Summary
Chronic fibrotic liver disease caused by viral or metabolic etiologies is a high-risk condition for developing hepatocellular carcinoma (HCC). Even after complete HCC tumor resection or ablation, the carcinogenic tissue microenvironment in the remnant liver can give rise to recurrent de novo HCC tumors, which progress into incurable, advanced-stage disease in the majority of patients. Thus, early detection and prevention of HCC development is, in principle, the most impactful strategy to improve patient prognosis. However, practice guideline-recommended “one-size-fits-all” HCC screening for early tumor detection is utilized in less than 20% of the target population, and performance of screening modalities, i.e., ultrasound and alpha-fetoprotein is suboptimal. Furthermore, optimal screening strategies for emerging at-risk patient populations such as chronic hepatitis C after viral cure and non-cirrhotic non-alcoholic fatty liver disease remain controversial. New HCC biomarkers and imaging modalities may improve sensitivity and specificity of HCC detection. Clinical and molecular HCC risk scores will enable precise HCC risk prediction followed by tailored HCC screening for individual patients to maximize its cost-effectiveness and optimize allocation of limited medical resources. Several etiology-specific and generic HCC chemoprevention strategies are evolving. Epidemiological and experimental studies have identified candidate chemoprevention targets and therapies, including statins, anti-diabetic drugs, and selective molecular targeted agents, although their clinical testing has been limited by the lengthy process of cancer development that requires long-term, costly studies. Individual HCC risk prediction is expected to overcome the challenge by enabling personalized chemoprevention targeting high-risk patients to achieve precision HCC prevention and substantially improve the dismal prognosis of HCC.
Keywords: Cancer screening, risk prediction, chemoprevention, cirrhosis, hepatocellular carcinoma, precision medicine
Introduction
Liver cancer, predominantly hepatocellular carcinoma (HCC) arising in the context of cirrhosis, is the second most lethal cancer worldwide with persistently increasing mortality in Europe, North/South America, and Africa in contrast to the decreasing trend in East Asia.1-3 Cirrhosis is estimated to cause over 1.2 million deaths (2% of global incidences) in 2013, increased by 47% since 1990.4 Cirrhosis and HCC are the major life-limiting consequences of progressive fibrotic liver diseases mainly caused by viral, i.e., hepatitis B virus (HBV) and hepatitis C virus (HCV), and metabolic, i.e., alcohol abuse and non-alcoholic fatty liver disease (NAFLD), etiologies.5 In the U.S., HCC is the fastest rising cause of cancer-related deaths; HCC mortality rate has been increasing across almost all counties over the past three decades particularly in HCV-infected white men aged 55 to 64 years old and Hispanics affected with NAFLD in the Texas region.6-8 In a model-based simulation forecasting until 2030, HCC incidence rate will continue increasing in the 1950-1959 birth cohorts, Hispanic men, and black women.9
HCC is highly refractory to therapeutic interventions. Even after surgical resection or ablation, 70% of patients experience tumor recurrence within 5 years,10 and once the tumors progress into advanced stage, currently available medical therapies yield only marginal survival benefit and are not cost-effective.11 Furthermore, the highly complex and heterogeneous genetic aberrations in HCC tumors hamper identification of therapeutic strategies despite the emerging breadth of molecular targeted anticancer agents.12 Thus, it seems sensible to consider preventing HCC development and progression in patients at risk rather than treating advanced-stage disease with limited health benefit. However, despite the clinical unequivocal predisposing factors for liver disease progression toward cirrhosis and HCC, cancer prevention in this setting remains a daunting task as evidenced by the still dismal HCC prognosis (5-year survival rate <15%13). In this review, we overview limitations of the currently available measures of HCC prevention and opportunities to develop individual cancer risk-based tailored preventive strategies in the era of precision medicine.
HCC prevention strategies
Cancer prevention encompasses a wide variety of medical interventions. Primary prevention focuses on preventing exposure to cancer-predisposing factors or eliminating them at an early stage by vaccination, lifestyle modification, or environmental interventions in an etiology specific manner. Secondary or tertiary prevention covers early detection and chemoprevention of HCC occurrence or recurrence, respectively, in patients already exposed to etiological agents.14 Tertiary prevention after radical HCC treatment aims to reduce either recurrence arisen from dissemination of residual tumor cells (disseminative recurrence) or de novo carcinogenesis in remnant fibrotic/cirrhotic livers (de novo recurrence).
Cancer chemoprevention discovery and development has been challenging and there has been scarce progress over the past decades due to elusive mechanisms of human carcinogenesis.15 It is not feasible to verify mechanisms of cancer initiation in patients that are inferred from pre-clinical studies, because it is ethically and logistically difficult to monitor cancer-free individuals with molecular assessment for long durations until a cancer develops. To overcome this challenge, a “reverse-engineering” approach has been proposed, in which clinically relevant targets are first identified in clinical cohorts with completed long-term follow-up, and subsequently validated in experimental systems.10 Another factor limiting chemoprevention development is the suboptimal animal models that may not resemble human disease, leading to false discovery of chemoprevention targets and biomarkers; these approaches may be improved by more sophisticated modeling strategies.16,17 Relatively difficult access to liver biospecimens is another limiting factor, contrasting with easier access to specimens from other tissues (for example skin, cervix, and gastrointestinal tract cancers) that have enabled more advanced chemoprevention development.18 Utilization of liquid biopsy may resolve the issue of sampling, although it is still uncertain whether any informative biomolecules are present in the circulation.19
A major difficulty is the design and conduct of chemoprevention clinical trials, which are generally very resource-intensive. Chemoprevention trials typically require large sample sizes and long observation periods for several reasons: suboptimal potency of chemoprevention agents that meet high safety requirements, insufficient enrichment of high-risk patient populations, and lack of reliable surrogate endpoints of definitive long-term clinical outcomes.18 For instance, two large HCC chemoprevention trials, enrolling patients with advanced fibrosis in Europe and the U.S., failed to demonstrate an effect of maintenance low-dose interferon on HCC incidence in the whole study subjects.20,21 However, in subgroup analyses of patents with more advanced disease (i.e., cirrhosis or portal hypertension), HCC incidence was reduced, supporting the concept of enrichment by identifying high-risk patients in chemoprevention trials to better capture their effects with smaller sample sizes compared to conventional “all-comer” enrollment. Interestingly, in retrospective and prospective assessments of interferon and other agents in HCV-infected patients, an HCC preventive effect started to emerge approximately 2 years after enrollment.20-23 This observation may indicate that the duration of the latent period in order for subclinical neoplastic clones to become clinically recognizable needs to be accounted for when designing HCC chemoprevention trials.24
Regular biannual HCC screening is recommended based on current HCC guidelines,25 but its implementation in clinical practice is far from satisfactory, as detailed in the next section. Furthermore, there is no established HCC chemoprevention therapy to date. Various precipitants of chronic liver diseases, for example hepatitis virus infection, hepatic inflammation and fibrosis, and metabolic syndrome, may serve as chemoprevention targets. However, the obstacles reviewed above hamper the discovery of such chemoprevention targets and biomarkers. In addition, these challenges obscure the optimal timing and duration of chemopreventive interventions during the lengthy natural history of fibrotic liver disease progression towards HCC, which typically lasts for decades (Figure 1). In addition, clinical validation of the findings could easily extend beyond the scope and timeframe of typical research studies and clinical trials, and may be less appealing for the pharmaceutical industry engagement, who may favor a faster return on investment.26
Figure 1. HCC-preventive interventions in the natural history of HCC development in progressive fibrotic liver diseases.
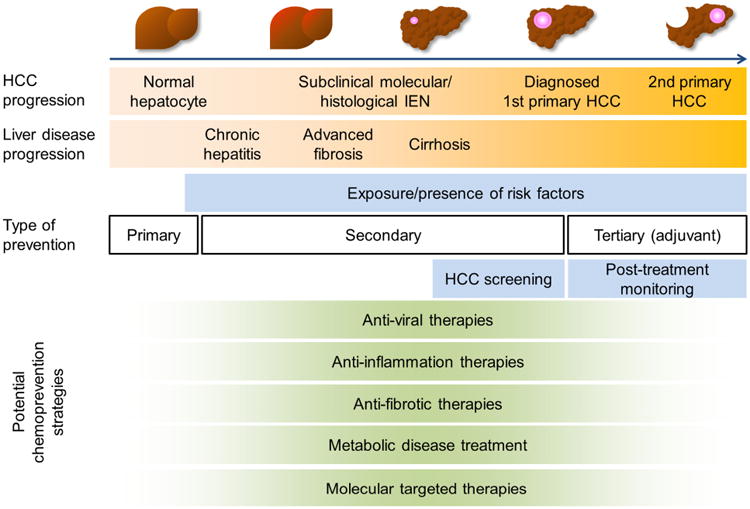
HCC-preventive strategy targeting each specific clinical context (i.e., etiology-specific or independent primary, secondary, and tertiary prevention) should be developed to ensure its clinically meaningful impact on the course of fibrotic liver disease progression toward HCC. IEN, intraepithelial neoplasia; HCC, hepatocellular carcinoma.
HCC screening
Screening is a vital component of cancer prevention. Current practice guidelines recommend regular HCC screening by biannual ultrasound with or without α-fetoprotein (AFP) in clinically identifiable population with HCC risk exceeding a certain threshold.25 A series of cohort studies and model-based simulation indicate that HCC screening is cost-effective and associated with improved early tumor detection, curative treatment rates, and survival, when it is available to more than 34% of patients at risk.27-31 However, the real-world utilization rate is below 20% due to multiple patient- and provider-related factors.32 Population-based interventions such as mailed outreach could improve the utilization rate to up to 50%.33 With the currently available resources, the vast size of the target population is another obstacle given that cirrhosis is estimated to affect 1-2% of global population and cause over 1.2 million (and 2% of total) deaths in 2013, increased by 47% since 1990.4 The magnitude of HCC risk for emerging populations, i.e., patients with non-cirrhotic NAFLD as well as after HCV cure, is yet to be determined, and screening strategies for these populations have not been established.32 These issues highlight the limitation of the current one-size-fits-all approach, which assumes uniform HCC risk across all patients with the same clinical condition (e.g., HCV cirrhosis) and results in often harmful over-or under-estimation of HCC risk for each patient. 34,35 Thus, prediction of individual HCC risk is critical to implementing effective and feasible HCC screening.
Clinical HCC risk scores
Combination of readily available clinical symptoms and laboratory variables have been evaluated to develop HCC risk-predictive scores, although their performance is somewhat limited and yet to be adopted in clinical practice (Table 1).32 Semi-quantitative histological fibrosis stage has been associated with future HCC risk, although sampling variability in liver biopsy hampers its robust determination.36 Quantification of collagen proportionate area may enable more reliable estimation of HCC risk.37-39 Hemodynamic measurement of portal hypertension, hepatic venous pressure gradient (HVPG), has been associated with HCC risk.40 Liver stiffness measurement (LSM) by ultrasound- or magnetic resonance imaging (MRI)-based elastography, by presumably capturing fibrotic and inflammatory tissue contents, has been associated with increased risk of HCC mostly in viral hepatitis, including cured HCV infection.41-44 Smoking has been associated with increased HCC risk (relative risk [RR], 1.51) in a meta-analysis of 38 cohort and 58 case-control studies,45 and incorporated in several HCC risk scores. The population attributable fraction (PAF) of smoking for HCC was 9% in the U.S.46 Passive smoking was also associated with HCC development (odds ratio [OR] at home, 4.86; OR at work, 2.44).47 Association of metabolic HCC risk factors is affected by smoking (interaction p=0.004).48 Alcohol exposure may also enhance risk, as suggested by characteristic somatic DNA aberrations.12
Table 1. Clinical HCC/fibrosis risk indicators.
