Abstract
Modifiable lifestyle factors, including exercise and activity energy expenditure (AEE), may attenuate the unfavorable health effects of obesity, such as risk factors of metabolic syndrome (MetS). However, the underlying mechanisms are not clear. In this study we sought to investigate whether the metabolite profiles of MetS and adiposity assessed by body mass index (BMI) and central obesity are inversely correlated with AEE and physical activity. We studied 35 men and 47 women, aged 30–60 years, using doubly labeled water to derive AEE and the Sedentary Time and Activity Reporting Questionnaire (STAR-Q) to determine the time spent in moderate and vigorous physical activity. Proton nuclear magnetic resonance spectroscopy was used for serum metabolomics analysis. Serine and glycine were found in lower concentrations in participants with more MetS risk factors and greater adiposity. However, serine and glycine concentrations were higher with increasing activity measures. Metabolic pathway analysis and recent literature suggests that the lower serine and glycine concentrations in the overweight/obese state could be a consequence of serine entering de novo sphingolipid synthesis. Taken together, higher levels of AEE and physical activity may play a crucial part in improving metabolic health in men and women with and without MetS risk factors.
Introduction
Obesity is a risk factor for the four most common chronic diseases worldwide: diabetes1, cardiovascular disease2, cancer3 and chronic respiratory disease such as asthma4. Together they contribute to substantial patient suffering, a serious economic cost to health care5 and lead to 30 million deaths annually6. Central (abdominal) obesity is of particular interest as it leads to the pathophysiology that results in metabolic syndrome (MetS)7,8, a cluster of risk factors (central obesity, hypertension, elevated fasting glucose and dyslipidemia) that, if untreated can lead to overt diabetes, cardiovascular disease and over the long-term to some cancers. The pathological development of MetS is characterized by an aberrant metabolism, in part consisting of a dysregulation in whole-body glucose and lipid metabolism, intra-organ lipid storage and systemic inflammation.
Since circulating metabolites are representative of systemic metabolism, they can reflect the metabolic health of an individual. Thus, metabolomics, the systematic study of a comprehensive set of metabolites in a biological compartment, has the potential to provide insight into the biological mechanisms underlying health and disease. To better understand the metabolic impact of obesity and its etiologic role in the development of diabetes and subsequent chronic diseases, attempts have been made to identify biomarkers using various ‘omics’ technologies, including metabolomics. Concentrations of branched chain amino acids (BCAA: leucine, isoleucine and valine) are the most extensively studied metabolic signatures of obesity and insulin signaling9,10 and increased plasma BCAA levels have been reported to be predictive of diabetes incidence years prior to diagnosis11,12. However, other metabolites may also play roles in the relation between obesity and obesity-related disorders.
The beneficial effects of physical activity as part of disease management and prevention have been clearly established for conditions such as type 2 diabetes, cardiovascular disease and certain types of cancer13. Yet, it is unclear through which mechanisms exercise and activity energy expenditure (AEE) attenuate the unfavorable effects of obesity, independent of weight reduction14. In this study we aimed to describe the metabolite profile associated with MetS and adiposity in relation to AEE and physical activity in weight stable men and women with and without MetS risk factors. Doubly labeled water (DLW), the gold standard methodology for assessing total energy expenditure15,16, was used to objectively assess AEE (AEEDLW) and physical activity level (PALDLW). In addition, the Sedentary Time and Activity Reporting Questionnaire (STAR-Q) was used to determine the time spent in moderate and vigorous physical activity. We hypothesized that increased adiposity and the presence of MetS risk factors would lead to gender-specific metabolic signatures17,18. We further hypothesized that some of these metabolites would be inversely associated with AEEDLW, AEE/kgDLW, PALDLW, moderate and/or vigorous physical activity, as exercise has previously been shown to influence the concentrations of circulating metabolites19–21.
Results
Data overview and characteristics of measured variables
Gender-stratification of the data was supported by the pronounced difference in the serum metabolome of men and women (R2 = 0.73, Q2 = 0.61) (Supplementary Fig. S1). Moreover, men had higher BMI, waist circumference, waist-to-hip ratio and body fat percentage compared to women (Supplementary Table S1) and presented with higher serum concentrations of BCAA and lower serum concentrations of serine and glycine, among other metabolites. No differences were seen for AEEDLW, AEE/kgDLW, PALDLW, moderate or vigorous physical activity. Information regarding all quantified metabolites can also be found in Supplementary Table S2.
The serum metabolite profiles of MetS and correlations with measures of adiposity
Men and women were dichotomized into categories representing presence of 1–3 MetS risk factors (MetSany) and absence of MetS risk factors (MetSzero). Participants with MetSany had significantly higher body measurements compared to their counterparts, and MetSany men were more likely to be overweight (BMI > 25) whereas MetSzero men were of normal weight (BMI 18.5–24.9) (Table 1). This is consistent with the known pathophysiology of MetS and its relation to adiposity22. There were no differences between groups in physical activity or DLW-derived measures of AEE.
Table 1.
Evaluation of differences in variables for MetSany compared to MetSzero for women and men separately.
| Variables | Women | Men | ||||
|---|---|---|---|---|---|---|
| MetSany Mean (SD) |
MetSzero Mean (SD) |
p-value | MetSany Mean (SD) |
MetSzero Mean (SD) |
p-value | |
| BMI (kg/m2) | 24.6 (2.8) | 21.6 (2.0) | <0.001 | 27.4 (2.5) | 23.4 (1.9) | <0.0001 |
| Body fat percentage (%) | 33.3 (5.8) | 25.2 (5.1) | <0.0001 | 24.8 (4.3) | 15.6 (3.7) | <0.0001 |
| Waist circumference (cm) | 86.9 (6.8) | 75.1 (4.5) | <0.0001 | 98.8 (8.1) | 84.7 (5.2) | <0.001 |
| Hip circumference (cm) | 103.2 (6.0) | 94.5 (5.4) | <0.0001 | 105.3 (4.3) | 97.7 (3.7) | <0.001 |
| Waist:hip ratio | 0.84 (0.04) | 0.79 (0.03) | <0.001 | 0.94 (0.06) | 0.87 (0.04) | <0.001 |
| AEEDLW (Kcal) | 1021.3 (460.0) | 948.5 (438.5) | 0.59 | 1163.0 (469.2) | 1419.8 (772.8) | 0.23 |
| AEE/kgDLW (Kcal/kg) | 15.1 (6.8) | 16.9 (7.6) | 0.42 | 13.4 (5.6) | 19.9 (10.7) | 0.024 |
| PALDLW (Kcal) | 1.9 (0.4) | 1.9 (0.4) | 0.99 | 1.8 (0.3) | 2.0 (0.5) | 0.080 |
| Moderate physical activity (hours/day) | 1.5 (1.3) | 1.6 (2.1) | 0.81 | 2.2 (2.1) | 0.9 (0.5) | 0.065 |
| Vigorous physical activity (hours/day) | 0.54 (0.6) | 0.55 (0.5) | 0.96 | 0.45 (0.5) | 0.82 (0.6) | 0.061 |
Significance was determined by two-tailed t-test, where the Benjamini and Hochberg corrected significance level was p < 0.028. Abbreviations are as follows in alphabetical order: AEEDLW, activity energy expenditure; BMI, body mass index; MetS, metabolic syndrome; PALDLW, physical activity level: SD, standard deviation.
