Abstract
Conventional cytotoxic cancer chemotherapy is often immunosuppressive and associated with drug resistance and tumor regrowth after a short period of tumor shrinkage or growth stasis. However, certain cytotoxic cancer chemotherapeutic drugs, including doxorubicin, mitoxantrone, and cyclophosphamide, can kill tumor cells by an immunogenic cell death pathway, which activates robust innate and adaptive anti-tumor immune responses and has the potential to greatly increase the efficacy of chemotherapy. Here, we review studies on chemotherapeutic drug-induced immunogenic cell death, focusing on how the choice of a conventional cytotoxic agent and its dose and schedule impact anti-tumor immune responses. We propose a strategy for effective immunogenic chemotherapy that employs a modified metronomic schedule for drug delivery, which we term medium-dose intermittent chemotherapy (MEDIC). Striking responses have been seen in preclinical cancer models using MEDIC, where an immunogenic cancer chemotherapeutic agent is administered intermittently and at an intermediate dose, designed to impart strong and repeated cytotoxic damage to tumors, and on a schedule compatible with activation of a sustained anti-tumor immune response, thereby maximizing anti-cancer activity. We also discuss strategies for combination chemo-immunotherapy, and we outline approaches to identify new immunogenic chemotherapeutic agents for drug development.
Keywords: anti-tumor immunity, immune suppression, immune memory, drug development, anti-cancer drug scheduling
Introduction
Cancer is a disease of malignant cells that interact with and co-opt their environment in complex ways, stimulating tumor growth, angiogenesis, invasion and metastasis and fostering an immune suppressive environment that counters the tumoricidal effects of many cytotoxic anti-cancer agents [1]. To be most effective, anti-cancer therapies need to take into account drug effects on the tumor microenvironment. This environment is dynamic and can be remodeled through interventions that alter the interactions between tumor cells and stromal cells, creating new therapeutic opportunities [2]. Certain conventional tumor cell cytotoxic and cytostatic cancer chemotherapeutic drugs have the potential to increase tumor cell immunogenicity by activating immunogenic cell death (ICD), an immunostimulatory form of cell death that activates innate immune responses and also elicits a tumor-specific adaptive immune response [3–5], with an increase in overall anti-tumor efficacy compared to tumor cell cytotoxicity alone [3,6]. In practice, however, the toxicity of these and many other cancer chemotherapeutic drugs to T cells, natural killer (NK) cells and dendritic cells (DCs) limits the extent of immune stimulation and can lead to immunosuppression [7,8]. Here we review studies on the actions of drugs that induce ICD, focusing on the dose and schedule dependence of conventional chemotherapy-activated immune responses and on combinations with immunotherapy, both in mouse models and in the clinic. We propose that anti-cancer chemo-immunotherapeutic responses to drugs that induce ICD can be optimized by using a modified metronomic schedule for drug delivery, which we term MEDIC, medium-dose intermittent chemotherapy. Finally, we outline approaches to identify novel lead immunogenic chemotherapeutic agents for drug development.
Chemotherapy-induced ICD
Doxorubicin, cyclophosphamide and several other cancer chemotherapeutic drugs have the capacity to induce ICD. Key events in this cell death pathway include: early translocation to the tumor cell surface of the endoplasmic reticulum chaperone protein calreticulin, which generates an essential “eat-me” signal for DC engulfment and tumor antigen uptake [9,10]; secretion of ATP from lysosomal stores, which stimulates macrophage recruitment and maturation [11], induces NK cell proliferation, and stimulates IFNγ production [12]; and post-apoptotic release of the nuclear chromatin binding protein HMGB1, which activates toll-like receptor 4 (TLR4) and mediates nucleic acid-activation of TLRs 3, 7 and 9 [13,14]. Certain ICD drugs can also activate type-I interferon signaling pathways in tumor cells, which may contribute to the downstream activation of host antitumor immunity [15].
ICD-induced translocation of HMGB1 from the nucleus to the cytoplasm is followed by HMGB1 release into the extracellular matrix of dying tumor cells. This release enables HMGB1 to interact with TLR4 expressed on DCs, thereby stimulating antigen presentation by DCs as well as DC production of IL1β, which activates CD8+ T cells [16,17]. ATP secreted from dying tumor cells can act on DC purinergic P2RX7 receptors to activate CD8+ T cells [16,18]. The importance of chemotherapy-induced ICD is highlighted by the low efficacy of chemotherapy in cells with loss-of-function alleles of TLR4 and P2RX7 [7,17,18]. Tumor cell surface molecules that present “don’t eat me” signals for DCs, including CD31, CD46, and CD47, are down regulated during ICD, allowing the eat-me signals to prevail and phagocytosis of apoptotic corpses to occur [19]. Molecular chaperones such as HSP90 appear on the tumor cell surface, enhancing DC-tumor cell adhesion and stimulating DC maturation [20]. Factors that inhibit ICD include: CD39/ENTPD1, which hydrolyzes extracellular ATP [21]; CD73/NT5E, which converts AMP into adenosine and is highly immunosuppressive of macrophages, NK cells and T cells [12]; and CD47, which counters the phagocytic signal of surface-expressed calreticulin [22].
Chemotherapy can also increase tumor cell immunogenicity by inducing expression of MHC-I molecules and tumor-specific antigens on the tumor cell surface [23]. Chemotherapy-induced stress may also activate NK cells by inducing expression of NK cell stimulatory ligands, such as NKG2D activating ligands [24,25] and by decreasing tumor cell surface levels of NK cell inhibitory ligands [26,27]. Death receptors present on the tumor cell surface, such as TRAIL receptor and mannose-6-phosphate receptor, can also be induced by chemotherapy, rendering tumor cells susceptible to immune cell attack [28,29].
Some of the stimulatory immune responses to cytotoxic anti-cancer drug treatment may result from the transient lymphopenia that many of these drugs induce, as seen in both animal models and in the clinic [30,31]. Lymphopenia is associated with up regulation of host danger-sensing and repair mechanisms, which lead to a “storm” of cytokines and chemokines, DC differentiation, maturation and homeostatic proliferation, T cell activation, and anti-tumor immune cell recruitment into tumors [32,33]. Depletion of chemotherapy-sensitive immune suppressive cells, such as myeloid-derived suppressor cells and circulating Tregs [34], can lead to restoration of NK cell effector function and T cell proliferation in patients [35] and contribute to the immune stimulatory effects of chemotherapy.
Dependence of ICD on choice of chemotherapeutic drug and tumor model
Anticancer drugs that induce ICD include cyclophosphamide, doxorubicin, epirubicin, idarubicin, mitoxantrone, and oxaliplatin [36–39]. The impact of ICD can be seen when immune competent mice are injected with tumor cells treated ex vivo with mitoxantrone, doxorubicin or idarubicin, which confers immunity against live tumor cell challenge on the opposite flank. Thus, the ICD drug-treated tumor cells immunize the host to the tumor and thus serve as an anti-cancer vaccine [9]. Other DNA-damaging agents, such as etoposide and mitomycin C, are non-immunogenic, and show little such vaccine activity when tested in the same experimental setting [9]. However, the immunogenicity of etoposide and mitomycin C becomes apparent when calreticulin is overexpressed or when protein phosphatase-1/GADD34 complex, a negative regulator of calreticulin exposure, is inhibited [9]. Poor calreticulin exposure is thus a critical determinant of the inability of these two drugs to induce ICD. While oxaliplatin and cisplatin both trigger HMGB1 release in colon cancer cells, oxaliplatin, but not cisplatin, stimulates calreticulin exposure and induces anticancer immunity in mice in vivo [40]. In other studies, the ICD drugs doxorubicin and idarubicin, but not the non-ICD drugs gemcitabine and etoposide, activate markers of ICD and stimulate various immune responses, including tumor cell uptake by DCs, DC maturation, and T cell activation [41]. Thus, non-ICD chemotherapeutic drugs may be non-immune stimulatory because of their inability to activate one or more of the cellular responses required to elicit ICD. Cell-based assays for the classic features of ICD (calreticulin exposure, HMGB1 release, etc.) can therefore be very useful, both from a mechanistic perspective and for their utility in screening for candidate ICD drugs (see below). However, evidence for a functional immunogenic response in vivo is ultimately required, for instance, by testing for the ability of ex vivo drug treated tumor cells, when injected on one flank of a mouse, to induce the rejection of live tumor cells injected on the opposite flank (vaccine activity assay) [9]. Such an assay can distinguish drugs (or drug-tumor cell combinations; see below) that show one or more hallmarks of ICD (e.g., calreticulin translocation or HMGB1 release) from those that additionally show a bona fide ICD response.
