Abstract
Purpose of Review
Intestinal epithelial cells show remarkable plasticity in regenerating the epithelium following radiation injury. In this review, we explore the regenerative capacity and mechanisms of various populations of intestinal stem cells (ISCs) in response to ionizing radiation.
Recent Findings
Ionizing radiation targets mitotic cells that include “active” ISCs and progenitor cells. Lineage-tracing experiments showed that several different cell types identified by a single or combination of markers are capable of regenerating the epithelium, confirming that ISCs exhibit a high degree of plasticity. However, the identities of the contributing cells marked by various markers require further validation.
Summary
Following radiation injury, quiescent and/or radioresistant cells become active stem cells to regenerate the epithelium. Looking forward, understanding the mechanisms by which ISCs govern tissue regeneration is crucial to determine therapeutic approaches to promote intestinal epithelial regeneration following injury.
Keywords: Intestinal epithelial cells, Stem cells, Irradiation, Regeneration
Introduction
Radiation therapy (RT) is a common treatment modality for malignant cancers and is used in approximately half of all cancer patients [1]. The major dose-limiting factor of RT is the damage to normal, non-cancerous tissues. Almost all patients undergoing RT to the abdomen, pelvis, or rectum develop acute enteritis [2]. In addition, 5–15% of the patients develop chronic enteritis due to fibrotic epithelium, ulceration, and damaged submucosa [3]. With the increasing cancer survival rate for all types of cancers [4], it is important to address the RT-induced side effects in patients. The major advances in radiation oncology have been in the increased precision of radiation dose delivery to reduce the damages to normal tissues and amplify effects on tumor cells [5]. Such advancements include image-guided radiation therapy and tumor-targeted radiosensitizers. However, currently available treatments for patients experiencing radiation enteritis are symptomatic cares for diarrhea, dehydration, malabsorption, and abdominal or rectal discomfort [1]. Amisfostine, an organic thiophosphate that can act as a free radical scavenger, is the only drug used in clinics to protect normal tissues from radiation-induced toxicity [1]. Considering a large number of cancer patients receiving radiotherapy and the critical functions it plays in cancer cures, the adverse effects of RT on the intestinal epithelial physiology warrant investigation.
Intestinal Stem Cells (ISCs)
The mammalian intestinal epithelium is a rapidly self-renewing tissues, with the entire epithelium replaced in approximately 3–5 days. Intestinal stem cells and progenitor cells that reside within the proliferative compartment of intestinal crypts are responsible for the rapid renewal of the tissue [6]. The cells within the crypts divide and differentiate as they migrate up the differentiated compartment of intestinal villi, which are primarily composed of absorptive enterocytes, goblet cells, enteroendocrine cells, and tuft cells [6].
Until now, two populations of ISCs have been identified. Active intestinal stem cells (aISCs), also called crypt base columnar (CBC) cells, rapidly divide to upwardly give rise to progenitor cells within the transit-amplifying (TA) zone [7]. The aISCs are major contributors to epithelial renewal, yet are sensitive to injuries incured from radiation or chemotherapy. Markers that identify aISCs include LGR5, ASCL2, OLFM4, SMOC2, PROM1, and SOX9lo [8–13]. Located at “+4 to +6” positions from the bottom of the crypts are quiescent stem cells that are resistant to stress but become activated upon perturbation to aISCs, thus named reserve intestinal stem cells (rISCs). This population was initially proposed by Potten and colleagues as the labeling-retaining cells (LRCs) that are long-living dormant cells but with proliferative potential [14]. Several proposed markers for rISCs include BMI1, MTERT, HOPX, LRIG1, SOX9hi, DCLK1, and KRT19 [15–22]. The development of lineage-tracing mouse models using promoter-driven expression of reporter genes, such as GFP, RFP, or LacZ, and inducible Cre recombinase system enormously contributed to identifying ISC markers. These mice showed that both active and reserve ISCs possess two major stem cell characteristics: the capacity to self-renew and to generate differentiated cells. In addition, the crypt TA zone harbors lineage-committed progenitors that express specific cell markers, such as ALPI for enterocyte progenitors [23], DLL1 or ATOH1 (MATH1) for secretory progenitors [24], and KRT19 for TA cells and rISCs [22]. Known ISC markers or those that regulate ISC function are summarized in Appendix Table 1.
Table 1.
Intestinal stem cell markers and functional proteins during homeostasis and post-irradiation regeneration
| Gene name | Name | Expression pattern | Functions during homeostasis and post-IR regeneration | References |
|---|---|---|---|---|
| Active ISC markers (CBCs) | ||||
| Lgr5 | Leucine-rich repeat-containing G protein-coupled receptor 5 | aISCs | ˗ Lgr5 is a WNT target gene that encodes a receptor for R-spondins that are involved in maintaining the expression of surface frizzled receptors to enhance WNT signaling ˗ Marks rapidly cycling stem cells that maintains the homeostasis of the intestinal epithelium with the capacity for multilineage differentiation and self-renewal ˗ LGR5+ cells are more susceptible to radiation injury and undergo apoptosis within 24 h following irradiation ˗ A subpopulation of LGR5+ cells can survive radiation injury and regenerate crypts |
[8, 25–30] |
| Olfm4 | Olfactomedin-4 | aISCs | ˗ Olfm4 encodes secretory glycoprotein olfactomedin 4, where the molecular function of OLFM4 is unknown. ˗ Olfm4 gene expression is WNT-independent; however, it is regulated by the NOTCH signaling pathway in CBCs and progenitor cells ˗ Lineage tracing with Olfm4-IRES-eGFPCreERT2 mice showed that OLFM4+ cells are long-lived and multipotent ˗ mTOR signaling is important for OLFM4+ aISC-driven crypt regeneration following irradiation |
