Abstract
Pathological changes to the tau protein, including conformational changes and aggregation, are major hallmarks of a group of neurodegenerative disorders known as tauopathies. Among the conformational changes are alterations involving the extreme amino terminus of the protein, known as the phosphatase-activating domain (PAD). Aberrant PAD exposure induces a signaling cascade that leads to disruption of axonal transport, a critical function for neuronal survival. Conformational display of PAD is an early marker of pathological tau in Alzheimer disease (AD), but its role in other tauopathies has yet to be firmly established. We used a relatively novel N-terminal, conformation-sensitive antibody, TNT2, to determine whether misfolding in the amino terminus (ie, PAD exposure) occurs in non-AD tauopathies. We found that TNT2 specifically labeled pathological tau in post-mortem human brain tissue from Pick disease, progressive supranuclear palsy, corticobasal degeneration, and chronic traumatic encephalopathy, but did not label nonpathological, parenchymal tau. Tau13, another N-terminal antibody, was not sensitive to pathological N-terminal conformations. Tau13 did not readily distinguish between normal (ie, parenchymal tau) and pathological tau species and showed a range of effectiveness at identifying tau pathologies in the non-AD tauopathies. These findings demonstrate that the conformational display of the PAD in tau represents a common pathological event in many tauopathies.
Dysfunction and aggregation of the tau protein are hallmarks of the group of neurodegenerative disorders known as tauopathies. Some of the diseases included in this group are Alzheimer disease (AD), Pick disease (PiD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and chronic traumatic encephalopathy (CTE). Although tau aggregation is a dominant neuropathological phenotype of these diseases, the morphology of these aggregates, the cell types affected, and/or their locations within the brain vary between diseases.1 For instance, in AD, tau pathology is first observed in the transentorhinal region before appearing in the hippocampus and then neocortical areas as the disease progresses.2 The dominant pathology takes the form of flame-shaped neurofibrillary tangles, neuropil threads, and neuritic plaques.3 Each of the non-AD tauopathies are differentiated by specific pathognomonic inclusions (as well as clinical presentations and brain regions affected) that include not only neuronal but also primarily glial lesions in some diseases.4 PiD is characterized by the presence of Pick bodies, spherical tau inclusions located within neurons of the dentate gyrus of the hippocampus as well as in the frontal and temporal cortices, in addition to some tau aggregation in glia.5, 6 PSP displays neuronal tau pathology in the form of round or globose tangles, along with neuropil threads.7 In addition, tau pathology is present in glial cells, in the form of extensive tufted astrocytes and oligodendrocytic coiled bodies.8, 9 In CBD, tau pathology is characterized by prominent astrocytic plaques along with occasional coiled bodies and neuronal inclusions.10 Similarly, CTE tau lesions affect both neurons and glial cells in the frontal and temporal cortices, particularly in perivascular regions and at the depths of sulci, as well as in limbic areas and the brainstem.11, 12
The pathological inclusions found in the various tauopathies are associated with conformation changes resulting in misfolded forms of tau. Antibodies such as Alz50 and MC1 recognize discontinuous, conformation-specific tau epitopes and provide evidence that these changes in conformation are early markers for the protein's dysfunction in disease and precede the formation of classic neurofibrillary tangles in AD.13, 14, 15, 16 Previously, we demonstrated that specific antibodies directed toward the amino terminus of tau (ie, TNT1 and TNT2) were effective at differentiating pathogenic forms of tau from normal forms of the protein in AD post-mortem brain tissue.17, 18 In contrast, other antibodies with nearby N-terminal epitopes (ie, Tau12 and Tau13) were unable to differentiate between normal and pathological forms of tau, indicating the conformational changes could be relatively subtle and confined to the TNT1 and TNT2 epitopes (eg, within amino acids 7 to 12). More important, we have demonstrated that the conformational changes detected by these antibodies may directly mediate the toxicity associated with pathological forms of the protein.
The region of tau identified by the TNT antibodies is located within the first 18 amino acids of the protein, in a region described as the phosphatase-activating domain (PAD).17 In pathogenic forms of the protein (eg, aggregated tau or tau phosphorylated at certain epitopes), this region is aberrantly exposed, leading to a disruption of kinesin-based anterograde fast axonal transport.17, 19, 20, 21 Exposure of this motif activates a signaling cascade involving protein phosphatase 1 and glycogen synthase kinase 3 that results in phosphorylation of the kinesin light chain and release of the cargo from kinesin-1.17 Although this mechanism has mostly been studied in the context of axonal transport, microtubule- and kinesin-dependent transport is critical throughout the somatodendritic compartment of neurons as well as within glial cells.22, 23, 24, 25 Thus, this toxic pathway may provide a potential mechanistic link between pathological conformations of tau and toxic effects across several tauopathies, where dysfunctional tau is found in multiple cell types.
Herein, we used TNT2 and Tau13 to determine whether conformational display of PAD occurs in the hallmark pathologies of a variety of non-AD tauopathies. All of the tauopathies tested herein, including PiD, CBD, PSP, and CTE, displayed robust TNT2 reactivity but varying degrees of reactivity with Tau13. These results suggest that PAD exposure is a common pathological event in several tauopathies, which may suggest that the disruption of kinesin-based transport is a common mechanism of toxicity among these diseases.