| Risk indicator |
Study design | Endpoint | Major etiology |
No. subjects |
Major race/et hnicit y |
Cirrhos is |
Variables | Validation | Referenc e |
|---|---|---|---|---|---|---|---|---|---|
| LSM-H CC score | Prospective, cohort | HCC (3/5y) | HBV | 1,035 + 520* | Asian | 32% + 31%* | LSM, age, albumin, HBV DNA | Internal | 262 |
| REACH-B | Prospective-retrospective, cohort | HCC (3/5/10y) | HBV | 3,584 + 1,505* | Asian | 0% + 18%* | Sex, age, ALT, HBeAg, HBV DNA | External | 263 |
| CU-HCC | Prospective-retrospective, cohort | HCC (5y) | HBV | 1,005 + 424* | Asian | 38% + 16%* | Age, albumin, bilirubin, HBV DNA, cirrhosis | External | 264 |
| Yang, et al. | Prospective-retrospective, cohort | HCC (5/10y) | HBV | 2,435 + 1,218* | Asian | n.a. | Sex, age, HCC family history, alcohol, ALT, HBeAg, HBV-DNA, HBV genotype C | Internal | 265 |
| Hung, et al. | Retrospective, cohort | HCC (10y) | HBV | 8,252 + 4,125* | Asian | n.a. | Age, sex, ALT, previous liver disease, HCC family history, smoking, HBV, HCV | External | 266 |
| PAGE-B | Retrospective, cohort | HCC (5y) | HBV | 1,325 + 490* | White | 20% + 48%* | Age, sex, platelet | External | 267 |
| Sohn, et al. | Retrospective, cohort | HCC (5y) | HBV | 990 + 1,071* | Asian | 39% + 35%* | Age, sex, cirrhosis | Internal | 268 |
| FIB-4 | Retrospective, cohort | HCC | HBV | 986 | Asian | 9.9% | AST, ALT, platelet, age | No | 269 |
| GAG-HCC | Retrospective, cohort | HCC (5/10y) | HBV | 820 | Asian | 15% | Age, sex, HBV DNA, core promoter mutations, cirrhosis | No | 270 |
| Shin, et al. | Retrospective, cohort | HCC (5y) | HBV | 227 | Asian | 50.3% | LSM, spleen diameter, platelet | No | 43 |
| Kim, et al. | Retrospective, cohort | HCC | HBV | 2,878 | Asian | 10% | LSM | No | 271 |
| Singal, et al. | Prospective-retrospective, cohort | HCC (3/5y) | HCV | 442 + 1,050* | White, black, Hispanic | 100% + 41%* | 23 clinical variables | External | 272 |
| REVEAL-HCV | Prospective-retrospective, cohort | HCC (5y) | HCV | 1,095 + 572* | Asian | 1.4% + 7.0%* | Age, ALT, AST/ALT, HCV RNA, cirrhosis, HCV genotype | External | 273 |
| Ganne-Carrié, et al. | Prospective-retrospective, cohort | HCC (3y) | HCV | 720 + 360* | n.a. | 100% | Age, past alcohol abuse, platelet, GGT, SVR | Internal | 274 |
| Lok, et al. | Prospective-retrospective, cohort | HCC (5y) | HCV | 1,005 | White, black, Hispanic | 40% (Ishak 5/6) | Age, race, platelet, ALP, esophageal varices, smoking | No | 275 |
| El-Serag, et al. | Retrospective, cohort | HCC | HCV | 5,586 + 5,760* | White, black | 100% | AFP, ALT, platelet, age | Internal | 276 |
| Huang, et al. | Retrospective, cohort | HCC | HCC | 533 | n.a. | 7% | CPA | No | 38 |
| Motosugi, et al. | Retrospective, case-control | HCC | HCV | 66:66 § | Asian | n.a. | LSM by MRE | No | 41 |
| Chang, et al. | Retrospective, cohort | HCC (5y) | HCV after interferon | 1,252 + 627* | Asian | 45% (F3/4) | Age, sex, platelet, AFP, advanced fibrosis, HCV genotype 1b, SVR | Internal | 277 |
| Ikeda, et al. | Retrospective, cohort | HCC | HCV after SVR | 1,056 | Asian | 10% | Age, AST, platelet before interferon treatment | No | 278 |
| scoreHCC | Retrospective, cohort | HCC | HCV after SVR | 871 | Asian | 30% | Age, AFP, platelet, advanced fibrosis | No | 279 |
| Wang, et al. | Retrospective, case-control | HCC | HCV after SVR | 21:355§ | Asian | 33.8% (F3/4) | LSM, advanced fibrosis, diabetes | No | 44 |
| ADRESS-HCC | Retrospective, cohort | HCC (1y) | HCV, alcohol, NASH/cryptogenic | 17,124 + 17,808* | White, Hispanic | 100% | Age, diabetes, race, etiology, sex, Child-Pugh score | External | 280 |
| Velázquez, et al. | Prospective, cohort | HCC (4y) | Alcohol, HCV | 295 + 168* | n.a. | 100% | Age, HCV, prothrombin time, platelet | Internal | 281 |
| VFMAP | Retrospective, cohort | HCC (5y) | Non-viral, HCV | 1,808 | Asian | 13% | LSM, fast plasma glucose, sex, age, AFP | No | 282 |
| Wen, et al. | Retrospective, cohort | HCC (10y) | General population | 428,584 | Asian | n.a. | Smoking, alcohol, physical activity, diabetes, AST, ALT, AFP, HBV, HCV | Internal | 155 |
| Singh, et al. | Meta-analysis of 9, 6 studies | HCC, fibrosis (decompensation) | HCV, HBV, Alcohol, NASH | 4,038, 2,410 | n.a. | n.a. | LSM by TE, MRE | n.a. | 42 |
| Konerman, et al. | Prospective-retrospective, cohort | Fibrosis (>2 Ishak score, 1.5/3.5y) | HCV | 184 | White | 0% | 25 clinical variables | Internal | 283 |
| Lens, et al. | Prospective-retrospective, cohort | Fibrosis (F4, 5/7/10y) | HCV | 832 + 457* | White | 8.9% + 12.7% (F3) * | Advanced fibrosis, age, AST, GGT, Forns score | Internal | 284 |
| FILI score | Prospective-retrospective, cohort | Fibrosis after lifestyle intervention (1y) | NASH | 261 | n.a. | 0% | HbA1c change, platelet, ALT normalization | No | 285 |
| ELF score | Prospective-retrospective, cohort | Fibrosis (F3-4) | HCV, HBV | 300 | n.a. | 25% (F3/4) | Hyaluronic acid, TIMP1, PIIINP | No | 286 |
| Tsochatz is, et al. | Retrospectiv e, cohort | Fibrosis (decompensation) | Alcohol, HCV | 69 | n.a. | 100% | CPA | No | 37 |
Validation: “Internal”, validation in patients from the same institution(s); “External”, validation in patients from independent institution(s).
Training + validation.
Case:control. n.a., not available/applicable.
“Prospective-retrospective” indicates retrospective analysis of prospectively collected cohort in the past.287 HCC, hepatocellular carcinoma; REACH-B, Risk estimation for hepatocellular carcinoma in chronic hepatitis B; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; LSM, liver stiffness measurement; ALT, alanine aminotransferase; HCV, hepatitis C virus; AST, aspartate aminotransferase; REVEAL-HCV, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HCV; GGT, γ-glutamyltransferase; SVR, sustained virologic response; ALP, alkaline phosphatase; AFP, α-fetoprotein; CPA, collagen proportionate area; MRE, magnetic resonance elastography; ADRESS-HCC, age, diabetes, race, etiology of cirrhosis, sex and severity of liver dysfunction-HCC; NASH, non-alocholic steatohepatitis; VFMAP, virtual touch quantification, fast plasma glucose, sex, age, and AFP; TE, transient elastography; FILI, fibrosis improvement after lifestyle interventions; HbA1c, glycosylated hemoglobin, type A1c; ELF, enhanced liver fibrosis; TIMP1, tissue inhibitor of metalloproteinase-1; PIIINP, propeptide of type III procollagen.
Molecular HCC risk scores
Molecular biomarkers of HCC risk have been actively explored as overviewed below. Some of them were combined with clinical prognostic factors to develop integrative HCC risk scores to complement clinical scoring systems to refine HCC risk prediction (Table 2). Several germline single nucleotide polymorphisms (SNPs) have been identified as indicators of elevated HCC risk with odds ratios of around 1.5 in prospective and retrospective cohorts: EGF (in HBV- or HCV-infected patients), MPO, DEPDC5, and MICA (in HCV-infected patients), region in 1p36.22, STAT4, and HLA-DQ (in HBV-infected patients), and PNPLA3 and TM6SF2 (in alcoholic liver disease and NAFLD patients).49-57 Shorter telomeres and germline mutations in TERT gene were observed in NAFLD-related HCC patients.58A SNP in MBOAT7 gene was linked to HCC in non-cirrhotic NAFLD patients.59A recent genome-wide association study identified a SNP in TLL1 gene associated with HCC risk after HCV cure.60 A 7-gene SNP panel (Cirrhosis Risk Score) was associated with fibrosis progression in HCV-infected individuals.61 Liver tissue-derived transcriptome signatures have been associated with HCC risk,. For example, a 32-gene signature in fibrotic liver has been validated as a pan-etiology HCC risk indicator in patients with chronic hepatitis B/C, alcohol abuse, and NASH.10 Abundance of serum/plasma proteins such as insulin-like growth factor 1 (IGF1) and osteopontin (OPN/SPP1) has also been associated with HCC risk in cirrhosis.62,63 The N-glycosylation pattern of total serum protein (GlycoHCCRiskScore) has identified a subset of compensated cirrhosis patients at HCC risk.64 Body fluid (e.g., blood, urine)-based biomarkers will enable less-invasive and more flexible prognostic prediction given the decrease of liver biopsies in clinical practice, although tissue acquisition will help ensure their relevance to liver disease at least during the process of establishing such assays. Scientifically rigorous biomarker validation following the predefined phases of biomarker development will help ensure clinical validity of the biomarkers.65 These biomarkers are promising candidates for clinical translation, although assay development and implementation, regulatory approval, and reimbursement are challenging obstacles.66
Table 2. Molecular feature-based HCC/fibrosis risk indicators.
| Risk indicator | Study design |
Endpoint | Major etiolo gy |
No. subjects | Major race/ethnicit y |
Cirrhosis | Combined clinical variables |
Validatio n |
Refere nce |
|---|---|---|---|---|---|---|---|---|---|
| Circulating microRNA signature | Prospective-retrospective, cohort | HCC | HBV | 373 | Asian | 34.6% | n.a. | No | 288 |
| IGF 1 (serum protein) | Prospective, cohort | HCC | HCV | 104 | White | 100% | n.a. | External | 63 |
| EGF 61*G (SNP, rs4444903) | Prospective-retrospective, cohort | HCC | HCV | 816 | White, black | 15.4% | Age, sex, smoking history, ALP, platelet | External | 289 |
| MPO -463*G (SNP, rs2333227) | Prospective-retrospective, cohort | HCC | HCV | 205 | White | 100% | n.a. | No | 50 |
| GlycoHCCRiskScore (serum glycome) | Prospective-retrospective, case-control | HCC | HCV | 125 | n.a. | 100% | n.a. | No | 64 |
| TLL1 (SNP, rs17047200) | Retrospective, case-control | HCC | HCV after SVR | 123:333 + 130:356*§ | Asian | 24.6% + 20.1%* (F3-4) | Age, albumin, AFP after SVR | External | 60 |
| PN PLA3 444*G (SNP, rs738409) | Prospective, cohort | HCC | Alcohol HCV | 532 | White | 100% | Age, sex, BMI | External | 290 |
| HFE C282Y (SNP, rs1800562) | Prospective, cohort | HCC | Alcohol HCV | 301 | White | 100% | n.a. | External | 291 |
| Osteopontin (serum protein) | Prospective-retrospective, nested case-control | HCC (2y) | Non-viral | 100:194§ | White | n.a. | AFP, AST, ALT, GGT | No | 62 |
| 186/32-gene signature (liver tissue transcriptome) | Prospective-retrospective, cohort | HCC, HCC recurrence | HCV, HBV, Alcohol, NASH | 82 + 225/216/145/263* | Asian + n.a./white/white/Asian* | 52.4% + n.a./100%/100%/43%* | AFP, vascular invasion, bilirubin, platelet, Child-Pugh class, AJCC stage | External | 10,292-294 |
| HSC signature (liver tissue transcriptome) | Experiment + retrospective, cohort | HCC, HCC recurrence | HCV, HBV | Mouse + 216/82 | White/Asian | 100%/100% | Bilirubin, platelet | No | 295 |
| Activated HSC signature (liver tissue transcriptome) | Retrospective, cohort | HCC recurrence | HBV | 247+ 226/72* | Asian | 90.7% | n.a. | External | 296 |
| HIR signature (liver tissue transcriptome) | Retrospective, cohort | Late HCC recurrence | HBV | 72 + 96/228* | Asian | 50% + 62.5%/92.5%* | n.a. | External | 297 |
| 65-gene signature (HCC tissue transcriptome) | Retrospective, cohort | Early HCC recurrence | HBV | 72 + 96/228* | Asian | 50% + 62.5%/92.5%* | n.a. | External | 297 |
| Cirrhosis Risk Score (serum glycome) | Retrospective, case-control | Fibrosis (5y) | HCV | 205:66§ | n.a. | 21.8% (F2) | n.a. | No | 298 |
Validation: “Internal”, validation in patients from the same institution(s); “External”, validation in patients from independent institution(s).
Training + validation.
Case:control.
“Prospective-retrospective” indicates retrospective analysis of prospectively collected cohort in the past.287 Biomarkers evaluated in prospective patient series and/or externally validated in >100 patients are included. HCC, hepatocellular carcinoma; HBV, hepatitis B virus; IGF 1, insulin-like growth factor 1; HCV, hepatitis C virus; ALP, alkaline phosphatase; SVR, sustained virologic response; AFP, α-fetoprotein; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; NASH, non-alcoholic steatohepatitis; AJCC, American Joint Committee on Cancer; HSC, hepatic stellate cell; HIR, hepatic injury and regeneration.
HCC screening modalities
Abdominal ultrasound and serum AFP have been widely used as the main HCC screening modalities. The suggested minimal sensitivity for an HCC screening test to be cost-effective is 42% assuming a screening access rate of 34%.31 The sensitivity of ultrasound and AFP for detection of early-stage HCC tumor exceeds the threshold (approximately 60%), although it is still considered suboptimal.67 Operator dependency and patient-related factors such as obesity are the major sources of variation in ultrasound sensitivity, which can be as low as 32%.68-70 Serum AFP levels can non-specifically rise due to chronic hepatitis-related liver regeneration, which raises concern about its clinical utility as a screening modality.71 New serum/plasma biomarkers have been explored as possible replacements for AFP, and some of them are awaiting larger clinical validation for further development and deployment (Table 3). Integrative scores combining serum biomarkers with clinical variables have been proposed to improve diagnostic performance.72,73 In addition, identification of specific clinical contexts (e.g., HCV cirrhosis with normal serum alanine aminotransferase [ALT] level) has been suggested as a strategy to achieve improved performance of AFP.74
Table 3. HCC diagnostic biomarkers and scores.
| Biomarker | Study design |
Major etiology |
HCC stage | Major race/ethnici ty |
Sensitivit y |
Specifici ty |
AUROC | Validation | Refere nce |
|---|---|---|---|---|---|---|---|---|---|
| - Biomarker in clinical use | |||||||||
| AFP | Meta-analysis (20 studies) | HCV | n.a. | Asian | 4-71% | 29-100% | 0.65 | n.a. | 299 |
| AFP-L3 | Meta-analysis (8 studies) | HCV | n.a. | Asian | 21-49% | 93-100% | 0.69 | n.a. | 299 |
| DCP | Meta-analysis (16 studies) | HCV | n.a. | Asian | 7-56% | 72-100% | 0.70 | n.a. | 299 |
| - Integrative score | |||||||||
| GALAD model | Prospective, case-control | Alcohol, HCV, HBV | 25% + 22%* | n.a. | 94% | 83% | 0.95 | External | 73 |
| Doylestown algorithm | Prospective-retrospective, nested case-control | HBV/HCVC + HCV/HBV/HCV* | 54%/79% + 48%/100%/33%* | n.a. | 53/58/63% | Fixed to 95% | 0.84/0.89/0.88 | External | 72 |
| - Experimental biomarker | |||||||||
| GPC3 | Meta-analysis (19 studies) | HBV, HCV | n.a. | n.a. | 55% | 84% | 0.76 | n.a. | 300 |
| microRNA panel | Retrospective, case-control | HBV | 78% + 75% (BCLC 0/A) * | Asian | 81% | 84% | 0.89 | External | 301 |
| DKK1 | Retrospective, case-control | HBV | 67.2% + 31.1% (BCLC 0/A) * | Asian | 71% | 87% | 0.86 | External | 302 |
| MDK | Retrospective, case-control | HBV | 49.2% + 100% (BCLC 0/A) * | Asian | 86% | 90% | n.a. | External | 303 |
| Annexin A2 | Retrospective, case-control | HBV | 81.7% (AJCC I/II) | Asian | 83% | 68% | 0.79 | No | 304 |
| GlycoHCC Test | Retrospective, case-control | HBV | 34.7% (AJCC I/II) | Asian | 57% | 88% | 0.81 | No | 305 |
| Osteopontin | Prospective-retrospective, case-control | HCV + HBV* | 60% (BCLC 0/A) + n.a.* | n.a. + Asian* | 93% | 61% | 0.93 | External | 306 |
| GP73 | Prospective-retrospective, case-control | HCV | 48% (UNOS TNM 1/2) | White | 69% | 86% | 0.79 | No | 307 |
| GlycoHCC RiskTest | Prospective-retrospective, case-control | HCV | n.a. | n.a. | n.a. | n.a. | 0.73 | No | 64 |
| Fucosylated kininogen | Prospective-retrospective, case-control | HCV | 60% (UNOS TNM 1/2) | n.a. | n.a. | n.a. | 0.88 | No | 308 |
Diagnostic performance measures (sensitivity, specificity, AUROC) were derived from meta-analysis or validation studies.
