Abstract
Background
Initiation of cigarette smoking during adolescence coincides with structural and cognitive neuromaturation. Thus, early onset smokers (EOS; initiated <16 years old) may be at unique risk of altered development of executive function relative to late onset smokers (LOS; initiated >16 years old). This study quantified the effects of age of smoking onset on response impulsivity and inhibitory control using a novel smoking Go/NoGo task (Luijten et al., 2011).
Methods
Nicotine deprived adult EOS (n=10) and LOS (n=10) and adult healthy non-smokers (HNS; n=10) were shown smoking-related and neutral images with either a blue (Go) or yellow (NoGo) frame. Participants were instructed to respond to blue-framed Go trials quickly and accurately, and withhold responding for yellow-framed NoGo trials.
Results
EOS made more Go response accuracy errors (p≤0.02) and failed more frequently to inhibit responses to NoGo trials (p<0.02) than LOS and HNS. EOS also made more errors in inhibiting responses to smoking-related (p≤0.02) and neutral (p≤0.02) NoGo trials. EOS reported greater baseline craving for cigarette smoking than LOS (p<0.04), and craving was significantly associated with greater omission errors (p≤0.04).
Conclusions
EOS exhibited greater difficulty than LOS in responding accurately to Go stimuli and withholding responses to both smoking and neutral NoGo stimuli, indicating greater response impulsivity, poor attention, and deficits in response inhibition. These findings suggest that EO smoking, in particular, contributes to diminished task-related attention and inhibitory control behaviors in adulthood and provide support for the tobacco-induced neurotoxicity of adolescent cognitive development (TINACD) theory (DeBry and Tiffany, 2008).
Keywords: early onset, smoking, nicotine, response inhibition, impulsivity, attention
1. Introduction
Initiation and persistent cigarette smoking during adolescence remains a threat to public health given that approximately 80% of adult chronic smokers report commencing their smoking behaviors prior to age 18 (Ng et al., 2014; CDC, 2014; 2015). Recent nationwide survey data indicates that approximately 850,000 adolescents, aged 12–17, reported initiating cigarette smoking while another 1.2 million adolescents reported ongoing cigarette smoking behaviors (SAMHSA, 2015). Importantly, initiation of cigarette smoking behaviors during earlier stages of adolescence, relative to later stages, has been associated with longer duration of use and increased difficulty quitting smoking during adulthood (Breslau and Peterson, 1996; Sussman, 2002).
Persistent nicotine exposure during adolescence, corresponding with critical maturational changes in brain development, disrupts sensitive neurobiological processes and elevates risks for developing nicotine dependence throughout adulthood (Dwyer et al., 2009; Singh et al., 2016). More specifically, early-onset smoking, which is defined as tobacco cigarette smoking initiated prior to age 16, may be particularly damaging given that active cortical synaptic pruning and rearrangement (Huttenlocher 1979, 1990) and white matter myelination (Paus et al., 1999) processes are transpiring in early adolescence as maturational changes in neurodevelopment begin to accelerate. Age 16 also has been used consistently in substance abuse research as a dividing mark for early- vs. late-onset drug use (e.g., Ehrenreich et al., 1999; Slade et al., 2008; Norberg et al., 2009; Becker et al., 2010; Gruber et al., 2011, Gruber et al., 2012a, 2012b; Sagar et al., 2015; Dahlgren et al., 2016).
Earlier smoking onset and persistent nicotine exposure also may interfere with normative cognitive developmental processes, resulting in suboptimal cortical efficiency that could linger in adulthood (e.g., Goriounova and Mansvelder, 2012). Though the effects of age of smoking onset on cognitive task performance have not been prospectively investigated, there is modest evidence from a retrospective analysis that suggests early onset cigarette smoking functionally alters cognitive processing. In this analysis, age of smoking onset was shown to relate to reduced working memory performance accuracy, indicating that adults who initiated smoking at a younger age, approximately 13 years old, performed poorly relative to adults who began smoking at a later age (Jacobsen et al., 2005). It is unclear, however, if early onset smoking exposure coinciding with the neural maturation of executive cognitive processing, occurring throughout adolescence and early adulthood (Gogtay et al., 2004), is related to measurable differences in other frontal cognitive domains in adult smokers.
Important executive cognitive functions such as sustained attention, decision-making and inhibitory control follow dynamic maturational trajectories during adolescence (Anderson et al., 2001; Conklin et al., 2007) and are known to be disrupted in adult smokers, particularly during nicotine deprivation (Spinella, 2002; Mitchell, 2004; Harrison et al., 2009; Billieux et al., 2010; Luijten et al., 2011; Xin et al., 2015). Sustained attention is the ability to maintain alertness and focus during extended monotonous tasks (Robinson et al., 2013) and inhibitory control reflects the behavioral and cognitive ability to regulate and suppress automatic or prepotent behaviors and responses (Roberts et al., 2011). Inhibitory control is a core executive function, critical for ignoring distracting yet irrelevant and unnecessary information (Friedman and Miyake, 2004). Inhibitory control can be tested by measuring response inhibition, which is the ability to effectively adapt to environmental contingencies by controlling and suppressing impulsive prepotent response behaviors (Groman et al., 2009).
Response inhibition is commonly measured using a frontal lobe-mediated Go/NoGo task, a multi-dimensional cognitive assessment tool that also activates sustained attention processes and tests impulsive responding (Erickson et al., 2005; Dinur-Klein et al., 2014). The task measures deficits in attention and response inhibition, as well as elevated impulsivity, by testing how quickly and accurately participants respond to frequently occurring ‘Go’ stimuli and their ability to inhibit prepotent responses to infrequently occurring novel ‘NoGo’ stimuli. Difficulty with inhibition in the Go/NoGo task is reflected by increased errors of commission, also noted as a high false alarm rate, made when failing to accurately withhold impulsive responding during infrequent NoGo trials. Difficulty with maintaining attention is reflected by increased errors of omission made when failing to respond accurately during frequent Go trials. Previous studies have reported impaired response inhibition, reflected by less response accuracy and more errors, in adult smokers, relative to non-smoking controls (Spinella, 2002; Luijten et al., 2011; Dinur-Klein et al., 2014). Importantly, however, previous studies did not account for how age of smoking onset in adult smokers could have influenced Go/NoGo response inhibition, attention, and task accuracy.
