Abstract
Objective
The Concussion in Sport Group guidelines recommend a multifaceted approach to help clinicians make return to sport decisions. The purpose of this study was to identify the most common multifaceted measures used to define clinical recovery from sport-related concussion in young athletes (high school and/or college level) and to summarise existing knowledge of criteria used to make return to sport decisions.
Design
Systematic review
Data Sources
The PubMed (MEDLINE), SPORTDiscus, and Embase electronic databases were searched from January 1, 2000 to March 1, 2017 by 3 independent reviewers.
Eligibility criteria
Inclusion criteria: elementary, high school and college age groups, and a specific definition of clinical recovery that required two or more measures. Exclusion criteria: review articles, articles using the same sample population, case studies, non-English language, and those that used one measure only or did not specify the recovery measures used.
Study quality
Study quality was assessed using the Downs and Black Criteria.
Results
Of 2,023 publications, 43 met inclusion criteria. Included articles reported the following measures of recovery: somatic symptom resolution or return to baseline (100%), cognitive recovery or return to baseline (86%), no exacerbation of symptoms on physical exertion (49%), normalisation of balance (30%), normal special physical examination (12%), successful return to school (5%), no exacerbation of symptoms with cognitive exertion (2%), and normalisation of cerebral blood flow (2%). Follow-up to validate the return to sport decision was reported in 8 (19%) articles. Most studies were case-control or cohort (Level of Evidence 4) and had significant risk of bias.
Conclusion
All studies of sport-related concussion use symptom reports to define recovery. A minority of studies used multiple measures of outcome or had clearly defined recovery criteria, the most common being a combination of a self-reported symptom checklist and a computerized neurocognitive test. Future studies ideally should define recovery a-priori using objective physiological measures in addition to symptom reports.
Prospero Systematic Review Registration
CRD42016032373
Key Terms: Sport-related concussion, recovery, mild traumatic brain injury, student athlete, return to play
Introduction
Concussion incidence is significant in contact sport and recreational activities.1 In 2006 1.8 to 3.8 million sport-related traumatic brain injuries were estimated to occur annually in the US. The majority of these are sport-related concussions.2 Although there is some ambiguity in the definitions of mild traumatic brain injury and concussion, the term concussion is generally used in sport-related injuries.3 Concussion occurs when sudden deceleration and rotational forces applied to the brain 4 trigger an acute and subacute pathophysiological metabolic response in the absence of gross brain lesions.5 Concussion results in somatic, cognitive and emotional symptoms, cognitive impairment, abnormal physical examination findings, behavioral issues, and sleep disturbance.6 Many patients with sport-related concussion recover within 7 – 10 days 7,8 although a recent study in adolescents, which defined recovery as normalisation of physiological, visual and balance function,9 reported that recovery typically required 3–4 weeks.
The most widely accepted guidelines for return to sport are the Concussion in Sport Group (CISG) guidelines. The most recent are from the Berlin 5th International Conference on Concussion in Sport that recommend a multifaceted evaluation to include physical examination, neuropsychological testing, and a graded return to activity to help determine recovery from concussion. The latest CISG guidelines state that athletes should return to a baseline level of symptoms but do not provide definitions to establish when an athlete is fully recovered physiologically and ready to return to sport. Resolution of symptoms is recognised as a critical part of recovery but symptom reporting alone is problematic because athletes often under-report symptoms,10 some concussion-related symptoms are reported in populations without concussion,11 and symptoms are not specific to concussion.12
While the CISG guidelines recommend that symptom and cognitive recovery must occur before athletes can return to sport, actual clinical practice may differ.13 Buckley et al.14 found that 65% of athletic trainers used a multifaceted assessment to establish recovery from sport-related concussion while 11% used only one or no assessment tool when deciding on return to sport. The most frequently used assessments were symptom reports (92%), clinical examination (86%), computerized neuropsychological testing (74%), balance testing (65%), and the Standard Assessment of Concussion (SAC) (54%).
An evidence-based definition of recovery from concussion is important given the risk of more severe consequences should repeat injury occur before resolution of the first concussion15 and increased awareness of possible long-term effects of concussion.16 The test-retest reliability and internal consistency of Immediate Post-concussion Assessment and Cognitive Testing (ImPACT),17 the Buffalo Concussion Treadmill Test (BCTT),18 and the Sport Concussion Assessment Tool (SCAT)19 have been reported on but not for other measures. The international consensus meetings on concussion in sport6,7 has stated that a single criterion may not be sufficient to define concussion recovery; hence, we included only research articles that used at least two measures to define recovery from sport-related concussion. We chose the age groups of elementary, high school and college athletes for this systematic review because of the abundance of research specific to them. There are not many publications on athletes older than college age or in athletes under the age of twelve. 20 The purpose of this systematic review was to summarise the criteria that have been used to define recovery after sport-related concussion. Study quality was assessed to identify areas of potential improvement for future studies.
Methods
This review was prospectively registered on 18 January 2016 in the PROSPERO database (registration number: CRD42016032373)21.
Selection criteria
We included articles published in English that described sport-related concussion and included elementary school, high school and college age athletes. Articles had to report the recovery criteria and use at least two measures. We included those articles that were published since 2000 because of the change of definition of concussion and recommendations for sport concussion management in 2001.22 Cohen’s Kappa was used to measure inter-rater reliability between reviewers for article selection.
Exclusion criteria: review articles, case reports, and articles that did not clearly define recovery measure(s) used. For articles reporting on the same sample population, we included the study that reported recovery measures. If all reported recovery measures, then only the earliest article was included. We excluded articles that measured the time it took for specific clinical symptoms to resolve or for cognition to return to baseline but did not state that the athlete had recovered or was ready to return to sport or to school since normalisation of symptoms is not the same as clinical recovery. We also excluded articles where a physician had documented recovery independent of the study but did not specify the basis for this clinical decision. We excluded articles that stated the CISG Guidelines were used but did not include sufficient detail about implementation of the return to sport protocol. Some patient samples were not exclusively sport-related concussion. Here, we identified the mechanism of injury and included the article only if the mechanism of injury for a majority (>50%) of subjects was sport-related concussion or similar to it and the participants received treatment similar to that used for sport-related concussion.
Literature search
We searched the PubMed (MEDLINE), SportDiscus and Embase electronic databases in March 2017. Search terms included: “Concussion AND recovery AND (athlete OR sport) AND (children OR youth OR teens OR teenagers OR college OR high school); Concussion AND symptoms AND (athlete OR sport) AND (children OR youth OR teens OR teenagers OR college OR high school); and Concussion AND resolution AND (athlete OR sport) AND (children OR youth OR teens OR teenagers OR college OR high school)”. Exact search syntax is provided. PRISMA23 flow chart was made.
Three reviewers independently screened the titles and abstracts of all articles identified in the electronic database search. If it was unclear from the title and/or abstract whether the article should be included, the full text of the article was obtained and independently screened by the 3 reviewers. Any discrepancies were resolved by consensus.
Data extraction
The following variables were independently extracted from each article by 3 reviewers: first author, year, study design, sample size, patient age, time to recovery, and the definition of recovery. Methods of assessing and conclusions pertaining to post-recovery follow-up were also extracted when reported.
Recovery measures were categorised as:
symptoms
cognitive performance at rest (computerised or paper-pen neurocognitive tests)
special physical examination
balance
symptom exacerbation during physical exertion
symptom exacerbation during cognitive exertion
ability to maintain academic performance
special tests (e.g. cerebral blood flow)
Risk of bias and level of evidence
We assessed risk of bias of included articles using the Downs and Black checklist for methodological quality24. Level of evidence was determined according to the guidelines by Melnyk et al.25 This system uses a seven level grading system that begins with systematic review of randomised controlled trials (Level 1) down to expert opinion (Level 7).
Results
Literature search
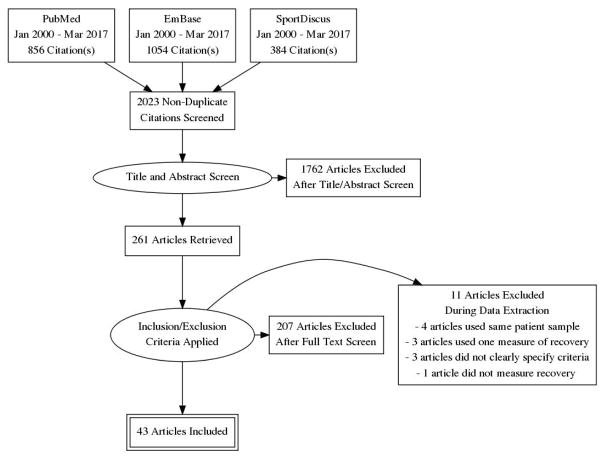
The literature search yielded a total of 2,294 articles (Figure 1). The titles and abstracts of 2,023 non-duplicate articles were screened and the full texts of 261 articles were evaluated. Two hundred and seven articles were excluded for not meeting recovery definition criteria and 11 articles were excluded during data extraction because their recovery criteria were not specific or were incomplete. Forty-three articles were included in this systematic review (Table 1). Cohen’s Kappa of inter-rater reliability for article selection was 0.61. Three articles had a minority (<50%) of subjects with concussion from non-sport activities. They were included because the authors specifically stated that the mechanism of injury and treatment of the non-sport-related concussion were similar to that of sport-related concussion.
Figure 1.
Table 1.
