Abstract
Photopolymerized hydrogels, such as gelatin methacryloyl (GelMA), have desirable biological and mechanical characteristics for a range of tissue engineering applications.
Objectives
This study aimed to optimize a new method to photopolymerize GelMA using a dental curing light (DL).
Methods
Lithium acylphosphinate photo-initiator (LAP, 0.05, 0.067, 0.1 % w/v) was evaluated for its ability to polymerize GelMA hydrogel precursors (10% w/v) encapsulated with odontoblast-like cells (OD21). Different irradiances (1650, 2300 and 3700 mW/cm2) and photo-curing times (5 to 20 s) were tested, and compared against the parameters typically used in UV light photopolymerization (45 mW/cm2, 0.1% w/v Irgacure 2959 as photoinitiator). Physical and mechanical properties of the photopolymerized GelMA hydrogels were determined. Cell viability was assessed using a live and dead assay kit.
Results
Comparing DL and UV polymerization methods, the DL method photopolymerized GelMA precursor faster and presented larger pore size than the UV polymerization method. The live and dead assay showed more than 80% of cells were viable when hydrogels were photopolymerized with the different DL irradiances. However, the cell viability decreased when the exposure time was increased to 20s using the 1650 mW/cm2 intensity, and when the LAP concentration was increased from 0.05 to 0.1%. Both DL and UV photocrosslinked hydrogels supported a high percentage of cell viability and enabled fabrication of micropatterns using a photolithography microfabrication technique.
Significance
The proposed method to photopolymerize GelMA cell-laden hydrogels using a dental curing light is effective and represents an important step towards the establishment of chair-side procedures in regenerative dentistry.
Keywords: hydrogel, biomedical and dental materials, bioengineering, visible light, regenerative medicine, endodontics, odontoblast
1. Introduction
Tissue engineering and regenerative medicine consist of delivering cells and bioactive agents (i.e. growth factors, nucleic acids) to injured sites to promote and restore tissue function [1–3]. Hydrogels, which are highly hydrated natural and synthetic biomaterials that closely replicate the structural and biological characteristics of the native extracellular matrix (ECM), have long been proposed as ideal candidates for cell delivery in regenerative medicine and dentistry [4]. Their characteristics, such as biocompatibility, biodegradability, tunable physical and chemical properties, and ease of fabrication, have made them attractive biomaterials for biomedical applications [5–7].
Various natural and synthetic materials have been chemically modified with photocrosslinkable functional groups, including gelatin, alginate, chitosan, collagen, polyethylene glycol, and many others (5). These materials can be mixed with a photoinitiator that absorbs an appropriate wavelength of light and decomposes into free radicals to initiate photopolymerization and form hydrogels [5]. Photocrosslinkable hydrogels allow control over mechanical properties, swelling ratios and degradation rates [6, 8, 9], while being compatible with cell encapsulation, which allows for precise tuning of the 3D microenvironment surrounding cells in tissue engineering constructs. This, in turn, enables precise regulation of cell behavior, which may lead to more predictable outcomes in regenerative strategies [8–10]. Gelatin methacryloyl (GelMA), in particular, has additional desirable properties for tissue engineering. GelMA has been shown to possess matrix metalloproteinase (MMP) and RGD (Arg-Gly-Asp) responsive peptide motifs, which are known to enhance cell-mediated matrix degradation and binding, respectively [7, 11, 12].
Although several photoinitiators have been proposed for hydrogel cell encapsulation and photocrosslinking, Irgacure 2959 (2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone) has been the most commonly used for cell encapsulation and tissue engineering applications [13–17]. However, in addition to its low water solubility, the requirement for exposure to light at ultra-violet (UV) (365 nm) wavelengths is a significant limitation. UV light has been shown to have potential detrimental consequences for both delivered cells and host tissues, hence, the formation of free radicals upon longer UV exposure may lead to DNA damage and impair cellular function [5, 14, 18–20]. As a result, photoinitiators that absorb light in the visible region are considered advantageous over conventional UV photoinitiators. It was demonstrated that the visible light photoinitiator lithium acylphosphinate salt (LAP) has high water solubility and permits cell encapsulation at lower photoinitiator concentrations and longer light wavelength (405 nm), enabling ef cient polymerization compared to Irgacure 2959 [14]. Also, visible light is expected to cause less damage to cells and to be more efficiently transmitted through tissues, allowing greater depth of cure [13, 21]. Moreover, many devices, such as dental lamps, endoscopic probes, microscope imaging lamps and lasers emit light in the short wavelength visible spectrum, but not in the UV spectrum [14]. Especially, dental curing light devices that use light emitting diode (LED) technology have become the dominant visible light source for photopolymerizations due to their high energy [22, 23].
Recently, we have demonstrated a novel strategy to engineer pre-vascularized, cell-laden hydrogel pulp-like tissue constructs in full-length root canals in vitro by sequential GelMA polymerization using UV-light [10]. Such techniques for oral regeneration can benefit from hydrogel polymerization using dental curing lights operating in the visible range (Figure 1).