Comparing early onset and late onset adult smokers on Go/NoGo task performance may reveal functional differences between these groups that affect impulsive decision-making, sustained attention, and inhibitory control and can potentially promote maintenance of nicotine dependence. It is hypothesized that initiation of cigarette smoking behavior during early adolescence, corresponding to important early neuromaturational trajectories, alters normal executive function development and consequently will have more lasting effects on cognitive processing relative to initiation of smoking during later stages of adolescence and corresponding neuromaturation. As such, it is predicted that early onset smokers will perform more poorly on task performance outcomes, such as making more errors of omission and commission and performing less accurately than late onset smokers and healthy non-smokers.
2. Material and Methods
2.1. Participants
Ten early onset cigarette smokers (EOS; 5 women), 10 late onset cigarette smokers (LOS; 5 women), and 10 healthy non-smokers (HNS; 5 women) were recruited from the Boston metropolitan area to participate in this experiment, which was conducted as part of a larger electroencephalography smoking cue reactivity study. Though the sample size is modest, the EOS and LOS groups were extremely well-characterized for nicotine dependence with extremely limited prior drug use history, assessed using a time line follow back procedure, and no history of psychiatric disorders. Volunteer recruitment resulted in over 500 thorough screenings of cigarette smokers, and those who qualified by meeting stringent inclusion/exclusion criteria (described below) were invited to participate. The factor that limited initial recruitment of smokers was that onset of smoking behavior had often overlapped with the onset of marijuana (MJ) use. Early onset MJ use (use initiated prior to age 16), relative to late onset use (use initiated at or after age 16), has been associated with poor cognitive executive function, particularly inhibitory processing, in adult MJ users (e.g., Gruber et al., 2011; Gruber et al., 2012a, 2012b). In order to account for the possibility that overlapping MJ and nicotine use would confound the hypothesized effects of smoking on response inhibition and attention, our criteria excluded smokers who had initiated any MJ use within five years of initiating cigarette smoking. Though this criterion is stringent and eliminated the majority of initial volunteers, it insured that any smokers who had used MJ began their exposure at least five years after smoking initiation. As such, their MJ use would be considered late-onset use, which has shown to be less detrimental to brain function (Gruber et al., 2011; Gruber et al., 2012a, 2012b).
As such, twenty-three smokers met criteria and, of those, twenty (10 EOS and 10 LOS) chose to participate in the study. All participants were between the ages of 22–40 years old, and smokers were asked to report their age of cigarette smoking onset, specifically defined as when they were routinely smoking cigarettes daily or near daily (approximately 5–7 days per week). To facilitate accuracy of recall, guided interview questions were utilized to determine age of onset (i.e., When did you first try a cigarette? Who were you with? When did you start smoking every day?) (Sagar et al., 2015). Smokers were then categorized as either early onset, if they reported daily or near daily smoking before age 16, or late onset if they reported daily or near daily smoking at or after age 16. All early and late onset adult smokers smoked between 10–20 cigarettes daily, met DSM-IV criteria for nicotine dependence and scored 5 or greater on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991), which is a standard measure for assessing physical dependence to nicotine. All HNS participants reported fewer than 5 lifetime uses of nicotine. Group demographics, including education, body mass index, socioeconomic status, age of smoking initiation, duration of smoking exposure, and frequency of past-month cigarette, alcohol, and marijuana use, are provided in Table 1.
Table 1.
Demographic and Substance Use Measures
| EOS (n=10) | LOS (n=10) | HNS (n=10) | p | |
|---|---|---|---|---|
| Age | 33.9 ± 3.3 | 31.2 ± 4.8 | 31.1 ± 3.9 | ns |
| % female | 50% | 50% | 50% | - |
| Education | 11.5 ± 4.2 | 16.2 ± 2.5 | 11.4 ± 8.0 | ns |
| FTND scores | 6.1 ± 1.0 | 5.4 ± 1.0 | - | ns |
| Age at 1st cigarette | 12.2 ± 1.6 | 15.8 ± 3.7 | - | 0.02 |
| Age began daily/near daily smoking | 13.2 ± 1.3 | 17.7 ± 1.2 | - | 0.000 |
| Daily cigarettes smoked | 18.5 ± 5.7 | 17.1 ± 5.5 | - | ns |
| Smoking exposure (years) | 20.8 ± 3.7 | 13.0 ± 4.6 | - | 0.000 |
| Expired CO levels at study visit | 8.7 ± 2.6 | 8.1 ± 3.3 | 2.3 ± 0.7 | 0.000 (EOS>HNS) (LOS>HNS) |
| Alcohol drinks/week | 0.8 ± 0.9 | 6.7 ± 5.7 | 1.7 ± 1.7 | 0.01 (LOS>EOS) (LOS>HNS) |
| MJ use/week | 0.3 ± 0.7 | - | - | - |
Clinical assessments of study participants were performed using the Structured Clinical Interview of DSM-IV Non-Patient Edition (SCID-1/NP) and all participants were free of current Axis I diagnoses, neurological illness, and severe medical problems. Further exclusion criteria included current or recent (past 5 years) substance dependence (other than nicotine), past diagnoses of bipolar disorder or schizophrenia, current or past psychoactive medication use, and prior episodes of loss of consciousness or head injury. One EOS and one LOS met criteria for past alcohol dependence (i.e., greater than, on average, 6 years ago).
In order to optimize conditions for measuring specific cognitive executive functions that are disrupted during nicotine deprivation (Evans et al., 2013), all smokers were instructed to abstain from smoking for approximately 12 hours prior to laboratory arrival (Cortese et al., 2015) and were asked to report the time of their last cigarette when they arrived for their study visit. All smokers reported that their last cigarette had been, on average, 11.5 hours ± 1.1 hours prior to their arrival. Compliance was assessed with carbon monoxide (CO) measurements (i.e., CO levels were < 12 ppm) in order to qualify for participation. All smokers were compliant with maintaining overnight abstinence. All procedures were approved by the McLean Hospital Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent and were compensated for their participation.
2.2. Procedures
2.2.1 Questionnaires
All eligible study participants completed a baseline clinical assessment battery of mood and craving measures, including the Profile of Mood State (POMS; McNair et al., 1981), Questionnaire on Smoking Urges (QSU-Brief; Tiffany and Drobes; 1991); Positive and Negative Affect Schedule (PANAS; Watson et al., 1988), State-Trait Anxiety Inventory (Spielberger et al., 1983); Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995); Hamilton Anxiety Scale (HAM-A; Hamilton, 1959); Beck Depression Inventory (BDI-II; Beck et al., 1996); the Wisconsin Smoking Withdrawal Scale (Welsch et al., 1999) and the FTND prior to the smoking Go/NoGo task. Following smoking Go/NoGo task performance, all EOS and LOS participants completed the WSWS and the QSU to assess end-of-day (EOD) withdrawal and craving.