Study Characteristics
| First Author | Year | Study Design | Sample Size (Control Sample Size) | Age in years (Controls age in years) | Time to return to sport in days (Controls return to sport time) |
|---|---|---|---|---|---|
| Anzalone 91 | 2017 | Prospective cohort study | 167 | 15 ± 2 | 19.9 ± 13.4 |
| Baker 92 | 2016 | Retrospective cohort study | 147 | 15.4 ± 1.5 | 16.5 |
| Black 64 | 2017 | Retrospective chart review | 759 | 19.34 ± 1.81 | Median = 11 |
| Broglio 93 | 2016 | Prospective cohort study | 24 (21) | 16.7 ± 2.5 (17.1 ± 2.9) | 26.2 ± 42.8 |
| Brooks 51 | 2016 | Retrospective cohort study | 75 (182) | 19.3 ± 1.3 (19.9 ± 1.3) | 21.0 |
| Brown 90 | 2014 | Prospective cohort study | 335 | 15 ± 2.6 | 43 ± 53 |
| Buckley 47 | 2015 | Prospective cohort study | 25 (25) | 19.8 ± 1.2 (19.4 ± 1.3) | 6.8 ± 4.6 (7.2 ± 5.8) |
| Clausen 41 | 2016 | Prospective experimental study | 6 (13) | 23 ± 6 (21 ± 3) | Not mentioned |
| Collins 48 | 2006 | Prospective cohort study | 1173 (968)* | 16.3 ± 1.1 (15.9 ± 1.3) | 10.9 (13.0) |
| Corwin 89 | 2015 | Retrospective cohort study | 200 (47) | 14 (range= 7–18) | 106 (29) |
| Crowe 42 | 2016 | Prospective cohort study | 10 | 14.6 (range= 11–17) | 30 (except 1 case) |
| Darling 35 | 2014 | Retrospective chart review | 117 | 15.5 ± 1.6 | 16 ± 15 |
| Elbin 53 | 2016 | Prospective cohort study | 35 (34) | 15.35 ± 1.73 (15.61 ± 1.65) | 44.4 ± 36.0 (22.0 ± 18.7) |
| Field 94 | 2003 | Case control study | HS: 19 (20), college: 35 (18) | HS= 15.9 (range= 14–18), college= 19.9 (range= 17 – 25) | HS did not recover by 7, college recovered by 3 |
| Gill 54 | 2017 | Prospective cohort study | 46 (37) | 19.1 ± 1.18 (18.7 ± 0.67) | 21.68 ± 42.99 |
| Henry 37 | 2015 | Prospective cohort study | 66 | 16.5 ± 1.9 | 21 to 28 |
| Hutchison 55 | 2016 | Prospective cohort study | 26 (26) | 21 ± 2.5 | 34.7 ± 37.7 |
| Iverson 95 | 2007 | Prospective cohort study | 114 (55 simple, 59 complex) | 16.2 ± 1.2 (13 – 16) | “Simple”= 4.5 ± 2.1, “Complex”= 18.9 ± 8.6 |
| Kontos 49 | 2013 | Prospective cohort study | 138 | 15.96 ± 1.18 | 63% < 7 days, 37% >21 |
| Kriz 57 | 2016 | Prospective cohort study | 145 | 15.4 ± 1.5 | 44.5 ± 48.7 |
| Lau 32 | 2011 | Prospective cohort study | 107 | 16.02 ± 1.22 | 13.26 ± 9.05 |
| Lax 96 | 2015 | Prospective cohort study | 25 | 11.8 ± 1.44 | 20.13 ± 31.2 |
| Lovell 97 | 2003 | Case control study | 64 (24) | Not mentioned | Return to sport for most: 4 – 7 |
| Lovell 59 | 2007 | Case control Study | 28 (13) | 16.6 ± 4 (18.3 ± 3.5) | 33.3 ± 33.8 |
| Lynall 60 | 2016 | Retrospective cohort study | 34 | 18.38 ± 0.78 | Not mentioned |
| Maerlender 40 | 2015 | Randomized control trial | 13 (15) | Not mentioned | Median = 15 (median = 13) |
| Makdissi 61 | 2010 | Prospective cohort study | 78 | Median= 22 | 4.8 |
| Maugans 81 | 2012 | Prospective cohort study | 12 (12) | 13.4 (13.4) | 14 |
| Mautner 44 | 2015 | Case control study | 70 (70) | 15.5 (15.7) | 16.5 (13.5) |
| McClincy 62 | 2006 | Prospective cohort study | 104 | 16.11 ± 2.22 | 63% recovered by 14 days |
| McDevit 98 | 2015 | Prospective cohort study | 87 | 19.47 ± 6.02 | 55.73 ± 85.79 |
| Mcgrath 45 | 2013 | Retrospective cohort study | 54 | 15.46 ± 1.48 | 13.33 ± 8.87 |
| Meehan 50 | 2013 | Prospective cohort study | 182 | 15 ± 3.04 | 73.6% recovered in ≤28 |
| Miller 99 | 2016 | Case control study | 294 | ||
| Morgan 100 | 2015 | Case control study | 40 (80) | 14.9 ± 2.1 (14.8 ± 2.0) | Control recovered ≤3 weeks |
| Murugavel 56 | 2014 | Prospective cohort study | 21 (21) | 20.19 ± 1.03 (19.9 ± 1.67) | All were recovered 2 months post-injury |
| Nelson 65 | 2016 | Prospective cohort study | HS = 405 (89) College = 213 (61) |
HS = 16.04 ± 0.99 (16.24 ± 0.73) College = 19.72 ± 1.47 (19.27 ± 1.46) |
Resolved by 7 days |
| Newsome 66 | 2016 | Prospective cohort study | 13 (13) | 16± 1.1 (16.4 ± 1.3) | All recovered by day 30 |
| Ono 101 | 2016 | Prospective cohort study | 176 | range= 10–18 | Not mentioned |
| Ransom 63 | 2015 | Cross-sectional study | 349 | 13.72 (range= 5 – 18) | 68.8% recovered ≤28 |
| Slobounov 84 | 2007 | Prospective cohort study | 160 | Male= 20.95, female= 21.42 | All recovered by day 10 |
| Slobounov 82 | 2012 | Prospective cohort study | 49 | range= 18 – 25 | 10 |
| Terwilliger 67 | 2016 | Case control study | 21 (21) | 14.9 ± 0.89 (14.9 ± 0.89) | Not mentioned |
Level of Evidence details: Level 2: evidence from one well-designed randomized control trial, Level 3: well-designed controlled trial without randomization, Level 4: well-designed case-control or cohort study, Level 6: single descriptive qualitative study.
= Athlete exposure from 2002 to 2004
Excluded articles
Out of 207 excluded articles, 28 used only one recovery measure (either symptoms or neurocognitive testing), 37 monitored symptom recovery but participants were not defined as clinically recovered in the study, 1 was a study of sport-related concussion in adults, and 141 either did not specify any recovery criteria or did not monitor recovery. The eleven articles excluded during data extraction were: Hang et al.26 and Kelty-Stephen et al.27, which used neurocognitive testing and symptoms to measure recovery but stated that recovery was defined as resolution of symptoms and that the neurocognitive tests were just being validated. Kontos et al.28 used neurocognitive tests, a mood test, or professional recommendation to describe recovery but did not mention the basis for the professional recommendation or how many of the participants were cleared exclusively due to the professional recommendation. Moser et al.29 used ImPACT, which includes neurocognitive measures and a symptom checklist, and mentioned that a portion of subjects had fully recovered by the time of their last visit but did not state whether ImPACT was the main tool to determine return to sport or if it was the physician’s decision. Madura et al.30 measured concussion severity rather than recovery. The Meier et al.31 study was excluded because it did not specify which aspect of the CISG Guidelines was used to determine recovery. Studies that used the same sample were excluded. Lau et al.32, Lau et al.33 and Lau et al.34 Darling et al.35 and Baker et al.36, and Henry et al.37 and Henry et al.38 used the same sample so only the earlier studies were included. Kostyun et al.39 examined several parameters (symptoms, cognitive impairment, return to learn, and a special physical examination) but defined recovery only by symptoms returning to baseline.
Risk of bias assessment and level of evidence
Thirty eight out of 43 studies were case-control or cohort studies (level of Evidence 4).25 Study quality according to the Downs and Black Criteria24 is presented in Table 2. Some of the Downs and Black questions did not apply to most of the studies since they were not randomized trials. Except for Maerlender40, only questions 1–3, 5–7, 10–12, 16–18, 20–22 and 25 were relevant to the majority of the studies. Most studies were of low quality (case-control or cohort, Level of Evidence 4) and had significant risk of bias (Downs and Black score < 14). Studies in general had well defined objectives (Q1), main outcomes (Q2), and patient characteristics (Q3). Principal confounders (Q5) were not documented in some of the studies. The main findings (Q6), the random variability (Q7), probability values (Q10) and source population (Q11) were clearly documented in almost all studies. Most studies did not mention the proportion of the potential participants who agreed to participate in the study (participant representation, Q12). Most studies had appropriate internal validity (Q16–18 and Q20–22) except that there was very little adjustment for confounding variables (Q25).
Table 2.
Level of Evidence and Downs and Black Criteria Study Quality Assessment
| First Author | LOE | Downs and Black Question | Total | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | |||
| Anzalone 91 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 0 | - | - | 9 |
| Baker 92 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 0 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 13 |
| Black 64 | 6 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 10 |
| Broglio 93 | 4 | 1 | 1 | 0 | - | 0 | 1 | 1 | - | - | 0 | 0 | 1 | - | - | - | 1 | 0 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 9 |
| Brooks 51 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 0 | 0 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 9 |
| Brown 90 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 0 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 1 | - | - | 10 |
| Buckley 47 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 13 |
| Clausen 41 | 3 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 0 | 0 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 1 | - | - | 11 |
| Collins 48 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 0 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 13 |
| Corwin 89 | 4 | 1 | 1 | 0 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 0 | 1 | 0 | - | 1 | 1 | 1 | - | - | 0 | - | - | 9 |
| Crowe 42 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 0 | - | 1 | 1 | 1 | - | - | 0 | - | - | 12 |
| Darling 35 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 0 | 1 | 1 | - | - | - | 0 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 11 |
| Elbin 53 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 14 |
| Field 94 | 4 | 1 | 1 | 1 | - | 2 | 1 | 0 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 14 |
| Gill 54 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 12 |
| Henry 37 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 11 |
| Hutchison 55 | 4 | 1 | 1 | 0 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 11 |
| Iverson 95 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 0 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 13 |
| Kontos 49 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 13 |
| Kriz 57 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 14 |
| Lau 32 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 11 |
| Lax 96 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 13 |
| Lovell 97 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 12 |
| Lovell 59 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 1 | - | - | 11 |
| Lynall 60 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 0 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 10 |
| Maerlender 40 | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | 25 |
| Makdissi 61 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 0 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 12 |
| Maugans 81 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 14 |
| Mautner 44 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 13 |
| McClincy 62 | 4 | 1 | 1 | 1 | - | 2 | 1 | 0 | - | - | 0 | 1 | 1 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 0 | - | - | 10 |
| McDevit 98 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 11 |
| Mcgrath 45 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 0 | 1 | 1 | - | 1 | 0 | 1 | - | - | 0 | - | - | 11 |
| Meehan 50 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 13 |
| Miller 99 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 10 |
| Morgan 100 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 0 | - | - | 12 |
| Murugavel 56 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 0 | - | - | 0 | - | - | 12 |
| Nelson 65 | 4 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 0 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 0 | - | - | 1 | - | - | 14 |
| Newsome 66 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 0 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 0 | - | - | 9 |
| Ono 101 | 4 | 1 | 1 | 1 | - | 0 | 0 | 1 | - | - | 0 | 0 | 0 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 9 |
| Ransom 63 | 6 | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | 1 | 1 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 0 | - | - | 11 |
| Slobounov 84 | 4 | 1 | 1 | 1 | - | 2 | 1 | 0 | - | - | 1 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 14 |
| Slobounov 82 | 4 | 1 | 1 | 1 | - | 2 | 1 | 1 | - | - | 0 | 1 | 1 | - | - | - | 1 | 1 | 1 | - | 1 | 1 | 1 | - | - | 1 | - | - | 14 |
| Terwilliger 67 | 4 | 1 | 1 | 1 | - | 0 | 1 | 1 | - | - | 1 | 1 | 0 | - | - | - | 1 | 0 | 1 | - | 1 | 0 | 0 | - | - | 0 | - | - | 10 |
Recovery measures
All 43 studies reported symptom recovery, thirty-seven studies (86%) used neurocognitive testing, twenty-one (49%) used a provocative exercise test, thirteen (30%) used normalisation of balance, five (12%) used a special physical examination, two (5%) used successful return to school, one (2%) used absence of symptoms during cognitive exertion, and one (2%) used normalisation of cerebral blood flow.