Cyclophosphamide, when given on a 6-day repeating schedule, induces robust innate anti-tumor immune responses leading to major tumor regression in glioma-bearing scid immunodeficient mice [42–45]. Tumor regression is abolished in NSG mice, where NK cells are absent and macrophages are dysfunctional, highlighting the essential role of the innate immune system in the overall anti-tumor response [42]. KM12 colon cancer xenografts given the same cyclophosphamide regimen do not show these responses, despite the intrinsic chemo-sensitivity of KM12 tumor cells to activated cyclophosphamide [46]. In C57BL/6 mice, which are fully immune competent, the every 6-day cyclophosphamide schedule cures GL261 gliomas by an NK cell- and CD8+ T cell-dependent mechanism. In contrast, the same treatment regimen effects only modest growth delay and little or no immune responses in LLC lung carcinoma and B16F10 melanoma models, despite their intrinsic sensitivity to cyclophosphamide cytotoxicity [47]. Thus, immune effects of an ICD drug, such as cyclophosphamide, can differ dramatically between tumor models and/or tumor types, and most likely, between individual cancer patients as well. Tumors unresponsive to the immunogenic actions of cyclophosphamide may be deficient in factors essential for ICD, such as stress ligands like MHC class I [23], or may express factors that confer resistance to ICD, such as PD-L1 [48]. Tumor mutational burden and the presence of neo-antigens [49] may also be a factor in the responsiveness of a tumor to an ICD drug. Tumor vascularity may also be a factor, as poorly perfused tumors could present a barrier to drug access and/or immune cell infiltration [50]. Given this tumor model dependence of ICD, it is important to identify biomarkers that distinguish ICD immune responsive from non-responsive tumors and patients [47].
Cancer chemotherapeutic agents characterized as non-immunogenic in one setting may nevertheless exert immune stimulatory functions in another context. For example, cisplatin, a non-ICD drug [40], can eliminate myeloid-derived suppressor cells and thereby relieve tumor immune suppression and augment the homing ability of exogenous and endogenous immune effector cells, as seen in B16 mouse melanoma [51]. Similarly, the non-ICD drug etoposide [9] can facilitate DC maturation [52]. Thus, it is likely that more drugs are capable of inducing anti-tumor immune responses, including ICD, than is currently recognized. Further, as discussed in the following section, a bona fide ICD drug may fail to elicit significant ICD or immune responses when given at a suboptimal dose or schedule.
Impact of chemotherapeutic drug dose and schedule
Cytotoxic cancer chemotherapeutic drugs are typically administered on a maximum tolerated dose (MTD) schedule, which may induce high host toxicity and can lead to tumor vasculature regrowth and selection of drug-resistant cell populations during the prolonged drug-free breaks required to recover from host toxicity [53]. In contrast, metronomic treatment schedules [54–57] deliver chemotherapeutic drugs at a lower dose than traditional MTD chemotherapy, but on a more frequent, or even a continuous (daily) schedule with less overall toxicity to the host. Several preclinical studies have investigated the impact of these and other treatment schedules on the strength and duration of immune anti-tumor responses.
Cyclophosphamide, which is employed in a large fraction (>40%) of clinical trials evaluating metronomic chemotherapy [39,58], elicits immune modulatory responses that are often dose and schedule dependent. In mouse glioma models, cyclophosphamide delivered on an MTD schedule induces transient innate immune responses, whereas that same drug given on an intermittent, 6-day repeating metronomic schedule stimulates sustained immune responses and a prolonged period of tumor regression [42–44]. The every 6-day metronomic schedule was also more effective in activating anti-tumor innate immune responses than when cyclophosphamide was delivered on an exposure dose-equivalent daily low-dose metronomic schedule [43]. The 6-day cyclophosphamide schedule induced a potent CD8+ T cell response leading to tumor ablation and acquisition of immune memory when tested in a fully immunocompetent, syngeneic glioma model [59]. When cyclophosphamide treatment was halted after two 6-day cyclophosphamide treatment cycles, there was a significant increase in Treg cells marked by Foxp3 in the tumor compartment and decreased expression of perforin, a cytotoxic immune effector [59]. Thus, in these experimental glioma models, a cyclophosphamide schedule with a drug-free break longer than 6 days leads to immune suppression.
In autochthonous mouse prostate tumors, a single injection of cyclophosphamide at 50 to 100 mg/kg induces tumor-specific T cell infiltration, while doses > 200 mg/kg are highly toxic to circulating CD4+ T, CD8+ T and CD19+ B cells [60]. In other studies, tumor-specific T cells were increased in tumor draining lymph nodes when cyclophosphamide was given at a dose of 100 mg/kg every 8 days, but were suppressed when the same total cyclophosphamide dose was delivered at a dose of 50 mg/kg every 4 days [61]. Metronomic cyclophosphamide treatments at either 10 mg/kg per day or 50 mg/kg per week synergize with tumor vaccines to induce tumor regression in an HPV tumor-bearing C57BL/6 mouse model, however, tumor-specific CD8+ T cell responses were reduced with the daily cyclophosphamide schedule as compared to the weekly schedule [62]. Similarly, when cyclophosphamide was combined with IL12 gene therapy in CT26 colorectal carcinoma-bearing BALB/c mice, a single injection of cyclophosphamide at 50 mg/kg induced a superior anti-tumor response compared to when cyclophosphamide was given three times per week at a dose of 25 mg/kg [63]. Thus, immune responses can be expected to be suboptimal when drug treatment is too frequent, as in low-dose daily metronomic chemotherapy, or when the break between treatments is too long, as in many MTD treatment schedules. Overall, these findings establish the principle that chemotherapy dose and schedule are both critically important determinants of the immune outcome, and that the optimal dose and precise schedule vary between tumor models.
Combination of chemotherapy with immunotherapy
Despite the strong, beneficial anti-tumor immune responses that can sometimes be achieved with ICD-inducing chemotherapy alone, initial anti-tumor responses are often followed by tumor regrowth [64]. One attractive approach to this problem is combination with immunotherapy, whereby the patient’s own immune system is stimulated to unleash its intrinsic anti-tumor potential. Immunotherapy is increasingly used for cancer treatment [65,66] and can be synergistic with chemotherapy. It may involve diverse treatments, ranging from use of tumor vaccines, TLR agonists, cytokines, and agents that counter of immunosuppression, including checkpoint inhibitors.
Immunotherapy may be synergistic with chemotherapy
Immunotherapy can be implemented using tumor vaccines that prime and stimulate anti-tumor T cell responses, either with or without co-administration of antigen-presenting DCs [67]. Adoptive transfer to the host of anti-tumor immune cells that are activated and expanded ex vivo is another approach [68]. T cells engineered to express T cell receptors or chimeric antigen receptors that recognize specific tumor antigens are often used in immune cell adoptive transfer studies [69]. Immune-stimulatory cytokines, such as GM-CSF, IFN-α, IL2, and IL12, can further improve the efficacy of immunotherapy [70,71]. Tumor vaccines and adoptive transfer are both subject to multiple tumor-derived immune suppressive mechanisms, including anergy and apoptosis of anti-tumor immune cells [72]. In some cases, peripheral immune cells cannot access the tumor [73]. Combination chemotherapy can address these issues by depleting or inhibiting immune suppressive cells, by producing immune-stimulatory cytokines and immune cell-recruiting chemokines, and/or by increasing the exposure of tumor antigen to immune cells [16], which may increase the responses of therapy-resistant cells [74].