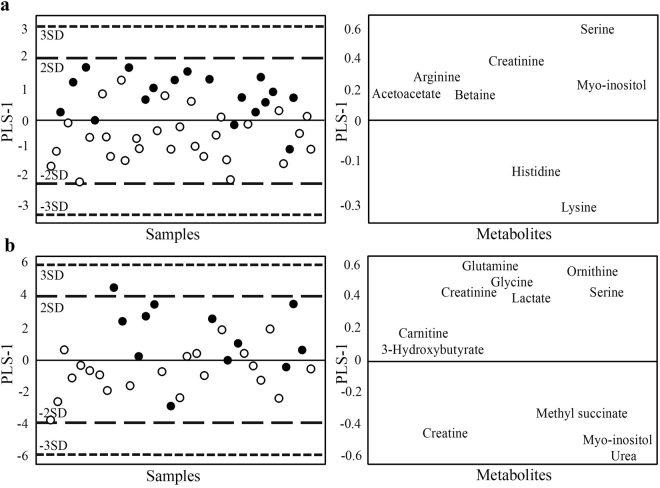
The metabolite profiles of MetS were unique for both genders, with the exception of serine and creatinine, which were lower in both MetSany men (R2 = 0.31, Q2 = 0.12) and MetSany women (R2 = 0.42, Q2 = 0.30) compared with the respective MetSzero groups (Fig. 1).
Figure 1.
Supervised OPLS-DA score scatter plots and loadings plots showing the separation between MetSany (circles) and MetSzero (dots) for (A)women and (B) men. Every dot/circle represents one participant. The score scatter plot (left) and loading plot (right) are superimposable and indicate which (VIP > 1) metabolites associate with which MetS group in women (R2 = 0.42, Q2 = 0.30) and in men (R2 = 0.31, Q2 = 0.12), respectively.
MetSany men had higher serum metabolite concentrations of methyl succinate, creatine, myo-inositol and urea and lower concentrations of glutamine, ornithine, glycine, serine, creatinine, lactate, carnitine and 3-hydroxybutyrate compared with the MetSzero group. Glycine and serine were also inversely correlated with all measures of adiposity, except that glycine was not associated with BMI class (VIP > 1, Supplementary Table S3). The observed negative correlation between glycine and the waist-to-hip ratio, a measure thought to best represent male adiposity and fat distribution, reached bivariate statistical significance (r = −0.47, p < 0.01) in addition to the positive correlation found between glycine and vigorous physical activity (r = 0.48, p < 0.01). Other trends included higher concentrations of arginine, creatine, methyl succinate, pyruvate and tyrosine with increasing body measures in men (Table 2).
Table 2.
Pearson correlation coefficients (r) and p-values (p) for significant (p < 0.05, VIP > 1) metabolites for each variable for women and men.
| Body fat percentage | r | p | BMI value | r | p | |||
|---|---|---|---|---|---|---|---|---|
| Women | ||||||||
| Serine | −0.45 | 0.0015 | Serine | −0.36 | 0.012 | |||
| Creatine | 0.31 | 0.034 | ||||||
| Hip circumference | r | p | Waist circumference | r | p | Waist:Hip ratio | r | p |
| Serine | −0.40 | 0.0049 | Serine | −0.49 | <0.001 | Serine | −0.38 | 0.0090 |
| Pyruvate | 0.30 | 0.043 | Myo-Inositol | −0.36 | 0.012 | Myo-Inositol | −0.31 | 0.032 |
| AEE DLW | Strenuous PA | r | p | |||||
| Myo-Inositol | −0.34 | 0.021 | Glutamine | −0.37 | 0.011 | |||
| Acetoacetate | 0.31 | 0.036 | Creatine | −0.32 | 0.030 | |||
| Phenylalanine | −0.32 | 0.030 | ||||||
| Carnitine | −0.29 | 0.045 | ||||||
| Men | ||||||||
| Pyruvate | 0.41 | 0.015 | Tyrosine | 0.48 | 0.013 | |||
| Creatine | 0.37 | 0.026 | Carnitine | 0.37 | 0.028 | |||
| Arginine | 0.36 | 0.036 | 3-Hydroxybutyrate | −0.36 | 0.032 | |||
| Waist circumference | r | p | Waist:Hip ratio | r | p | |||
| Methyl succinate | 0.40 | 0.017 | Glycine | −0.47 | 0.0049 | |||
| Pyruvate | 0.37 | 0.031 | Arginine | 0.46 | 0.0059 | |||
| Arginine | 0.34 | 0.042 | Pyruvate | 0.36 | 0.033 | |||
| AEE DLW | Moderate PA | r | p | Strenuous PA | r | p | ||
| Carnitine | 0.42 | 0.011 | Carnitine | 0.41 | 0.013 | Glycine | 0.48 | 0.024 |
| Lactate | 0.42 | 0.012 | Lactate | 0.37 | 0.017 | Taurine | −0.35 | 0.043 |
| Lysine | 0.40 | 0.018 | Methionine | 0.32 | 0.048 | Acetate | 0.35 | 0.048 |
Abbreviations are as follows in alphabetical order: AEEDLW, activity energy expenditure; BMI, body mass index; PA, physical activity.
MetSany women had higher levels of histidine and lysine and lower levels of serine, creatinine, myo-inositol, arginine, acetoacetate and betaine compared with MetSzero women. The lower serine concentrations in MetSany women were also significant with univariate analysis (p < 0.001). Serine was furthermore found to be lower with increasing values of all body measurements in women and as the number of MetS risk factors increased (Supplementary Table S4). Of note, overweight and increasing MetS risk factors were associated with lower glycine concentrations in women (VIP > 1, Supplementary Table S4).