All of the included studies used symptom recovery according to the following checklists: 31 used either the Post-Concussion Symptom Scale (PCSS, which is part of the Sport Concussion Assessment Tool [SCAT]), or the symptom checklist from ImPACT, 1 used CogSport for Kids, 2 used the Graded Symptom Checklist (GSC), 1 used the Post-Concussion Symptom Scale-Revised (PCS-R), 1 used CNS Vital Signs, 2 used the Post-Concussion Symptom Inventory (PCSI), 1 used the Subjective Symptom Rating scale, 1 used the Rivermead Post-concussion Symptom Questionnaire, and 3 listed patient-reported symptoms or did not specify the symptom instrument used. Seven studies32,36,41–45 used a cut off of a minimal symptom score (less than 7 out of a maximum of 132), 14 studies37,46–58 used the cut off of “symptom-free” (symptom score = 0), and 10 studies40,59–67 used “return to baseline symptoms” as their recovery measure. The other studies did not specify what qualified as a normal level of symptoms or did not use a 22-symptom Likert-scale checklist.
The most common computerised cognitive test was ImPACT (27 studies), 1 used CogSport, 1 used Axon Computerized Cognitive Assessment Tool, 1 used CNS Vital Signs, 5 used a paper-pencil test, and two did not specify. One study35 initially used the Automated Neurophysiological Assessment Metrics (ANAM) but changed to ImPACT. Seventeen studies used a non-specific definition of provocative exercise to test for exacerbation of symptoms, i.e., ‘no exacerbation of symptoms on exertion’. Four studies systematically assessed exercise tolerance: 3 used the Buffalo Concussion Treadmill Test (BCTT) and 1 clearly described the return to sport protocol from the 2012 CISG guidelines. For balance measures, 11 used the Balance Error Scoring System (BESS, which is also part of the SCAT), 1 used the Sensory Organization Test (SOT), and 1 did not specify how balance was assessed.
Combined recovery criteria
Table 4 is the contingency table for the recovery criteria (see data extraction) employed in each study. Eleven out of 43 studies used a combination of somatic symptom scales and cognitive performance to assess recovery representing the most common multi-modal recovery battery employed. The remaining 32 studies used some combination of the 8 recovery measures.
Table 4.
Frequency of Recovery Criteria
| Recovery Criteria | Number of Studies | Studies |
|---|---|---|
| Somatic symptoms and cognitive performance | 11 | 42,44,45,57,63,66,82,84,94,97,101 |
| Somatic symptoms and physical exertion test | 4 | 41,51,55,100 |
| Somatic symptoms and cognitive exertion test | 1 | 96 |
| Somatic symptoms, cognitive performance and physical exertion test | 10 | 32,48,49,53,56,59,61,62,92,95 |
| Somatic symptoms, cognitive performance and balance | 5 | 40,47,65,93,114 |
| Somatic symptoms, cognitive performance and special physical exam | 2 | 37,91 |
| Somatic symptoms, cognitive performance, balance and physical exertion test | 4 | 35,50,54,90 |
| Somatic symptoms, cognitive performance, balance and special physical exam | 2 | 60,98 |
| Somatic symptoms, cognitive performance, balance and return to learn | 1 | 67 |
| Somatic symptoms, cognitive performance, physical exertion test and cerebral blood flow | 1 | 81 |
| Somatic symptoms, cognitive performance, physical exertion test, special physical exam and return to learn | 1 | 89 |
| Somatic symptoms, balance and physical exertion test | 1 | 99 |
Post-recovery follow-up
Eight articles out of 43 (19%) followed up with subjects after recovery. The details of post-recovery follow-up and results are in Table 5. We note that it is not standard clinical practice to perform post-recovery surveillance because return to sport without symptoms is considered to be a successful outcome.
Table 5.
Methods of post-recovery follow-up and conclusion
| First author | Method of post-recovery follow-up | Conclusion |
|---|---|---|
| Crowe 42 | On day 30 when the athletes were recovered (except 1), the BRIEF-P/SR and CHQ were completed to measure executive function in day to day environments and functional health status and well-being respectively. | The questionnaires did not identify persistent impairments in the participants after recovery had taken place, with majority of the results falling in normal range. |
| Darling 35 | A structured telephone follow-up interview of the athletes was conducted a minimum of 2 months after they were cleared for return to play. The interview asked about difficulty in school after they returned and presence of concussion symptoms. | All had successfully returned to sport without recurrent symptoms. 38.5% of the athletes reported new or increased problems in school, mainly decreased ability to concentrate, but the timing of cognitive symptoms was not assessed (may have been during natural recovery phase). |
| Lynall 60 | Repeat baseline testing was performed on the athletes 169.5 (range 37–333) days after last post-injury test. Repeat baseline testing included computerized neurocognitive testing, balance testing and a graded symptom checklist. Scores in repeat baseline testing were compared with original pre-season baseline testing. | There is limited usefulness for repeat baseline testing in concussion management. It is time consuming and costly and has no significant difference to original pre-season baseline scores. |
| Maugans 81 | Although not included in the criteria for recovery, MRI, diffusion tensor imaging, H-MRS and phase contrast angiography was performed at days 3, 14 and 30 post-injury. | There was no evidence of structural or metabolic injury in MRI or H-MRS. In 36% of the participants the CBF values had not normalised by day 30 post-injury. |
| McGrath 45 | Post exertion ImPACT testing was done after recovery and compared with baseline pre-injury ImPACT scores. | 27.7% of the concussed student athletes who were determined to be recovered exhibited cognitive decline following moderate physical exertion. |
| Newsome 66 | Functional connectivity was measured 1 month post-sport-related concussion in athletes who were cleared to return to play using the Hopkins Verbal Learning Test during fMRI. | The injured and control groups did not differ in verbal memory after 1 month but differed in functional connectivity. |
| Slobounov 84 | Postural sway was assessed using a virtual reality device that displayed a “moving room” condition on day 30. | Balance problems (trunk sway during the “moving room” condition) persisted 30 days after injury, indicating that symptom and cognitive recovery did not coincide with balance recovery. Balance problems persisted significantly longer in those sustaining two concussions within 30 days when compared with a single concussion. |
| Slobounov 82 | EEG was performed alongside the measures used to determine recovery at day 7, 15 and 30 and months 6 and 12. EEG was recorded while sitting, standing on a force plate and then on a foam base of support with eyes open/closed conditions. | There was no significant change in neurological assessment and symptoms after the return to play decision was made but 85% of those who showed suppression in the acute phase did not return to pre-injury baseline up to 12 months post injury. |
Discussion
We systematically reviewed the literature for the most common measures used to make the return to sport decision after sport-related concussion. Given that the definition of concussion varies across the literature, it was not surprising that investigators varied in what criteria they used for return to sport. There are many studies that describe recovery from concussion but most do not indicate what measures were used to objectively make the return to sport decision. This is important for possible replication. It has been suggested that multiple measures should be used to make the return to sport decision from concussion but our search revealed that most studies used only one outcome measure (usually symptom resolution). The studies that used more than one recovery measure, those that qualified for inclusion in this review, used different combinations of measures.
Level of Evidence and Study Quality
Most of the studies were cohort or case-control studies. For study quality, the major source of bias was lack of documentation of principal confounders. History of previous concussions, for example, may be associated with longer duration of recovery and should always be documented.68,69 This could be due to the studies not documenting confounding variables or that appropriately matched controls were not employed.
Symptom resolution
Symptom resolution was the most common measure used to make the return to sport decision-all included studies used some version of a symptom assessment. The challenge for clinicians is that symptom reports are non-specific and may not coincide with brain recovery since physiological abnormalities (e.g., CBF, DTI) persisting beyond reported symptom resolution have been reported in multiple studies of sport-related concussion.70 Symptom recovery was not, however, defined always as an “asymptomatic state”. Healthy adolescents have been reported to have symptom severity scores of up to 6 (out of a maximum of 132) when given concussion symptom checklists;71 hence, several studies used a cut off score of less than 7 to define symptom recovery. Some studies used “return to baseline” and some used the terms “asymptomatic” or “symptom score of 0” to define symptom recovery. We found only two articles72,73 that did not use symptoms as a recovery criterion but since they used only one measure to define recovery (i.e., neurocognitive testing), they were not included in the final sample. Symptoms are usually assessed by symptom checklists. Concussion symptoms, however, are not specific to concussion. Leddy et al.12, for example, found no difference in the symptom patterns reported by those with concussion when compared with those who had cervical and vestibular issues.