Training + validation. “Prospective-retrospective” indicates retrospective analysis of prospectively collected cohort in the past.287 HCC, hepatocellular carcinoma; AUROC, area under the receiver operating characteristic curve; AFP, α-fetoprotein; HCV, hepatitis C virus; AFP-L3, lens culinaris agglutinin-reactive fraction of AFP; DCP, des-gamma-carboxy prothrombin ; GALAD, gender, age, AFP-L3, AFP, des-carboxy prothrombin; HBV, hepatitis B virus; GPC3, glypican 3; BCLC, Barcelona Clinic Liver Cancer; DKK1, Dickkopf-1; MDK, midkine; AJCC; American Joint Committee on Cancer; GP73, Golgi protein-73; UNOS, United Network of Organ Sharing.
Computed tomography (CT) and MRI may serve as alternatives to ultrasound with better performance, and are free from inter-operator variability. Indeed, CT and MRI can double the lesion-based sensitivity for small HCC tumors (up to 86%), although the high costs and irradiation (for CT) preclude their use as practical widespread options for HCC screening.75-77 An abbreviated contrast-enhanced MRI (AMRI) has been developed as an option that is specifically designed for regular HCC screening at half the cost of a full MRI, while maintaining a high sensitivity (81%) and specificity (96%).78
Individual risk-based tailored HCC screening
The heterogeneous individual HCC risk among the patients captured by clinical and/or molecular scores will enable rational allocation of the limited HCC screening resources to the high-risk patients who most need the intervention, and avoid ineffective and wasteful distribution of the demanding screening efforts to low-risk individuals. The currently recommended HCC screening interval of 6 months was determined based on estimated tumor volume doubling time.79,80 Uniformly longer or shorter intervals did not improve HCC detection.81,82 However, given that high-risk subjects likely develop HCC at a high frequency and in a multicentric manner, altering HCC screening intensity according to estimated individual HCC risk may enable more efficient early tumor detection (Figure 2).34 Such a personalized risk-based cancer screening strategy has been successfully implemented in other tumor types such as colorectal and breast cancers.83,84 In addition, education programs targeting high-risk communities with specific HCC risks based on etiology, for example African-born immigrants in New York City with a high prevalence of HBV infection, may efficiently improve uptake of high-risk individuals to HCC screening.85
Figure 2. Individual risk-based tailored HCC screening and chemoprevention.
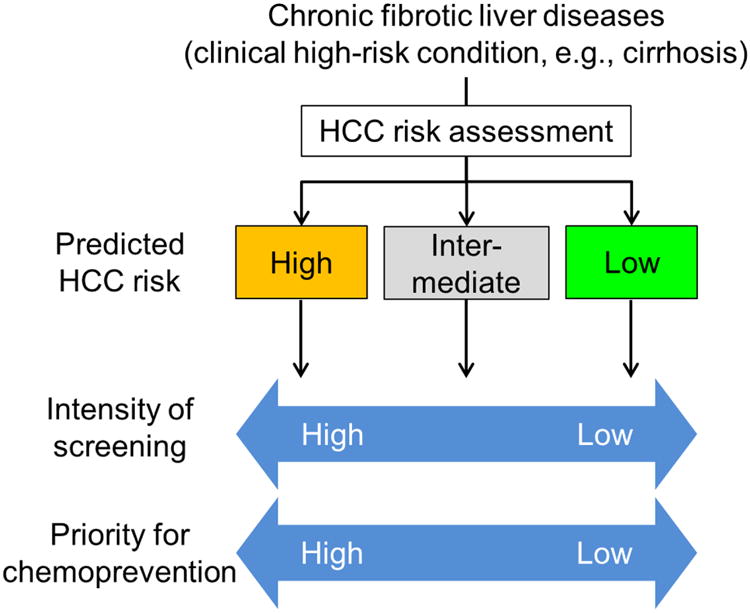
Individual HCC risk assessment with clinical and/or molecular risk indicators (see Tables 1 and 2) will enable personalized HCC screening and chemoprevention strategies to optimize allocation of limited medical resources and maximize cost-effectiveness of the interventions by tailoring intensity of screening (i.e., frequency and choice of modalities) and prioritizing a subset of patients with higher HCC risk for chemopreventive therapies.
The net benefit of HCC screening is determined as a function of multiple factors, including screening interval, performance of screening modalities, HCC incidence in the target population, and screening access rate, which has been evaluated by model-based cost-effectiveness analysis. A recent comprehensive assessment of individual risk-based tailored HCC screening strategies indeed revealed superior cost-effectiveness of personalized screening compared to the currently recommended uniform biannual screening of all patients.86 For instance, exclusive screening of high-risk subjects using AMRI is a robustly cost-effective strategy. More frequent screening, i.e., four times per year, is cost-effective when annual HCC incidence is greater than 3%. Although these results need to be clinically verified, testing of such strategies is now technically feasible with the HCC risk tests and new screening modalities already available in the clinical setting.
Etiology-specific HCC prevention
Hepatitis B
Chronic HBV infection has been the dominant etiology in Southeast Asia and sub-Saharan Africa, although the incidence of HBV-induced HCC is declining.87 Co-infection with hepatitis delta virus, food contamination with aflatoxin B1, microcystins, and metabolic risk factors facilitate fibrosis and/or HCC development.48,88-90 HBV DNA integration into the host genome is a unique feature that may lead to direct cis/trans activation of oncogenic signals and carcinogenesis without requiring a fibrotic tissue microenvironment.91 Serum HBV DNA levels, certain HBV strains (e.g., genotype C in Asian and genotype F in Alaskan), and mutations in the HBV genome (e.g., pre-Core and basal core promoter regions) are associated with increased HCC risk.91-94
Universal HBV vaccination is effective as a primary HCC prevention measure by reducing neonatal HBV vertical transmission.95 In a 20-year follow-up of a national vaccination program, the annual HCC incidence was significantly lower among vaccinated children, aged 6-19 years, compared with unvaccinated cohorts (RR, 0.31).96 Antiviral therapies have been evaluated as secondary prevention. In a meta-analysis of 12 clinical trials, involving 2,082 patients, interferon-based regimens decreased cirrhosis and HCC development (RRs, 0.65 and 0.59, respectively).97 Suppression of HBV replication by nucleot(s)ide analogs (NAs) reduced HCC incidence from 7.4% to 3.9% (hazard ratio [HR], 0.49) in a prospective trial enrolling 651 Taiwanese patients, and from 13.3% to 1.1% in a retrospective survey of 2,795 Japanese patients.98-100 Retrospective studies conducted mainly in Asia reported HCC risk reduction with newer generation first-line NAs, entecavir and tenofovir, by approximately 30% in cirrhotic and 80% in non-cirrhotic patients, although evidence in Western patients is still limited.101-103 A cohort study of 330 Taiwanese patients suggested that interferon may better prevent HCC development than NAs.104 In the setting of tertiary prevention (i.e., adjuvant therapy after curative resection or ablation of primary HCC tumors), a meta-analysis of 13 trials with 6,350 patients reported that use of NAs is associated with lower recurrence-free survival (HR, 0.66),105 which was confirmed in a more recent clinical trial (HR, 0.65).106 Of note, the HCC incidence after achieving virologic response with HBV DNA level consistently < 2,000 IU/mL to NAs was significantly higher than the HCC incidence in inactive chronic hepatitis B, indicating residual cancer risk not eliminated by current antiviral therapies.107 Even a low-level viremia (<2,000 IU/mL) during entecavir treatment increases HCC risk (HR, 1.98), especially in patients with cirrhosis (HR, 2.20) compared to patients with undetectable HBV DNA.108
Hepatitis C
Globally, 71 million individuals are affected with viremic HCV infection (prevalence, 1%).109 The incidence and mortality of HCV-related HCC keep rising in specific subpopulations such as the 1945-65 birth cohort (baby boomers) and veterans in the U.S.110 HCV clearance by antiviral therapies with a sustained virologic response (SVR) significantly reduces HCC incidence.111 However, interferon-based regimens had no impact on the incidence at a population level due to low treatment uptake (1-3% annually) and a modest SVR rate (50%) in a regional study in Australia.112 Despite the improved SVR rate with direct-acting antivirals (DAAs), HCC incidence is predicted to further increase until 2035 unless the treatment uptake rate is increased more than five-fold by 2018.113,114 HCV elimination is hampered by the high DAA costs and lack of comprehensive HCV screening linked to treatment programs.115 Undiagnosed HCV infection is estimated to represent 50% or more of the whole infected pool. In addition, high-risk populations such as inmates and injection drug users contribute to the 3-4 million new infections each year, and serves as a reservoir that maintains the pool of HCV-infected subjects via new and re-infection, and will lead to a sustained high disease burden in the next decade even in developed countries.114,116,117
Development of a prophylactic HCV vaccine as primary prevention has been challenging due to the high viral genetic variability, although there is promising progress.118 New experimental models such as HCV-related hepacivirus-infected rats may facilitate vaccine development.119 Targeting host genes/proteins such as viral entry factors may be an alternative or complementary strategy.120 In cirrhotic patients, DAA treatment to prevent re-infection after transplantation is cost-effective according to disease severity.121 HCV screening targeting high-risk populations is expected to boost uptake to antiviral treatment and subsequent HCC screening as needed.122
Secondary/tertiary chemoprevention could be achieved by antiviral therapies as suggested by retrospective studies consistently reporting reduction of annual HCC incidence from 1-8% to 0.07-1.2% by interferon-based SVR.111 Recent trials have shown that DAAs are better-tolerated compared to interferon even in compensated and decompensated cirrhotic patients.123,124 HCC incidence and recurrence rates after DAA-induced SVR are yet to be fully determined.111 Recent large cohort studies reported a comparable magnitude of reduction in HCC incidence between interferon- and DAA-induced SVR (HR, 0.28-0.29).124,125 Our understanding of post-SVR HCC risk drivers is still limited to several host factors, including advanced liver fibrosis, older age, accompanying metabolic diseases such as diabetes, persisting hepatic inflammation, and elevated AFP, and viral factors, including core protein variants and genotype 3.111 A liver transcriptome signature may enable more precise post-SVR HCC risk prediction.10,126 Clinical and experimental observations suggest there are DAA-specific modulations of host immunity and oncogenesis, for example reactivation of co-infected viruses, remission of follicular lymphoma, and rapidly restored function/differentiation of HCV-specific CD8+ T cells, memory T cells, and normalized NK cells.111 A cell culture-based study reported restoration of p53 function and ER stress response by interferon, but not by DAA.127 Further studies are needed to determine clinical utility and underlying mechanisms of action of DAAs as an HCC chemoprevention strategy.
Alcohol
Alcohol abuse remains a major and rising HCC etiology in several regions such as northern and central Europe, whereas alcohol consumption and HCC mortality are decreased in some countries such as France.128 A meta-analysis of 19 cohort studies showed a dose-dependent increase of HCC risk (HR, 1.16).129 Excessive alcohol drives hepatocarcinogenesis by increasing a mutagenic ethanol metabolite, acetaldehyde, oxidative stress, and DNA damage, and by generating a carcinogenic tissue microenvironment, which can synergize with viral hepatitis and metabolic syndrome.130-132 HCC genome DNA sequencing has identified recurrent mutations in genes encoding alcohol metabolizing enzymes, e.g., ADH1B.133 The magnitude of risk reduction by abstinence has not yet been established due to limited evidence. A meta-analysis of four cohort studies showed that abstinence reduced HCC risk by 6-7% annually, despite a large uncertainty in the estimate and more than two decades required to normalize the risk to the level of never drinkers when cirrhosis is present.134-136
NAFLD, obesity, and metabolic syndrome
One-fourth of the global population is affected by NAFLD, and among biopsied NAFLD patients, approximately 60% have non-alcoholic steatohepatitis (NASH), developing HCC annually at a rate of 5.29 per 1,000 person-years.137 Obesity, diabetes, and the metabolic syndrome are present in 51%, 23%, and 43% of NAFLD patients, respectively, suggesting highly heterogeneous pathogenesis across patients.137 Obesity and type 2 diabetes with insulin resistance are independent risk factors of HCC. In 5.24 million individuals registered in the Clinical Practice Research Datalink, high body-mass index (BMI) was significantly associated with liver cancer risk (HR, 1.19 per BMI 5 kg/m2).138 In a meta-analysis of 13 case-control and 13 cohort studies, diabetes was associated with increased HCC risk (OR, 2.5 and HR, 2.5, respectively).139 A more recent meta-analysis of 23 cohort studies reported a pooled RR of 2.0.140 Metabolic risk factors also increase HCC risk in viral hepatitis patients.48 The absence of established cirrhosis is more frequently associated with HCC in NAFLD compared to other etiologies such as HCV and alcohol abuse, which suggests NAFLD-specific mechanisms of carcinogenesis that are less dependent on hepatic fibrosis.141 Dysregulated hepatic and circulating pro-inflammatory cytokines and adipokines, oxidative and endoplasmic reticulum stress, and changes in intestinal microbiota (dysbiosis) are likely associated with obesity-related hepatocarcinogenesis.142-145 Bacterial metabolite (deoxycholic acid)-induced senescence-associated secretory phenotype (SASP)-mediated hepatic stellate cell activation that promotes tumors,146 disruption of circadian rhythm,147 depletion of anti-tumor CD4+ T-cells by linoleic acid from hepatocytes,148 induction of metabolic inflammation-associated interleukin 17A (IL17A),149 and prostaglandin E2 (PGE2)-mediated suppression of antitumor immunity by gut microbiota150 are all potential mechanisms of NAFLD carcinogenesis. Clinically relevant animal models of NAFLD fibrosis and/or HCC will allow more reliable preclinical assessment of experimental therapies.10,151-153
Lifestyle intervention may serve as secondary prevention as suggested by observational studies. A meta-analysis of 19 studies, involving 1,290,045 individuals, reported that increased intake of vegetables, but not fruits, may reduce HCC risk (RR, 0.72).154 In a prospective cohort of 428,584 subjects (HBV and HCV were positive in 15.7% and 2.6%, respectively), higher physical activity (metabolic equivalent tasks ≥ 7.5/hr) was associated with lower HCC risk (HR, 0.69).155
Statins
In experimental systems, statins elicit a variety of pleotropic anti-neoplastic, in addition to cholesterol-lowering, effects. Statins inhibit oncogenic pathways, including Myc,156 Akt,157,158 integrin and Rho-dependent kinase,159 nuclear factor κB (NF-κB), and tumor necrosis factor (TNF)-mediated IL6 production,160 and Hippo pathway effector TAZ, and extracellular signal–regulated kinase 1/2 (ERK1/2),161 whereas adenosine monophosphate-activated protein kinase (AMPK) and p38/mitogen-activated protein kinase (MAPK) pathways are activated,.162,163 and p53-dependent apoptosis is induced164 (Figure 3). Statins also limit fibrogenic hepatic stellate cell activation via nitric oxide synthase,165 paracrine signals from hepatocytes166 and endothelial cells,167 induction of sterol regulatory element-binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor (PPAR)-γ,168 and reduce portal hypertension via non-canonical hedgehog signaling.169
Figure 3. Molecular targets of potential HCC chemoprevention therapies.