2.2.2. Smoking Go/NoGo task paradigm
All study participants completed a smoking-related Go/NoGo task (Luijten et al., 2011), a behavioral paradigm that measures inhibitory control in a computerized test. In the Go/NoGo task, participants were asked to respond quickly and accurately to frequently occurring ‘Go’ stimuli and to inhibit responding to infrequently occurring ‘NoGo’ stimuli. The task paradigm was developed by Luijten and colleagues and has previously been described in full (Luijten et al., 2011). Briefly, participants were shown a series of pictures with either smoking-related or non-smoking-related stimuli content. Each image was displayed for 200 ms and was outlined with either a blue or yellow frame.
The frame color, not the type of image stimulus, determined whether the picture was a Go or NoGo trial. Images framed in blue, regardless of smoking- or non-smoking-related stimuli content, were Go trials, and participants were instructed to press a specific button as quickly as possible. Images framed in yellow were NoGo trials, and participants were instructed to withhold any response. The Go/NoGo paradigm contained 112 different smoking-related images displaying smoking stimuli (e.g., a lighter, an ashtray, cigarettes) as well as scenes of individuals engaging in smoking behavior. There were also 112 non-smoking-related pictures displaying neutral stimuli (e.g., a pen, a book, a feather) and scenes of individuals engaging in non-smoking behavior, such as typing or socializing. Each image was shown a total of four times during the task, once as a yellow-framed NoGo stimulus and three times as a blue-framed Go stimulus, for a total of 896 trials. The proportion of smoking- and non-smoking-related (‘neutral’) images shown in the task was equal; there were 112 NoGo trials and 336 Go trials for smoking-related images, and 112 NoGo trials and 336 Go trials for neutral images. Collectively, 25% of all trials (i.e., 224 trials) were NoGo trials and 75% (i.e., 672 trials) were Go trials. The order of stimuli content (smoking-related versus neutral) was completely randomized and the order of trial type (Go versus NoGo) was quasi randomized. Prior to starting the task, participants completed a set of 23 practice trials with an independent set of neutral pictures to ensure they understood the contingency to respond to blue-framed images and withhold responding to yellow framed images (Luijten et al., 2011).
2.3. Statistical analyses
Demographic and baseline clinical assessment measures were analyzed using analyses of variance (ANOVAs) to compare groups. Primary outcomes for the smoking Go/NoGo task were omission errors (failing to respond accurately to Go trials), commission errors (failing to inhibit responding to NoGo trials), overall task accuracy, Go trial accuracy (hit rate), NoGo trial accuracy, and average task reaction time (RT).
Secondary outcomes included: smoking image Go and NoGo accuracy; neutral image Go and NoGo accuracy; smoking image average overall RT (reflects correct Go RT and incorrect NoGo RT), Go RT, and NoGo RT; and neutral image average overall RT (reflects correct Go RT and incorrect NoGo RT), Go RT, and NoGo RT.
To compare between groups for each primary and secondary Go/NoGo outcome measure, separate one-way a priori analyses of covariance (ANCOVAs), with duration of smoking exposure and sex as covariates, were used. Post hoc pairwise comparisons to examine any group differences were performed only in the presence of a main-effect significant at the 0.05 level. Effect size f (ES) was calculated for significant main effects and interactions using G*power (Version 3.1.9.2; Faul, Erdfelder, Lang, and Buchner, Dusseldorf, Germany). Correlations of significant Go/NoGo task performance outcomes and group differences in withdrawal/craving and mood assessment ratings were assessed using Pearson’s r correlation coefficients. All statistical analyses were conducted using SPSS V19.0, with α set at 0.05.
3. Results
3.1. Participants
3.1.1 Demographics and substance use assessments
Participant demographic and substance use variables are reported in Table 1. Participants were well-matched, with no significant differences in age and education, and an equal number of males and females in each group. Smokers did not exhibit differences in FTND scores. As age of smoking onset is a focus in the current study, and participants were grouped by either early or late onset smoking, there are significant differences between smokers in age at first cigarette, age at regular cigarette smoking, and in duration of smoking exposure. Duration of smoking history is a critical factor in assessing differences between EOS and LOS in cognitive task performance, thus duration of smoking exposure, in years, was used as a covariate in all Go/NoGo outcome ANCOVA analyses.
There were no differences in CO levels at study baseline between EOS and LOS. LOS also reported more alcohol use per week than EOS and HNS.
3.1.2 Mood, smoking withdrawal and craving assessments
All mood measures are reported in Table 2, and all withdrawal and craving assessment variables measured at study baseline and/or end-of-day (EOD), following Go/NoGo task performance, are reported in Table 3. Some group differences in baseline mood measures did emerge between smokers and healthy non-smoking participants. Specifically, relative to HNS, EOS and LOS reported higher BDI, BIS motor and total impulsivity, POMS Tension, and STAI State scores. Relative to EOS and HNS, LOS reported higher HAM-A scores. Furthermore, relative to HNS, LOS reported higher POMS Anger and Confusion as well as PANAS Negative scores. A difference in baseline craving also emerged between smokers; EOS reported higher scores for similar QSU questions “Nothing would be better than smoking a cigarette right now” and “All I want right now is a cigarette.”
Table 2.