Materials and Methods
Human Subjects
Hippocampal and frontal cortical sections from control cases [n = 3; male (M), 77 years; female (F), 84 years; F, 82 years], Pick disease (n = 4; M, 75 years; F, 74 years; M, 66 years; M, 55 years), corticobasal degeneration (n = 4; M, 57 years; M, 61 years; F, 60 years; F, 64 years), and progressive supranuclear palsy (n = 5; M, 57 years; F, 62 years; M, 60 years; F, 55 years; M, 54 years) cases were obtained from the Brain Bank of the Cognitive Neurology and Alzheimer Disease Center at Northwestern University. Frontal cortical sections from chronic traumatic encephalopathy cases (n = 5; M, 66 years; M, 82 years; M, 85 years; M, 87 years; M, 76 years) were obtained from the Boston University Center for the Study of Traumatic Encephalopathy. Further details on human subjects, including Braak or CTE stages and post-mortem intervals, can be found in Supplemental Table S1.
Tissue Immunohistochemistry
Brain sections from the cases described above were used to evaluate the pattern of immunohistochemical staining with TNT2 and Tau13 using previously established methods.17, 18, 26 TNT2 and Tau13, both mouse IgG1 monoclonal antibodies, were generated as described previously.17, 18, 27 The primary antibodies were diluted in 2% goat serum–tris-buffered saline (150 mmol/L NaCl, 50 mmol/L Tris, pH 7.4) from 1 mg/mL stocks at 1:200,000 for TNT2 (final concentration, 5 ng/mL) and 1:450,000 for Tau13 (final concentration, 2.2 ng/mL) through intermediate dilutions (ie, 1:100 in antibody diluent) to confirm the accuracy of the final concentrations. Initial titering experiments ensured all antibodies were used at optimal dilutions. Immunoreactivity was detected using biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), avidin biotin complex solution (Vector Laboratories), and 3,3′-diaminobenzidine (8-minute development; Sigma, St. Louis, MO), as described previously.17, 18, 26 Tissue sections from each case were processed simultaneously for each antibody staining to eliminate interrun staining variability. Omission of primary antibody was used to ensure that the specificity of the immunohistochemical signal was because of tau antibody reactivity, and, as expected, no signal was observed with antibody omission (data not shown), as previously noted.28 All images are representative of the typical pathological findings in each disease group.
Results
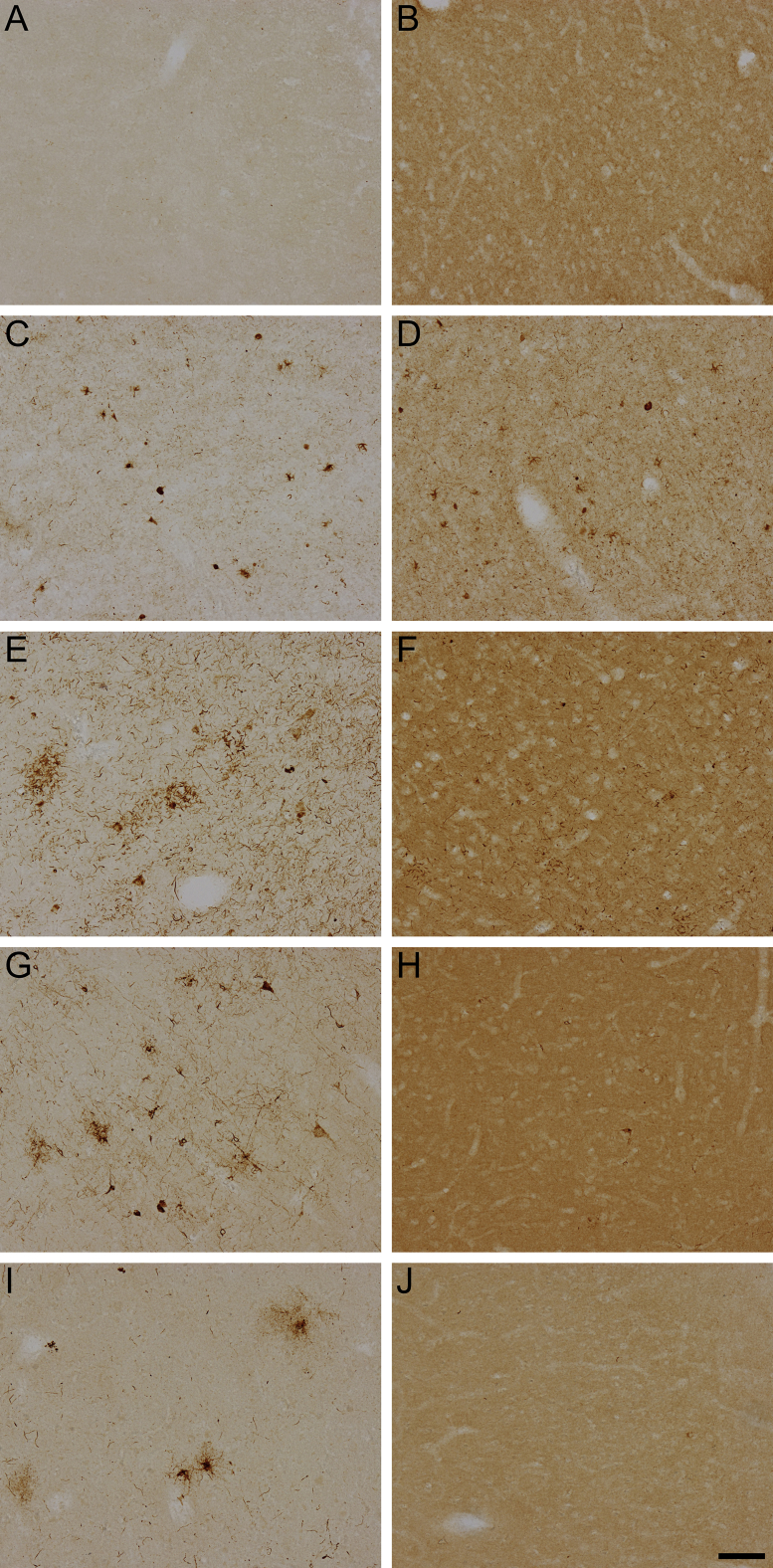
Our goal was to provide a detailed examination of whether exposure of PAD, a pathological conformation involving the N terminus of tau previously linked to a toxic mechanism, is a disease-related modification common to several non-AD tauopathies in humans. The TNT2 antibody was used to identify PAD-exposed tau, and Tau13 was used for comparisons to another N-terminal antibody that does not distinguish between normal and pathological forms of the protein. These antibodies were used in post-mortem human brain tissue sections from neuropathologically diagnosed cases of PiD, CBD, PSP, and CTE as well as control cases. In general, the degree of TNT2 reactivity and the pathologies it labeled were similar between cases within each disease diagnosis. Neither antibody detected tau pathology in the frontal cortex of control brains (Supplemental Figure S1, A and B).