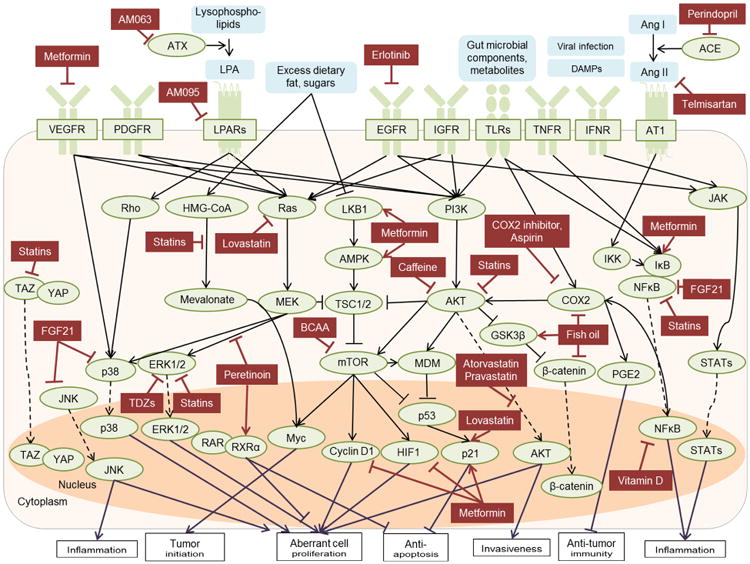
Intra- and extracellular targets of potential HCC chemopreventive therapies are summarized. Solid line with arrowhead or bar indicates activation or inhibition, respectively. Dotted line with arrowhead indicates translocation between intracellular compartments.
ACE, angiotensin-converting enzyme; AMPK, adenosine monophosphate-activated protein kinase; Ang, angiotensin; ASK1, apoptosis signal-regulating kinase 1; AT1, angiotensin type 1 receptor; CCR, C-C chemokine receptor; COX2, cyclooxygenase 2; DAMPs, damage-associated molecular pattern; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; ERK, extracellular signal–regulated kinase; FGF21, fibroblast growth factor 21; GSK3, glycogen synthase kinase 3; HIF, hypoxia inducible factor; HMG-CoA, 3-hydroxy-3nethyl-glutaryl-coenzyme A; IFNR, interferon receptor; IGFR, insulin-like growth factor 1 receptor; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LKB1, liver kinase B1; LPAR, lysophosphatidic acid receptor; MDM, mouse double minute; mTOR, mammalian target of rapamycin; NFκB, nuclear factor-kappa B; PDGFR, platelet-derived growth factor receptor; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; RAR, retinoic acid receptor; ROS, reactive oxygen species; RXR, retinoid X receptor; STAT, signal transducers and activator of transcription; TLR, toll-like receptor; TNFR, tumor necrosis factor receptor; TSC, tuberous sclerosis complex; VEGFR, vascular endothelial growth factor receptor; YAP, Yes-associated protein.
A dose-dependent reduction of HCC incidence was observed in Korean diabetic patients (ORs, 0.32 to 0.53)170 as well as Taiwanese patients infected with HBV (HR, 0.34 to 0.66) and HCV (HR, 0.33 to 0.66).171,172 In 7,248 HCV-infected persons in the U.S. ERCHIVES database, statin use was associated with less frequent progression to cirrhosis (HR, 0.6) and HCC (HR, 0.51).173 Fibrosis progression was reduced in the HALT-C cohort,174 and decompensation, mortality, and HCC were reduced in Taiwanese patients with HBV-, HCV-, and alcohol-related cirrhosis (HRs, 0.39, 0.46, and 0.52, respectively).175. In 18,080 non-cirrhotic NAFLD patients, even higher HCC suppressive effects were suggested (HR, 0.29).176 However, the protective effect was not observed in meta-analyses of 27 prospective studies involving 175,000 individuals in multiple cancer types including HCC.177,178 Differential effects between statins have also been suggested. In a systematic pair-wise comparison, fluvastatin was shown to be more effective in reducing HCC risk (RR, 0.55) compared to other statins.179 Atorvastatin and fluvastatin were associated with more significant anti-fibrotic effects compared to lovastatin, pravastatin, rosuvastatin, and simvastatin.173 Randomized clinical trials are currently ongoing to determine the role of statins in HCC chemoprevention (Table 4). Secondary preventive effect of simvastatin in patients with cirrhosis is being tested in a phase 2 trial, seeking for a change in AFP-L3% (NCT02968810). Atorvastatin is being evaluated for tertiary prevention after complete HCC resection or ablation (Statin for preventing HCC recurrence after curative treatment [SHOT] trial, NCT03024684).
Table 4. Ongoing clinical trials of HCC chemoprevention.
| Trial number | Agent/interventio n |
Type of agent/intervent ion |
Phase | Type of prevention |
Target population |
Primary endpoint |
No. planne d subject s |
Estimated completion data |
|---|---|---|---|---|---|---|---|---|
| NCT02224456 | Tenofovir disoproxil fumarate | Anti-viral | 4 | Secondary | CHB with advanced fibrosis | HCC development | 240 | 1/2021 |
| NCT02968810 | Simvastatin | Statin | 2 | Secondary | Cirrhosis | Change in AFP-L3 | 80 | 1/2020 |
| NCT02306070 | Metformin | Anti-diabetic | 2 | Secondary | CHC with diabetes | Change in LSM | 60 | 9/2017 |
| NCT02779465 | Vitamin D3 | Nutritional supplement | 4 | Secondary | CHB on anti-viral | Change in serum levels of 25(OH)D | 1,500 | 12/2026 |
| NCT02098785 | Caffeine | Nutritional supplement | 1 | Secondary | Healthy | VAP-1 serum levels | 63 | 9/2018 |
| NCT02273362 | Erlotinib | Kinase inhibitor | 1 | Secondary | Cirrhosis | Safety, pEGFR, 186-gene signature | 45 | 1/2018 |
| NCT03024684 | Atorvastatin | Statin | 4 | Tertiary | HCC (BCLC 0/A) after curative ablation or hepatectomy | HCC recurrence | 240 | 1/2022 |
| NCT03184493 | Metformin, celecoxib | Anti-inflammation, Anti-diabetic | 3 | Tertiary | HCC after hepatectomy | HCC recurrence | 200 | 6/2021 |
| NCT02281266 | Thymalfasin | Immune modulator | 4 | Tertiary | HBV-HCC after hepatectomy | DFS | 360 | 10/2018 |
| NCT02686372 | HBV antigen specific TCR redirected T cell | Immune modulator | 1 | Tertiary | CHB after transplantation | Safety | 10 | 11/2020 |
| NCT01924624 | Thalidomide | Immune modulator, anti-angiogenesis | 4 | Tertiary | HCC after hepatectomy | DFS | 140 | 12/2019 |
| NCT02447679 | Thalidomide, tegafururacil | Immune modulator, anti-angiogenesis, cytotoxic agent | 2 | Tertiary | HCC after hepatectomy | HCC recurrence | 40 | Completed, not reported |
| NCT03178929 | SAMe | Nutritional supplement | n.a. | Tertiary | HCC (BCLC 0/A) after radical treatment | HCC recurrence | 207 | 8/2020 |
| NCT01770431 | Huaier granule | Traditional herbal medicine | 4 | Tertiary | HCC (BCLC A/B) after hepatectomy | HCC recurrence, metastasis | 1,080 | 12/2016 |
| NCT02399033 | Xihuang Capsules | Traditional herbal medicine | 4 | Tertiary | HCC (BCLC 0-B) after hepatectomy | HCC recurrence | 1,000 | 12/2019 |
| NCT01717066 | Ginsenoside Rg | Chemo-sensitizer, anti-angiogenesis | 3 | Tertiary | HCC (BCLC A) after hepatectomy | HCC recurrence | 480 | Completed, not reported |
| NCT00116454 | 131 I-lipiodol | Cytotoxic agent | 3 | Tertiary | Viral/alcohol-HCC after hepatectomy or ablation (≤2 tumors) | HCC recurrence | 73 | Completed, not reported |
| NCT02767375 | Hepatic arterial infusion chemotherapy | Cytotoxic agent | 2∼3 | Tertiary | HCC after DFS hepatectomy | DFS | 192 | 12/2018 |
HCC, hepatocellular carcinoma; CHB, chronic hepatitis B; AFP-L3, lens culinaris agglutinin-reactive fraction of α-fetoprotein; CHC, chronic hepatitis C; LSM, liver stiffness measurement; 25(OH)D, 25-hydroxy vitamin D; VAP-1, vascular adhesion protein 1; BCLC, Barcelona clinic liver cancer; HBV, hepatitis B virus; DFS, disease-free survival; TCR, T cell receptor; SAMe, S-adenosylmethionine.
Metformin
Given the elevated HCC risk in association with type 2 diabetes, anti-diabetic therapies may be rational HCC chemopreventive strategies. Metformin, a biguanide derivate, inhibits gluconeogenesis and improves peripheral tissue insulin sensitivity, and also elicits various anti-neoplastic effects. Metformin inhibits the mammalian target of rapamycin (mTOR) pathway via activation of AMPK and its upstream regulator, LKB1,180 inhibits angiogenesis via suppression of hypoxia inducible factor 1 α (HIF1A) and vascular endothelial growth factor (VEGF),181 blocking cell cycle by decreasing cyclin D1 expression,182 suppresses cell survival-conferring NF-κB signaling by upregulating IκBα,180 and induces apoptosis via p53-independent mechanism183 and CCAAT/enhancer-binding protein δ (CEBPD)-induced autophagy184 (Figure 3) Metformin suppresses progenitor/stem cell activation and reduces HCC burden in a rat model of cirrhosis-driven carcinogenesis, although the HCC preventive effect is observed only when metformin treatment is started before development of cirrhosis.185
A meta-analysis of 19 studies involving 550,882 diabetic subjects suggested that metformin use reduced HCC incidence (OR, 0.52) compared to non-users.186 In exploratory subgroup analysis, metformin remained protective in patients with HBV/HCV infection (OR, 0.50), cirrhosis (OR, 0.49), and obesity (OR, 0.42). However, pooled results of two randomized controlled trials enrolling 8,798 patients found no significant difference in HCC risk according to metformin use (OR, 0.84; p=0.87). A phase 3 trial was initiated to evaluate secondary HCC chemopreventive effect of metformin in compensated HCV cirrhosis and insulin resistance in France, however the trial was terminated by the decision of investigator (NCT02319200). A phase 2 trial is planned to evaluate change in liver fibrosis by metformin in HCV-infected patients with or without HIV (NCT02306070).
Fibroblast growth factor 21 (FGF21)
FGF21 is a pleiotropic hormone with various beneficial effects on glucose metabolism and sugar intake and preference, which can be regulated by a variety of mechanisms such as adipose-derived circulating miRNAs and genetic polymorphism (rs838133).187-189 Lack of FGF21 accelerates the development of NASH and HCC in diabetic mice.190 FGF21 inhibits mTOR and improve insulin resistance as a candidate treatment for type 2 diabetes.191 A synthetic FGF21 protein, LY2405319, reduces transforming growth factor β1 (TGFβ1) and collagen I expression as well as NF-κB p65, c-Jun N-terminal kinase 1/2 (JNK1/2), and p38 phosphorylation, and inhibits NASH progression in leptin-deficient ob/ob mice fed methionine-and choline-deficient (MCD) diet, suggesting that FGF21 may have a role in chemoprevention of NAFLD cirrhosis and/or HCC.192
Generic chemoprevention strategies
Anti-inflammatory and immunomodulatory therapies
Chronic hepatic inflammation is a well-established driver of hepatocarcinogenesis.14 The HCC preventive effect of low-dose maintenance interferon therapy in HCV cirrhosis patients20,21 is likely due to reduced hepatic inflammation, so called “biochemical response”, instead of viral clearance, although the drug's intolerability hampers its wider use.24 Hepatic expression of an interferon-stimulated gene, retinoic acid-inducible gene-I (RIG-I), and downstream STAT1 signaling are suppressed in association with increased HCC risk, which possibly contributes to the higher HCC incidence in men compared to women.193 There is somewhat conflicting epidemiological evidence about the HCC preventive effect of non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin.194 A pooled analysis of 10 U.S.-based cohorts (679 HCC cases among 1,084,133 individuals) suggested a protective effect of aspirin use (HR, 0.68).195 Intestinal microbiota can induce innate immune signaling via Toll-like receptor 4 (TLR4) and support promotion of transformed neoplastic cells in the liver, which can be inhibited by gut sterilization in mice.196 Overexpression of cyclooxygenase 2 (COX2) has been implicated in hepatocarcinogenesis in experimental models.197 COX2 expression in hepatocytes is sufficient to induce HCC through inducing promoter hypermethylation by reducing tet methylcytosine dioxygenase 1 (TET1) expression, silencing tumor suppressor genes and activating oncogenic pathways.198 Hepatic translocation of lipoteichoic and deoxycholic acids from gut microbiota enhances SASP of hepatic stellate cells to upregulate COX2-mediated PGE2 production via TLR2, and suppresses anti-tumor immunity in a mouse model of obesity/NAFLD-associated HCC.150 lncRNA HULC stabilizes COX2 protein and promotes HCC cell growth.199 Activated hepatic stellate cells enhance immunosuppressive cell populations, including myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) via COX2-PGE2-EP4 signaling.200 Co-administration of a COX2 inhibitor, NS398, and simvastatin synergistically reduces proliferation and enhances apoptosis of HCC lines.201
In a clinical trial enrolling 232 patients who underwent curative HCC resection or ablation (i.e., tertiary prevention), a COX2 inhibitor, meloxicam, did not reduce overall and disease-free survival, although subgroup analyses suggested a possible chemopreventive effect in non-viral HCC.202 A phase 3 trial of another COX2 inhibitor, celecoxib, with or without metformin for tertiary prevention in patients who underwent curative HCC resection is now recruiting participants (NCT03184493). In a phase 3 trial conducted in Korea, adjuvant immunotherapy with activated cytokine-induced killer (CIK) cells (a natural killer [NK] cell subset incubated with patient-derived peripheral blood mononuclear cells, IL2, and CD3 antibody) reduced recurrence-free survival (HR, 0.63) and overall death (0.21) in HCC patients treated with resection or ablation.203 Thymalfasin, an immune modulator with pleiotropic activities towards T cells, NK cells, and dendritic cells, prolonged the time to HCC recurrence and survival as adjuvant therapy in HBV HCC patients in several pilot studies.204 A multicenter clinical trial is planned to evaluate the effect of thymalfasin 2-year recurrence-free survival rate and tumor immune microenvironment in patients with curatively treated HBV HCC (NCT02281266).