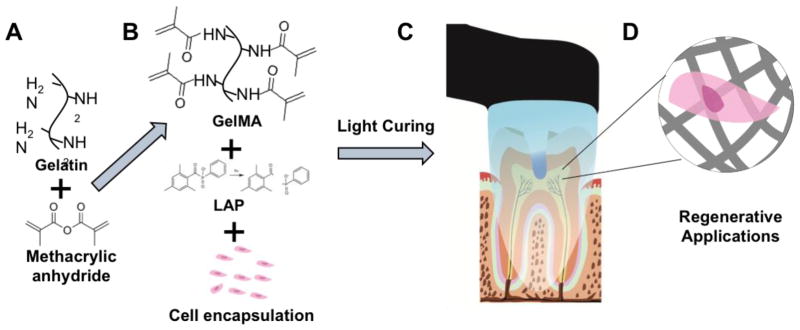
Figure 1.
Example of application of GelMA hydrogel in regenerative dentistry. Synthesis of GelMA macromer (A), cell encapsulation (B), example intracanal hydrogel loading and photopolymerization, (C), and the resulting cell-laden hydrogel material (D).Note that although the schematic depicts an example for regenerative endodontics, the material can be used for any application of intra-oral regeneration, such guided periodontal regeneration, alveolar bone growth and others.
Therefore, to bring this new tissue engineering strategy in regenerative dentistry a step closer to clinical practice, a polymerization process using visible light dental curing devices (DL), which are FDA approved and well established in dental practice, is desirable. Considering the advantage of visible light relative to UV light, we carried out a study comparing the effect of visible DL and UV light polymerization methods on GelMA encapsulating odontoblast-like cells (OD21). First, we evaluated the effect of dental light irradiance, exposure time and photoinitiator concentration on OD21s encapsulated in GelMA. After determining the optimized condition to photopolymerize cell-laden GelMA with a DL instrument, we then compared the physical and mechanical properties of DL and UV light photopolymerized GelMA hydrogels. Lastly, we compared the viability of OD21 cells encapsulated in GelMA hydrogels polymerized with either DL or UV light polymerization.
2. Materials and methods
2.1. Gelatin methacryloyl (GelMA) synthesis
The syntheses of GelMA was performed as described previously [11]. Type A gelatin (10% w/v) from porcine skin (Sigma, St Louis, MO, USA) was dissolved in Dulbecco’s phosphate buffered saline (DPBS, Sigma). While stirring, the solution was heated to 50°C. Then, 8% (v/v) methacrylic anhydride (Sigma) was added to the solution in a dropwise manner, allowing the reaction to proceed for 2 hours at 50°C, and subsequently stopping it a 5x dilution of 40°C DPBS. The solutions were dialyzed against distilled water and 12–14 kDa dialysis tubing at 45±5°C for five days with two water changes per day. The solution was then stored at −80°C overnight before being lyophilized for 5 days and stored at room temperature.
2.2. Cell Culture
OD21 cells were cultured in DMEM containing 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin [24]. Cells were maintained in a humidified, 37°C, 5% CO2 incubator, and the media changed every two days with two cells passages per week.
2.3. Hydrogel preparation and polymerization
GelMA macromer at a concentration of 10% (w/v) was dissolved in DPBS with either LAP (Tokyo Chemical Industry, L0290) photoinitiator at 0.05, 0.067, 0.1% (w/v), or 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959, Tokyo Chemical Industries) photoinitiator at 0.1% (w/v). GelMA hydrogels were fabricated by dispensing the hydrogel precursors into molds and exposing the samples to either a visible light dental curing device (DL) or UV light at different photo-curing times, as described below.
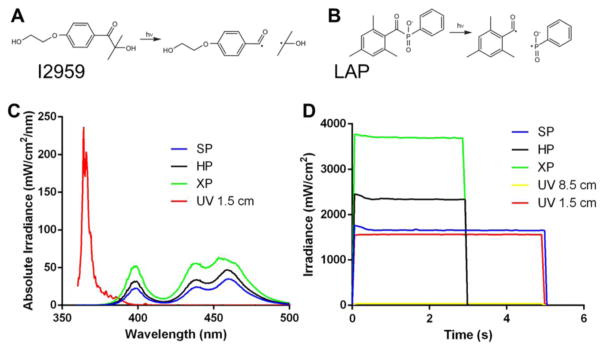
GelMA hydrogels prepared with LAP photoinitiator were photopolymerized using a VALO cordless dental curing light unit (Ultradent Products, 395–480 nm, 10.5 mm curing tip), which has different polymerization presets: standard power (SP), which can be used for 5, 10, 15 or 20 s; high power (HP), which can be used for 1, 2, 3 or 4 s; and Xtra power (XP) which is preset for 3 s of polymerization only. GelMA hydrogels prepared with Irgacure, on the other hand, were polymerized using UV light (EXFO Acticure 4000, 365 nm, 5 mm light guide diameter) from either 5, 10, 15 or 20 s. The spectrum of the DL and UV light (Figure 2), and their respective intensities were measured using a Marc Resin Calibrator (Bluelight, 4 mm sensor diameter). SP, HP and XP were measured holding the Valo DL unit at 0.5 cm away from the sensor, while the UV light intensity was measured at either 8.5 or 1.5 cm of distance between the UV light guide and the sensor. The DL unit emits light at three different peak wavelengths over a spectrum of 395 to 480 nm. The DL irradiance for each mode at 0.5 cm distance were: SP 1650 mW/cm2, HP 2300 mW/cm2, and XP 3700 mW/cm2. The UV light unit generates one peak wavelength at 365 nm, and the light intensities at 8.5 and 1.5 cm distance were 45 and 1560 mW/cm2, respectively (Figure 2).