Participant Mood Measures
| EOS (n=10) | LOS (n=10) | HNS (n=10) | p | |
|---|---|---|---|---|
| HAM-A | 0.6 ± 0.8 | 2.0 ± 1.9 | 0.8 ± 0.9 | 0.05 (LOS>HNS) (LOS>EOS) |
| BDI | 4.2 ± 4.2 | 5.1 ± 5.3 | 0.6 ± 1.3 | 0.05 (EOS>HNS) (LOS>HNS) |
| BIS Cognitive | 22.3 ± 3.2 | 23.6 ± 2.6 | 22.7 ± 1.6 | ns |
| Motor | 26.1 ± 2.6 | 24.9 ± 2.5 | 21.5 ± 2.6 | 0.01 (EOS>HNS) (LOS>HNS) |
| Non-planning | 24.8 ± 2.1 | 25.5 ± 2.5 | 25.4 ± 2.2 | ns |
| Total | 73.1 ± 2.7 | 74.0 ± 3.0 | 69.6 ± 4.2 | 0.02 (EOS>HNS) (LOS>HNS) |
| POMS Tension | 7.9 ± 5.4 | 9.0 ± 5.5 | 2.2 ± 2.1 | .01 (EOS>HNS) (LOS>HNS) |
| Depression | 3.0 ± 3.7 | 2.8 ± 2.5 | 0.6 ± 0.8 | ns |
| Anger | 3.5 ± 4.9 | 6.3 ± 5.7 | 0.4 ± 1.0 | 0.02 (LOS>HNS) |
| Vigor | 14.1 ± 7.3 | 14.8 ± 8.5 | 16.9 ± 6.2 | ns |
| Fatigue | 3.1 ± 3.9 | 4.9 ± 2.9 | 1.9 ± 2.6 | ns |
| Confusion | 3.9 ± 3.0 | 6.0 ± 2.7 | 2.1 ± 1.2 | 0.01 (LOS>HNS) |
| PANAS Positive | 31.3 ± 7.9 | 34.1 ± 7.5 | 35.5 ± 7.0 | ns |
| Negative | 12.8 ± 4.2 | 14.3 ± 3.4 | 11.0 ± 1.7 | 0.03 (LOS>HNS) |
| STAI State | 36.9 ± 9.9 | 37.8 ± 10.9 | 26.5 ± 4.6 | 0.02 (EOS>HNS) (LOS>HNS) |
| Trait | 36.1 ± 10.0 | 36.2 ± 8.5 | 29.3 ± 6.7 | ns |
Table 3.
Participant Withdrawal and Craving Assessments
| EOS (n=10) | LOS (n=10) | p | |
|---|---|---|---|
| At Baseline | |||
| WSWS Urge to Smoke | 2.7 ± 0.9 | 2.9 ± 0.7 | ns |
| Anxiety | 1.7 ± 0.8 | 1.5 ± 1.1 | ns |
| Irritability | 1.3 ± 0.9 | 1.3 ± 0.9 | ns |
| Sadness | 1.1 ± 0.6 | 1.0 ± 0.8 | ns |
| Increased appetite | 1.9 ± 0.7 | 2.0 ± 0.8 | ns |
| Difficulty concentrating | 1.5 ± 0.8 | 1.3 ± 0.5 | ns |
| Insomnia | 1.9 ± 0.5 | 1.5 ± 0.8 | ns |
| QSU Desire a cigarette right now | 5.0 ± 0.0 | 4.8 ± 0.4 | ns |
| Nothing better than smoking a cig right now | 4.5 ± 0.7 | 2.7 ± 1.3 | 0.001 |
| If possible I would smoke right now | 4.4 ± 1.3 | 4.3 ± 1.3 | ns |
| Could control things better if I could smoke | 3.0 ± 1.7 | 2.3 ± 1.1 | ns |
| All I want right now is a cigarette | 4.4 ± 0.7 | 3.1 ± 1.2 | 0.01 |
| Urge for a cigarette | 4.5 ± 1.1 | 4.6 ± 0.5 | ns |
| Cigarette would taste good right now | 4.5 ± 1.3 | 4.4 ± 1.0 | ns |
| I would do almost anything for a cigarette | 2.3 ± 1.6 | 1.8 ± 0.8 | ns |
| Smoking would make me less depressed | 1.9 ± 1.3 | 1.9 ± 1.3 | ns |
| Going to smoke as soon as possible | 4.7 ± 0.9 | 4.9 ± 0.3 | ns |
| Total | 42.0 ± 12.0 | 34.8 ± 5.0 | 0.05 |
| At End of Day (following Go/NoGo Task Performance) | |||
| WSWS Urge to Smoke | 2.4 ± 1.6 | 3.3 ± 0.5 | ns |
| Anxiety | 1.6 ± 0.9 | 2.1 ± 0.7 | ns |
| Irritability | 1.1 ± 1.0 | 1.8 ± 1.1 | ns |
| Sadness | 1.2 ± 0.4 | 1.3 ± 0.8 | ns |
| Increased appetite | 2.4 ± 0.8 | 2.4 ± 0.7 | ns |
| Difficulty concentrating | 1.6 ± 0.7 | 1.9 ± 0.6 | ns |
| Insomnia | 1.7 ± 0.7 | 1.4 ± 0.6 | ns |
| QSU Desire a cigarette right now | 4.9 ± 0.3 | 4.7 ± 0.5 | ns |
| Nothing better than smoking a cig right now | 4.1 ± 0.9 | 3.3 ± 1.6 | ns |
| If possible I would smoke right now | 4.6 ± 0.5 | 4.6 ± 1.3 | ns |
| Could control things better if I could smoke | 2.4 ± 1.4 | 3.0 ± 1.0 | ns |
| All I want right now is a cigarette | 4.3 ± 0.8 | 3.8 ± 1.4 | ns |
| Urge for a cigarette | 4.7 ± 0.5 | 4.6 ± 0.5 | ns |
| Cigarette would taste good right now | 4.5 ± 1.3 | 4.6 ± 0.7 | ns |
| I would do almost anything for a cigarette | 2.7 ± 1.6 | 2.7 ± 1.1 | ns |
| Smoking would make me less depressed | 1.5 ± 1.3 | 2.0 ± 0.9 | ns |
| Going to smoke as soon as possible | 4.6 ± 0.7 | 5.0 ± 0.0 | ns |
| Total | 38.3 ± 6.3 | 38.3 ± 5.5 | ns |
3.2. Primary outcomes
One-way a priori ANCOVA analyses of group performance on the smoking Go/NoGo task, controlling for sex and duration of smoking exposure, revealed significant main effects of omission errors (F(4,25) = 7.6, p≤0.001, ES=1.1), commission errors (F(4,25) = 7.02, p≤0.002, ES=1.1), overall task accuracy (F(4,25) = 10.29, p≤0.01, ES=1.3), Go trial accuracy (F(4,25) = 5.93, p≤0.002, ES=.94), and NoGo trial accuracy (F(4,25) = 6.83, p≤0.01, ES=1.1). There was no significant main effect of average task RT (F(4,25) = 0.27, n.s.). There were also no significant interactions between any primary outcomes and sex or duration of smoking exposure.
