsay E. TBX1 is required for inner ear morphogenesis. Hum Mol Genet. 2003;12:2041–2048. doi: 10.1093/hmg/ddg216. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct Lineage Specification Roles for NANOG, OCT4, and SOX2 in Human Embryonic Stem Cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–67. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Zou D, Silvius D, Rodrigo-Blomqvist S, Enerbäck S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Developmental Biology. 2006;298:430–441. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