Figure 2.
Chemical structures and cleavage of the photoinitiator 1-[4-(2-hydroxyethoxy)-phenyl]-2hydroxy-2-methyl-1-propanone (I2959) (A) and lithium phenyl-2,4,6-trimethyl- benzoylphosphinate (LAP) (B). Spectrum of UV and dental curing lights (C), and measurement of light intensities (D) used in this study. Note that light irradiance is sustained and stable over the time. SP - standard power, HP–high power, XP–extra power, and UV at 1.5 and 8.5 cm distance.
2.4. Cell encapsulation and viability
OD21 cells were trypsinized and counted using Countess™ II FL automated cell counter (Life Technologies), and resuspended in GelMA, containing either LAP or Irgacure, at 5 × 106 cells/ml. Cell-laden hydrogel constructs were fabricated by dispensing 5 μl of a cell-laden GelMA hydrogel precursor on plastic Petri dish, and compressed with TMSPMA ([3-(Methacryloyloxy)propyl]trimethoxysilane, Sigma) coated glass slides supported by two parallel cover slips to form 100 μm thick GelMA constructs, as described previously [10]. We first sought to optimize the DL polymerization conditions that resulted in enhanced cell viability. To that end, we evaluated the effect of the DL polymerization presets (SP for 5 and 20 s, HP for 3 s or XP for 3 s), on the viability OD21s embedded in GelMA hydrogels (10% GelMA, 0.067% LAP photoinitiator). The reason we selected 10% GelMA in this study is because we have demonstrated that OD21 cells showed higher survival rates in stiffer gels polymerized with UV light even at early time points [10]. The 0.067% LAP was initially selected based on Fairbanks et al., where they showed that this LAP concentration maintained high viability during direct encapsulation of cells [14]. The rationale for the use of these four-different light curing conditions was to test different radiant exposure, which is the radiant energy received by a surface per unit area (J/cm2). Therefore, the radiant exposure for each condition was: 8.25 J/cm2 (SP 5s), 16.5 J/cm2 (SP 10s), 24.75 J/cm2 (SP 15s), 33 J/cm2 (SP 20s), 6.9 J/cm2 (HP 3s), and 11.1 J/cm2 (XP 3s). Next, we tested the effect of LAP photoinitiator concentration on OD21 cell viability in GelMA hydrogels. For that, cell-laden hydrogel constructs were polymerized using the SP 5s mode with LAP photoinitiator concentrations of either 0.05, 0.067, or 0.1%. After determining the best combination of photopolymerization mode, exposure time, and LAP photoinitiator concentration, the following experiments compared GelMA hydrogels polymerized using the best combination of parameters for DL polymerization versus the common settings used for UV polymerization. UV polymerization used 45 mW/cm2 of light irradiance at 8.5 cm of distance. Samples were polymerized for 5 s, 10 s, 15 s, and 20 s. Finally, we compared the effect of DL and UV light at similar irradiance (DL at 1650 mW/cm2 and UV 1560 mW/cm2) for 5 s.
The viability of OD21 cells in GelMA hydrogels was determined using a membrane permeability based live/dead assay kit (Molecular Probes) under a uorescence microscope (EVOS FL Auto, Life Technologies). The live and dead cells were counted using ImageJ software based on at least 3 locations of triplicate samples after 1, 3, and 5 days. The percentage of viable cells was then calculated based on the number of live relative to the total (live and dead) cells in the construct.
2.5. Physical and mechanical properties
Next, we determined the physical and mechanical properties of the GelMA hydrogels photopolymerized using either a DL or UV light, samples were prepared using 10% GelMA with either LAP at 0.05% (w/v) or with Irgacure 2959 at 0.1% (w/v). GelMA hydrogel discs measuring 5 mm in diameter and 2.5 mm in height were fabricated by dispensing the hydrogel precursors in Poly(dimethylsiloxane) (PDMS, Sigma) molds and exposing samples to DL at SP or UV light for 5, 10, 15 and 20 s (exposed to the air).
The polymerization time of the GelMA prepolymer was investigated using a vial tilting method, as described previously [13]. 0.2 mL of GelMA prepolymer was dispensed into a 1 mL clear glass vial and irradiated using either a DL or UV light at the determined exposure times (5, 10, 15 or 20 s). Polymerization was determined to be satisfactory when flow was not observed during inversion of the vial.
Hydrogel pore structure and morphology was analyzed via scanning electron microscopy (SEM). GelMA hydrogels (n=3) were cross-sectioned, flash frozen in liquid nitrogen and lyophilized overnight (Labconco, Freezone 4.5). Samples were then coated with gold/palladium and imaged using a FEI Quanta 200 SEM at 20.0 kV. Hydrogel degradation was determined by incubating GelMA hydrogel disks (n=4) for 2, 5, and 24 hours at 37 C in a 1 U/ml collagenase solution (MP Biomedical). After incubation, the non-degraded hydrogel fragments were retrieved and lyophilized overnight. Degradation percentage was determined by calculating the weight ratio of degraded versus intact hydrogel samples at each time point.