MetS-associated metabolites are inversely associated with measures of AEEDLW and physical activity in men and women
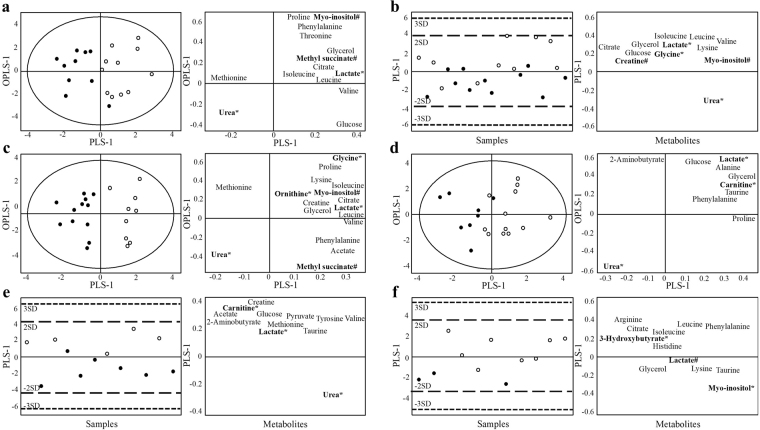
Next, we were interested in examining whether MetS-associated metabolites were inversely related with AEEDLW, AEE/kgDLW, PALDLW, moderate or vigorous physical activity in comparison to MetS using multivariate analysis. The highest 50% (high groups) and lowest 50% (low groups) of each activity measurement were compared within the respective MetSany and MetSzero groups of men and women. The supervised analysis showed MetSany men to have higher concentrations of glycine and lactate with the higher levels of PALDLW (R2 = 0.86, Q2 = 0.46) and AEE/kgDLW (R2 = 0.34, Q2 = 0.24) compared with the low PALDLW and AEE/kgDLW groups (Fig. 2). Lactate was furthermore positively correlated with AEEDLW (R2 = 0.67, Q2 = 0.36) and moderate physical activity (R2 = 0.38, Q2 = 0.21) in MetSany men and with moderate (R2 = 0.62, Q2 = 0.51) and vigorous physical activity (R2 = 0.57, Q2 = 0.25) in MetSzero men. Of note, none of these variables were correlated with body measures in MetSany or MetSzero men as assessed by bivariate Pearson correlation on the continuous values.
Figure 2.
Supervised OPLS-DA score scatter plots and loadings plots for men, showing AEEDLW and physical activity variables stratified for MetSany (A–D) and MetSzero (E–F). Every dot/circle represents one participant, with circles indicating participants with the highest 50% (circles) and the lowest 50% (dots) of each variable. The score scatter plots (left) and loading plots (right) are superimposable and show (VIP > 1) metabolites. Only variables that resulted in models are shown. (A) AEEDLW (R2 = 0.67, Q2 = 0.36), (B) AEE/kgDLW (R2 = 0.34, Q2 = 0.24), (C) PALDLW (R2 = 0.86, Q2 = 0.46), (D) moderate physical activity (R2 = 0.38, Q2 = 0.21) for MetSany and (E) moderate physical activity (R2 = 0.62, Q2 = 0.51) and (F) vigorous physical activity (R2 = 0.57, Q2 = 0.25) for MetSzero.
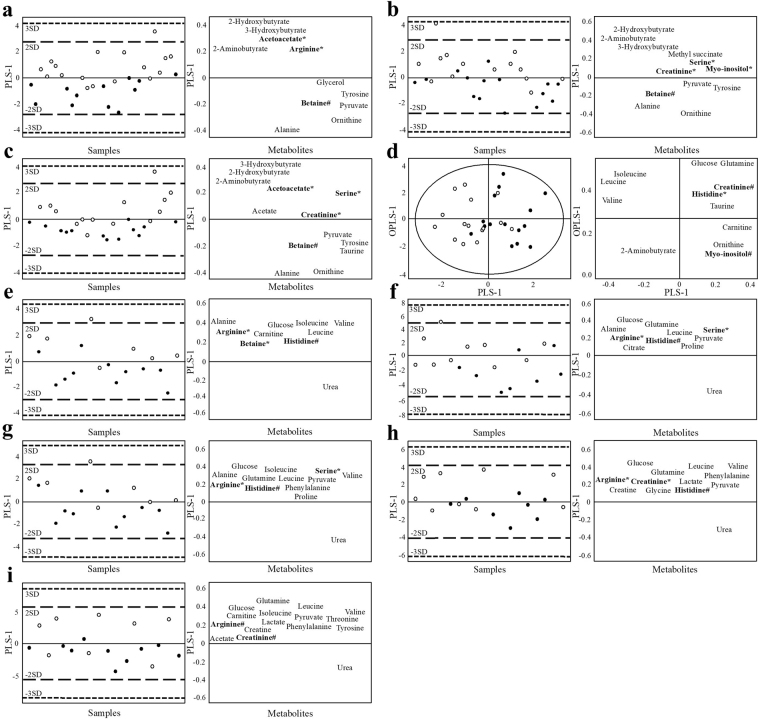
For MetSzero women, serine and arginine were found to be higher with high levels of AEE/kgDLW (R2 = 0.32, Q2 = 0.21) and PALDLW (R2 = 0.40, Q2 = 0.33) compared with low levels in MetSzero women (Fig. 3). Arginine and betaine were also higher with AEEDLW (R2 = 0.43, Q2 = 0.37). For MetSany women, AEEDLW, AEE/kgDLW and PALDLW were positively correlated with several metabolites that had been found to be lower in the presence of MetS risk factors (Fig. 3). For example, higher levels of AEE and AEE/kg were associated with higher serum concentrations of acetoacetate and arginine as well as creatinine, myo-inositol and serine, respectably, compared with lower levels (Fig. 3). The high PALDLW group had higher concentrations of acetoacetate, creatinine and serine compared to the low PALDLW group. Notably, women in the MetSzero and MetSany groups of higher levels of physical activity, AEEDLW, AEE/kgDLW and PALDLW had lower body measures overall (Supplementary Table S4). Thus, the impact of the higher activity measurements on the serum metabolome cannot be defined as independent of the lower body measures. Yet, these patterns are consistent with known correlations of lower body weight with higher AEE and physical activity23,24.
Figure 3.
Supervised OPLS-DA score scatter plots and loadings plots for women, showing AEEDLW and physical activity variables stratified for MetSany (A–D) and MetSzero (E–I). Every dot/circle represents one participant, with circles indicating participants with the highest 50% (circles) and the lowest 50% (dots) of each variable. The score scatter plots (left) and loading plots (right) are superimposable and show VIP > 1 metabolites. Only variables that resulted in models could be shown. The variables presented include (A) AEEDLW (R2 = 0.45, Q2 = 0.27), (B) AEE/kgDLW (R2 = 0.33, Q2 = 0.11), (C) PALDLW (R2 = 0.44, Q2 = 0.26), (D) vigorous physical activity (R2 = 0.43, Q2 = 0.22), for MetSany and (E) AEEDLW (R2 = 0.43, Q2 = 0.37), (F) AEE/kgDLW (R2 = 0.32, Q2 = 0.21), (G) PALDLW (R2 = 0.40, Q2 = 0.33), (H) moderate physical activity (R2 = 0.28, Q2 = 0.14) and (I) vigorous physical activity (R2 = 0.28, Q2 = 0.16) for MetSzero.