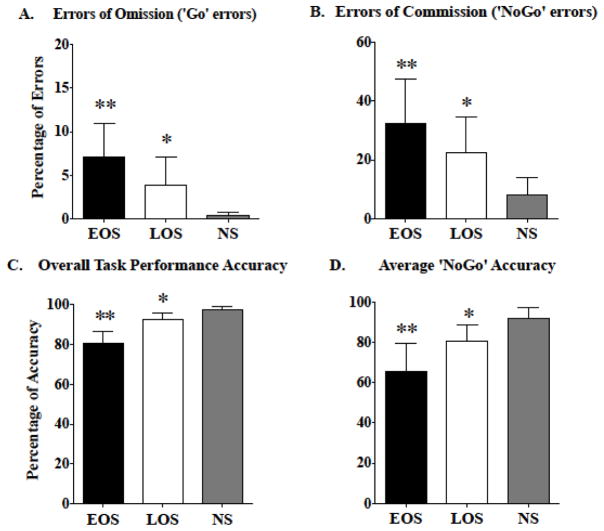
Post hoc group comparisons revealed that, compared to LOS and HNS, EOS made more errors in failing to respond to Go trials (omission errors; p≤0.02), failing to withhold responses to NoGo trials (commission errors; p≤0.02), overall task accuracy (p≤0.01), and NoGo trial accuracy (p≤0.02) (Figure 1, panels A–D).
Figure 1.
Significant differences in primary Go/NoGo task outcomes among early onset smokers (EOS), late onset smokers (LOS), and healthy non-smokers (HNS) are shown. (A) Group differences in errors of omission (‘Go’ errors). (B) Group differences in errors of commission (‘NoGo’ errors). (C) Group differences in overall Go/NoGo task performance accuracy. (D) Group differences in average ‘NoGo’ trial accuracy. All values are the means ± SD. ** p≤0.02, EOS relative to LOS and HNS. * p≤0.05, LOS relative to HNS.
The significant group difference in number of alcohol drinks reported per week could have influenced task performance in this study, as previous reports indicate that frequent alcohol exposure is associated with poor performance on a Go/NoGo task (Ames et al., 2014). Thus, additional ANCOVAs, controlling for sex, duration of smoking exposure, and number of drinks per week were performed on primary Go/NoGo task outcomes. Analyses revealed significant main effects of omission errors (F(5,24) = 5.89, p≤0.01, ES=1.1), commission errors (F(5,24) = 6.82, p≤0.000, ES=1.2), overall task accuracy (F(5,24) = 9.24, p≤0.000, ES=1.4), Go trial accuracy (F(5,24) = 4.32, p≤0.01, ES=.94), and NoGo trial accuracy (F(5,24) = 8.24, p≤0.000, ES=1.3). There was no significant main effect of average task RT (F(5,24) = 0.36, n.s.).
Significant interactions were observed between the number of commission errors × duration of smoking exposure (F(1,24) = 6.35, p≤0.02, ES=.52) and of commission errors × number of drinks per week (F(1,24) = 5.58, p≤0.03, ES=.40). Similarly, significant interactions were found between average NoGo trial accuracy × duration of smoking exposure (F(1,24) = 5.94, p≤0.03, ES=.52) and NoGo trial accuracy × number of alcohol drinks per week (F(1,24) = 5.93, p≤0.03, ES=.44). There were no other significant interactions between primary outcomes and smoking exposure or number of drinks per week. There were also no significant interactions between any primary outcomes and sex.
Post hoc group comparisons indicated that, compared to LOS and HNS, EOS made more errors in failing to respond to Go trials (omission errors; p≤0.03), failing to withhold responses to NoGo trials (commission errors; p≤0.01), overall task accuracy (p≤0.01), and NoGo trial accuracy (p≤0.01).
3.3. Secondary outcomes
ANCOVA analyses, controlling for sex and duration of smoking exposure, revealed significant main effects of smoking Go accuracy (F(4,25) = 6.16, p≤0.001, ES=.99), smoking NoGo accuracy (F(4,25) = 6.58, p≤0.001, ES=1.0), neutral Go accuracy (F(4,25) = 4.78, p≤0.01, ES=.87), neutral NoGo accuracy (F(4,25) = 5.6, p≤0.002, ES=.95), smoking NoGo RT (F(4,25) = 7.22, p≤0.001, ES=1.1), and neutral NoGo RT (F(4,25) = 6.54, p≤0.001, ES=1.0). Statistically significant interactions for secondary outcomes and smoking exposure were observed for neutral NoGo RT × smoking exposure (F(1,25) = 5.02, p≤0.05, ES=.45) and for smoking NoGo RT × smoking exposure (F(1,25) = 9.37, p≤0.01, ES=.61). There were no significant interactions for any secondary outcomes and sex.
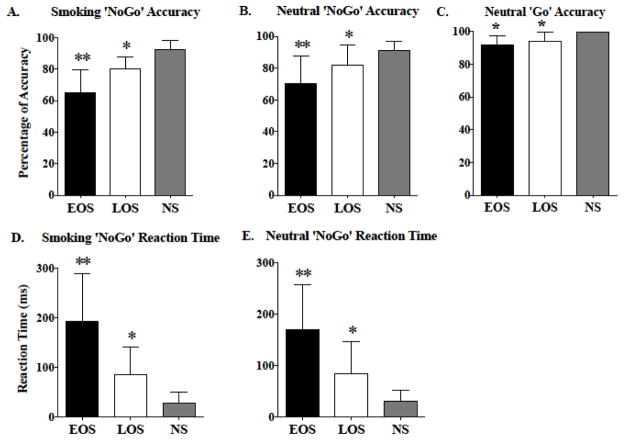
Post hoc group comparisons revealed that, compared to LOS and HNS, EOS made more errors in inhibiting responses to smoking NoGo trials (p≤0.02) and neutral NoGo trials (p≤0.02) (Figure 2, panels A and B, respectively). To that end, EOS also exhibited greater neutral NoGo RT (p≤0.01) and greater smoking NoGo RT (p≤0.001) (Figure 2, panels D and E, respectively). Compared to HNS, EOS (p≤0.03) and LOS (p≤0.03) made more errors in responding accurately to neutral Go trials (Figure 2, panel C).
Figure 2.
Significant differences in secondary Go/NoGo task outcomes among early onset smokers (EOS), late onset smokers (LOS), and healthy non-smokers (HNS) are shown. (A) Group differences in smoking-related ‘NoGo’ trial accuracy. (B) Group differences in neutral ‘NoGo’ trial accuracy. (C) Group differences in neutral ‘Go’ trial accuracy. (D) Group differences in smoking ‘NoGo’ response reaction time. (E) Group differences in neutral ‘NoGo’ response reaction time. All values are the means ± SD. ** p≤0.03, relative to LOS and HNS. * p≤0.05, relative to HNS.