Neurocognitive testing
The second most common measure used for the return to play decision was return to baseline using neurocognitive testing, usually with a computer test like ImPACT. ImPACT assesses symptoms and aspects of cognition including visual memory, verbal memory, visual motor speed, and reaction time that can be compared with individual pre-injury or age-normative values. The other computerised neurocognitive tests used were ANAM74, Axon Computerized Cognitive Assessment Tool, CogSport for Kids75 and CNS Vital Signs76, which are similar to ImPACT in that they measure different aspects of cognition and have a symptom checklist. Computerised neurocognitive tests can be administered in a 25 to 30-minute sitting and are used widely throughout the world. However, there are limitations of these tests with respect to re-test reliability. For example, the intraclass correlation coefficient for ImPACT has been reported to range between 0.15 to 0.39.77 Pen and paper tests (Children’s Color Trails, Rey Auditory Verbal Learning Test, Rey Complex Figure Test, Stroop Color and Word Test Children’s version, Symbol Digit Modalities Test, Trail Making Test-B, and Digit Symbol Substitution Test) have the limitation that they need a neuropsychologist to interpret the results and are not reliable when used in succession due to the learning effect.42
Physical exertion testing
The CISG Guidelines’ Graduated return to sport strategy is a clinical guideline that can be adapted to specific sports. Exacerbation of symptoms at any level is a reason to return to the previous step until the athlete can exercise without symptoms at the level of exertion required for that sport. The principle of return of normal exercise tolerance has been used in 21 studies to establish physiological recovery from concussion. Three of the studies systematically evaluated exercise tolerance after concussion using the Buffalo Concussion Treadmill Test (BCTT), all from the institution where the test was developed. This test is considered to be a clinical measure of autoregulation of cerebral blood flow (CBF) during exercise.41 It has been used to establish physiological recovery from concussion in adolescents after sport-related concussion.35 The BCTT is the only functional test that has been shown to safely78 and reliably18 diagnose and establish recovery from exercise intolerance35 after sport-related concussion and is used throughout the world in the assessment of athletes after head injury.
Balance and special physical examination tests
We included those measures that are considered to be more concussion-specific, e.g., the vestibular-ocular examination.79 Balance is an important criterion for the return to sport decision since persisting and untreated balance deficits could lead to future injuries on the playing field.32 The most common balance measure used was the BESS test, which is part of the SCAT. Vestibular components of the physician physical examination that measure balance, such as tandem gait, might have been used but the studies that utilised a physical examination did not provide a description of the elements performed. There is a clear need for a sensitive and a specific physical examination that includes the elements most related to the injury that produces concussion; specifically, the cervical, oculomotor and vestibular systems. A concussion-relevant physical examination could help clinicians not only with diagnosis but also to establish recovery from concussion.80
Physiological measures
fMRI with N-Back testing59, resting CBF 81, and EEG 82 have been used to try to establish concussion recovery using physiological parameters that do not involve motivation or effort. There is discordance between normalisation of these tests and symptom resolution. Physiological assessment of concussion has the potential to establish objective “physiological biomarkers” of recovery. Physiological tests, however, require validation in larger and more diverse samples because some participants had abnormal fMRI, CBF and brain electrophysiological measures despite reporting symptom resolution. The clinical significance of these measures and whether they represent ongoing neuronal or cerebrovascular damage, recovery or adaptation remains unknown.
Studies assessing return to sport follow-up and their implications for concussion assessment
Validation of the return to sport decision made by clinicians can be established if athletes who were returned to sport after concussion did not have return of symptoms while playing their sport. Eight articles in this systematic review evaluated the return to sport decision and they were diverse in terms of the length of time to follow up. Among high school athletes, 39% who returned to sport (after apparent recovery based on symptom resolution and normalisation of cognitive performance, balance, and exercise tolerance on the BCTT) reported new or increased problems upon return to school35 (although it was not clear whether return to school occurred within or beyond the typical timeframe for recovery). Over a quarter (28%) of athletes diagnosed as being recovered from concussion demonstrated cognitive impairment on ImPACT after moderate physical exertion45,suggesting that physical exertion precipitated cognitive problems not identified at rest. On the other hand, physical exertion appears to adversely affect ImPACT scores83, suggesting that it might not be appropriate to administer the test immediately after training or games. Multimodal testing on a weekly basis for up to 1 month following sport-related concussion (including general health questionnaires, symptoms, neurocognitive and balance testing, and exertion testing) may the best approach for determining recovery from concussion in young athletes. 84,42,60,66 However, more research on multimodal measures of recovery after concussion is needed.
Limitations
A limitation of this study is the inability to also perform a meta-analysis with the data. Table 4 shows the heterogeneity in the return to sport criteria used across studies thus preventing a meta-analysis of the 43 studies. Furthermore, while some of the studies employed similar return to sport criteria (e.g., Row 1 of Table 4), they did not provide individual patient level data but merely summary statistics with little uniformity in the provided summary statistics. These reasons make a meaningful meta-analysis impossible. Average return to sport times reported for the same set of criteria used by different studies are not truly comparable given the different study inclusion criteria and variable or undefined treatment.
Our systematic review is at risk of publication bias since we only included published, peer-reviewed articles and we could not search all the grey literature. There is also a risk for language bias since we only included English language articles. In addition, some researchers may have used additional measures for the return to sport decision that were not mentioned in their studies. Recent research has identified the frequency and importance of oculomotor dysfunction as an objective indicator of concussion.85 The King Devick Test for visual tracking has been shown to be sensitive for diagnosing concussion86–88. Oculomotor testing, however, has been used primarily to diagnose concussion and not as much to establish concussion recovery. Return to baseline on neurocognitive testing can be determined using a Reliable Change Index (RCI). ImPACT uses RCI but studies using other neurocognitive tests did not report using an RCI.
Three articles included concussions that were not exclusively sport-related. Corwin et al.89 and Crowe et al. 42 had 23% and 40% non-sport-related concussion subjects, respectively. They clearly mentioned that the mechanisms of injury were similar to sport-related concussion and that the concussions were treated like sport-related concussion. Brown et al. 90 included a “majority of sport-related concussion” and said that their sample was treated like sport-related concussion.
Future research
A more objective definition of sport-related concussion is needed so that we may better establish valid return to sport criteria. Future studies must define their return to sport criteria a-priori, use validated measures whenever possible, and until a universally accepted definition of concussion is reached, use a multi-modal approach to decide readiness to return to sport. This could encompass return to a baseline or normal level of symptoms, return to baseline or to normal cognitive performance, a normal physical examination (based on physical examination elements pertinent to concussion) and, in certain cases, demonstrating normal exercise tolerance on a graded physical exertion test.
Conclusion
There has been much written and studied about recovery from concussion, especially in the last ten to fifteen years. Only a minority of studies used multiple measures of recovery and had clearly defined return to sport criteria. The most common combination was self-reported symptom checklists and computerized neurocognitive tests. Second was the combination of self-reported symptoms, a computerized neurocognitive test, and a physical exertion test. We conclude that there are disparate measures of recovery being used in sport-related concussion research, that the research is of limited quality and subject to bias, and that it has led to conclusions with limited applicability. We recommend that a consensus be reached in the discipline regarding a set of reliable measures to make the return to sport decision. This would promote consistent study design in sport-related concussion research as well as uniform and safe applications of return to sport strategies.
Table 3.
Definitions of Return to Sport
| Article | Return to Sport Criteria | Definition of Return to Sport | Special considerations |
|---|---|---|---|
| Anzalone 91 | 3 = somatic symptoms, cognitive performance and special physical exam. | A patient was cleared to begin the graduated return to play protocol after he or she had remained symptom free for a minimum of 48 hours, displayed a normal physical examination, exhibited a normal VOMS, and achieved neurocognitive testing scores that were at the patient’s baseline or within normal limits. | Different recovery times for each part of the vestibulo-ocular exam. |
| Baker 92 | 3 = somatic symptoms, cognitive performance and physical exertion test. | Recovery from sport-related concussion was self-assessment of asymptomatic or minimal symptoms (PCSS1= 0–6), then confirmed by computerized cognitive test (ANAM3 or ImPACT4) and no symptom exacerbation on voluntary exhaustion on BCTT2. | Only those student athletes that recovered during the 2–3 month time period were included in the analysis. |
| Black 64 | 3 = somatic symptoms, cognitive performance and balance. | Once all concussion-related symptoms on the SCAT had resolved, athletes were allowed to attempt the ImPACT. The athlete was recovered if the ImPACT scores were considered passable by the sports medicine doctor or certified athletic trainer. | |
| Broglio 93 | 3 = somatic symptoms, cognitive performance and balance. | Axon Sports Computerized Cognitive Assessment Tool (CCAT) for neurocognition and SCAT for symptoms were used to measure recovery following sport concussion. | Electroencephalogram was performed during auditory oddball and go/no-go tasks but no significant differences were seen between injured and controls. |
| Brooks 51 | 2 = somatic symptoms and physical exertion test. | After resolution of clinical symptoms and signs, athletes gradually increased activity under supervision of a team physician. Athletes who remained asymptomatic throughout rehabilitation were cleared for return to play. | |
| Brown 90 | 4 = somatic symptoms, cognitive performance, balance, and physical exertion test. | Athletes were considered recovered when they were symptom-free at rest, symptom-free with exertion and after discontinuing medicines prescribed for post-concussion symptoms, their BESS5 scores were back to baseline where available and their computerized neurocognitive test (ImPACT) scores were at or above baseline values. | Did not include participants who recovered in less than 3 weeks. Included some participants who had concussions that were similar to sport-related concussion; for example, falling off a jungle gym. |
| Buckley 47 | 3 = somatic symptoms, cognitive performance, and balance. | Recovery from sport related concussion was self-reported asymptomatic (GSC6= 0) and baseline values on ImPACT, BESS and SAC7. | The study measured recovery from sport related concussion for those recommended rest after concussion versus those not recommended rest after concussion. |
| Clausen 41 | 2 = somatic symptoms and physical exertion test. | Recovery was defined as self-reported asymptomatic (PCSS= 0–6) and ability to exercise to voluntary exhaustion without exacerbation of concussion symptoms on the BCTT. Secondary outcomes of recovery were recovery of cerebral blood flow velocity measured by Transcranial Doppler in the MCA9 and exercise to exhaustion without symptom exacerbation while achieving 85% of maximal heart rate and 90% of predicted VO2 max. | Females with PCS8 lasting for more than 6 weeks but less than 12 weeks completed a sub-symptom threshold aerobic exercise treatment program with physiological measures before and after treatment. |
| Collins 48 | 3 = somatic symptoms, cognitive performance and physical exertion test | All athletes needed to exhibit an asymptomatic presentation at rest and with physical exertion, as well as intact neurocognitive test performance on ImPACT | Measured the efficacy of new helmet design. |
| Corwin 89 | 5 = somatic symptoms, cognitive performance, academic performance, physical exertion test, and special physical exam. | Both clinical and computerized neurocognitive testing (ImPACT) were used. Clinical clearance for sports participation included ability to carry full cognitive workload in school, be asymptomatic with physical exertion and have normal VOMS10 Assessment. | The sample (200 participants) had vestibular signs after concussion whereas the controls did not have vestibular signs after concussion. Only 77% of the entire sample were sports related concussions, rest were similar injuries. |