[10, 31–34] |
| Smoc2 | SPARC-related modular calcium binding 2 | aISCs | ˗ SMOC2 is a matricellular protein involved in matrix assembly ˗ Lineage-tracing with Smoc2-EGFP-ires-CreERT2 mice showed that SMOC2+ cells are long-lived and multipotent cells ˗ The function of SMOC2+ cells in IR response is not determined |
[11] |
| Ascl2 | Achaete scute-like 2 | aISCs | ˗ Ascl2 is a WNT target gene, where its expression is enriched in LGR5+ aISCs ˗ It maintains the stemness of LGR5+ aISC ˗ Ascl2 gene is expressed in the entire crypt during crypt regeneration, but the function of ASCL2+ cells in IR response is not determined |
[9, 26, 35] |
| Rnf43 and Znrf3 | Ring finger protein 43/zinc and ring finger 3 | aISCs | ˗ RNF43/ZNRF3 are RING-type E3 ubiquitin ligases that negatively regulate the WNT signaling pathway by targeting frizzled receptors for degradation ˗ Their roles in IR response are not determined |
[36] |
| Tnfrsf19 (Troy) | TNF (tumor necrosis factor) receptor superfamily, member 19 | aISCs | ˗ Tnfrsf19 is a WNT target gene that encodes a receptor tyrosine kinase required for cell migration and positioning in the intestinal crypts ˗ Lineage tracing with Troy-CreERT2;Rosa-LacZ mice shows that TROY+ cells are long-lived and multipotent ˗ Troy negatively regulates LGR5-mediated signaling ˗ Its role in IR response is not determined |
[37] |
| Reserve ISC markers | ||||
| Lrig1 | Leucine-rich repeats and immunoglobulin-like domains 1 | qISCs (+4 position) | ˗ LRIG1 is a negative inhibitor of ERBB signaling and functions to regulate aberrant proliferation by stem cells ˗ LRIG1+ cells are slowly cycling and long-living ˗ LRIG1+ cells are radioresistant and capable of crypt regeneration following 8-Gy irradiation |
[19, 38] |
| Hopx | Homeodomain-only protein | rISCs (+4 to +7 position) | ˗ Hopx encodes a homeodomain-only protein, but the function of this protein in ISCs is unknown ˗ HOPX+ cells are slow-cycling stem cells found at +4 position and capable of giving rise to rapidly cycling ISCs (CBCs) ˗ mRNA and protein expressions detected in the CBCs; however, Hopx-driven reporter proteins are usually found at +4 to +7 positions ˗ HOPX + cells have differential expressions of CD24/CD44 compared to Lgr5 + cells ˗ HOPX + ISCs contribute to crypt regeneration following 12-Gy irradiation |
[11, 20, 39–41] |
| mTert | Mouse telomerase reverse transcriptase | rISCs (+4 position) | ˗ mTert encodes mouse telomerase reverse transcriptase that regulates telomerase activity ˗ mTERT+ cells are slow-cycling and multipotent cells found at +4 position ˗ mTERT+ cells are radioresistant and capable of surviving 1- or 10-Gy irradiation and contributing to crypt regeneration |
[17, 42] |
| Markers with differential expression pattern | ||||
| Bmi1 | Polycomb group RING finger protein 4 | Reserve ISCs (+4 position) and predetermined enteroendocrine cells | ˗ BMI1 is a member of polycomb group of transcription repressors that are expressed in slow-cycling stem cells found at +4 position ˗ BMI1+ cells can replace aISCs when LGR5+ cells are depleted ˗ BMI1+ cells are radioresistant and capable of regenerating crypts following 12-Gy irradiation ˗ Notably, the Bmi1-lineage tracing model (Bmi1-CreER) has a penetrance of 10% and is limited to the proximal region of the small intestine ˗ mRNA expressions detected in LGR5+ and TA cells; however, Bmi1-driven reporter proteins are usually found at supra-Paneth positions (+1 to +6 positions) ˗ Transcriptome and open chromatin structure analyses showed that Bmi1 + cells may be predetermined enteroendocrine cells |
[11, 25, 26, 39, 43, 44, 45••] |
| EphB2 | EPH receptor B2 | Highest in active ISCs and gradually decreased as cells differentiate | ˗ EphB2 is a WNT target gene and a receptor tyrosine kinase that is required for cell migration and positioning in the intestinal crypts ˗ EphB2hi cells express mRNA of ISC-specific markers, such as Lgr5 and Ascl2, capable of forming in vitro organoid ˗ Its role in IR response is not determined |
[46–49] |
| Prom1 (CD133) | Prominin-1 | Active ISCs and TA cells | ˗ Prominin-1 is a transmembrane protein ˗ PROM1+ cells that co-expresses Lgr5 are capable of self-renewal and multilineage differentiation ˗ Prom1-CreERT2-IRES-nLacZ-PGK-Neo mice showed that PROM1 + cells are long-living and multipotent cells ˗ Its role in IR response is not determined |
[12, 50] |
| Msi1 | Musashi-1 | Active ISCs and reserve ISCs (+4 position) | ˗ Msi1 encodes an RNA-binding protein that regulates proliferation through activation of WNT and NOTCH signaling pathways ˗ Using Msi1-eGFP mice, GFPHi cells co-express mRNA of rISC markers mTert, Hopx, and Lrig1, whereas GFPlo cells express high levels of Lgr5, Ascl2, Olfm4, and Smoc2 ˗ MSI1 and MSI2 expressions in rISCs are required for effective regeneration following 12-Gy irradiation. |
[51–53] |
| Sox9 | SRY (sex-determining region Y)-box 9 | Sox9 EGFPLO (using Sox9 EGFP transgenic mouse) in active ISCs; Sox9 EGFPHI in +4 to +7 reserve ISCs; also expressed in enteroendocrine cells | ˗ SOX9 is a transcription factor that has dose-dependent functions in stem cells and precursor cells ˗ SOX9 EGFPLO cells are expressed in rapidly cycling stem cells that reside at the crypt base at a relatively low level ˗ Sox9 EGFPHI cells are slow-cycling and are enriched for Bmi1 and Hopx ˗ Sox9 EGFPHI cells are radioresistant, and SOX9 is required for crypt regeneration following 12-Gy irradiation |
[13, 18, 54] |
| Mex3a | Mex-3 RNA-binding family member A | Subpopulation of slow-cycling LGR5+ cells at +3 to +4 positions | ˗ MEX3A is an RNA-binding protein that regulates a transcription factor CDX2 ˗ LGR5+MEX3Ahigh cells are a slow-cycling and multipotent subpopulation of LGR5+ cells ˗ LGR5+MEX3Ahigh cells are radioresistant and survive at 48 h following 12-Gy irradiation |
[55, 56••] |