Additional ANCOVAs also controlling for number of drinks per week were performed on secondary Go/NoGo task outcomes. Analyses revealed significant main effects of smoking Go accuracy (F(5,24) = 4.76, p≤0.01, ES=.99), smoking NoGo accuracy (F(5,24) = 6.91, p≤0.001, ES=1.2), neutral Go accuracy (F(5,24) = 3.67, p≤0.02, ES=.87), neutral NoGo accuracy (F(5,24) = 6.43, p≤0.001, ES=1.2), smoking NoGo RT (F(5,24) = 7.56, p≤0.000, ES=1.25), and neutral NoGo RT (F(5,24) = 7.42, p≤0.000, ES=1.3).
Significant interactions were observed between: smoking NoGo accuracy × smoking exposure (F(1,24) = 10.10, p≤0.01, ES=.65) and smoking NoGo accuracy × number of drinks per week (F(1,24) = 4.52, p≤0.01, ES=.43); neutral NoGo accuracy × smoking exposure (F(1,24) = 5.82, p≤0.02, ES=.49) and neutral NoGo accuracy × number of drinks per week (F(1,24) = 5.60, p≤0.03, ES=.48); smoking NoGo RT × smoking exposure (F(1,24) = 13.33, p≤0.001, ES=.75) and smoking NoGo RT × number of drinks per week (F(1,24) = 4.66, p≤0.05, ES=.44); and neutral NoGo RT × number of drinks per week (F(1,24) = 5.85, p≤0.02, ES=.49). There were no significant interactions between any secondary outcomes and sex.
Post hoc group comparisons revealed that, compared to LOS and HNS, EOS made more errors in inhibiting responses to smoking NoGo trials (p≤0.01) and neutral NoGo trials (p≤0.01). To that end, EOS also exhibited greater neutral NoGo RT (p≤0.01) and greater smoking NoGo RT (p≤0.001). Compared to HNS, EOS (p≤0.04) and LOS (p≤0.03) made more errors in responding accurately to neutral Go trials.
3.4. Correlations
3.4.1. Task performance outcomes and withdrawal/craving measures
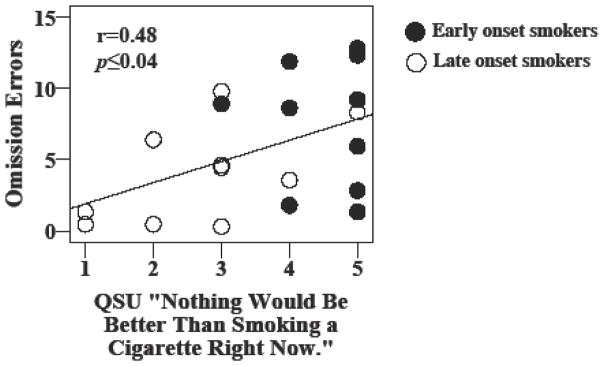
Importantly, significant positive correlations were found between baseline craving measured by the QSU-Brief, specifically the question “Nothing would be better than smoking a cigarette right now,” and number of omission errors (r=0.48, p≤0.04) (Figure 3). No other significant relationships between task performance outcomes and withdrawal/craving or mood measures were observed.
Figure 3.
A scatterplot representing individual early onset smokers (EOS) and late onset smokers (LOS) is shown that exhibits a significant relationship between omission errors and baseline Questionnaire on Smoking Urges (QSU) craving scores.
4. Discussion
EOS made more errors, took more time to respond, and performed less accurately than LOS and HNS on nearly every response accuracy and response inhibitory outcome measured during the smoking Go/NoGo task. Primary and secondary task performance outcome results suggest that EOS exhibited clear deficits in maintaining sustained attention and general inhibitory control, regardless of smoking-related or neutral image category. Furthermore, all analyses controlled for duration of smoking exposure, suggesting that these differences in task performance are more impacted by age of cigarette smoking onset. Overall, poor performance outcomes in adult EOS and LOS, relative to HNS, are consistent with previous reports of impaired response inhibition and less accuracy in adult smokers, relative to non-smokers (Spinella, 2002; Luijten et al., 2011; Dinur-Klein et al., 2014). Current findings, however, are the first to report the influence of smoking exposure during early or late stages of adolescence on the magnitude of impulsivity and lack of inhibitory control and attentional processing (behaviors that are typically refined during adolescent development) that endures into adulthood. Greater inaccuracy during task completion and poor performance outcomes in EOS suggests that cigarette smoking initiation during early stages of adolescence, coinciding with critical early stages of brain maturation, has a more significant impact on the normal development of executive functions such as inhibitory control and sustained attention.
Relative to LOS and HNS, EOS showed difficulty in responding accurately during frequent blue-framed Go trials, regardless of smoking or neutral image stimulus category, suggesting that it was challenging for this group to maintain attention during the task. Furthermore, when required to withhold responses to infrequent yellow-framed NoGo images, EOS were less successful than both LOS and HNS in inhibiting the prepotent button press response and, as such, exhibited less overall response accuracy for NoGo trials. When evaluating NoGo trial performance by stimulus category, EOS also made more errors than LOS and HNS in withholding responses to both neutral and smoking NoGo trials, suggesting that it was uniquely harder for this group to engage inhibitory control and hold back the impulsive response, regardless of category. The consequence of these impulsive NoGo responses was longer RT shown by EOS for both neutral and smoking NoGo trials. Statistical analyses revealed evidence of an interaction indicating that neutral and smoking NoGo trial RT, but not accuracy, was affected by duration of smoking exposure. It is possible that slower visuomotor processing and consequently longer NoGo RT produced (erroneously) by EOS may have been influenced by the longer duration of smoking exposure in this group. Conversely, response accuracy for neutral and smoking NoGo trials may be mediated more specifically by differences between EOS and LOS in developing and engaging inhibitory control.
Groups differed significantly in their alcohol consumption per week; LOS reported more drinks per week than either EOS or HNS groups. In accounting for this difference in alcohol consumption with additional analyses, several main effect interactions emerged. Namely, alcohol consumption and duration of smoking exposure interacted with commission errors as well as NoGo (overall, smoking, and neutral) trial accuracy and RT. Overall, greater monthly alcohol consumption, primarily observed in LOS, combined with duration of smoking exposure may have collectively contributed to the poor task performance also observed in LOS. Consistent with this interpretation, other studies have shown that long-term combined nicotine and alcohol consumption was significantly and inversely related to neurocognitive performance on tasks measuring attention, RT, response inhibition, and visuoperceptual processing (Friend et al., 2005; Glass et al., 2009; Ames et al., 2014). All post hoc comparisons continued to indicate, however, that EOS performed more poorly on the majority of primary and secondary task outcomes than LOS, suggesting that smoking exposure initiated during early adolescence and maintained into adulthood likely played an influential role in disturbing normative cognitive proficiency development (Counotte et al., 2009).