| Crowe 42 | 2 = somatic symptoms and cognitive performance. | Return to baseline on CogSport for Kids11. Supplementary test= BRIEF-P and BRIEF-SR12 and CHQ13 were also completed on day 30. CogSport symptom scale > 7 was considered significant. | Six out of 10 participants were injured during contact sports, the remaining were injured due to falls or blows. |
| Darling 35 | 4 = somatic symptoms, cognitive performance, balance, physical exertion test. | Recovery was defined as no or minimal symptoms (PCSS) and normalization of balance (BESS) on the SCAT-2, return to baseline on neurocognitive testing (ImPACT or ANAM) and no exacerbation of symptoms on BCTT. | This study was a retrospective chart review and follow-up via telephonic interview after two months to check for exacerbation of symptoms or difficulties with school after the return to play decision was made. Only 91 put of 117 had follow-ups. |
| Elbin 53 | 3 = somatic symptoms, cognitive performance and physical exertion test. | Athletes were required to be symptom-free (PCSS) at rest and after physical exertion before receiving medical clearance. Athletes were also required to demonstrate neurocognitive performance (ImPACT) within normal limits (i.e., 80% confidence intervals using reliable change indices) of their own baseline scores after exertion. When symptom-free at rest, athletes were asked to schedule a clearance appointment, which included neurocognitive testing and a standardized exertion test. | |
| Field 94 | 2 = somatic symptoms and cognitive performance | The PCSS was used to measure symptoms and a 25-minute battery of neuropsychological testing (paper-pencil tests) to measure neurocognitive ability after concussion. | |
| Gill 54 | 4 = somatic symptoms, cognitive performance, physical exertion test and balance | Athletes had to be asymptomatic at rest and with each step of the return to play progression before returning to their sport. In addition, cognition (ImPACT) and postural stability (BESS) had to be at preinjury levels. | There was a concussed athlete group vs a non-concussed athlete group. There was also a second control group of non-athletes. |
| Henry 37 | 3 = somatic symptoms, cognitive performance, and special physical exam. | Recovery defined as symptom free (PCSS=0), return to baseline on ImPACT, DHI14 and vestibular-oculomotor exam except near-point convergence. Scores from DHI and vestibular-oculomotor exam were combined to form an aggregate score. | |
| Hutchison 55 | 2 = somatic symptoms and exertion test. | Clinical recovery was defined as symptom free on Rivermead Post Concussion Symptom Questionnaire and no symptoms during the return to play protocol (exertion testing). | Other tests performed were Profile of Mood States – Short Form and Perceived Stress Scales. They were not used as a marker of recovery. |
| Iverson 95 | 3 = somatic symptoms, cognitive performance and physical exertion test. | The athlete must not have had an ImPACT neurocognitive composite score that was significantly worse than baseline or below the 10th percentile. They also had to be asymptomatic at rest on PCSS (PCSS<7) and after light aerobic exercise. | |
| Kontos 49 | 3 = somatic symptoms, cognitive performance, physical exertion test. | Recovery determined by a trained clinician by being symptom free at rest, back to baseline cognitive performance (ImPACT) at rest and symptom free after exertion. | The sample was divided into three groups based on post-concussion headaches and recovery time for each was given separately. |
| Kriz 57 | 2 = somatic symptoms and cognitive performance. | PCSS was measured at initial and follow-up assessments. Pre-injury baseline (when available) and post-injury neurocognitive function was assessed with ImPACT and used in decision-making regarding return to school and return to play for injured student-athletes. | This study compared the level of physical development before and after puberty to recovery from sport-related concussion. |
| Lau 32 | 3 = somatic symptoms, cognitive performance and physical exertion text. | Recovery was defined as clearance to return to play which was defined as asymptomatic at both rest and after exertion protocols (Zurich Guidelines), PCSS less than 7, the athlete must have had 2 ImPACT scores that were statistically lower than baseline or age-normative data and athlete’s neurocognitive composite score must have been above the tenth percentile for his age. | The study sample was divided into groups based on recovery times. Rapid recovery group recovered in 4.31 ±1.74 days and protracted group recovered in 29.61 ±6.65 days. |
| Lax 96 | 2 = somatic symptoms and cognitive exertion test. | Recovery was defined at resolution of symptoms on PCS-R15 and then ability to perform a neurocognitive battery in one sitting without symptom exacerbation. The neurocognitive battery consisted of Children’s Color Trails, Rey Auditory Verbal Learning Test, Rey Complex Figure Test, Stroop Color and Word Test Children’s version, Symbol Digit Modalities Test and verbal fluency component of NEPSY-II16. | Although all these neurocognitive tests were performed, the only goal was to complete them without exacerbation of symptoms. |
| Lovell 97 | 2 = somatic symptoms and cognitive performance. | Recovery was monitored using ImPACT’s PCSS and neurocognitive testing. | Only mild concussions were included in this study (i.e, no loss of consciousness) |
| Lovell 59 | 3 = somatic symptoms, cognitive performance and physical exertion test. | For an athlete to be recovered, all ImPACT composite scores were required to be above baseline levels or within the normal range and athletes had to be asymptomatic at rest and during graduated aerobic exercises. | fMRI was also done and compared with clinical recovery. |
| Lynall 60 | 4 = somatic symptoms, cognitive performance, balance and special physical exam. | After concussion, the athlete has to complete a clinical battery of tests including computerized neurocognitive testing, graded symptom checklist (both part of CNS Vital Signs) and SOT17 and these results had to be comparable to baseline values. Recovery was complemented by a thorough clinical exam by physician. Additional testing may be conducted if requested by the physician. | This study tried to see if there were any difference in preseason baseline testing scores before and after a concussion hence they described the time from recovery to next preseason baseline. |
| 3 = somatic symptoms, cognitive performance, and balance. | When ImPACT, balance and symptoms (PCSS) had returned to baseline, the athlete was determined to be recovered. Exercise intolerance was also measured in this study but was not a criterion for recovery. | Method of assessing balance was not specified. This randomized control trial evaluated the efficacy of moderate physical exercise on a bike versus rest as a treatment modality after acute concussion. | |
| Makdissi 61 | 3 = somatic symptoms, cognitive performance and physical exertion test. | Clinical recovery was defined as return to full training or competitive play, it included symptom resolution both at rest and exertion, and recovery of cognitive function (DSST18, TMT-B19). The team doctor also had to make the return to play decision. CogSport was also used to measure cognitive recovery, but due to time and resource limitations, not all teams were able to use it as part of the testing protocol. | |
| Maugans 81 | 4 = somatic symptoms, cognitive performance, physical exertion test and cerebral blood flow. | Clinical recovery was defined as symptom resolution on the ImPACT, return to normal physical and cognitive activity. Recovery of cerebral blood flow was defined as return to control values. MRI20 and H-MRS21 were performed during this study, but were not statistically significant and were not used to measure clinical recovery. | 27% of patients recovered cerebral blood flow within 14 days and > 64% after 30 days but clinical recovery was within 14 days. |
| Mautner 44 | 2 = somatic symptoms and cognitive performance. | Post-concussion recovery was defined as a return to equivalent baseline neurocognitive score on ImPACT and concussion symptom score of less than or equal to 7. | In this study, patients with self-reported ADHD plus sport-related concussion were compared to controls without ADHD but with sport-related concussion. |
| McClincy 62 | 3 = somatic symptoms, cognitive performance and physical exertion test. | To determine when an athlete was completely recovered from concussion, their post-concussion data were compared to their baseline data. All athletes diagnosed with an in season concussion did not return to play until they were symptom free at rest and with exertion and their ImPACT data had returned to baseline levels. | On day 14, only verbal memory scores were significantly different from baseline. |
| McDevit 98 | 4 = somatic symptoms, cognitive performance, balance and special physical exam. | Full return to play was determined by treating physician which included objective screening (including vestibular-ocular assessments), balance (BESS) and ImPACT. Gene testing for GRIN2A promoter polymorphs was also done to see if it would have any correlation to recovery time. | This study tested the relationship of the gene and the duration of post concussive symptoms. |
| Mcgrath 45 | 2 = somatic symptoms and cognitive performance. | The athletic trainer made the decision that participant was symptom free (this does not mean PCSS=0), ImPACT scores returned to baseline at rest. After the patients were symptom free at rest, they participated in a physical exertion protocol and neurocognitive tests were retested. This did not affect the already made diagnosis of clinically recovered. | The participants were classified as post physical exertion neurocognitive test pass or fail. |
| Meehan 50 | 4 = somatic symptoms, cognitive performance, balance and physical exertion test. | Recovery is defined as symptom free (PCSS=0) at rest and exertion after discontinuing any medication for post concussive symptoms, ImPACT scores at or above baseline, BESS at baseline. Baseline score were not available for majority of the participants, so they were compared to age normative date. | |
| Miller 99 | 2 = somatic symptoms, balance and physical exertion test | Recovery was defined as patients who were free of symptoms on the SCAT2 both at rest and with exertion. | Participants injured in motor sports like motocross were also included. |
| Morgan 100 | 2 = somatic symptoms and physical exertion test. | The controls were defined as recovered by clearance from a trained health care provider of symptom resolution (PCSS) at rest and exertion. They did not use the Likert Scale on the PCSS, instead scored them as present or absent. | In this study, the controls were the ones who recovered from a concussion within 3 weeks and the study sample developed PCS. |
| Murugavel 56 | 3 = somatic symptoms, cognitive performance and physical exertion test. | Athletes were cleared to return to full contact play once they were symptom free at rest, had successfully completed the exertional program and were neurocognitively functioning at baseline levels on ImPACT. | |
| Nelson 65 | 3 = somatic symptoms, cognitive performance and balance. | Recovery was defined in two ways: (1) GSC, BESS, SAC and traditional paper-pencil neurocognitive tests at each baseline and post-injury time point to look for evidence of differential recovery patterns across high school and collegiate athletes, and (2) compared concussed athletes (for high school and collegiate groups separately) with uninjured control participants at each time point. | |
| Newsome 66 | 2 = somatic symptoms and cognitive performance. | Resolution of post-concussion symptoms, recovery of cognitive performance on ImPACT to preseason level, and clearance by a licensed health provider to return to play by day 30 post-injury. | After the athlete was recovered, functional connectivity was assessed 1 month after sport-related concussion. |
| Ono 101 | 2 = somatic symptoms and cognitive performance. | Recovery was measured as the number of days to return to baseline neurocognitive and symptom scores on ImPACT | |
| Ransom 63 | 2 = somatic symptoms and cognitive performance. | Recovered participants were those that had no elevation of symptoms on PCSI22 (was adjusted by subtracting post injury scores by baseline scores) and no impairment on neurocognitive testing (age 5–12 used Multimodal Assessment of Cognition and Symptoms for Children and age 13–18 used ImPACT). Participants and their parents also completed the CLASS23 to measure post injury academic experiences but that was not part of the recovery criteria. | The participants were labeled as recovered or not on average of 28th day from their concussion based on the tests. |
| Slobounov 84 | 2 = somatic symptoms and cognitive performance. | All patients were cleared for sport participation 10–15 days post-injury. Postural responses to visual field motion in a virtual reality environment were recorded up to 30 days after concussion. | Despite clinical recovery, all athletes had persistent abnormalities of balance (incoherence with visual field motion responses) during a “moving room” condition displayed on a virtual reality device, consistent with subtle ongoing abnormal postural control. Postural control was significantly worse and took longer to recover in those suffering a second concussion during the study period. |
| Slobounov 82 | 2 = somatic symptoms and cognitive performance. | All the athletes were cleared for sports participation based on Subjective Symptom Rating scale, DSST18, and Trails “B” Test. EEG24 was done on day 7, 15, 30, 6 months and 12 months but did not contribute to the return to play decision. | |
| Terwilliger 67 | 4 = somatic symptoms, cognitive performance, balance and academic performance. | Date of recovery was determined by clinician based on child and parent report of the resolution of symptoms (PCSI-SR13 and PCSI-P) plus cognition, balance, and school performance having returned to normal functioning. | They divided the participants into single and repeat injury groups and measured the difference in their return to play time. |
PCSS= Post Concussion Symptom Scale, part of the Sport Concussion Assessment Tool (SCAT)71 and ImPACT.
