Abstract
Background
Herpes simplex virus, type 1 (HSV-1) infects over 3.4 billion people, world-wide. Though it can cause encephalitis, in the vast majority it is asymptomatic, with lifelong latent infection in neurons. HSV-1 infected individuals have greater cognitive dysfunction than uninfected individuals, particularly persons with schizophrenia – even without encephalitis. We investigated whether HSV-1 related cognitive dysfunction is progressive or remediable.
Methods
In a prospective naturalistic follow up sample (PNFU), temporal changes in cognitive functions were analyzed in relation to baseline HSV-1 infection in persons with/without schizophrenia (N=226). Independently, in a randomized controlled trial (RCT), HSV-1 infected, clinically stabilized SZ outpatients received Valacyclovir (VAL, an HSV-1 specific antiviral, 1.5 G twice daily for 16 weeks) or placebo (PLA) added to standard antipsychotic treatment, using a stratified randomization design, following placebo run-in (N=67). In both samples, HSV-1 infection (seropositivity) was estimated using serum IgG antibodies. Clinical evaluations were blinded to HSV-1 or treatment status. Standardized Z scores for accuracy on eight cognitive domains were analyzed for temporal trajectories using generalized linear models (PNFU) and VAL/PLA differences compared with intent to treat analyses (RCT).
Results
PNFU: At baseline, HSV-1 infected participants had significantly lower accuracy scores for Emotion Identification and Discrimination (EMOD), Spatial memory and Spatial ability, regardless of SZ diagnosis (p=0.025, 0.029, 0.046, respectively). They also had significantly steeper temporal worsening for EMOD (p=0.03). RCT: EMOD improved in VAL-treated patients (p=0.048, Cohen’s d=0.43).
Conclusions
A proportion of age related decline in EMOD is attributable to HSV-1 infection.
Keywords: cognition, herpes virus, HSV-1, schizophrenia, emotion, memory, Valacyclovir
1. Introduction
HSV-1 causes lifelong infection in approximately 3.4 billion people (Looker et al., 2015). It produces sporadic productive, lytic eruptions on mucosal surfaces, interspersed with latent, apparently asymptomatic infection restricted to neurons (Steiner et al., 2007). HSV-1 induced encephalitis is rare (0.004%) (Steiner et al., 2007), but many cross-sectional studies indicate cognitive dysfunction in working memory, attention and related domains in seropositive persons (Dickerson et al., 2003; Dickerson et al., 2004; Dickerson et al., 2008; Dickerson et al., 2012; Prasad et al., 2007; Prasad et al., 2012; Schretlen et al., 2010; Shirts et al., 2008; Strandberg et al., 2003; Tarter et al., 2014; Thomas et al., 2013; Watson et al., 2013; Yolken et al., 2011). Among healthy children, HSV-1 seropositivity is associated with lower reading and spatial reasoning test scores (Tarter et al., 2014). The associations are detectable among persons without a history of encephalitis and after accounting for potential socio-economic and infectious confounding factors (Studies cited above). Fruchter et al (2015) studied associations between cognitive function and HSV-1 exposure among healthy young soldiers and found that HSV-1 exposed individuals were significantly more impaired. Other studies of non-psychiatric samples also reported that cognitive functions were more significantly impaired among HSV-1 exposed persons (Strandberg et al (2003); Tarter et al. (2014); Jonker et al. (2014).
Cortical volume reductions in frontotemporal regions are also detectable in HSV-1 infected persons (Prasad et al., 2007). Though HSV-1 has not been etiologically linked with SZ (Thomas et al., 2013), the cognitive dysfunction is observed frequently among persons with SZ (Dickerson et al., 2003b; McGrath et al., 1997; Yolken et al., 2011). In sum, the cognitive dysfunction associated with persistent HSV-1 infection is distinct from the global, severe cognitive dysfunction among survivors of HSV-1 encephalitis (Hokkanen and Launes, 2007; McGrath et al., 1997; Prasad et al., 2007). It is reminiscent of ‘cognitive aging’, i.e., minor cognitive dysfunction among otherwise healthy adults that can compromise or magnify other physical and mental disabilities (Blazer et al., 2011). Like cognitive aging, varying trajectories of temporal cognitive decline have also been reported (Table 1). To test whether the HSV-1 associated cognitive dysfunction is remediable, we previously conducted a small randomized controlled adjunctive trial (RCT, N=24) among US SZ patients with HSV-1 infection and found that 18-week adjunctive treatment with valacyclovir (VAL), an antiviral drug with high specificity for HSV-1 infection significantly improved verbal memory, working memory and visual object learning, compared with placebo augmentation of antipsychotic treatment (Prasad et al., 2013). Valacyclovir is highly specific as an antiviral agent, and studies have shown that it is very safe drug (Tyring et al. 2002). To evaluate the prognosis of HSV-1 associated cognitive dysfunction, we used a prospective, naturalistic follow up (PNFU) design to assess patterns of temporal change in cognitive functions among persons with and without HSV-1 infection. As our initial RCT was relatively small (Prasad et al., 2013), we separately evaluated a larger, independent sample using the same protocol.
Table 1.