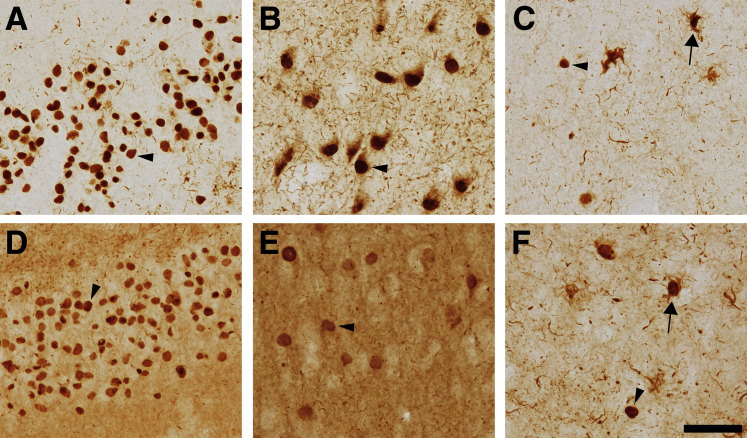
In PiD brains, TNT2 and Tau13 both clearly labeled Pick bodies in the dentate gyrus (Figure 1, A and D) as well as in pyramidal neurons of the hippocampus (Figure 1, B and E). The pyramidal neurons containing Pick bodies also displayed diffuse cytoplasmic patterns of staining surrounding the Pick bodies that were more prevalent with TNT2 than with Tau13. Pick bodies were also evident in the frontal cortex using each of the N-terminal tau antibodies (Figure 1, C and F, and Supplemental Figure S1, C and D). In addition, TNT2 detected neuritic threads as well as inclusions resembling ramified astrocytes, characterized by their thick processes and cortical localization, but did not label parenchymal tau (Figure 1C).5, 29 Tau13 robustly labeled parenchymal tau in all examined areas of the brain, as well as Pick bodies, threads, and ramified astrocytes. The difference in parenchymal staining supports the observation that Tau13 is unable to differentiate normal tau from pathological forms, whereas TNT2 specifically labels pathological tau.
Figure 1.
Pick disease pathology contains PAD-exposed tau. Post-mortem human brain tissue slices from PiD were examined with TNT2 and Tau13 antibodies. A–C: TNT2, a marker of PAD exposure, labels Pick bodies (arrowheads) and neuropil threads in the dentate gyrus (A) and pyramidal neurons (B) of the hippocampus, as well as the frontal cortex (C). C: TNT2-reactive pathology resembling ramified astrocytes is present in the frontal cortex (arrow). D and E: Tau13 labels Pick bodies (arrowheads) in the dentate gyrus (D) and pyramidal cell layers (E) and robustly labels parenchymal tau, which tends to obscure thread pathologies. F: Glial pathology is noted in the frontal cortex with Tau13 as well (arrow). n = 4. Scale bar = 50 μm.
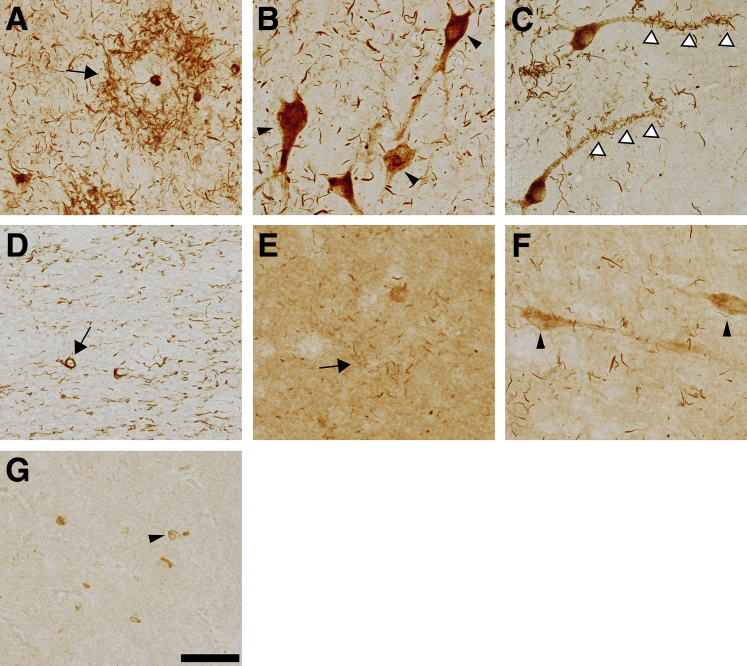
In CBD cases, TNT2 produced robust reactivity with the pathognomonic astrocytic tau plaques in the frontal cortex (Figure 2A and Supplemental Figure S1E). Tau13 detected tau pathology in CBD, but the labeling was not robust and the relatively high parenchymal tau staining with Tau13 may obscure finer thread inclusions (Figure 2E and Supplemental Figure S1F). In the hippocampus, TNT2 labeled diffuse pretangles in neurons (Figure 2B) but only rarely identified mature compact tangles. TNT2 also identified extensive neuropil threads in addition to thread pathology clustered along the apical dendrites of neurons in several of the observed cases (Figure 2C). Tau13 only weakly displayed diffuse pretangle pathology in the hippocampus along with relatively limited recognition of thread pathology (Figure 2F). TNT2 identified thread pathology in white matter of the frontal cortex along with occasional inclusions resembling oligodendritic coiled bodies (Figure 2D). Tau13 recognized cellular inclusions in white matter of the frontal cortex, but did not clearly identify the thread pathology in this area (Figure 2G).