Important group differences in overall mood emerged at baseline, although these differences were not significantly associated with specific task outcomes. Relative to their HNS counterparts, EOS and LOS reported higher BDI scores, though these did fall within the threshold for minimal depression (0–13; Beck et al., 1996). Consistent with their impulsive NoGo responses, EOS and LOS also reported high scores for motor and total impulsivity, indicating that their task performance was likely influenced by their reported tendency to act quickly without thought or reflection on consequences. While there were no differences between EOS and LOS in BIS motor impulsivity, EOS did make more omission errors and perform more poorly on overall task accuracy than LOS, thus, reported impulsiveness in EOS may have more acutely affected task performance. Elevated POMS Tension subscale and STAI State scores also suggest that both groups of smokers were experiencing greater tension and acute anxiety than HNS during the study visit, which also may have interfered with their ability to maintain attention and inhibitory control.
Acute nicotine deprivation in smokers has previously been associated with elevated anxiety and stress levels and decreased alertness, as well as shorter latency to post-deprivation smoking resumption, suggesting that avoidance of deprivation-induced negative affect contributes to the maintenance of smoking behavior (Parrott, 1995, 1998; Zvolensky et al., 2014). While EOS did not report experiencing greater tension or anxiety than LOS, these negative mood measures may have impacted the performance of both groups during the task. Anxiety and stress diminishes the efficiency of frontal cortical networks involved in inhibitory and attentional processing and attention allocation that direct task-appropriate behavior (Eysenck et al., 2007; Ansari and Derakshan, 2011). Furthermore, anxiety disrupts efficient prefrontal attention processing that withstands task-irrelevant distractor stimuli, increasing the likelihood of distractibility and poor prepotent response inhibition (Bishop, 2009; Ansari and Derakshan, 2011). Sub-optimal cognitive efficiency in maintaining attention and withholding prepotent responses is reflected in current findings of poor task accuracy and greater errors of omission and commission. Based on their poor response accuracy and inhibitory control, as well as potentially altered frontal cortical processing efficiency related to their early onset smoking, EOS may be uniquely more vulnerable to the additional influence of anxiety on disrupting focus and yielding impulsive responding.
Smoking craving also influenced and disrupted EOS attention, contributing to sub-optimal task performance by this group. EOS reported greater baseline craving than LOS, indicating higher scores at similar QSU-Brief statements “Nothing would be better than smoking a cigarette right now” and “All I want right now is a cigarette.” Importantly, a significant positive correlation was observed between reported scores on “Nothing would be better than smoking a cigarette right now” and the number of omission errors emitted in the Go/NoGo task. Thus, the higher the reported craving score, the higher the number of errors in responding accurately to blue-framed Go trials, suggesting that craving disrupted the sustained attention required for responding accurately. Of note, there were no differences reported between EOS and LOS in any acute withdrawal or craving measures at either baseline or at end of day (EOD). EOS, relative to LOS, likely experienced and therefore reported more acute craving at baseline, following overnight nicotine deprivation, which persisted throughout the session and influenced task performance. LOS cigarette craving was higher toward the EOD but there were no differences observed between groups.
Our findings of poor sustained attention and response accuracy as well as elevated impulsive responding on a frontally-mediated cognitive task in adult early onset smokers provides support for the tobacco-induced neurotoxicity of adolescent cognitive development (TINACD) theory (DeBry and Tiffany, 2008). One assumption of the TINACD theory that is particularly germane to the current findings is the idea that the earlier the initiation of smoking, the greater the likelihood of suboptimal executive functioning and poor management of impulsivity and decision-making in adult smokers (DeBry and Tiffany, 2008; Counotte et al., 2009, 2011). As such, current findings of suboptimal cognitive task performance in EOS may be related to maladaptive neural effects of early adolescent smoking. TINACD proposes that the earlier the smoking initiation, the earlier the damage to maturing neurons and the structure of frontal cognitive networks that regulate executive function (DeBry and Tiffany, 2008).
TINACD also posits that during periods of great stress or affective instability, effective oversight over impulsive behavior, inhibitory control, and impaired judgment may be compromised, particularly in smokers who initiated use during early adolescence (DeBry and Tiffany, 2008). Thus, EOS experiencing intense affective states may have additional trouble regulating their attention and impulsivity and exercising inhibitory control. One such intensely affective state is elevated craving, which was greater in EOS at baseline, relative to LOS. To this end, greater smoking craving may have exacerbated poor task performance and resulted in less sustained attention, poor response accuracy, and more impulsive errors than LOS.
Ongoing neuroimaging investigations of early and late onset smokers will reveal if early onset smoking is associated with altered frontal cortical structure in adulthood, which could provide empirical support for the TINACD theory that EOS exhibit altered frontal cortical architecture. Future investigations should also explore the influence of sex-specific neuromaturational differences in Go/NoGo task performance and outcomes in early and late onset smokers. A limitation of the current investigation is the inability to closely measure sex-specific cognitive performance differences within the EOS and LOS groups. Furthermore, it was not possible in the current study to determine the degree to which the effects of nicotine deprivation, relative to the effects of long-term nicotine dependence, influenced cognitive task performance in groups. Smokers did not report any significant withdrawal symptoms. While craving in nicotine-deprived smokers was associated with greater omission errors, no other relationships between craving and task outcomes were observed, suggesting that age of onset was the driving influence on overall differences in task performance. The inclusion of a nicotine-satiety condition in which all smokers smoke a cigarette prior to completing the Go/NoGo task in future investigations of executive function task performance in EOS and LOS will help to clarify and distinguish the effects of deprivation from the effects of long-term nicotine dependence on task outcomes. An additional potential limitation is that participants were required to provide self-reports that contributed to determining mood, craving, and past and current drug and alcohol use histories. Participants may be reluctant to respond accurately or may be concerned about the consequences of admissibility, which can compromise accurate estimation and disclosure (Del Boca and Darkes, 2003). Participants in the current investigation were assured that their responses to all questionnaires and measures would be kept secure and confidential, and were given adequate time to complete their self-reports. Targeted questions were utilized to help participants accurately recall and report their past nicotine and other drug and alcohol use. Collectively, these approaches increased our confidence in the participant self-reports and the reliability of the data.