| Dclk1 | Doublecortin and CaM kinase-Like-1 | rISCs (+4 position) and differentiated Tuft cells | ˗ DCLK1 is expressed in a subpopulation of MSI1+ cells at +4 position and long-lived intestinal Tuft cells ˗ In situ hybridization showed that mRNA of Dclk1 is also expressed in a subpopulation of Lgr5 + cells at the crypt base ˗ DCLK1+ Tuft cells are capable of self-renewing and functioning as stem cells following dextran sodium sulfate-induced injury ˗ Inhibition of the NOTCH pathway following 12-Gy irradiation decreases DCLK1+ population and reduces crypt regeneration ˗ The protein kinase ataxia-telangiectasia mutated (ATM)-mediated DNA repair following radiation injury requires interaction with DCLK1 |
[21, 26, 57–62] |
| Nkx2.2 | NK2 homeobox 2 | Subset of enteroendocrine cells, BMI1+ cells, and LGR5+ cells | ˗ Nkx2.2 encodes the transcription factor NK2 homeobox 2 that plays a critical function in enteroendocrine cell fate determination ˗ NKX2.2+ cells retain stem cell-like characteristics, such as the capacity to multilineage differentiation ˗ NKX2.2+ cells are radioresistant to 12-Gy irradiation |
[63] |
| Krt19 | Keratin-19 | Reserve ISCs and TA cells | ˗ Keratin-19 is an intermediate filament that maintains the cytoskeleton ˗ Lineage tracing from Krt19-CreERT mice showed that KRT19+ cells are a distinct population from LGR5+ cells ˗ KRT19+ cells are able to regenerate the epithelium following radiation injury in colon |
[22] |
| Dll1 | Delta-like 1 | Secretory precursor cells and differentiated secretory cells | ˗ DLL1 inhibits neighboring cells become secretory cells by stimulating NOTCH signaling pathways ˗ DLL1+ cells are capable of regenerating crypts following radiation injury |
[64–66] |
| Math1 (Atoh1) | Atonal BHLH transcription factor | Secretory precursor cells | ˗ MATH1 is a basic helix-loop-helix transcription factor that regulates secretory cell fate determination ˗ Deletion of Math1 results in loss of Paneth cell, without apparent consequences on CBCs during homeostasis or regeneration following radiation injury |
[67, 68] |
| Klf4 | Krüppel-like factor 4 | Subpopulation of BMI1+ rISCs at +4 position and differentiated cells | ˗ KLF4 is a transcription factor that regulates cell lineage differentiation and maintains epithelial homeostasis ˗ BMI1+ and KLF4+ cells are located at +4 position ˗ KLF4 is critical for crypt regeneration following 12-Gy irradiation |
[69, 70] |
| Stat5 | Signal transducer and activator of transcription 5 | Intestinal epithelial cells, including LGR5+ cells | ˗ STAT5 is transcription factor that plays a functional role in maintenance of intestinal epithelial cell integrity and response to gut injury ˗ STAT5 expression in LGR5+ cells is required for aISC proliferation during homeostasis ˗ STAT5 is required for crypt regeneration following 12- and 15-Gy irradiation by Lgr5 + aISCs |
[71, 72] |
Intestinal Epithelial Tissue Response to Radiation Injury
The clinically relevant γ-irradiation doses of 8 to 15 Gy are sufficient to induce intestinal epithelial injury in mice, although these doses are lethal due to bone marrow failure [73]. The mouse intestinal epithelium is capable of regenerating when exposed to total body irradiation (TBI) at doses below 14 Gy, as indicated by the increase in survival with bone marrow transplant or partial bone marrow shielding [73, 74]. However, doses above 15 Gy result in gastrointestinal acute radiation syndrome (GI-ARS) and mortality even with bone marrow transplant [74]. The regenerative responses by the intestinal epithelium can be divided into three phases: apoptotic phase, proliferative phase, and normalization phase (Fig. 1a). The apoptotic phase consists of first 2 days following irradiation. Histology and immunostaining analysis of irradiated mouse intestines show continuous crypt loss, shrinkage in crypt size, and shortening of the villi during this phase [73]. The regenerative phase follows the apoptotic phase between 2 to 4 days post irradiation, where surviving crypt cells regenerate entire crypts. While the overall number of crypts decrease in both small and large intestines, the regenerative crypts enlarge in size due to an approximately twofold increase in the number of proliferating cells. During the normalization phase, the size of the crypts and the length of the villi are restored to “homeostatic” (pre-irradiation) condition.
Fig. 1.
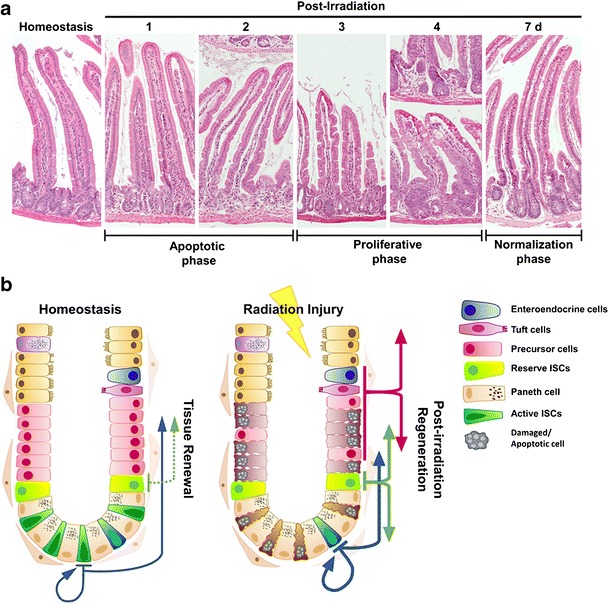
a The regenerative responses by the intestinal epithelium can be divided into three phases, apoptotic phase (first 2 days), proliferative phase (2 to 4 days), and normalization phase (7 days) following radiation injury. b The intestinal epithelial cells demonstrate remarkable plasticity during regeneration following irradiation, where multiple populations of cells contribute to regenerative responses. (Fig. 1 b) Adapted from Kuruvilla et al. with permission from Elsevier) [70]
Intestinal Epithelial Cellular Response to Radiation Injury
Recent studies using lineage-tracing mouse models demonstrated remarkable plasticity within ISCs and progenitor cells in their capability to drive regeneration of the intestinal epithelium upon injury (Fig. 1b). The LGR5+ stem cell population is highly susceptible to ionizing radiation and is diminished upon injury [25]. One prominent source of regenerative responses are rISCs, regarded as a radioresistant population owing to its quiescence. When LGR5+ aISCs are specifically depleted, rISCs enter the cell cycle, repopulate the crypt, and give rise to aISCs [44]. Of note, colonic and small intestine LGR5+ cells respond differentially to irradiation damage. Under low- to medium-dose radiation, small intestinal LGR5+ cells tolerate better compared to colonic LGR5+ cells in terms of DNA damage repair and cell proliferation at 72 h following 0.1-, 1-, or 4-Gy irradiation [75]. On the other hand, colonic LGR5+ aISCs are more resilient to high-dose irradiation injury compared to small intestine LGR5+ aISCs and survive better following 19-Gy irradiation with complete DNA double-strand break repair [76].