HSV-1 exposure and temporal trajectory of cognitive function in published studies.
| First author / Reference | Study sample (N) | Cognitive tests | Follow up duration | Key results |
|---|---|---|---|---|
| Strandberg (Strandberg et al., 2003) | Random sample of Helsinki residents with cardiovascular disease (383) | MMSE, CDR | 1 year | Reduction in MMSE scores proportional to viral burden due to HSV-1, CMV and HSV-2 |
| Aiello (Aiello et al., 2008) | Community-dwelling elderly Latino sample (1204) | MMSE, episodic memory (word list-learning test) | 4 years | Rate of cognitive decline significantly related to CMV titers, but not HSV-1 titers |
| Prasad (Prasad et al., 2012) | First-episode antipsychotic-naive SZ patients (26); Healthy subjects (38) | WCST | 1 year | Greater reduction in perseverative errors in HSV-1 seronegative SZ patients than in the HSV-1-seropositive patients. No significant associations with CMV, or in healthy subjects. |
| Barnes (Barnes et al., 2014) | 3 cohorts of elderly community members (N=849) | MMSE, tests of working memory, episodic memory, visuospatial function | 5 years | No significant temporal associations with HSV-1 status. CMV seropositivity associated with greater risk of Alzheimer disease and faster rate of cognitive decline in global cognition. |
| Nimgaonkar (Nimgaonkar et al., 2016) | Representative community sample of elders | MMSE, tests of complex attention, executive functions, memory, language, visuospatial function | 5 years | IgG titers of antibodies to CMV, HSV-2 and Tox associated with differing patterns of greater cognitive decline. No temporal associations with HSV-1. |
MMSE: Mini-Mental State Examination; CDR: Clinical Dementia Rating scale; HSV-1: herpes simplex virus, type 1 (HSV-1); CMV: cytomegalovirus; HSV-2: herpes simplex virus, type 2 (HSV-2); Tox: Toxoplasma Gondii.
2. Methods
The study was conducted at Dr. Ram Manohar Lohia Hospital, Delhi, India (RMLH). All participants were assessed with the Diagnostic Interview for Genetic Studies and consensus diagnoses were assigned as described (Thomas et al., 2013). Cognitive functions were assessed using the validated Penn Cognitive Neuropsychological Battery (PennCNB), which estimates accuracy and speed estimates for ten cognitive domains (Gur et al., 2001a; Gur et al., 2001b; ;Watson et al., 2013). As the accuracy and speed estimates are correlated, only age standardized accuracy scores were analyzed here (Bhatia et al., 2012). HSV-1 infection was estimated using standard immunoassays at certified clinical laboratories. Highly specific IgG antibodies are normally produced following HSV-1 infection, so elevated HSV-1 antibody levels in the serum were used to assess HSV-1 exposure. Antibodies to HSV-1 were assayed using Euroimmun anti-HSV-1 Type specific glycoprotein C1 Elisa (IgG) kits by certified laboratories (SRL and Quest Diagnostics, India (http://www.srl.in/srl/srl.asp) (www.QuestDiagnostics.in). The reference ranges were: for SRL; less than 16.0 international units (IU, HSV-1 seronegative), 16 to 100 (ambiguous) and above 100 (seropositive, HSV-1 infected). At Quest diagnostics, the cutoff values were: less than 0.9 units (negative, HSV-1 uninfected), 0.9 to 5.0 (ambiguous) and above 5 (positive, HSV-1 infected). Data from patients with ambiguous exposure were not analyzed in either sample.
Quality control for serological assays
Randomly selected samples were analyzed in duplicate (N=28). The duplicate samples matched completely with regard to HSV-1 seropositive status using the reference ranges for positive, negative or equivocal results. There were significant correlations for quantitative antibody titers between the pairs of samples, all of which were analyzed blind (r=0.92, p< 0.001). Samples with equivocal antibody titers based on predetermined cutoff values were not analyzed further (SZ, N = 9, non-psychotic, N= 10).
2.1 Sample 1, PNFU
Individuals with SZ and individuals without psychoses were included and re-assessed after 1–3 years (ages 18–50 years). Persons with substance abuse, medical or neurological disorders and those unable to complete cognitive tests were excluded.
2.2. Sample 2, RCT
All inclusion/exclusion criteria and RCT protocols were identical to our earlier US study (Prasad et al., 2013) (Figure 1). The sample comprised of consenting HSV-1 seropositive patients with early course SC (ECSZ, over 7 years of illness), aged 18–50 years, receiving stable doses of antipsychotic drugs for ≥ one month at study entry, with a score of ≥ 4 on any item of the Positive and Negative Symptoms Scale (PANSS) (Kay et al., 1987). Stratified randomization software was used to balance the VAL/PLA groups on gender and age categories (<30, ≥30years). If a consenting patient fulfills all inclusion criteria, she/he is randomised using block randomized stratified lists generated by an online randomization program (http://www.randomization.com). Randomization procedure is mentioned in details in our earlier RCT (Bhatia et al. 2014).