The mechanical properties of the GelMA hydrogels were characterized using a DHR-1 rheometer (TA Instruments) with 8 mm parallel-plate geometry. The measurement gap between the plates was set at 2.5 mm and allowed to relax until the normal force was zero. In order to determine the storage (G′) modulus of GelMA hydrogel groups, oscillatory frequency sweep tests were performed at a constant oscillatory strain (0.5%) by logarithmically varying the angular frequency between 0.1 to 100 rad/s. The temperature was maintained at 25°C throughout the measurement period. The elastic modulus was calculated from the storage modulus using the equation K = 2G(1+ν)/3(1–2ν) where, K, G and ν are Elastic modulus, storage modulus and Poisson’s ratio (assumed to be 0.44), respectively.
2.6. Data Analysis
Statistical analysis was performed using GraphPad Prism 6. The values represent averages ± standard deviations. One-way/two-way ANOVA followed by Tukey post-hoc test (α = 0.05) was used to analyze the differences between different light irradiances, LAP concentrations, and culture time.
3. Results
3.1. Effect of dental light irradiance and exposure time
The effect of dental light irradiance and exposure time on OD21 cells encapsulated in GelMA was assessed using a live/dead assay kit (Figure 3).
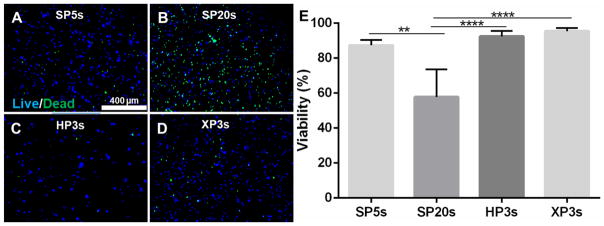
Figure 3.
Effect of dental light intensities and exposure time (LAP at 0.067%,). Cell viability of OD21 encapsulated GelMA after 24 hours. The viability assay showed that cells were viable (>80%) when photopolymerized with SP5s (A), HP3s (C) and XP3s (D). The cell viability decreased when photo-curing time was increased to SP20s. Scale bar 400 μm.
By keeping the photoinitiator LAP at 0.067%, the following DL irradiances and exposure times were compared: SP-5s, SP-20s, HP-3s and XP-3s. The cells remained viable (>85%) when exposed to SP-5s, HP-3s and XP-3s, with no significant differences between them. However, the cell viability decreased significantly when the polymerization time for SP was increased to 20 seconds.
3.2. Effect of photo-initiator concentration
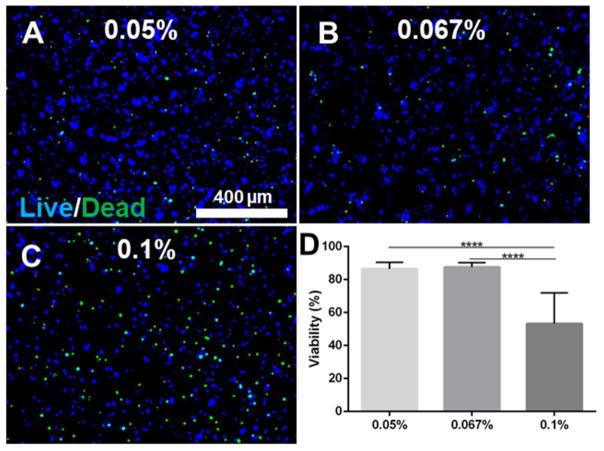
SP5s and XP3s were used to determine the effect of LAP concentration (0.05, 0.067, and 0.1% w/v) on the viability of encapsulated OD21 cells. The cell viability assay showed that cells were more than 80% viable when photopolymerized using 0,05% and 0,067% (w/v) LAP concentration after 24 hours (Figure 4). However, the cell viability decreased to approximately 50% when LAP concentration was increased to 0.1%. Significant differences were observed between 0.05% vs 0.1% and 0.067 vs 0.1% LAP concentration (p<0.0001). A similar trend was observed when OD21 cell-laden GelMA was photopolymerized with XP3s (supplementary Figure 1).
Figure 4.
Effect of photo initiator concentration. Cell viability of OD21 encapsulated GelMA after 24 hours. The viability assay showed that cells were viable (>80%) when photo-cured with SP5s and 0,05 (A) and 0,067% (B) (w/v) LAP. The cell viability decreased when LAP concentration was increased to 0.1% (C, D). Scale bar 400 μm. p<0.0001 (****).
3.3. GelMA polymerization and photolithography using DL and UV light
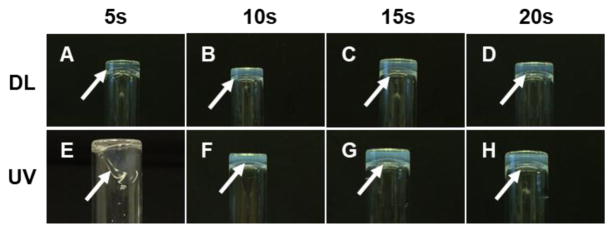
After determining the effect of DL intensities and LAP photoinitiator concentration on OD21 cell-laden GelMA, we evaluated the gelation time, the ability to create micropatterns, gel morphology, degradation, and mechanical properties of GelMA precursors exposed to either DL or UV light both for 5, 10, 15 and 20 s. GelMA hydrogels containing LAP photoinitiator set after 5 seconds of DL exposure (Figure 5); whereas, UV treated GelMA hydrogels photopolymerize after at least 10s of UV light exposure. We then created 120 μm GelMA micropatterns using a photolithography technique, using either a DL or UV light (supplementary Figure 2). The images suggest that a defined pattern was obtained for DL polymerized GelMA after 20s, whereas, UV light requires at least 50s of light exposure to obtain comparable patterns.