Figure 2.
PAD exposure occurs in hallmark tau pathologies of corticobasal degeneration. Immunohistochemical staining of corticobasal degeneration (CBD) post-mortem human brain tissue sections was performed with TNT2 and Tau13 antibodies. A: TNT2 clearly labels the hallmark astrocytic plaques (arrow) characteristic of CBD in the frontal cortex, as well as extensive thread pathology. B: In the hippocampus, TNT2 labels diffuse, granular pretangle neuronal pathology (arrowheads), but few classic neurofibrillary tangles (not depicted). C: Some hippocampal neurons display clusters of thread pathology that appears to surround the apical dendrites of pyramidal cells (arrowheads). D: TNT2 stains coiled bodies (arrow) in the white matter of the frontal cortex as well as extensive thread pathology. E: Astrocytic plaques (arrow) are occasionally and weakly identified by Tau13 in the frontal cortex but are not clearly evident because of relatively strong labeling of parenchymal tau. F: Tau13 labels some neuritic threads and neuronal pathology (arrowheads) in the hippocampus. G: Tau13 identifies some cellular pathology (arrowhead) in the frontal cortex but is not effective at labeling thread pathology in this region. n = 4. Scale bar = 50 μm.
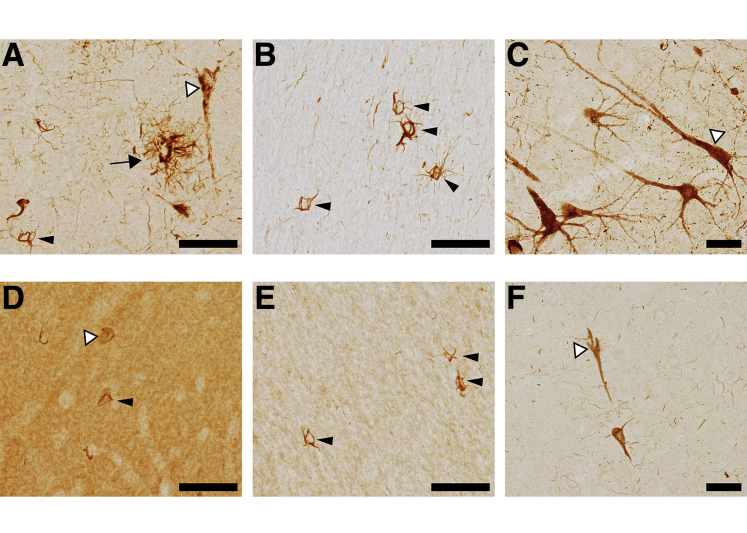
In PSP cases, TNT2 robustly labeled PAD exposure in the pathognomonic glial tau pathologies, including tufted astrocytes and coiled bodies, as well as early pretangle neuronal tau pathology that was largely diffuse and granular with some areas of more compact inclusions (Figure 3A and Supplemental Figure S1G). In the white matter of the frontal cortex, TNT2 identified large numbers of coiled bodies, a classic hallmark of PSP (Figure 3B). TNT2 also clearly detected diffuse tau pathology in hippocampal neurons and their proximal projections (Figure 3C). Tau13 strongly labeled parenchymal tau in the frontal cortical gray matter and showed relatively weak labeling of the neuronal and glial tau pathologies (Figure 3D and Supplemental Figure S1H). In the white matter, Tau13 labeled coiled bodies but with minimal labeling of thread pathology (Figure 3E). Neuronal pathology in the hippocampus was identified by Tau13, but the staining of projections was weak (Figure 3F).
Figure 3.
Progressive supranuclear palsy pathology is composed of PAD-exposed tau proteins. Post-mortem human brain tissue from progressive supranuclear palsy (PSP) cases were used for immunohistochemistry with TNT2 and Tau13. A: TNT2 labels various forms of tau pathology associated with PSP, including those resembling tufted astrocytes (arrow), coiled bodies (black arrowhead), and diffuse neuronal tau inclusions (white arrowhead) in frontal cortical gray matter. B: TNT2 also labels coiled bodies in frontal cortical white matter (arrowheads). C: Pretangle neuronal inclusions in the pyramidal layer of the hippocampus (white arrowhead) are identified by TNT2 and the pathology extended far into the neuronal processes. D: Tau13 poorly labels only a few coiled bodies (black arrowhead) and neuronal inclusions (white arrowhead) and strongly labels parenchymal tau in the gray matter of the frontal cortex. E: Coiled bodies (arrowheads) are also detected by Tau13 in the white matter of the frontal cortex. F: Neuronal inclusions (white arrowhead) in the hippocampus are also lightly stained by Tau13. n = 5. Scale bar = 50 μm.