Multiple studies have reported that rISCs are responsible for the regenerative response following radiation injury using Bmi1, mTert, Lrig1, or Hopx promoters as the lineage-tracing drivers [17, 19, 25, 39] (Appendix Table 1). These studies confirmed that cells expressing these markers were slow-cycling, distinguishably expressed in “position +4 to +6,” and able to regenerate the epithelium by their lineages. Supporting the regenerative functions of non-LGR5-expressing stem cells, KRT19+-expressing cells at “position +4” were also determined to have stem cell functions, distinguished from CBCs, and able to regenerate the epithelium following radiation injury in colon [22].
Maintenance of quiescence in rISCs is a crucial mechanism of radioresistance. A subpopulation of BMI1+ rISCs also express KLF4, a zinc-finger transcription factor that is involved in regulation of the cell cycle by decelerating the rate of cell proliferation [70, 77]. Mice in which Klf4 is deleted from BMI1+ rISCs failed to regenerate the epithelium following 12-Gy irradiation, suggesting that the quiescence regulation by KLF4 also regulates cellular responses to radiation damage. In addition to KLF4, the transcription factor SOX9 [18] and RNA-binding proteins MSI1/2 [53] are involved in regulation of rISC quiescence via differential expression levels. The intestinal epithelial crypt cells with high levels of Sox9 expression (EGFPHI) maintain quiescence through SOX9-mediated WNT signaling suppression [18]. Lineage-tracing experiments using Sox9-CreERT2 showed that under 12-Gy irradiation, where aISCs are supposedly depleted due to their radiosensitivity, SOX9+ cells were able to regenerate crypts, whereas conditional deletion of Sox9 in the intestinal epithelium impaired this regeneration. On the contrary, MSI proteins in rISCs are dispensable in homeostatic condition, but their increased levels are required to exit from quiescence during regeneration following 12-Gy irradiation [53]. Collectively, these studies confirm our understanding that quiescence in stem cell population provides a regenerative mechanism for the intestinal epithelium to restore homeostasis when aISCs are depleted.
In addition to rISCs, contributors to regeneration following radiation injury include the short-lived secretory progenitor cells in the TA zone. Abrogation of LGR5+ aISCs in diphtheria toxin-treated Lgr5-DTR mice did not alter the epithelial structure, indicating that the intestinal epithelial renewal was maintained without LGR5+ aISCs, but the number of enteroendocrine cells in the crypts doubled [44]. The increase in secretory cells suggested that secretory progenitor cells may serve as a source of tissue renewal in the absence of LGR5+ cells. Using markers for lineage-committed secretory or enterocyte progenitor cells, lineage-tracing experiments showed that DLL1+ secretory progenitor cells participate in regeneration of the epithelium when the homeostasis is disrupted with 6-Gy radiation injury [24]. The progenitor cells stop proliferating once they are committed to secretory lineages, and they stimulate NOTCH signaling pathways in neighboring cells by expressing NOTCH ligands to induce their fate to non-secretory lineages [78]. The stalled proliferation may also provide advantages to cell survival following irradiation injury, allowing them to de-differentiate to stem cells.
While multiple studies have reported that aISCs are more susceptible to radiation injury and undergo apoptosis within approximately 24 h following irradiation [22, 25], other studies suggest that LGR5+ aISCs also contribute to tissue regeneration [30, 79]. According to Hua et al., not all LGR5+ cells undergo apoptosis within 24 h following 10- and 12-Gy irradiation, and surviving LGR5+ cells at 48 h are an important source of tissue regeneration [28]. The authors suggest that LGR5+ cells are more efficient in DNA damage repair by homologous recombination compared to progenitor and villi cells, although further studies are necessary to confirm this observation. Supporting the notion that surviving LGR5+ cells are involved in the regenerative response, depletion of LGR5+ cells using the Lgr5-DTR mouse model immediately after 10-Gy irradiation decreased the number of regenerating crypts [30]. This study demonstrates that high-dose irradiation does not completely eliminate LGR5+ cells, allowing surviving LGR5+ cells to replenish the intestinal epithelium.
The contribution of LGR5+ aISCs in the regenerative response is reasonable, considering aISCs have relatively slow proliferation rate of one cell cycle every 21.5 h, whereas progenitor cells divide once every 12 h [80]. It may be that the surviving LGR5+ aISCs were at a cell cycle phase that is less sensitive to DNA damage. Another hypothesis is that there are subpopulations of cells within LGR5+ cells identified in Lgr5-reporter mice that are differentially responsive to radiation injury. Indeed, single-cell transcript profiling of LGR5+ aISCs isolated from Lgr5-EGFP mice showed that cells with high levels of GFP expressions are not exclusively aISCs but also include early progenitors derived from aISCs [27]. Interestingly, these early progenitor cells expressed high levels of cell cycle inhibitors, such as Cdkn1a, compared to “true” aISCs, indicating that these cells proliferate at a relatively slower rate. Recently, a subpopulation of slow-cycling LGR5+ cells was identified, which expresses high levels of the RNA-binding protein Mex3a and is capable of organoid formation in ex vivo culture [56••]. Upon 12-Gy irradiation, LGR5+MEX3Ahigh cells survived and regenerated crypts. Collectively, these data demonstrate the existence of slow-cycling LGR5+ cell populations that are resistant to irradiation and may play a role during regeneration.