Several new and clinically effective immunotherapies target immune checkpoints molecules, notably CTLA-4 and PD-1, which are inhibitory receptors expressed on T cells and other immune cells [75]. Antagonist antibodies used to block these inhibitory receptors allow for activation of anti-tumor immune responses that would otherwise be strongly suppressed. Cytotoxic drugs may be particularly effective when administered in the context of checkpoint blockade, when the tumor cells are already under T cell attack and may be more susceptible to drug toxicity [76]. Cytotoxic drugs administered at this point may also kill tumor cells that escape T cell attack, and may block the increases in Tregs and other immune suppressive cells that often follow immune stimulation. Checkpoint blockade applied following a cycle of chemotherapy is also expected to be effective, in particular for drugs that induce ICD: as chemotherapy-induced tumor cell cytotoxicity and anti-tumor immune responses wane and pro-tumor immune responses rebound, the checkpoint inhibitors may suppress the pro-tumor immune responses and thereby prolong, and perhaps augment immune responses activated by the immunogenic chemotherapy. Agents used to enhance NK cell cytotoxicity, such as anti-killer-cell immunoglobulin-like receptor [77], and anti-TGF-β [78], to inhibit immune suppression, can be expected to synergize with ICD-active chemotherapeutic drugs in a similar manner.
Tumor cells may be sensitized to chemotherapy by treatment with TLR agonists [79], such as CpG oligonucleotides, which can induce de novo immunity or boost chemotherapy-stimulated immunity by providing an alternative or complementary route to innate immune cell activation [80]. TLRs are prominently expressed by many immune cells; they recognize pathogen-associated molecular pattern molecules, such as unmethylated CpG oligonucleotides (TLR9 agonists), bacterial lipopolysaccharide (LPS, a TLR4 agonist) and viral-derived dsRNA (e.g., TLR3 agonist poly(I:C)) [81–83]. TLRs also recognize endogenous signals released by stressed and dying tumor cells, such as HMGB1, an activator of TLR4 discussed above [84]. Studies from this laboratory exemplify this approach using CpG-1826, a class B CpG oligonucleotide [85], which induces strong anti-tumor immune responses, complementing and enhancing the immune stimulatory actions of low dose, metronomic cyclophosphamide treatment in a syngeneic mouse glioma model [86]. While CpG-1826 treatment alone increased tumor-infiltrating macrophages markedly, an apparently synergistic increase in CD8+ cytotoxic T cells was achieved when CpG-1826 was combined with cyclophosphamide, resulting in long-term tumor ablation and resistance to tumor rechallenge, indicative of immune memory [86].
Combination of chemotherapy with immunotherapy in mouse models
Chemotherapeutic agents capable of eliminating or modulating immune suppressive cells in the tumor microenvironment are good candidates as preconditioning agents for immunotherapy [37,38,87]. Cyclophosphamide and paclitaxel can inhibit or eliminate Treg cells [38], while gemcitabine and 5-flurouracil are effective at depleting myeloid-derived suppressor cells [88]. Doxorubicin (5 mg/kg) boosts anti-tumor responses when given 7 days after vaccination in neu-transgenic FVB/n female mice, in part by polarizing macrophages to an anti-tumor M1 activation status [89]. Of note, chemotherapeutic drug dose and schedule can have a large effect on immunomodulatory activity. For example, cyclophosphamide can deplete Treg cells when administered at a low dose on a daily schedule (10–20 mg/kg per day in mice; 50 mg/day, orally, in humans) or at a higher dose given as a single injection (50–100 mg/kg ip in mice; 200–300 mg/m2 iv in humans) [37,87]. However, the high-dose cyclophosphamide treatment may limit tumor-reactive T cell responses [62,87]. Likewise, in a rat glioma model, temozolomide decreases the Treg/CD4+ T cell ratio when given at a low dose (0.5 or 2 mg/kg per day, 5 days/week for 3 weeks), but not at a high dose (30 mg/kg/day for 5 days, or 10 mg/kg/day, 5 days/week for 3 weeks). This is most likely due to the non-selective toxicity of high doses of temozolomide to lymphocyte populations [90].
Cyclophosphamide-induced Treg cell depletion is often transient [60,62], which necessitates repeated cyclophosphamide treatment or direct follow-up with an active immunotherapy regimen. In an autochthonous prostate cancer mouse model, a single injection of cyclophosphamide at 50 mg/kg induced transient depletion of Treg cells in the prostate tumor draining lymph nodes [60]. Interestingly, when a GM-CSF-secreting tumor vaccine was given one day after cyclophosphamide, Treg deletion was prolonged compared to when cyclophosphamide was given in the absence of vaccine [60]. Two cycles of cyclophosphamide then vaccine combination therapy, spaced one week apart, stimulated much stronger tumor-specific CD8+ T cell responses compared to a single cycle [60].
Low-dose cyclophosphamide can also stimulate host danger-sensing and repair mechanisms, which generate immune-stimulatory cytokines and chemokines that facilitate DC maturation, anti-tumor T cell proliferation and tumor infiltration by cytotoxic immune cells [32,87]. Therefore, the administration of immunotherapy needs to be optimally timed to take advantage of the “space” previously occupied by tumor tolerant or pro-tumor immune cells, as well as dynamic production of cytokines and chemokines generated by chemotherapy [32,33]. Adoptive immunotherapy given either 5 hours or one day after a single injection of cyclophosphamide (83 mg/kg) showed maximum anti-tumor activity in 3Cl-8 Friend leukemia-bearing DBA/2 mice, whereas splenocytes derived from tumor-immunized mice transferred at least 3 days after cyclophosphamide treatment showed no anti-tumor activity [32]. In other studies, vaccines given one day after a single injection of cyclophosphamide at 50 mg/kg induced the highest level of tumor-specific CD8+ T cells in an autochthonous prostate cancer mouse model [60].
Higher doses of chemotherapy may induce different immune modulatory effects, as exemplified by cyclophosphamide. For example, immature DCs that rebound and peak 12 days after a single lymphodepleting dose of cyclophosphamide (160 mg/kg) are functional and can mediate enhanced prime-boost vaccination anti-tumor responses when stimulated at the 12-day time point with a TLR3 agonist in a tolerogenic pmel-1 TCR transgenic C57BL/6 mouse model [91]. This suggests that the restoration phase (days 5 to 18) following the early lymphopenia phase (days 1 to 4) has a distinct immune stimulation value, which should be considered when designing combination chemo-immunotherapy. A single low-dose injection of cyclophosphamide (100 mg/kg) can also spare bone marrow DC precursors and stimulate DC differentiation and activation beginning 3 days after cyclophosphamide injection [10]. In contrast, cyclophosphamide given at a myeloablative dose (200 mg/kg) depletes bone marrow DC precursors [92]. Thus, the immune perturbation function of cyclophosphamide is highly dose-dependent and the timing of combinational immunotherapy needs to take into consideration the chemotherapeutic drug dose.
Combination of chemotherapy with immunotherapy in clinical trials
Many clinical trials have explored the immune stimulatory potential of chemotherapy. Consistent with findings in mouse models, low-dose cyclophosphamide was found to be immunostimulatory in several human clinical trials. For example, low-dose metronomic cyclophosphamide treatment of end-stage cancer patients (50 mg orally, b.i.d., 1 week on, and 1 week off, for 1 month or more) strongly curtailed immunosuppressive Treg cells, leading to a restoration of peripheral T cell proliferation and innate immune cell killing activities [35]. Several successful clinical trials have examined low-dose metronomic chemotherapy as part of an immune induction regimen. In a prospective, randomized trial, patients with advanced, unresectable pancreatic adenocarcinoma, non-small cell lung cancer, or prostate cancer were given either standard chemotherapy (control group) or were additionally given low-dose metronomic cyclophosphamide (50 mg/day, orally) combined with G-CSF, a sulfhydryl donor, a Cox2 inhibitor, and a preparation of autologous tumor antigens (experimental group). The experimental group displayed higher anti-tumor immunity after three months and a significantly longer mean survival time [93]. In another study, 28 progressive metastatic melanoma patients were treated with low-dose metronomic cyclophosphamide (50 mg/twice daily, orally; 1 week on, and 1 week off) together with celecoxib (200 mg daily), followed by vaccination with DCs [94]. A general increase in immune responses was seen, including induction of antigen-specific immune responses. The number of patients with stable disease more than doubled and 6-month survival was increased significantly compared to a previous trial without cyclophosphamide and celecoxib [94].