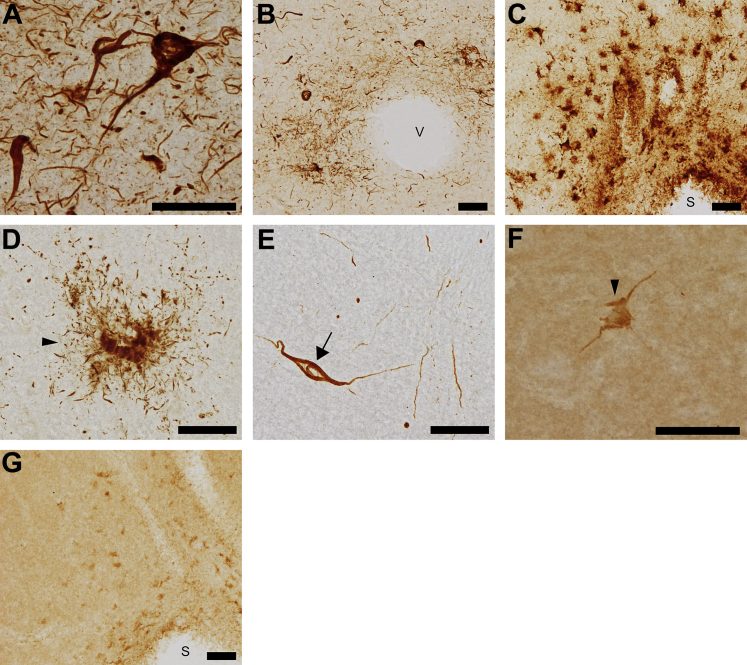
In cases of CTE, TNT2 labeled the pathognomonic tau pathologies of CTE in the frontal cortex. Numerous neuronal tangles and pretangles were identified along with pervasive thread pathology (Figure 4A and Supplemental Figure S1I). Two hallmarks of CTE are the presence of concentrated perivascular tau pathology and inclusions at the depths of sulci.30 TNT2 stained multiple forms of this pathology, including neuronal tangles, astrocytic plaques, and a large volume of threads, surrounding blood vessels (Figure 4B) as well as in the depths of sulci (Figure 4C) of the frontal cortex. TNT2 also detected PAD exposure in glia, including those resembling astrocytic plaques (Figure 4D) and occasional coiled bodies in white matter of the frontal cortex (Figure 4E), as well as cortical thread pathology. Tau13 did not label the tau inclusions in CTE well, which was evident by poor labeling of a neuronal inclusion (Figure 4F) and pathologies at the depth of sulci (Figure 4G). Again, Tau13 produced parenchymal tau staining and did not effectively label thread pathology in the frontal cortex of CTE cases (Figure 4, F and G, and Supplemental Figure S1J).
Figure 4.
PAD exposure is present in the characteristic tau pathology of chronic traumatic encephalopathy. Chronic traumatic encephalopathy human brain tissue was analyzed by TNT2 and Tau13 immunohistochemistry. A: Extensive tangle and thread pathology is detected by TNT2 in the frontal cortex. B: Tau-positive tangles, plaques, and threads in proximity to blood vessels (v) of the frontal cortex are detected by TNT2. C: TNT2 labels tau pathology concentrated at the depth of a sulcus (s) within the frontal cortex. D: Astrocytic plaques (arrowhead) are detected by TNT2 in the frontal cortex. E: TNT2 identifies PAD-exposed tau in thread pathology as well as coiled bodies (arrow) within white matter of the frontal cortex. F: Neuronal tau pathology (arrowhead) is weakly detected by Tau13, but the antibody also labels high levels of parenchymal tau. G: Tau13 faintly identifies tau pathology near a sulcus of the frontal cortex. n = 5. Scale bar = 50 μm.
Discussion
Misfolding and aggregation of tau are the common hallmarks of all tauopathies, including AD, PiD, CBD, PSP, and CTE, among others.1 However, there is significant diversity in inclusion morphology and location across this group of neurodegenerative diseases. Subtle differences in the conformation of the protein at a molecular level are potential causes of this morphological diversity. Conformational changes associated with pathological tau are of particular importance because they can serve as indicators of tau dysfunction and, more critically, can also play a specific role in tau toxicity. A growing body of evidence supports the hypothesis that one such conformational change, the aberrant exposure of PAD in the extreme N terminus of tau, can mediate toxicity through a disruption in anterograde fast axonal transport.17, 26, 31 Given that tau may display variable conformations among these diseases, we aimed to determine whether PAD exposure was part of the pathological sequelae of multiple non-AD tauopathies. Using immunohistochemistry and a conformation-specific antibody, we demonstrated that PAD exposure in the amino terminus of tau is common among the pathognomonic inclusions of PiD, CBD, PSP, and CTE. This indicates that the PAD of tau is exposed in multiple cell types and that this conformational change could play a role in tau-mediated toxicity in each of these diseases, similar to AD.18
TNT2 specifically identifies a conformational change at the amino terminus in aggregated recombinant tau, soluble and sarkosyl-insoluble fractions purified from AD frontal cortex lysates, as well as pretangle and tangle inclusions in post-mortem human tissue sections.17, 18 Much of this previous work was accomplished using the TNT1 antibody.17 Like TNT2, TNT1 is a conformation-specific antibody that also detects PAD exposure in pathological forms of tau. Herein, we reported data from TNT2 because TNT2 was determined to be a more sensitive reagent for detecting these changes in nondenaturing assays as well as in human AD brain tissue18 and both antibodies produced similar findings in the non-AD tauopathies examined (data not shown).