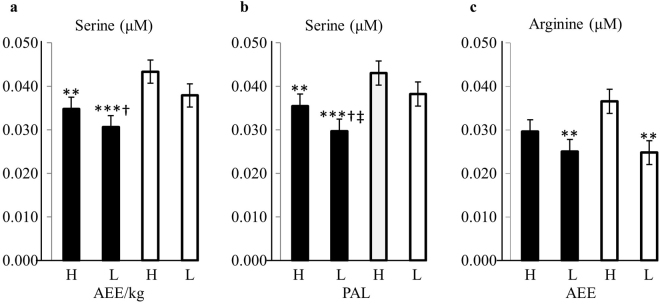
Finally, since serine and arginine had correlated with DLW measurements in both MetSany and MetSzero women, we compared the serum concentrations for the 4 groups of high and low activity measurements of MetSany and MetSzero women, respectively (Fig. 4). For serine there were 3 main findings; 1) MetSzero women with high PALDLW and AEE/kgDLW had the highest concentration of serine 2) for MetSany women, higher PALDLW, but not AEE/kgDLW, was associated with higher serine concentrations and 3) MetSany women with high PALDLW and AEE/kgDLW have comparable serine concentrations to the MetSzero women with low PALDLW and AEE/kgDLW. Arginine concentrations were significantly higher in MetSzero women with high AEEDLW compared to both the MetSany and the MetSzero women with low AEEDLW (p < 0.01).
Figure 4.
Serum serine and arginine concentrations for MetSany (black bars) and MetSzero women (white bars) with high (H) or low (L) levels of AEE/kgDLW and PALDLW, and AEEDLW respectively. Significant difference compared to MetSzero-H (**p < 0.01 ***p < 0.001), MetSzero-L (†p < 0.05) and MetSany-H (‡p < 0.05) is indicated. Serum concentrations were normalized to the total sum for each sample to assure normal distribution and comparability across samples.
Sphingolipid metabolism potential pathway for MetS
To gain further insight into the biological mechanisms underlying the reported metabolite findings, pathway analysis was performed on all analyzed metabolites. Two pathways were significant when comparing MetSany to MetSzero women, both involving serine; ‘sphingolipid metabolism’ (serine, p < 0.001) and ‘methane metabolism’ (serine and glycine, p < 0.05) (Supplementary Table S6).
No statistically significant pathways could be identified for MetS for men or for BMI, waist circumference, AEEDLW, PALDLW or physical activity measurements in either gender after correcting for false discovery rate. However, there were pathways that had significant unadjusted p-values. For example, waist circumference associated with multiple pathways involving serine and/or glycine in men, including sphingolipid metabolism, glycine, serine and threonine metabolism, methane metabolism, sulfur metabolism, cysteine and methionine metabolism and cyanoamino metabolism (punadjusted < 0.05, data not shown).
Discussion
Although much is known about the physiological impact of exercise on immediate fuel utilization, body coordination, cognitive function and cardiorespiratory fitness, there is a large gap in the understanding of how these and other changes are linked to long-term disease prevention and amelioration14. Metabolomics and other “omics” approaches can potentially provide insight to the mechanisms linking exercise and health.
In this study we describe the serum metabolite signatures associated with adiposity in relation to AEEDLW, AEE/kgDLW, PALDLW and moderate and vigorous physical activity in men and women with or without MetS risk factors. The most consistent metabolomics trend consisted of the lower serum concentrations of serine in the presence of MetS risk factors and greater adiposity in both men and women. Glycine was also found at lower concentrations with a higher number of MetS risk factors in both genders and with higher adiposity in men. In contrast, DLW-assessed measurements i.e. AEE/kgDLW and PALDLW, but not self-reported moderate or vigorous physical activity, were associated with higher concentration of serine in MetSany women and glycine in MetSany men. Interestingly, higher PALDLW levels in MetSany women were associated with serine levels comparable to the less active MetSzero women. This correlation was partly dependent on the positive correlation between BMI and PALDLW in the high PALDLW MetSany group. We speculated that this may be an indication of leanness since 1) the BMI was lower in the high PALDLW group compared to the low group, with most women in the high PAL group having a BMI within the normal range and since 2) BMI was inversely correlated with serine levels in women in the low PALDLW and AEE/kgDLW groups, yet without statistical significance. The highest serine concentrations were seen for MetSzero women with the highest PALDLW, attesting to the importance of engaging in physical activity even for normal weight and healthy women. Our results also suggest that high PAL may mitigate the impact of adiposity and MetS risk factors on the serum metabolome in women. Of note, exercise has also been shown by others to influence the human serum and plasma metabolite profiles19–21. This is supported in animal models, showing exercise to mitigate the metabolite profile associating with diet-induced obesity25 and diabetes26.
Similar to our findings, others have found decreasing serine and glycine concentrations with obesity, insulin resistance, diabetes and MetS risk factors27–36. For example, glycine concentrations have previously been shown to be linked with whole body glucose uptake and thus insulin responsiveness, as assessed by hyperinsulinemic-euglycemic glucose clamps29. Moreover, lower serine and glycine levels were reported to be instrumental in differentiating between insulin sensitive, insulin resistant and diabetic adults29. Serine and glycine have also been reported to positively correlate with insulin action, as assessed by repeated glucose tolerance tests in overweight and obese sedentary men and women32. In addition, serine has been shown to be correlated with impairments in fasting glycemia in type 2 diabetic adults, while fasting concentrations of glycine have been reported to be lower in diabetic compared to normoglycemic adults31. A metabolic signature consisting of glycine in combination with phenylalanine, hexose, sphingomyelin 16:1 and phosphatidylcholines has furthermore been able to predict diabetes with high accuracy 7 years prior to clinical manifestation36. Finally, Batch et al. reported that the combination of reduced serine, glycine and ornithine concentrations had the potential to distinguish between metabolically well and unwell (>2 MetS risk factors) adults, after adjusting for BMI30. Importantly, none of these studies assessed physical activity or objective measures of AEE to determine their relation to these metabolites.