4.1. Conclusions
EOS exhibited poor inhibitory control and made more impulsive inaccurate responses, relative to LOS and HNS, on a frontally-mediated smoking Go/NoGo cognitive task (Luijten et al., 2011). These findings are the first to measure the influence of age of smoking onset on differences in cognitive task performance in smokers, and also provide behavioral testing support for the TINACD theory proposed by DeBry and Tiffany (2008). It is possible that initiation of cigarette smoking behaviors early in adolescence, coinciding with critical neurodevelopmental processes that culminate in refined frontal lobe executive function, results in suboptimal cognitive processing. Thus, accounting for age of smoking onset may be an important factor in individualizing treatment approaches that are designed to improve executive function in adult smokers participating in smoking cessation programs.
Highlights.
Quantified effects of early onset (EO) smoking on inhibitory control and attention
Adult smokers, grouped by either early or late onset (LO), performed Go/NoGo task
EO smokers were less accurate and more impulsive in task responding than LO smokers
Greater baseline smoking craving in EO correlated with higher omission errors
EO smoking contributes to diminished attention and inhibitory control in adulthood
Acknowledgments
This investigation was supported by the National Institute on Drug Abuse grant K01 DA034028 (YM). The authors wish to thank Drs. Maartje Luijten and Ingmar Franken for generously allowing use of their novel smoking Go/NoGo task paradigm for the current investigation. We also wish to thank Ms. Mary Kathryn (Kate) Dahlgren for her help in providing technical and data organization support.
Footnotes
Contributors
YM and SEL designed the study. JB and SLF oversaw participant recruitment and cognitive task performance, as well as data organization. YM conducted the data analysis and wrote the manuscript. SEL, JB, and SLF made contributions and edited the manuscript. YM consolidated edits from coauthors. All authors approved the final manuscript.
Conflict of Interest
No conflict declared.
Role of funding sources
The study sponsor (National Institutes of Health, National Institute on Drug Abuse) had no role in study design, data acquisition, data analysis, or manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav Brain Res. 2014;274:382–389. doi: 10.1016/j.bbr.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Ansari TL, Derakshan N. The neural correlates of impaired inhibitory control in anxiety. Neuropsychol. 2011;49:1146–1153. doi: 10.1016/j.neuropsychologia.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, Khazaal Y, Zullino D, Van der Linden M. Lack of inhibitory control predicts cigarette smoking dependence: Evidence from a non-deprived sample of light to moderate smokers. Drug Alcohol Depend. 2010;112:164–167. doi: 10.1016/j.drugalcdep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Current Cigarette Smoking Among Adults - United States, 2005–2014. Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- CDC. The health consequences of smoking-50 years of progress. US Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31:103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cortese BM, Uhde TW, LaRowe SD, Stein SV, Freeman WC, McClernon FJ, Brady KT, Hartwell KJ. Olfactory cue reactivity in nicotine-dependent adult smokers. Psychol Addict Behav. 2015;29:91–96. doi: 10.1037/adb0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacol. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Dahlgren MK, Sagar KA, Racine MT, Dreman MW, Gruber SA. Marijuana use predicts cognitive performance on tasks of executive function. J Stud Alcohol Drugs. 2016;77:298–308. doi: 10.15288/jsad.2016.77.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBry SC, Tiffany ST. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): A proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res. 2008;10:11–25. doi: 10.1080/14622200701767811. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Kertzman S, Rosenberg O, Kotler M, Zangen A, Dannon PN. Response inhibition and sustained and attention in heavy smokers versus non-smokers. Isr J Psychiatry Relat Sci. 2014;51:240–246. [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacol (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, Charney DS, Sahakian BJ. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacol. 2013;38:2525–2531. doi: 10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friend KB, Malloy PF, Sindelar HA. The effects of chronic nicotine and alcohol use on neurocognitive function. Addict Behav. 2005;30:193–202. doi: 10.1016/j.addbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Glass JM, Buu A, Adams KM, Nigg JT, Puttler LI, Jester JM, Zucker RA. Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction. 2009;104:38–48. doi: 10.1111/j.1360-0443.2008.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb Perspect Med. 2012;2:a012120. doi: 10.1101/cshperspect.a012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. 2012a;511:89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012b;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Coppola S, McKee SA. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Exp Clin Psychopharmacol. 2009;17:91–98. doi: 10.1037/a0015657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychol. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Luijten M, Littel M, Franken IH. Deficits in inhibitory control in smokers during a Go/NoGo task: An investigation using event-related brain potentials. PLoS One. 2011;6:e18898. doi: 10.1371/journal.pone.0018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. EDITS manual for the Profile of Mood States. Educational and Industrial Service; San Diego: 1981. [Google Scholar]
- Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- Norberg KE, Bierut LJ, Grucza RA. Long-term effects of minimum drinking age laws on past-year alcohol and drug use disorders. Alcohol Clin Exp Res. 2009;33:2180–2190. doi: 10.1111/j.1530-0277.2009.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90:233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Nesbitt’s Paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychol (Amst) 2011;138:419–428. doi: 10.1016/j.actpsy.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: Perspectives from human threat of shock studies. Front Hum Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar KA, Dahlgren MK, Gonenc A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci. 2015;16:84–92. doi: 10.1016/j.dcn.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Behavioral health trends in the United States: Results from the 2014. National Survey on Drug Use and Health; Rockville, MD: 2015. [Google Scholar]
- Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students - United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- Slade EP, Stuart EA, Salkever DS, Karakus M, Green KM, Ialongo N. Impacts of age of onset of substance use disorders on risk of adult incarceration among disadvantaged urban youth: A propensity score matching approach. Drug Alcohol Depend. 2008;95:1–13. doi: 10.1016/j.drugalcdep.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the Stait-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press, Inc; Palo Alto: 1983. [Google Scholar]
- Spinella M. Correlations between orbitofrontal dysfunction and tobacco smoking. Addict Biol. 2002;7:381–384. doi: 10.1080/1355621021000005964. [DOI] [PubMed] [Google Scholar]
- Sussman S. Effects of sixty-six adolescent tobacco use cessation trials and seventeen prospective studies of self-initiated quitting. Tob Induc Dis. 2002;1:35–81. doi: 10.1186/1617-9625-1-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Xin Z, Ting LX, Yi ZX, Li D, Bao ZA. Response inhibition of cigarette-related cues in male light smokers: Behavioral evidence using a two-choice oddball paradigm. Front Psychol. 2015;6:1506. doi: 10.3389/fpsyg.2015.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, Leventhal AM. Anxiety sensitivity as an amplifier of subjective and behavioral tobacco abstinence effects. Drug Alcohol Depend. 2014;142:224–230. doi: 10.1016/j.drugalcdep.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]