In several clinical trials, low-dose cancer chemotherapeutic agents were used as preconditioning agents for immunotherapy. In a study combining chemotherapy with HER2-positive, allogeneic, GM-SCF-secreting tumor vaccine in 28 metastatic breast cancer patients, HER2-specific antibody responses were enhanced by 200 mg/m2 cyclophosphamide (given 1 day prior to vaccination) and 35 mg/m2 doxorubicin (given 7 days after vaccination), but were suppressed by higher cyclophosphamide doses (250 or 350 mg/m2) [95]. In another study, a cohort of patients with advanced pancreatic cancer was treated with cyclophosphamide at 250 mg/m2 one day before treatment with a GM-CSF-secreting tumor vaccine [96]. This combination regimen induced tumor-specific CD8+ T cells and led to longer median survival (4.3 months vs. 2.3 months in patients treated by the tumor vaccine alone) [96].
In other cases, however, low-dose cyclophosphamide in combination with immunotherapy did not induce objective responses. For example, cyclophosphamide (300 mg/m2, on day 1) plus escalating doses of an IL2 conjugate with antibody to the cell surface adhesion molecule EpCAM, on days 2, 3, 4 of each 21-day cycle, was used to treat EpCAM-positive advanced solid tumors. Ten of 26 patients (38%) treated for up to 6 cycles had stable disease as the best response, but in only 3 patients did this response last longer than 4 treatment cycles [97]. In a randomized phase II trial of early stage melanoma patients, four mixed modified HLA-class I tumor vaccines were given in combination with cyclophosphamide (300 mg/m2) and low-dose IL2. While vaccination induced a rapid and persistent increase in specific effector memory CD8+ T cells, cross-recognition of native vaccine and reaction to melanoma cells was limited [98]. Finally, a phase II trial of advanced hepatocellular carcinoma patients treated with cyclophosphamide (300 mg/m2, on day -3) in combination with GM-CSF and a telomerase peptide vaccine, GV101, on days 1, 3, 5, 8, 15, 22, and 36, followed by 4 weekly injections, did not induce complete or partial responses in any patients, and no GV101-specific immune responses were detected after vaccination [99].
While different reasons might account for each failed clinical trial, the immune-based effects and overall performance of low-dose chemotherapy, either given as a single injection or in a metronomic manner, is best described as moderate, even though statistically significant increases in immune responses were found in some cases. A systematic review of low-dose metronomic chemotherapy based on 80 published clinical trials up to 2012 (65% involving combination therapy) found that the mean response rate was only 26%, with a median progression-free survival of only 4.6 months [58]. Since in many cases low-dose metronomic chemotherapeutic agents are used as “maintenance” or “consolidation” treatment for elderly and frail patients because of their ease of administration and comparatively low-degree of toxicity [100], randomized phase III trials are needed to definitively assess the immune modulation function of low-dose chemotherapy. However, based on available results, we do not expect that current low-dose chemotherapy-based treatments will generally result in substantial immune-based improvements in cancer treatment. These findings indicate a need for new treatment strategies and regimens.
Medium-dose Intermittent Chemotherapy (MEDIC)
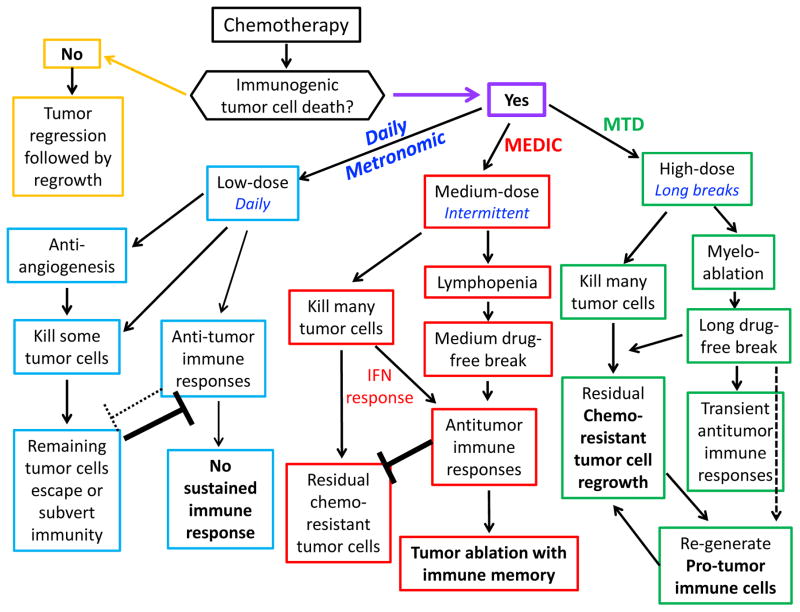
A key goal of ICD-based chemotherapy is to take advantage of the synergistic effects of combining tumor cell cytotoxicity with ICD-induced activation of the patient’s immune system in order to eliminate tumor cells, in particular, tumor cells that may be resistant to conventional chemotherapy. Ideally, this would be achieved in a way that activates both the innate and the adaptive immune system and leads to tumor ablation with long-term anti-tumor immune memory [59]. To achieve this, we propose to combine immunotherapy with a modified metronomic schedule, termed MEDIC (medium-dose intermittent chemotherapy; Fig. 1; red), which uses an immunogenic cancer chemotherapeutic agent given at a dose between that of a low dose daily regimen (Fig. 1; blue) and an MTD dose (Fig. 1; green). Thus, MEDIC employs an intermediate or medium drug dose given on a schedule with an intermediate-length drug-free break. A MEDIC regimen is designed to achieve two important goals: 1) to impart strong and repeated cytotoxic damage to tumors, in order to kill a substantial fraction of tumor cells; and 2) to activate a sustained anti-tumor immune response. In addition, a MEDIC regimen with its expected low host toxicity compared to MTD chemotherapy may allow for prolonged treatments with improve patient quality of life, and thus have wider application in the clinic. The key features of MEDIC are its dose and schedule, which are interrelated; indeed, the total amount of drug delivered on a MEDIC schedule need not be all that different from that of a ‘low dose daily’ metronomic schedule, or from that of an MTD schedule, e.g., for cyclophosphamide in mouse models: 20–25 mg/kg for low dose daily, 140 mg/kg every 6 days for medium dose intermittent (MEDIC), and 150–170 mg/kg × 2 or 3 consecutive days, every 21 days, for MTD dosing [43].
Fig. 1. Cancer chemotherapy: Immune effects of low-dose, medium-dose, high-dose options.
Cancer chemotherapeutic drugs may be non-immunogenic (yellow rectangles) or immunogenic; the latter may be further divided into three categories based on the dose and schedule: Low-dose daily metronomic chemotherapy (blue), medium-dose and intermediate-length intermittent chemotherapy schedule (MEDIC, a modified form of metronomic chemotherapy, red), and high-dose with long drug-free break schedule (MTD, green). The impact of each treatment regimen on tumor cell killing and immune responses is shown.
To achieve a robust anti-tumor response, it is important to select a chemotherapeutic drug capable of inducing ICD and/or depleting immune suppressive cells, and to administer it at a dose that can eradicate a large fraction of tumor cells [90]. In the absence of ICD-stimulated immune responses, the effectiveness of single agent chemotherapy is often limited by a failure to substantially reduce the tumor burden and by the emergence of tumor cell-evolved drug resistance (Fig. 1; yellow). Some chemotherapy regimens, when given at a dose too low to inhibit tumor growth (even when given on a daily schedule), might be able to kill some tumor cells via an anti-angiogenic mechanism [56] or perturb immune cell populations in a beneficial manner, e.g., by decreasing the Treg/CD4+ T cell ratio, but do not activate a robust anti-tumor immune response [90] (Fig. 1; blue). Thus, low-dose daily regimens may not effectively reduce tumor volume significantly [56]. Tumor burden often shows a negative correlation with the potency of immunotherapy, presumably because immune suppressive signals derived from a large and expanding tumor mass are sufficiently strong to override the stimulatory effects of immunotherapy [101]. Therefore, non-immunogenic regimens of chemotherapy, or immunogenic chemotherapy regimens that are given at a dose too low to inhibit tumor growth, are not ideal candidates to combine with immunotherapy.