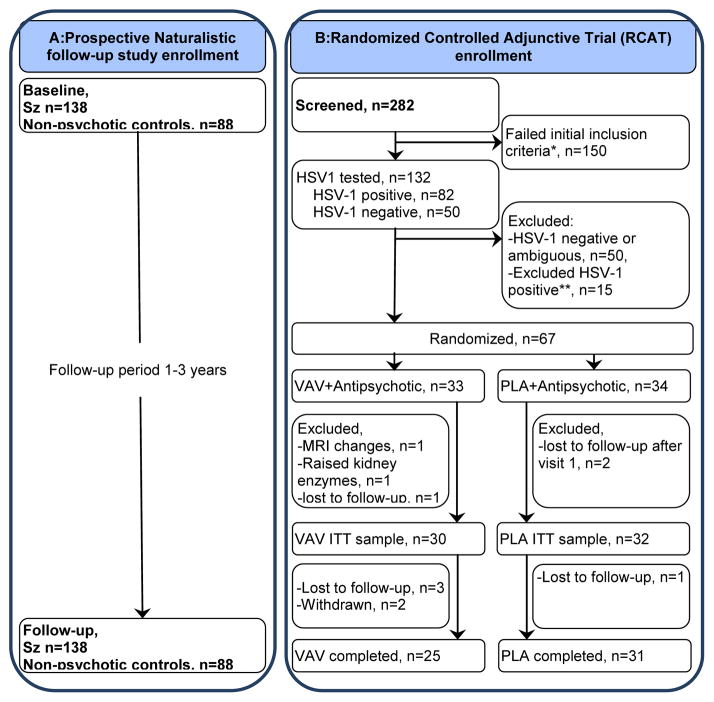
Figure 1. Flow of participants in Both Studies.
A: Prospective Naturalistic follow-up study enrollment B: Randomized Controlled Adjunctive Trial (RCAT) enrollment D
Treatment trial exclusions: aReasons for exclusion after screening: symptomatic n=5; transport problems n=8; refused, n=8; could not be contacted after screening, n=36; medical illness, n=4; comorbidity, n=6; illness >7yrs, n=16; age <18 years, n=1; age >50years, n=14; not stable on medication, n=18; illness <one year, n=7; other, n=35. bReasons for exclusion after HSV-1 assays: Raised liver enzymes, N=2, Raised uric acid levels, N=2, Refused, N=4, Lost contact, N=3, Diagnosed with gall bladder stones, N=1, Dystonia, N=1, Worsened symptoms, N=1, Age above 50, N=1
Following a two-week placebo run in, HSV-1 seropositive patients were randomized to VAL (1.5 g by mouth, twice daily) + antipsychotic treatment as usual (APTAU) or placebo (PLA) + APTAU for 16 weeks. All participants were subsequently followed up without VAL/PLA for 4 weeks. Therapists maintained stable doses of psychoactive medications if feasible, and medication changes were noted.
2.3. Clinical Evaluations
Participants in both samples were evaluated using an overlapping set of assessments with differing schedules identical to our prior RCT (Prasad et al., 2013). Cognitive parameters at all timepoints of both PNFU and RCT samples are presented as supplementary tables S1 and S2 respectively. The study was approved by the Institutional Ethics Committee at PGIMER, RMLH and the University of Pittsburgh Institutional Review Boards. All participants provided written, informed consent, with additional videotaped consent for RCT participants. The RCT was registered at Clinicaltrials.gov (NCT01794897) and the Indian clinical trial registry (CTRI/2013/10/004078). A Data Safety Monitoring Board monitored the RCT.
2.4. Data analysis
Data errors / missing data were reconciled.
2.4.1. PNFU sample
A fully conditional specification (FCS) was used to perform multiple imputations (van Buuren, 2007). Missing data were imputed in 10 dataset iterations and pooled analysis of the imputed datasets was performed using generalized linear models (GLM). Baseline comparisons utilized GLM with HSV-1 seropositive state as the predictor and diagnostic status, sex and age as covariates. For temporal trajectory analyses, GLM analyses were conducted for each cognitive domain using as the dependent variable the change in z score between baseline and follow-up divided by the duration of follow up for the respective cognitive domain (i.e., slope of cognitive change). HSV-1 status was used as the predictor and the correlates included diagnostic status (SZ/not SZ), sex, age and the baseline measure for the relevant cognitive domain. An interaction term between diagnostic status and HSV-1 status was added in a subsequent model using Statistical Package for Social Sciences (SPSS v21)(IBM Corp, Released 2012.).
2.4.2. RCT sample
We used intention to treat analysis. With three time-points of assessment, after randomization (visit 2), after completing VAL/PLA (visit 7) and one month after completing VAL/PLA (visit 8), we used a mixed model repeated measures analytical method. The model included arms (VAL, PLA), time (visit 2, 7, 8), time*arm interaction, gender, age and SES. For the two intervention groups (VAL, PLA), effect sizes for the changes in cognitive domains at different assessment points in relation to visit 2 values were calculated by Cohen’s D, adjusted for the covariates age, gender and SES. Changes from visit 2 were examined using mixed model analysis.
3. Results
3.1 PNFU sample
3.1.1. Comparison between HSV-1 infected and HSV-1 uninfected participants at study entry
We recruited 412 participants after informed consent (patients n=265 and controls n=146) at baseline and blood samples were drawn. Out of these 65 individuals (cases 44, control 21) were equivocal on lab testing and we could not get report of 24 individuals. The sample with follow-up information comprised 138 patients with SZ and 88 individuals without psychosis. The sample included some participants from our published cross-sectional study (overlap: SZ, N = 29, non-psychotic, N = 46) (Thomas et al., 2013). HSV-1 infected individuals were significantly older than the uninfected individuals (Table 2). There were no significant differences in the proportions of individuals with/without SZ in the HSV-1 infected and uninfected groups. There were no significant differences by gender or SES between the HSV-1 infected/uninfected groups. HSV-1 positive and negative participants were compared on cognitive domains separately for schizophrenia and healthy samples and are presented as supplementary tables S3. S4.