The pathway analysis suggested sphingolipid metabolism as a potential underlying mechanism to the detected inverse correlations between serine and MetS risk factors. This finding is supported by recent reports indicating that obesity, insulin resistance and metabolic syndrome are associated with lower concentrations of serine28,29,32,37 and glycine27,29–31,38 (substrates) and higher concentrations of sphingolipid species39–64 (products) in humans. Interestingly, the opposite trend was seen for physical activity65–74, with some studies reporting conflicting results28,45,67. Dubé et al. also showed that exercise, but not dietary restriction, reduced ceramide and sphingosine levels, while both approaches improved on insulin sensitivity65. It is thus possible that serine is used as a precursor for sphingolipids in the obese state, leading to the accumulation of the bioactive lipid ceramide in insulin sensitive tissues, such as muscle and liver, where it can contribute to the development of insulin resistance75. In contrast, serine may be spared with physical activity, perhaps by the downregulation of serine palmitoyltransferase76, the enzyme that catalyzes the first step of de novo sphingolipid synthesis utilizing serine and palmitoyl-CoA to produce 3-ketosphinganine. An exercise intervention study for participants with and without MetS investigating de novo sphingolipid metabolism with isotope labeled precursors and liquid chromatography mass spectrometry metabolomics could complement the present work. However, it is also possible for circulating serine concentrations to be dependent on other factors including diet, protein and phospholipid degradation or synthesis from 3-phosphoglycerate77.
Although serine and glycine were prominent features of this study, other metabolites may also play a crucial part in adiposity and MetS. Among the key findings were the lower levels of arginine with MetS risk factors in women and the higher levels with AEE/kgDLW and PALDLW in both MetSany and MetSzero women. Arginine has multiple functions related to glucose and insulin concentrations; it is a gluconeogenic amino acid and has the ability to activate AMPK and mTOR, resulting in higher insulin secretion and glucose uptake77. However, arginine levels were positively correlated with body measures in men. Furthermore, creatinine was found to be lower in MetSany men and women and to be positively correlated to AEE/kgDLW, PALDLW and moderate physical activity in MetSany women as well as vigorous physical activity in MetSzero women. Creatinine is a breakdown product of muscular creatine phosphate, and may thus reflect the higher levels of physical activity in these subjects. Finally, lactate concentrations were lower in the presence of MetS risk factors in men and higher with PALDLW, AEEDLW and moderate physical activity in MetSany men and with moderate and vigorous physical activity in MetSzero men. Concentrations of lactate are best known to increase during physical activity and to return to normal after approximately 30 minutes of active or passive recovery78. Lactate levels are also commonly found to be higher as a consequence of cancer and critical illness, however none of the participants in the present study had any chronic or acute illnesses. Interestingly, BCAA did not contribute significantly to the metabolite profiles of MetS or adiposity in our work, with the exception that BCAA concentrations were higher in men (who had significantly higher BMI, waist circumference and waist-to-hip ratio) compared to women. These results may reflect the natural variation between populations and habits in free-living humans, as BCAA concentrations have been reported to be influenced by factors such as BMI, diabetes and diet (proportion of fat and carbohydrates)12,29.
The limitations of the study need to be addressed. The relatively small sample size and lower proportion of male participants restricted statistical analyses and while creating subgroups increased the homogeneity of the groups it also decreased the power to detect true associations. For this reason, our findings need to be validated in larger well-controlled studies that investigate other possible metabolic pathways of interest. Studies containing 25–30 participants per group should suffice to detect key metabolites with modest differences in abundance levels between groups79,80. Another limitation included the reliance on an estimation of RMR (resting metabolic rate) using an equation based on gender, age and anthropometric measures, rather than an actual measure using indirect calorimetry, which may have resulted in reducing the precision of the AEEDLW estimation (see Equation 1). As well, moderate and vigorous physical activity levels were estimated from self-report, known to be associated with measurement error. We have previously shown, however, that there is substantial agreement between vigorous physical activity estimated from the STAR-Q and prospectively collected 7-day activity diaries indicating that the estimate may be informative for the ranking of subjects according to vigorous physical activity levels81. On the other hand, a major strength of our study includes the use of DLW, the gold standard for TEE measurement, which allowed for objective estimations of AEEDLW, AEE/kgDLW and PALDLW. To our knowledge this is the first study to examine the relation between DLW-derived AEEDLW and PALDLW estimates and metabolites in subjects with and without risk factors of MetS. This study also included anthropometric measures that were assessed by trained staff rather than having to rely on participant self-report. The multiple measures allowed for the investigation of the effect of site-specific adiposity with respect to certain metabolite profiles. Notably, AEEDLW and BMI in men were positively correlated reaching statistical significance, potentially as a consequence of the higher ‘energy cost’ of movement with excess body mass82 as the men in the present study were overweight on average. Standardizing AEEDLW to bodyweight partly reduced this association and AEE/kgDLW was negatively correlated to waist circumference.
In conclusion, we observed for the first time that higher levels of DLW- derived PAL and AEE measures were inversely correlated with MetS and adiposity-associated metabolites. While not conclusive, our findings provide evidence to support the promising potential effects of physical activity and overall energy expenditure in mitigating the negative effects of obesity on metabolism. We suggest that serine and glycine, through the involvement in de novo synthesis of sphingolipids such as ceramides may play an important part in the development of metabolic disease from obesity. To the best of our knowledge, this is the first study to examine metabolite profiles associated with DLW-derived AEE and PAL in humans.
Methods
Study design and ethical approval
This study was comprised of a convenience sample of volunteer participants who were recruited to a validation study designed to examine the measurement properties of the STAR-Q83. The study design, recruitment and data collection procedures, excluding the metabolomics procedures, have previously been described81.
Of 102 participants in the STAR-Q validation study81, 82 (35 men and 47 women) provided overnight fasting blood samples from which quantitative metabolomics data (see below) were generated. Recruitment to the original study was based on the following inclusion criteria: 1) 30–60 years of age; 2) living in the Calgary, Alberta, Canada area; 3) weight stability (≤2.5 kilogram weight change for at least 3 months) and; 4) body mass index (BMI) ≤35. Pregnant or breastfeeding women, participants with metabolic disorders (e.g., diabetes and thyroid dysfunction) or individuals taking medications affecting water balance were not eligible for the study. All participants provided informed consent as well as detailed reports of their medical history. Ethical approval for the study was obtained from the Alberta Cancer Research Ethics Committee of Alberta Health Services and the Conjoint Health Research Ethics Board of the University of Calgary, Calgary, Alberta, Canada. All methods were performed in accordance with relevant guidelines and regulations.