For chemo-immunotherapy to be effective, the dose of chemotherapy needs to be lower than the threshold dose that induces severe myeloablation and host toxicity; doses higher than this would necessitate a prolonged drug-free break, such as the break required in a classical MTD drug schedule, which could allow for the development of immune suppression, overriding any transient anti-tumor immune responses [42] and enabling drug-resistant tumor cell clones to emerge [102] (Fig. 1; green). Once drug-resistant tumor cells expand to form a significant tumor mass, the growing tumor cells may evolve additional mechanisms to subvert or escape the immune system and undermine the effectiveness of immunotherapy.
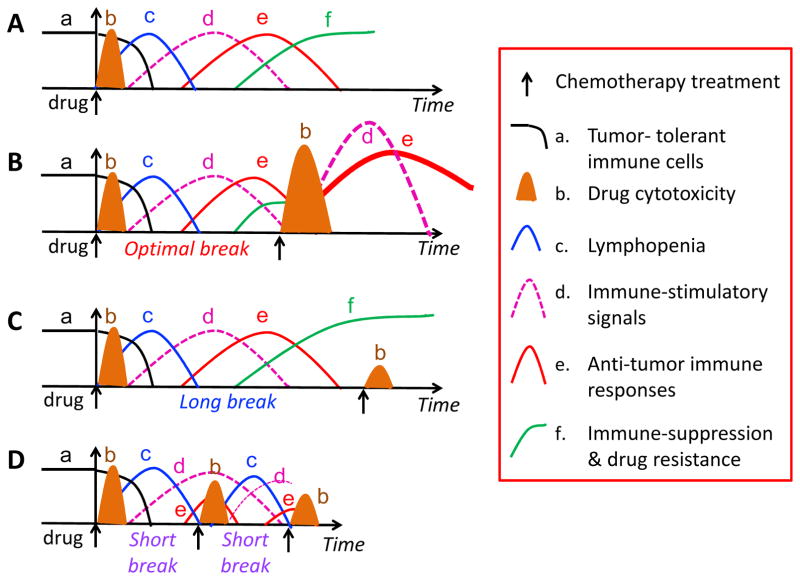
The length of the drug-free break needs to be properly timed to achieve the right balance between cytotoxicity to tumor cells and a sustained immune response (Fig. 2). Typically, a single injection of a cancer chemotherapeutic drug may induce multiple responses, including a reduction of tumor-tolerant immune cells (Fig. 2A, black line) and an increase in drug cytotoxicity (Fig. 2B, brown cone) to tumor cells and immune cells, e.g., lymphopenia (Fig. 2C, blue line), and immune-stimulatory signals (Fig. 2D, pink line). The immune stimulation signals activate anti-tumor immune responses (Fig. 2E, red line), which are followed by increased immune suppression and drug resistance (Fig. 2F, green line). An optimal drug-free break will allow for rebound of endogenous tumor-reactive immune cells and give sufficient time to expand the immunotherapy-activated immune response (Fig. 2B). A drug-free break that is too long (e.g., ≥ 9-days in intermittent cyclophosphamide treatment regimens [44,59,103]) may lead to immune suppression and drug resistance (Fig. 2C), while a drug-free break that is too short (e.g., ≤ 3–4-days on those same cyclophosphamide regimens [43,59,61–63]), will ablate responding anti-tumor immune cells (Fig. 2D). We thus propose to employ an intermediate-length drug-free break, such as the 6-day MEDIC schedule that is highly effective in glioma models [42,43,45,47,59]. Multiple cycles of combination treatment will likely be needed to eradicate sufficient numbers of tumor cells and to achieve strong, sustained anti-tumor immune responses, while preventing the development of immune suppression and the emergence of drug resistant tumor cell populations.
Fig. 2. Chemo-immunotherapy schedules.
(A) A single injection of a cancer chemotherapeutic drug may induce multiple events, including changes in the number of tumor-tolerant immune cells (a, black line), chemotherapy treatment-induced drug cytotoxicity (b, brown cone), which is often followed by lymphopenia (c, blue line) and immune-stimulatory signals (d, pink line). The immune stimulatory signals activate anti-tumor immune responses (e, red line), which are followed by increased immune suppression and drug resistance (f, green line). (B) An optimal drug-free break allows for an overall increase in anti-tumor immune response. A second treatment with chemotherapy may have greater effect than the first treatment due to synergism with the anti-tumor immune responses activated by the first drug treatment, and can circumvent or interrupt the emergence of immune suppression. (C) A drug-free break that is too long may enable the development of immune suppression and the emergence of drug resistance, thereby countering the effectiveness of the second drug treatment; (D) A drug-free break that is too short ablates anti-tumor immune responses prematurely, thereby reducing the efficacy of subsequent treatments with chemotherapy.
An intermittent, every 6-day repeating medium-dose cyclophosphamide schedule (6-day MEDIC regimen) at 90 to 140 mg/kg per injection can activate robust and sustained innate and adaptive immune responses following an initial transient lymphopenia (Fig. 1; red) [59]. These mouse doses correspond to an adult human dose range of 7.3–11.4 mg cyclophosphamide/kg body weight (270–420 mg/m2) based on a species-adjusted per body surface area dose conversion [104]. The 6-day MEDIC cyclophosphamide regimen is well tolerated in the mouse model with no need for special palliative care, indicating its low toxicity. The total dose of cyclophosphamide on the 6-day MEDIC schedule, when calculated on a per day basis, is similar to that used in MTD cyclophosphamide schedules in mouse models [42,105]. The essential difference is that the length of the drug-free break is substantially shorter on the MEDIC schedule. Whereas MTD schedules of cyclophosphamide induce transient immune responses associated with substantial tumor growth rebound [42], the 6-day cyclophosphamide schedule provides a good balance between maximizing tumor cell toxicity and minimizing the frequency of immune cell ablation [59]. Thus, the preclinical studies from our laboratory and by others support the use of MEDIC schedules [42–44,61,62]. Further work is needed to extend these studies to other tumor types and a broader range of tumor models, including orthotopic tumors and genetically engineered mouse models. Such studies may include PDX (patient-derived xenograft) models grown in scid (adaptive immune-deficient) mice [106,107], insofar as a significant component of the immune response to the 6-day cyclophosphamide MEDIC regimen involves the innate immune system, which is sufficient to induce major immune-based tumor regression, even in the absence of the adaptive immune system [42].
It will be challenging to finding the right balance between a chemotherapy treatment interval that is not ‘too short’ and one that is not ‘too long’ for effective MEDIC, as it will be to identify the optimal MEDIC dose when targeting different tumor types with different chemotherapeutics. In this regard, the discovery of predictive biomarkers of responsiveness may be critical for clinical translation. It will also be important to ascertain whether MEDIC schedules show superior efficacy compared to other doses and schedules in the clinic, either alone or when combined with immunotherapy. To investigate this question, we reviewed all clinical trials from 12/01/2012 to 01/15/2014, as cited by Vacchelli [39], to identify trials that employed cyclophosphamide and monitored an immunogenic response. We also examined 264 other clinical trials involving cyclophosphamide registered at the NIH clinical trial website (clinicaltrials.gov) for the subsequent 2-year period. We found only 12 clinical trials where cyclophosphamide was used on a MEDIC-type schedule, most of which were based on a 7-day drug-free break (Supplementary Table 1). Unfortunately, none of these trials compared MEDIC schedules with other cyclophosphamide schedules (e.g., low dose daily metronomic or traditional MTD schedules), and none determined whether the cyclophosphamide-based treatments activated an immune response. Further, in all 12 trials, cyclophosphamide was given together with dexamethasone, a glucocorticoid anti-emetic with immune suppressive activity [108], including CD4+ and CD8+ T cell depletion and Treg activation [109], which may compromise MEDIC-activated anti-tumor immune responses. These findings, together with the moderate performance of many low-dose metronomic chemotherapy trials, summarized above, indicate a critical need for suitably designed clinical trials to properly evaluate the therapeutic utility of MEDIC schedules and their efficacy, both alone and in combination with immunotherapy and other co-medications used in cancer patients.