Table 2.
Demographic details of participants.
| Prospective Naturalistic Follow up Study (Sample 1) | Randomized Controlled Adjunctive Trial (Sample 2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HSV-1 exposure status | T or X2 | p | Randomization status | T- or X2 value | p | ||
| Seronegative (N=95, 42.03%) | Seropositive (N=131, 57.03%) | VAV (N=30) | Placebo (N=32) | |||||
| Age (years) | 31.47±8.86 | 35.01±9.30 | −2.88 | 0.004 | 31.77±8.55 | 30.75±8.68 | −0.46 | 0.644 |
| Gender (male/female) | 65/30 | 74/57 | 3.31 | 0.07 | 15/15 | 18/14 | 0.24 | 0.622 |
| HOH Occupation (1/2/3/4) | 8/18/17/48 | 5/18/22/79 | 3.93 | 0.28 | 2/0/7/21 | 1/4/6/21 | 4.35 | 0.273 |
| Diagnostic status (SZ/not SZ) | 55/40 | 83/48 | 0.69 | 0.41 | 30/0 | 32/0 | - | - |
| Duration of follow up (years) | 1.96±1.03 | 1.92±1.09 | 0.26 | 0.79 | NA | NA | NA | NA |
| Duration of Illness (weeks) | 254.42 ±104.89 | 258.47 ±122.42 | 0.14 | 0.89 | ||||
HOH: Head of the household. HOH occupation categories: 1. managerial and professional specialty occupations; 2. technical, sales and administrative support occupations; 3. service occupations (household, protective); 4. other occupations (farming, forestry, fishing, mechanic, construction, transportation, laborers, armed services, homemaker, student, unemployed, retired).
All the patients with schizophrenia received psychotropic drugs, whereas none of the control individuals were taking such drugs. In the multivariate analyses that included diagnostic status (schizophrenia / not schizophrenia), it is therefore difficult to disentangle possible associations with psychotropic drug effects. Nevertheless, we found that cognitive dysfyunction worsened over time to a greater extent in the HSV-1 exposed group in the entire sample, regardless of diagnostic group status. This point is now emphasized in the discussion section.
3.1.2. Cognitive functions among HSV-1 infected and HSV-1 uninfected participants at study entry
Participants were encouraged to complete all cognitive assessments, but some participants could not do so. Six cognitive outcomes had data missing rates ranging from 1–8%, except for the cognitive domains of spatial ability (31%) and attention (27%); these data were imputed. After co-varying for age, sex and SZ diagnosis, GLM analysis indicated significantly reduced accuracy scores for EMOD (B = −0.28, p = 0.018), SMEM (B = −0.25, p = 0.035), SPA (B = −0.20, p = 0.050) among HSV-1 infected participants compared with HSV-1 uninfected participants (Supplementary Table S5). When cognitive domains were analyzed in relation to SZ status, with age, sex and HSV-1 exposure as covariates, individuals with a diagnosis of SZ had significantly lower scores in all cognitive domains (data not shown).
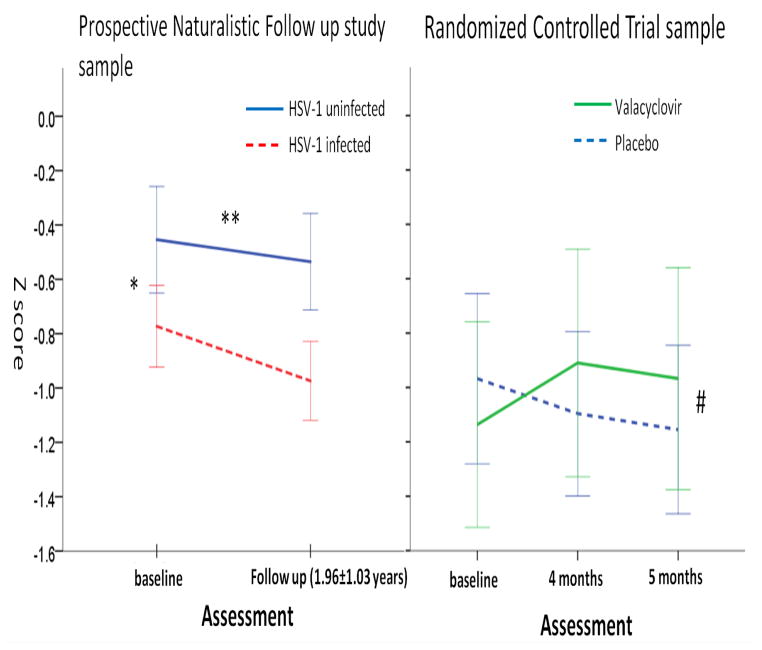
3.1.3. Temporal trajectories of cognitive change in relation to HSV-1 exposure
The mean follows up duration was 1.93 years (Standard deviation, SD = 1.07). The temporal slope of accuracy for EMOD was significantly greater among HSV-1 infected participants, compared with HSV-1 uninfected participants, co-varying for SZ status, sex, age and baseline accuracy score for EMOD (B=−0.15, p=0.033), suggesting that temporal decline in EMOD performance was greater among infected individuals (Fig 2, Supplementary Table S5). In this model, accuracy estimates for EMOD at baseline and SZ diagnosis were also significantly associated with the temporal slope for EMOD. When an interaction term between diagnostic group and HSV-1 exposure status was added to this model, it did not attain statistical significance (data not shown). Significant associations between HSV-1 exposure and temporal trajectories of change were not observed for any of the other cognitive domains (data not shown).