Figure 5.
Comparing DL and UV photopolymerization methods. DL (A, B, C, D) photopolymerized GelMA hydrogels at close distance faster than UV at 8.5 cm (E, F, G, H). GelMA was not photopolymerized after UV exposure to 5s.
3.4. Physical and mechanical properties of GelMA hydrogels
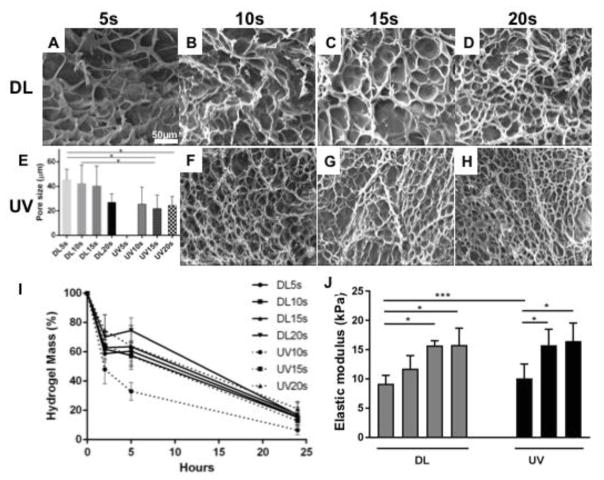
Figure 6 shows SEM images of cross-sectioned GelMA hydrogels after exposure to DL and UV from 5 to 20 s. SEM images show microporous structures formed within the hydrogel during polymerization. The pore size decreased as the polymerization time increased for GelMA hydrogels photopolymerized with DL, but no significant differences were observed between the exposure times. Comparing with the pore size of the UV photopolymerized hydrogels (25 ± 10 μm), GelMA hydrogels photopolymerized with DL showed larger pore size (40 ± 13 μm). Significant differences (p<0.05) were observed between low DL exposure times (5s and 10s) and high UV light exposure times (15 and 20s). No differences were observed between the hydrogels polymerized with UV light. In terms of area of porosity, significant differences were observed between different DL exposure times (Supplementary Figure 3).
Figure 6.
Morphology (DL images A to D, UV images F to H) and pore size (E), degradation (I) and elastic modulus (J) of GelMA hydrogels. SEM images showed microporous structure induced during polymerization of GelMA hydrogels. The porous structure of DL photopolymerized hydrogels at different time points are larger than UV photopolymerized hydrogels. UV photopolymerized GelMA hydrogels degraded faster than DL photopolymerized GelMA hydrogels after 10s exposure. The elastic modulus increased with the exposure time for both DL and UV photopolymerized GelMA hydrogels. p<0.05 (*).
In terms of degradation, DL photopolymerized GelMA hydrogels underwent approximately 15% degradation within 2 hours (Figure 6I). No significant differences were observed between the different exposure times. Comparing with UV photopolymerized GelMA hydrogels, UV10s underwent 52% degradation within 2 hours. After 5 hours, DL photopolymerized GelMA hydrogels underwent approximately 40% degradation, and significant differences were observed between low and high DL exposure times. Comparing with UV photopolymerized GelMA hydrogels, UV10s underwent 67% degradation. After 24 hours, GelMA hydrogels underwent more than 80% degradation and no significant differences were observed between the DL and UV photopolymerized GelMA hydrogels. The elastic modulus (elasticity) increased with exposure time for both DL and UV photopolymerized GelMA hydrogels (Figure 6J). Comparing DL vs UV photopolymerized GelMA hydrogels, no significant differences were observed between DL and UV light at high exposure times. UV 5s did not polymerize the gels.
3.5. Cell viability of DL and UV photopolymerized hydrogels
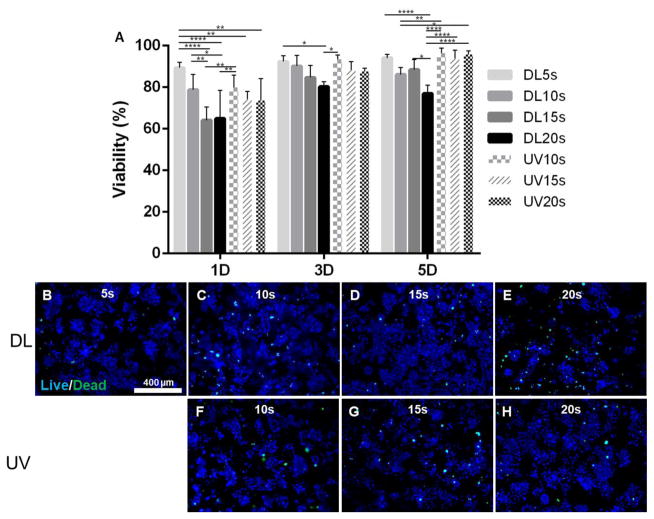
Figure 7 shows the cell viability of OD21 cells encapsulated in GelMA hydrogels photopolymerized with DL and UV light for 5 to 20 seconds, and cultured in vitro for 1, 3 and 5 days. Results showed that GelMA hydrogels encapsulating OD21 cells photopolymerized with DL5s displayed significantly higher cell viability than DL15s and DL20s at day 1. Representative fluorescent images of OD21 cells cultured in vitro on days 1 and 3 are shown in Supplementary Figure 4. Cell viability decreased when the exposure time increased for the DL. At day 3, the cell viability increased for all conditions. However, significant differences were observed between and DL5s and DL20s. At day 5 significant differences were observed between DL5s and DL20s. This result shows that DL20s had a negative effect on cells. Comparing with the cell viability of UV photopolymerized GelMA hydrogels, significant differences were observed between high DL exposure times (15s and 20s) and UV light exposure times (p<0.0001).