Dual chemotherapy-based chemo-immunotherapy
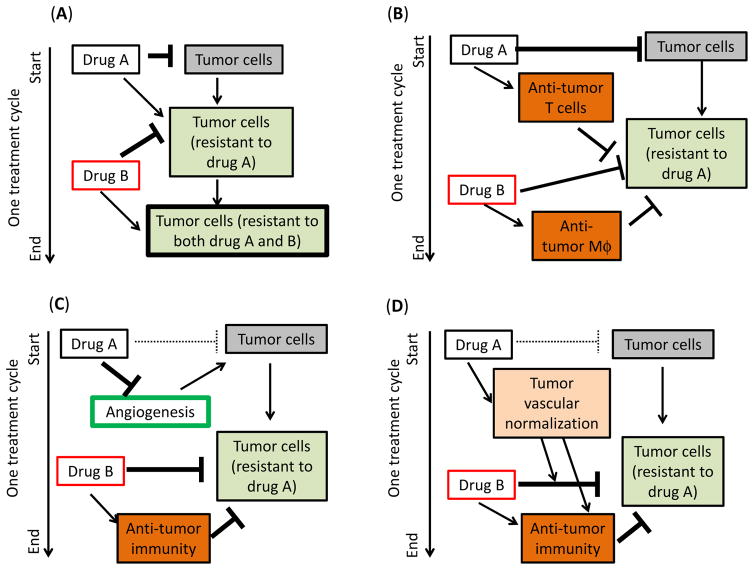
Immunotherapy combined with two cytotoxic cancer chemotherapeutic drugs might be more effective than a single chemotherapeutic drug-based regimen, for the following reasons (Fig. 3). First, inclusion of a second cytotoxic agent may minimize the selection of drug-resistant tumor cell clones (Fig. 3A). Second, different cancer chemotherapeutic drugs may stimulate different anti-tumor immune populations (Fig. 3B). For example, cyclophosphamide and paclitaxel can each boost tumor vaccine-mediated T cell responses when given at lymphodepleting doses one day prior to vaccination, whereas doxorubicin can spare T cells and activate macrophages when given 7 days after vaccine injection [89]. Third, one chemotherapeutic drug may be used to inhibit tumor growth by an anti-angiogenic mechanism or reduce tumor burden by its intrinsic tumor cell cytotoxicity, while a second drug may complement the first drug by activating ICD (Fig. 3C). However, caution should be exercised when using such a strategy, since VEGFR2-targeting anti-angiogenesis agents can block the strong immune cell recruitment stimulated by a 6-day cyclophosphamide MEDIC schedule [42,45]. Finally, to address the leakiness of the tumor vasculature, which results in a high interstitial pressure and a low rate of drug penetration [110], it may be beneficial to use one drug to normalize the tumor vasculature, and thereby improve the uptake of a second chemotherapeutic drug, resulting in more effective tumor cell killing and altering the tumor microenvironment in a way that favors anti-tumor immune responses (Fig. 3D).
Fig. 3. Four strategies for cancer chemotherapeutic drug combinations.
(A) Drug A synergizes with Drug B by minimizing the survival of drug-resistant tumor cell clones. (B) Drug A and Drug B can each stimulate or induce different anti-tumor immune populations. (C) Drug A inhibits tumor growth by its intrinsic anti-tumor cytotoxicity or by an anti-angiogenic mechanism, while Drug B complements Drug A by activating ICD. (D) Drug A normalizes the tumor vasculature and thereby improves the uptake of Drug B, which activates ICD.
In terms of drug schedule, the same principles that we discussed for MEDIC using a single chemotherapeutic agent will apply when two or more chemotherapeutic drugs are combined with immunotherapy. However, special consideration will need to be given to dose and schedule optimization, as well as drug sequencing, all of which can be expected to complicate MEDIC trial design and clinical evaluation.
Implications for drug development
In vitro tumor cell cytotoxicity assays are commonly used to screen for novel anti-tumor drugs [111,112]. While this approach can be used to identify novel cytotoxic agents, it is not an effective way to identify novel inducers of ICD. It is thus important to incorporate new criteria, such as ICD or immune modulation, when screening for anti-cancer drugs. The clinical efficacy of imatinib mesylate (Gleevec) in treating gastrointestinal stromal tumors (GIST) that lack the activating mutations in KIT and PDGFRA, normally targeted by imatinib, is a good example that supports this approach [113]. These GIST cells do not respond to imatinib in vitro due to their lack of an imatinib target. In this case, imatinib blocks KIT signaling in host DCs, which leads to DC-mediated NK cell activation and inhibition of tumor cell growth [113]. Further, in the case of some KIT-expressing GIST tumors, imatinib functions in a non-cell autonomous manner by restoring the anti-tumor functions of CD8+ T cells and NK cells by inhibiting the KIT-ETV4-IDO-Treg axis between tumor cells and Treg cells [114]. In both cases, key stromal cell components are required for any cell culture-based detection of imatinib activity.
There are several ways to assess the capacity of chemotherapeutic agents to modulate immune cell function and induce immunogenic tumor cell death. One way is to engineer a fluorescent marker to track the expression of immunogenic tumor cell death. For example, U2OS cells that stably express a calreticulin-GFP fusion protein has been used to monitor the cellular location of calreticulin protein [115]. This system was used to ascertain that cisplatin, which does not induce ICD, failed to induce translocation of calreticulin from the endoplasmic reticulum to the cell surface, in contrast to the translocation activity seen with the ICD drugs oxaliplatin and mitoxantrone [116]. This assay was used to screen a broad range of compounds and led to the discovery that thapsigargin, an inducer of endoplasmic reticulum stress, can synergize with cisplatin to induce translocation of calreticulin to the plasma membrane and activate ICD [116]. Reporters for HMGB1 release can also be used for immunogenic anticancer drug screening and development.
Assays for chemotherapy-mediated immune cell modulation can readily be implemented in a high throughput in vitro screen format. In one example, an engineered IL1 promoter was used to drive the expression of a fluorescent reporter protein in a murine DC cell line, and served as a surrogate marker for DC maturation in a screen for immune stimulatory chemotherapeutic drugs [52]. DC function can be affected by many non-cell autonomous factors, including eat-me signals coming from immunogenic tumor cell death and cytokines secreted by tumor cells or other stromal cells [10]. Therefore, the utility of such a screen might be greatly improved by adding tumor cell-conditioned medium or tumor cells, and/or stromal cells, to mimic the in vivo composition of a specific tumor type. By incorporating tumor cells into the assay, tumor cell ICD may be indirectly read out using the high-throughput reporter for DC activation. Mixed tumor cell populations designed to mimic the in vivo tumor composition may also be used to screen for other immune modulatory features of cancer chemotherapeutic drugs. These include the ability to induce critical immune stimulatory cytokines or chemokines, activate NK cell or T cell effectors, deplete immune suppressive cells, or increase death receptor expression on tumor cells. Gene expression profiles of untreated tumors that reflect the immunogenicity of tumor cells, the composition of stromal cells, and the immune cell activation status [117–120] may also help identify targets of immunotherapy and design suitable in vitro screens for new and effective chemotherapeutic drugs.
Conclusion
We have reviewed the dependence of ICD on the choice of chemotherapeutic drug and tumor model and the impact of chemotherapeutic drug dose and schedule on the ability to achieve robust anti-tumor immune responses. We have proposed a modified metronomic schedule, termed MEDIC, for effective immunogenic chemotherapy and for combination chemo-immunotherapy. MEDIC schedules offer several advantages over conventional MTD and low-dose daily (metronomic) schedules; these include lower host toxicity compared to MTD schedules, higher peak drug levels and greater cytotoxicity to tumor cells than low-dose daily metronomic schedules, and a drug-free break that is designed to maximize anti-tumor immune responses. The ultimate clinical utility and effectiveness of MEDIC scheduling in unknown and awaits rigorous testing in clinical trials. The discovery of biomarkers of immune responsiveness may facilitate clinical translation by helping to address the challenge of finding an optimal dosing schedule and the right dose for each MEDIC regimen, tumor type and chemotherapeutic – immunotherapeutic combination. Finally, immune activation-based assays can be expected to stimulate the discovery of novel immunogenic anti-cancer drugs that spur the development of novel and more effective cancer treatments.