Figure 2. Changes in Accuracy scores for Emotion Identification in each sample.
The prospective naturalistic follow up sample included patients with schizophrenia and non-psychotic control individuals. The randomized controlled trial sample included only HSV-1 infected patients with schizophrenia. *: p = 0.025; HSV-1 infected persons performed significantly worse than HSV-1 uninfected persons at baseline. ** p = 0.03; Temporal change in HSV-1 infected persons significantly different from HSV-1 uninfected persons. # p = 0.048; Temporal change in Valacyclovir treated persons significantly different from placebo treated persons.
3.2. RCT sample
The flow of participants is illustrated in Figure 1. Among patients referred by therapists (N=282), 132 individuals were evaluated for HSV-1 exposure following assessment of inclusion criteria. Among patients with elevated HSV-1 antibody titers (N=82), 15 declined further participation. Among 67 patients who were randomized (N=67, total, VAL: 33, PLA: 34), 5 did not complete the PLA run in, leaving an intention to treat (ITT) sample of 62 patients (VAL 30; PLA 32). Six participants dropped out during the study (VAL, n=5 total; three lost to follow up and two withdrawn; PLA, N=1, lost to follow up). Thus, a total 56 participants completed the study (VAL 25; PLA 31). There were no significant differences between the randomized and non-randomized participants with regard to demographic or clinical variables, except duration of illness, which was significantly longer in the non-randomized group (Supplementary table S6).
There were no significant differences between the VAL and PLA participants with regard to demographic or clinical variables at baseline (Table 2). There were no significant differences between two groups with regard to cognitive functions at baseline, apart from spatial ability (SPA); the VAL group performed better on the accuracy index (p=0.021) (Supplementary table S7). The domains of SPA and ATT have relatively high missing rates (47% and 32%), but all the other cognitive functions are almost complete (less than 2% missing). There were a few missing cognitive evaluations (7th visit, N=1, symptomatic participant; 8th visit, N=1, technical problems). No participant reported diabetes, hypertension and cardiac disorders. On baseline or follow up lab investigations also, no report of raised glucose level was observed in the RCT sample. On baseline or follow up lab investigations also, elevated blood glucose levels were not observed in the RCT sample.
3.2.1. Changes in cognitive functions following VAL/PLA treatment
Compared with PLA, VAL treated participants had significantly greater improvement in the accuracy index of EMOD, using analysis of the ITT sample or only the sample that completed the entire treatment (p=0.048, Cohen’s D=0.43; analysis of RCT completers) (Figure 2). There were no significant differences between the VAL and PLA groups for the remaining cognitive functions assessed with the CNB, or the TMT. The sample had over 80% power to detect effect size of 0.8, the mean value for the significant effect sizes in a prior RCT sample (ranges 0.34–1.14) (Prasad et al., 2013).
3.2.2.Clinical Severity, Social Functions
No significant change was reported on any of the positive, negative global psychopathology symptoms, CGI scores, ILSS-I, ILSS-SR, Quality of life, GAF and Sheehan Disability Scale in any group from visit 1 to visit 7 and 8.
3.2.3. Adverse effects
Vomiting and nausea were reported more frequently in the VAL group but there was no significant VAL-PLA difference (VAL, n = 15, PLA, n = 9; p = 0.18). Dizziness was reported more frequently in the PLA group (VAL, n = 4, PLA, n = 12; p = 0.049). Other complaints included constipation (VAL, n=2, PLA, n=3), body ache (VAL, n = 2; PLA, n=1) and ‘rolling of eyes’ (PLA, n=1).
3.2.4. Antipsychotics and other drugs
All the participants received antipsychotic drugs throughout the study; there were no differences in the proportions receiving atypical antipsychotic drugs between the VAL and PLA groups (VAL 63%; PLA 75%). Chlorpromazine equivalents were calculated for all antipsychotics and it ranged between 100mg to 2350mg and there was no significant difference between VAL and PLA group on doses of antipsychotics. Majority (46/67 (68%); VAL 23; PLA23) of the participants were prescribed trihexyphenidyl. In the RCT sample there were similar proportions of drug adjunct or placebo treated patients who reported taking Vitamin B12 (N=12, Valacyclovir group; N= 8, placebo group), two (PLA) and one (VAL) participants were on folic acid and none on Vitamin D.
4. Discussion
At study entry in the PNFU sample, significant dysfunction was observed among HSV-1 infected participants in three of eight cognitive domains, consistent with prior cross-sectional studies. In particular, HSV-1 infected persons not only performed worse at study entry with regard to EMOD, but they also had a greater decline in EMOD over time. The greater temporal EMOD decline is notable because it also improved significantly following VAL treatment in the RCT sample. Taken together, the results suggest a novel link between HSV-1 infection and EMOD, which estimates the ability to discriminate between emotions and is thus considered an important component of social cognition (Gur et al., 2010). Emotional identification and discrimination, also known as emotion perception (EP), is an important aspect of social cognition and is associated with social function and outcome of patients with schizophrenia (Schneider et al., 2006; Couture et al., 2006). EP could mediate the relationship between neurocognition and functioning outcome and, as such, is highly relevant for daily lives of individuals with SZ.