Anti-fibrotic therapies
Anti-fibrotic therapies may serve as HCC chemoprevention by halting progression of fibrotic liver diseases toward carcinogenesis as suggested by experimental studies and recent clinical trials.5 Hepatocyte apoptosis due to chronic injury leads to release of inflammation-mediating damage-associated molecular patterns (DAMPs), including TNF, IL6, IL1β, reactive oxygen species (ROS), and hedgehog ligands, and triggers fibrogenic hepatic stellate cell activation.5 Apoptosis signal-regulating kinase 1 (ASK1) activates JNK and p38 MAPK in response to various cellular stresses. A phase 2 trial of ASK1 inhibitor, selonsertib (GS-4997), reduced liver fibrosis (> 1 stage) in 43% of NASH patients (NCT02466516). Cenicriviroc, a dual inhibitor of fibrosis-promoting CCR2/CCR5 reduced liver fibrosis in a phase 2 trial (CENTAUR trial),205 and is now being tested in a follow-up phase 3 trial (AURORA, NCT03028740). PPARs, nuclear receptors for various fatty acids and derivatives, transcriptionally regulate metabolic processes to maintain energy homeostasis.5 A dual PPARα/δ agonist, elafibranor, stopped fibrosis progression in non-cirrhotic NASH in a phase 2 trial,206 and a follow-up phase 3 trial has been initiated (RESOLVE-IT, NCT02704403). Despite the promising results, the framework to assess anti-fibrotic therapies for clinically meaningful HCC chemopreventive effects is not yet established. Therapeutically amenable cancer risk biomarkers such as HCC risk gene signatures may serve as surrogate endpoints to complete clinical trials within a realistic time frame and with an achievable trial size. In addition, drug development pipelines are largely designed to assess either anti-fibrotic or anti-cancer effects but not both in the same trial, posing a logistical difficulty in justifying evaluation of agents from anti-fibrotics pipeline in the context of cancer.
Dietary and nutritional agents
In large-scale cohort or population-based studies, intake of unsaturated fat (HR, 0.71), n-3 polyunsaturated fatty acids (PUFAs) (HR, 0.64), eicosapentaenoic acid (EPA) (HR, 0.56), docosapentaenoic acid (DPA) (HR, 0.64), and docosahexaenoic acid (DHA) (HR, 0.56) are associated with lower HCC risk.207,208 Omega-3 PUFAs, DHA, and EPA inhibit HCC growth through inhibition of COX2 and GSK-3β-mediated β-catenin degradation.209 PUFA-forming Fat-1 transgenic mouse is protected from diethylnitrosamine (DEN)-induced hepatocarcinogenesis with reduced TNF and COX2 expression.210 Intake of white meat (chicken, turkey, and fish) was associated with a lower risk of HCC (HR, 0.52), whereas red meat (beef and pork) was associated with a higher risk (HR, 1.74) in the NIH-AARP cohort.211
Higher vitamin D, 25(OH)D, levels have been associated with reduced risk of HCC (RR, 0.51).212 Low serum levels of 25(OH)D3 are associated with adverse outcomes, including HBV-related HCC (HR, 1.90).213 Vitamin D3 up-regulated protein 1 (VDUP1) suppresses TNF and NF-κB signaling, and protects mice from DEN-induced hepatocarcinogenesis.214 Expression of KLF4 sensitizes HCC cells to the anti-proliferative effects of 25(OH)D3.215 p62/SQSTM1 promotes heterodimerization of the vitamin D receptor (VDR) with retinoid X receptor (RXR), and inhibits liver fibrosis and HCC.216 A clinical trial of vitamin D3 is planned for prevention of HCC in chronic hepatitis B patients on nucleos(t)ide analog treatment (VDHCC trial, NCT02779465).
Excessive dietary iron and/or genetic polymorphisms such as HFE C282Y and H63D variants can induce oxidative DNA damage and inflammation that increase HCC risk independently or with other etiologies, including HCV and alcohol.14 Liver-specific β-catenin knockout increases susceptibility to dietary iron and steatohepatitis, fibrosis, and HCC via AKT, ERK, and NF-κB pathways in mice, which is protected by N-Acetyl-L-(+)-cysteine (NAC).217 Long-term phlebotomy can lower serum ALT level and incidence of HCV-related HCC.218 An oral iron chelator, deferasirox, suppresses N-nitrosodiethylamine-induced murine liver carcinogenesis, and upregulates expression of hepcidin, transferrin receptor 1, and hypoxia inducible factor-1α, but its use in humans is limited by dose-limiting toxicities.219
Branched-chain amino acid (BCAA), used for hepatic encephalopathy, enhances mTOR signaling-mediated cellular senescence, and reduces liver fibrosis and HCC in DEN-treated rats.220,221 In HCV-transgenic mice, BCAA reduces hepatic iron and reactive oxygen species with elevated hepcidin-25, which is also observed in HCV fibrosis patients.222 In high-fat diet-fed atherogenic NASH mice, BCAA represses TGFβ1-stimulated pro-fibrogenic gene expression in hepatic stellate cells, protects hepatocytes from apoptosis, and reduces transformation of WB-F344 rat liver epithelial stem-like cells in an mTOR-dependent manner.223 In C57BL/KsJ-db/db obese mice, BCAA increases expression of PPARγ, p21CIP1, and p27KIP1, suppresses expression of IL6, IL1β, IL18, and TNF, reduces inflammation in both liver and white adipose tissues, and inhibits spontaneous hepatic carcinogenesis.224 In an observational study of 299 Japanese cirrhotic patients, BCAA supplementation was associated with less frequent HCC development (RR, 0.45).225
Coffee consumption (>2 cups/day) has been associated with reduced HCC risk in a meta-analysis of 18 cohorts, involving 2,905 HCC cases (RR, 0.71) and 8 case-control studies, involving 1,825 HCC cases (RR, 0.53).226 Caffeinated and decaffeinated coffee was associated with 27% and 14% reduced HCC risk, respectively. The reduced HCC risk was partly attributed to reduced hepatocellular injury measured by IL6, ALT, aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT).227,228 In a European prospective cohort including 201 HCC cases, tea intake was also associated with reduced HCC risk to a lesser extent (HR, 0.41) than coffee (HR, 0.28).229 A caffeine analog, CGS 15943, inhibits HCC cell growth by targeting phosphoinositide 3-kinase (PI3K)/AKT pathway.230 A phase 1 trial of caffeine is planned to assess its effect on serum vascular adhesion protein 1 (VAP-1) linked to hepatic inflammation and fibrosis in NASH5 (NCT02098785).
Dietary phytochemicals such as curcumin (turmeric extract), resveratrol (polyphenol in grapes, red wine, and berries), silymarin (herbal flavonoid), and carotenoids have been evaluated as potential HCC chemoprevention agents by activating cytoprotective mechanisms such as Keap1/Nrf2 pathway in mostly carcinogen-induced rodent models, although supporting clinical evidence is lacking.14 This class of compounds may need careful assessment given the recent classification of curcumin as pan-assay interference compound (PANIS) that likely shows false experimental activity.231 Reduction of DNA damage biomarkers such as urine 8-hydroxydeoxyguanosine (8-OHdG) was observed for epigallocatechin gallate (EGCG, green tea polyphenol) and broccoli sprout in human, although their HCC-preventive effect is undetermined.14 Glycyrrhizin, licorice root extract, reduced HCC incidence when ALT was normalized (HR, 0.39).232 S-adenosylmethionine (SAMe), a ubiquitous major methyl donor, is reduced in rodent HCC models, and SAMe treatment suppresses HCC development.14 In a phase 2 trial, 24 weeks of SAMe treatment in 87 HCV cirrhosis subjects did not alter AFP level and biomarkers of liver injury and oxidative stress.233
Molecular targeted therapies
PI3K/AKT/mTOR pathway is involved in cell cycle and proliferation, and is an appealing candidate HCC chemoprevention target.14 AKT was indeed identified as a key HCC risk driver in a human liver transcriptome meta-analysis.10 Retrospective studies and their meta-analysis suggest that mTOR inhibitor-based immunosuppression reduces post-transplant HCC recurrence,234 but adverse effect of an mTOR inhibitor, sirolimus, including hepatic artery thrombosis and decreased patient and graft survival have been noted.235 In animal models of chemical- and obesity-driven HCC development, sirolimus activates IL6/STAT3 and enhances HCC development, despite a transient reduction of steatosis.236 To determine the benefit or harm of mTOR inhibition, sirolimus and everolimus, have been tested in prospective trials for prevention of post-transplantation HCC recurrence.237 A phase 3 trial of sirolimus after transplantation enrolling 525 patients (viral hepatitis, 48%; alcoholic, 31%) did not improve recurrence-free survival beyond 5 years (HR, 0.84), although subgroup analyses suggested that patients with less advanced HCC tumors within Milan criteria or younger age may benefit from the therapy (SiLVER trial).238
Bioactive lipids, e.g., lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), transmit cellular signals via G-protein-coupled receptors, and regulate cell survival, differentiation, proliferation, and migration.239 In liver, expression of autotaxin (ATX), which converts lysophosphatidylcholine into LPA, increases HCV replication and is elevated in serum of HCV-infected patients in association with hepatic fibrosis and HCC.240 In NAFLD, lipotoxic lipids, including LPA, are generated from excess dietary fat and sugars, and induce phenotypic manifestation of NASH, fibrosis, and HCC in the Substrate Overload Lipotoxic Liver Injury (SOLLI) model of NAFLD pathogenesis.142 In a transcriptome-based meta-analysis of 523 human fibrotic livers, LPA receptor 1 (LPAR1) signaling was identified as a pan-etiology HCC risk driver, and Rho kinase and Ras/MAPK/ERK, but not PI3K/AKT/mTOR signaling, were identified as its downstream effector pathways in cirrhotic livers.10 Genetic knockout of ATX as well as pharmacological inhibition of ATX or LPAR1 ameliorates liver fibrosis and HCC in multiple rodent models,10,241 reinforcing the LPA pathway as a promising chemoprevention target.
The renin-angiotensin system is involved in liver fibrosis and carcinogenesis.14 Angiotensin II-mediated NF-kB activation promotes fibrogenic myofibroblast survival, which can be inhibited by an angiotensin-converting enzyme (ACE) inhibitor, captopril.242 Hepatic stellate cell-targeted delivery of an angiotensin II type 1 receptor blocker (ARB), losartan, reduce liver fibrosis by inhibiting expression of NADPH oxidase and collagen type I.243 An ARB, telmisartan, prevents hepatic carcinoma in CDAA-induced NASH fibrosis and HCC in rats.244 In a retrospective single center clinical study, the use of ARBs was associated with longer time to HCC recurrence and increased survival after radiofrequency ablation.245 An ACE inhibitor, perindopril, combined with vitamin K2 reduced HCC recurrence after curative therapy.246 A clinical trial of perindopril in combination with BCAA reduced serum VEGF level and post-ablation HCC recurrence in 54 patients with insulin resistance.247
A synthetic acyclic retinoid (vitamin A analogue), peretinoin, inhibits multiple cellular signaling, including Wnt and platelet-derived growth factor (PDGF) pathways; it also induces differentiation and apoptosis of hepatic stem cells, and is assumed to suppresses neoplastic clones.248-250 Peretinoin also inhibits HCV replication and infectious virus release in cultured cells.251 In atherogenic high-fat diet-fed mice, peretinoin activates autophagy and suppresses NASH and HCC development.252 In a phase 3 trial of peretinoin in 377 patients with curatively treated HCV-related HCC, a lower trend of HCC recurrence was observed for the entire study period (HR, 0.73), and also after 2 years of randomization (HR, 0.27).23 A follow-up survey reported a longer overall survival of patients treated with higher dose of peretinoin compared to untreated controls (HR, 0.58; p=0.03).253 Prospective trials in cured HBV-related HCC patients are ongoing.248
Several kinase inhibitors initially developed and evaluated for treatment of advanced-stage cancers have also been tested as adjuvant therapies in HCC. Activation of epidermal growth factor receptor (EGFR) signaling in hepatic stellate cells and macrophages promotes HCC development in rodent models.254,255 A small molecule EGFR inhibitor, erlotinib, reversed a high-risk pattern of the liver transcriptome and suppressed HCC development in rodent models of fibrosis-driven carcinogenesis.256 Based on these animal studies, a phase 1 HCC chemoprevention trial was initiated using the transcriptome signature as a companion biomarker (NCT02273362). A multi-kinase inhibitor, sorafenib, did not alter recurrence-free survival (HR, 0.94) after complete resection or ablation of primary HCC tumors in a phase 3 trial.257
The estrogen pathway is deemed to play a key role in the sex disparity in HCC risk.258 In a meta-analysis of 87 studies, variations in estrogen receptor 1 (ESR1) gene were associated with increased HCC risk.259 In a case-control study of 234 female patients with treated HCC and 282 healthy controls, estrogen replacement (as menopause hormone therapy) was associated with a reduced risk of HCC (OR, 0.53, 0.32) and prolonged survival (HR 0.55).260 Systemic delivery of mi-R101, down-regulated in HCC tissue, inhibits some of the candidate HCC chemoprevention targets, e.g., COX2 and Rho-GTPase, and suppresses tumorigenesis in mice.261
Conclusions
Clinical evaluation and implementation of HCC preventive strategies, encompassing HCC screening and chemopreventive intervention, will not be successful and/or feasible without individual risk-based tailored approaches. Comprehensive, multi-omics, and multi-cell type characterization of diseased liver tissue microenvironment at risk of cancer development will facilitate cataloging of candidate chemoprevention targets. Such coordinated efforts will lead to tailored intervention for each individual according to specific molecular risk mechanism and chemoprevention targets. However, requirement of large sample size and long observation period are the major logistical challenges in chemoprevention clinical trials that diminish physicians' motivation to engage asymptomatic individuals and adhere to the protocol. Diversity in HCC incidence according to etiology, patient race/ethnicity, and clinical context (e.g., post-SVR cirrhosis) needs to be taken into account in assessing clinical utility and real-world effectiveness of preventive interventions. Drug safety in cirrhotic patients is another critical factor. The precision medicine approaches rely on molecular information derived from biospecimens. Although liver tissue is deemed as the most reliable source to interrogate pathogenic molecular dysregulation, transition to less invasive types of biospecimen during the process of clinical translation will help its wider applicability. Sampling bias and robustness in molecular readout should also be determined in preclinical and clinical studies. Once these issues are resolved and the preventive strategies are clinically implemented, the tailored approach will enable more cost-effective and precise preventive intervention in the clinical care of patients at HCC risk, which will substantially improve the dismal prognosis of HCC.
Key points.
HCC mortality keeps increasing in several regions in Europe, Africa, and the U.S. in contrast to a decreasing trend in traditionally endemic areas such as East Asia.