BCTT= Buffalo Concussion Treadmill Test,102 a progressive, graded treadmill test which leads to voluntary exhaustion.
ANAM= Automated Neuropsychological Assessment Metrics,74 a computerized neurocognitive test.
ImPACT= Immediate Post-concussion Assessment and Cognitive Test,43 a computerized neurocognitive test.
BESS= Balance Error Scoring System,103 a test for static balance also part of the SCAT.
GSC= Graded Symptom Checklist,104 a 17 item Likert style symptom list promoted by the National Athletic Trainer Association.
SAC= Standard Assessment of Concussion,105 a standardized onsite tool for checking concussion symptoms.
PCS= Post-Concussion Syndrome.
MCA= Middle Cerebral Artery.
VOMS= Vestibular/Ocular Motor Screening Assessment, a standardized concussion screening assessment validated by the University of Pittsburg.106
CogSport for Kids= A computerized neurocognitive test with includes a post-concussion symptom checklist (PCSC).75
BRIEF-P/SR= Behavioral Rating Inventory of Executive Function – Parent/Self-Report107 is a measure to assess executive function in day to day environments for ages 12 and greater.
CHQ= Child Health Questionnaire108 is a measure of functional health status and well-being.
DHI= Dizziness Handicap Inventory,109 a measure to assess general dizziness.
PCS-R= Post Concussion Symptom Scale-Revised,110 a 22 item self-report symptom questionnaire with a Likert scale.
NEPSY-II= Developmental Neuropsychological Assessment Second Edition,111 a comprehensive neuropsychological battery designed to create neuropsychological profiles for children.
SOT= Sensory Organization Test performed on the NeuroCom Smart Balance Master.112
DSST= Digit Symbol Substitution Test.
TMT= Trail Making Test. CNS Vital Signs= A computerized neurocognitive test which includes a graded symptom checklist.76
MRI= Magnetic Resonance Imaging.
H-MRS= Hydrogen Magnetic Resonance Spectroscopy.
PCSI= Post-Concussion Symptom Inventory,113 a self-report form for concussion symptoms.
CLASS= Concussion Learning Assessment and School Survey,63 a tool to measure post injury academic experiences.
EEG= Electroencephalogram.
Summary Box.
What are the new findings?
Since the year 2000, 43 papers that defined recovery from sport-related concussion using two or more measures were identified.
All of the included articles used symptom recovery, 86% used cognitive recovery, 49% used response to physical exertion, 30% used balance testing, 12% used a special physical examination, 5% used return to full academic activities, 2% used a cognitive exertion test, and 2% used normalisation of cerebral blood flow.
How might it impact on clinical practice in the near future?
Researchers and physicians are encouraged to use standardized multiple criteria to establish recovery from sport-related concussion. Examples of these include normalisation of symptoms, a concussion-relevant physical examination, cognitive performance, and exercise tolerance.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS094444. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided by the Ralph and Mary Wilson Foundation and the Robert Rich Family Foundation.
Footnotes
Declarations
Ethical Approval: Not applicable.
Competing interests: The authors declare no conflict of interest.
Author contribution: Mohammad Haider, John Leddy, John Baker, Sonja Pavlesen, Melissa Kluczynski and Barry Willer contributed to the conception and design of the research, collection of data and writing, editing and approval of the manuscript. Jeffrey Miecznikowski contributed to data analysis and editing and approval of the manuscript.
Contributor Information
John J Leddy, Email: leddy@buffalo.edu.
Sonja Pavlesen, Email: pavlesen@buffalo.edu.
Melissa Kluczynski, Email: mk67@buffalo.edu.
John G Baker, Email: jgbaker@buffalo.edu.
Jeffrey C Miecznikowski, Email: jcm38@buffalo.edu.
Barry S Willer, Email: bswiller@buffalo.edu.
References
- 1.Tommasone BA, McLeod TCV. Contact sport concussion incidence. Journal of athletic training. 2006;41(4):470. [PMC free article] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 2006;21(5):375–78. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Anderson T, Heitger M, Macleod A. Concussion and mild head injury. Practical Neurology. 2006;6(6):342–57. [Google Scholar]
- 4.Barth JT, Freeman JR, Broshek DK, et al. Acceleration-Deceleration Sport-Related Concussion: The Gravity of It All. Journal of athletic training. 2001;36(3):253–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Signoretti S, Lazzarino G, Tavazzi B, et al. The pathophysiology of concussion. PM&R. 2011;3(10):S359–S68. doi: 10.1016/j.pmrj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. British journal of sports medicine. 2013;47(5):250–58. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 7.McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on Concussion in Sport–the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. South African Journal of Sports Medicine. 2009;21(2) doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- 8.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. Jama. 2003;290(19):2556–63. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 9.Covassin T, Elbin R, Harris W, et al. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. The American journal of sports medicine. 2012;40(6):1303–12. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 10.Chrisman SP, Quitiquit C, Rivara FP. Qualitative study of barriers to concussive symptom reporting in high school athletics. Journal of Adolescent Health. 2013;52(3):330–35e3. doi: 10.1016/j.jadohealth.2012.10.271. [DOI] [PubMed] [Google Scholar]
- 11.Alla S, Sullivan SJ, McCrory P. Defining asymptomatic status following sports concussion: fact or fallacy? British journal of sports medicine. 2012;46(8):562–69. doi: 10.1136/bjsm.2010.081299. [DOI] [PubMed] [Google Scholar]
- 12.Leddy JJ, Baker JG, Merchant A, et al. Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/vestibular injury. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2015;25(3):237–42. doi: 10.1097/jsm.0000000000000128. published Online First: 2014/07/23. [DOI] [PubMed] [Google Scholar]
- 13.Castile L, Collins CL, McIlvain NM, et al. The epidemiology of new versus recurrent sports concussions among high school athletes, 2005–2010. British journal of sports medicine. 2012;46(8):603–10. doi: 10.1136/bjsports-2011-090115. [DOI] [PubMed] [Google Scholar]
- 14.Buckley TA, Burdette G, Kelly K. Concussion-Management Practice Patterns of National Collegiate Athletic Association Division II and III Athletic Trainers: How the Other Half Lives. Journal of athletic training. 2015;50(8):879–88. doi: 10.4085/1062-6050-50.7.04. published Online First: 2015/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dessy AM, Rasouli J, Yuk F, et al. Second Impact Syndrome: A Rare, Devastating Consequence of Repetitive Concussions. Contemporary Neurosurgery. 2015;37(20):1–5. [Google Scholar]
- 16.Control CfD, Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged≤ 19 years---United States, 2001--2009. MMWR: Morbidity and mortality weekly report. 2011;60(39):1337–42. [PubMed] [Google Scholar]
- 17.Brett BL, Smyk N, Solomon G, et al. Long-term Stability and Reliability of Baseline Cognitive Assessments in High School Athletes Using ImPACT at 1-, 2-, and 3-year Test–Retest Intervals. Archives of clinical neuropsychology. 2016;31(8):904–14. doi: 10.1093/arclin/acw055. [DOI] [PubMed] [Google Scholar]
- 18.Leddy JJ, Baker JG, Kozlowski K, et al. Reliability of a graded exercise test for assessing recovery from concussion. Clinical Journal of Sport Medicine. 2011;21(2):89–94. doi: 10.1097/JSM.0b013e3181fdc721. [DOI] [PubMed] [Google Scholar]
- 19.Chin EY, Nelson LD, Barr WB, et al. Reliability and Validity of the Sport Concussion Assessment Tool–3 (SCAT3) in High School and Collegiate Athletes. The American journal of sports medicine. 2016 doi: 10.1177/0363546516648141. 0363546516648141. [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117(4):1359–71. doi: 10.1542/peds.2005-0994. [DOI] [PubMed] [Google Scholar]
- 21.University of York CfRaD. [accessed 2/19 2016];PROSPERO: International prospective register of systematic reviews. Available from: http://www.crd.york.ac.uk/PROSPERO/
- 22.Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the first International Conference on Concussion in Sport, Vienna 2001. British journal of sports medicine. 2002;36(1):6–7. doi: 10.1136/bjsm.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–69. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of epidemiology and community health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing & healthcare: A guide to best practice. Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 26.Hang B, Babcock L, Hornung R, et al. Can Computerized Neuropsychological Testing in the Emergency Department Predict Recovery for Young Athletes With Concussions? Pediatric emergency care. 2015;31(10):688–93. doi: 10.1097/pec.0000000000000438. published Online First: 2015/10/03. [DOI] [PubMed] [Google Scholar]
- 27.Kelty-Stephen DG, Qureshi Ahmad M, Stirling L. Use of a tracing task to assess visuomotor performance for evidence of concussion and recuperation. Psychological Assessment. 2015;27(4):1379–87. doi: 10.1037/pas0000122. [DOI] [PubMed] [Google Scholar]
- 28.Kontos AP, Covassin T, Elbin R, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Archives of physical medicine and rehabilitation. 2012;93(10):1751–56. doi: 10.1016/j.apmr.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Moser RS, Schatz P, Glenn M, et al. Examining prescribed rest as treatment for adolescents who are slow to recover from concussion. Brain injury. 2015;29(1):58–63. doi: 10.3109/02699052.2014.964771. published Online First: 2014/10/04. [DOI] [PubMed] [Google Scholar]
- 30.Madura SA, McDevitt JK, Tierney RT, et al. Genetic variation in SLC17A7 promoter associated with response to sport-related concussions. Brain injury. 2016;30(7):908–13. doi: 10.3109/02699052.2016.1146958. published Online First: 2016/04/01. [DOI] [PubMed] [Google Scholar]