Reserve ISCs were first identified as label-retaining cells (LRCs) that incorporated labels in DNA and resided at position +4 [81]. A study using H2B-YFP mice for nuclear labeling showed that LRCs are secretory progenitor cells that ultimately differentiate to Paneth and enteroendocrine lineages, but with retained ability to de-differentiate into stem cells when homeostasis is perturbed [82]. Under 6-Gy irradiation, these LRCs were also capable of regenerating the crypts. Further supporting the notion that rISCs may be secretory progenitor cells, the transcriptome and open chromatin structure analyses of GFP+ cells from transgenic reporter Bmi1-GFP mice showed strong similarities to secretory progenitor cells and enteroendocrine cells, suggesting Bmi1-GFP+ cells are predetermined enteroendocrine cells [45••]. Additionally, Bmi1-GFP+ cells were enriched for enteroendocrine marker messenger RNAs (mRNAs), such as Gip, Pax6, Chga, and Chgb, confirming that these cells are enteroendocrine lineages with the plasticity to convert to regenerative population after radiation injury [45••, 83••]. Interestingly, these cells also exhibited open chromatin structures in regions that are lineage-restricted, which potentially explains the high plasticity of these cells in response to aISC depletion. These observations provide first mechanistic explanations of how BMI1+ rISCs are capable of regenerating crypts following irradiation injury.
Beside the secretory progenitors, mRNAs of putative rISC markers, Bmi1, mTert, Hopx, and Lrig1, have been found to be expressed in LGR5+ cells using transcriptome analysis and in situ hybridization [11]. Curiously, the lineage-tracing mouse models using some of these rISC markers as driving promoters did not show tracings that occurred from CBCs or with similar mitotic kinetics. This may be that the lineage tracing of cells expressing these markers does not solely depend on the mRNA or protein levels.
A comparison study using Lgr5-EGFP, Bmi1-CreERT2, and Hopx-CreERT2 transgenic mice with reporter genes and inducible Cre system showed that long-term LRCs are homogenous Paneth or enteroendocrine lineage that have lost stem cell functions, thus distinct from aISC and rISC populations [39]. In addition, LRCs have differentially expressed cell cycle genes that were distinct from rISCs identified using reporter mouse models. This suggests that position +4 are variably occupied by LRCs or rISCs, which may be two different cell populations.
Secretory progenitor cells limit their numbers in the crypts through a process called “lateral inhibition,” which induces neighboring cells to commit to highly proliferative absorptive progenitor cells [78]. On the contrary, absorptive progenitor cells are the dominant residents in the crypts, and ALPI+ absorptive progenitor cells are capable of de-differentiating to give rise to the epithelium under circumstances that obliterate crypt aISCs [23]. Despite the highest proliferation rate, which may render them more sensitive to radiation injury, absorptive progenitor cells may contribute to regeneration following radiation injury, and further studies are warranted.
Mesenchymal Stem Cells in Intestinal Stem Cell Proliferation and Regeneration
Mesenchymal stem cells (MSCs) are undifferentiated fibroblast-like cells with the capacity to differentiate into various cell types, including muscle, bone, cartilage, and fat cells [84]. Recently, MSCs have been identified as a potential treatment for regenerative disease due to their ability to regulate inflammation, angiogenesis, and regeneration [85]. In the intestinal epithelium, MSCs also function as a critical mediator of injury repair by creating microenvironments that are suitable for ISCs and progenitor cells to regenerate crypts. For example, systemic administration of MSCs in irradiated mice resulted in improved tissue regeneration by increasing animal survival and reducing colonic ulceration [86, 87] and stimulating production of growth factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) that induce anti-inflammation and stimulate angiogenesis [87, 88]. The supportive function of MSCs in the intestinal epithelial regeneration is not specific to subpopulation of cells, but rather a global effect. It is important to understand the cell-to-cell and tissue-to-tissue interactions in studying tissue regeneration in order to account for complex environments of organs. The supporting function of MSCs is a potential therapeutic target for alleviating side effects of irradiation injury. MSCs can be pre-activated by pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and nitric oxide (NO), in amplifying the paracrine effects of MSCs on the intestinal epithelial regeneration after irradiation [89]. Priming local MSCs in preparation for radiation therapy may be advantageous for patients in recovering faster from the GI symptoms.
Following up with the involvement of pro-inflammatory cytokines in potentially creating a beneficial environment for the intestinal epithelial regeneration, signal transducer and activator of transcription 5 (STAT5) activation by cytokines are critical for crypt regeneration by ISCs after irradiation [72]. While Stat5 deletion in LGR5+ aISCs resulted in impaired crypt regeneration, Stat5 activation in LGR5+ cells resulted in increased proliferation and crypt regeneration following 15 Gy irradiation. In addition to MSCs, other cell types involved in the regenerative response following irradiation injury include endothelial cells [74, 90] and immune cells [91]. Collectively, these data indicate that external environments created by supporting cells are crucial to ISC proliferation and regeneration.
Factors Involved in Intestinal Epithelial Regeneration
As previously discussed, global effects of non-epithelial cells play critical functions in the regenerative response following radiation injury. Several factors have been implicated in acceleration of the regenerative process. Such factors include general inducer of proliferation, including R-spondin 1 [92, 93] and Slit guidance ligand 2 (Slit2) [93], secretory proteins that cooperatively promote the WNT/β-catenin signaling, growth factors insulin-like growth factor 1 (IGF-1) [94] and bFGF [95] that promote growth of the regenerative crypts and impair radiation-induced apoptosis through inhibition of PUMA expressions, and prostaglandins [96]. Other factors involved in the regenerative response after irradiation injury are Pectin [97] and glucagon-like peptide 2 (GLP-2) that directly stimulates BMI1+ rISCs to promote stem cell proliferation during homeostasis and crypt regeneration following 10-Gy radiation injury. We can postulate that many other external modulatory factors are available to enhance the regeneration of the tissue. These factors may be dietary or secretory proteins contributed by epithelial or non-epithelial cells. It is of great interest to study natural molecules involved in maintenance of tissue homeostasis and regeneration.
Cellular Response to Radiation-Induced DNA Damage
The lethal effect of radiotherapy arises from cellular and molecular responses to DNA damage induced by free radicals causing double-strand breaks [98]. Ionizing radiation induces water radiolysis in cells, which generates reactive oxygen species (ROS) that become the major source of DNA damage. This type of damage is more detrimental to cells in the early DNA synthesis [99], which undergo apoptosis at a higher incidence than cells in other stages of the cell cycle. In the intestinal epithelium, aISCs and progenitor cells in the crypts are more radiosensitive. The ultimate fate of these cells can be predicted based on the presence of apoptotic factors and DNA damage repair proteins. Indeed, stem cell populations with large accumulation of DNA damages may be more detrimental to overall health of the tissue, while survival of cells capable of DNA damage repairs and tissue regeneration is crucial.