Similar to the Indian sample, the domains that improved with VAL in our prior US RCT sample (verbal memory, working memory and visual object learning) also worsened over time in another US sample (Prasad et al., 2011). To understand reasons for the different patterns of improvement, we compared the US and Indian RCT samples. The Hindi Penn CNB battery used in the Indian RCT did not assess verbal memory, one of the domains that improved significantly in the US RCT study. Working memory estimates in the US study were based on the 2-back test, but Indian participants found it difficult to complete this test, leading to relatively high rates of missing data. There were no significant differences in demographic variables between the samples, but the US sample generally rated worse on accuracy measures (data not shown). Thus, the distinctive improvement patterns could be attributed partially to the different versions of the Penn CNB used, but additional variables like HSV-1 strain differences in US and India need to be investigated. We need to compare HSV-1 stains in the Indian and the US sample.
While the effect size for the cognitive dysfunction is relatively small in the PNFU sample, the relatively high prevalence of HSV-1 infection increases its potential public health impact. The temporal change in cognitive dysfunction could contribute to ‘cognitive aging’ (Nimgaonkar et al., 2016), a process that is arousing public health concern (Blazer et al., 2011). At the other end of the lifespan, even subtle temporal cognitive changes in children related to HSV-1 exposure (Tarter et al., 2014) could markedly alter the trajectory of neurodevelopment and increase risk for childhood psychiatric disorders (Hotez, 2014). The effects of chronic infections such as HSV-1 could be long lasting and more ominous than acute infections that can cause temporary cognitive dysfunction. Valacyclovir is highly HSV-1 specific antiviral agent, and studies have shown that it is very safe drug (Tyring et al. 2002). We checked drug interaction checker on drugs.com for interaction of valacyclovir with antipsychotics, there was no mild, moderate or high interaction with any known antipsychotics. On pubmed search also there was no study found showing any interaction.
Several mechanisms are plausible for the observed cognitive dysfunction. Primary HSV-1 infection typically targets the oro-nasal or genital mucosa, followed by retrograde transport of virions into sensory ganglia where a latent stage ensues in sensory ganglia, including the trigeminal ganglion. Latent infection in the brain could also occur via the olfactory epithelium, with further progression to the frontotemporal region (Becker, 1995). Viral DNA is detectable in over a third of post-mortem brain samples from individuals without prior encephalitis (Karatas et al., 2008) and rodent studies indicate that latent HSV-1 infection can occur in the brain (Yao et al., 2014). Viral replication is suspended during latency and gene expression limited to a few non-translated sequences, but three mechanisms could explain the present associations. First, recurrent cycles of latency and recurrence in relatively circumscribed brain regions could cumulatively impair cognitive function. This mechanism could explain the patchy cognitive dysfunction and the differences in dysfunctional cognitive domains in the published studies and the cortical gray matter volume reduction (Table 1) ( Prasad et al., 2011; Prasad et al., 2012). Second, initial exposure in utero or in childhood could cause sub-clinical brain damage that alters the trajectory of neurodevelopment, compromising cognitive function over time. Third, repeated reactivation outside the blood brain barrier could disrupt cognitive functions through release of cytokines that can cross the blood brain barrier ( Kennelly, 2013; Prasad et al., 2012; Sparkman et al., 2006).
Some limitations of the studies should be noted. The improvement in EMOD with VAL attained only modest statistical significance. On the other hand, the temporal decline in the same variable among HSV-1 infected individuals in the PNFU sample suggests that VAL-related benefit suggests a link with its antiviral effects. A variable correlated with HSV-1 infection could arguably be the primary source of the observed dysfunction in EMOD in the PNFU sample. Though SES was included as a covariate in the analyses, other infectious agents that frequently cause chronic neuro-viral infections, such as cytomegalovirus (CMV) and herpes simplex virus, type 2 (HSV-2) could also explain the associations (Watson et al., 2013). Exposure to HSV-2 is relatively low in the Indian population (11.3% in females, 8.9% in males) (Looker et al. 2008; Sgaier et al., 2011) and CMV infection is high (95%) (Chakravarti et al., 2009), so these infections are unlikely to explain the present associations. The effects of psychotropic medications were not examined, but there is no evidence for different patterns of medication prescription for HSV-1 infected/ uninfected patients. The faces used in Penn-CNB for emotions are not from Indian population. However, the emotions and faces used in Penn-CNB are culture neutral. Beupre and Hess (2005) compared emotion recognition in three cultures and found that there are no significant differences across cultures when their members see equivalent emotional expressions from persons of their own culture or of a different culture. When members of different cultural groups decode equivalent prototypical expressions from members of their own or other cultural groups, highly similar judgments are made.