Patients with active or cured HCV infection and individuals with NAFLD or metabolic disorders are emerging populations for HCC development, awaiting customized HCC screening strategies.
Regular HCC screening is significantly underutilized due to multiple patient- and provider-related barriers. This challenge may be overcome by multi-level clinical and community-based interventions as well as individual risk-based personalized HCC screening.
New HCC screening modalities, including serum biomarkers, integrative scores, and imaging techniques, are under development or clinical evaluation for improved early HCC tumor detection.
A variety of etiology-specific and independent interventions are evolving as potential HCC chemopreventive measures, although the framework of their clinical testing and implementation needs to be developed.
Anti-viral therapies can be effective etiology-specific HCC chemopreventive interventions, although viral cure does not eliminate HCC risk, and therefore requires continued risk assessment, screening, and/or additional chemopreventive interventions.
Anti-inflammatory, immunomodulatory, anti-fibrotic, metabolic, dietary, physical, and molecular targeted interventions may serve as generic HCC chemoprevention therapies.
Acknowledgments
Financial supports: This work is supported by Uehara Memorial Foundation (to N.F.), and U.S. NIH/NIDDK R01 DK099558, European Union ERC-2014-AdG-671231 HEPCIR, Irma T. Hirschl Trust, and U.S. Department of Defense W81XWH-16-1-0363 (to Y.H.).
Footnotes
Conflict of interest statement: No conflict of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Mohamed EA, Aziz AO, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol. 2017;2:103–11. doi: 10.1016/S2468-1253(16)30161-3. [DOI] [PubMed] [Google Scholar]
- 4.GBD. Mortality and Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980-2014. JAMA. 2017;317:388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812–20 e5. doi: 10.1053/j.gastro.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–37. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787–94. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa S, Wei L, Song WM, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–90. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh ND, Singal AG, Hutton DW. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer. 2017 doi: 10.1002/cncr.30863. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network. Electronic address wbe, Cancer Genome Atlas Research N. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327–41 e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens N, Sun X, Hoshida Y. Molecular classification of hepatocellular carcinoma: potential therapeutic implications. Hepat Oncol. 2015;2:371–9. doi: 10.2217/hep.15.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges. Curr Cancer Drug Targets. 2012;12:1129–59. [PMC free article] [PubMed] [Google Scholar]
- 15.Bode AM, Dong Z. Cancer prevention research - then and now. Nat Rev Cancer. 2009;9:508–16. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman JE, Grandis J. Oral Cancer Chemoprevention--The End of EPOC, the Beginning of an Epoch of Molecular Selection. JAMA Oncol. 2016;2:178–9. doi: 10.1001/jamaoncol.2015.4637. [DOI] [PubMed] [Google Scholar]
- 17.Le Magnen C, Dutta A, Abate-Shen C. Optimizing mouse models for precision cancer prevention. Nat Rev Cancer. 2016;16:187–96. doi: 10.1038/nrc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maresso KC, Tsai KY, Brown PH, Szabo E, Lippman S, Hawk ET. Molecular cancer prevention: Current status and future directions. CA Cancer J Clin. 2015;65:345–83. doi: 10.3322/caac.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spira A, Yurgelun MB, Alexandrov L, et al. Precancer Atlas to Drive Precision Prevention Trials. Cancer Res. 2017;77:1510–41. doi: 10.1158/0008-5472.CAN-16-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Poynard T, Colombo M, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–9. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–9. doi: 10.1053/j.gastro.2010.11.050. quiz e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. International Interferon-alpha Hepatocellular Carcinoma Study Group. Lancet. 1998;351:1535–9. [PubMed] [Google Scholar]
- 23.Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191–202. doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79–90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimbach J, Kulik LM, Finn R, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2017 doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 26.Meyskens FL, Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila) 2011;4:311–23. doi: 10.1158/1940-6207.CAPR-09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol. 2016;65:1148–54. doi: 10.1016/j.jhep.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med. 2017 doi: 10.1016/j.amjmed.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Cadier B, Bulsei J, Nahon P, et al. Early detection and curative treatment of hepatocellular carcinoma: A cost-effectiveness analysis in France and in the United States. Hepatology. 2017;65:1237–48. doi: 10.1002/hep.28961. [DOI] [PubMed] [Google Scholar]
- 31.Mourad A, Deuffic-Burban S, Ganne-Carrie N, et al. Hepatocellular carcinoma screening in patients with compensated hepatitis C virus (HCV)-related cirrhosis aware of their HCV status improves survival: a modeling approach. Hepatology. 2014;59:1471–81. doi: 10.1002/hep.26944. [DOI] [PubMed] [Google Scholar]
- 32.Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140–51. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singal AG, Tiro JA, Marrero JA, et al. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology. 2017;152:608–15 e4. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goossens N, Bian CB, Hoshida Y. Tailored algorithms for hepatocellular carcinoma surveillance: Is one-size-fits-all strategy outdated? Curr Hepatol Rep. 2017;16:64–71. doi: 10.1007/s11901-017-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65:1196–205. doi: 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germani G, Hytiroglou P, Fotiadu A, Burroughs AK, Dhillon AP. Assessment of fibrosis and cirrhosis in liver biopsies: an update. Semin Liver Dis. 2011;31:82–90. doi: 10.1055/s-0031-1272836. [DOI] [PubMed] [Google Scholar]
- 37.Tsochatzis E, Bruno S, Isgro G, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60:948–54. doi: 10.1016/j.jhep.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, de Boer WB, Adams LA, MacQuillan G, Bulsara MK, Jeffrey GP. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J Hepatol. 2014;61:22–7. doi: 10.1016/j.jhep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Wang TH, Chen TC, Teng X, Liang KH, Yeh CT. Automated biphasic morphological assessment of hepatitis B-related liver fibrosis using second harmonic generation microscopy. Sci Rep. 2015;5:12962. doi: 10.1038/srep12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suk KT, Kim EJ, Kim DJ, et al. Prognostic Significance of Hemodynamic and Clinical Stages in the Prediction of Hepatocellular Carcinoma. Journal of clinical gastroenterology. 2017;51:285–93. doi: 10.1097/MCG.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 41.Motosugi U, Ichikawa T, Koshiishi T, et al. Liver stiffness measured by magnetic resonance elastography as a risk factor for hepatocellular carcinoma: a preliminary case-control study. European radiology. 2013;23:156–62. doi: 10.1007/s00330-012-2571-6. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573–84 e1-2. doi: 10.1016/j.cgh.2013.07.034. quiz e88-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin SH, Kim SU, Park JY, et al. Liver stiffness-based model for prediction of hepatocellular carcinoma in chronic hepatitis B virus infection: comparison with histological fibrosis. Liver Int. 2015;35:1054–62. doi: 10.1111/liv.12621. [DOI] [PubMed] [Google Scholar]
- 44.Wang JH, Yen YH, Yao CC, et al. Liver stiffness-based score in hepatoma risk assessment for chronic hepatitis C patients after successful antiviral therapy. Liver Int. 2016;36:1793–9. doi: 10.1111/liv.13179. [DOI] [PubMed] [Google Scholar]
- 45.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 46.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–65. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu J, Lin Y, Guo Z, Niu M, Su C. The Epidemiological Investigation on the Risk Factors of Hepatocellular Carcinoma: A Case-Control Study in Southeast China. Medicine. 2016;95:e2758. doi: 10.1097/MD.0000000000002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-related Death in Men with Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Zhong JH, You XM, Gong WF, et al. Epidermal growth factor gene polymorphism and risk of hepatocellular carcinoma: a meta-analysis. PLoS One. 2012;7:e32159. doi: 10.1371/journal.pone.0032159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nahon P, Sutton A, Rufat P, et al. A variant in myeloperoxidase promoter hastens the emergence of hepatocellular carcinoma in patients with HCV-related cirrhosis. J Hepatol. 2012;56:426–32. doi: 10.1016/j.jhep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Zhai Y, Hu Z, et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755–8. doi: 10.1038/ng.638. [DOI] [PubMed] [Google Scholar]
- 52.Jiang DK, Sun J, Cao G, et al. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet. 2013;45:72–5. doi: 10.1038/ng.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar V, Kato N, Urabe Y, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455–8. doi: 10.1038/ng.809. [DOI] [PubMed] [Google Scholar]
- 54.Miki D, Ochi H, Hayes CN, et al. Variation in the DEPDC5 locus is associated with progression to hepatocellular carcinoma in chronic hepatitis C virus carriers. Nat Genet. 2011 doi: 10.1038/ng.876. [DOI] [PubMed] [Google Scholar]
- 55.Huang CF, Huang CI, Yeh ML, et al. Diversity of the association of serum levels and genetic variants of MHC class I polypeptide-related chain A with liver fibros is in chronic hepatitis C. Oncotarget. 2017;8:32618–25. doi: 10.18632/oncotarget.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, Qiao K, Trieu C, et al. Genetic Polymorphism of Epidermal Growth Factor rs4444903 Influences Susceptibility to HCV-Related Liver Cirrhosis and Hepatocellular Carcinoma in a Chinese Han Population. Clin Lab. 2017;63:845–50. doi: 10.7754/Clin.Lab.2016.161203. [DOI] [PubMed] [Google Scholar]
- 57.Trepo E, Romeo S, Zucman-Rossi J, Nahon P. PNPLA3 gene in liver diseases. J Hepatol. 2016;65:399–412. doi: 10.1016/j.jhep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Donati B, Pietrelli A, Pingitore P, et al. Telomerase reverse transcriptase germline mutations and hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancer Med. 2017 doi: 10.1002/cam4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donati B, Dongiovanni P, Romeo S, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuura K, Sawai H, Ikeo K, et al. Genome-Wide Association Study Identifies TLL1 Variant Associated With Development of Hepatocellular Carcinoma After Eradication of Hepatitis C Virus Infection. Gastroenterology. 2017;152:1383–94. doi: 10.1053/j.gastro.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 61.Besheer T, El-Bendary M, Elalfy H, et al. Prediction of Fibrosis Progression Rate in Patients with Chronic Hepatitis C Genotype 4: Role of Cirrhosis Risk Score and Host Factors. J Interferon Cytokine Res. 2017;37:97–102. doi: 10.1089/jir.2016.0111. [DOI] [PubMed] [Google Scholar]
- 62.Duarte-Salles T, Misra S, Stepien M, et al. Circulating Osteopontin and Prediction of Hepatocellular Carcinoma Development in a Large European Population. Cancer Prev Res (Phila) 2016;9:758–65. doi: 10.1158/1940-6207.CAPR-15-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazziotti G, Sorvillo F, Morisco F, et al. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer. 2002;95:2539–45. doi: 10.1002/cncr.11002. [DOI] [PubMed] [Google Scholar]
- 64.Verhelst X, Vanderschaeghe D, Castera L, et al. A Glycomics-Based Test Predicts the Development of Hepatocellular Carcinoma in Cirrhosis. Clin Cancer Res. 2017;23:2750–8. doi: 10.1158/1078-0432.CCR-16-1500. [DOI] [PubMed] [Google Scholar]
- 65.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–8. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4:256–69. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1927–33 e2. doi: 10.1016/j.cgh.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 70.Simmons O, Fetzer D, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2017;45:169–77. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song PP, Xia JF, Inagaki Y, et al. Controversies regarding and perspective s on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016;22:262–74. doi: 10.3748/wjg.v22.i1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M, Devarajan K, Singal AG, et al. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev Res (Phila) 2016;9:172–9. doi: 10.1158/1940-6207.CAPR-15-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson PJ, Pirrie SJ, Cox TF, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–53. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 74.Yang JD, Dai J, Singal AG, et al. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol Biomarkers Prev. 2017;26:1085–92. doi: 10.1158/1055-9965.EPI-16-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–7. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Pocha C, Dieperink E, McMaken K, Knott A, Thuras P, Ho S. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography–a randomised study. Alimentary pharmacology & therapeutics. 2013;38:303–12. doi: 10.1111/apt.12370. [DOI] [PubMed] [Google Scholar]
- 77.Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdominal Radiology. 2016:1–12. doi: 10.1007/s00261-016-0841-5. [DOI] [PubMed] [Google Scholar]
- 79.Furlan A, Marin D, Agnello F, et al. Hepatocellular Carcinoma Presenting at Contrast-Enhanced Multi–Detector-Row Computed Tomography or Gadolinium-Enhanced Magnetic Resonance Imaging as a Small (≤ 2 cm), Indeterminate Nodule: Growth Rate and Optimal Interval Time for Imaging Follow-Up. Journal of computer assisted tomography. 2012;36:20–5. doi: 10.1097/RCT.0b013e31823ed462. [DOI] [PubMed] [Google Scholar]
- 80.Taouli B, Goh JS, Lu Y, et al. Growth rate of hepatocellular carcinoma: evaluation with serial computed tomography or magnetic resonance imaging. Journal of computer assisted tomography. 2005;29:425–9. doi: 10.1097/01.rct.0000164036.85327.05. [DOI] [PubMed] [Google Scholar]
- 81.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. Journal of hepatology. 2010;53:291–7. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–97. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 83.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology*†. CA: a cancer journal for clinicians. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 84.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Annals of internal medicine. 2008;148:337–47. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bolutayo K, van Manh AL, Cohen N, Ndiaye D, Jandorf L, Perumalswami PV. Reducing Liver Cancer Risk in African-Born Immigrants Through Culturally Targeted Hepatitis B Group Education Programs. J Cancer Educ. 2017 doi: 10.1007/s13187-017-1231-6. [DOI] [PubMed] [Google Scholar]
- 86.Goossens N, Singal AG, King LY, et al. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. doi: 10.1038/ctg.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534–45. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chu YJ, Yang HI, Wu HC, et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141:711–20. doi: 10.1002/ijc.30782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng C, Zeng H, Lin H, et al. Serum microcystins level positively linked with risk of hepatocellular carcinoma: a case-control study in Southwest China. Hepatology. 2017 doi: 10.1002/hep.29310. [DOI] [PubMed] [Google Scholar]
- 90.Abbas Z, Abbas M, Abbas S, Shazi L. Hepatitis D and hepatocellular carcinoma. World journal of hepatology. 2015;7:777–86. doi: 10.4254/wjh.v7.i5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84–101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 92.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 93.Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–43. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Livingston SE, Simonetti JP, McMahon BJ, et al. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5–11. doi: 10.1086/509894. [DOI] [PubMed] [Google Scholar]
- 95.Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012;57:730–5. doi: 10.1016/j.jhep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 96.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–55. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 97.Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265–71. doi: 10.1111/j.1365-2893.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 98.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 99.Yuen MF, Seto WK, Chow DH, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–303. [PubMed] [Google Scholar]
- 100.Matsumoto A, Tanaka E, Rokuhara A, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–84. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–67. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Su TH, Hu TH, Chen CY, et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755–64. doi: 10.1111/liv.13253. [DOI] [PubMed] [Google Scholar]
- 103.Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631–8. doi: 10.1002/cncr.29537. [DOI] [PubMed] [Google Scholar]