- 31.Meier TB, Bellgowan PS, Singh R, et al. Recovery of cerebral blood flow following sports-related concussion. JAMA neurology. 2015;72(5):530–8. doi: 10.1001/jamaneurol.2014.4778. published Online First: 2015/03/03. [DOI] [PubMed] [Google Scholar]
- 32.Lau BC, Kontos AP, Collins MW, et al. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? The American journal of sports medicine. 2011;39(11):2311–18. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- 33.Lau BC, Collins MW, Lovell MR. Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery. 2012;70(2):371–79. doi: 10.1227/NEU.0b013e31823150f0. [DOI] [PubMed] [Google Scholar]
- 34.Lau B, Lovell MR, Collins MW, et al. Neurocognitive and symptom predictors of recovery in high school athletes. Clinical Journal of Sport Medicine. 2009;19(3):216–21. doi: 10.1097/JSM.0b013e31819d6edb. [DOI] [PubMed] [Google Scholar]
- 35.Darling SR, Leddy JJ, Baker JG, et al. Evaluation of the Zurich guidelines and exercise testing for return to play in adolescents following concussion. Clinical Journal of Sport Medicine. 2014;24(2):128–33. doi: 10.1097/JSM.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 36.Baker JG, Leddy JJ, Darling SR, et al. Factors Associated With Problems for Adolescents Returning to the Classroom After Sport-Related Concussion. Clinical pediatrics. 2015;54(10):961–8. doi: 10.1177/0009922815588820. published Online First: 2015/06/19. [DOI] [PubMed] [Google Scholar]
- 37.Henry LC, Elbin RJ, Collins MW, et al. Examining Recovery Trajectories After Sport-Related Concussion With a Multimodal Clinical Assessment Approach. Neurosurgery. 2015 doi: 10.1227/neu.0000000000001041. published Online First: 2015/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry LC, Tremblay S, De Beaumont L. Long-Term Effects of Sports Concussions: Bridging the Neurocognitive Repercussions of the Injury with the Newest Neuroimaging Data. Neuroscientist. 2016 doi: 10.1177/1073858416651034. published Online First: 2016/05/18. [DOI] [PubMed] [Google Scholar]
- 39.Kostyun RO, Hafeez I. Protracted recovery from a concussion: a focus on gender and treatment interventions in an adolescent population. Sports health. 2015;7(1):52–7. doi: 10.1177/1941738114555075. published Online First: 2015/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maerlender A, Rieman W, Lichtenstein J, et al. Programmed Physical Exertion in Recovery From Sports-Related Concussion: A Randomized Pilot Study. Developmental neuropsychology. 2015;40(5):273–8. doi: 10.1080/87565641.2015.1067706. published Online First: 2015/08/01. [DOI] [PubMed] [Google Scholar]
- 41.Clausen M, Pendergast DR, Willer B, et al. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. The Journal of head trauma rehabilitation. 2015 doi: 10.1097/htr.0000000000000145. published Online First: 2015/06/23. [DOI] [PubMed] [Google Scholar]
- 42.Crowe L, Collie A, Hearps S, et al. Cognitive and physical symptoms of concussive injury in children: a detailed longitudinal recovery study. Br J Sports Med. 2015 doi: 10.1136/bjsports-2015-094663. published Online First: 2015/10/03. [DOI] [PubMed] [Google Scholar]
- 43.Iverson GL, Brooks BL, Collins MW, et al. Tracking neuropsychological recovery following concussion in sport. Brain injury. 2006;20(3):245–52. doi: 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- 44.Mautner K, Sussman WI, Axtman M, et al. Relationship of Attention Deficit Hyperactivity Disorder and Postconcussion Recovery in Youth Athletes. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2015;25(4):355–60. doi: 10.1097/jsm.0000000000000151. published Online First: 2014/10/30. [DOI] [PubMed] [Google Scholar]
- 45.McGrath N, Dinn WM, Collins MW, et al. Post-exertion neurocognitive test failure among student-athletes following concussion. Brain injury. 2013;27(1):103–13. doi: 10.3109/02699052.2012.729282. [DOI] [PubMed] [Google Scholar]
- 46.Brown DA, Elsass JA, Miller AJ, et al. Differences in Symptom Reporting Between Males and Females at Baseline and After a Sports-Related Concussion: A Systematic Review and Meta-Analysis. Sports medicine (Auckland, NZ) 2015;45(7):1027–40. doi: 10.1007/s40279-015-0335-6. published Online First: 2015/05/15. [DOI] [PubMed] [Google Scholar]
- 47.Buckley TA, Munkasy BA, Clouse BP. Acute Cognitive and Physical Rest May Not Improve Concussion Recovery Time. The Journal of head trauma rehabilitation. 2015 doi: 10.1097/htr.0000000000000165. published Online First: 2015/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins M, Lovell MR, Iverson GL, et al. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three-year prospective cohort study. Neurosurgery. 2006;58(2):275–86. doi: 10.1227/01.NEU.0000200441.92742.46. [DOI] [PubMed] [Google Scholar]
- 49.Kontos AP, Elbin R, Lau B, et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. The American journal of sports medicine. 2013 doi: 10.1177/0363546513488751. 0363546513488751. [DOI] [PubMed] [Google Scholar]
- 50.Meehan WP, Mannix RC, Stracciolini A, et al. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. The Journal of pediatrics. 2013;163(3):721–25. doi: 10.1016/j.jpeds.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brooks MA, Peterson K, Biese K, et al. Concussion Increases Odds of Sustaining a Lower Extremity Musculoskeletal Injury After Return to Play Among Collegiate Athletes. The American journal of sports medicine. 2016;44(3):742–7. doi: 10.1177/0363546515622387. published Online First: 2016/01/21. [DOI] [PubMed] [Google Scholar]
- 52.Anzalone AJ, Blueitt D, Case T, et al. A Positive Vestibular/Ocular Motor Screening (VOMS) Is Associated With Increased Recovery Time After Sports-Related Concussion in Youth and Adolescent Athletes. American Journal of Sports Medicine. 2017;45(2):474–79. doi: 10.1177/0363546516668624. [DOI] [PubMed] [Google Scholar]
- 53.Elbin RJ, Sufrinko A, Schatz P, et al. Removal From Play After Concussion and Recovery Time. Pediatrics. 2016;138(3) doi: 10.1542/peds.2016-0910. published Online First: 2016/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gill J, Merchant-Borna K, Jeromin A, et al. Acute plasma tau relates to prolonged return to play after concussion. Neurology. 2017;88(6):595–602. doi: 10.1212/WNL.0000000000003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchison MG, Mainwaring L, Senthinathan A, et al. Psychological and Physiological Markers of Stress in Concussed Athletes Across Recovery Milestones. The Journal of head trauma rehabilitation. 2016 doi: 10.1097/htr.0000000000000252. published Online First: 2016/09/08. [DOI] [PubMed] [Google Scholar]
- 56.Murugavel M, Cubon V, Putukian M, et al. A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. Journal of neurotrauma. 2014;31(22):1860–71. doi: 10.1089/neu.2014.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kriz PK, Stein C, Kent J, et al. Physical Maturity and Concussion Symptom Duration among Adolescent Ice Hockey Players. The Journal of pediatrics. 2016;171:234, 9e1–2. doi: 10.1016/j.jpeds.2015.12.006. published Online First: 2016/01/20. [DOI] [PubMed] [Google Scholar]
- 58.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. Journal of neurosurgery Pediatrics. 2016;17(4):491–6. doi: 10.3171/2015.8.peds14332. published Online First: 2015/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovell MR, Pardini JE, Welling J, et al. Functional Brain Abnormalities Are Related To Clinical Recovery and Time To Return-To-Play in Athletes. Neurosurgery. 2007;61(2):352–60. doi: 10.1227/01.NEU.0000279985.94168.7F. [DOI] [PubMed] [Google Scholar]
- 60.Lynall RC, Schmidt JD, Mihalik JP, et al. The Clinical Utility of a Concussion Rebaseline Protocol After Concussion Recovery. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2015 doi: 10.1097/jsm.0000000000000260. published Online First: 2015/11/19. [DOI] [PubMed] [Google Scholar]
- 61.Makdissi M, Darby D, Maruff P, et al. Natural history of concussion in sport markers of severity and implications for management. The American journal of sports medicine. 2010;38(3):464–71. doi: 10.1177/0363546509349491. [DOI] [PubMed] [Google Scholar]
- 62.McClincy MP, Lovell MR, Pardini J, et al. Recovery from sports concussion in high school and collegiate athletes. Brain injury. 2006;20(1):33–39. doi: 10.1080/02699050500309817. [DOI] [PubMed] [Google Scholar]
- 63.Ransom DM, Vaughan CG, Pratson L, et al. Academic effects of concussion in children and adolescents. Pediatrics. 2015;135(6):1043–50. doi: 10.1542/peds.2014-3434. published Online First: 2015/05/13. [DOI] [PubMed] [Google Scholar]
- 64.Black AM, Sergio LE, MacPherson AK. The Epidemiology of Concussions: Number and nature of concussions and time to recovery among female and male canadian varsity athletes 2008 to 2011. Clinical Journal of Sport Medicine. 2017;27(1):52–56. doi: 10.1097/JSM.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 65.Nelson LD, Guskiewicz KM, Barr WB, et al. Age Differences in Recovery After Sport-Related Concussion: A Comparison of High School and Collegiate Athletes. Journal of athletic training. 2016;51(2):142–52. doi: 10.4085/1062-6050-51.4.04. published Online First: 2016/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newsome MR, Li X, Lin X, et al. Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report. Frontiers in neurology. 2016;7:116. doi: 10.3389/fneur.2016.00116. published Online First: 2016/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terwilliger VK, Pratson L, Vaughan CG, et al. Additional Post-Concussion Impact Exposure May Affect Recovery in Adolescent Athletes. Journal of neurotrauma. 2016;33(8):761–5. doi: 10.1089/neu.2015.4082. published Online First: 2015/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colvin AC, Mullen J, Lovell MR, et al. The role of concussion history and gender in recovery from soccer-related concussion. The American journal of sports medicine. 2009;37(9):1699–704. doi: 10.1177/0363546509332497. [DOI] [PubMed] [Google Scholar]