The present studies suggest several avenues for future research, because the search for even modest risk factors for cognitive dysfunction is important from a public health perspective. Additional prospective cohort studies are needed to evaluate a possible causal relationship between non-encephalitic HSV-1 exposure and cognitive dysfunction. To evaluate causality between common risk factors and chronic disorders, Bradford Hill proposed nine guidelines (Hill, 1965), of which five were fulfilled by prior studies of HSV-1 exposure and cognitive dysfunction (Prasad et al., 2012). New biomarker studies or antibody assays for other HSV-1 antigens could be informative. Rigorous studies are also needed to evaluate whether, in addition to cognitive dysfunction, HSV-1 infected persons experience greater functional decline and whether they are at risk for other neuropsychiatric disorders. Additional RCTs are also needed to further explore the novel cognitive changes with VAL adjunctive treatment among persons without SZ.
In conclusion, HSV-1 exposure was associated with dysfunction in the cognitive domains of EMOD, sensorimotor function and spatial ability at study entry among individuals regardless of SZ diagnosis. HSV-1 infection was also associated with greater temporal decline in EMOD. The RCT suggests improvement in EMOD among HSV-1 infected persons with SZ, following VAL treatment. The results suggest a new approach for treating EP deficits in schizophrenia.
Supplementary Material
Acknowledgments
Role of Funding Source
Funds for this research are from Grants from the Department of Science and Technology, Government of India, Delhi to SND (SR/CSI/63/2010(G); the Stanley Medical Research Institute, Bethesda MD to RHY, VLN (07R-1712) and FBD (07R-1690); NIH, Bethesda MD to VLN (MH63480; D43 TW009114). The funding agencies are not responsible for the design and conduct of the study; collection, management, analysis, or interpretation of the data; nor for preparation, review, or approval of the manuscript.
We thank our research participants and their families, as well as our dedicated research team members N.N.Mishra, PhD, Gyan Deepak Shah, MSc, Hemant Pande, Deepak Malik, and Nupur Kumari. We also thank Dr. Ashutosh, Dr.Rahul Saha, Dr. Shrikant Sharma, Dr. Kiran Jakhar, Dr. Mona Chowdhary, Dr. Aastha Sharma, Dr. Aishwarya George and all other clinicians at the Dept. of Psychiatry, PGIMER-Dr. RML Hospital; study consultant Dr.Rajesh Nagpal, MD (Manobal Klinik, New Delhi, India); our DSMB colleagues Drs. Naseem Shah (chair), Dr V. Sreenivas,, Dr. Jaspreet Brar and Dr. S. Dayal.
Footnotes
Contributors:
Triptish Bhatia: Review of literature, design, retrieving data, preparing first draft.
Joel Wood: Statistical analysis and drafting and reviewing manuscript.
Satish Iyengar: Designing statistical analyses, review and interpreting results.
Rampratap Beniwal: Revision of the manuscript and review of literature.
Sreelatha S. Narayanan: Retrieving data and review of literature.
Konasale M Prasad: Critical revision of the manuscript and design.
Kehui Chen: Statistical analyses, interpreting the data and designing analyses.
Robert H Yolken: Critical revision of the manuscript for important intellectual content
Faith Dickerson: Designing the protocol and Critical revision of the manuscript.
Ruben C Gur: Critical revision of the manuscript for important intellectual content especially in cognitive assessment.
Raquel E Gur: Critical revision of the manuscript for important intellectual content especially in designing cognitive tools.
Smita N Deshpande: Study design and critical review.
Vishwajit L Nimgaonkar: Study concept, design, review and editing.
Conflict of interest
There is no conflict of interest to be reported by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63(6):610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Capuano AW, Aiello AE, Turner AD, Yolken RH, Torrey EF, Bennett DA. Cytomegalovirus Infection and Risk of Alzheimer Disease in Older Black and White Individuals. J Infect Dis. 2014;211(2):230–237. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y. HSV-1 brain infection by the olfactory nerve route and virus latency and reactivation may cause learning and behavioral deficiencies and violence in children and adults: a point of view. Virus Genes. 1995;10(3):217–226. doi: 10.1007/BF01701811. [DOI] [PubMed] [Google Scholar]
- Bhatia T, Agarwal A, Shah G, Wood J, Richard J, Gur RE, Gur RC, Nimgaonkar VL, Mazumdar S, Deshpande SN. Adjunctive cognitive remediation for schizophrenia using yoga: an open, non-randomized trial. Acta Neuropsychiatr. 2012;24(2):91–100. doi: 10.1111/j.1601-5215.2011.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Yaffe K, Siverman CT. Cognitive Aging: Progress in Understanding and Opportunities for Action. National Academies Press; 2011. [PubMed] [Google Scholar]
- Chakravarti A, Kashyap B, Matlani M. Cytomegalovirus infection: an Indian perspective. Indian J Med Microbiol. 2009;27(1):3–11. [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83–88. doi: 10.1016/j.schres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Sullens A, Origoni A, Leister F, Krivogorsky B, Yolken R. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008;22(7):1103–1107. doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, Krivogorsky B, Yolken RH. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry. 2004;55(6):588–593. doi: 10.1016/j.biopsych.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60(5):466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Fruchter E, Goldberg S, Fenchel D, Grotto I, Ginat K, Weiser M. The impact of Herpes simplex virus type 1 on cognitive impairments in young, healthy individuals - A historical prospective study. Schizophr Res. 2015;168(1–2):292–6. doi: 10.1016/j.schres.2015.08.036. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001a;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001b;25(5):777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokkanen L, Launes J. Neuropsychological sequelae of acute-onset sporadic viral encephalitis. Neuropsychol Rehabil. 2007;17(4–5):450–477. doi: 10.1080/09602010601137039. [DOI] [PubMed] [Google Scholar]