- 104.Liang KH, Hsu CW, Chang ML, Chen YC, Lai MW, Yeh CT. Peginterferon Is Superior to Nucleos(t)ide Analogues for Prevention of Hepatocellular Carcinoma in Chronic Hepatitis B. J Infect Dis. 2016;213:966–74. doi: 10.1093/infdis/jiv547. [DOI] [PubMed] [Google Scholar]
- 105.Sun P, Dong X, Cheng X, Hu Q, Zheng Q. Nucleot(s)ide analogues for hepatitis B virus-related hepatocellular carcinoma after curative treatment: a systematic review and meta-analysis. PLoS One. 2014;9:e102761. doi: 10.1371/journal.pone.0102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261:56–66. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 107.Cho JY, Paik YH, Sohn W, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014;63:1943–50. doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- 108.Kim JH, Sinn DH, Kang W, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335–43. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 109.Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 110.Torres HA, Shigle TL, Hammoudi N, et al. The oncologic burden of hepatitis C virus infection: A clinical perspective. CA Cancer J Clin. 2017 doi: 10.3322/caac.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baumert TF, Juhling F, Ono A, Hoshida Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017;15:52. doi: 10.1186/s12916-017-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waziry R, Grebely J, Amin J, et al. Trends in hepatocellular carcinoma among people with HBV or HCV notification in Australia (2000-2014) J Hepatol. 2016 doi: 10.1016/j.jhep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Sievert W, Razavi H, Estes C, et al. Enhanced antiviral treatment efficacy and uptake in preventing the rising burden of hepatitis C-related liver disease and costs in Australia. J Gastroenterol Hepatol. 2014;29(1):1–9. doi: 10.1111/jgh.12677. [DOI] [PubMed] [Google Scholar]
- 114.Harris RJ, Thomas B, Griffiths J, et al. Increased uptake and new therapies are needed to avert rising hepatitis C-related end stage liver disease in England: Modelling the predicted impact of treatment under different scenarios. J Hepatol. 2014;61:530–7. doi: 10.1016/j.jhep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Nasrullah M, Sergeenko D, Gamkrelidze A, Averhoff F. HCV elimination - lessons learned from a small Eurasian country, Georgia. Nat Rev Gastroenterol Hepatol. 2017;14:447–8. doi: 10.1038/nrgastro.2017.100. [DOI] [PubMed] [Google Scholar]
- 116.Stasi C, Silvestri C, Voller F, Cipriani F. The epidemiological changes of HCV and HBV infections in the era of new antiviral therapies and the anti-HBV vaccine. J Infect Public Health. 2016;9:389–95. doi: 10.1016/j.jiph.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 117.Chhatwal J, Wang X, Ayer T, et al. Hepatitis C Disease Burden in the United States in the Era of Oral Direct-Acting Antivirals. Hepatology. 2016 doi: 10.1002/hep.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fauvelle C, Colpitts CC, Keck ZY, Pierce BG, Foung SK, Baumert TF. Hepatitis C virus vaccine candidates inducing protective neutralizing antibodies. Expert Rev Vaccines. 2016:1–10. doi: 10.1080/14760584.2016.1194759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Billerbeck E, Wolfisberg R, Fahnoe U, et al. Mouse models of acute and chronic hepacivirus infection. Science. 2017;357:204–8. doi: 10.1126/science.aal1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99–105. doi: 10.1016/j.coviro.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samur S, Kues B, Ayer T, et al. Cost-Effectiveness of Pre versus Post Liver Transplant Hepatitis C Treatment with Direct-Acting Antivirals. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asselah T, Perumalswami PV, Dieterich D. Is screening baby boomers for HCV enough? A call to screen for hepatitis C virus in persons from countries of high endemicity. Liver Int. 2014;34:1447–51. doi: 10.1111/liv.12599. [DOI] [PubMed] [Google Scholar]
- 123.Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–7. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 124.Nahon P, Bourcier V, Layese R, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142–56 e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 125.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct Acting Antiviral Agents. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 126.Ono A, Goossens N, Finn RS, et al. Persisting risk of hepatocellular carcinoma after HCV cure monitored by a liver transcriptome signature. Hepatology. 2017 doi: 10.1002/hep.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aydin Y, Chatterjee A, Chandra PK, et al. Interferon-alpha-induced hepatitis C virus clearance restores p53 tumor suppressor more than direct-acting antivirals. Hepatol Commun. 2017 doi: 10.1002/hep4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.La Vecchia C, Bosetti C, Bertuccio P, Castro C, Pelucchi C, Negri E. Trends in alcohol consumption in Europe and their impact on major alcohol-related cancers. Eur J Cancer Prev. 2014;23:319–22. doi: 10.1097/CEJ.0b013e32836562f1. [DOI] [PubMed] [Google Scholar]
- 129.Turati F, Galeone C, Rota M, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25:1526–35. doi: 10.1093/annonc/mdu020. [DOI] [PubMed] [Google Scholar]
- 130.Stickel F. Alcoholic cirrhosis and hepatocellular carcinoma. Adv Exp Med Biol. 2015;815:113–30. doi: 10.1007/978-3-319-09614-8_7. [DOI] [PubMed] [Google Scholar]
- 131.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–17. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 132.Vandenbulcke H, Moreno C, Colle I, et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J Hepatol. 2016;65:543–51. doi: 10.1016/j.jhep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 133.Totoki Y, Tatsuno K, Covington KR, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–73. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 134.Heckley GA, Jarl J, Asamoah BO, U GG. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11:446. doi: 10.1186/1471-2407-11-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Day CP. Treatment of alcoholic liver disease. Liver Transpl. 2007;13:S69–75. doi: 10.1002/lt.21336. [DOI] [PubMed] [Google Scholar]
- 136.La Vecchia C. Alcohol and liver cancer. Eur J Cancer Prev. 2007;16:495–7. doi: 10.1097/CEJ.0b013e3280145b5d. [DOI] [PubMed] [Google Scholar]
- 137.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 138.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–48. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 141.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124–31 e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–45. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 145.Nakagawa H, Umemura A, Taniguchi K, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–43. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 147.Kettner NM, Voicu H, Finegold MJ, et al. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30:909–24. doi: 10.1016/j.ccell.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–7. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomes AL, Teijeiro A, Buren S, et al. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2016;30:161–75. doi: 10.1016/j.ccell.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 150.Loo TM, Kamachi F, Watanabe Y, et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer discovery. 2017;7:522–38. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 151.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017 doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asgharpour A, Cazanave SC, Pacana T, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579–88. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Teufel A, Itzel T, Erhart W, et al. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues From Patients. Gastroenterology. 2016;151:513–25 e0. doi: 10.1053/j.gastro.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 154.Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031–42. doi: 10.1053/j.gastro.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 155.Wen CP, Lin J, Yang YC, et al. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J Natl Cancer Inst. 2012;104:1599–611. doi: 10.1093/jnci/djs372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cao Z, Fan-Minogue H, Bellovin DI, et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71:2286–97. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roudier E, Mistafa O, Stenius U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Molecular cancer therapeutics. 2006;5:2706–15. doi: 10.1158/1535-7163.MCT-06-0352. [DOI] [PubMed] [Google Scholar]
- 158.Ghalali A, Martin-Renedo J, Hogberg J, Stenius U. Atorvastatin Decreases HBx-Induced Phospho-Akt in Hepatocytes via P2X Receptors. Mol Cancer Res. 2017;15:714–22. doi: 10.1158/1541-7786.MCR-16-0373. [DOI] [PubMed] [Google Scholar]
- 159.Relja B, Meder F, Wang M, et al. Simvastatin modulates the adhesion and growth of hepatocellular carcinoma cells via decrease of integrin expression and ROCK. Int J Oncol. 2011;38:879–85. doi: 10.3892/ijo.2010.892. [DOI] [PubMed] [Google Scholar]
- 160.Wang J, Tokoro T, Higa S, Kitajima I. Anti-inflammatory effect of pitavastatin on NF-kappaB activated by TNF-alpha in hepatocellular carcinoma cells. Biological & pharmaceutical bulletin. 2006;29:634–9. doi: 10.1248/bpb.29.634. [DOI] [PubMed] [Google Scholar]
- 161.Higashi T, Hayashi H, Kitano Y, et al. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33:123. doi: 10.1007/s12032-016-0845-6. [DOI] [PubMed] [Google Scholar]
- 162.Yang PM, Liu YL, Lin YC, Shun CT, Wu MS, Chen CC. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 2010;70:7699–709. doi: 10.1158/0008-5472.CAN-10-1626. [DOI] [PubMed] [Google Scholar]
- 163.Sutter AP, Maaser K, Hopfner M, Huether A, Schuppan D, Scherubl H. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J Hepatol. 2005;43:808–16. doi: 10.1016/j.jhep.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 164.Kah J, Wustenberg A, Keller AD, et al. Selective induction of apoptosis by HMG-CoA reductase inhibitors in hepatoma cells and dependence on p53 expression. Oncol Rep. 2012;28:1077–83. doi: 10.3892/or.2012.1860. [DOI] [PubMed] [Google Scholar]
- 165.Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PLoS One. 2013;8:e76538. doi: 10.1371/journal.pone.0076538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chong LW, Hsu YC, Lee TF, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15:22. doi: 10.1186/s12876-015-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Marrone G, Russo L, Rosado E, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 168.Marinho TS, Kawasaki A, Bryntesson M, et al. Rosuvastatin limits the activation of hepatic stellate cells in diet-induced obese mice. Hepatol Res. 2016 doi: 10.1111/hepr.12821. [DOI] [PubMed] [Google Scholar]
- 169.Uschner FE, Ranabhat G, Choi SS, et al. Statins activate the canonical hedgehog-signaling and aggravate non-cirrhotic portal hypertension, but inhibit the non-canonical hedgehog signaling and cirrhotic portal hypertension. Sci Rep. 2015;5:14573. doi: 10.1038/srep14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim G, Jang SY, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int J Cancer. 2017;140:798–806. doi: 10.1002/ijc.30506. [DOI] [PubMed] [Google Scholar]
- 171.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623–30. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 172.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514–21. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 173.Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47–57. doi: 10.1002/hep.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017 doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 176.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically non-cirrhotic non-alcoholic fatty liver disease. Int J Cancer. 2017 doi: 10.1002/ijc.30784. [DOI] [PubMed] [Google Scholar]
- 177.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–32. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 178.Cholesterol Treatment Trialists C. Emberson JR, Kearney PM, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou YY, Zhu GQ, Wang Y, et al. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7:21753–62. doi: 10.18632/oncotarget.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zheng L, Yang W, Wu F, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372–80. doi: 10.1158/1078-0432.CCR-13-0203. [DOI] [PubMed] [Google Scholar]
- 181.Zhou X, Chen J, Yi G, et al. Metformin suppresses hypoxia-induced stabilization of HIF-1alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7:873–84. doi: 10.18632/oncotarget.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–15. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 183.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 184.Tsai HH, Lai HY, Chen YC, et al. Metformin promotes apoptosis in hepatocellular carcinoma through the CEBPD-induced autophagy pathway. Oncotarget. 2017;8:13832–45. doi: 10.18632/oncotarget.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DePeralta DK, Wei L, Ghoshal S, et al. Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer. 2016;122:1216–27. doi: 10.1002/cncr.29912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine. 2017;96:e6888. doi: 10.1097/MD.0000000000006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–5. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Soberg S, Sandholt CH, Jespersen NZ, et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017;25:1045–53 e6. doi: 10.1016/j.cmet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 189.von Holstein-Rathlou S, BonDurant LD, Peltekian L, et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016;23:335–43. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu X, Zhang P, Martin RC, et al. Lack of fibroblast growth factor 21 accelerates metabolic liver injury characterized by steatohepatities in mice. Am J Cancer Res. 2016;6:1011–25. [PMC free article] [PubMed] [Google Scholar]
- 191.Gong Q, Hu Z, Zhang F, et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64:425–38. doi: 10.1002/hep.28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee JH, Kang YE, Chang JY, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8:4750–63. [PMC free article] [PubMed] [Google Scholar]
- 193.Hou J, Zhou Y, Zheng Y, et al. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25:49–63. doi: 10.1016/j.ccr.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 194.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer Prev Res (Phila) 2015;8:1156–62. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hu KQ. Cyclooxygenase 2 (COX2)-prostanoid pathway and liver diseases. Prostaglandins Leukot Essent Fatty Acids. 2003;69:329–37. doi: 10.1016/j.plefa.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 198.Chen H, Cai W, Chu ES, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017 doi: 10.1038/onc.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xiong H, Li B, He J, Zeng Y, Zhang Y, He F. lncRNA HULC promotes the growth of hepatocellular carcinoma cells via stabilizing COX-2 protein. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.06.103. [DOI] [PubMed] [Google Scholar]
- 200.Xu Y, Zhao W, Xu J, et al. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget. 2016;7:8866–78. doi: 10.18632/oncotarget.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee SJ, Hwang JW, Yim H, et al. Synergistic effect of simvastatin plus NS398 on inhibition of proliferation and survival in hepatocellular carcinoma cell line. J Gastroenterol Hepatol. 2014;29:1299–307. doi: 10.1111/jgh.12503. [DOI] [PubMed] [Google Scholar]
- 202.Takami Y, Eguchi S, Tateishi M, et al. A randomised controlled trial of meloxicam, a Cox-2 inhibitor, to prevent hepatocellular carcinoma recurrence after initial curative treatment. Hepatol Int. 2016;10:799–806. doi: 10.1007/s12072-016-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–91 e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 204.Qiu SJ, Zhou ZG, Shen F, et al. A multicenter, randomized, observation-controlled clinical trial to evaluate the efficacy and safety of thymalfasin adjuvant therapy in patients with HBV-related HCC after curative resection - first announcement of the protocol. Expert Opin Biol Ther. 2015;15(1):S133–7. doi: 10.1517/14712598.2015.1039979. [DOI] [PubMed] [Google Scholar]
- 205.Friedman SL, Ratziu V, Harrison SA, et al. A Randomized, Placebo-Controlled Trial of Cenicriviroc for Treatment of Nonalcoholic Steatohepatitis with Fibrosis. Hepatology. 2017 doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–59 e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 207.Duarte-Salles T, Fedirko V, Stepien M, et al. Dietary fat, fat subtypes and hepatocellular carcinoma in a large European cohort. Int J Cancer. 2015;137:2715–28. doi: 10.1002/ijc.29643. [DOI] [PubMed] [Google Scholar]
- 208.Sawada N, Inoue M, Iwasaki M, et al. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468–75. doi: 10.1053/j.gastro.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 209.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Molecular cancer therapeutics. 2009;8:3046–55. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weylandt KH, Krause LF, Gomolka B, et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis. 2011;32:897–903. doi: 10.1093/carcin/bgr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Freedman ND, Cross AJ, McGlynn KA, et al. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354–65. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fedirko V, Duarte-Salles T, Bamia C, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60:1222–30. doi: 10.1002/hep.27079. [DOI] [PubMed] [Google Scholar]