AEEDLW, PALDLW and physical activity measures
Doubly labeled water (DLW: deuterium and oxygen-18 (18O)) was used to derive total energy expenditure (TEEDLW). In brief, saliva and urine samples were obtained after an overnight fast for the determination of the background isotope levels (day 0). Participants were then orally administered 0.18 g 99 atom percent deuterium and 2.5 g 10 atom percent 18O per kilogram of estimated total body water. Post-dose saliva samples were collected at 3 and 4 hours and second-void urine samples were collected on days 1, 8 and 14. The samples were batch-analyzed in duplicate using an Isoprime Stable Isotope Ratio Mass Spectrometer (Isoprime Ltd., Cheadle Hulme, United Kingdom) to measure the decline in isotope enrichment. TEEDLW was calculated according to the method of Racette et al.84 using a modified Wier equation and an assumed respiratory quotient of 0.85. AEEDLW (and in extension AEEDLW/kg) was estimated from TEEDLW using Equation 1 below where resting metabolic rate (RMR) was estimated using the Schofield equation85 and hours of sleep as reported in the STAR-Q:
AEEDLW = 0.9TEEDLW – ((RMR/24) × (24 - hrs of sleep)) + (0.9(RMR/24) × hrs of sleep)
Activities were self-reported for the previous month using the self-administered STAR-Q completed on day 14 of the DLW protocol. All activities were assigned activity codes and values for metabolic equivalents of task were obtained from the Compendium of Physical Activities86,87. Estimates of time spent in moderate (3.0–6.0 metabolic equivalents of task) and vigorous intensity physical activity (>6.0 metabolic equivalents of task) were then derived81. The physical activity level (PALDLW) was estimated as the ratio of TEEDLW to RMR.
MetS risk factors and anthropometric measurements
MetS is defined as a cluster of risk factors comprising high blood pressure (systolic ≥ 130 mm Hg, diastolic ≥ 85 mm Hg), abdominal obesity (men ≥ 102 cm, women ≥ 88 cm)88,89, raised fasting glucose (≥ 5.6 mmol/l) and dyslipidemia, defined as low HDL (men < 1.0 mmol/l, women < 1.3 mmol/l) and high triglycerides (≥ 1.7 mmol/l) or alternatively, medication for any of the mentioned conditions7. Prescription drug use was ascertained at the study center visit at which time participants were requested to bring in medication containers. Triglycerides, HDL cholesterol, total cholesterol and fasting glucose were determined at the Calgary Laboratory Services using established protocols and fasting whole blood.
Anthropometric measures and blood pressure were determined at the study center by a certified exercise physiologist. Waist circumference was measured midway between the iliac crest and the lowest rib, as per Canadian guidelines90. Both waist and hip circumference were recorded to the nearest 0.5 cm. Weight and height were measured to nearest 0.1 kg and 0.1 cm, respectively, for BMI calculations (kg/m2). BMI was analyzed as a continuous and a binary variable that was dichotomized to represent healthy body weight (BMI < 24.9 kg/m2) and overweight and obese categories (BMI ≥ 25.0 kg/m2). Body fat percentage was determined using the TBF-310 Tanita Body Composition Analyzer and Scale (Tanita Corporation of America, Preston, USA). Blood pressure was assessed following the recommendations by the Canadian Hypertension Educational Program91.
Serum metabolomics analysis using proton nuclear magnetic resonance spectroscopy
Whole blood was collected in Red Top vacutainers following a 10 hour fast, processed by centrifuge to yield serum and stored in aliquots of 0.5 mL cryovials in −80 °C until further analysis. At the time of analysis, samples were thawed on ice and larger molecules (e.g., proteins and lipid assemblies) were removed using 3-kDa Nanosep centrifugal filters and analyzed as previously described92. In brief, the filtrate volume was brought up to 650 μL, using a DSS-containing sodium phosphate buffer (130 μL, 0.5 M), the antibacterial compound sodium azide (10 μL, 1 M) and deuterium oxide. In addition, pH was adjusted to 7 ± 0.01 using sodium hydroxide and hydrochloride. Proton nuclear magnetic resonance spectroscopy (1H NMR) was used to acquire 1-dimensional spectra using a standard pulse program (prnoesy1d) on a Bruker Avance 600 spectrometer (600.22 MHz, 5 mm TXI Probe) at 298 °K. All samples were analyzed in automatic mode using Bruker NMRCase sample changer after shimming the first sample of each batch. Each sample was then processed using Chenomx NMR Suite 7.5 software (Chenomx Inc., Edmonton, Canada), comprised of line broadening (0.5 Hz), baseline and phase correction, water region deletion, shimming and concentration calibration. Quantitative metabolic profiling was performed using the NMR Suite profiling module employing the Chenomx library assisted by the human metabolome database (www.hmdb.ca) as well as 2-dimensional heteronuclear single quantum coherence spectroscopy (1H,13C HSQC) and total correlation spectroscopy (1H,1H TOCSY). All samples were coded with sample ID and prepared, analyzed and profiled in a randomized order.
Statistical analysis
Serum metabolites and study variables were analyzed by univariate (two-tailed t-test assuming unequal variance), bivariate (Pearson correlation analysis) and multivariate statistical analysis (as described below). Statistical significance was defined as a p-value < 0.05 for uni- and bivariate statistical analysis and a variable importance in the projection-value (VIP) > 1.0, for the multivariate statistical analysis. Univariate and bi-variate p-values were corrected for false discovery rate (FDR) according to Benjamini and Hochberg93.
The analysis of the metabolomics data is based on work by Goodacre et al.94, and their standards for analysis. In brief, sample normalization of the metabolomics data was performed to ensure Gaussian distribution. The measured concentration of each metabolite in each sample was divided with the total concentration sum of that sample. Glucose, glycerol and lactate, which consistently had the highest concentrations in the samples, were excluded from the total sum in order to not dominate the normalization procedure. The normalized 1H NMR data was then used for the uni- and bivariate (Pearson correlation) statistical analysis using Microsoft Excel and imported into the SIMCA-P + software (version 12.01, Umetrics AB, Umeå, Sweden) for multivariate statistical analysis. In brief, for the multivariate analysis, data were further mean centered and scaled using unit variance scaling and statistical models were created based on 7-fold cross-validation. Unsupervised principal component analysis (PCA) was conducted to identify possible groupings of the data and to identify outliers, defined as samples outside the default 95 percent confidence interval. Outliers were excluded when creating supervised models. Only the models that were based on the most influential metabolites (VIP > 1) are shown. Partial least squares regression analysis (PLS) was applied for continuous Y-variables whereas PLS discriminant analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA) were applied for qualitative Y-variables with more than three classes (e.g., the number of MetS risk factors, MetS0-3) and two classes (e.g., BMI class and MetSany vs Metszero), respectively. All models were evaluated based on R2 and Q2 values, representing the explained variance and the predictive ability of the models, respectively. Multivariate statistical models are generally believed to not be over-fit if the R2Y and the Q2 values are within <0.3 of each other80,95. Analyses were carried out separately in men and women, and further stratified by MetS when indicated i.e., MetSany (1–3 MetS risk factors) and MetSzero (0 MetS risk factors).