Figure 7.
Cell viability of 3D GelMA hydrogels encapsulating OD21 cells after exposure to DL and UV and cultured in vitro for 1, 3 and 5 days. Cell viability increased after 5 days in vitro culture (A). Representative uorescent images of OD21 cells stained for live (blue) and dead (green) cells on day 5 (DL images B to E, UV images F to H). UV 5s did not polymerize. p<0.05 (*), p<0.01(**), p<0.001(***), p<0.0001(****).
3.6. Effect of UV light irradiance
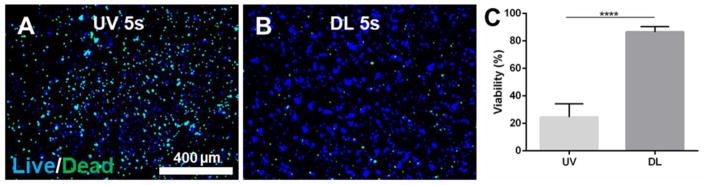
When we set the irradiance of both DL and UV light to a comparable value (DL 1650 mW/cm2 and UV 1560 mW/cm2) by adjusting the distance of the light guide to the sample, results showed that OD21 cells encapsulated in GelMA were viable when exposed to DL (Figure 8), but drastically decreased (25%) when exposed to UV (p<0.0001).
Figure 8.
Effect of UV light intensity. Cell viability of OD21 encapsulated GelMA exposed to UV light (A) at 1560 mW/cm2, and DL (B) at 1560 mW/cm2 for 5 and 10 seconds after 24 hours. The viability assay (C) showed that cells were viable when photopolymerized with DL for 5 s, and decreased when exposed to UV for 5 s. p<0.0001(****). Scale bar 400 μm.
4. Discussion
In this study, we optimize a method to photopolymerize cell-laden GelMA hydrogels using visible DL, as compared to the standard UV photopolymerization. Photopolymerization is the process used for the solidification of the hydrogel from pre-gel precursors, which allows for encapsulation of cells at near physiological conditions, and various light sources are used accordingly for different clinical applications [16]. The photoinitiator is the light reactive ingredient that allows for the formation of photopolymerized hydrogels. When exposed to specific wavelengths of light, the photoinitiator absorbs the energy and forms radicals that can subsequently convert liquid macromer solutions to a polymerized network. Different factors in the polymerization process can affect cell viability, including the macromer concentration, exposure to different wavelengths, heat production, photoinitiator type, free radical concentration and cell type [13, 16, 25–27]. Different cell types have distinct responses to the same concentrations of the same photoinitiator, and it has been shown that cell lines that divided more quickly were more sensitive to photoinitiator Irgacure 2959 [16]. The visible light photoinitiator LAP (405 nm) is a possible alternative for UV photoinitiator Irgacure 2959 (365 nm) [14, 25]. It was demonstrated that exposure to inactivated LAP (photoinitiator without any monomer to react) did not affect cell viability, while exposure to inactivated Irgacure 2959 affected cell viability [25]. In the same study, it was demonstrated that light exposure alone (365 nm and 405 nm at 10 mW/cm2 irradiance) after 30 and 120 seconds, 0.3 J/cm2 and 1.2 J/cm2 respectively, did not affect cell viability. However, when exposed to an activated photoinitiator, cell viability decreased for both Irgacure 2959 and LAP [25]. This result is explained by the fact that without a macromer to react with, activation of photoinitiators releases free radicals that interact with cells (i.e. cell membranes, proteins and nucleic acids), thus, affecting their viability [13]. However, when combined with GelMA precursors, the viability of the cells seeded on GelMA was not affected [25]. In another study, Wilkens et al. reported the effect of Irgacure 2959 at different concentrations (0.2%, 0.5% and 1% w/v), and Irgacure 2959 alone did not affect significantly the viability or proliferation of HUVEC cultured in 2D [26]. However, combined exposure of Irgacure 2959 and UV significantly decreased the cell viability, confirming that free radicals generated by the photoinitiator after UV irradiation is the main cause of cell toxicity [26].