Supplementary Material
Highlights.
Chemotherapy-induced anti-tumor immunity is highly dependent on dose and schedule
Combination therapies need to balance cytotoxicity to tumor cells vs. immune cells
Medium-dose intermittent chemotherapy can greatly enhance anti-tumor immune responses
Strategies to identify new immunogenic chemotherapeutics are identified
Acknowledgments
Funding: This work was supported in part by National Institutes of Health grant CA049248 (to DJW).
Abbreviations
- DC
dendritic cell
- ICD
immunogenic cell death
- IFN
interferon
- MEDIC
medium-dose intermittent chemotherapy
- MTD
maximum tolerated dose
- NK
natural killer
- TLR
toll-like receptor
- Treg
regulatory T cells
Footnotes
Authors’ Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis. 2013;4:e688. doi: 10.1038/cddis.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2:e23510. doi: 10.4161/onci.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg AD, De Ruysscher D, Agostinis P. Immunological metagene signatures derived from immunogenic cancer cell death associate with improved survival of patients with lung, breast or ovarian malignancies: A large-scale meta-analysis. Oncoimmunology. 2016;5:e1069938. doi: 10.1080/2162402X.2015.1069938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 8.Shurin MR, Naiditch H, Gutkin DW, Umansky V, Shurin GV. ChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agents. Curr Med Chem. 2012;19:1792–1803. doi: 10.2174/092986712800099785. [DOI] [PubMed] [Google Scholar]
- 9.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 10.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D’Urso MT, Belardelli F, Gabriele L, Proietti E, Bracci L. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 11.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 15.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nature Medicine. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 16.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 17.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 18.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 19.Martins I, Kepp O, Galluzzi L, Senovilla L, Schlemmer F, Adjemian S, Menger L, Michaud M, Zitvogel L, Kroemer G. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann N Y Acad Sci. 2010;1209:77–82. doi: 10.1111/j.1749-6632.2010.05740.x. [DOI] [PubMed] [Google Scholar]
- 20.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 24.Khallouf H, Marten A, Serba S, Teichgraber V, Buchler MW, Jager D, Schmidt J. 5-Fluorouracil and interferon-alpha immunochemotherapy enhances immunogenicity of murine pancreatic cancer through upregulation of NKG2D ligands and MHC class I. J Immunother. 2012;35:245–253. doi: 10.1097/CJI.0b013e31824b3a76. [DOI] [PubMed] [Google Scholar]
- 25.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 27.Fine JH, Chen P, Mesci A, Allan DS, Gasser S, Raulet DH, Carlyle JR. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res. 2010;70:7102–7113. doi: 10.1158/0008-5472.CAN-10-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramakrishnan R, Huang C, Cho HI, Lloyd M, Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E, Gabrilovich DI. Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res. 2012;72:5483–5493. doi: 10.1158/0008-5472.CAN-12-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S, Larma I, Prosser A, Robinson BW, Smyth MJ, Scalzo AA, Degli-Esposti MA, Lake RA. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Perez L, Suryadevara CM, Choi BD, Reap EA, Sampson JH. Leveraging chemotherapy-induced lymphopenia to potentiate cancer immunotherapy. Oncoimmunology. 2014;3:e944054. doi: 10.4161/21624011.2014.944054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Moschella F, Valentini M, Arico E, Macchia I, Sestili P, D’Urso MT, Alessandri C, Belardelli F, Proietti E. Unraveling cancer chemoimmunotherapy mechanisms by gene and protein expression profiling of responses to cyclophosphamide. Cancer Res. 2011;71:3528–3539. doi: 10.1158/0008-5472.CAN-10-4523. [DOI] [PubMed] [Google Scholar]
- 33.Moschella F, Torelli GF, Valentini M, Urbani F, Buccione C, Petrucci MT, Natalino F, Belardelli F, Foa R, Proietti E. Cyclophosphamide induces a type I interferon-associated sterile inflammatory response signature in cancer patients’ blood cells: implications for cancer chemoimmunotherapy. Clin Cancer Res. 2013;19:4249–4261. doi: 10.1158/1078-0432.CCR-12-3666. [DOI] [PubMed] [Google Scholar]
- 34.Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78:661–671. doi: 10.1007/s00280-016-3152-1. [DOI] [PubMed] [Google Scholar]
- 35.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautes-Fridman C, Fucikova J, Galon J, Spisek R, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3:e27878. doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 41.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 42.Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72:1103–1115. doi: 10.1158/0008-5472.CAN-11-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CS, Doloff JC, Waxman DJ. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84–96. doi: 10.1593/neo.131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Waxman DJ. Metronomic cyclophosphamide schedule-dependence of innate immune cell recruitment and tumor regression in an implanted glioma model. Cancer Lett. 2014;353:272–280. doi: 10.1016/j.canlet.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doloff JC, Chen CS, Waxman DJ. Anti-tumor innate immunity activated by intermittent metronomic cyclophosphamide treatment of 9L brain tumor xenografts is preserved by anti-angiogenic drugs that spare VEGF receptor 2. Mol Cancer. 2014;13:158. doi: 10.1186/1476-4598-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia L, Waxman DJ. Thrombospondin-1 and pigment epithelium-derived factor enhance responsiveness of KM12 colon tumor to metronomic cyclophosphamide but have disparate effects on tumor metastasis. Cancer Lett. 2013;330:241–249. doi: 10.1016/j.canlet.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Jordan M, Waxman DJ. Metronomic cyclophosphamide activation of anti-tumor immunity: tumor model, mouse host, and drug schedule dependence of gene responses and their upstream regulators. BMC Cancer. 2016;16:623. doi: 10.1186/s12885-016-2597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer. 2016;99:166–171. doi: 10.1016/j.lungcan.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Lu YC, Robbins PF. Targeting neoantigens for cancer immunotherapy. Int Immunol. 2016;28:365–370. doi: 10.1093/intimm/dxw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, Phung TL, Mani SA, Stossi F, Sreekumar A, Mancini MA, Decker WK, Zong C, Lewis MT, Zhang XH. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Huang X, Huang G, Chen Y, Chen L, Song H. Preconditioning chemotherapy with cisplatin enhances the antitumor activity of cytokine-induced killer cells in a murine melanoma model. Cancer Biother Radiopharm. 2012;27:210–220. doi: 10.1089/cbr.2011.1116. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka H, Matsushima H, Mizumoto N, Takashima A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–6986. doi: 10.1158/0008-5472.CAN-09-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 54.Pantziarka P, Hutchinson L, Andre N, Benzekry S, Bertolini F, Bhattacharjee A, Chiplunkar S, Duda DG, Gota V, Gupta S, Joshi A, Kannan S, Kerbel R, Kieran M, Palazzo A, Parikh A, Pasquier E, Patil V, Prabhash K, Shaked Y, Sholler GS, Sterba J, Waxman DJ, Banavali S. Ecancermedicalscience; Next generation metronomic chemotherapy-report from the Fifth Biennial International Metronomic and Anti-angiogenic Therapy Meeting; 6–8 May 2016; Mumbai. 2016. p. 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussein MM, Gaafar RM, Abdel-Warith AM, Ahmed WA, Allahloubi NMA, Salem SE, Abdel-Salam IM. Efficacy and Toxicity of Metronomic Chemotherapy in Metastatic Breast Cancer: Egyptian Experience. Clin Breast Cancer. 2017 doi: 10.1016/j.clbc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Andre N, Tsai K, Carre M, Pasquier E. Metronomic Chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer. 2017;3:319–325. doi: 10.1016/j.trecan.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358:100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49:3387–3395. doi: 10.1016/j.ejca.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Waxman DJ. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8+ T cell responses and immune memory. Oncoimmunology. 2015;18:13. doi: 10.1080/2162402X.2015.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, Demarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tongu M, Harashima N, Monma H, Inao T, Yamada T, Kawauchi H, Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2013;62:383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng S, Lyford-Pike S, Akpeng B, Wu A, Hung CF, Hannaman D, Saunders JR, Wu TC, Pai SI. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol Immunother. 2013;62:171–182. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malvicini M, Alaniz L, Bayo J, Garcia M, Piccioni F, Fiore E, Atorrasagasti C, Aquino JB, Matar P, Mazzolini G. Single low-dose cyclophosphamide combined with interleukin-12 gene therapy is superior to a metronomic schedule in inducing immunity against colorectal carcinoma in mice. Oncoimmunology. 2012;1:1038–1047. doi: 10.4161/onci.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Connor R. A review of mechanisms of circumvention and modulation of chemotherapeutic drug resistance. Curr Cancer Drug Targets. 2009;9:273–280. doi: 10.2174/156800909788166583. [DOI] [PubMed] [Google Scholar]