Metabolic pathway analysis
Metaboanalyst (www.metaboanalyst.ca) was used for the pathway analysis. The normalized data were imported and scaled and mean centered and the analysis specified for humans. Pathways were evaluated based on p-values corrected for false discovery rate93 with p < 0.05 indicating statistical significance.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge the Alberta Tomorrow Project and Calgary community volunteers who participated in the original validation study of the STAR-Q. The study was funded by the Canadian Institutes of Health Research (grant MOP-86632 awarded to I.C., K.A.K. and P.J.R.) and the Alberta Cancer Foundation (grant 24265 awarded to I.C., K.A.K. and P.J.R.). R.R-L. was the recipient of a Chercheur-Boursier Senior salary award from Fonds de Recherche du Québec. H.J.V. was the holder of the Armstrong Chair for Molecular Cancer Epidemiology Research.
Author Contributions
I.C. and H.J.V. conceptualized the metabolomics study design. R.A.S. performed the 1H NMR experiments. R.R-L. and D.M. performed the DLW isotope analyses and estimated the DLW-derived TEE. M.S.A.P. performed the quantitative metabolic profiling, planned and performed all statistical analysis and interpreted the data. K.A.K. provided expertise for the statistical analysis and guided the reporting of data. M.S.A.P. and I.C. wrote the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21585-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hans J. Vogel, Email: vogel@ucalgary.ca
Ilona Csizmadi, Email: icsizmad@ucalgary.ca.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Morley TS, Kim M, et al. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiria LO, Martins MA, Saad MJ. Obesity and asthma: beyond T(H)2 inflammation. Metabolism. 2015;64:172–181. doi: 10.1016/j.metabol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Gerteis J et al. in AHRQ Publications No, Q14-0038. Rockville, MD: Agency for Healthcare Research and Quality (2014).
- 6.Folsom AR, et al. Hormonal replacement therapy and morbidity and mortality in a prospective study of postmenopausal women. American Journal of Public Health. 1995;85:1128–1132. doi: 10.2105/AJPH.85.8_Pt_1.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 8.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 9.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durstine JL, Gordon B, Wang Z, et al. Chronic disease and the link to physical activity. JSHS. 2013;2:3–11. [Google Scholar]
- 14.Neufer PD, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Speakman JR. The history and theory of the doubly labeled water technique. Am J Clin Nutr. 1998;68:932S–938S. doi: 10.1093/ajcn/68.4.932S. [DOI] [PubMed] [Google Scholar]
- 16.Schoeller DA. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J Nutr. 1999;129:1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 17.Krumsiek J, et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittelstrass, K. et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet7, 10.1371/journal.pgen.1002215 (2011). [DOI] [PMC free article] [PubMed]
- 19.Wientzek A, et al. Serum metabolites related to cardiorespiratory fitness, physical activity energy expenditure, sedentary time and vigorous activity. Int J Sport Nutr Exerc Metab. 2014;24:215–226. doi: 10.1123/ijsnem.2013-0048. [DOI] [PubMed] [Google Scholar]
- 20.Lewis, G. D. et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2, 10.1126/scitranslmed.3001006 (2010). [DOI] [PMC free article] [PubMed]
- 21.Pechlivanis A, et al. 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum. J Proteome Res. 2013;12:470–480. doi: 10.1021/pr300846x. [DOI] [PubMed] [Google Scholar]
- 22.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9:819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berntzen, B. et al. Physical activity, cardiorespiratory fitness, and metabolic outcomes in monozygotic twin pairs discordant for body mass index. Scandinavian Journal of Medicine & Science in Sports, n/a-n/a, 10.1111/sms.12975. [DOI] [PubMed]
- 24.Levine JA, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 25.Duggan GE, et al. Metabolomic response to exercise training in lean and diet-induced obese mice. J Appl Physiol (1985) 2011;110:1311–1318. doi: 10.1152/japplphysiol.00701.2010. [DOI] [PubMed] [Google Scholar]
- 26.Xiang L, et al. Plasma metabolic signatures reveal the regulatory effect of exercise training in db/db mice. Mol Biosyst. 2015;11:2588–2596. doi: 10.1039/C5MB00363F. [DOI] [PubMed] [Google Scholar]
- 27.Lustgarten, M. S., Price, L. L., Phillips, E. M. & Fielding, R. A. Serum glycine is associated with regional body fat and insulin resistance in functionally-limited older adults. PLoS One8, 10.1371/journal.pone.0084034 (2013). [DOI] [PMC free article] [PubMed]
- 28.Zhou Y, et al. Obesity and diabetes related plasma amino acid alterations. Clin Biochem. 2013;46:1447–1452. doi: 10.1016/j.clinbiochem.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 29.Thalacker-Mercer AE, et al. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes. 2014;63:791–800. doi: 10.2337/db13-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batch BC, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62:961–969. doi: 10.1016/j.metabol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mook-Kanamori, D. O. et al. Type 2 diabetes is associated with postprandial amino acid measures. Arch Biochem Biophys, 10.1016/j.abb.2015.1008.1003 (2015). [DOI] [PubMed]
- 32.Huffman KM, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Würtz P, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35:1749–1756. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laferrere, B. et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med3, 10.1126/scitranslmed.3002043 (2011). [DOI] [PMC free article] [PubMed]
- 35.Bain JR. Targeted metabolomics finds its mark in diabetes research. Diabetes. 2013;62:349–351. doi: 10.2337/db12-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Floegel A, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertea M, et al. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 2010;16:84. doi: 10.1186/1476-511X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huffman KM, et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care. 2011;34:174–176. doi: 10.2337/dc10-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coen PM, et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 2013;21:2362–2371. doi: 10.1002/oby.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blachnio-Zabielska AU, Koutsari C, Tchkonia T, et al. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring) 2012;20:2341–2347. doi: 10.1038/oby.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Badeanlou L, Bielawski J, et al. Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance. Am J Physiol Endocrinol Metab. 2014;06:E756–768. doi: 10.1152/ajpendo.00549.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baranowski M, et al. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. Journal of lipid research. 2010;51:74–80. doi: 10.1194/jlr.M900002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowalski GM, Carey AL, Selathurai A, et al. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8:e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Górska M, Dobrzyń A, Baranowski M. Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med Sci Monit. 2005;11:CR35–38. [PubMed] [Google Scholar]
- 45.Blachnio-Zabielska AU, et al. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. Journal of cellular physiology. 2012;227:550–557. doi: 10.1002/jcp.22745. [DOI] [PubMed] [Google Scholar]