In our study, we first investigated the effect of DL irradiances (SP 1650, HP 2300 and XP 3700 mW/cm2) and exposure times (3, 5 and 20 s) on OD21 cells encapsulated in GelMA using visible LAP photoinitiator (Figure 3). Although we used high light intensity, the cell viability was not significantly affected when exposed to dental light SP5s, HP3s or XP3s (8.25, 6.9 and 11.1 J/cm2, respectively). However, when the exposure time was increased the free radical formation and potentially heat production also increased, affecting the cell viability, which explains the low cell viability of SP20s (33 J/cm2). In another study, prolonged visible light irradiation (500 mW/cm2) from 40s to 600s (20 to 300 J/cm2) improved the mechanical properties of methacrylated hydrogels, but drastically reduced cell viability [13]. Here, we also show that the concentration of photoinitiator LAP (0.05% to 0.1% w/v) affects the cell viability (Figure 4). High cell viability was observed for low LAP concentration, which is in accordance with previous work by Fairbanks et al [14]. Moreover, our results showed that the exposure time has more negative effect on cell viability than the dental light intensity at high concentration of LAP photoinitiator.
For further analysis, we compared the physical and mechanical properties of GelMA hydrogel polymerized with DL versus UV light. UV treated GelMA hydrogels did not photopolymerize after 5 seconds and could not be tested (Figure 5). Importantly, we have demonstrated that it is possible to create micropatterns using the DL photopolymerization method similar to the UV photopolymerization method. Photolithography techniques have been developed to photo-encapsulate cells and they have been useful tissue engineering [28, 29].
Photopolymerization of hydrogels under certain reaction conditions creates porous network structures about the size of mammalian cells [30]. We found that GelMA hydrogels photopolymerized with low DL exposure times showed larger pore size than GelMA hydrogels photopolymerized with high UV light exposure time. It is known that in the presence of an excess of water, GelMA undergoes photopolymerization induced phase separation via syneresis, where the excess of water is squeezed out of the formed polymer to reach its equilibrium swelling concentration [30]. Network porosity, pore size, wall thickness, degradation and mechanical properties are directly related to the rate of polymerization, which depends on GelMA/photoinitiator concentration, degree of methacrylation, light intensity and exposure time [11, 31–33]. Therefore, fabrication conditions can be adjusted in order to improve the properties of GelMA hydrogels. If the photoinitiator concentration is too low, photocrosslinking reactivity of the polymer solution is low as well, creating large porous structures. Our results show that the pore size decreased as the polymerization time increased for GelMA hydrogels photopolymerized with DL; whereas, no significant differences were observed for GelMA hydrogels photopolymerized with UV (Figure 6A).
Due to the biodegradable properties of gelatin, cells degrade the porous structure and migrate through the network. Our results confirm the susceptibility of GelMA hydrogels to be degraded by collagenase (Figure 6B). GelMA hydrogels photopolymerized with DL showed a slower degradation profile than GelMA hydrogels photopolymerized with UV exposure, indicating a higher crosslinking density and reduced penetration and accessibility of the collagenase into hydrogels photopolymerized with DL. This result is explained by the fact that GelMA hydrogels photopolymerized with DL have more porous structure and the same modulus as the UV cured, suggesting that cross-link density may be higher for the DL which is corroborated by the higher degradation resistance [13].
It is well known that mechanical properties of the 3D microenvironments influence cell function and differentiation [11, 31–33]. Our results show that the elastic modulus (elasticity) increased with exposure time for both DL and UV photopolymerized GelMA hydrogels (Figure 6C). Although, 5s UV exposure did not fully form hydrogels, similar elastic modulus was observed between DL5s and UV10s, and subsequent polymerization times.
An experiment was performed to test cell viability after encapsulation for at least 5 days (Figure 7). Our results showed that the cell viability was not affected when cells were exposed to dental light for 5 seconds. At day 5, except for DL20s, the cell viability increased for all the conditions, demonstrating that short time exposure to dental light or UV did not cause significant long-term damage to the cells. This result is also in accordance with previous reports [10, 13, 14, 25, 27]. Finally, we compared the effect of UV light and DL at similar light irradiances (Figure 8), and the cell viability drastically decreased when OD21 cells were exposed to UV light at 1560 mW/cm2 for 5 seconds, but cells were viable when polymerized with DL 1650 mW/cm2 for same period of time. This result suggests that UV polymerization has a more negative effect on cells than DL polymerization method at similar light energy dose. Therefore, the UV wavelength has a more detrimental effect on cells than visible wavelengths. Wilkens et al reported similar results on the effect of UV light on HUVECs encapsulated in GelMA [26]. The dental light method using LAP as photoinitiator has advantages of being convenient for dental tissue regeneration. It can be used at close distance to focus the light at the injured site where the encapsulated cells would be delivered. Therefore, one can specifically direct the energy only to the desired area, avoiding light exposure to the surrounding tissues and decreasing heat generation.
Dental tissue engineering efforts are focused on bioengineering dental supporting tissues using stem cell populations [1, 10, 34–37]. To the best of our knowledge, GelMA hydrogels remain poorly explored regarding their application in regenerative dentistry despite their excellent potential as regenerative dental materials. The combination of dental cells with hydrogels photopolymerized with visible light is a promising strategy for regenerative endodontics, for instance, where the long-term goal is to create pulp-like cell-laden tissue constructs in full-length root canals in vivo. We believe that this study represents an important step towards achieving this goal, and the establishment of chair-side regenerative procedures in dental care.