In these tauopathies, TNT2 precisely labeled tau pathologies, whereas Tau13, another antibody that binds to a partially overlapping region of the extreme N terminus of tau, did not. TNT2 recognizes an epitope within amino acids 7 to 12, whereas Tau13 binds to a discontinuous epitope slightly downstream of the TNT2 epitope at amino acids 8 to 9 and 13 to 21.18 Despite the close proximity of these epitopes, a notable difference in the ability of these antibodies to recognize normal and pathological tau was observed in the tauopathies. TNT2 does not label parenchymal tau (ie, normal tau), whereas Tau13 does so well, as noted previously in AD tissue.18 These differences in reactivity suggest that the conformational change leading to PAD display may be confined to a relatively small region within the protein.
Aside from differences in clinical presentation, the major underlying distinctions between tauopathies are the brain regions and cell types affected, the characteristic inclusions, and the tau isoforms involved in pathology.1 The detection of PAD exposure by TNT2 in all of the major pathological hallmarks of these tauopathies, including both neuronal and glial pathologies, reinforces the commonality of change in this region of tau across the multiple cell types affected. These findings support a role for PAD exposure as a mediator of tau-induced toxicity across multiple tauopathies as well. Varying ratios of tau isoforms comprise the pathologies of tauopathies, and we recently confirmed that all six adult human central nervous system isoforms of tau exhibit PAD exposure, oligomerization, and transport impairment, further supporting the potential common role of PAD exposure in the toxic effects of dysfunctional tau.20, 32, 33, 34, 35
In this model, aberrantly exposed PAD improperly initiates a protein phosphatase 1–glycogen synthase kinase 3 signaling cascade that disrupts kinesin-based motor activities by inducing the release of its cargo.17 Previously, this series of events has been considered in the context of disruptions to fast axonal transport in neurons, but the results presented herein demonstrate PAD exposure is present in glial cells as well (eg, in PSP, CBD, and CTE). Although we did not directly confirm the glial nature of the inclusions identified by TNT2 and Tau13, they closely resembled the pathognomonic glial inclusions in non-AD tauopathies.4, 5, 8, 10, 28, 36 Like neurons, glial cells also depend on kinesin-based transport for their normal functions, and altering kinesin activity in glia can negatively affect neuronal activity and myelination.23, 37 In addition, there is a growing awareness of the robust levels of communication and material transfer that exists between glial cells and neurons, a process that could potentially introduce pathological forms of glial tau into the axons of nearby neurons through the transfer of exosomes.38, 39 Further research into the mechanisms of tau toxicity in non-AD tauopathies could elucidate some of these questions, but it would not be surprising if disruptions of kinesin-based transport in glia could severely alter normal brain functions.
Although TNT2 recognition of PAD exposure allowed the antibody to be a robust identifier of pathological tau, Tau13 was less effective in most of the tauopathies. It is interesting that Tau13 was unable to readily detect pathology in CBD, PSP, and CTE because it did effectively label pathology in PiD and AD, even if the amount of parenchymal tau staining often obscured some of the less intense staining.18 Tau13 is a remarkably sensitive tau antibody with no previously demonstrated changes to affinity because of alterations in tau. Tau13's poor reactivity with tau inclusions in some tauopathies could indicate the existence of another unidentified conformational modification that obscures its epitope. This is in agreement with previous observations that Tau12, which binds the same N-terminal epitope as Tau13, also displays weak reactivity to pathology in CBD and PSP despite evidence that the amino terminus is intact in many of the inclusions (eg, TNT2 and Alz50 reactivity).40, 41
Alternatively, differences in post-translational modifications, such as phosphorylation status, of individual proteins or changes to tau isoform composition within the inclusion could further accentuate the TNT2 epitope or help to mask the Tau13 epitope in these tauopathies. The patterns of some modifications are already known to differ between AD and non-AD tauopathies. For example, nitration of the tyrosine 18 residue, located within the epitope of Tau13, is present in AD tau pathology, but antibodies directed toward nitrated tyrosine 18 did not label pathology in CBD or PSP brains.18, 42 This observation could be the result of conformation changes in the region, and further studies are needed to elucidate and clarify how the amino terminus of tau is modified among these various tauopathies. Interestingly, phosphorylation at tyrosine 18 occurred in more compact TNT1-reactive aggregates in AD brains and was found to prevent toxic effects of aberrant PAD exposure.26 Little is known about this modification and any potential differences in non-AD tauopathies.
Other modifications to the amino terminus may also explain why TNT2 primarily displayed pretangles and early neuronal tangles in CBD cases despite intense staining of mature, compact tangles by the PHF1 antibody (data not shown). Cleavage of the N-terminus is known to occur in mature neurofibrillary tangles of AD, and a similar effect may be occurring in CBD tangles, although many of the typical C-terminal truncations associated with AD tangles (recognized by Tau-C3 and MN423) are reduced or absent in many non-AD tauopathies.28, 43, 44, 45 The proteolytic processing of tau is reportedly different between CBD and PSP, but little is known about the progression of this process in non-AD tauopathies and what potential implications it might have for toxicity.46, 47
The TNT2 antibody, a reagent known to identify tau when PAD is conformationally displayed, was used in this study to demonstrate that PAD is aberrantly exposed in the major pathological hallmarks of multiple non-AD tauopathies, including both neuronal and glial pathologies. This supports PAD exposure as a common mechanism of tau dysfunction across several, if not all, tauopathies, and that PAD-induced alterations in kinesin-based transport could affect neuronal and glial functions.