- 69.Brooks BL, McKay CD, Mrazik M, et al. Subjective, but not objective, lingering effects of multiple past concussions in adolescents. Journal of neurotrauma. 2013;30(17):1469–75. doi: 10.1089/neu.2012.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? Systematic review. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-097464. bjsports-2016-097464. [DOI] [PubMed] [Google Scholar]
- 71.McCrory P, Meeuwisse W, Johnston K, et al. SCAT2. British Journal of Sports Medicine. 2009;43(Suppl_1):i85–i88. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- 72.McCrea M, Kelly JP, Randolph C, et al. Immediate neurocognitive effects of concussion. Neurosurgery. 2002;50(5):1032–42. doi: 10.1097/00006123-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Ravdin LD, Barr WB, Jordan B, et al. Assessment of cognitive recovery following sports related head trauma in boxers. Clinical Journal of Sport Medicine. 2003;13(1):21–27. doi: 10.1097/00042752-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Cernich A, Reeves D, Sun W, et al. Automated neuropsychological assessment metrics sports medicine battery. Archives of Clinical Neuropsychology. 2007;22:101–14. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Collie A, Maruff P, Makdissi M, et al. CogSport: reliability and correlation with conventional cognitive tests used in postconcussion medical evaluations. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2003;13(1):28–32. doi: 10.1097/00042752-200301000-00006. published Online First: 2003/01/25. [DOI] [PubMed] [Google Scholar]
- 76.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Archives of Clinical Neuropsychology. 2006;21(7):623–43. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Elliott R. Test-retest reliability of computerized concussion assessment programs. Journal of athletic training. 2007;42(4):509. [PMC free article] [PubMed] [Google Scholar]
- 78.Leddy JJ, Kozlowski K, Donnelly JP, et al. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clinical Journal of Sport Medicine. 2010;20(1):21–27. doi: 10.1097/JSM.0b013e3181c6c22c. [DOI] [PubMed] [Google Scholar]
- 79.Matuszak JM, McVige J, McPherson J, et al. A Practical Concussion Physical Examination Toolbox Evidence-Based Physical Examination for Concussion. Sports Health: A Multidisciplinary Approach. 2016;8(3):260–69. doi: 10.1177/1941738116641394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellis MJ, Leddy JJ, Willer B. Physiological, vestibulo-ocular and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain injury. 2015;29(2):238–48. doi: 10.3109/02699052.2014.965207. published Online First: 2014/10/15. [DOI] [PubMed] [Google Scholar]
- 81.Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slobounov S, Sebastianelli W, Hallett M. Residual brain dysfunction observed one year post-mild traumatic brain injury: combined EEG and balance study. Clinical Neurophysiology. 2012;123(9):1755–61. doi: 10.1016/j.clinph.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Covassin T, Weiss L, Powell J, et al. Effects of a maximal exercise test on neurocognitive function. British Journal of Sports Medicine. 2007;41(6):370–74. doi: 10.1136/bjsm.2006.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slobounov S, Slobounov E, Sebastianelli W, et al. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery. 2007;61(2):338–44. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- 85.Sussman ES, Ho AL, Pendharkar AV, et al. Clinical evaluation of concussion: the evolving role of oculomotor assessments. Neurosurg Focus. 2016;40(4):E7. doi: 10.3171/2016.1.focus15610. published Online First: 2016/04/02. [DOI] [PubMed] [Google Scholar]
- 86.King D, Gissane C, Hume PA, et al. The King-Devick test was useful in management of concussion in amateur rugby union and rugby league in New Zealand. Journal of the neurological sciences. 2015;351(1–2):58–64. doi: 10.1016/j.jns.2015.02.035. published Online First: 2015/03/10. [DOI] [PubMed] [Google Scholar]
- 87.Seidman DH, Burlingame J, Yousif LR, et al. Evaluation of the King-Devick test as a concussion screening tool in high school football players. Journal of the neurological sciences. 2015;356(1–2):97–101. doi: 10.1016/j.jns.2015.06.021. published Online First: 2015/06/22. [DOI] [PubMed] [Google Scholar]
- 88.Tjarks BJ, Dorman JC, Valentine VD, et al. Comparison and utility of King-Devick and ImPACT® composite scores in adolescent concussion patients. Journal of the neurological sciences. 2013;334(1–2):148–53. doi: 10.1016/j.jns.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 89.Corwin DJ, Wiebe DJ, Zonfrillo MR, et al. Vestibular Deficits following Youth Concussion. The Journal of pediatrics. 2015;166(5):1221–5. doi: 10.1016/j.jpeds.2015.01.039. published Online First: 2015/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown NJ, Mannix RC, O’Brien MJ, et al. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299–e304. doi: 10.1542/peds.2013-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anzalone AJ, Blueitt D, Case T, et al. A Positive Vestibular/Ocular Motor Screening (VOMS) Is Associated With Increased Recovery Time After Sports-Related Concussion in Youth and Adolescent Athletes. The American journal of sports medicine. 2017;45(2):474–79. doi: 10.1177/0363546516668624. published Online First: 2016/10/30. [DOI] [PubMed] [Google Scholar]
- 92.Baker JG, Leddy JJ, Darling SR, et al. Gender Differences in Recovery From Sports-Related Concussion in Adolescents. Clinical pediatrics. 2015 doi: 10.1177/0009922815606417. published Online First: 2015/09/18. [DOI] [PubMed] [Google Scholar]
- 93.Broglio SP, Rettmann A, Greer J, et al. Investigating a Novel Measure of Brain Networking Following Sports Concussion. International journal of sports medicine. 2016;37(9):714–22. doi: 10.1055/s-0042-107250. published Online First: 2016/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. The Journal of pediatrics. 2003;142(5):546–53. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 95.Iverson G. Predicting slow recovery from sport-related concussion: the new simple-complex distinction. Clinical Journal of Sport Medicine. 2007;17(1):31–37. doi: 10.1097/JSM.0b013e3180305e4d. [DOI] [PubMed] [Google Scholar]
- 96.Lax ID, Paniccia M, Agnihotri S, et al. Developmental and gender influences on executive function following concussion in youth hockey players. Brain injury. 2015;29(12):1409–19. doi: 10.3109/02699052.2015.1043344. published Online First: 2015/09/13. [DOI] [PubMed] [Google Scholar]
- 97.Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. Journal of neurosurgery. 2003;98(2):296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- 98.McDevitt J, Tierney RT, Phillips J, et al. Association between GRIN2A promoter polymorphism and recovery from concussion. Brain injury. 2015;29(13–14):1674–81. doi: 10.3109/02699052.2015.1075252. published Online First: 2015/10/28. [DOI] [PubMed] [Google Scholar]
- 99.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: A case-control study. Journal of Neurosurgery: Pediatrics. 2016;17(4):491–96. doi: 10.3171/2015.8.PEDS14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. Journal of neurosurgery Pediatrics. 2015;15(6):589–98. doi: 10.3171/2014.10.peds14356. published Online First: 2015/03/10. [DOI] [PubMed] [Google Scholar]
- 101.Ono KE, Burns TG, Bearden DJ, et al. Sex-Based Differences as a Predictor of Recovery Trajectories in Young Athletes After a Sports-Related Concussion. The American journal of sports medicine. 2015 doi: 10.1177/0363546515617746. published Online First: 2015/12/17. [DOI] [PubMed] [Google Scholar]
- 102.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current sports medicine reports. 2013;12(6):370–6. doi: 10.1249/jsr.0000000000000008. published Online First: 2013/11/15. [DOI] [PubMed] [Google Scholar]
- 103.Iverson GL, Kaarto ML, Koehle MS. Normative data for the balance error scoring system: implications for brain injury evaluations. Brain injury. 2008;22(2):147–52. doi: 10.1080/02699050701867407. [DOI] [PubMed] [Google Scholar]
- 104.Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association position statement: management of sport-related concussion. Journal of athletic training. 2004;39(3):280. [PMC free article] [PubMed] [Google Scholar]
- 105.McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. The Journal of head trauma rehabilitation. 1998;13(2):27–35. doi: 10.1097/00001199-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Mucha A, Collins MW, Elbin RJ, et al. A Brief Vestibular/Ocular Motor Screening (VOMS) Assessment to Evaluate Concussions: Preliminary Findings. The American journal of sports medicine. 2014;42(10):2479–86. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function: BRIEF. Psychological Assessment Resources; Odessa, FL: 2000. [Google Scholar]
- 108.Landgraf J, Abetz L, Ware J. The CHQ user’s manual. Boston: The Health Institute, New England Medical Center; 1996. pp. 235–9. [Google Scholar]
- 109.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of otolaryngology--head & neck surgery. 1990;116(4):424–7. doi: 10.1001/archotol.1990.01870040046011. published Online First: 1990/04/01. [DOI] [PubMed] [Google Scholar]
- 110.Lovell MR, Collins MW. Neuropsychological assessment of the college football player. The Journal of head trauma rehabilitation. 1998;13(2):9–26. doi: 10.1097/00001199-199804000-00004. published Online First: 1998/05/09. [DOI] [PubMed] [Google Scholar]
- 111.Davis JL, Matthews RN. NEPSY-II Review. Journal of Psychoeducational Assessment. 2010;28(2):175–82. [Google Scholar]
- 112.NeuroCom International I. NeuroCom System Operator’s Manual. 2001. [Google Scholar]
- 113.Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Archives of clinical neuropsychology. 2014;29(4):348–63. doi: 10.1093/arclin/acu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Black AM, Sergio LE, Macpherson AK. The Epidemiology of Concussions: Number and Nature of Concussions and Time to Recovery Among Female and Male Canadian Varsity Athletes 2008 to 2011. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2017;27(1):52–56. doi: 10.1097/jsm.0000000000000308. published Online First: 2016/02/11. [DOI] [PubMed] [Google Scholar]