- Hotez PJ. Neglected infections of poverty in the United States and their effects on the brain. JAMA Psychiatry. 2014;71(10):1099–1100. doi: 10.1001/jamapsychiatry.2014.1045. [DOI] [PubMed] [Google Scholar]
- IBM Corp, A., NY: IBM Corp. BM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; Released 2012. [Google Scholar]
- Karatas H, Gurer G, Pinar A, Soylemezoglu F, Tezel GG, Hascelik G, Akalan N, Tuncer S, Ciger A, Saygi S. Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis. J Neurol Sci. 2008;264(1–2):151–156. doi: 10.1016/j.jns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Jonker I, Klein HC, Duivis HE, Yolken RH, Rosmalen JGM, et al. Association between Exposure to HSV-1 and Cognitive Functioning in a General Population of Adolescents. The TRAILS Study. PLoS ONE. 2014;9(7):e101549. doi: 10.1371/journal.pone.0101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kennelly SP. Cognitive dysfunction: an important extrahepatic manifestation of hepatitis C infection? Postgrad Med J. 2013;89(1054):431–432. doi: 10.1136/postgradmedj-2012-131337. [DOI] [PubMed] [Google Scholar]
- Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86(10):805–812, A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, Hess U. Cross-cultural emotion recognition among Canadian ethnic groups. Journal of Cross-cultural Psychology. 2005;36(3):355–377. [Google Scholar]
- McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63(3):321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgaonkar VL, Yolken RH, Wang T, Chang CC, McClain L, McDade E, Snitz BE, Ganguli M. Temporal Cognitive Decline Associated With Exposure to Infectious Agents in a Population-based, Aging Cohort. Alzheimer Dis Assoc Disord. 2016;30(3):216–222. doi: 10.1097/WAD.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, Nimgaonkar VL. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. Am J Psychiatry. 2011;168(8):822–830. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Keshavan MS, Yolken RH, Iyengar S, Nimgaonkar VL. Antiherpes virus-specific treatment and cognition in schizophrenia: a test-of-concept randomized double-blind placebo-controlled trial. Schizophr Bull. 2013;39(4):857–866. doi: 10.1093/schbul/sbs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12(1):105–113. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Watson AM, Dickerson FB, Yolken RH, Nimgaonkar VL. Exposure to herpes simplex virus type 1 and cognitive impairments in individuals with schizophrenia. Schizophr Bull. 2012;38(6):1137–1148. doi: 10.1093/schbul/sbs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Koch K, Backes V, Amunts K, Shah NJ, Bilker W, Gur RE, Habel U. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163(3):442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Vannorsdall TD, Winicki JM, Mushtaq Y, Hikida T, Sawa A, Yolken RH, Dickerson FB, Cascella NG. Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophr Res. 2010;118(1–3):224–231. doi: 10.1016/j.schres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Sgaier SK, Mony P, Jayakumar S, McLaughlin C, Arora P, Kumar R, Bhatia P, Jha P. Prevalence and correlates of Herpes Simplex Virus-2 and syphilis infections in the general population in India. Sex Transm Infect. 2011;87(2):94–100. doi: 10.1136/sti.2010.043687. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106(2–3):268–274. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26(42):10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6(11):1015–28. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34(9):2126–2131. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- Tarter KD, Simanek AM, Dowd JB, Aiello AE. Persistent viral pathogens and cognitive impairment across the life course in the third national health and nutrition examination survey. J Infect Dis. 2014;209(6):837–844. doi: 10.1093/infdis/jit616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Bhatia T, Gauba D, Wood J, Long C, Prasad K, Dickerson FB, Gur RE, Gur RC, Yolken RH, Nimgaonkar VL, Deshpande SN. Exposure to herpes simplex virus, type 1 and reduced cognitive function. J Psychiatr Res. 2013;47(11):1680–1685. doi: 10.1016/j.jpsychires.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring SK, Baker D, Snowden W. Valacyclovir for Herpes Simplex Virus Infection: Long-Term Safety and Sustained Efficacy after 20 Years’ Experience with Acyclovir. J Infect Dis. 2002;186(Supplement_1):S40–S46. doi: 10.1086/342966. [DOI] [PubMed] [Google Scholar]
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- Watson AM, Prasad KM, Klei L, Wood JA, Yolken RH, Gur RC, Bradford LD, Calkins ME, Richard J, Edwards N, Savage RM, Allen TB, Kwentus J, McEvoy JP, Santos AB, Wiener HW, Go RC, Perry RT, Nasrallah HA, Gur RE, Devlin B, Nimgaonkar VL. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol Med. 2013;43(5):1023–1031. doi: 10.1017/S003329171200195X. [DOI] [PubMed] [Google Scholar]
- Yao HW, Ling P, Tung YY, Hsu SM, Chen SH. In vivo reactivation of latent herpes simplex virus 1 in mice can occur in the brain before occurring in the trigeminal ganglion. J Virol. 2014;88(19):11264–11270. doi: 10.1128/JVI.01616-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128(1–3):61–65. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.