Acknowledgments
We thank Tessa Grabinski for her technical assistance in purifying tau antibodies; and the Neuropathology Core in the Alzheimer Disease Core Center at Northwestern University (Chicago, IL) and the Boston University Center for the Study of Traumatic Encephalopathy (Boston, MA) for providing the human brain tissue.
Footnotes
Supported by NIH/National Institute on Aging grant R01 AG044372 (N.M.K.), NIH/National Institute of Neurological Diseases and Stroke grant R01 NS082730 (N.M.K.), the Jean P. Schultz Biomedical Research Endowment (N.M.K.), the Secchia Family Foundation Research Fund (N.M.K.), BrightFocus Foundation grant A2013364S (N.M.K.), the Department of Veterans Affairs, NIH/National Institute of Neurological Diseases and Stroke grant 1U01NS086659-01, National Institute of Aging Boston University Alzheimer's Disease Center grant P30AG13846 (supplement 0572063345-5), Northwestern Alzheimer's Disease Center grant AG13854, NADC Neuropathology Core Tissue Bank, and the Michigan Alzheimer's Disease Center grant P30AG053760.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.01.019.
Supplemental Data
Supplemental Figure S1.
Lower-magnification images of TNT2 and Tau13 reactivity in control and non-Alzheimer disease tauopathies. Images from immunohistochemical staining of frontal cortical sections by TNT2 and Tau13 were obtained using a 10× objective lens. In general, neither TNT2 (A) nor Tau13 (B) identifies tau pathology in control brain sections from the frontal cortex. C: TNT2 specifically labels tau pathology in Pick's disease (PiD) brains. D: Tau13 labels tau pathology and parenchymal tau in the PiD frontal cortex. E: TNT2 detects pathological tau in corticobasal degeneration (CBD). F: Tau13 is less able to recognize tau pathology in the frontal cortex of CBD but displays robust parenchymal tau staining. G: TNT2 identifies a variety of neuronal and glial forms of tau pathology in progressive supranuclear palsy (PSP). H: Tau13 does not demonstrate any specificity for pathological tau in PSP. I: In cases of chronic traumatic encephalopathy (CTE), TNT2 is able to specifically recognize pathognomonic tau pathology J: Tau13 is generally unable to identify tau pathology with any specificity in CTE cases. Scale bar = 100 μm (A–J).
References
- 1.Kovacs G.G. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41:3–23. doi: 10.1111/nan.12208. [DOI] [PubMed] [Google Scholar]
- 2.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Braak E., Braak H., Mandelkow E.M. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 4.Komori T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick's disease. Brain Pathol. 1999;9:663–679. doi: 10.1111/j.1750-3639.1999.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwatsubo T., Hasegawa M., Ihara Y. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol. 1994;88:129–136. doi: 10.1007/BF00294505. [DOI] [PubMed] [Google Scholar]
- 6.Probst A., Tolnay M., Langui D., Goedert M., Spillantini M.G. Pick's disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol. 1996;92:588–596. doi: 10.1007/s004010050565. [DOI] [PubMed] [Google Scholar]
- 7.Pollock N.J., Mirra S.S., Binder L.I., Hansen L.A., Wood J.G. Filamentous aggregates in Pick's disease, progressive supranuclear palsy, and Alzheimer's disease share antigenic determinants with microtubule-associated protein, tau. Lancet. 1986;2:1211. doi: 10.1016/s0140-6736(86)92212-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T., McGeer P.L., McGeer E.G. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135:99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 9.Hauw J.J., Verny M., Delaere P., Cervera P., He Y., Duyckaerts C. Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy: basic differences with Alzheimer's disease and aging. Neurosci Lett. 1990;119:182–186. doi: 10.1016/0304-3940(90)90829-x. [DOI] [PubMed] [Google Scholar]
- 10.Feany M.B., Dickson D.W. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146:1388–1396. [PMC free article] [PubMed] [Google Scholar]
- 11.McKee A.C., Stein T.D., Nowinski C.J., Stern R.A., Daneshvar D.H., Alvarez V.E., Lee H.S., Hall G., Wojtowicz S.M., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., Cantu R.C. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geddes J.F., Vowles G.H., Nicoll J.A., Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 13.Carmel G., Mager E.M., Binder L.I., Kuret J. The structural basis of monoclonal antibody Alz50's selectivity for Alzheimer's disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 14.Hyman B.T., Van Hoesen G.W., Wolozin B.L., Davies P., Kromer L.J., Damasio A.R. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 1988;23:371–379. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- 15.Jicha G.A., Bowser R., Kazam I.G., Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Jicha G.A., Berenfeld B., Davies P. Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer's disease. J Neurosci Res. 1999;55:713–723. doi: 10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Kanaan N.M., Morfini G.A., LaPointe N.E., Pigino G.F., Patterson K.R., Song Y., Andreadis A., Fu Y., Brady S.T., Binder L.I. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combs B., Hamel C., Kanaan N.M. Pathological conformations involving the amino terminus of tau occur early in Alzheimer's disease and are differentially detected by monoclonal antibodies. Neurobiol Dis. 2016;94:18–31. doi: 10.1016/j.nbd.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiernan C.T., Combs B., Cox K., Morfini G., Brady S.T., Counts S.E., Kanaan N.M. Pseudophosphorylation of tau at S422 enhances SDS-stable dimer formation and impairs both anterograde and retrograde fast axonal transport. Exp Neurol. 2016;283:318–329. doi: 10.1016/j.expneurol.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox K., Combs B., Abdelmesih B., Morfini G., Brady S.T., Kanaan N.M. Analysis of isoform-specific tau aggregates suggests a common toxic mechanism involving similar pathological conformations and axonal transport inhibition. Neurobiol Aging. 