- 65.Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12:631–645. doi: 10.1517/14740338.2013.795944. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mondino A, Vella G, Icardi L. Targeting the tumor and its associated stroma: One and one can make three in adoptive T cell therapy of solid tumors. Cytokine Growth Factor Rev. 2017;36:57–65. doi: 10.1016/j.cytogfr.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Redeker A, Arens R. Improving Adoptive T Cell Therapy: The Particular Role of T Cell Costimulation, Cytokines, and Post-Transfer Vaccination. Front Immunol. 2016;7:345. doi: 10.3389/fimmu.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu C, Zhang Y, Rolfe PA, Hernandez VM, Guzman W, Kradjian G, Marelli B, Qin G, Qi J, Wang H, Yu H, Tighe R, Lo KM, English JM, Radvanyi L, Lan Y. Combination Therapy with NHS-muIL12 and Avelumab (anti-PD-L1) Enhances Antitumor Efficacy in Preclinical Cancer Models. Clin Cancer Res. 2017;23:5869–5880. doi: 10.1158/1078-0432.CCR-17-0483. [DOI] [PubMed] [Google Scholar]
- 72.Herber DL, Nagaraj S, Djeu JY, Gabrilovich DI. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res. 2007;67:5067–5069. doi: 10.1158/0008-5472.CAN-07-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beatty PL, Cascio S, Lutz E. Tumor immunology: basic and clinical advances. Cancer Res. 2011;71:4338–4343. doi: 10.1158/0008-5472.CAN-11-0717. [DOI] [PubMed] [Google Scholar]
- 74.Kareva I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33:4623–4631. doi: 10.1038/onc.2013.432. [DOI] [PubMed] [Google Scholar]
- 76.Parra K, Valenzuela P, Lerma N, Gallegos A, Reza LC, Rodriguez G, Emmenegger U, Di Desidero T, Bocci G, Felder MS, Manciu M, Kirken RA, Francia G. Impact of CTLA-4 blockade in conjunction with metronomic chemotherapy on preclinical breast cancer growth. Br J Cancer. 2017;116:324–334. doi: 10.1038/bjc.2016.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inoges S, Melero I, Berraondo P. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95:347–355. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 78.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 79.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirota H, Tross D, Klinman DM. CpG Oligonucleotides as Cancer Vaccine Adjuvants. Vaccines (Basel) 2015;3:390–407. doi: 10.3390/vaccines3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gosu V, Basith S, Kwon OP, Choi S. Therapeutic applications of nucleic acids and their analogues in Toll-like receptor signaling. Molecules. 2012;17:13503–13529. doi: 10.3390/molecules171113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Billod JM, Lacetera A, Guzman-Caldentey J, Martin-Santamaria S. Computational Approaches to Toll-Like Receptor 4 Modulation. Molecules. 2016;21 doi: 10.3390/molecules21080994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Glavan TM, Pavelic J. The exploitation of Toll-like receptor 3 signaling in cancer therapy. Curr Pharm Des. 2014;20:6555–6564. doi: 10.2174/1381612820666140826153347. [DOI] [PubMed] [Google Scholar]
- 84.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 85.Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther. 2012;22:77–89. doi: 10.1089/nat.2012.0340. [DOI] [PubMed] [Google Scholar]
- 86.Jordan M, Waxman DJ. CpG-1826 immunotherapy potentiates chemotherapeutic and anti-tumor immune responses to metronomic cyclophosphamide in a preclinical glioma model. Cancer Lett. 2016;373:88–96. doi: 10.1016/j.canlet.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 88.Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 90.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, Luznik L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59:137–148. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lasalvia-Prisco E, Goldschmidt P, Galmarini F, Cucchi S, Vazquez J, Aghazarian M, Lasalvia-Galante E, Golomar W, Gordon W. Addition of an induction regimen of antiangiogenesis and antitumor immunity to standard chemotherapy improves survival in advanced malignancies. Med Oncol. 2012;29:3626–3633. doi: 10.1007/s12032-012-0301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellebaek E, Engell-Noerregaard L, Iversen TZ, Froesig TM, Munir S, Hadrup SR, Andersen MH, Svane IM. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer Immunol Immunother. 2012;61:1791–1804. doi: 10.1007/s00262-012-1242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connor JP, Cristea MC, Lewis NL, Lewis LD, Komarnitsky PB, Mattiacci MR, Felder M, Stewart S, Harter J, Henslee-Downey J, Kramer D, Neugebauer R, Stupp R. A phase 1b study of humanized KS-interleukin-2 (huKS-IL2) immunocytokine with cyclophosphamide in patients with EpCAM-positive advanced solid tumors. BMC Cancer. 2013;13:20. doi: 10.1186/1471-2407-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, Maurichi A, Cova A, Rigamonti G, Giardino F, Di Florio A, Asioli M, Frati P, Sovena G, Squarcina P, Maio M, Danielli R, Chiarion-Sileni V, Villa A, Lombardo C, Tragni G, Santinami M, Parmiani G, Rivoltini L. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin Cancer Res. 2012;18:6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 99.Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. doi: 10.1186/1471-2407-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loven D, Hasnis E, Bertolini F, Shaked Y. Low-dose metronomic chemotherapy: from past experience to new paradigms in the treatment of cancer. Drug Discov Today. 2013;18:193–201. doi: 10.1016/j.drudis.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 101.Liseth K, Ersvaer E, Hervig T, Bruserud O. Combination of intensive chemotherapy and anticancer vaccines in the treatment of human malignancies: the hematological experience. J Biomed Biotechnol. 2010;2010:692097. doi: 10.1155/2010/692097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 103.Stathopoulos A, Pretto C, Devillers L, Pierre D, Hofman FM, Kruse C, Jadus M, Chen TC, Schijns VE. Development of immune memory to glial brain tumors after tumor regression induced by immunotherapeutic Toll-like receptor 7/8 activation. Oncoimmunology. 2012;1:298–305. doi: 10.4161/onci.19068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB Journal. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 105.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 106.Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10:106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, McNary TJ, Churakova Y, Cheung C, Triscott J, Pisapia D, Rao R, Mosquera JM, Robinson B, Faltas BM, Emerling BE, Gadi VK, Bernard B, Elemento O, Beltran H, Demichelis F, Kemp CJ, Grandori C, Cantley LC, Rubin MA. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017;7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cook AM, McDonnell AM, Lake RA, Nowak AK. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo943 immunotherapy strategies. Oncoimmunology. 2016;5:e1066062. doi: 10.1080/2162402X.2015.1066062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 112.Holbeck SL. Update on NCI in vitro drug screen utilities. Eur J Cancer. 2004;40:785–793. doi: 10.1016/j.ejca.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 113.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, Le Cesne A, Chung-Scott V, Lazar V, Tchou I, Crepineau F, Lemoine F, Bernard J, Fletcher JA, Turhan A, Blay JY, Spatz A, Emile JF, Heinrich MC, Mecheri S, Tursz T, Zitvogel L. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, Besmer P, Guo T, Antonescu CR, Taguchi T, Yuan J, Wolchok JD, Allison JP, DeMatteo RP. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, Kroemer G. Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology. 2014;3:e28473. doi: 10.4161/onci.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, Kroemer G. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 117.Li B, Li T, Pignon JC, Wang B, Wang J, Shukla SA, Dou R, Chen Q, Hodi FS, Choueiri TK, Wu C, Hacohen N, Signoretti S, Liu JS, Liu XS. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48:725–732. doi: 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356 e1316. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.