5. Conclusions
The focus of this study was to optimize a new method to photopolymerize GelMA using a visible light dental curing unit with parameters similar to those used in the dental office, without affecting the desirable biological and physical properties of cell-laden GelMA hydrogels. We evaluated the effect of irradiance and photoinitiator concentration on GelMA encapsulating OD21 cells. Our results show that short-time exposure to DL did not significantly affect cell survival. We also compare this new method with the well-established UV light polymerization method. Both DL and UV produced hydrogels that were able to support viable cell populations and create micropatterns using a photolithography technique. In conclusion, DL hydrogel polymerization method enables cells to be readily encapsulated and polymerized within the hydrogel and can be directly established in regenerative procedures in dental care.
Supplementary Material
HIGHLIGHTS.
The chemistry of gelatin methacryloyl (GelMA) was adjusted to enable photopolimerization of cell-laden hydrogels using a dental curing light.
The light parameters used preserved the viability of odontoblast-like cells
This approach will be useful for chair-side procedures in regenerative dentistry
Acknowledgments
This work was supported by funding from the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institutes of Health (NIH) (R01DE026170 to LEB), the Medical Research Foundation of Oregon (MRF to LEB), the OHSU Fellowship for Diversity and Inclusion in Research (OHSU-OFDIR to CMF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monteiro N, Yelick P. Advances and Perspectives in Tooth Tissue Engineering. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2134. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrie Aronin CE, Kuhn NZ, Tuan RS. Tissue Engineering and Selection of Cells. In: Paul D Editor-in-Chief, editor. Comprehensive Biomaterials. Oxford: Elsevier; 2011. pp. 81–93. [Google Scholar]
- 3.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nature Nanotechnology. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10:1646–62. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85–123. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–71. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin R-Z, Chen Y-C, Moreno-Luna R, Khademhosseini A, Melero-Martin JM. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:6785–96. doi: 10.1016/j.biomaterials.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6:024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–11. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgeley C, et al. A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like. Sci Rep. 2017 doi: 10.1038/s41598-017-02532-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichol JW, Koshy S, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, et al. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng Part A. 2012;18:2453–65. doi: 10.1089/ten.tea.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Hou Y, Park H, Choi B, Hou S, Chung A, et al. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012;8:1730–8. doi: 10.1016/j.actbio.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–7. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–57. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 16.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–8. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Fedorovich NE, Oudshoorn MH, van Geemen D, Hennink WE, Alblas J, Dhert WJ. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–53. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Kappes UP, Luo D, Potter M, Schulmeister K, Runger TM. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol. 2006;126:667–75. doi: 10.1038/sj.jid.5700093. [DOI] [PubMed] [Google Scholar]
- 19.Jones CA, Huberman E, Cunningham ML, Peak MJ. Mutagenesis and cytotoxicity in human epithelial cells by far- and near-ultraviolet radiations: action spectra. Radiat Res. 1987;110:244–54. [PubMed] [Google Scholar]
- 20.Kielbassa C, Roza L, Epe B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18:811–6. doi: 10.1093/carcin/18.4.811. [DOI] [PubMed] [Google Scholar]
- 21.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96:3104–7. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campregher UB, Samuel SM, Fortes CB, Medina AD, Collares FM, Ogliari FA. Effectiveness of second-generation light-emitting diode (LED) light curing units. J Contemp Dent Pract. 2007;8:35–42. [PubMed] [Google Scholar]
- 23.Stahl F, Ashworth SH, Jandt KD, Mills RW. Light-emitting diode (LED) polymerisation of dental composites: flexural properties and polymerisation potential. Biomaterials. 2000;21:1379–85. doi: 10.1016/s0142-9612(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 24.Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, et al. Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res. 1998;37:233–49. doi: 10.3109/03008209809002442. [DOI] [PubMed] [Google Scholar]
- 25.BioBots. LAP and Irgacure Viability with GelMA. 2016. [Google Scholar]
- 26.Wilkens CA, Rivet CJ, Akentjew TL, Alverio J, Khoury M, Acevedo JP. Layer-by-layer approach for a uniformed fabrication of a cell patterned vessel-like construct. Biofabrication. 2016;9:015001. doi: 10.1088/1758-5090/9/1/015001. [DOI] [PubMed] [Google Scholar]
- 27.Sabnis A, Rahimi M, Chapman C, Nguyen KT. Cytocompatibility studies of an in situ photopolymerized thermoresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. J Biomed Mater Res A. 2009;91:52–9. doi: 10.1002/jbm.a.32194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selimović Š, Oh J, Bae H, Dokmeci M, Khademhosseini A. Microscale Strategies for Generating Cell-Encapsulating Hydrogels. Polymers. 2012;4:1554. doi: 10.3390/polym4031554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu VA, Bhatia SN. Three-Dimensional Photopatterning of Hydrogels Containing Living Cells. Biomedical Microdevices. 2002;4:257–66. [Google Scholar]
- 30.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–30. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 33.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khayat A, Monteiro N, Smith E, Angstadt S, Yelick PC. GelMA Encapsulated HDPSCs and HUVECs for Dental Pulp Regeneration. J Dent Res. 2016 doi: 10.1177/0022034516682005. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottino MC, Yassen GH, Platt JA, Labban N, Windsor LJ, Spolnik KJ, et al. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med. 2015;9:E116–23. doi: 10.1002/term.1712. [DOI] [PubMed] [Google Scholar]
- 36.Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29:97–102. doi: 10.1016/j.dental.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilipchuk SP, Plonka AB, Monje A, Taut AD, Lanis A, Kang B, et al. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater. 2015;31:317–38. doi: 10.1016/j.dental.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.