P53/P21/PUMA
The transcription factor P53 is the key regulator of DNA damage response in the intestinal crypt. Following high-dose ionizing radiation, activated P53 upregulates the expressions of P21 and PUMA [100]. The protein P21 is a cyclin-dependent kinase (CDK) inhibitor that blocks the G1-to-S phase transition of the cell cycle and allows DNA repairs in the crypts [101]. On the other hand, activation of PUMA results in aISC and progenitor cell apoptosis following DNA damage, whereas the absence of PUMA leads to improved survival of intestinal stem cells and crypt regeneration [101]. Modulation of p53 activity following irradiation increases the cell survival. KLF4-mediated inhibition of p53 resulted in suppression of apoptosis and proliferation, thus allowing DNA damage repair [102, 103]. In addition to this observation, the pro-survival effects of GSK-3 inhibitor CHIR99021 on in vitro 3D culture of LGR5+ aISCs following radiation was due to inhibition of p53-dependent induction of PUMA [104]. This suggests that inhibition of apoptotic pathways may be a therapeutic target for radiation-induced injuries.
NOTCH
The cell-to-cell NOTCH signaling in ISCs and progenitor cells has been shown to be important for stem cell functions and lineage commitments in the intestinal epithelium [33]. Deletion of Notch1 increased the number of post-mitotic goblet cells, whereas deletion of Notch2 had no effect on the lineage commitment [33]. However, inhibition of NOTCH with difluorophenacetyl-l-alanyl-S-phenylglycine t-butyl ester (DAPT) or deletions of Notch1 or Notch2 impaired crypt regeneration following irradiation injury due to absence of proliferation, suggesting that NOTCH receptors play important functions that are beyond cell fate determination, and strong NOTCH signaling is required for tissue regeneration [31, 33, 59]. Further investigations are necessary to elucidate the effect of irradiation on intestinal NOTCH signaling and its potential role in post-IR regeneration.
PI3K-AKT-mTORC1
Another important regulatory signaling pathway in intestinal epithelial tissue homeostasis is PI3K-AKT-mTROC1 signaling cascade. PTEN is a negative regulator of the PI3K-AKT-mTORC1 signaling pathway implicated in various cellular functions, such as proliferation and metabolism. PTEN is also implicated in maintenance of quiescence of mTERT+ cells at “position +4” in the crypt, and deletion of Pten resulted in loss of quiescent ISCs [105], potentially due to aberrant proliferation and exhaustion of stem cells. Interestingly, PTEN functions to regulate dormancy of mTERT+ rISCs. The GFP+ cells’ population obtained from the mTert-GFP mouse model showed lack of expression of inactive form of PTEN (P-PTEN) during homeostasis [17]. Yet, when these mice were irradiated with dose of 16 Gy, which is sufficient to eliminate the aISCs, the intestinal epithelium was able to regenerate 96 h later, possibly due to activation of rISCs, whereas Pten-deleted mice showed significantly less regenerative colonies [105]. Of note, the radiation dose used in this study induces GI-ARS, resulting in complete loss of intestinal epithelial regeneration. Thus, it is difficult to conclude that mTERT+PTEN+ rISCs play critical functions in regenerative response after irradiation, unless lower doses are used to study during the actual regenerative process.
The functions of the PI3K-AKT-mTORC1 pathway in the intestinal crypts may be cell type-specific. While the inhibition of this pathway is important for maintenance of quiescence in rISCs, mTOR plays critical functions in lineage generation and intestinal stem cell physiology of OLFM4+ aISCs through mechanisms that are not mediated by WNT activity [34]. During intestinal crypt regeneration after 10-Gy irradiation injury, deletion of mTOR in the intestinal epithelium resulted in impaired regeneration, as well as decreased level of Olfm4. This result is interesting, because the expressions of Ascl2 and mTert, which are WNT target genes and putative aISC markers, increase even with mTOR deletion [34]. This suggests that the putative stem cell markers may be indicators of active signaling pathways, and there is no bona fide single marker for ISCs, just as there are multiple essential signaling pathways involved in the maintenance of stem cell functions.
Conclusions
Radiotherapy is an essential part of cancer treatment, although clinical advancements in addressing its adverse effects on non-cancerous tissues have been insufficient. Understanding the mechanisms of intestinal epithelial regenerative processes is a critical step in developing therapeutic approaches for protection of normal tissue and may be beneficial to reestablish homeostasis post injury. The application of lineage-tracing mouse models has greatly contributed to determining cell populations responsible for the tissue homeostasis and regeneration following irradiation (Fig. 1b). However, the attempts to use a single marker to identify a cell population have resulted in an intricate list of cell markers and types. The recent focuses have been placed on delineating ISC or progenitor markers using various methods, such as transgenic reporter mouse models, RNA sequencing, in situ hybridization, and chromatin structure analysis. Looking forward, it may be worthwhile to understand the global effects on the intestinal epithelium instead of targeting a cell population that contributes to regeneration, at least in achieving our goal to develop therapeutic approaches to ameliorate the adverse effects on normal tissue by radiotherapy. This will include determining the inter- and intra-cellular key mediators of the tissue regeneration that globally influence the tissue homeostasis and regeneration.
Acknowledgments
Work from our laboratory was supported by grants from the National Institutes of Health (DK052230, DK093680, CA084197, and CA172113 awarded to V.W.Y.).