- 46.Mamtani M, et al. Lipidomic risk score independently and cost-effectively predicts risk of future type 2 diabetes: results from diverse cohorts. Lipids Health Dis. 2016;15:67. doi: 10.1186/s12944-016-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meikle PJ, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 2013;8:e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mamtani M, et al. Plasma dihydroceramide species associate with waist circumference in Mexican American families. Obesity (Silver Spring) 2014;22:950–956. doi: 10.1002/oby.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez X, Goldfine AB, Holland WL, et al. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013;26:995–998. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 50.Weir JM, et al. Plasma lipid profiling in a large population-based cohort. Journal of lipid research. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumdar I, Mastrandrea LD. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine. 2012;41:442–449. doi: 10.1007/s12020-011-9589-4. [DOI] [PubMed] [Google Scholar]
- 52.Liang H, et al. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol. 2013;591:2897–2909. doi: 10.1113/jphysiol.2012.247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haus JM, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straczkowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 55.Sajan MP, Ivey RA, III, Farese RV. BMI-related progression of atypical PKC-dependent aberrations in insulin signaling through IRS-1, Akt, FoxO1 and PGC-1alpha in livers of obese and type 2 diabetic humans. Metabolism. 2015;64:1454–1465. doi: 10.1016/j.metabol.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Maza MP, et al. Skeletal muscle ceramide species in men with abdominal obesity. J Nutr Health Aging. 2015;19:389–396. doi: 10.1007/s12603-014-0548-7. [DOI] [PubMed] [Google Scholar]
- 57.Blachnio-Zabielska AU, et al. Increased bioactive lipids content in human subcutaneous and epicardial fat tissue correlates with insulin resistance. Lipids. 2012;47:1131–1141. doi: 10.1007/s11745-012-3722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amati F, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thrush AB, Brindley DN, Chabowski A, et al. Skeletal muscle lipogenic protein expression is not different between lean and obese individuals: a potential factor in ceramide accumulation. J Clin Endocrinol Metab. 2009;94:5053–5061. doi: 10.1210/jc.2008-2565. [DOI] [PubMed] [Google Scholar]
- 60.Moro C, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab. 2009;94:3440–3447. doi: 10.1210/jc.2009-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams JM, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 62.Galgani JE, et al. Enhanced skeletal muscle lipid oxidative efficiency in insulin-resistant vs insulin-sensitive nondiabetic, nonobese humans. J Clin Endocrinol Metab. 2013;98:E646–653. doi: 10.1210/jc.2012-3111. [DOI] [PubMed] [Google Scholar]
- 63.Coen, P. M. et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 59, 80–88, 10.2337/ (2010). [DOI] [PMC free article] [PubMed]
- 64.Straczkowski M, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53:1215–1221. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]
- 65.Dube JJ, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–1156. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao, Q. et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int J Epidemiol. pii: dyw033. [Epub ahead of print] (2016). [DOI] [PMC free article] [PubMed]
- 67.Bergman BC, et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309:E398–408. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baranowski M, Charmas M, Dlugolecka B, et al. Exercise increases plasma levels of sphingoid base-1 phosphates in humans. Acta Physiol (Oxf) 2011;203:373–380. doi: 10.1111/j.1748-1716.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 69.Baranowski M, Górski J, Klapcinska B, et al. Ultramarathon run markedly reduces plasma sphingosine-1-phosphate concentration. Int J Sport Nutr Exerc Metab. 2014;24:148–156. doi: 10.1123/ijsnem.2013-0093. [DOI] [PubMed] [Google Scholar]
- 70.Kasumov T, et al. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 2015;23:1414–1421. doi: 10.1002/oby.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coen PM, et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes. 2015;64:3737–3750. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helge JW, Dobrzyn A, Saltin B, et al. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol. 2004;89:119–127. doi: 10.1113/expphysiol.2003.002605. [DOI] [PubMed] [Google Scholar]
- 73.Dubé JJ, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruce CR, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 75.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith IJ, Huffman KM, Durheim MT, et al. Sex-specific alterations in mRNA level of key lipid metabolism enzymes in skeletal muscle of overweight and obese subjects following endurance exercise. Physiol Genomics. 2009;36:149–157. doi: 10.1152/physiolgenomics.90216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wishart DS, et al. HMDB 3.0–The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menzies P, et al. Blood lactate clearance during active recovery after an intense running bout depends on the intensity of the active recovery. J Sports Sci. 2010;28:975–982. doi: 10.1080/02640414.2010.481721. [DOI] [PubMed] [Google Scholar]
- 79.Tibshirani R. A simple method for assessing sample sizes in microarray experiments. BMC Bioinformatics. 2006;7:106. doi: 10.1186/1471-2105-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyamundanda G, Gormley IC, Fan Y, et al. MetSizeR: selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinformatics. 2013;14:338. doi: 10.1186/1471-2105-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Csizmadi I, et al. The Sedentary Time and Activity Reporting Questionnaire (STAR-Q): Reliability and Validity Against Doubly Labeled Water and 7-Day Activity Diaries. Am J Epidemiol. 2014;180:424–435. doi: 10.1093/aje/kwu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeLany JP, Kelley DE, Hames KC, et al. High energy expenditure masks low physical activity in obesity. Int J Obes (Lond) 2013;37:1006–1011. doi: 10.1038/ijo.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neilson HK, Ullman R, Robson PJ, et al. Cognitive testing of the STAR-Q: insights in activity and sedentary time reporting. J Phys Act Health. 2013;10:379–389. doi: 10.1123/jpah.10.3.379. [DOI] [PubMed] [Google Scholar]
- 84.Racette SB, et al. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267:E585–590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 85.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39:5–41. [PubMed] [Google Scholar]
- 86.Ainsworth BE, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 87.Ainsworth BE, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 88.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 89.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. National Institutes of Health. Obes Res. 6, 51–209 (1998). [PubMed]
- 90.Health Canada. Canadian Guidelines for Body Weight Classification in Adults-Quick Reference Tool for Professionals, Available from: http://www.hc-sc.gc.ca/fn-an/nutrition/weights-poids/guide-ld-adult/cg_quick_ref-ldc_rapide_ref-eng.php. Accessed 4 January 2016 (Accessed on January 4 2016).
- 91.Spitzer WO, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 92.Weljie AM, Dowlatabadi R, Miller BJ, et al. An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J Proteome Res. 2007;6:3456–3464. doi: 10.1021/pr070123j. [DOI] [PubMed] [Google Scholar]
- 93.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 94.Goodacre R, et al. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics. 2007;3:231–241. doi: 10.1007/s11306-007-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trygg J, Holmes E, Lundstedt T. Chemometrics in Metabonomics. Journal of Proteome Research. 2007;6:469–479. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.