- 213.Wong GL, Chan HL, Chan HY, et al. Adverse effects of vitamin D deficiency on outcomes of patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2015;13:783–90 e1. doi: 10.1016/j.cgh.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 214.Kwon HJ, Won YS, Suh HW, et al. Vitamin D3 upregulated protein 1 suppresses TNF-alpha-induced NF-kappaB activation in hepatocarcinogenesis. J Immunol. 2010;185:3980–9. doi: 10.4049/jimmunol.1000990. [DOI] [PubMed] [Google Scholar]
- 215.Li Q, Gao Y, Jia Z, et al. Dysregulated Kruppel-like factor 4 and vitamin D receptor signaling contribute to progression of hepatocellular carcinoma. Gastroenterology. 2012;143:799–810 e1-2. doi: 10.1053/j.gastro.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Duran A, Hernandez ED, Reina-Campos M, et al. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Preziosi ME, Singh S, Valore EV, et al. Mice lacking liver-specific beta-catenin develop steatohepatitis and fibrosis after iron overload. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kato J, Miyanishi K, Kobune M, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830–6. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 219.Saeki I, Yamamoto N, Yamasaki T, et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:8967–77. doi: 10.3748/wjg.v22.i40.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cha JH, Bae SH, Kim HL, et al. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8:e77899. doi: 10.1371/journal.pone.0077899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nakano M, Nakashima A, Nagano T, Ishikawa S, Kikkawa U, Kamada S. Branched-chain amino acids enhance premature senescence through mammalian target of rapamycin complex I-mediated upregulation of p21 protein. PLoS One. 2013;8:e80411. doi: 10.1371/journal.pone.0080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Korenaga M, Nishina S, Korenaga K, et al. Branched-chain amino acids reduce hepatic iron accumulation and oxidative stress in hepatitis C virus polyprotein-expressing mice. Liver Int. 2015;35:1303–14. doi: 10.1111/liv.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Takegoshi K, Honda M, Okada H, et al. Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget. 2017;8:18191–205. doi: 10.18632/oncotarget.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Terakura D, Shimizu M, Iwasa J, et al. Preventive effects of branched-chain amino acid supplementation on the spontaneous development of hepatic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Carcinogenesis. 2012;33:2499–506. doi: 10.1093/carcin/bgs303. [DOI] [PubMed] [Google Scholar]
- 225.Kawaguchi T, Shiraishi K, Ito T, et al. Branched-chain amino acids prevent hepatocarcinogenesis and prolong survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1012–8 e1. doi: 10.1016/j.cgh.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 226.Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7:e013739. doi: 10.1136/bmjopen-2016-013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Aleksandrova K, Bamia C, Drogan D, et al. The association of coffee intake with liver cancer risk is mediated by biomarkers of inflammation and hepatocellular injury: data from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2015;102:1498–508. doi: 10.3945/ajcn.115.116095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xiao Q, Sinha R, Graubard BI, Freedman ND. Inverse associations of total and decaffeinated coffee with liver enzyme levels in National Health and Nutrition Examination Survey 1999-2010. Hepatology. 2014;60:2091–8. doi: 10.1002/hep.27367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bamia C, Lagiou P, Jenab M, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 2015;136:1899–908. doi: 10.1002/ijc.29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Edling CE, Selvaggi F, Ghonaim R, Maffucci T, Falasca M. Caffeine and the analog CGS 15943 inhibit cancer cell growth by targeting the phosphoinositide 3-kinase/Akt pathway. Cancer Biol Ther. 2014;15:524–32. doi: 10.4161/cbt.28018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017;60:1620–37. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Veldt BJ, Hansen BE, Ikeda K, Verhey E, Suzuki H, Schalm SW. Long-term clinical outcome and effect of glycyrrhizin in 1093 chronic hepatitis C patients with non-response or relapse to interferon. Scand J Gastroenterol. 2006;41:1087–94. doi: 10.1080/00365520600641365. [DOI] [PubMed] [Google Scholar]
- 233.Morgan TR, Osann K, Bottiglieri T, et al. A Phase II Randomized, Controlled Trial of S-Adenosylmethionine in Reducing Serum alpha-Fetoprotein in Patients with Hepatitis C Cirrhosis and Elevated AFP. Cancer Prev Res (Phila) 2015;8:864–72. doi: 10.1158/1940-6207.CAPR-15-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Duvoux C, Toso C. mTOR inhibitor therapy: Does it prevent HCC recurrence after liver transplantation? Transplant Rev (Orlando) 2015;29:168–74. doi: 10.1016/j.trre.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 235.Massoud O, Wiesner RH. The use of sirolimus should be restricted in liver transplantation. J Hepatol. 2012;56:288–90. doi: 10.1016/j.jhep.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 236.Umemura A, Park EJ, Taniguchi K, et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab. 2014;20:133–44. doi: 10.1016/j.cmet.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol. 2015;21:8753–68. doi: 10.3748/wjg.v21.i29.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116–25. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Binder BY, Williams PA, Silva EA, Leach JK. Lysophosphatidic Acid and Sphingosine-1-Phosphate: A Concise Review of Biological Function and Applications for Tissue Engineering. Tissue Eng Part B Rev. 2015;21:531–42. doi: 10.1089/ten.teb.2015.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Erstad DJ, Tager AM, Hoshida Y, Fuchs BC. The autotaxin-lysophosphatidic acid pathway emerges as a therapeutic target to prevent liver cancer. Mol Cell Oncol. 2017;4:e1311827. doi: 10.1080/23723556.2017.1311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kaffe E, Katsifa A, Xylourgidis N, et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology. 2017;65:1369–83. doi: 10.1002/hep.28973. [DOI] [PubMed] [Google Scholar]
- 242.Oakley F, Teoh V, Ching ASG, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology. 2009;136:2334–44 e1. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 243.Moreno M, Gonzalo T, Kok RJ, et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942–52. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- 244.Tamaki Y, Nakade Y, Yamauchi T, et al. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J Gastroenterol. 2013;48:491–503. doi: 10.1007/s00535-012-0651-7. [DOI] [PubMed] [Google Scholar]
- 245.Facciorusso A, Del Prete V, Crucinio N, et al. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J Gastroenterol Hepatol. 2015;30:1643–50. doi: 10.1111/jgh.12988. [DOI] [PubMed] [Google Scholar]
- 246.Yoshiji H, Noguchi R, Toyohara M, et al. Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma. J Hepatol. 2009;51:315–21. doi: 10.1016/j.jhep.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 247.Yoshiji H, Noguchi R, Ikenaka Y, et al. Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: a randomized control trial. Oncol Rep. 2011;26:1547–53. doi: 10.3892/or.2011.1433. [DOI] [PubMed] [Google Scholar]
- 248.Tan CK. Peretinoin as an adjuvant therapy for hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2016;10:1201–10. doi: 10.1080/17474124.2016.1238303. [DOI] [PubMed] [Google Scholar]
- 249.Okada H, Honda M, Campbell JS, et al. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0028. [DOI] [PubMed] [Google Scholar]
- 250.Guan HB, Nie YZ, Zheng YW, et al. Acyclic retinoid induces differentiation and apoptosis of murine hepatic stem cells. Stem Cell Res Ther. 2015;6:51. doi: 10.1186/s13287-015-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shimakami T, Honda M, Shirasaki T, et al. The acyclic retinoid Peretinoin inhibits hepatitis C virus replication and infectious virus release in vitro. Sci Rep. 2014;4:4688. doi: 10.1038/srep04688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Okada H, Takabatake R, Honda M, et al. Peretinoin, an acyclic retinoid, suppresses steatohepatitis and tumorigenesis by activating autophagy in mice fed an atherogenic high-fat diet. Oncotarget. 2017;8:39978–93. doi: 10.18632/oncotarget.18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Okita K, Izumi N, Ikeda K, et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol. 2015;50:667–74. doi: 10.1007/s00535-014-0996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lanaya H, Natarajan A, Komposch K, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nature cell biology. 2014;16:972–81. 1–7. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Komposch K, Sibilia M. EGFR Signaling in Liver Diseases. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–90. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 258.Zheng B, Zhu YJ, Wang HY, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575–84. doi: 10.1007/s11427-016-9043-9. [DOI] [PubMed] [Google Scholar]
- 259.Sun H, Deng Q, Pan Y, et al. Association between estrogen receptor 1 (ESR1) genetic variations and cancer risk: a meta-analysis. J BUON. 2015;20:296–308. [PubMed] [Google Scholar]
- 260.Hassan MM, Botrus G, Abdel-Wahab R, et al. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zheng F, Liao YJ, Cai MY, et al. Systemic delivery of microRNA-101 potently inhibits hepatocellular carcinoma in vivo by repressing multiple targets. PLoS genetics. 2015;11:e1004873. doi: 10.1371/journal.pgen.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339–45. doi: 10.1016/j.jhep.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 263.Yang HI, Yuen MF, Chan HL, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–74. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 264.Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660–5. doi: 10.1200/JCO.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 265.Yang HI, Sherman M, Su J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol. 2010;28:2437–44. doi: 10.1200/JCO.2009.27.4456. [DOI] [PubMed] [Google Scholar]
- 266.Hung YC, Lin CL, Liu CJ, et al. Development of risk scoring system for stratifying population for hepatocellular carcinoma screening. Hepatology. 2015;61:1934–44. doi: 10.1002/hep.27610. [DOI] [PubMed] [Google Scholar]
- 267.Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–6. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 268.Sohn W, Cho JY, Kim JH, et al. Risk score model for the development of hepatocellular carcinoma in treatment-naive patients receiving oral antiviral treatment for chronic hepatitis B. Clinical and molecular hepatology. 2017;23:170–8. doi: 10.3350/cmh.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology. 2015;61:1261–8. doi: 10.1002/hep.27654. [DOI] [PubMed] [Google Scholar]
- 270.Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–8. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 271.Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–9. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 272.Singal AG, Mukherjee A, Elmunzer BJ, et al. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723–30. doi: 10.1038/ajg.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee MH, Lu SN, Yuan Y, et al. Development and validation of a clinical scoring system for predicting risk of HCC in asymptomatic individuals seropositive for anti-HCV antibodies. PLoS One. 2014;9:e94760. doi: 10.1371/journal.pone.0094760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ganne-Carrie N, Layese R, Bourcier V, et al. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir) Hepatology. 2016;64:1136–47. doi: 10.1002/hep.28702. [DOI] [PubMed] [Google Scholar]
- 275.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249–55 e1. doi: 10.1053/j.gastro.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chang KC, Wu YY, Hung CH, et al. Clinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapy. Br J Cancer. 2013;109:2481–8. doi: 10.1038/bjc.2013.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ikeda M, Fujiyama S, Tanaka M, et al. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–56. doi: 10.1007/s00535-004-1519-2. [DOI] [PubMed] [Google Scholar]
- 279.Chang KC, Hung CH, Lu SN, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. The Journal of antimicrobial chemotherapy. 2012;67:2766–72. doi: 10.1093/jac/dks269. [DOI] [PubMed] [Google Scholar]
- 280.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. 2014;120:3485–93. doi: 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–7. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 282.Aoki T, Iijima H, Tada T, et al. Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J Gastroenterol. 2017;52:104–12. doi: 10.1007/s00535-016-1228-7. [DOI] [PubMed] [Google Scholar]
- 283.Konerman MA, Zhang Y, Zhu J, Higgins PD, Lok AS, Waljee AK. Improvement of predictive models of risk of disease progression in chronic hepatitis C by incorporating longitudinal data. Hepatology. 2015;61:1832–41. doi: 10.1002/hep.27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lens S, Torres F, Puigvehi M, et al. Predicting the development of liver cirrhosis by simple modelling in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2016;43:364–74. doi: 10.1111/apt.13472. [DOI] [PubMed] [Google Scholar]
- 285.Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, et al. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int. 2017 doi: 10.1111/liv.13480. [DOI] [PubMed] [Google Scholar]
- 286.Irvine KM, Wockner LF, Shanker M, et al. The Enhanced liver fibrosis score is associated with clinical outcomes and disease progression in patients with chronic liver disease. Liver Int. 2016;36:370–7. doi: 10.1111/liv.12896. [DOI] [PubMed] [Google Scholar]
- 287.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang C, Hann HW, Ye Z, et al. Prospective evidence of a circulating microRNA signature as a non-invasive marker of hepatocellular carcinoma in HBV patients. Oncotarget. 2016 [Google Scholar]
- 289.Abu Dayyeh BK, Yang M, Fuchs BC, et al. A Functional Polymorphism in the Epidermal Growth Factor Gene Is Associated With Risk for Hepatocellular Carcinoma. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Guyot E, Sutton A, Rufat P, et al. PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J Hepatol. 2013;58:312–8. doi: 10.1016/j.jhep.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 291.Nahon P, Sutton A, Rufat P, et al. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology. 2008;134:102–10. doi: 10.1053/j.gastro.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 292.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hoshida Y, Villanueva A, Sangiovanni A, et al. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology. 2013;144:1024–30. doi: 10.1053/j.gastro.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.King LY, Canasto-Chibuque C, Johnson KB, et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut. 2015;64:1296–302. doi: 10.1136/gutjnl-2014-307862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang DY, Goossens N, Guo J, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65:1754–64. doi: 10.1136/gutjnl-2015-309655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ji J, Eggert T, Budhu A, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62:481–95. doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kim JH, Sohn BH, Lee HS, et al. Genomic predictors for recurrence patterns of hepatocellular carcinoma: model derivation and validation. PLoS Med. 2014;11:e1001770. doi: 10.1371/journal.pmed.1001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Marcolongo M, Young B, Dal Pero F, et al. A seven-gene signature (cirrhosis risk score) predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;50:1038–44. doi: 10.1002/hep.23111. [DOI] [PubMed] [Google Scholar]
- 299.Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jia X, Liu J, Gao Y, Huang Y, Du Z. Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: a systematic review with meta-analysis. Arch Med Res. 2014;45:580–8. doi: 10.1016/j.arcmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 301.Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–8. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 302.Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–26. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 303.Zhu WW, Guo JJ, Guo L, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19:3944–54. doi: 10.1158/1078-0432.CCR-12-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sun Y, Gao G, Cai J, et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis. 2013;34:595–604. doi: 10.1093/carcin/bgs372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Liu XE, Desmyter L, Gao CF, et al. N-glycomic changes in hepatocellul a r carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426–35. doi: 10.1002/hep.21855. [DOI] [PubMed] [Google Scholar]
- 306.Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–90. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Marrero JA, Romano PR, Nikolaeva O, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–12. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 308.Wang M, Sanda M, Comunale MA, et al. Changes in the Glycosylation of Kininogen and the Development of a Kininogen-Based Algorithm for the Early Detection of HCC. Cancer Epidemiol Biomarkers Prev. 2017;26:795–803. doi: 10.1158/1055-9965.EPI-16-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]