Abbreviations
- ALPI
Alkaline phosphatase
- ASCL2
Achaete-scute family BHLH transcription factor 2
- ATOH1 (MATH1)
Atonal BHLH transcription factor 1
- BMI1
B lymphoma Mo-MLV insertion region 1 homolog polycomb ring finger proto-oncogene
- DCLK1
Doublecortin like kinase 1
- DLL1
Delta-like canonical notch ligand 1
- DTR
Diphtheria toxin receptor
- GFP
Green fluorescent protein
- HOPX
HOP homeobox
- KLF4
Krüppel-like factor 4
- KRT19
Keratin 19
- LGR5
Leucine-rich repeat-containing G protein-coupled receptor 5
- LRIG1
Leucine-rich repeats and immunoglobulin like domains 1
- MEX3A
Mex-3 RNA binding family member A
- mTERT
Telomerase reverse transcriptase
- OLFM4
Olfactomedin 4
- PROM1
Prominin 1
- PUMA
p53 Upregulated modulator of apoptosis
- RFP
Red fluorescent protein
- SMOC2
SPARC-related modular calcium binding 2
- SOX9
SRY-box 9
- STAT5
Signal transducer and activator of transcription 5
Appendix
Compliance with Ethical Standards
Conflict of Interest
Chang-Kyung Kim, Vincent W. Yang, and Agnieszka B. Bialkowska declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Radiation Biology and Stem Cells
A correction to this article is available online at https://doi.org/10.1007/s40778-018-0121-0.
Contributor Information
Chang-Kyung Kim, Email: Chang.kim@stonybrookmedicine.edu.
Vincent W. Yang, Email: Vincent.Yang@stonybrookmedicine.edu
Agnieszka B. Bialkowska, Phone: (631) 638-2161, Email: Agnieszka.Bialkowska@stonybrookmedicine.edu
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15(4):360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gastrointestinal Complications (PDQ(R)) Health Professional Version. Bethesda: PDQ Cancer Information Summaries; 2002. [Google Scholar]
- 3.Yu J. Intestinal stem cell injury and protection during cancer therapy. Transl Cancer Res. 2013;2(5):384–396. [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16(4):234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 6.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 7.Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11(4):452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 9.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136(5):903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 10.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31(14):3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1108–G1118. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115(Pt 11):2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 15.Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One. 2011;6(10):e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108(1):179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149(6):1553–1563. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26(3):630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 22.Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, et al. Krt19(+)/Lgr5(−) cells are radioresistant cancer-initiating stem cells in the colon and intestine. Cell Stem Cell. 2015;16(6):627–638. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18(2):203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 24.van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14(10):1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109(2):466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. 2011;14(1):106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TH, Saadatpour A, Guo G, Saxena M, Cavazza A, Desai N, et al. Single-cell transcript profiles reveal multilineage priming in early progenitors derived from Lgr5(+) intestinal stem cells. Cell Rep. 2016;16(8):2053–2060. doi: 10.1016/j.celrep.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143(5):1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Huang YF, Kek C, Bulavin DV. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12(3):298–303. doi: 10.1016/j.stem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14(2):149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 31.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139(3):488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuijers J, van der Flier LG, van Es J, Clevers H. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Rep. 2014;3(2):234–241. doi: 10.1016/j.stemcr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402(1):98–108. doi: 10.1016/j.ydbio.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson LL, Davis AK, Grogg MW, Zheng Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. 2016;30(3):1263–1275. doi: 10.1096/fj.15-278606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V, et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 2015;16(2):158–170. doi: 10.1016/j.stem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 37.Fafilek B, Krausova M, Vojtechova M, Pospichalova V, Tumova L, Sloncova E, et al. Troy, a tumor necrosis factor receptor family member, interacts with lgr5 to inhibit wnt signaling in intestinal stem cells. Gastroenterology. 2013;144(2):381–391. doi: 10.1053/j.gastro.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 38.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14(4):401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse label-retaining cells are molecularly and functionally distinct from reserve intestinal stem cells. Gastroenterology. 2016;151(2):298–310. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, et al. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 2013;31(9):2024–2030. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Yousefi M, Nakauka-Ddamba A, Jain R, Tobias J, Epstein JA, et al. Single-cell analysis of proxy reporter allele-marked epithelial cells establishes intestinal stem cell hierarchy. Stem Cell Rep. 2014;3(5):876–891. doi: 10.1016/j.stemcr.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, et al. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci U S A. 2008;105(30):10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jadhav U, Saxena M, O'Neill NK, Saadatpour A, Yuan GC, Herbert Z, et al. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell. 2017;21(1):65–77 e5. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111(2):251–263. doi: 10.1016/S0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 47.Koo BK, Lim HS, Chang HJ, Yoon MJ, Choi Y, Kong MP, et al. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology. 2009;137(1):145–155. doi: 10.1053/j.gastro.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 48.Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8(5):511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Jarde T, Kass L, Staples M, Lescesen H, Carne P, Oliva K, et al. ERBB3 positively correlates with intestinal stem cell markers but marks a distinct non proliferative cell population in colorectal cancer. PLoS One. 2015;10(9):e0138336. doi: 10.1371/journal.pone.0138336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136(7):2187–2194. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535(1–3):131–135. doi: 10.1016/S0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 52.Maria Cambuli F, Rezza A, Nadjar J, Plateroti M. Brief report: musashi1-eGFP mice, a new tool for differential isolation of the intestinal stem cell populations. Stem Cells. 2013;31(10):2273–2278. doi: 10.1002/stem.1428. [DOI] [PubMed] [Google Scholar]