2016;47:113–126. doi: 10.1016/j.neurobiolaging.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson K.R., Ward S.M., Combs B., Voss K., Kanaan N.M., Morfini G., Brady S.T., Gamblin T.C., Binder L.I. Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport. Biochemistry. 2011;50:10300–10310. doi: 10.1021/bi2009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marszalek J.R., Weiner J.A., Farlow S.J., Chun J., Goldstein L.S. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J Cell Biol. 1999;145:469–479. doi: 10.1083/jcb.145.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt I., Thomas S., Kain P., Risse B., Naffin E., Klambt C. Kinesin heavy chain function in Drosophila glial cells controls neuronal activity. J Neurosci. 2012;32:7466–7476. doi: 10.1523/JNEUROSCI.0349-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson J.H., Worboys K., Ainger K., Barbarese E. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil Cytoskeleton. 1997;38:318–328. doi: 10.1002/(SICI)1097-0169(1997)38:4<318::AID-CM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Potokar M., Kreft M., Li L., Daniel Andersson J., Pangrsic T., Chowdhury H.H., Pekny M., Zorec R. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanaan N.M., Morfini G., Pigino G., LaPointe N.E., Andreadis A., Song Y., Leitman E., Binder L.I., Brady S.T. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33:826.e15–826.e30. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoshal N., Garcia-Sierra F., Wuu J., Leurgans S., Bennett D.A., Berry R.W., Binder L.I. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 28.Kanaan N.M., Cox K., Alvarez V.E., Stein T.D., Poncil S., McKee A.C. Characterization of early pathological tau conformations and phosphorylation in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2015;75:19–34. doi: 10.1093/jnen/nlv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feany M.B., Mattiace L.A., Dickson D.W. Neuropathologic overlap of progressive supranuclear palsy, Pick's disease and corticobasal degeneration. J Neuropathol Exp Neurol. 1996;55:53–67. doi: 10.1097/00005072-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Stein T.D., Alvarez V.E., McKee A.C. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther. 2014;6:4. doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaPointe N.E., Morfini G., Pigino G., Gaisina I.N., Kozikowski A.P., Binder L.I., Brady S.T. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buee L., Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol. 1999;9:681–693. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrer I., Lopez-Gonzalez I., Carmona M., Arregui L., Dalfo E., Torrejon-Escribano B., Diehl R., Kovacs G.G. Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol. 2014;73:81–97. doi: 10.1097/NEN.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 34.Sergeant N., Wattez A., Delacourte A. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem. 1999;72:1243–1249. doi: 10.1046/j.1471-4159.1999.0721243.x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–470. doi: 10.1111/j.1440-1789.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 36.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyons D.A., Naylor S.G., Scholze A., Talbot W.S. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat Genet. 2009;41:854–858. doi: 10.1038/ng.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fruhbeis C., Frohlich D., Kuo W.P., Kramer-Albers E.M. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman B.M., Hill A.F. Extracellular vesicles: their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol. 2015;40:89–96. doi: 10.1016/j.semcdb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Berry R.W., Sweet A.P., Clark F.A., Lagalwar S., Lapin B.R., Wang T., Topgi S., Guillozet-Bongaarts A.L., Cochran E.J., Bigio E.H., Binder L.I. Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol. 2004;33:287–295. doi: 10.1023/B:NEUR.0000044190.96426.b9. [DOI] [PubMed] [Google Scholar]
- 41.Feany M.B., Ksiezak-Reding H., Liu W.K., Vincent I., Yen S.H., Dickson D.W. Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol. 1995;90:37–43. doi: 10.1007/BF00294457. [DOI] [PubMed] [Google Scholar]
- 42.Reyes J.F., Reynolds M.R., Horowitz P.M., Fu Y., Guillozet-Bongaarts A.L., Berry R., Binder L.I. A possible link between astrocyte activation and tau nitration in Alzheimer's disease. Neurobiol Dis. 2008;31:198–208. doi: 10.1016/j.nbd.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickson D.W., Ksiezak-Reding H., Liu W.K., Davies P., Crowe A., Yen S.H. Immunocytochemistry of neurofibrillary tangles with antibodies to subregions of tau protein: identification of hidden and cleaved tau epitopes and a new phosphorylation site. Acta Neuropathol. 1992;84:596–605. doi: 10.1007/BF00227736. [DOI] [PubMed] [Google Scholar]
- 44.Bondareff W., Harrington C., Wischik C.M., Hauser D.L., Roth M. Immunohistochemical staging of neurofibrillary degeneration in Alzheimer's disease. J Neuropathol Exp Neurol. 1994;53:158–164. doi: 10.1097/00005072-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Guillozet-Bongaarts A.L., Glajch K.E., Libson E.G., Cahill M.E., Bigio E., Berry R.W., Binder L.I. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta Neuropathol. 2007;113:513–520. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- 46.Arai T., Ikeda K., Akiyama H., Tsuchiya K., Yagishita S., Takamatsu J. Intracellular processing of aggregated tau differs between corticobasal degeneration and progressive supranuclear palsy. Neuroreport. 2001;12:935–938. doi: 10.1097/00001756-200104170-00014. [DOI] [PubMed] [Google Scholar]
- 47.Arai T., Ikeda K., Akiyama H., Nonaka T., Hasegawa M., Ishiguro K., Iritani S., Tsuchiya K., Iseki E., Yagishita S., Oda T., Mochizuki A. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–79. doi: 10.1002/ana.10793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.