- 53.Yousefi M, Li N, Nakauka-Ddamba A, Wang S, Davidow K, Schoenberger J, et al. Msi RNA-binding proteins control reserve intestinal stem cell quiescence. J Cell Biol. 2016;215(3):401–413. doi: 10.1083/jcb.201604119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1111–G1132. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira B, Sousa S, Barros R, Carreto L, Oliveira P, Oliveira C, et al. CDX2 regulation by the RNA-binding protein MEX3A: impact on intestinal differentiation and stemness. Nucleic Acids Res. 2013;41(7):3986–3999. doi: 10.1093/nar/gkt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barriga FM, Montagni E, Mana M, Mendez-Lago M, Hernando-Momblona X, Sevillano M, et al. Mex3a marks a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell. 2017;20(6):801-16 e7. doi: 10.1016/j.stem.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137(6):2179–2180. doi: 10.1053/j.gastro.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 58.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, et al. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 2014;32(3):822–827. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu D, May R, Sureban SM, Weygant N, Chandrakesan P, Ali N, et al. Inhibition of Notch signaling reduces the number of surviving Dclk1+ reserve crypt epithelial stem cells following radiation injury. Am J Physiol Gastrointest Liver Physiol. 2014;306(5):G404–G411. doi: 10.1152/ajpgi.00088.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124(3):1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, et al. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget. 2015;6(31):30876–30886. doi: 10.18632/oncotarget.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandrakesan P, May R, Weygant N, Qu D, Berry WL, Sureban SM, et al. Intestinal tuft cells regulate the ATM mediated DNA damage response via Dclk1 dependent mechanism for crypt restitution following radiation injury. Sci Rep. 2016;6:37667. doi: 10.1038/srep37667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gross S, Balderes D, Liu J, Asfaha S, Gu G, Wang TC, et al. Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential. Am J Physiol Gastrointest Liver Physiol. 2015;309(12):G975–G987. doi: 10.1152/ajpgi.00244.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140(4):1230–40 e1-7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamataki D, Holder M, Hodgetts C, Jeffery R, Nye E, Spencer-Dene B, et al. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One. 2011;6(9):e24484. doi: 10.1371/journal.pone.0024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakata T, Shimizu H, Nagata S, Ito G, Fujii S, Suzuki K, et al. Data showing proliferation and differentiation of intestinal epithelial cells under targeted depletion of Notch ligands in mouse intestine. Data Brief. 2017;10:551–556. doi: 10.1016/j.dib.2016.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132(7):2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 68.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci U S A. 2012;109(23):8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129(11):2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuruvilla JG, Kim CK, Ghaleb AM, Bialkowska AB, Kuo CJ, Yang VW. Kruppel-like factor 4 modulates development of BMI1(+) intestinal stem cell-derived lineage following gamma-radiation-induced gut injury in mice. Stem Cell Rep. 2016;6(6):815–824. doi: 10.1016/j.stemcr.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med. 2012;4(2):109–124. doi: 10.1002/emmm.201100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbert S, Nivarthi H, Mayhew CN, Lo YH, Noah TK, Vallance J, et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015;4(2):209–225. doi: 10.1016/j.stemcr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103(4):383–399. doi: 10.1097/HP.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 75.Otsuka K, Suzuki K. Differences in radiation dose response between small and large intestinal crypts. Radiat Res. 2016;186(3):302–314. doi: 10.1667/RR14455.1. [DOI] [PubMed] [Google Scholar]
- 76.Hua G, Wang C, Pan Y, Zeng Z, Lee SG, Martin ML, et al. Distinct levels of radioresistance in Lgr5+ colonic epithelial stem cells versus Lgr5+ small intestinal stem cells. Cancer Res. 2017;77(8):2124–2133. doi: 10.1158/0008-5472.CAN-15-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15(2):92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sancho R, Cremona CA, Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep. 2015;16(5):571–581. doi: 10.15252/embr.201540188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cazzoli D, Muri RM, Schumacher R, von Arx S, Chaves S, Gutbrod K, et al. Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain. 2012;135(Pt 11):3426–3439. doi: 10.1093/brain/aws182. [DOI] [PubMed] [Google Scholar]
- 80.Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30(6):1104–1109. doi: 10.1038/emboj.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: where are they? Cell Prolif. 2009;42(6):731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495(7439):65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 83.Yan KS, Gevaert O, GXY Z, Anchang B, Probert CS, Larkin KA, et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21(1):78–90 e6. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 85.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14(9):2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semont A, Demarquay C, Bessout R, Durand C, Benderitter M, Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One. 2013;8(7):e70170. doi: 10.1371/journal.pone.0070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, et al. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. doi: 10.1038/cddis.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6(9):e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, et al. Pre-activation of mesenchymal stem cells with TNF-alpha, IL-1beta and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. doi: 10.1038/srep08718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122(5):1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francois A, Milliat F, Guipaud O, Benderitter M. Inflammation and immunity in radiation damage to the gut mucosa. Biomed Res Int. 2013;2013:123241. doi: 10.1155/2013/123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4(11):e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature. 2013;501(7465):107–111. doi: 10.1038/nature12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Landeghem L, Santoro MA, Mah AT, Krebs AE, Dehmer JJ, McNaughton KK, et al. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 2015;29(7):2828–2842. doi: 10.1096/fj.14-264010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2010;29(11):1622–1632. doi: 10.1038/onc.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stenson WF. Prostaglandins and epithelial response to injury. Curr Opin Gastroenterol. 2007;23(2):107–110. doi: 10.1097/MOG.0b013e3280143cb6. [DOI] [PubMed] [Google Scholar]
- 97.Sureban SM, May R, Qu D, Chandrakesan P, Weygant N, Ali N, et al. Dietary pectin increases intestinal crypt stem cell survival following radiation injury. PLoS One. 2015;10(8):e0135561. doi: 10.1371/journal.pone.0135561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lewanski CR, Gullick WJ. Radiotherapy and cellular signalling. Lancet Oncol. 2001;2(6):366–370. doi: 10.1016/S1470-2045(00)00391-0. [DOI] [PubMed] [Google Scholar]
- 99.Withers HR, Mason K, Reid BO, Dubravsky N, Barkley HT, Jr, Brown BW, et al. Response of mouse intestine to neutrons and gamma rays in relation to dose fractionation and division cycle. Cancer. 1974;34(1):39–47. doi: 10.1002/1097-0142(197407)34:1<39::AID-CNCR2820340107>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 100.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3(2):117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 101.Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang L, Yu J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res. 2011;9(5):616–625. doi: 10.1158/1541-7786.MCR-11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghaleb AM, Katz JP, Kaestner KH, Du JX, Yang VW. Kruppel-like factor 4 exhibits antiapoptotic activity following gamma-radiation-induced DNA damage. Oncogene. 2007;26(16):2365–2373. doi: 10.1038/sj.onc.1210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talmasov D, Xinjun Z, Yu B, Nandan MO, Bialkowska AB, Elkarim E, et al. Kruppel-like factor 4 is a radioprotective factor for the intestine following gamma-radiation-induced gut injury in mice. Am J Physiol Gastrointest Liver Physiol. 2015;308(2):G121–G138. doi: 10.1152/ajpgi.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Wei L, Cramer JM, Leibowitz BJ, Judge C, Epperly M, et al. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci Rep. 2015;5:8566. doi: 10.1038/srep08566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richmond CA, Shah MS, Deary LT, Trotier DC, Thomas H, Ambruzs DM, et al. Dormant intestinal stem cells are regulated by PTEN and nutritional status. Cell Rep. 2015;13(11):2403–2411. doi: 10.1016/j.celrep.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


